Abstract
This study investigated the antimicrobial and antioxidant activity of three Spirulina extracts (methanol, acetone, and hexane) and the biological selenium nanoparticles (SeNPs) fabricated by Bacillus subtilis AL43. The results showed that Spirulina extracts exhibited antimicrobial activity against tested pathogens. Besides, Spirulina extracts significantly scavenged ABTS and DPPH radicals in a dose-dependent manner. The methanolic extract had higher total phenolic content, antimicrobial activity, and antioxidant activity than other extracts. The selenium nanoparticles were synthesized by Bacillus subtilis AL43 under aerobic conditions and were characterized as spherical, crystalline with a size of 65.23 nm and a net negative charge of −22.7. We evidenced that SeNPs possess considerable antimicrobial activity against three gram-positive, three gram-negative bacteria, and three strains from both Candida sp. and Aspergillus sp. Moreover, SeNPs were able to scavenge ABTS and DPPH radicals in a dose-dependent manner. An association was found between the total phenolic content of Spirulina and SeNPs and their biological activities. Our results indicate that Spirulina and SeNPs with significant antimicrobial and antioxidant activities seem to be successful candidates for safe and reliable medical applications.
Keyword: Spirulina platensis, Green nanotechnology, Biogenic SeNPs, Bacillus subtilis, Antimicrobial, Antioxidant
1. Introduction
Foodborne diseases have increased worldwide, with a noticeable public health concern (Abd El-Hack et al., 2021, Abd El-Hack et al., 2020c). Besides, the development and outbreaks of antibiotic-resistance microbes threaten human and animal health and cause a global health crisis (Abdelhady et al., 2021, Nour et al., 2021). Additionally, the antioxidant defenses in biological systems are not fully able to counter oxidative stress due to the wide variety of stressors and free radical inducers (El-Tarabily et al., 2021). Among the novel approaches for tackling this problem are natural products, as antibiotic alternatives, that have antioxidant and antimicrobial activities (Abd El-Hack et al., 2020b, Abd El-Hack et al., 2020d, Abou-Kassem et al., 2021b, Alagawany et al., 2021a, Reda et al., 2021a). These natural products may contribute to mitigating oxidative stress via enhancing enzymic and non-enzymic antioxidants (Abd El-Hack et al., 2020c, Abdel-Moneim et al., 2021a, Abdel-Moneim et al., 2020c, Abdelnour et al., 2020a, Abdelnour et al., 2020b, Saad et al., 2021c, Saad et al., 2020b).
Spirulina platensis is an edible blue-green alga. The beneficial role of Spirulina in human food and domestic animal feed has received increased attention across several disciplines in recent years (Abdel-Moneim et al., 2021b, De La Jara et al., 2018, EL-Sabagh et al., 2014, Holman and Malau-Aduli, 2013). It contains high protein levels with all essential amino acids, essential fatty acids, minerals, pigments, carotenoids, and vitamins (Abdel-Moneim et al., 2021b, Mendiola et al., 2007). Spirulina was found to act as a probiotic and antioxidant agent (Abdel-Moneim et al., 2021b, Abdelkhalek et al., 2015, Bhowmik et al., 2009). Therefore, Spirulina is supplemented to human food and animal diets to prevent gut dysbiosis and pathogens colonization and improve antioxidant status.
Selenium is an essential trace element and has received considerable attention due to its essential functions in the biological system. Selenium is the key component of selenoproteins that is well known to be involved in animal cells' antioxidant defense system. Moreover, the antimicrobial activity of this micronutrient metalloid has been demonstrated (Cremonini et al., 2016). Nanotechnology is a burgeoning interdisciplinary approach in multiple fields of academic research. It has the ability to facilitate ground-breaking applications in human and animal health, involving pathogens resistance, antioxidant, toxin degradation, nutrient efficiency, etc. (Abd El-Hack et al., 2020b, El-Saadony et al., 2020a, Reda et al., 2021b, Reda et al., 2020). Because it is widely thought that selenium in nano form has more effective and affordable antimicrobial and antioxidant activities and safer than other forms (Abbas et al., 2021, Forootanfar et al., 2014, Ibrahim et al., 2020), it has gained much attention and wide applications in recent years. Biogenic selenium nanoparticles (SeNPs) can be synthesized using bacteria as biological catalysts, giving a safe and environmental innovation strategy for producing metal/metalloid nanoparticles with high bioactivity and low cytotoxicity and without the need to reducing and stabilizing agents (Abbas et al., 2021, Sheiha et al., 2020, Xu et al., 2018). To the best of our knowledge, limited investigations have been done to evaluate both antioxidant and antimicrobial activities of Spirulina and the biogenic SeNPs. Indeed, antibiotics and other chemical antimicrobials can inhibit the growth of the pathogens; however, with concerning the advantages of the high bioavailability and lower cytotoxicity to humans and animals (Cremonini et al., 2016, Kata et al., 2018), Spirulina and SeNPs present novel antibiotic alternatives with high potential for preventing infection in the future. The current study was undertaken to assess the antioxidant and antimicrobial activities of Spirulina and biogenic SeNPs in order to evaluate the potential of using them as therapeutic candidates.
2. Materials and methods
2.1. Isolation of Spirulina
Zarrouks medium was used to isolate and cultivate the pure culture of Spirulina as follow; 10 mL of 5 d old Spirulina was mixed with 250 mL of Zarrouks medium pH 9.5 in screw bottles then incubated at 25 °C for 10 d under continuous illumination (600–800 lx) (Zarrouk, 1966). The pure culture of Spirulina was obtained by streaking method on Zarrouks medium to get a single culture from this isolate. The plates were incubated at 25◦C under continuous illumination (600 lx). Developed colonies were picked up and microscopically examined, and those composed of Spirulina cells were preserved on slants containing Zarrouks medium.
2.2. Preparation of Spirulina platensis extracts
The Spirulina platensis was obtained from Soda lake in Wadi El-Natrun, Monufia Governorate, Egypt, then was dried and powdered. Forty grams of Spirulina powder were homogenized in 200 mL of solvents (methanol, acetone, and hexane) and were stirred for 2 h, and the supernatants were obtained (Hassanin et al., 2020, Saad et al., 2020a). The rotary evaporator retained the solvents, and the residues were stored at 4 °C for further analysis. All chemicals used in this work were of analytical grade.
2.3. Preparation of SeNPs
2.3.1. Isolation, screening, and identification of Se-tolerant bacterium
0.85 g of sodium selenite was dissolved in one liter of sterilized water to prepare a stock solution of sodium selenite 5 mM concentration. 1 mM and 2 mM concentrations were prepared by taking 200 and 400 mL of stock solution and diluted to a liter with sterilized water. All solutions were stored to use in further experiments. The heavy metal contaminated soil was collected from Abu-Hammad City, Wady El-Moulak village, Sharkia governorate, Egypt. 10 gm of soil were homogenized in 90 mL peptone buffer and stirred for 15 min to obtain 10−1 dilution. Serial dilutions were prepared to 10−7 (Desoky et al., 2020a, Desoky et al., 2020b, Hassan et al., 2021). 100 µL of each dilution was spread over Mueller Hinton agar (MHA) plates supplemented with different sodium selenite concentrations (1, 2, and 5 mM), then incubated at 30 °C for 24 h and observed the colonies in each plate (El-Saadony et al., 2020b).
The Se-tolerant bacterium was identified based on the morphological, biochemical, and physiological tests in Bergey’s Manual (DeVos et al., 2011). The identification was confirmed by and MALDI-TOF mass spectrometry (bioMérieux, Marcy l’Etoile, France) (Singhal et al., 2015).
2.3.2. Biosynthesis and characterization of biogenic SeNPs
Sodium selenite (0.17 g) was homogenized in Luria-Bertani Broth (100 mL) containing 100 µL of bacterial isolate inoculum. The conditions were adjusted to obtain the best yield of SeNPs: pH 7.2, incubation temperature 30 °C, reaction time 24 h under agitation at 150 rpm in shaking incubator. Luria-Bertani Broth (LBB) without sodium selenite was considered a control (El-Saadony et al., 2021e, Fesharaki et al., 2010, Yadav et al., 2008). The produced SeNPs were characterized using six advanced instruments. UV–Vis spectroscopy was used to estimate the optical property of the SeNPs mixture (El-Saadony et al., 2020a, Saad et al., 2021a). Fourier Transform-Infrared (FT-IR) spectroscopy (“Bruker Tensor 37, Kaller”, Germany) was used to identify the potential active compounds in the SeNPs mixture (Beekes et al., 2007, El-Saadony et al., 2021b). Powder X-ray diffraction (XRD) was used to identify the crystalline nature of SeNPs (El-Saadony et al., 2021g). The shape and size of SeNPs were measured by Transmission Electron Microscopy (TEM) (JEOL 1010, Japan) (Akl et al., 2020). Size distribution and Zeta potential were estimated by Zeta sizer analysis (Nano “Z2 Malvern, Malvern Hills, UK”) (El-Saadony et al., 2021c, El-Saadony et al., 2021f, Saad et al., 2021b).
