Abstract
Nemours effective management tactics were used to reduce world crop losses caused by plant-parasitic nematodes. Nowadays the metallic nanoparticles are easily developed with desired size and shape. Nanoparticles (NPs) technology becomes a recognized need for researchers. Ecofriendly and biosafe SiNPs are developed from microorganisms. Recently, silicon nanoparticles (SiNPs) have gained novel pesticide properties against numerous agricultural pests. This study assessed the biosynthesis of SiNPs from Fusarium oxysporum SM5. The obtained SiNPs were spherical with a size of 45 nm and a negative charge of −25.65. The nematocidal effect of SiNPs against egg hatching and second-stage juveniles (J2) of root-knot nematode (RKN) (Meloidogyne incognita) was evaluated on eggplant,Solanum melongena L. plants. In vitro, all tested SiNPs concentrations significantly (p ≤ 0.05) inhibited the percentage of egg hatching at a different time of exposure than control. Meanwhile, after 72 h, the percent mortality of J2 ranged from 87.00 % to 98.50 %, with SiNPs (100 and 200 ppm). The combination between SiNPs and the half-recommended doses (0.5 RD) of commercial nematicides namely, fenamiphos (Femax 40 % EC)R, nemathorin (Fosthiazate 10 % WG) R, and fosthiazate (krenkel 75 % EC) R confirmed the increase of egg hatching inhibition and J2 mortality after exposure to SiNPs (100 ppm) mixed with 0.5 RD of synthetic nematicides. The findings suggest that the combination between SiNPs, and 0.5 RD of nematicides reduced nematode reproduction, gall formation, egg masses on roots and final population of J2 in the soil. Therefore, improving the plant growth parameters by reducing the M. incognita population.
Keywords: Meloidogyne incognita, Silicon nanoparticles, Nematicidal activity, Synthetic nematicides, Plant growth, Eggplant
1. Introduction
Yield production, mainly in tropical and subtropical areas, has been negatively affected by plant parasitic nematodes, which cause almost 20 % of crop losses (Sasser, 1987, Gohar and Maareg, 2005). The root-knot nematodes (RKNs) (Meloidogyne spp.), which have over 100 legal species, are the most damaging nematodes (Trinh et al., 2019). They cause an estimated $100 billion loss/year worldwide (Khan et al., 2008). The RKNs have a high reproductive potential and a wide host range, therefore, their control is partly hard (Hussain et al., 2016). Traditional control methods were used to manage plant-parasitic nematodes, i.e., fallow, crop rotation, cultivation of resistant varieties, and chemical nematicides. However, they are both expensive and environmentally unfriendly methods, however using alternative natural materials were effective, safe (El-Saadony et al., 2021, Saad et al., 2015, Saad et al., 2021) and have several biological activities (Saad et al., 2021, Swelum et al., 2020). Alternatives approaches were used in controlling nematodes such as plant growth-promoting rhizobacteria (PGPR) as a safe and sustainable method (Khan et al., 2012, El-Ashry et al., 2019, Hegazy et al., 2019, El-Saadony et al., 2021a). As well as nematicidal effects of some botanical plants against Meloidogyne spp. (Refaat et al.,2020), organic soil amendments (Oka et al., 2000, Siddiqui and Futai, 2009), antagonistic fungi and a PGPR (Siddiqui and Akhtar, 2008).
Actually, eco-friendly and environmentally safe methods are increased by using available nanotechnology materials, which offer promising results in managing plant diseases like RKN nematode, M. incognita (Sharon et al., 2010).
The use of nanotechnology for the controlled delivery of agrochemicals has been used to suppressed the development of phytopathogens (Khan and Rizvi, 2014). Additionally, the ultra-small size and large surface area of NPs reduce the nutrient loss in fertilization and reduce the amount of sprayed chemicals (Prasad et al., 2017, El-Saadony et al., 2021b). Nanoparticles are also identified to promote plant growth and development (El-Argawy et al., 2017, El-Saadony et al., 2021b). Metal nanoparticles play an important role in biological applications due to their unique properties (Sheiha et al., 2020, Abd El-Hack et al., 2021, El-Saadony et al., 2021c, El-Saadony et al., 2021d). The synthesis of nanomaterials is one of the most demanding and highest increasing nanotechnology sectors (El-Saadony et al., 2020a, Reda et al., 2021). Physical, chemical, and biological methods are available for nanoparticle synthesis. Both physical and chemical methods may effectively synthesize pure and well-defined nanoparticles, but these methods are expensive and considered unsafe to the environment (Prasad et al., 2017, El-Saadony et al., 2021e, El-Saadony et al., 2021f). The use of biological mass such as bacteria, fungi, yeast, plant extract or plant biomass, and algae extract, or biomass could be an alternative to these methods for the synthesis of nanoparticles in an eco-friendly manner, safe, less time consuming, and low cost (El-Saadony et al., 2019, Reda et al., 2020, El-Saadony et al., 2021g, Abdel-Moneim et al., 2022).
Recent studies showed the nematocidal effect of several nanoparticles, i.e., silver nanoparticles (Saad et al., 2021), gold nanoparticles (Thakur et al. 2018), silica carbide nanoparticles (Al Banna et al. 2018) and copper nanoparticles (Mohamed et al. 2019) were used against root-knot nematodes. The nematicidal effect of silver nanoparticles was well-established. Hence it was proposed as a viable alternative nematicide (Cromwell et al. 2014).
SiNPs exhibit great potential in agriculture (Tripathi et al., 2015, Abdel-Haliem et al., 2017, Cui et al., 2017), where they have a direct impact on plant growth. SiNPs can also be used as nano pesticides, nano herbicides, and nano fertilizers. Furthermore, they may be used as carriers for delivering molecules like proteins, nucleotides, and other chemicals in plants (Rastogi et al., 2019, Bapat et al., 2020). Moreover, the multifunction of SiNPs on the plant, its evaluation for controlling the agricultural pests has been demonstrated. Bapat et al. (2020) found that the higher concentration of SiNPs retarded the larval growth of Helicoverpa armigera. Furthermore, SiNPs had an entomotoxic effect against rice weevil Sitophilus oryzae (Debnath et al. 2011). Slight studies on SiNPs, which were chemically synthesized, were assessed against nematodes, exhibited incompatible findings.
In this regard, the present investigation aimed to biosynthesize SiNPs from Fusarium oxysporum, evaluating their nematocidal potential on egg hatching and juvenile mortality of RKN M. incognita. Moreover, the combination of SiNPs with 0.5 RD of three traditional nematicides, fenamiphos (Femax 40 % EC)R, nemathorin (Fosthiazate 10 % WG) R, and fosthiazate (krenkel 75 %EC) R was evaluated, and its impact on eggplant growth (brinjal/aubergine), Solanum melongena L. under greenhouse conditions.
2. Materials and methods
2.1. Isolation and identification of fungal isolate
Soil samples were collected from several regions in Sharkia Governorate, Egypt, and kept in plastic bags, then transferred to the laboratory. In brief, 10 g of soil were homogenized in 90 ml sterile peptone buffer (1 g peptone, 8.5 g NaCl) to obtain 10-1 dilution (Alagawany et al., 2021a, El-Saadony et al., 2022). Serial dilutions from the previous dilution (10-1) were prepared up to 10-7 (Abdelnour et al., 2020, Ashour et al., 2020, Desoky et al., 2020a, Desoky et al., 2020b, El-Saadony et al., 2020b, Alagawany et al., 2021b). 100 µL of each dilution was inoculated to a sterilized solidified Potato Dextrose agar (PDA) plates supplemented with different concentrations of potassium silicafloride (K2SiF6) (10, 20, 30, 40, and 50 mg L-1) (Ahmad et al. 2006). The plates were incubated at 28 °C for five days and were monitored daily for the appearance of fungal colonies, according to maximum tolerable concentration (MTC). The MTC was considered the maximum concentration of the metal ions that allowed fungal growth. The highest metal tolerant fungus was identified by morphological tests and MALDI-TOF (Schumaker et al., 2012, Sauget et al., 2017).
2.2. Biosynthesis and characterization of silica nanoparticles
The isolated fungus was inoculated in Malt extract Glucose Yeast extract Peptone (MGYP) media (3 g malt extract, 10 g glucose, 3 g yeast extract and 5 g peptone) and incubated at shaking incubator (250 rpm /30 °C) for four days. Later, the flask was centrifuged under cooling at (5000 rpm /20 min) and the fungal mycelium was obtained and washed trice with sterile deionized water. The collected mycelia mass (20 g) was used for the SiNPs biosynthesis (Ahmad et al. 2003) with some modifications, the harvested mycelia mass (20 g) was homogenized in 100 ml aqueous solutions of 40 mg/L K2SiF6 (pH 4) and incubated at shaking incubator (250 rpm/ 30 °C). After incubation, SiNPs biosynthesis in the reaction mixture was filtrated to obtain the supernatant containing SiNPs.
The absorption of obtained SiNPs was estimated by UV–vis spectrophotometer and freeze-drying at −60℃ for 2d. Further characterization by Transmission electron microscope (TEM), DLS analysis (Zeta sizer and Zeta potential) and Energy Dispersive X-Ray Analysis (EDX). In control experiments, the fungal biomass was resuspended in sterile deionized water in the absence of an aqueous solution of K2SiF6 and the filtrate obtained after that was characterized by UV–vis spectrophotometer. In another control experiment, the aqueous solution of K2SiF6 in sterile deionized water in the absence of fungal biomass, SiNPs, didn’t obtain in both reactions.
2.3. Optimization of the physiochemical parameters for SiNPs biosynthesis
The size of SiNPs was optimized by various parameters one at a time according to El-Saadony et al. (2021e) i.e. pH (2, 4, 6, 8, and 10), temperature (15, 20, 25, 30, 35 °C), K2SiF6 concentration (10, 20, 30, 40, and 50 mg/L), reaction time (1, 2, 3, 4, and 5d), media type (Potato dextrose broth (PDB), Malt extract Glucose Yeast extract Peptone (MGYP), Sabouraud’s broth (SB), Czapeck Dox medium (CDM), and Richard medium (RM), fungal biomass (10, 15, 20, 25, and 30 g), movement (static, and shacking), and agitation speed (100, 150, 200, 250, and 300 rpm). The particle size of the biosynthesized SiNPs was determined using the Dynamic light scattering (DLS) technique.
2.4. Nematicidal assessment
Current experiments were conducted at the Nematodes Laboratory of Plant Protection Department, Faculty of Agriculture, Zagazig University. Eggplants (cv. Local) provided by Salhyia Company for Agriculture Investments, Egypt. Eggplants were grown under greenhouse conditions, and the plants were exposed to M. incognita inoculum or tested nanoparticles with a temperature of 25 ± 4 °C, photoperiod 18:6, D, L, and relative humidity of 68 ± 5 %. The replicated experiments were carried out at the beginning of summer 2020. All treatments and controls in this study were in five replicates.
