Abstract
The non-enzymatic and enzymatic antioxidant defense systems play a major role in detoxification of pro-oxidant endobiotics and xenobiotics. The possible involvement of beetle non-enzymatic [α-tocopherol, glutathione (GSH), and ascorbic acid] and enzymatic [catalase (CAT), superoxide dismutase (SOD), peroxidase (POX), and polyphenol oxidase (PPO)] antioxidant defense system on the insecticidal activity of synthetic insecticides (cypermethrin, 2,2-dicholorovinyl dimethyl phosphate, and λ-cyhalothrin) and ethanolic plant extracts of Tithonia diversifolia, Cyperus rotundus, Hyptis suaveolens leaves, and Jatropha Curcas seeds was investigated. 2,2-Dicholorovinyl dimethyl phosphate (DDVP; 200 ppm, LC50 = 13.24 ppm) and T. diversifolia (20,000 ppm) resulted in 100% beetle mortality at 96-hour post-treatment. The post-treatments significantly increased the beetle α-tocopherol and GSH contents. Activities of CAT, SOD, POX, and PPO were modulated by the synthetic insecticides and bioinsecticides to diminish the adverse effect of the chemical stresses. Quantitative and qualitative allelochemical compositions of bioinsecticides and chemical structure of synthetic insecticides possibly account and for modulation of their respective enzyme activities. Altogether, oxidative stress was enormous enough to cause maladaptation in insects. This study established that oxidative imbalance created could be the molecular basis of the efficacy of both insecticides and bio-insecticides. Two, there was development of functional but inadequate antioxidant defense mechanism in the beetle.
Keywords: Callosobruchus maculatus Cowpea storage beetles, oxidative stress, antioxidant enzymes, insecticides
Introduction
Cowpea [Vigna unguiculata (L.) Walp.] is a leguminous crop and a source of revenue that is popularly grown in various parts of tropical and subtropical regions of Africa. Seeds of the crop serve as a source of dietary protein to naturally and nutritionally complement starchy cereals and tuber crops where the per capital daily intake of animal proteins is low, thus alleviating protein malnutrition in humans.1,2 The green parts of the plant can be used as vegetables or as fodder for cattle. Cowpea is susceptible to many insect pests both on the field and during storage. 3 The major constraint facing effective and efficient storage of the grain legume is the insect pest depredation. Cowpea storage beetle (CSB), Callosobruchus maculatus (Fabr.) (Coleoptera: Chrysomelidae), is a principal storage pest of cowpea and poses a great challenge to the process of assuring food security in developing tropical countries like Nigeria.4,5 Infestation by this insect begins from the field when mature adults lay eggs on drying cowpea pods or seeds. The eggs hatch into larvae, which use their mouthparts to penetrate the pod wall or the seed testa. This feeding process ultimately reduces the weight of the seeds and enhances mold growth, poor germination viability, and hampered seed marketability. 6 The post-harvest loss could be 100% within two to three months after harvest. Tropical temperatures and humidity are reported to be responsible for rapid increase in the number of insect population. 7 Unfortunately, Cowpea genome lacks insect resistant gene loci to be induced to produce insect-resistant cultivar. 8
Over the years, diverse methods have been used to protect the stored cowpea against the pulse beetle (CSB). Hermetic storage, synthetic insecticides and/or plant materials application, gamma irradiation, and freezing and heating the beans are some of the documented control methods.9,10 Substantial progress has been made on the effective and efficient control of this pulse beetle, which focused on the use of synthetic insecticides and plant products.5,11 The use of insecticides remains the most effective means of controlling beetle in large-scale storages. Indiscriminate and misguided use and undesirable side effects have limited the usage of synthetic insecticides. Currently, plant materials as bioinsecticides have also proven their efficacy as insecticides.12,13 The responses of the pulse beetle (CSB) to insecticides and bioinsecticides have been informative on ovicidal and oviposition effects, adult emergence, and avoidance and repellence to insecticides and the bioinsecticides. All these have not provided useful baseline information for strategizing effective control and resistance management. The main threat to the success of this control approach is the possible development of resistance to synthetic insecticides and bioinsecticides after prolong usage without prior and absolute understanding of the molecular basis of their respective efficacy. The current and reported understandings of the molecular basis of efficacy of the insecticidal plants and some selected insecticides on CSB have been inadvertently scanty. In CSB, study of acetylcholine esterase (AChE), 14 cytochrome P450, 8 and glutathione (GSH) transferase 15 is motivated by the perception that they have a role in insecticide or bioinsecticide toxicity.
The efficacy of the insecticidal plants and insecticides on the CSB could be based on several mechanisms to exert their toxic effect despite adaptive and compensatory responses that could be induced as a result of exposure. Some reports have posited that some pesticides and bergapten (an allelochemical) cause oxidative stress, characterized by exposure to in vivo excessive reactive oxygen species (ROS).8,16,17 ROS is involved in the pathogenesis of some diseases. It is doubtful, however, in cowpea storage bruchid, if oxidative stress may be a consequence of detoxification enzymes' inhibitions, a separate effect, or a concurrent event. Inconsistent and preliminary results exist regarding this line of thought. Considerable work has been done on antioxidant enzyme systems and detoxification systems in insects. There have been no linkages of insects' antioxidant enzyme systems and detoxification enzymes.