2.4. Chemical studies
2.4.1. Total phenolic content
The total phenolic contents of S. platensis (Table 1) and biogenic SeNPs suspension (Table 2) were estimated using the Folin-Ciocalteu method (Kalagatur et al., 2018). 50 µL of each evaluated Spirulina extract or SeNPs suspension was mixed with 50 µL of sodium carbonate (Na2CO3 7.5%, w/v) and 25 µL of diluted Folin-Ciocalteu reagent with water (1:10, v/v). The microtiter plate was placed in a microtiter plate reader (BioTek Elx808, USA) and the absorbance was read at 750 nm after 30 min. The total phenolic content was expressed as Gallic acid equivalent (µg GAE/mL).
Table 1.
Total phenolic component in Spirulina extracts.
| Solvents | Concentration (mg/mL) | Total polyphenols (µg GAE/mL) |
|---|---|---|
| Methanol | 2.5 | 1120c,A,* |
| 5 | 1403b,A,** | |
| 10 | 1592a,A,*** | |
| Acetone | 2.5 | 645f,B,* |
| 5 | 747e,B,** | |
| 10 | 928d,B,*** | |
| Hexane | 2.5 | 337 h,C,* |
| 5 | 463 g,C,** | |
| 10 | 519 g,C,*** | |
| SEM | 80.53 | |
| P-values | ||
| Solvent | <0.001 | |
| Concentration | <0.001 | |
| Solvent × Concentration | <0.001 |
GAE, gallic acid equivalent, SEM, standard error of means, means in the same column with different lowercase letters indicating significant differences of the interaction, different uppercase letters indicating significant differences between Spirulina extracts, but different *, ** and *** indicating significant differences between concentrations of the same extract.
Table 2.
Total phenolic component in biogenic selenium nanoparticles (SeNPs).
| SeNPs Conc. (µg/mL) | Total polyphenols (µg GAE/mL) |
|---|---|
| 100 | 569.0e |
| 200 | 852.3d |
| 300 | 994.0c |
| 400 | 1140.7b |
| 500 | 1381.3a |
| SEM | 72.97 |
| P-value | <0.001 |
GAE, gallic acid equivalent, SEM, standard error of means, means in the same column with different letters are significantly different.
2.5. Microbial studies
2.5.1. Bacterial and fungal strains
Bacterial isolates (Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes, Salmonella typhi, Escherichia coli, and Klebsiella pneumonia) and fungal isolates (Candida tropicalis, Candida albicans, Candida glabrata, Aspergillus niger, Aspergillus flavus, and Aspergillus fumigates) were obtained from Microbiology department, Faculty of Agriculture, Zagazig University, Zagazig, Egypt. Both bacterial and fungal isolates were stored at 4 °C on nutrient agar (NA) slants and Sabouraud’s dextrose agar (SDA) slants, respectively.
2.5.2. Preparation of bacterial and fungal inoculum
A streak of tested bacteria slant was mixed in 5 mL of nutrient broth and then incubated at 30 °C until 1.5x108 CFU/mL (Alagawany et al., 2021b). A loop of tested fungi slant was mixed in 5 mL of SDA and incubated at 28 °C until 0.5 McFarland standards 2.3 × 103 (El-Saadony et al., 2019).
2.6. Antimicrobial activity
2.6.1. Disc diffusion method
The antibacterial and antifungal activities of Spirulina extracts and SeNPs were estimated by the disc diffusion method (El-Saadony et al., 2021d). Spirulina extracts were prepared at three concentrations (2.5, 5, and 10 mg/mL). 100 µL of each concentration was dissolved in 1 mL of DMSO 5%. Sterilized paper discs (6 mm) were prepared and saturated with Spirulina extracts. 100 µL of bacterial inoculum (1x108 CFU/mL) and fungal spore suspension (2.3 × 103 CFU/mL) were spread on MHA and SDA plates surface, respectively. The previously saturated discs (6 mm) were placed on both sides of MHA and SDA plates. The discs saturated with ciprofloxacin or diniconazole were positive controls. The MHA plates were incubated at 37 °C for a day, and SDA plates were incubated at 28 °C for five days (fungi). The same procedure was repeated with SeNPs concentrations (100, 200, 300, 400, and 500 µg/mL). The inhibition zones (mm) around discs were measured by the transparent ruler.
2.6.2. Minimum inhibitory concentration (MIC)
The MIC of Spirulina extracts and SeNPs were estimated by the microdilution method according to Ericsson and Sherris (1971). Spirulina extracts and SeNPs concentrations were dissolved in 5% DMSO. 500 µL of spirulina extracts and SeNPs concentrations were homogenized in Mueller Hinton broth (MHB) and Sabouraud dextrose broth (SDB) tubes that inoculated with 100 µL of bacterial (1.5 × 108 CFU/mL) and standard size of fungal spore suspension (3 × 103 CFU/mL). The controls were MHB and SDB tubes inoculated with tested microorganisms. All tubes were incubated for a day at 37 °C and 5 days at 28 °C, respectively. The MIC values were recorded as the lowest concentration of antibacterial agents that prevented the growth of bacteria or fungi (Ashour et al., 2020, El-Saadony et al., 2021a).
2.7. Antioxidant assay
2.7.1. ABTS assay
The ABTS• radical scavenging activity (RSA) was determined by the ability of antioxidant agents to eliminate the ABTS• radical. The scavenging activities of Spirulina extracts and SeNPs were estimated (Gil et al., 2002) with some modifications. 3 mL of 0.1 mM ABTS• was added to 1 mL of Spirulina extracts (2.5, 5, and 10 mg/mL) and SeNPs concentrations (100, 200, 300, 400, and 500 µg/mL). The tubes were left for 30 min, then the absorbance was read at 745 nm by spectrophotometer. The control was ABTS• solution, and Tret-Butyl hydroquinone (TBHQ) was used as an antioxidant reference. The ABTS•RSA (%) of Spirulina extracts and SeNPs was calculated as the following equation:
2.7.2. DPPH assay
The antioxidant activity of Spirulina extracts and SeNPs concentrations was estimated by the scavenging DPPḢ radical as compared to a positive control (TBHQ). 2 mL ethanolic DPPH was added to 1 mL of each concentration and incubated in the dark for 30 min. The absorbance was estimated at 517 nm. DPPH• reagent was a control (Hassanin et al., 2020). TBHQ was used as an antioxidant reference, and DPPḢ scavenging activity (%) was measured as the following:
2.8. Statistical analysis
All experiments were performed in triplicate, and data were recorded and analyzed with SPSS package (v 20, SPSS Inc., Chigaco, IL, USA). The Two-way ANOVA test was used to examine the effect of solvent type and concentration on the total phenolic component of Spirulina platensis and its antimicrobial and antifungal activities. One-way ANOVA was performed to analyze the rest of the parameters and to compare the concentrations of Spirulina platensis within different solvents with the positive control. LSD test was used to compare the statistically significant differences among mean at P < 0.05.
3. Results
3.1. Isolation, screening, and identification of Se-resistant isolate
Thirty-three bacterial isolates were obtained from soil samples at PCA plates supplemented with sodium selenite (1 mM). Only five bacterial isolates were recovered at a 2 mM concentration, and coded as (AL17, AL26, AL39, AL43, and AL51), one isolate was tolerated with sodium selenite (5 mM), and called AL43, and it was considered Se-resistant bacteria. Based on the biochemical tests in the Bergy manual, the screened isolate was gram-negative, motile, short rod, and non-spore-forming under a light microscope and aerobic conditions, and it was identified as Bacillus subtilis AL43. The obtained findings showed a maximum similarity of 99% to several Bacillus spp., predominantly Bacillus subtilis. Thus, the local screened bacterial isolate (Bacillus subtilis AL43) was similar to Bacillus subtilis spp subtilis DSM 10 T DSM.