2.4.1. Nematicides
The three commercial nematicides are available in Egyptian markets, includingfenamiphos (Femax 40 % EC)R, nemathorin (Fosthiazate 10 % WG) R, and fosthiazate (krenkel 75 %EC) R and applied at the rates of 0.2 ml/plant, 12.5 kg/fed and 20 kg/Fed, respectively. The tested nematicides were obtained from the Central Laboratory of Pesticides, Dokki, Giza, Egypt.
2.4.2. Meloidogyne incognita inoculum Preparation
A single egg mass was used to establish a population of root-knot nematode, Meloidogyne incognita on eggplants (Solanum melongena L.) susceptible cultivar Super Strain B for the current experiments. Nematode species were identified based on the Perineal pattern region RKN females and infective juveniles (IJs) measurements (Jepson 1987). Free eggs and J2 were extracted from the infected roots for inoculation of plants (El-Ashry et al. 2019).
2.5. In vitro assessment of SiNPs
2.5.1. SiNPs assay on eggs hatching
In vitro experiments were conducted to test toxicity of different concentrations (50, 100, 150, 200, 250, 300, and 350 ppm) of SiNPs on free eggs hatching. Number of free eggs was adjusted to 2000 eggs/ ml by letting eggs settle naturally for 10 min. An aliquot of 0.1 ml containing about 200 eggs were pipetted into 5 cm sterilizer Petri dishes containing 10 ml of SiNPs concentrations and kept at 24 ± 2˚C for ten days. Petri dishes were examined daily for egg hatching inhibition for 12, 24, 48, 72, 120 and 168 h. Viable eggs and active J2 juveniles were scored as live members while immotile eggs or J2 were allowed to recover in tap water for 5 h and the number of hatched J2 was expressed as a cumulative number of viable J2. The number of hatching eggs in control petri dishes received only 10 ml of distilled water. The effect of SiNPs treatment on egg hatching inhibition were calculated compared to control treatment according to the following equation:
| (1) |
2.5.2. SiNPs assay on M. incognita Juveniles
The nematocidal effect of SiNPs concentrations (50, 100, 150, 200, 250, 300, and 350 ppm) was tested on J2 of M. incognita at 24 ± 2˚C. 100 µL of J2 suspension (200 J2) was added to 10 ml of SiNPs concentrations in petri plates, additionally, 10 ml of distilled water in control plates. Juvenile mortality was evaluated at interval of (12, 24, 48, 72, and 120 h) post-treatment. J2 showed inactive straight posture or did not show any movement after prodding was considered dead (De Nardo & Grewal 2003). Mortality counts were observed under 100 X magnification in 1 ml over the specified periods. Immobile juveniles were sieved, collected and allowed to recover by washing in distilled water for 5 h (Saad et al., 2021). The mortality percent was calculated according to the following equation:
| (2) |
2.6. Interactive effects of SiNPs and nematicides mixtures in Vitro
The interactive effects of SiNPs and 0.5 RD of three nematicides fenamiphos (Femax 40 % EC)R, nemathorin (Fosthiazate 10 % WG) R, and fosthiazate (krenkel 75 %EC) R were evaluated against free eggs hatching and juveniles’ mortality of M. incognita.
2.6.1. Nematocidal effect of mixtures on free eggs
Following experiments were used to assess the nematocidal effect of 0.5 RD of tested nematicides mixed with SiNPs concentrations compared with RD of nematicides on egg hatching of M. incognita.
The free eggs from infected roots were extracted by Hussey (1973). About 200 nematode eggs in 0.1 ml of distilled water were exposed to 10 ml of RD or 0.5 RD of nematicides SiNPs mixture (50, 100, 175, 200, 250, 300, 350 ppm). Immotile eggs or J2 were scored as dead, any movement counted as viable. Control treatment dishes were received only 10 ml of nematicides RD. In comparison, petri dishes of other treatments contained 0.5 RD of 10 ml tested nematicides and SiNPs (1: 1, v: v) and allowed to immotile eggs or J2 to recover for 5 h and the number of hatched J2 was expressed as a cumulative number of viable J2 (Talavera-Rubia et al., 2020). All treatments were left under room temperature (24 ± 3 ˚C). Percentages of egg hatching inhibition were calculated by equation (2).
2.6.2. The nematicidal effect on juvenile mortality
Control or treated juveniles were left under room temperature (24 ± 3 ˚C) to assess the effect of nematicides and SiNPs on juvenile mortality. Tested materials were observed daily for J2 mortality. Second-stage juveniles (J2) showed inactive straight posture or did not show any movement after prodding was considered dead (De Nardo & Grewal 2003). Mortality observed under 100 X magnification in 1 ml over the specified periods. The mortality percentages were calculated as equation (2).
2.7. Experimental design of in vivo SiNPs activity against M. incognita
When seedlings were nearby 20 cm tall with four leaves of local variety of eggplants, seedlings transplanted to in plastic pots of 15 cm diameter containing 1.5 kg mixture of sterilized sandy soil (75 % sand, 15 % clay, 5 % silt), 50 g peat moss and 3 mg urea fertilizer per kg of soil were added to pots. After one week, each seedling was inoculated with 1000 newly hatched IJs from a pure culture of M. incognita by pipetting 2 ml of the inoculum suspension into 4 holes around the root system, which were directly covered with 5 g of moist sandy soil. All plastic pots treatments were arranged in a completely randomized design with five replicates.
Negative control treatment (healthy plants) was without nematode, SiNPs and nematicides whereas, positive control treatment contained plants infected by IJs of M. incognita only. In the current investigation, plants are grouped into 5 treatments. 30 ml of RD of three nematicides fenamiphos (Femax 40 % EC)R, nemathorin (Fosthiazate 10 % WG) R, and fosthiazate (krenkel 75 %EC) R were incorporated with the upper 5 cm of soil around each plant. As well as 30 ml of 0.5 RD of the mentioned nematicides mixed separately with four concentrations (50, 100, 150, 200 ppm) of SiNPs (1:1; v: v) were incorporated with the upper 5 cm of soil around each plant. All plants in the greenhouse were incubated at 24 ± 4 °C, and all received similar horticultural treatments.
After 60 days, plant growth parameters (fresh shoot weight, fresh root weight, stem diameter, number of leaves and plant height and the nematode parameters (number of galls, number of egg masses and number of IJs /250 g soil and, root gall index (RGI), egg masses index (EI) and reproduction factor (RF) were evaluated. To calculate the reproduction factor (RF), the final population of nematode (the number of eggs and number of J2 in soil) divided into the initial population (1000 IJs). Gall diameter measurement was assessed according to El-Deeb et al. (2018). Also, samples of 100 g soil were processed for nematode extraction using a combination of sieving and Baermann trays technique (Hooper 1990).
All uprooted eggplants were wrapped in tissue paper to avoid their dryness and numbers of galls and egg masses were counted. The parameters changing the percentage of increase or decrease imputed to “negative or positive” control values and the current equations were used.
| (3) |
| (4) |
2.8. Statistical analysis
A completely randomized design was used in the experiment. All the data were subjected to one-way analysis of variance (ANOVA) using Gen Stat Package version released in 2009 (12th edition) version 12.1.0.3278 (www.vsni.co.uk). Means were compared by Duncan’s multiple range test at p ≤ 0.05 probability (Duncan 1955).
3. Results
3.1. Isolation and identification of fungal isolate
Table 1 showed eleven fungal isolates appeared in PDA plates supplemented with K2SiF6 concentration (10, 20, and 30 mg L-1), six fungal isolates were obtained at PDA plates supplemented with K2SiF6 concentration (40 mg L-1), and one fungal isolate was appeared in PDA plates supplemented with K2SiF6 concentration (50 mg L-1), this isolate coded as SM5. According to morphological and biochemical tests, this isolate was identified as Fusarium oxysporum. The identification was confirmed with MALDI-TOF technique where the obtained results showed that selected fungi had maximum similarity to several Fusarium species (99%), especially Fusarium oxysporum. Thus, the local screened fungal isolate Fusarium oxysporum SM5, is similar to Fusarium oxysporum DSM 841.
Table 1.
Fungal growth on PDA medium supplemented with K2SiF6 concentrations.
| Isolate code | Fungal growth on PDA / K2SiF6 concentration (mg L-1) |
||||
|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | |
| SM1 | +++ | +++ | ++ | – | – |
| SM2 | +++ | +++ | ++ | + | – |
| SM3 | +++ | +++ | + | – | – |
| SM4 | +++ | +++ | ++ | – | – |
| SM5 | +++ | +++ | +++ | ++ | + |
| SM6 | +++ | +++ | + | + | – |
| SM7 | +++ | +++ | ++ | – | – |
| SM8 | +++ | +++ | + | + | – |
| SM9 | +++ | +++ | ++ | + | – |
| SM10 | +++ | +++ | ++ | + | + |
| SM11 | +++ | +++ | + | – | – |
+++, excellent growth; ++, very good growth; +, good growth; - no growth.
3.2. Optimization and characterization of silica nanoparticles
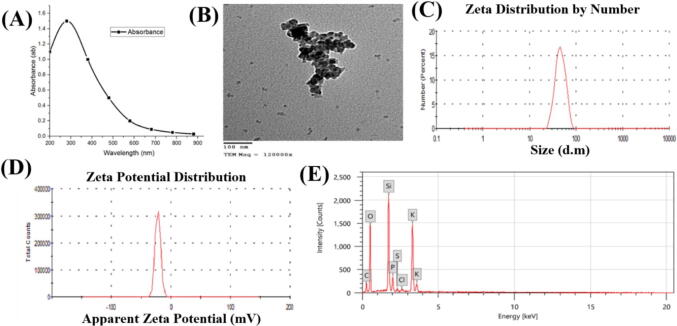
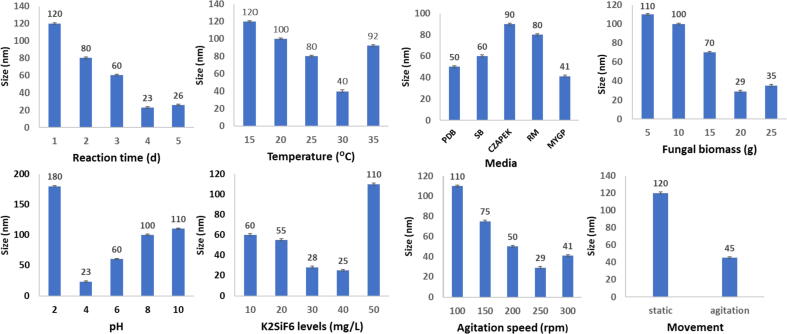
The optimum condition for SiNPs manufacture was cleared in Fig. 1. The best conditions were 30 mg L-1 substrate mixed with 20 g fungal biomass in MGYP media, pH 4, temperature 30 °C, in shacking incubator (250 rpm) for four days. These optimal conditions give SiNPs ranged from 25 to 38 nm and complete oxidation of the K2SiF6 ions by F. oxysporum SM5 occurs after nearly 96 h of reaction.