3.2. SeNPs characterization
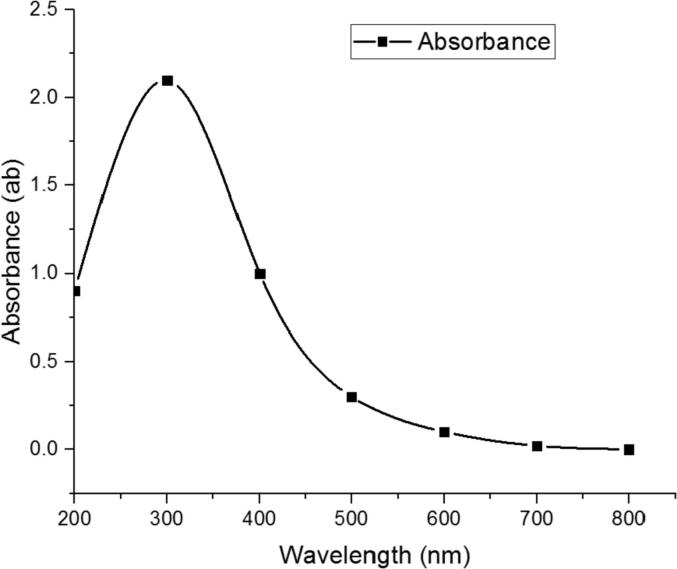
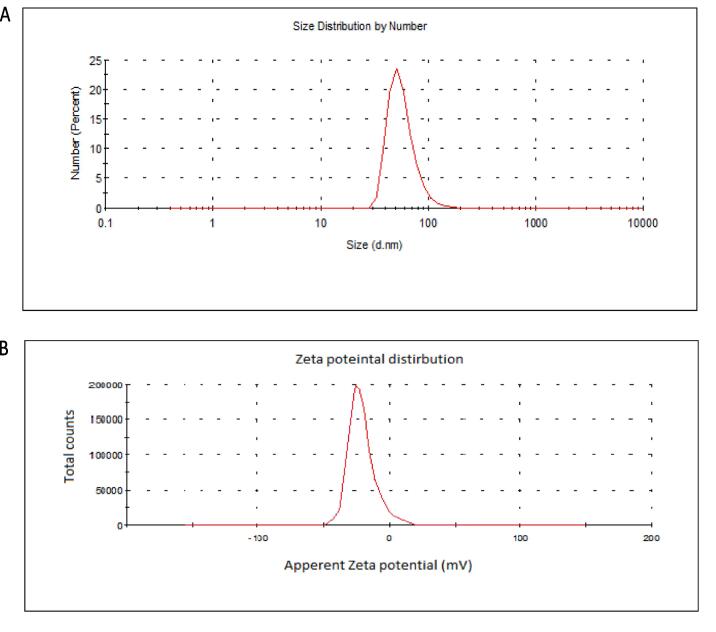
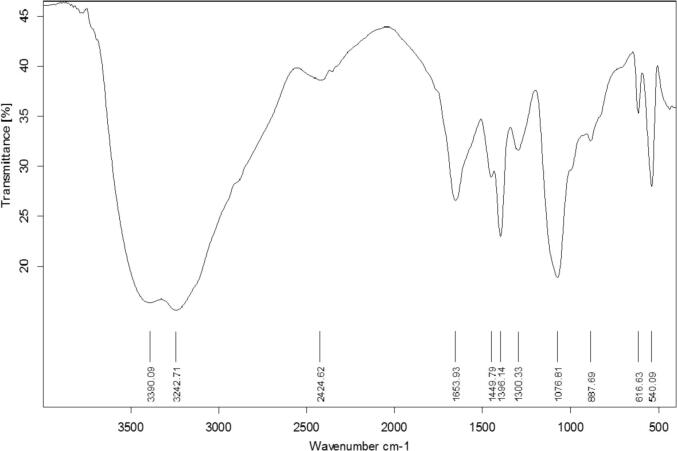
The biological SeNPs were fabricated by homogenizing sodium selenite with selected isolate B. subtilis AL43 under optimal conditions. After incubation, the appearance of red color in the culture flask suggested the formation of SeNPs. The maximum UV–visible absorption of SeNPs was found at 300 nm (Fig. 1). The TEM observed that the mean diameter of the produced SeNPs was 45–80 nm, indicating that B. subtilis AL43 could synthesize intracellular SeNPs (Fig. 2). XRD showed that the biosynthesized nanoparticles are crystalline with a spherical structure. The crystalline size of SeNPs was in the range of 32–86 nm. Zeta seizer and Zeta potential results indicated that the average SeNPs size was 65.24 nm and the Zeta potential was −22.7 mV (Fig. 3). FTIR spectrum showed that the bands at 3242.71 cm−1 and 3390.09 cm−1 matched the O—H and N—H stretching vibration. The hydrogen-bonded SH stretching vibration appears at 2424.62 cm−1. The band at 1653.93 cm−1 is related to C O. The bands around 1076.81 cm−1 are probably correlated to the C—O stretching vibrations. 887.69 cm−1 is probably the vibration absorption peak due to the C—O—C. The bands at 540.09 cm−1 and 616.63 cm−1 indicated the stretching vibration of C—S bond. The increase in depth and width of peaks may result from the C—O stretching vibrations of phenolic compounds attached to SeNPs. These results confirmed the presence of functional biomolecules (protein, phenols, and polysaccharides) attached to the SeNPs surface (Fig. 4).
Fig. 1.
UV–Vis spectra of SeNPs synthesized by B. subtilis AL43.
Fig. 2.
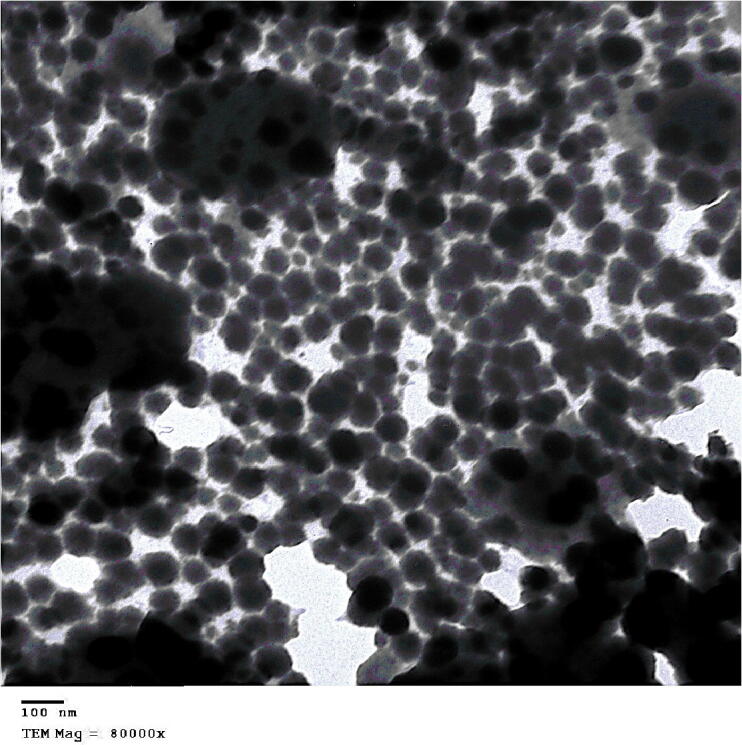
TEM image of biogenic selenium nanoparticles (SeNPs) synthesized by Bacillus subtilis AL43.
Fig. 3.
Size distribution (A) and zeta potential (B) of SeNPs synthesized by B. subtilis AL43.
Fig. 4.
FT-IR spectrum of SeNPs synthesized by B. subtilis AL43.
3.3. Chemical studies
The values of total phenolic content in Spirulina extracts showed significant differences, as shown in Table 1. Methanol extract exhibited higher values for all the tested levels, followed by acetone extract and then hexane extract. Table 2 presents the values of the total phenolic content of the different concentrations of SeNPs, which increased in a concentration-dependent manner.
3.4. Microbial studies
3.4.1. Antimicrobial activity of Spirulina
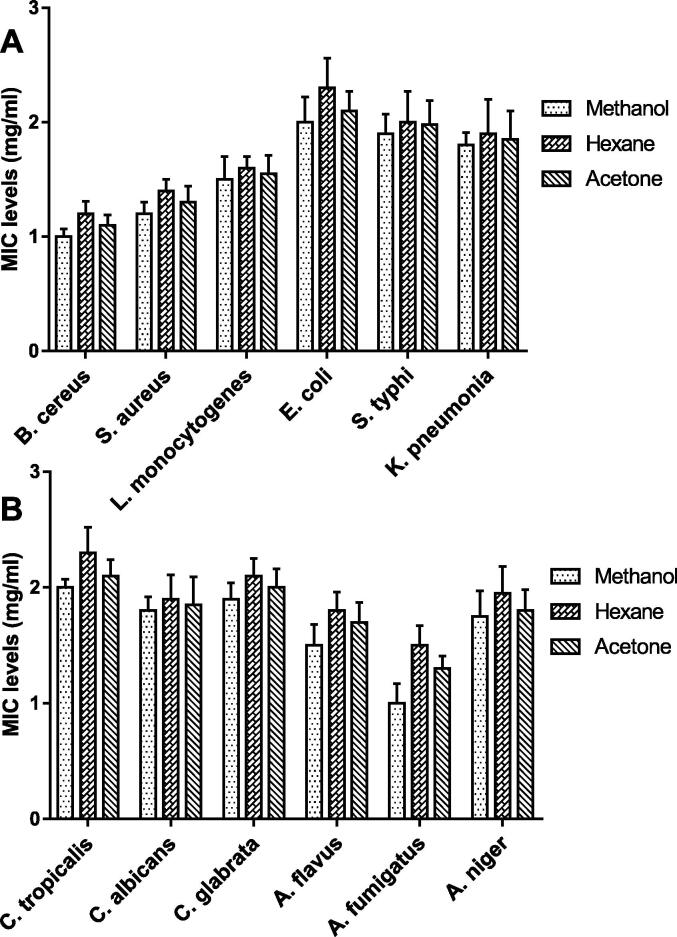
Table 3, Table 4 show the inhibition zones diameter (IZD) of spirulina extracts (methanol, acetone, and hexane) against tested bacteria and fungi compared to ciprofloxacin and diniconazole, respectively. The IZD values increased in a concentration-dependent manner. Gram-positive bacteria (B. cereus, S. aureus, and L. monocytogenes) showed higher sensitivity to Spirulina extracts than Gram-negative bacteria. Our results showed that the antimicrobial effect against all tested bacteria and fungi was varied among the different extracts. Spirulina methanolic extract had higher antimicrobial activity than other extracts, with inhibition zones ranging from 17 to 22 mm for 10 mg/mL concentration. Results of MIC confirmed these results (Fig. 5) where Spirulina methanolic extract exhibited the lower MIC (1–2 mg/mL) against tested microorganisms compared to other extracts. On the other hand, the methanolic extract of Spirulina had higher antifungal activity than other extracts with IZDs ranged from 15 to 21 mm as compared to diniconazole. Disc diffusion and MIC results showed that Candida sp. were more resistant to spirulina extracts than Aspergillus sp.