Fig. 1.
Characterization of silica nanoparticles fabricated by Fusarium oxysporum SM5, (A) UV absorbance at 280 by UV Spectrophotometer, (B) SiNPs size ranged 20–55 nm by TEM, (C) size of SiNPs 45 nm by zeta seizer, (D) SiNPs Charge of −25.56 mV, (E) the element that accompanied SiNPs in media detected by EDX.
Fig. 2 showed that the myogenic SiNPs supernatant absorbed UV–Vis spectra at 280 nm. The crystalline SiNPs have an average size range of 30–55 nm was obtained by TEM. Based on DLS analysis, the zeta potential of SiNPs has a net negative charge on their surface (-25.65 mV) and 45 nm. EDX showed various elements, i.e., O, C, Cl, Si, P beside SiNPs, in the media used in fungal growth
Fig. 2.
Optimization of the physiochemical parameters for SiNPs biosynthesis by Fusarium oxysporum SM5.
3.3. In vitro assessment
3.3.1. Larvicidal and ovicidal effect of SiNPs on M. incognita J2 and eggs
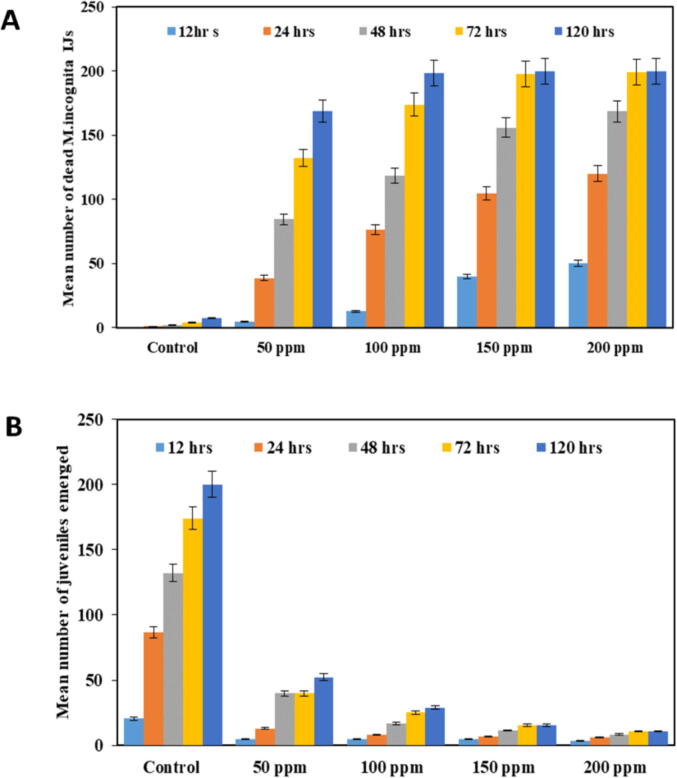
The obtained results from single treatment of SiNPs showed that M. incognita J2 mortality increased gradually with increasing of SiNPs concentrations and exposure time (Fig. 3A). After 12 h, the mortality percent of IJs was 25% at 300 ppm. As the exposure time increased from 24 h to 72 h after treatments, the larvicidal effect of SiNPs was increased. After 72 h, concentrations of 100, 150 and 200 ppm exhibited the perfect larvicidal effect on J2 mortality ranged from 87.00 %, 98.8 %, and 99.70 %, respectively in petri dishes. After 120 h of treatment, the percent mortality reached 98 and 100 % with 100 and 150 ppm concentrations (Fig. 3a). On the other hand, non-positive correlation was found between the larvicidal effect of SiNPs and the used concentrations higher than 200 ppm.
Fig. 3.
Mean number of dead M. incognita IJs and number of juveniles emerged treated with 50 ,100 ,150 ,200, 250, 300 and 350 ppm of SiNPs after 12 ,24, 48, 72 and 120 h.
As shown in (Fig. 3b), the results indicated that egg hatching inhibition was proportioned with tested SiNPs concentration at different exposure time intervals. The number of emerged J2 decreased as SiNPs concentrations increased compared with control. Mainly, effective concentrations ranged from 100 to 250 ppm exhibiting significant hatching inhibition of more than 50 %.
3.3.2. Comparative effects of SiNPs mixed with 0.5 RD of nematicides
3.3.2.1. Larvicidal efficiency against M. incognita J2
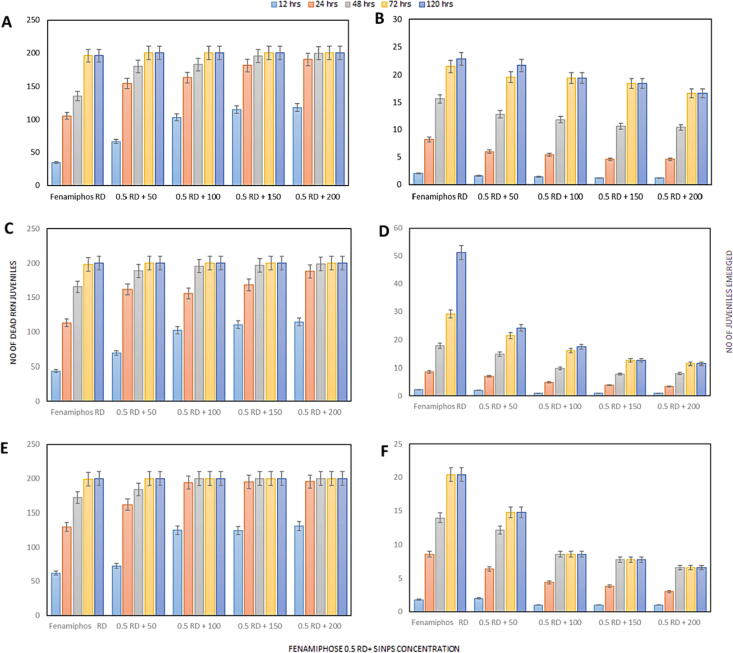
Fig. 4a showed the larvicidal effect of SiNPs concentrations when mixed with 0.5 RD of three nematicides included fenamiphos, fosthiazate and krenkel, compared with their RD on J2 of M. incognita at different times of exposure.
Fig. 4.
Comparative effect of different concentrations of SiNPs mixed with 0.5 RD of nematicides, fenamiphos, fosthiazate and Krenkel on mean number of dead M. incognita IJs (a1, a2, a3) and number of juveniles emerged (b1, b2, b3) after 12 ,24, 48, 72 and 120 h. after treated.
Generally, after 12 h, all treatments mixtures showed a significant increase of J2 mortality compared with RD of the nematicides. In particular, the concentration 200 ppm for the three nematicides exhibited an increase over 50% of J2 mortality. On the other hand, the larvicidal effect of the tested SiNPs was directly proportional to concentration and exposure time. For example, after 24 h, treatments of SiNPs (100, 175 and 200 ppm) mixed with fenamiphos (Fig. 4a1) exhibited J2 mortality of 97.00 %, 97.50 % and 97.70 %, respectively. While J2 mortality were 77.80%, 84.20% and 93.90 %, respectively, when same concentration of SiNPs mixed with fosthiazate (Fig. 4a2) and the equivalent values with SiNPs and krenckel mixtures were 81.70 %, 90.60 % and 95.10 %, respectively (Fig. 4a3).
As well as the exposure time increased from 24 to 48 h after treatments, the larvicidal effect of the concentration 200 ppm of SiNPs alone was increased from 60.10 % (24 h) to 84.30 % (48 h) as shown in (Fig. 3a), however when same concentration mixed with 0.5 RD of the three nematicides separately, the fast action in 100% killing of M. incognita J2 was 48 h later (Fig. 4a1,2,3).
3.3.2.2. Ovicidal efficiency against M. incognita eggs
SiNPs with 0.5 RD of nematicides mixtures showed a significantly superior effect against the egg hatching of M. incognita more than RD rate of nematicides alone. After 12 h of exposure, the RD of nematicides (fenamiphos, fosthiazate and krenkel) showed ovicidal effect recording emerged juveniles of 1.80, 2.20 and 2.00, respectively. While the emerged juveniles number decreased gradually when 0.5 RD of the three nematicides mixed individually with SiNPs concentrations reaching to 0.4 at 350 ppm (Fig. 4b1.2,3).
After 24 h of treatment, all tested RD of nematicides showed little reduction in egg hatching inhibition comparing with the 0.5 RD mixed with the highest concentration of SiNPs. Egg hatching inhibition percent of SiNPs and 0.5 RD fenamiphos mixtures ranged from 25.58 in treatment of 50 ppm to 86.04 in treatment of 350 ppm (Fig. 4b1). The parallel values of egg hatching inhibition percentage with fosthiazate and krenkel were 18.6 (26.83) in treatment of 50 ppm of SiNPs to 81.39 (68.29) in treatment of 350 ppm of SiNPs, respectively (Fig. 4b2,3). The same trend was observed after.
Generally, the rate of egg hatching inhibition was proportionated with concentration and time of exposure. On the other hand, as exposure time increased after 12 h to 24 h of treatments, ovicidal effect and egg hatching inhibition percent reached the peak then obviously decreased gradually from 48 h to 120 h at all the tested SiNPs concentrations mixtures except fosthiazate.
Our results indicated that counting the number of emerged juveniles from eggs in Petri dishes treated with SiNPs and nematicides as shown in (Fig. 4b), at all tested times, significantly reduced at all tested concentrations compared with the control (RD of fenamiphos, fosthiazate, and krenkel).
3.4. In vivo activity of nematicides and various concentrations of SiNPs on J2 infection and plant growth (pot experiments)
3.4.1. Combined use of SiNPs with 0.5 RD of fenamiphos
Table 2, Table 3, Table 4, Table 5, Table 6, Table 7 illustrate the effects of four SiNPs concentrations (50, 100, 150 and 200 ppm) mixed with 0.5 RD of the three tested nematicides compared to their RD were evaluated on M. incognita J2 infection and plant growth of eggplant (Solanum melongena L.) in pots under greenhouse conditions.
Table 2.
Effect of various SiNPs concentrations in comparison with fenamiphos RD and their biomass changes of eggplant infected with Meloidogyne incognita in the greenhouse tests.
| Treatments | Fresh root weight |
Fresh shoot weight |
Number of leaves/plant |
Stem diameter (mm or cm) |
Plant height (cm) |
|---|---|---|---|---|---|
| (Increase %) | (Increase %) | (Increase %) | (Increase %) | (Increase %) | |
| Healthy plants | 9.46 a | 20.29 a | 13.00a | 8.60 a | 24.40a |
| Plants infected with M. incognita | 5.11e | 10.77d | 6.40d | 5.00e | 16.80f |
| M. incognita + RD fenamiphos | 8.26b | 17.48b | 12.00a | 7.40bc | 22.80b |
| (61.64) | (62.30) | (87.50) | (48.00) | (35.71) | |
| M. incognita + 50 ppm SiNPs + 0.5 RD fenamiphos | 6.36 d | 15.50c | 9.20c | 5.80d | 18.60e |
| (24.46) | (43.91) | (43.75) | (16.00) | (10.71) | |
| M. incognita + 100 ppm SiNPs + 0.5 RD fenamiphos | 6.94 cd | 15.90c | 10.40b | 6.80c | 20.00d |
| (35.81) | (47.63) | (62.50) | (36.00) | (19.04) | |
| M. incognita + 150 ppm SiNPs + 0.5 RD fenamiphos | 7.18c | 16.94b | 11.40b | 7.80b | 21.20c |
| (40.50) | (57.28) | (78.12) | (56.00) | (26.19) | |
| M. incognita + 200 ppm SiNPs + 0.5 RD fenamiphos | 7.92b | 17.13b | 11.60a | 8.60a | 22.60b |
| (54.99) | (59.05) | (81.24) | (72.00) | (34.52) |
*Each value is a mean of five replicates.
Means followed by the same letter (s) in each column indicates no significant differences at p ≤ 0.05 according to Duncan's multiple range test.
Table 3.
Effect of various SiNPs concentrations in comparison with RD of fenamiphos on galling and reproduction of Meloidogyne incognita infected eggplant in relation to total weight under greenhouse tests.
| Treatments |
Total plant weight |
Root parameters |
Soil parameters |
||||
|---|---|---|---|---|---|---|---|
| No. of galls |
No. of egg masses |
Root Gall Index (RGI) and Egg mass Index (EI) |
Final population/250 soil |
Reproduction factor(RF = Pf/Pi) |
|||
| (Increase %) | (Reduction %) | (Reduction %) | RGI | EI | (Reduction) | ||
| Plants infected with M. incognita | 15.88d | 122.20a | 175.20a | 5.00a | 5.00a | 456.00a | 2.73 |
| M. incognita RD of fenamiphos | 25.75a | 24.20 e | 35.40 de | 3.00d | 3.80b | 136.60b | 0.81 |
| (62.15) | (80.19) | (79.79) | (70.04) | ||||
| M. incognita + 50 ppm SiNPs + 0.5 RD fenamiphos | 22.06c | 50.60b | 63.60b | 4.00b | 4.00b | 88.80c | 0.53 |
| (38.91) | (58.59) | (63.69) | (80.52) | ||||
| M. incognita + 100 ppm SiNPs + 0.5 RD fenamiphos | 22.84c | 39.60c | 47.20c | 4.00b | 4.00b | 70.20d | 0.42 |
| (43.82) | (67.59) | (73.05) | (84.60) | ||||
| M. incognita + 150 ppm SiNPs + 0.5 RD fenamiphos | 24.12b | 32.00 d | 40.00 d | 3.60c | 4.00b | 42.20e | 0.25 |
| (51.88) | (73.81) | (77.16) | (90.74) | ||||
| M. incognita + 200 ppm SiNPs + 0.5 RD fenamiphos | 25.05 a | 25.60 e | 32.40 e | 3.00d | 3.75b | 29.00e | 0.17 |
| (57.74) | (79.05) | (81.50) | (93.64) | ||||
0: No galls or egg masses, 1: 1–2 galls or egg masses, 2: 3–10 galls or egg masses, 3: 11–30 galls or egg masses, 4: 31–100 galls or egg masses and 5: more than 100 galls or egg masses, according to the scale given by Taylor and Sasser (1978).
*Each value is a mean of five replicates.
Means followed by the same letter (s) in each column indicates no significant differences at p ≤ 0.05 according to Duncan's multiple range test.
Table 4.
Effect of various SiNPs concentrations in comparison with fosthiazate RD and their biomass changes of eggplant infected with Meloidogyne incognita in the greenhouse tests.
| Treatments | Fresh root weight |
Fresh shoot weight |
Number of leaves/plant |
Stem diameter (mm) |
Plant height (cm) |
|---|---|---|---|---|---|
| (Increase %) | (Increase %) | (Increase %) | (Increase %) | (Increase %) | |
| Healthy plants | 9.46a | 20.29a | 12.00a | 8.60 a | 24.40 a |
| Plants infected with M. incognita | 5.11 d | 10.77 e | 6.40 e | 5.00 d | 16.80f |
| M. incognita + fosthiazate RD | 7.86b | 17.26b | 11.80ab | 7.40b | 22.00 bc |
| (53. 81) | (60.25) | (84.37) | (48.00) | (30.95) | |
| M. incognita + 50 ppm SiNPs + 0.5 RD | 5.44d | 14.56d | 8.60 d | 5.60 d | 18.60 e |
| (6.45) | (35.19) | (34.37) | (12.00) | (10.71) | |
| M. incognita + 100 ppm SiNPs + 0.5 RD | 6.16c | 15.96c | 9.40d | 6.60c | 20.00 d |
| (20.54) | (48.18) | (46.87) | (32.00) | (19.04) | |
| M. incognita + 150 ppm SiNPs + 0.5 RD | 6.42c | 16.49 bc | 10.60c | 7.40b | 21. 20 cd |
| (25.63) | (53.11) | (65.62) | (48.00) | (26.19) | |
| M. incognita + 200 ppm SiNPs + 0.5 RD | 6.71c | 16.86b | 11.80ab | 7.60b | 22.60b |
| (31.31) | (56.54) | (84.37) | (52.00) | (34.52) |
*Each value is a mean of five replicates.
Means followed by the same letter (s) in each column indicates no significant differences at P ≤ 0.05 according to Duncan's multiple range test.
Table 5.
Effect of various SiNPs concentrations in comparison with RD of fosthiazate on galling and reproduction of Meloidogyne incognita infected eggplant in relation to total weight under greenhouse tests.
| Treatments |
Total plant weight |
Root parameters |
Soil parameters |
||||
|---|---|---|---|---|---|---|---|
| No. of galls |
No. of egg masses |
RGI and EI |
Final population /250 soil |
Reproduction factor (RF = Pf/Pi) |
|||
| (Increase %) | (Reduction %) | (Reduction %) | RGI | EI | (Reduction %) | ||
| Plants infected with M. incognita | 15.43e | 122.20 a | 175.20 a | 5.00 a | 5.00a | 456.00 a | 2.73 |
| M. incognita + RD of fosthiazate | 25.53a | 33.00 e | 49.80 e | 3.60b | 4.00b | 144.20b | 0.86 |
| (65.45) | (72.99) | (71.57) | (68.37) | ||||
| M. incognita + 50 ppm SiNPs + 0.5 RD | 17.43d | 88.00b | 103.60b | 4.00b | 4.80a | 146.00b | 0.87 |
| (12.96) | (27.98) | (40.86) | (67.98) | ||||
| M. incognita + 100 ppm SiNPs + 0.5 RD | 18.76c | 65.00c | 77.00c | 4.00b | 4.00b | 90.40c | 0.54 |
| (21.58) | (46.80) | (56.05) | (80.17) | ||||
| M. incognita + 150 ppm SiNPs + 0.5 RD | 20.23b | 48.60 d | 61.00 d | 4.00b | 4.00b | 66.00 cd | 0.39 |
| (31.10) | (60.22) | (65.18) | (85.52) | ||||
| M. incognita + 200 ppm SiNPs + 0.5 RD | 21.02b | 33.60 e | 44.00 e | 3.60b | 4.00b | 53.00d | 0.31 |
| (36.22) | (72.50) | (74.88) | (88.37) | ||||
0: No galls or egg masses, 1: 1–2 galls or egg masses, 2: 3–10 galls or egg masses, 3: 11–30 galls or egg masses, 4: 31–100 galls or egg masses and 5: more than 100 galls or egg masses, according to the scale given by Taylor and Sasser (1978).
*Each value is a mean of five replicates.
Means followed by the same letter (s) in each column indicates no significant differences at p ≤ 0.05 according to Duncan's multiple range test.
Table 6.
Effect of various SiNPs concentrations in comparison with krenkel RD and their biomass changes of eggplant infected with Meloidogyne incognita in the greenhouse tests.
| Treatments | Fresh root weight |
Fresh shoot weight |
Number of leaves/ plant |
Stem diameter (mm or cm) |
Plant height (cm) |
|---|---|---|---|---|---|
| (Increase %) | (Increase %) | (Increase %) | (Increase %) | (Increase %) | |
| Healthy plants | 9.46 a | 20.29 a | 13.00 a | 8.60 a | 24.40 a |
| positive control infected with RNK M. incognita | 5.11e | 10.77 e | 6.40 e | 5.00c | 16.80d |
| M. incognita + RD of krenkel | 7.95b | 16.65b | 11.40b | 7.00b | 21.00b |
| (55.57) | (54.59) | (78.12) | (40.00) | (25.00) | |
| M. incognita + 50 ppm SiNPs + 0.5 RD | 5.86 d | 13.04d | 8.40 d | 6.80b | 17.00 d |
| (14.67) | (21.07) | (31.25) | (36.00) | (1.19) | |
| M. incognita + 100 ppm SiNPs + 0.5 RD | 67 | 14.92c | 9.40c | 7.40b | 18.60c |
| (19.56) | (38.52) | (46.87) | (48.00) | (10.71) | |
| M. incognita + 150 ppm SiNPs + 0.5 RD | 7.03c | 16.14b | 11.80b | 8.60 a | 20.80b |
| (37.57) | (49.86) | (76.56) | (72.00) | (23.80) | |
| M. incognita + 200 ppm SiNPs + 0.5 RD | 7.10c | 15.90b | 11.80b | 8.40 a | 21.20b |
| (38.94) | (47.63) | (76.56) | (68.0) | (26.19) |
*Each value is a mean of five replicates.
Means followed by the same letter (s) in each column indicates no significant differences at p ≤ 0.05 according to Duncan's multiple range test.