Table 3.
Antibacterial activity of Spirulina extracts expressed as inhibition zones diameters (mm).
| Solvents | Conc. (mg/mL) | Bacillus cereus | Staphylococcus aureus | Listeria monocytogenes | Escherichia coli | Salmonella typhi | Klebsiella pneumonia |
|---|---|---|---|---|---|---|---|
| Methanol | 2.5 | 19.0de | 18.3 cd | 16.7de | 15.2de | 16.6de | 17.0c |
| 5 | 20.0 cd | 19.0bc | 18.0bc | 16.4c | 18.0c | 16.6c | |
| 10 | 22.0b | 20.3b | 19.0b | 18.7b | 19.7b | 19.0b | |
| Acetone | 2.5 | 14.7 h | 13.3 h | 12.3 g | 10.6 h | 12.0i | 11.7 g |
| 5 | 15.6 h | 14.0gh | 13.3 fg | 12.0 g | 13.4 h | 12.6 fg | |
| 10 | 17.0 g | 15.0 fg | 14.4f | 13.0f | 14.3gh | 13.5ef | |
| Hexane | 2.5 | 17.7 fg | 16.3ef | 15.6e | 13.7f | 14.6 fg | 14.2de |
| 5 | 18.7ef | 17.3de | 16.4e | 14.6e | 15.7ef | 15.0d | |
| 10 | 20.3c | 18.7 cd | 17.6 cd | 16.0 cd | 17.0 cd | 16.3c | |
| Positive control (Ciprofloxacin) | 10 | 30.0a | 28.0a | 25.0a | 22.7a | 24.0a | 20.3a |
| SEM | 0.447 | 0.465 | 0.427 | 0.457 | 0.456 | 0.446 | |
| P-values | |||||||
| Solvent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Concentration | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Solvent × Concentration | 0.927 | 0.966 | 0.939 | 0.151 | 0.852 | 0.503 | |
| Treatments vs. Positive control | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
SEM, standard error of means, means in the same column with different letters are significantly different
Table 4.
Antifungal activity of Spirulina extracts expressed as inhibition zones diameters (mm).
| Solvents | Conc. (mg/mL) | Candida tropicalis | Candida albicans | Candida glabrata | Aspergillus flavus | Aspergillus fumigatus | Aspergillus niger |
|---|---|---|---|---|---|---|---|
| Methanol | 2.5 | 15.0 cd | 17.0 cd | 16.0de | 18.0 cd | 19.0 cd | 17.0 cd |
| 5 | 16.1bc | 18.0bc | 18.0bc | 19.0bc | 20.0bc | 18.0bc | |
| 10 | 17.0b | 19.2b | 18.8b | 20.0b | 21.0b | 19.0b | |
| Acetone | 2.5 | 11.0 g | 13.1 g | 13.0 g | 14.0 g | 15.0 g | 13.0 g |
| 5 | 12.3 fg | 14.3 fg | 14.5 fg | 15.4 fg | 16.3 fg | 14.3 fg | |
| 10 | 13.2ef | 15.6ef | 15.0ef | 16.0ef | 17.0ef | 15.0ef | |
| Hexane | 2.5 | 13.1ef | 15.2ef | 15.4ef | 16.0ef | 17.0ef | 15.0ef |
| 5 | 14.4de | 16.4de | 16.3de | 17.3de | 18.3de | 16.3de | |
| 10 | 15.0 cd | 17.0 cd | 17.7 cd | 18.0 cd | 19.0 cd | 17.0 cd | |
| Positive control (Diniconazole) | 10 | 20.3a | 22.3a | 23.4a | 25.3a | 28.3a | 23.3a |
| SEM | 0.381 | 0.273 | 0.136 | 0.564 | 0.431 | 0.412 | |
| P-values | |||||||
| Solvent | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Concentration | 0.001 | 0.001 | <0.001 | 0.001 | 0.001 | 0.001 | |
| Solvent × Concentration | 0.996 | 0.996 | 0.871 | 0.996 | 0.802 | 0.374 | |
| Treatments vs. Positive control | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
SEM, standard error of means, means in the same column with different letters are significantly different.
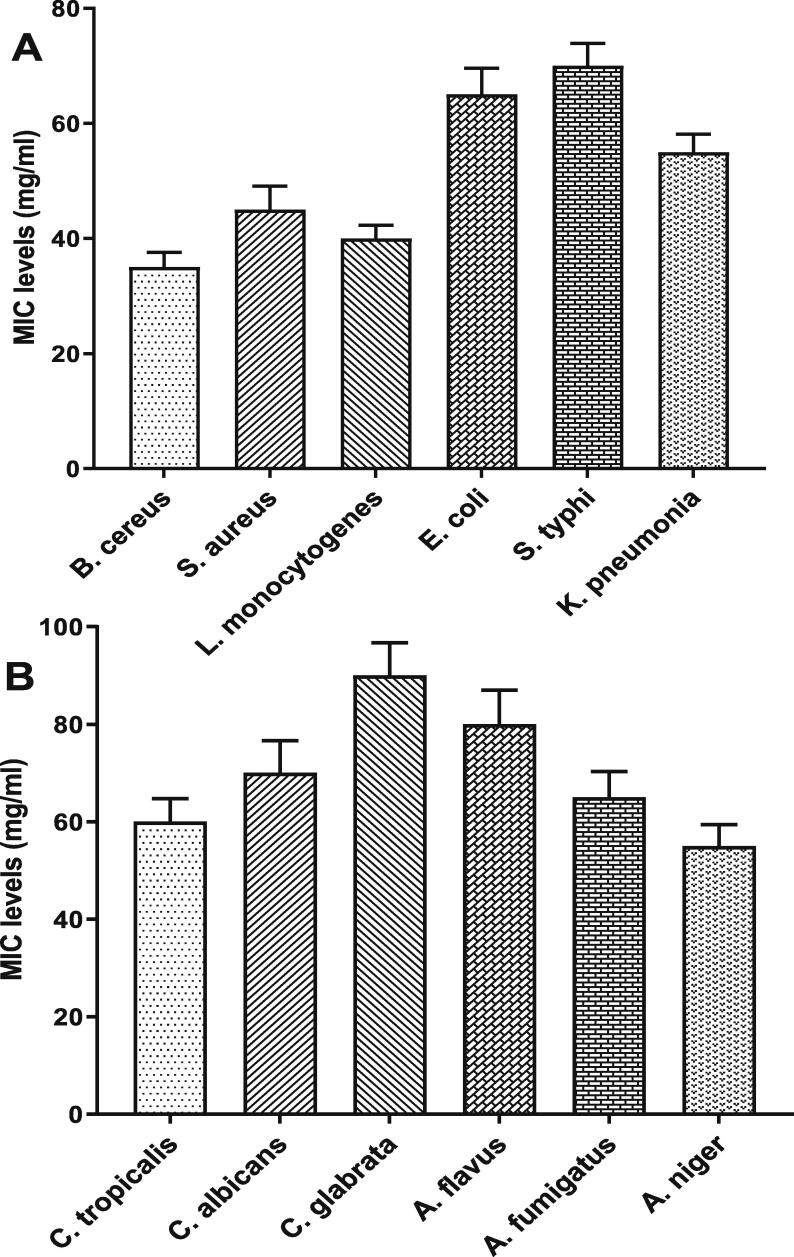
Fig. 5.
MIC levels of Spirulina extracts (methanol, hexane, and acetone) against (A) tested bacteria, and (B) tested fungi, data are presented as mean ± SE.
3.4.2. Antimicrobial activity of SeNPs
Table 5, Table 6 present the antimicrobial activity of SeNPs against three gram-positive bacteria and three gram-negative bacteria and six fungal isolates. The results showed that the values of IZD increased gradually in a concentration-dependent manner. B. cereus exhibited a higher sensitivity to the SeNPs, followed by L. monocytogenes and then S. aureus. Gram-negative bacteria showed higher resistance than gram-positive bacteria, with the lowest value of IZD for S. typhi (13.3–22.4 mm). Concerning the antifungal activity, SeNPs showed higher potential against Aspergillus spp. compared to Candida spp. The results of IZD showed that A. niger had a higher value (27.5 mm), and C. glabrata had a lower value (9.9 mm). Further analysis using the MIC test (Fig. 6A) showed significant differences among the tested microbes. The lower MIC value (35 µg/mL) was observed with B. cereus, while the higher value (70 µg/mL) was detected with S. typhi. A significant difference was found in the MIC level of SeNPs between the fungal isolates. As shown in Fig. 6B, the MIC value of SeNPs against A. niger (55 µg/mL) was lower than that detected against other strains, while the higher value (90 µg/mL) was observed against C. glabrata.