Table 7.
Effect of various SiNPs concentrations in comparison with RD of krenkel on galling and reproduction of Meloidogyne incognita infected eggplant in relation to total weight under greenhouse tests.
| Treatments |
Total plant weight |
Root parameters |
Soil parameters |
||||
|---|---|---|---|---|---|---|---|
| No. of galls |
No. of egg masses |
Root Gall Index (RGI) and Egg mass Index (EI) |
Final population /250 soil |
Reproduction factor (RF = Pf/Pi) |
|||
| (Increase %) | (Reduction %) | (Reduction %) | RGI | EI | (Reduction %) | (Reduction %) | |
| Plants infected with M. incognita | 15.88 e | 122.20a | 175.20 a | 5.00a | 5.00a | 456.00a | 2.73 |
| M. incognita RD of krenkel | 24.58 a | 32.60d | 41.00 e | 3.80b | 4.00b | 136.60b | 0.81 |
| (54.78) | (73.32) | (76.50) | (70.04) | ||||
| M. incognita + 50 ppm SiNPs + 0.5 RD | 18.93d | 56.00b | 82.40b | 4.00b | 4.00b | 95.60c | 0.57 |
| (19.20) | (54.17) | (52.96) | (79.03) | ||||
| M. incognita + 100 ppm SiNPs + 0.5 RD | 20.79c | 46.80c | 63.60c | 4.00b | 4.00b | 74.20 d | 0.44 |
| (30.91) | (61.70) | (63.69) | (83.72) | ||||
| M. incognita + 150 ppm SiNPs + 0.5 RD | 23.15b | 42.60c | 53.60 d | 4.00b | 4.00b | 61.00 de | 0.36 |
| (45.78) | (65.13) | (69.40) | (86.62) | ||||
| M. incognita + 200 ppm SiNPs + 0.5 RD | 23.08b | 28.60d | 36.00 e | 3.00c | 3.80b | 51.80 e | 0.31 |
| (45.34) | (76.59) | (79.45) | (88.64) | ||||
0: No galls or egg masses, 1: 1–2 galls or egg masses, 2: 3–10 galls or egg masses, 3: 11–30 galls or egg masses, 4: 31–100 galls or egg masses and 5: more than 100 galls or egg masses, according to the scale given by Taylor and Sasser (1978).
*Each value is a mean of five replicates.
Means followed by the same letter (s) in each column indicates no significant differences at p ≤ 0.05 according to Duncan's multiple range test.
Table 2 presented the application of SiNPs concentrations combined with 0.5 RD of Fenamiphos significantly (p ≤ 0. 05) increased the growth of eggplants (as specified by fresh root weight, fresh shoot weight, number of leaves, stem diameter and plant height) compared to eggplants infected with M. incognita alone.
Pots treated with RD of fenamiphos surpassed those treated with SiNPs concentrations combined with 0.5 RD. Among the tested SiNPs concentrations, 200 ppm combined with 0.5 RD displayed the best results, followed by 150 ppm, while 50 ppm concentration showed the least plant growth.
Fresh root and shoot weight in pots treated with RD of fenamiphos were 8.26 and 17.48 and pots treated with 200 ppm of SiNPs mixtures were 7.9 and 17.13, respectively. On the other hand, these values in pots with infected plants were 5.11 and 10.77 and in pots treated with 50 ppm of SiNPs mixture were 6.36 and 15.50, respectively.
The morphological properties of treated eggplant with fenamiphos i.e., stem diameter and plant height recorded 48 and 72 % as compared to SiNPs (200 ppm) mixture recorded 35.71% and 34.52%, respectively. For plant growth parameters, it was clear that combined use of 200 ppm SiNPs concentration enhanced the eggplant's growth to a certain extent compared to RD of fenamiphos whereas, it significantly surpassed fenamiphos action in stem diameter (Table 2).
Eggplants’ seedlings grown in soil which treated with different concentrations of SiNPs combined with 0.5 RD of fenamiphos significantly (p ≤ 0. 05) suppressed M. incognita galling (number of galls) and reproduction (number of J2 in pot soil and reproduction factor) when compared with the control and RD of fenamiphos (Table 3). Average over the two runs of five replicates, the reduction percent of galls number was increased from 58.59% to 79.05% after the individual treatments of 50 ppm and 200 ppm of SiNPs mixtures with fenamiphos as compared to treatments of fenamiphos alone was 80.19%. In the same way, the egg masses were reduced from 63.69% to 81.50% as compared to treatments of fenamiphos alone was 79.79% as shown in (Table 3).
Root gall index (RGI) and egg mass index (EI) in pots treated with RD fenamiphos and 200 ppm SiNPs were 3.00 (3.80) and 3.0 (3.75), respectively. All tested SiNPs and fenamiphos mixtures' concentrations exceeded fenamiphos treatment alone in reducing final population J2 in soil. Percent reduction in the final J2 population in treatments of four SiNPs concentrations compared with RD of fenamiphos were 80.52, 84.60, 90.74, 93.64 and 70.04%, respectively. Also, the reproduction factor (RF) decreased significantly from 2.73 in control treatment to 0.81and 0.17 in RD of fenamiphos and 200 ppm of SiNPs, respectively. Among the tested SiNPs concentrations, 200 ppm gave the best results, followed by 150 ppm, while 50 ppm showed lower nematicidal effect than fenamiphos alone.
3.4.2. Combined use of SiNPs with 0.5 RD of fosthiazate
Treatments of eggplants affected the infection by M. incognita J2. All treatments of SiNPs concentrations significantly (P ≤ 0. 05) enhanced the plant growth with respect to the control under greenhouse experiments (Table 4).
Up to 50% increase was achieved in fresh shoot weight and stem diameter by applying 200 ppm of SiNPs concentration combined with 0.5 RD of fosthiazate. RD of fosthiazate alone exceeded all treatments of SiNPs combined with 0.5 RD of fosthiazate in fresh root weight and fresh shoot weight of eggplants.
The treatment of SiNPs (200 ppm) combined with 0.5 RD displayed the best results, followed by 150 ppm, while 50 ppm concentration showed the lowest plant growth.
A maximum increase in plant growth parameters was obtained in stem when pots treated with 200 ppm of SiNPs combined with 0.5 RD fosthiazate compared to other treatments, which was 52.00%. Also, percent increase in treatment of eggplants height compared to infected plants was 34.52 and 30.95 % with 200 ppm SiNPs and RD of Fosthiazate, respectively (Table 4).
All SiNPs concentrations combined with 0.5 RD of fosthiazate significantly (P ≤ 0. 05) inhibited M. incognita galling (number of galls) and reproduction (IJs in soil and reproduction factors) and varied according to tested concentrations (Table 5). From obtained results, the increased SiNPs concentration from 50 ppm to 200 ppm reduced number of galls (% reduction) from 27.98% to 72.50%. The parallel values of reduction percent with egg masses were 40.86% and 74.88%. Concerning RGI and EI, pots treated with 200 ppm of SiNPs gained the same index with RD of fosthiazate which were 3.60 for RGI and 4.00 for EI. Moreover, all four SiNPs concentrations surpassed the RD of fosthiazate in decreasing the final J2 population in soil, the percent reductions were 67.98, 80.17, 85.52, 88.37 and 68.37 %, respectively.
The tested SiNPs concentrations decreased the reproduction of M. incognita. The reproduction factor (RF) in infected eggplants was 2.73 while those in 50 and 200 ppm were 0.87 and 0.31. Among the tested SiNPs concentrations, 200 ppm gave the best results followed by 150 ppm while 50 ppm showed the lowest nematicidal effect as compared to fosthiazate alone. Even though the decrease in root infection parameters and soil infection parameters, the total plant weight in fosthiazate-treated pots overwhelmed those treated with SiNPs combined with 0.5 RD of fosthiazate.
3.4.3. Combined use of SiNPs with 0.5 RD of krenkel
As can be detected from Table 6, the application of krenkel caused a significant increase (P ≤ 0. 05) in fresh root weight surpassed the mixtures of SiNPs concentrations and 0.5 RD of krenkel. The percent increase of fresh root weight for RD of krenkel and tested SiNPs concentrations i.e., 50, 100, 150 and 200 ppm were 55.57, 14.67, 19.56, 37.57 and 38.94%, respectively. Similarly, fresh shoot weight in RD krenckel treatments exceeded other treatments, as shown in (Table 6).
On the other hand, slightly insignificant differences were detected in percent increase of leaves number between RD of krenkel treatment and SiNPs 200 ppm combined with 0.5 RD of krenkel when they were compared which they were 78.12 and 76.56%, respectively. It is worth mentioning that, among the applied SiNPs concentrations, 200 ppm sustained the best results in plant growth parameters besides, no significant difference with krenckel treatment. It was followed descending by 150 ppm SiNPs concentration.
Data in Table 7 showed the effect of the four mentioned SiNPs concentrations compared to RD of Krenkel on RKN, M. incognita reproduction. It was found that all treatments significantly (P ≤ 0.05) reduced numbers of galls and egg masses compared to check treatment. Krenkel treatment surpassed all applications except 200 ppm SiNPs concentration with insignificant variations. Conversely, other concentrations slightly decreased the number of galls and egg masses. Significant differences were detected between the other three concentrations under investigation treatments compared with each other and check control.
Reduction percent in descending order for 200 ppm SiNPs, Krenkel, and 150 ppm SiNPs were 76.59 (79.45%), 73.32 (76.50 %) and 65.13(69.40%) with galls and egg masses, respectively.
Regarding the effects on RGI and EI, krenckel treatment and SiNPs concentrations significantly decreased the RGI and EI numbers compared to infected eggplants. Percent reduction in final J2 population ranged from 70.04% in krenkel treatment to 88.64 % in the treatment of 200 ppm SiNPs concentration. Also, the reproduction factor (RF) decreased significantly from 0.81 in krenkel treatment to 0.31 in the treatment of 200 ppm of SiNPs. Contrarily, total plant weight in pots treated with krenkel overwhelmed those treated with SiNPs combined with 0.5 RD of krenckel.
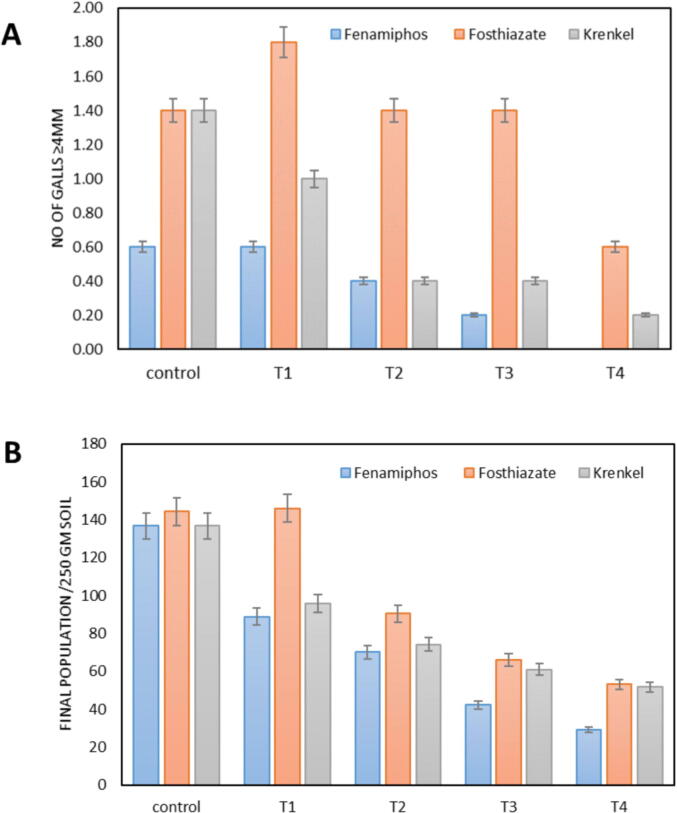
Generally, the treatment of SiNPs combined with 0.5 RD of fenamiphos, fosthiazate and krenkel and their RD significantly (p ≤ 0. 05) reduced galls formation (galls ≥ 4 mm) and final nematode population (Fig. 5). In treatments of RD of nematicides (fenamiphos, fosthiazate and krenkel), several galls ≥ 4 mm were 0.6, 1.4 and 1.4, respectively. The treated eggplant's roots with 100, 150 and 200 ppm combined with 0.5 RD of fenamiphos showed a reduction in the number of galls ≥ 4 mm recording 0.4, 0.2 and 0.0, respectively. The parallel values in pots treated with fosthiazate were 1.4, 1.4 and 0.6, respectively and with krenkel were 0.4, 0.4 and 0.2, respectively. To minimize the number of galls ≥ 4 mm, galling was reduced the most (0.0, 0.6 and 0.2) when 200 ppm of SiNPs was used with the treated nematicides. Regarding the effects of SiNPs, on the final population of M. incognita in pot soils, all the tested SiNPs concentrations, significantly minimized their numbers to 29, 53 and 51.8 in treatments of 200 ppm SiNPs with fenamiphos, fosthiazate and krenkel, respectively (Fig. 5).
Fig. 5.
Comparative effects of SiNPs concentrations (50, 100, 150 and 200 ppm) combined with 0.5 RD of three traditional nematicides with their RDs on number of galls ≥ 4 mm and final population (Pf). Control (RKN + RD), T1 (RKN + 0.5RD + Si50), T2 (RKN + 0.5RD + Si100), T3 (RKN + 0.5RD + Si150), T4 (RKN + 0.5RD + Si200).
4. Discussion
4.1. Biosynthesis, optimization and characterization of SiNPs from Fusarium oxysporum SM5
Microorganisms such as bacteria, fungus, and yeast play a precious role in the biosynthesis of metal and metal oxide NPs. Various recent stuides employing diverse microorganism models in nanoparticles fabrication (Akl et al., 2020, El-Saadony et al., 2020a, El-Saadony et al., 2021e, El-Saadony et al., 2021f, Abd El-Hack et al., 2021). In this study the tolerant fungus isolate with K2SiF6 concentration (50 mg L-1), was identified as Fusarium oxysporum SM5 (Gaddeyya et al. 2012), and has the ability to produce silicon nanoparticles. The resultant NPs by fungal enzyme were clear from fungal cells, besides, they were mono-dispersed (Raliya and Tarafdar, 2013).
The optimum conditions to produce silicon nanoparticles must be considered to obtain the maximum yield of nanoparticles (El-Saadony et al., 2018). Ahmed et al. (2016), and Shahzad et al. (2019) stated that the increase of fungal biomass leads to an increase of the reaction time and that lead to the increase of nanoparticles size because of the extensive content of fungal enzyme reduced all available silica ions in the media. Also, the nanoparticles characterization is important step to ensure the biotransformation of silicon nanoparticles (El-Saadony et al.,2021e). Nematodes cause biotic stresses such as salinity stress in plants (Elrys et al, 2019; Elrys et al., 2020). In our study, besides the effect of RD of nematicides, Si NPs have a strong lethal effect on M. incognita J2 and a parasitic efficiency when used with minimum effective dose of nematicides and confirmed by number of galls, egg masses and final population of J2 in pot soils as evidence to M. incognita reproduction (Shekoohi et al, 2021). Results of this study is one of the numerous attempts to use new available nanotechnology to increase treasured of current nematicides against the top of five major plant pathogens (Meloidogyne spp.) and the first among of the ten most important genera of plant parasitic nematodes in the world (Mukhtar et al., 2018).
4.2. Nematicidal efficiency of Si NPs against M. incognita
Our results showed that the bio-synthesized SiNPs with size of 30–55 nm induced the mortality of free eggs and J2 of the RKN, M. incognita, in vitro. The obtained results from SiNPs showed that M. incognita J2 mortality increased gradually as SiNPs concentrations increased. Conversely, Al Banna et al., (2018) reported that silicon carbide nanoparticles (SiC NPs) of the size of 50 nm ± 21.5 (with a concentration of 172 mg/L) did not exhibit a lethal effect even on J2 or egg hatching of M. incognita. However, SiC NPs affected the viability of first-stage larvae of C. elegans (Al Banna et al., 2018). Likewise, Ardakani, (2013) reported that silica oxide nanoparticles (SiO2 NPs) did not exhibit any M. incognita J2 mortality in laboratory experiments.
On the other hand, the positive effect of the different nanoparticles against M. incognita has been reported in several investigations. For instance, silver nanoparticles (30–150 μg/mL) caused inactive J2 of M. incognita (Cromwell et al. 2014). Taha and Abo-Shady (2016) found the larvicidal activity of a high concentration of AgNPs (1500 ppm) achieved 96.5% mortality after 72 h (Roh et al., 2009, Lim et al., 2012).
These contradictory results may attribute to nanoparticle toxicity depends on the physicochemical characteristics (Pourchez et al. 2012) and the synthesis origin, whether chemical or biological synthesis of the nanoparticles (El-Saadony et al. 2020a). Besides, the agglomeration of chemically synthesized nanoparticles may be involved in the lack of lethal efficiency of the nanoparticles (Gudikandula & Charya Maringanti 2016). Furthermore, the toxicity mechanisms of SiNPs were investigated on the nematode C. elegans. SiNPs induced the premature aging phenotype of C. elegans by accumulating insoluble proteins and amyloid-like proteins and reducing pharyngeal pumping (Scharf et al., 2013, Scharf et al., 2016). This toxicity mechanism is also supported by Liang et al. (2020), who demonstrated that the high exposure concentration of mesoporous silica nanoparticles induced neurotoxicity in C. elegans nematodes. The toxicity of nanoparticles is not a species-specific (Hamed et al. 2019); hence, these findings on C. elegans may contribute to explain our results on M. incognita.
4.3. Nematicidal efficiency of combined synthetic nematicides (SN) and Si NPs
The combination between SiNPs and 0.5 RD of nematicides illustrated that all applied concentrations of SiNPs increased egg hatching inhibition rate and induced a marked J2 mortality of M. incognita, in vitro. Our results represented that the action of 0.5 RD and SiNPs is exceeded the RD of synthetic nematicides efficacy, similar observation was reported by Rastogi et al. (2019). The obtained results revealed that the bio-synthesized SiNPs could improve the synthetic nematicides efficacy and facilitate their delivery (Rai and Ingle 2012). The mode of action of nanoparticles was attributed to cellular mechanisms malfunction which permit cell wall penetration of the nematode eggs (Sharon et al. 2010). The same inhibitory effect was obtained from other nanoparticles, when AgNPs combined with the commercial nematicides fenamiphos and oxamyl (Hassan et al. 2016). AgNPs synthesis by natural fabrication of nanoparticles such as microorganisms or plant extracts could be used as an eco-friendly alternative for chemical and physical approaches reducing the use of synthetic nematicides (Ahmed et al. 2016). In Egypt, up till now, synthetic nematicides are utilized in controlling plant-parasitic nematodes (PPN). So, nanoparticles have numerous advantages for RKN, M. incognita management, such as their greater chemical reactivity (Bhattacharyya et al. 2010).
4.4. The effect of SiNPs combined with synthetic nematicides (SN) on plant growth and M. incognita parameters
Our results under the greenhouse conditions suggest that the efficacy of SiNPs and traditional nematicide combination on the reduction of root infection parameters (number of galls and egg masses) and soil infection parameters (final J2 population and reproduction factor). A similar observation was reported by Cromwell et al., 2014, Hassan et al., 2016, which AgNPs reduced root galling and J2 population in infected soil. The results from combination of SiNPs and fenamiphos are in agreement with the results from Hassan et al. (2016), who demonstrated that the combination of fenamiphos and silver nanoparticles showed a significant increase in growth parameters of tomato seedlings as a consequence of the reduction of J2 population and reproduction factor. Conversely, the decrease in root infection and soil infection parameters, total plant weight in pots treated with RD of fosthiazate overwhelmed those treated with SiNPs combined with 0.5 RD of fosthiazate. With regard to krenkel, combined use of SiNPs concentrations enhanced the efficiency of the nematicide and declined root infection by M. incognita, also diminished the reproduction of M. incognita under field tests (Shekoohi et al, 2021).
The results of the current study indicated that the tested treatments exhibited a considerable nematicidial activity and significantly minified final populations of M. incognita. A few studies were conducted to study the effect of SiNPs combined with the synthetic nematicides on M. incognita. The tested concentrations had a negative effect on IJ2 development and reproduction. Application by 0.5 RD of nematicides and SiNPs reduced nematode parameters such as gall formation, egg masses on roots and final population of J2 in the soil. As well, it improved plant growth parameters by reduced populations of M. incognita (Danish et al, 2021).
Our results show the efficiency of SiNPs in single treatments or combined with different nematicides in eggplant growth. These results were the same observation detected for SiO2-NPs that increased maize plant growth as reported by Yuvakkumar et al. (2011). The results also observed the same trend as other studies, which showed that SiNPs enhances plant growth by interacting with plant morphology and physiology (Strout et al., 2013, Siddiqui and Al-Whaibi, 2014, Sun et al., 2016). Reduction in the population of M. incognita was associated either with the ability of J2 to infect roots of eggplants or subsequently formation of galls and egg masses.