Table 5.
Antibacterial activity of biogenic selenium nanoparticles (SeNPs) expressed as inhibition zones diameters (mm).
| SeNPs (µg/mL) | Bacillus cereus | Staphylococcus aureus | Listeria monocytogenes | Escherichia coli | Salmonella typhi | Klebsiella pneumonia |
|---|---|---|---|---|---|---|
| 100 | 20.1f | 18.2ef | 19.8f | 15.2de | 13.3e | 16.8de |
| 200 | 23.4e | 20.4e | 22.3e | 17.5d | 16.8d | 19.6d |
| 300 | 25.7d | 23.5 cd | 24.3d | 20.0c | 19.4c | 21.5c |
| 400 | 28.1c | 25.0c | 27.5c | 21.2c | 20.3c | 22.7c |
| 500 | 31.5b | 27.6b | 30.8b | 23.7b | 22.4b | 24.2b |
| Positive control (Ciprofloxacin, 20 mg/mL) | 37.2a | 33.1a | 35.4a | 28.3a | 26.0a | 29.2a |
| SEM | 0.532 | 0.325 | 0.223 | 0.323 | 0.411 | 0.316 |
| P-values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
SEM, standard error of means, means in the same column with different letters are significantly different.
Table 6.
Antifungal activity of biogenic selenium nanoparticles (SeNPs) expressed as inhibition zones diameters (mm).
| SeNPs (µg/mL) | Candidatropicalis | Candida albicans | Candida glabrata | Aspergillus flavus | Aspergillusfumigatus | Aspergillusniger |
|---|---|---|---|---|---|---|
| 100 | 11.8f | 13.1f | 9.9f | 14.4f | 12.8e | 15.6f |
| 200 | 13.6e | 15.5e | 11.6de | 16.7e | 15.9de | 18.3e |
| 300 | 15.4d | 17.2d | 13.2d | 18.5d | 17.7d | 20.8d |
| 400 | 18.3c | 19.5bc | 17.8bc | 21.2c | 19.9c | 23.5c |
| 500 | 21.4b | 20.9b | 19.3b | 23.2b | 22.7b | 27.5b |
| Positive control (Diniconazole, 20 mg/mL) | 28.8a | 27.9a | 25.1a | 29.1a | 27.4a | 33.6a |
| SEM | 0.257 | 0.316 | 0.389 | 0.276 | 0.311 | 0.226 |
| P-values | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
SEM, standard error of means, means in the same column with different letters are significantly different.
Fig. 6.
MIC levels of biogenic selenium nanoparticles (SeNPs) against (A) tested bacteria, and (B) tested fungi, data are presented as mean ± SE.
3.5. Antioxidant activity
3.5.1. Antioxidant activity of Spirulina
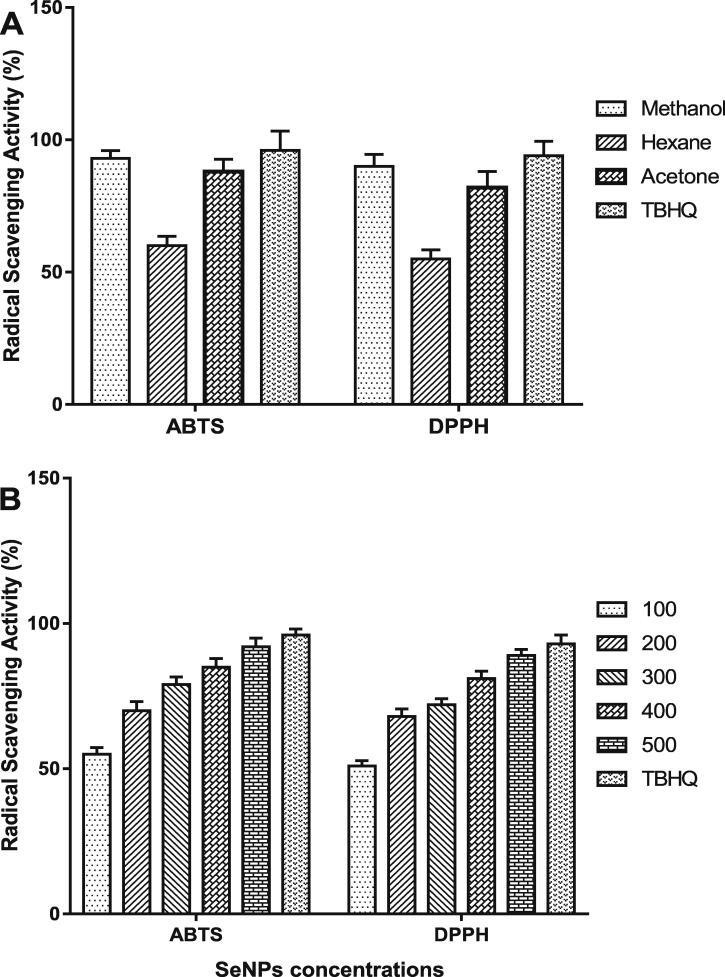
The scavenging activity of Spirulina extracts (methanol, hexane, and acetone) was evaluated by the antioxidant assays: ABTS• and DPPH• methods (Fig. 7A). All Spirulina extracts showed considerable ABTS and DPPH radical scavenging activities. Among Spirulina extracts, the methanolic extract exhibited higher ABTS and DPPH radical inhibition (93% and 90%, respectively), followed by acetone extract (88% and 82%, respectively), and hexane extract (60% and 55%, respectively), compared to the standard compound (96% and 94%, respectively).
Fig. 7.
Scavenging activity of (A) Spirulina extracts and (B) biogenic selenium nanoparticles (SeNPs, μg/mL) against ABTS• and DPPH• radicals at room temperature, data are presented as mean ± SE.
3.5.2. Antioxidant activity of SeNPs
ABTS• and DPPH• methods were applied to evaluate the antioxidant activity of SeNPs concentrations (100, 200, 300, 400, and 500 µg/mL) (Fig. 7B). SeNPs significantly scavenged the ABTS and DPPH radicals. The results showed that the antioxidant activity of SeNPs increased in a concentration-dependent manner. The standard compound showed the highest scavenging activity against ABTS and DDPH radicals (96% and 93%, respectively). The level of 500 µg/mL showed higher ABTS and DPPH radical inhibition (92% and 89%, respectively) compared to the other tested concentrations.
4. Discussion
4.1. Antimicrobial activity of Spirulina
Spirulina has long been used as a functional additive in a number of animal feeds and health food. Therefore, the commercial production of Spirulina has gained importance worldwide due to its multiple benefits. The antimicrobial activity of Spirulina has long been a question of great interest in a wide range of fields during the last decades. Spirulina can suppress several microorganisms' growth due to its rich content of bioactive ingredients with antimicrobial activity. An objective of this study was to investigate the antimicrobial activity of Spirulina against three gram-positive, three gram-negative bacteria, and three strains from both Candida spp. and Aspergillus spp. The present study results revealed that Spirulina extracts had a higher potential to inhibit gram-positive bacteria's growth than gram-negative bacteria. This effect may be attributed to the complicated structure of the cell wall (the outer membrane) of gram-negative bacteria (Breijyeh et al., 2020).
Elshouny et al. reported that Spirulina had stronger antimicrobial activity against S. aureus, E. coli, P. aeruginosa, Salmonella spp., and Shigella spp. than Chlorella vulgaris, Saragassum wightii, and Saragassum latifolium (Elshouny et al., 2021). Additionally, Spirulina methanolic extract was the most effective against tested microorganisms (Gheda and Ismail, 2020). The strong antimicrobial activity of methanolic extract may be attributed to its high total phenolic content. It has been reported that pathogens colonize humans and animals gut with the same mechanism of dhesion and invasion, and the antimicrobial activity of Spirulina might be attributed to its potential to disrupt attachment and invasion, motility, biofilm formation, and quorum sensing of pathogens (Abd El-Hack et al., 2019, Abd El-Hack et al., 2020c, Abd El-Hack et al., 2020d, Abdel-Moneim et al., 2020b, Abou-Kassem et al., 2021a, Saleh et al., 2021). The bioactive compounds in Spirulina can impair bacterial cell integrity and increase cell permeability, which leads to cytoplasmic content leakage. Cultures of Campylobacter jejuni treated with some plant-derived compounds showed a reduction in the activity of the autoinducer AI-2, swarm motility and biofilm formation (90% and 35–75%, respectively) (Castillo et al., 2014).
4.2. Antimicrobial activity of SeNPs
The application of metal‐based antimicrobial strategies and nanoparticles presents one of the extremely promising approaches to prevent diseases caused by antibiotic-resistant microbes (Chudobova et al., 2014). Our results showed that SeNPs synthesized by the strain B. subtilis AL43 exhibited antimicrobial effect towards both gram-positive and gram-negative bacteria, and even antifungal activity against both Candida spp. and Aspergillus spp. The antimicrobial activity of biologically and chemically synthesized SeNPs was evaluated before, but with different methodologies and particle sizes (Cremonini et al., 2016, Tran and Webster, 2011, Zonaro et al., 2015). Nevertheless, the biogenic SeNPs showed stronger antimicrobial activity than the chemically synthesized SeNPs (Cremonini et al., 2016). Furthermore, SeNPs were found to have twice as much IZD against Staphylococcus aureus as silver nanoparticles (7 and 3 mm, respectively) (Chudobova et al., 2014). It has been reported that the antimicrobial effect of SeNPs exhibits size-dependent responses (Zonaro et al., 2015). The small size of nanoparticles results in increasing surface-to-volume ratio, which improves the biological reactivity of the nanoparticles.