Root infestation might be affected by the repellent activity of the nematocidal compounds absorbed by roots from the soil (Premachandra et al. 2014) or activated the plant defense systems (Gao et al. 2016).
The current results showed that the biosynthesis of SiNPs alone or combined with the nematicides disclosed effectiveness against the nematodes M. incognita. Several research pieces reported that SiNPs were applied as insecticides on a range of insect pests such as Tribolium castaneum (Herbst), Sitophilus oryzae L., Rhizopertha dominica, aphids, and cotton leafworm (Barik et al., 2008, Yang et al., 2009, El-Naggar et al., 2020).
5. Conclusion
The utilization of SiNPs for controlling M. incognita is considered a promising tool even used alone. Their toxicity against eggs and J2 was found depending on the concentration and exposure time. Combination of SiNPs plus 0.5 RD of fenamiphos, fosthiazate and krenkel synergistic their effect against M. incognita and improvement plant growth parameters as compared with RD of available commercial nematicides under greenhouse tests. This study indicated that the SiNPs are effective nematicide and can be used either alone or to increase the commercial nematicides' efficiency and facilitate their delivery. However, further studies need to be conducted to verify these results, and other studies are needed to understand the specific mode of action of SiNPs against M. incognita.
Acknowledgement
The current work was funded by Taif University Researchers Supporting Project number (TURSP − 2020/221), Taif University, Taif, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohamed T. El-Saadony, Email: m_tlatelsadony@yahoo.com.
Nashwa Elshaer, Email: Nashwa.elshaer@yahoo.com.
References
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11(7):1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Haliem M.E., Hegazy H.S., Hassan N.S., Naguib D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017;99:282–289. [Google Scholar]
- Abdel-Moneim, A. M. E., El-Saadony, M. T., Shehata, A. M., Saad, A. M., Aldhumri, S. A., Ouda, S. M., & Mesalam, N. M., 2022. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 29 (2), 1197–1209. [DOI] [PMC free article] [PubMed]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Ahmad A., Mukherjee P., Senapati S., Mandal D., Khan M.I., Kumar R., Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf. B: Biointerfaces. 2003;28:313–318. [Google Scholar]
- Ahmad I., Ansari M.I., Aqil F. Biosorption of Ni, Cr and Cd by metal tolerant Aspergillus niger and Penicillium sp. using single and multi-metal solution. Indian J. Exp. Biol. 2006;44(1):73–76. [PubMed] [Google Scholar]
- Ahmed S., Ahmad M., Swami B.L., Ikram S. A review on plants extracts mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 2016;7:17–28. doi: 10.1016/j.jare.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akl B., Nader M.M., El-Saadony M.T. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020;11:1–8. [Google Scholar]
- Al Banna L., Salem N., Ghrair A., Habash S. Impact of silicon carbide nanoparticles on hatching and survival of soil nematodes Caenorhabditis elegans and Meloidogyne incognita. Appl. Ecol. Environ. Res. 2018;16:2651–2662. [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F.M. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100(6) doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Ardakani A.S. Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology. 2013;15:671–677. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Bapat G., Zinjarde S., Tamhane V. Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Colloids Surf. B: Biointerfaces. 2020;193 doi: 10.1016/j.colsurfb.2020.111079. [DOI] [PubMed] [Google Scholar]
- Barik T., Sahu B., Swain V. Nanosilica—from medicine to pest control. Parasitol. Res. 2008;103:253–258. doi: 10.1007/s00436-008-0975-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A., Bhaumik A., Rani P.U., Mandal S., Epidi T.T. Nano-particles-A recent approach to insect pest control. Afr. J. Biotechnol. 2010;9:3489–3493. [Google Scholar]
- Cromwell W., Yang J., Starr J., Jo Y.-K. Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. J. Nematol. 2014;46(3):261–266. [PMC free article] [PubMed] [Google Scholar]
- Cui J., Liu T., Li F., Yi J., Liu C., Yu H. Silica nanoparticles alleviate cadmium toxicity in rice cells: mechanisms and size effects. Environ. Pollut. 2017;228:363–369. doi: 10.1016/j.envpol.2017.05.014. [DOI] [PubMed] [Google Scholar]
- Danish M., Altaf M., Robab M.I., Shahid M., Manoharadas S., Hussain S.A., Shaikh H. Green synthesized silver nanoparticles mitigate biotic stress induced by Meloidogyne incognita in Trachyspermum ammi (L.) by improving growth, biochemical, and antioxidant enzyme activities. ACS omega. 2021;6(17):11389–11403. doi: 10.1021/acsomega.1c00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardo E.A., Grewal P.S. Compatibility of Steinernema feltiae (Nematoda: Steinernematidae) with pesticides and plant growth regulators used in glasshouse plant production. Biocontrol. Sci. Technol. 2003;13:441–448. [Google Scholar]
- Debnath N., Das S., Seth D., Chandra R., Bhattacharya S.C., Goswami A. Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.) J. Pest Sci. 2011;84:99–105. [Google Scholar]
- Desoky E.-S.-M., Merwad A.-R.-M., Semida W.M., Ibrahim S.A., El-Saadony M.T., Rady M.M. Heavy metals-resistant bacteria (HM-RB): Potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198 doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- Desoky E.-S.-M., Saad A.M., El-Saadony M.T., Merwad A.-R.-M., Rady M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020;30 [Google Scholar]
- Duncan D.B. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- El-Argawy E., Rahhal M., El-Korany A., Elshabrawy E., Eltahan R. Efficacy of some nanoparticles to control damping-off and root rot of sugar beet in El-Behiera Governorate. Asian J. Plant Pathol. 2017;11:35–47. [Google Scholar]
- El-Ashry R., Ali A., ElSobki A. Nematicidal properties of three adjuvants for management of southern root-knot nematode, Meloidogyne incognita in vitro and under greenhouse conditions. J. Plant Prot. Res. Pathol. 2019;10:511–519. [Google Scholar]
- El-Deeb A., El-Ashry R., El-Marzoky A. Nematicidal activities of certain animal manures and biopesticides against Meloidogyne incognita infecting cucurbit plants under greenhouse conditions. J. Plant Prot. Res. Pathol. 2018;9:265–271. [Google Scholar]
- El-Naggar M.E., Abdelsalam N.R., Fouda M.M., Mackled M.I., Al-Jaddadi M.A., Ali H.M., Siddiqui M.H., Kandil E.E. Soil application of nano silica on maize yield and its insecticidal activity against some stored insects after the post-harvest. Nanomaterials. 2020;10:739. doi: 10.3390/nano10040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrys, A.S., Desoky, E.M., Abo El-Maati, M.F., Elnahal, A.S., Abdo, A.I., Raza, S., Zhou, J., 2019. Can secondary metabolites extracted from 1 Moringa seeds suppress ammonia oxidizers to increase nitrogen use efficiency and reduce nitrate contamination in potato tubers? Ecotoxicol. Environ. Saf., 185. [DOI] [PubMed]
- Elrys A.S., Abdo A.I.E., Abdel-Hamed Enas M.W., Desoky E.M. Integrative application of licorice root extract or lipoic acid with fulvic acid improves wheat production and defenses under salt stress conditions. Ecotoxicol. Environ. Saf. 2020;190 doi: 10.1016/j.ecoenv.2019.110144. [DOI] [PubMed] [Google Scholar]
- El-Saadony, M. T., Abuljadayel, D. A., Shafi, M. E., Albaqami, N. M., Desoky, E. S. M., El-Tahan, A. M., ... & Saad, A. M., 2021a. Control of foliar phytoparasitic nematodes through sustainable natural materials: Current progress and challenges. Saudi J. Biol. Sci. 28 (12), 7314–7326. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony, M. T., ALmoshadak, A. S., Shafi, M. E., Albaqami, N. M., Saad, A. M., El-Tahan, A. M., ... & Helmy, A. M., 2021b. Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review. Saudi J. Biol. Sci. 28 (12), 7349–7359. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Desoky E.S.M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony, M. T., Saad, A. M., El-Akkad, H. A., El-Tahan, A. M., Alshahrani, O. A., Alshilawi, M. S., ... & Ahmed, A. I., 2022. Flavoring and extending the shelf life of cucumber juice with aroma compounds-rich herbal extracts at 4°C through controlling chemical and microbial fluctuations. Saudi J. Biol. Sci. 29 (1), 346–354. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: Biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Hassan M.A. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi. J. Biol. Sci. 2021;28(8):4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony, M. T., Saad, A. M., Taha, T. F., Najjar, A. A., Zabermawi, N. M., Nader, M. M., ... & Salama, A., 2021g. Selenium nanoparticles from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi as a new source from human breast milk. Saudi J. Biol. Sci. 28 (12), 6782–6794. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69 [Google Scholar]
- El-Saadony, M.T., Abd El-Hack, M.E., Mohamed, E., Taha, A.E., Fouda, M.M., Ajarem, J.S., N Maodaa, S., Allam, A.A., Elshaer, N., 2020a. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials 10, 587. [DOI] [PMC free article] [PubMed]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah A., Mahgoub S. Biosynthesis, optimization and characterization of silver nanoparticles biosynthesized BY Bacillus subtilis ssp spizizenii MT5 isolated from heavy metals polluted soil. Zagazig J. Agric. Res. 2018;45:2439–2454. [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- Gaddeyya G., Niharika P.S., Bharathi P., Kumar P.R. Isolation and identification of soil mycoflora in different crop fields at Salur Mandal. Adv. Appl. Sci. Res. 2012;3:2020–2026. [Google Scholar]
- Gao H., Qi G., Yin R., Zhang H., Li C., Zhao X. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohar I., Maareg M. Relationship between crop losses and initial population densities of root-knot nematode, Meloidogyne incognita in soil of sugar beet grown in West Nubariya district. Egypt. J. Agric. Res. 2005;83:1315–1328. [Google Scholar]
- Gudikandula K., Charya Maringanti S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016;11:714–721. [Google Scholar]
- Hamed S.M., Hagag E.S., El-Raouf N.A. Green production of silver nanoparticles, evaluation of their nematicidal activity against Meloidogyne javanica and their impact on growth of faba bean. Beni-Suef Univ. J. Basic Appl. Sci. 2019;8(9):1–12. [Google Scholar]
- Hassan M., Zawam H., El-Nahas S., Desoukey A. Comparison study between silver nanoparticles and two nematicides against Meloidogyne incognita on tomato seedlings. Plant Pathol. J. (Faisalabad) 2016;15:144–151. [Google Scholar]
- Hegazy M.I., Salama A.S., El-Ashry R.M., Othman A.E.I. Serratia marcescens and Pseudomonas aeruginosa are promising candidates as biocontrol agents against root-knot nematodes (Meloidogyne spp.) Middle East J. Agric. Res. 2019;8:828–838. [Google Scholar]
- Hooper D. Extraction and processing of plant and soil nematodes. Plant parasitic nematodes in subtropical and tropical agriculture. 1990:45–68. [Google Scholar]
- Hussain M.A., Mukhtar T., Kayani M.Z. Reproduction of Meloidogyne incognita on resistant and susceptible okra cultivars. Pak. J. Agric. Sci. 2016;53(02):371–375. [Google Scholar]
- Hussey R. A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973;57:1025–1028. [Google Scholar]
- Jepson, S.B., 1987. Identification of root-knot nematodes (Meloidogyne species).