These results suggest a probable mechanism of antimicrobial activity of SeNPs involves the generation of reactive oxygen species (ROS) (Galić et al., 2020, Tiwari et al., 2018), penetration of the nanoparticles into the cell, and disruption of cell survival pathways. Nanomaterial-induced ROS plays a fundamental role in cellular toxicity and apoptosis. SeNPs could interact with DNA and impair zntR gene amplified from bacteria (Chudobova et al., 2014). However, the low cytotoxic effect of biogenic SeNPs has been reported (Abbas et al., 2021, Forootanfar et al., 2014). Generation of ROS elevates oxidative DNA damage and membrane lipid peroxidation and subsequently increases cytoplasmic content leakage and damaging cell wall. Estevez et al. found that SeNPs exhibited antimicrobial activity against Mycobacterium tuberculosis via impairing their cell envelope integrity (Estevez et al., 2020). Furthermore, the antimicrobial activity of SeNPs appears to be linked to the nanoparticles and the organic cap surrounding biogenic nanoparticles (Cremonini et al., 2016). Several studies demonstrated that the protein could bind to the SeNPs surface, either through free cysteine or amine group in protein, and act as a capping agent for stabilization (El-Saadony et al., 2020a). Similar results reported that bioactive compounds, e.g., phenolics, can attach to the surface of metal nanoparticles (Cheng et al., 2017, Xu et al., 2018).
4.3. Antioxidant activity of Spirulina
Besides the high nutritional value of Spirulina, several studies provided robust evidence for its potential therapeutic applications. Spirulina contains distinctive natural antioxidants, such as polyphenols, carotenoids, and phycocyanin (Abdel-Moneim et al., 2021b, Estrada et al., 2001, Park et al., 2018). In the current study, the antioxidant activity of different Spirulina extracts (methanol, hexane, and acetone) was evaluated by ABTS and DPPH assays. Results of the current study revealed that all spirulina extracts have a strong antioxidant effect against ABTS and DDPH radicals. This scavenging activity of Spirulina is associated with the rich content of bioactive compounds with multiple biological activities. The values of the total phenolic content of Spirulina extracts were associated with the relative polarity of each extract. The more polar extract (methanol) had higher total phenolic content, followed by acetone and then hexane (Akkari et al., 2016). A previous study observed a positive association between the bioactive substances content and DPPH and ABTS radical scavenging activity (Park et al., 2018). These bioactive compounds have the potential to scavenge single oxygen, superoxide, and hydroxyl radicals (Abd El-Hack et al., 2019, Abo Ghanima et al., 2020, Aladaileh et al., 2020, Attia et al., 2020, Elbaz et al., 2021, Mesalam et al., 2021, Naiel et al., 2020, Yaqoob et al., 2021). Spirulina methanol extract exhibited the highest antioxidant activity compared to other tested extracts, which may be attributed to the high concentration of the bioactive substances in the methanolic extract. A recent study demonstrated that Spirulina methanolic extract had stronger antioxidant activity compared to ethyl acetate, hexane extracts (Gheda and Ismail, 2020). The strong antioxidant effect of methanolic extract appears to be associated with its high total phenolic content. Several previous studies (Abd El-Hack et al., 2019, Abd El-Hack et al., 2020a, Abd El-Moneim and Sabic, 2019, Abd El-Moneim et al., 2019, Abdel-Moneim et al., 2020c, Elbaz et al., 2021) proposed synergetic effect between the bioactive compounds, particularly polyphenols, flavonoids, pigments, polysaccharides, chlorophyll, and polyunsaturated fatty acids. Despite the strong antioxidant activity of polyphenols and flavonoids, the total carotenoid content showed a higher positive correlation with the antioxidant activity than the total phenolic and flavonoid content in some extracts of Spirulina products (Park et al., 2018). Besides, Spirulina act as a probiotic (Abdel-Moneim et al., 2021b, Bhowmik et al., 2009), and the antioxidant effect of probiotics has been well-documented (Abd El-Moneim and Sabic, 2019, Abd El-Hack et al., 2020d, Abdel-Moneim et al., 2020a, Abdel-Moneim et al., 2020b, Amaretti et al., 2013). Some bioactive metabolites from Spirulina have been reported to mediate the activity of the antioxidant enzymes in a cell line model (Bermejo-Bescós et al., 2008). Furthermore, evidence from in vivo studies reported a positive correlation between the antioxidant activity of Spirulina and the anti-inflammatory effect (Abdel-Daim et al., 2016). These findings are important insights for aggregating the practical and functional importance of these natural products.
4.4. Antioxidant activity of SeNPs
Recently, there has been renewed interest in the biosynthesis of nanoparticles owing to the prospect of using them in the future to make nanomedicine. Several studies have aimed to develop new, functional, and cost-effective antioxidants with lower toxicity (Forootanfar et al., 2014). Biogenic synthesis of selenium nanoparticles by microorganisms holds significant potential to be used as an antioxidant agent due to its eco-friendly, low cytotoxicity, low cost, and does not involve organic solvents (Xu et al., 2018). In the current study, the antioxidant activity of SeNPs was investigated by ABTS and DDPH radical scavenging assays. Our results showed that SeNPs exhibited dose-dependent antioxidant activity against ABTS and DDPH radicals. It has been demonstrated that SeNPs synthesized by Lactobacillus casei ATCC 393 (Xu et al., 2018) and Bacillus paralicheniformis SR14 (Cheng et al., 2017) for in vivo use decreased lipid peroxidation and improved the activity of antioxidant enzymes. Besides, SeNPs synthesized by lactic acid bacteria could attenuate H2O2-induced oxidative injury and apoptosis of human normal epithelial cells (NCM460) (Xu et al., 2018). It was also reported that SeNPs synthesized by Bacillus mitigated H2O2-induced oxidative damage in porcine jejunum epithelial (IPEC-J2) (Cheng et al., 2017). The organic cap surrounding biogenic nanoparticles was found to play a crucial role in the potency of SeNPs to scavenge the free radicals (Cheng et al., 2017, Sheiha et al., 2020, Xu et al., 2018). Consistent with the literature, this research confirmed that enhanced antioxidant activity of SeNPs is correlated to the concentration of the total phenolic content (Akkari et al., 2016, Sheiha et al., 2020, Xu et al., 2018).
5. Conclusion
The obtained results showed that all tested Spirulina extracts and SeNPs concentration exhibited antimicrobial and antioxidant activities, which increased in a concentration-dependent manner. Furthermore, antimicrobial and antioxidant activities of Spirulina methanolic extract were observed to be the most potent. This potency of methanolic extract may be attributed to its high total phenolic content. We conclude that Spirulina and SeNPs may act as promising antimicrobial agents as well as natural antioxidant substitutes. Therefore, they can be utilized as alternatives to antibiotics and traditional chemical drugs.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge their respective institutes and universities. The authors are thankful to Taif University Research Supporting Project number (TURSP-2020/315), Taif University, Taif, Saudi Arabia, for providing the financial support and research facilities
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas H.S., Abou Baker D.H., Ahmed E.A. Cytotoxicity and antimicrobial efficiency of selenium nanoparticles biosynthesized by Spirulina platensis. Arch. Microbiol. 2021;203:523–532. doi: 10.1007/s00203-020-02042-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Abdelnour S.A., Abd El-Moneim A.E., Arif M., Khafaga A., Shaheen H., Samak D., Swelum A.A. Putative impacts of phytogenic additives to ameliorate lead toxicity in animal feed. Environ. Sci. Pollut. Res. 2019;26:23209–23218. doi: 10.1007/s11356-019-05805-8. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alagawany M., Abdel-Moneim A.-M.-E., Mohammed N.G., Khafaga A.F., Bin-Jumah M., Othman S.I., Allam A.A., Elnesr S.S. Cinnamon (Cinnamomum zeylanicum) oil as a potential alternative to antibiotics in poultry. Antibiotics. 2020;9:210–221. doi: 10.3390/antibiotics9050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.-E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;1(164):2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. (Berl) 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.-E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2020;28(5):4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Moneim A.E., Sabic E.M. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J. Anim. Feed Sci. 2019;28:52–61. [Google Scholar]
- Abd El-Moneim A.E., Sabic E.M., Abu-Taleb A.M. Influence of dietary supplementation of irradiated or non-irradiated olive pulp on biochemical profile, antioxidant status and immune response of Japanese quails. Biol. Rhythm Res. 2019:1–16. [Google Scholar]
- Abdel-Daim M., El-Bialy B.E., Rahman H.G.A., Radi A.M., Hefny H.A., Hassan A.M. Antagonistic effects of Spirulina platensis against sub-acute deltamethrin toxicity in mice: biochemical and histopathological studies. Biomed. Pharmacother. 2016;77:79–85. doi: 10.1016/j.biopha.2015.12.003. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.-E., Selim D.A., Basuony H.A., Sabic E.M., Saleh A.A., Ebeid T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 2020;52:671–680. doi: 10.1007/s11250-019-02055-1. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.-E., Shehata A.M., Khidr R.E., Paswan V.K., Ibrahim N.S., El-Ghoul A.A., Aldhumri S.A., Gabr S.A., Mesalam N.M., Elbaz A.M. Nutritional manipulation to combat heat stress in poultry–A comprehensive review. J. Therm. Biol. 2021;98 doi: 10.1016/j.jtherbio.2021.102915. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.-E., Shehata A.M., Mohamed N.G., Elbaz A.M., Ibrahim N. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace Elem. Res. 2021:1–12. doi: 10.1007/s12011-021-02662-w. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.E., Elbaz A.M., Khidr R.E., Badri F.B. Effect of in ovo inoculation of Bifidobacterium spp. on growth performance, thyroid activity, ileum histomorphometry and microbial enumeration of broilers. Probiot. Antimicrob. Proteins. 2020;12:873–882. doi: 10.1007/s12602-019-09613-x. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.M.E., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Swelum A.A., Arif M., Fayyaz M., Abd El-Hack M.E. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. (Berl) 2020;104:1851–1855. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- Abdelhady A.Y., El-Safty S.A., Hashim M., Ibrahim M.A., Mohammed F.F., Elbaz A.M., Abdel-Moneim A.-M.-E. Comparative evaluation of single or combined anticoccidials on performance, antioxidant status, immune response, and intestinal architecture of broiler chickens challenged with mixed Eimeria species. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelkhalek N.K., Ghazy E.W., Abdel-Daim M.M. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: impact on lipid peroxidation and oxidative stress. Environ. Sci. Pollut. Res. 2015;22:3023–3031. doi: 10.1007/s11356-014-3578-0. [DOI] [PubMed] [Google Scholar]
- Abdelnour S., El-Saadony M., Saghir S., Abd El-Hack M., Al-Shargi O., Al-Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abo Ghanima M.M., Abd El-Hack M.E., Othman S.I., Taha A.E., Allam A.A., Abdel-Moneim A.-M.-E. Impact of different rearing systems on growth, carcass traits, oxidative stress biomarkers and humoral immunity of broilers exposed to heat stress. Poult. Sci. 2020;99:3070–3078. doi: 10.1016/j.psj.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D., Elsadek M., Abdel-Moneim A., Mahgoub S., Elaraby G., Taha A., Elshafie M., Alkhawtani D., Abd El-Hack M., Ashour E. Growth, carcass characteristics, meat quality and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum) Poult. Sci. 2021;100:84–93. doi: 10.1016/j.psj.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Akkari H., Hajaji S., B’chir F., Rekik M., Gharbi M. Correlation of polyphenolic content with radical-scavenging capacity and anthelmintic effects of Rubus ulmifolius (Rosaceae) against Haemonchus contortus. Vet. Parasitol. 2016;221:46–53. doi: 10.1016/j.vetpar.2016.03.007. [DOI] [PubMed] [Google Scholar]
- Akl B., Nader M.M., El-Saadony M. Biosynthesis of silver nanoparticles by Serratia marcescens ssp sakuensis and its antibacterial application against some pathogenic bacteria. J. Agric. Chem. Biotechnol. 2020;11:1–8. [Google Scholar]
- Aladaileh S.H., Khafaga A.F., Abd El-Hack M.E., Al-Gabri N.A., Abukhalil M.H., Alfwuaires M.A., Bin-Jumah M., Alkahtani S., Abdel-Daim M.M., Aleya L. Spirulina platensis ameliorates the sub chronic toxicities of lead in rabbits via anti-oxidative, anti-inflammatory, and immune stimulatory properties. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.134879. [DOI] [PubMed] [Google Scholar]
- Alagawany M., El-Saadony M., Elnesr S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: Antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Amaretti A., Di Nunzio M., Pompei A., Raimondi S., Rossi M., Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013;97:809–817. doi: 10.1007/s00253-012-4241-7. [DOI] [PubMed] [Google Scholar]
- Ashour E.A., El-Hack M.E.A., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Attia Y.A., Alagawany M.M., Farag M.R., Alkhatib F.M., Khafaga A.F., Abdel-Moneim A.-M.-E., Asiry K.A., Mesalam N.M., Shafi M.E., Al-Harthi M.A. Phytogenic products and phytochemicals as a candidate strategy to improve tolerance to coronavirus. Front. Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.573159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekes M., Lasch P., Naumann D. Analytical applications of Fourier transform-infrared (FT-IR) spectroscopy in microbiology and prion research. Vet. Microbiol. 2007;123:305–319. doi: 10.1016/j.vetmic.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Bermejo-Bescós P., Piñero-Estrada E., del Fresno Á.M.V. Neuroprotection by Spirulina platensis protean extract and phycocyanin against iron-induced toxicity in SH-SY5Y neuroblastoma cells. Toxicol. In Vitro. 2008;22:1496–1502. doi: 10.1016/j.tiv.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Bhowmik D., Dubey J., Mehra S. Probiotic efficiency of Spirulina platensis-stimulating growth of lactic acid bacteria. World J. Dairy Food Sci. 2009;4:160–163. [Google Scholar]
- Breijyeh Z., Jubeh B., Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo S., Heredia N., Arechiga-Carvajal E., García S. Citrus extracts as inhibitors of quorum sensing, biofilm formation and motility of Campylobacter jejuni. Food Biotechnol. 2014;28:106–122. [Google Scholar]
- Cheng Y., Xiao X., Li X., Song D., Lu Z., Wang F., Wang Y. Characterization, antioxidant property and cytoprotection of exopolysaccharide-capped elemental selenium particles synthesized by Bacillus paralicheniformis SR14. Carbohydr. Polym. 2017;178:18–26. doi: 10.1016/j.carbpol.2017.08.124. [DOI] [PubMed] [Google Scholar]
- Chudobova D., Cihalova K., Dostalova S., Ruttkay-Nedecky B., Merlos Rodrigo M.A., Tmejova K., Kopel P., Nejdl L., Kudr J., Gumulec J. Comparison of the effects of silver phosphate and selenium nanoparticles on Staphylococcus aureus growth reveals potential for selenium particles to prevent infection. FEMS Microbiol. Lett. 2014;351:195–201. doi: 10.1111/1574-6968.12353. [DOI] [PubMed] [Google Scholar]
- Cremonini E., Zonaro E., Donini M., Lampis S., Boaretti M., Dusi S., Melotti P., Lleo M.M., Vallini G. Biogenic selenium nanoparticles: characterization, antimicrobial activity and effects on human dendritic cells and fibroblasts. Microb. Biotechnol. 2016;9:758–771. doi: 10.1111/1751-7915.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Jara A., Ruano-Rodriguez C., Polifrone M., Assunçao P., Brito-Casillas Y., Wägner A., Serra-Majem L. Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: main health targets and systematic review. J. Appl. Phycol. 2018;30:2403–2423. [Google Scholar]
- Desoky E.-S.-M., Merwad A.-R.-M., Semida W.M., Ibrahim S.A., El-Saadony M.T., Rady M.M. Heavy metals-resistant bacteria (HM-RB): potential bioremediators of heavy metals-stressed Spinacia oleracea plant. Ecotoxicol. Environ. Saf. 2020;198 doi: 10.1016/j.ecoenv.2020.110685. [DOI] [PubMed] [Google Scholar]
- Desoky E.-S.-M., Saad A.M., El-Saadony M.T., Merwad A.-R.-M., Rady M.M. Plant growth-promoting rhizobacteria: Potential improvement in antioxidant defense system and suppression of oxidative stress for alleviating salinity stress in Triticum aestivum (L.) plants. Biocatal. Agric. Biotechnol. 2020;30(101878) [Google Scholar]
- DeVos, P., Garrity, G., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.-H., Whitman, W.B. 2011. Bergey's manual of systematic bacteriology: Volume 3: The Firmicutes. Springer Science & Business Media.