- Khan A., Sayed M., Shaukat S., Handoo Z. Efficacy of four plant extracts on nematodes associated with papaya in Sindh. Pakistan. Nematol. Medit. 2008;2008(36):93–98. [Google Scholar]
- Khan M., Khan M., Anwer M., Haque Z. Laboratory and field performance of some soil bacteria used as seed treatments on Meloidogyne incognita in chickpea. Nematol. Medit. 2012;40:143–151. [Google Scholar]
- Khan M.R., Rizvi T.F. Nanotechnology: scope and application in plant disease management. Plant Pathol. J. 2014;13:214–231. [Google Scholar]
- Liang X., Wang Y., Cheng J., Ji Q., Wang Y., Wu T., Tang M. Mesoporous silica nanoparticles at predicted environmentally relevant concentrations cause impairments in gabaergic motor neurons of nematode Caenorhabditis elegans. Chem. Res. Toxicol. 2020;33:1665–1676. doi: 10.1021/acs.chemrestox.9b00477. [DOI] [PubMed] [Google Scholar]
- Lim, D., Roh, J.y., Eom, H.j., Choi, J.Y., Hyun, J., Choi, J., 2012. Oxidative stress‐related PMK‐1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environ. Toxicol. Chem. 31, 585–592. [DOI] [PubMed]
- Mohamed E.A., Elsharabasy S.F., Abdulsamad D. Evaluation of in vitro nematicidal efficiency of copper nanoparticles against root-knot nematode Meloidogyne incognita. South Asian J. Parasitol. 2019:1–6. [Google Scholar]
- Mukhtar T., Jabbar A., Raja M.U., Javed H. Management of root-knot nematode, Meloidogyne incognita, in tomato with two Trichoderma species. Pakistan J. Zool. 2018;50:1195–1198. [Google Scholar]
- Oka Y., Koltai H., Bar-Eyal M., Mor M., Sharon E., Chet I., Spiegel Y. New strategies for the control of plant-parasitic nematodes. Pest Manag. Sci.: formerly. Pesticide Sci. 2000;56:983–988. [Google Scholar]
- Pourchez J., Forest V., Boumahdi N., Boudard D., Tomatis M., Fubini B., Herlin-Boime N., Leconte Y., Guilhot B., Cottier M. In vitro cellular responses to silicon carbide nanoparticles: impact of physico-chemical features on pro-inflammatory and pro-oxidative effects. J. Nanopart. Res. 2012;14:1–12. [Google Scholar]
- Prasad R., Bhattacharyya A., Nguyen Q.D. Nanotechnology in sustainable agriculture: recent developments, challenges, and perspectives. Front. Microbiol. 2017;8:1014. doi: 10.3389/fmicb.2017.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premachandra W.D., Mampitiyarachchi H., Ebssa L. Nemato-toxic potential of betel (Piper betle L.) (Piperaceae) leaf. Crop Prot. 2014;65:1–5. [Google Scholar]
- Rai M., Ingle A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012;94:287–293. doi: 10.1007/s00253-012-3969-4. [DOI] [PubMed] [Google Scholar]
- Rastogi A., Tripathi D.K., Yadav S., Chauhan D.K., Živčák M., Ghorbanpour M., El-Sheery N.I., Brestic M. Application of silicon nanoparticles in agriculture. 3. Biotech. 2019;9:1–11. doi: 10.1007/s13205-019-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20(1):324–335. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaat M.M., Mahrous M.E., El-Ashry R.M., El-Marzoky A.M. Nematicidal potential of some botanicals against Meloidogyne Incognita in vitro and in vivo. Biosci. Res. 2020;17(1):157–164. [Google Scholar]
- Roh J.-Y., Sim S.J., Yi J., Park K., Chung K.H., Ryu D.-Y., Choi J. Ecotoxicity of silver nanoparticles on the soil nematode Caenorhabditis elegans using functional ecotoxicogenomics. Environ. Sci. Technol. 2009;43:3933–3940. doi: 10.1021/es803477u. [DOI] [PubMed] [Google Scholar]
- Saad A.M., Elmassry R.A., Wahdan K.M., Ramadan F.M. Chickpea (Cicer arietinum) steep liquor as a leavening agent: effect on dough rheology and sensory properties of bread. Acta Period. Technol. 2015;46:91–102. doi: 10.2298/APT1546091S. [DOI] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28:5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El‐Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. 2021;56(7):3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT. 2021;148:111668. [Google Scholar]
- Sasser J. A world perspective on nematology: the role of the society. Vistas on Nematol. 1987:7–14. [Google Scholar]
- Sauget M., Valot B., Bertrand X., Hocquet D. Can MALDI-TOF mass spectrometry reasonably type bacteria? Trends Microbiol. 2017;25:447–455. doi: 10.1016/j.tim.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Scharf A., Güh K.-H., von Mikecz A. Anti-amyloid compounds protect from silica nanoparticle-induced neurotoxicity in the nematode C. elegans. Nanotoxicology. 2016;10:426–435. doi: 10.3109/17435390.2015.1073399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf A., Piechulek A., von Mikecz A. Effect of nanoparticles on the biochemical and behavioral aging phenotype of the nematode Caenorhabditis elegans. ACS nano. 2013;7:10695–10703. doi: 10.1021/nn403443r. [DOI] [PubMed] [Google Scholar]
- Schumaker S., Borror C.M., Sandrin T.R. Automating data acquisition affects mass spectrum quality and reproducibility during bacterial profiling using an intact cell sample preparation method with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2012;26:243–253. doi: 10.1002/rcm.5309. [DOI] [PubMed] [Google Scholar]
- Shahzad A., Saeed H., Iqtedar M., Hussain S.Z., Kaleem A., Abdullah R., Sharif S., Naz S., Saleem F., Aihetasham A. Size-controlled production of silver nanoparticles by Aspergillus fumigatus BTCB10: likely antibacterial and cytotoxic effects. J. Nanomater. 2019;2019(7) [Google Scholar]
- Sharon M., Choudhary A.K., Kumar R. Nanotechnology in agricultural diseases and food safety. J. Phytolo. 2010;2 [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekoohi S.S., Charehgani H., Abdollahi M., Rajabi H.R. Combined effect of β-aminobutyric acid and silver nanoparticles on eggplants, Solanum melongena, infected with Meloidogyne javanica. Nematology. 2021;1(aop):1–16. [Google Scholar]
- Siddiqui M.H., Al-Whaibi M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.) Saudi J. Biol. Sci. 2014;21:13–17. doi: 10.1016/j.sjbs.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui Z.A., Akhtar M.S. Synergistic effects of antagonistic fungi and a plant growth promoting rhizobacterium, an arbuscular mycorrhizal fungus, or composted cow manure on populations of Meloidogyne incognita and growth of tomato. Biocontrol. Sci. Technol. 2008;18:279–290. [Google Scholar]
- Siddiqui Z.A., Futai K. Biocontrol of Meloidogyne incognita on tomato using antagonistic fungi, plant-growth-promoting rhizobacteria and cattle manure. Pest Manag. Sci.: formerly Pesticide Sci. 2009;65:943–948. doi: 10.1002/ps.1777. [DOI] [PubMed] [Google Scholar]
- Strout G., Russell S.D., Pulsifer D.P., Erten S., Lakhtakia A., Lee D.W. Silica nanoparticles aid in structural leaf coloration in the Malaysian tropical rainforest understorey herb Mapania caudata. Ann. Bot. 2013;112:1141–1148. doi: 10.1093/aob/mct172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Hussain H.I., Yi Z., Rookes J.E., Kong L., Cahill D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere. 2016;152:81–91. doi: 10.1016/j.chemosphere.2016.02.096. [DOI] [PubMed] [Google Scholar]
- Swelum A.A., Shafi M.E., Albaqami N.M., El-Saadony M.T., Elsify A., Abdo M., Taha A.E., Abdel-Moneim A-M., Al-Gabri N.A., Almaiman A.A., Al-wajeeh A.S., Tufarelli V., Staffa V.N., Abd El-Hack M.E. COVID-19 in Human, Animal, and Environment: A Review. Front. Vet. Sci. 2020;7:578. doi: 10.3389/fvets.2020.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha E.H., Abo-Shady N.M. Effect of silver nanoparticles on the mortality pathogenicity and reproductivity of entomopathogenic nematodes. Int. J. Zool. Res. 2016;12:47–50. [Google Scholar]
- Talavera-Rubia M., Vela-Delgado M.D., Verdejo-Lucas S. Nematicidal efficacy of milbemectin against root-knot nematodes. Plants. 2020;9(7):839. doi: 10.3390/plants9070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur R.K., Dhirta B., Shirkot P. Studies on effect of gold nanoparticles on Meloidogyne incognita and tomato plants growth and development. BioRxiv. 2018;428144 [Google Scholar]
- Trinh Q., Le T., Nguyen T., Nguyen H.T., Liebanas G., Nguyen T. Meloidogyne daklakensis n. sp. (Nematoda: Meloidogynidae), a new root-knot nematode associated with Robusta coffee (Coffea canephora Pierre ex A. Froehner) in the Western Highlands. Vietnam. J. Helminthol. 2019;93:242–254. doi: 10.1017/S0022149X18000202. [DOI] [PubMed] [Google Scholar]
- Tripathi D.K., Singh V.P., Prasad S.M., Chauhan D.K., Dubey N.K. Silicon nanoparticles (SiNP) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015;96:189–198. doi: 10.1016/j.plaphy.2015.07.026. [DOI] [PubMed] [Google Scholar]
- Yang F.-L., Li X.-G., Zhu F., Lei C.-L. Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) J. Agric. Food Chem. 2009;57:10156–10162. doi: 10.1021/jf9023118. [DOI] [PubMed] [Google Scholar]
- Yuvakkumar R., Elango V., Rajendran V., Kannan N.S., Prabu P. Influence of nanosilica powder on the growth of maize crop (Zea mays L.) Int. J. Green Nanotechnol. 2011;3:180–190. [Google Scholar]