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28(8):4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.-S.-M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Hack A., Mohamed E., Taha A.E., Fouda M.M., Ajarem J.S., Maodaa N.S., Allam A.A., Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10:587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., El-Wafai N.A., El-Fattah H.I.A., Mahgoub S.A. Biosynthesis, optimization and characterization of silver nanoparticles using a soil isolate of Bacillus pseudomycoides MT32 and their antifungal activity against some pathogenic fungi. Adv. Anim. Vet. Sci. 2019;7:238–249. [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice’s quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28(8):4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.-S.M., Fouda S.E., El-Tahan A.M. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28(8):4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28(8):6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69 [Google Scholar]
- EL-Sabagh M.R., Abd Eldaim M.A., Mahboub D., Abdel-Daim M. Effects of Spirulina platensis algae on growth performance, antioxidative status and blood metabolites in fattening lambs. J. Agric. Sci. 2014;6:92. [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28(9):5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A.M., Ibrahim N.S., Shehata A.M., Mohamed N.G., Abdel-Moneim A.-M.E. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021;53:1–10. doi: 10.1007/s11250-021-02554-0. [DOI] [PubMed] [Google Scholar]
- Elshouny W.A.E.-F., El-Sheekh M.M., Sabae S.Z., Khalil M.A., Badr H.M. Antimicrobial activity of Spirulina platensis against aquatic bacterial isolates. J. Microbiol. Biotechnol. Food sci. 2021;2021:1203–1208. [Google Scholar]
- Ericsson H.M., Sherris J.C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol. Microbiol. Scand B Microbiol. Immunol. 1971;11(Suppl 217):217. [PubMed] [Google Scholar]
- Estevez H., Palacios A., Gil D., Anguita J., Vallet-Regi M., González B., Prados-Rosales R., Luque-Garcia J.L. Antimycobacterial effect of selenium nanoparticles on Mycobacterium tuberculosis. Front. Microbiol. 2020;11:800. doi: 10.3389/fmicb.2020.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada J.P., Bescós P.B., Del Fresno A.V. Antioxidant activity of different fractions of Spirulina platensis protean extract. Il Farmaco. 2001;56:497–500. doi: 10.1016/s0014-827x(01)01084-9. [DOI] [PubMed] [Google Scholar]
- Fesharaki P.J., Nazari P., Shakibaie M., Rezaie S., Banoee M., Abdollahi M., Shahverdi A.R. Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process. Braz. J. Microbiol. 2010;41:461–466. doi: 10.1590/S1517-838220100002000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forootanfar H., Adeli-Sardou M., Nikkhoo M., Mehrabani M., Amir-Heidari B., Shahverdi A.R., Shakibaie M. Antioxidant and cytotoxic effect of biologically synthesized selenium nanoparticles in comparison to selenium dioxide. J. Trace Elem. Med. Biol. 2014;28:75–79. doi: 10.1016/j.jtemb.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Galić E., Ilić K., Hartl S., Tetyczka C., Kasemets K., Kurvet I., Milić M., Barbir R., Pem B., Erceg I. Impact of surface functionalization on the toxicity and antimicrobial effects of selenium nanoparticles considering different routes of entry. Food Chem. Toxicol. 2020;144 doi: 10.1016/j.fct.2020.111621. [DOI] [PubMed] [Google Scholar]
- Gheda S.F., Ismail G.A. Natural products from some soil cyanobacterial extracts with potent antimicrobial, antioxidant and cytotoxic activities. Anais da Academia Brasileira de Ciências. 2020;92 doi: 10.1590/0001-3765202020190934. [DOI] [PubMed] [Google Scholar]
- Gil M.I., Tomás-Barberán F.A., Hess-Pierce B., Kader A.A. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J. Agric. Food Chem. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- Hassan M.A., El-Saadony M.T., Mostafa N.G., El-Tahan A.M., Mesiha P.K., El-Saadony F.M., Hassan A.M., El-Shehawi A.M., Ashry N.M. The use of previous crops as sustainable and eco-friendly management to fight Fusarium oxysporum in sesame plants. Saudi J. Biol. Sci. 2021;28(10):5849–5859. doi: 10.1016/j.sjbs.2021.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanin A.A., Saad A.M., Bardisi E.A., Salama A., Sitohy M.Z. Transfer of anthocyanin accumulating delila and rosea1 genes from the transgenic tomato micro-tom cultivar to moneymaker cultivar by conventional breeding. J. Agric. Food Chem. 2020;68:10741–10749. doi: 10.1021/acs.jafc.0c03307. [DOI] [PubMed] [Google Scholar]
- Holman B., Malau-Aduli A. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. (Berl) 2013;97:615–623. doi: 10.1111/j.1439-0396.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim N.S., Sabic E.M., Wakwak M.M., El-Wardany I.E., El-Homosany Y.M., El-Deen Mohammad N. In-ovo and dietary supplementation of selenium nano-particles influence physiological responses, immunological status and performance of broiler chicks. J. Anim. Feed Sci. 2020;29:46–58. [Google Scholar]
- Kalagatur N.K., Kamasani J.R., Mudili V. Assessment of detoxification efficacy of irradiation on zearalenone mycotoxin in various fruit juices by response surface methodology and elucidation of its in-vitro toxicity. Front. Microbiol. 2018;9:2937. doi: 10.3389/fmicb.2018.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kata F.S., Athbi A.M., Manwar E.Q., Al-Ashoor A., Abdel-Daim M.M., Aleya L. Therapeutic effect of the alkaloid extract of the cyanobacterium Spirulina platensis on the lipid profile of hypercholesterolemic male rabbits. Environ. Sci. Pollut. Res. 2018;25:19635–19642. doi: 10.1007/s11356-018-2170-4. [DOI] [PubMed] [Google Scholar]
- Mendiola J., Jaime L., Santoyo S., Reglero G., Cifuentes A., Ibañez E., Señoráns F. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007;102:1357–1367. [Google Scholar]
- Mesalam N.M., Aldhumri S.A., Gabr S.A., Ibrahim M.A., Al-Mokaddem A.K., Abdel-Moneim A.-M.E. Putative abrogation impacts of Ajwa seeds on oxidative damage, liver dysfunction and associated complications in rats exposed to carbon tetrachloride. Mol. Biol Rep. 2021 doi: 10.1007/s11033-021-06544-1. In Press. [DOI] [PubMed] [Google Scholar]
- Naiel M.A., Shehata A.M., Negm S.S., Abd El-Hack M.E., Amer M.S., Khafaga A.F., Bin-Jumah M., Allam A.A. The new aspects of using some safe feed additives on alleviated imidacloprid toxicity in farmed fish: a review. Rev. Aquac. 2020;12:2250–2267. [Google Scholar]
- Nour M.A., El-Hindawy M.M., Qattan S.Y., Abou-Kassem D.E., Ashour E.A., Aboelenin S.M., Soliman M.M., Abdel-Moneim A.-M.E. Effect of graded levels of dietary Bacillus toyonensis and Bifidobacterium bifidum supplementation on growth, carcass traits and ileal histomorphometry and microbiota of growing quails. Saudi J. Biol. Sci. 2021:4532–4541. doi: 10.1016/j.sjbs.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Lee S., Kim I. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018;97:2451–2459. doi: 10.3382/ps/pey093. [DOI] [PubMed] [Google Scholar]
- Reda F., El-Saadony M., El-Rayes T., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021:101266. doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate Sustainable Silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol Sci. 2021;28(10):5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad A.M., El-Saadony M.T., Mohamed A.S., Ahmed A.I., Sitohy M.Z. Impact of cucumber pomace fortification on the nutritional, sensorial and technological quality of soft wheat flour-based noodles. Int. J. Food Sci. Technol. 2021;56:3255–3268. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT-Food Sci. Technol. 2021;148 [Google Scholar]
- Saad A.M., Mohamed A.S., Ramadan M.F. Storage and heat processing affect flavors of cucumber juice enriched with plant extracts. Int. J. Veg. Sci. 2020:1–11. [Google Scholar]
- Saad A.M., Osman A.O.M., Mohamed A.S., Ramadan M.F. Enzymatic hydrolysis of Phaseolus vulgaris protein isolate: characterization of hydrolysates and effect on the quality of minced beef during cold storage. I Int. J. Pept. Res. Ther. 2020;26:567–577. [Google Scholar]
- Saleh A.A., Shukry M., Farrag F., Soliman M.M., Abdel-Moneim A.-M.E. Effect of feeding wet feed or wet feed fermented by Bacillus licheniformis on growth performance, histopathology and growth and lipid metabolism marker genes in broiler chickens. Animals. 2021;11:83. doi: 10.3390/ani11010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., El-Hack A., Mohamed E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal N., Kumar M., Kanaujia P.K., Virdi J.S. MALDI-TOF mass spectrometry: an emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015;6:791. doi: 10.3389/fmicb.2015.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari V., Mishra N., Gadani K., Solanki P.S., Shah N., Tiwari M. Mechanism of anti-bacterial activity of zinc oxide nanoparticle against carbapenem-resistant Acinetobacter baumannii. Front. Microbiol. 2018;9:1218. doi: 10.3389/fmicb.2018.01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran P.A., Webster T.J. Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomedicine. 2011;6:1553. doi: 10.2147/IJN.S21729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Qiao L., Guo Y., Ma L., Cheng Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr. Polym. 2018;195:576–585. doi: 10.1016/j.carbpol.2018.04.110. [DOI] [PubMed] [Google Scholar]
- Yadav, V., Sharma, N., Prakash, R., KK, R., Bharadwaj, L., Prakash, N.T., 2008. Generation of selenium containing nano-structures by soil bacterium Pseudomonas Aeruginosa. Biotechnol. 7, 299–304.
- Yaqoob M., Abd El-Hack M., Hassan F., El-Saadony M., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021:101143. doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrouk C. University of Paris; France: 1966. Contribution a l'etude d'une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina mixima. Thesis. [Google Scholar]
- Zonaro E., Lampis S., Turner R.J., Qazi S.J.S., Vallini G. Biogenic selenium and tellurium nanoparticles synthesized by environmental microbial isolates efficaciously inhibit bacterial planktonic cultures and biofilms. Front. Microbiol. 2015;6:584. doi: 10.3389/fmicb.2015.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]