Given the present suggestion that synthetic insecticides and plant-derived insecticides may cause oxidative stress, the present study was undertaken to characterize their role in oxidative stresses and cellular antioxidant defense mechanism in their possible toxicity or otherwise. Ethanolic extracts of Tithonia diversifolia, Cyperus rotundus, Hyptis suaveolens leaves, and Jatropha Curcas seeds as well as some selected synthetic insecticides on the bruchid non-enzymatic antioxidants contents and antioxidant enzymes [superoxide dismutase (SOD), catalase (CAT), peroxidase (POX), and polyphenol oxidase (PPO)] was studied.
Materials and Methods
Materials
Catechol, 5,5-dithio-bis-2-nitrobenzoic acid (DTNB), Pyrogallol, and O-phthalaldehyde were purchased from Sigma-Aldrich Chemie GmbH. Bradford reagent (#500-006) and BSA protein standard (#500-0206) were from Bio-Rad Laboratories. Cyperforce (Cypermethrin) was a product of Gharda Chemicals Ltd, and Master (λ-cyhalothrin) was purchased from Sinochem Ningbo Ltd. Sniper (2,2-dicholorovinyl dimethyl phosphate; DDVP) was manufactured by Hubei Sanonda Co. Ltd and supplied by Saro AgroSciences Limited. All other chemicals were of analytical grade. Absolute ethanol was used as a solvent in the preparation of the pesticides and biopesticide samples.
Preparation of Plant-Derived Insecticides
Mature fresh leaves of T. diversifolia, C. rotundus, and H. suaveolens; and the seeds of J. curcas were collected from the Teaching and Research Farm of the Federal University of Technology, Akure, Nigeria in July 2012. The climatic condition of the location is tropical sub-Sahara. The plant materials were rinsed with autoclaved distilled water, air-dried to constant weight, and then ground into fine powder. Chemical extraction was carried out as described earlier. 18 Extracts were stored at —20°C prior to use. Here, extracted plant materials were referred to as bioinsecticides.
Insect Culture
C. maculatus adults used for the establishment of the insect colony were obtained from a local market in Akure, Nigeria. The beetles were cultured on clean uninfested susceptible cowpea seeds in an ambient laboratory temperature of 25 ± 1°C, a relative humidity of 75 ± 5%, and a 12-hour photoperiod. New generation of CSB that was used for subsequent experiments was derived from the stock culture by infesting clean uninfested beans with 20 copulating pairs of teneral adult CSB.
Mortality Response
In a typical mortality response study, disinfested cowpea seeds weighing 100 g put in sterile petri dishes (9 cm diameter) were serially treated with 2.0 mL of Cyperforce (cypermethrin), Master (λ-cyhalothrin), and Sniper (DDVP) at concentrations of 0, 50, 100, 150 and 200 ppm. In another set of dishes, the seeds were thoroughly mixed with an aliquot of 2 mL of 5,000, 10,000, 15,000, and 20,000 ppm ethanolic extracts of plant materials. The dishes were agitated manually for five minutes and then allowed to air-dry for four hours. Each experimental dish, prepared in triplicates, was infested with copulating adult beetle (20 males and 20 females). The mortality of the beetle because of the insecticides and the bioinsecticides was monitored for four days. Here, the LC50 values were derived from the best-fit line obtained from regression equation (Y = % mortality; X = concentration) using Microsoft Excel (for Mac 2011).
Enzyme Extraction and Assays
Frozen 700 mg of whole insects were homogenized in ice cold buffer [50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA] and centrifuged at 10,000 g for 20 minutes at 4°C. Protein contents were quantified by Bradford's method, using bovine serum albumin as a standard. 19 Unless otherwise stated, Shimadzu UV 1800 Double Beam Spectrophotometer was used for all colorimetric assays, while Hitachi F-4500 Fluorescence Spectrophotometer was used for fluorimetric assays.
Determination Ascorbic Acid Content
Ascorbic acid content was estimated by following exact procedure described by Omaye et al 20 where 1 g of insect was ground with 5 mL of 10% trichloroacetic acid (TCA) and the mixture was centrifuged at 5,000 g for 20 minutes at 4°C. An aliquot of 1.0 mL of 2,4-dinitrophenyl hydrazine-thio-urea-CuSO4 (DTC) reagent was added to 0.5 mL of the supernatant, and the mixture was allowed to stand for three hours at 37°C. The reaction was terminated with 0.75 mL of ice-cold 65% H2SO4. After incubation at 30°C for 30 minutes, absorbance was read at 520 nm against blank. The result of the ascorbic acid content was expressed in milligrams per gram of fresh weight.
Determination Reduced GSH Content
The method of Ellman 21 was used for the assay. In brief, 200 mg of insects was homogenized with 2 mL of 5% (w/v) TCA in 1 mM EDTA and then centrifuged at 10,000 g for 20 minutes at 4°C. One milliliter of the reaction mixture, containing 150 μL extract, 800 μL of 0.1 M phosphate buffer (pH 8.0), and 50 μL of DTNB (0.01% in 0.1 M phosphate buffer, pH 8.0), was mixed thoroughly and then incubated at 25°C for 20 minutes. The absorbance of the reaction mixture was 412 nm. The GSH content was determined from a GSH standard curve, and the result was expressed in micrograms per gram of fresh weight.
Determination of α-Tocopherol Content
α-Tocopherol content was estimated as described elsewhere. 22 2,2 Dipyridyl was used as chromophore, and absorbance was read at 520 nm. α-Tocopherol content was read from α-tocopherol standard curve and later expressed in micrograms per gram of fresh weight.
Determination of SOD Activity
SOD activity was assayed according to the method of Fridovich, 23 which uses a tetrazolium salt to detect superoxide radicals generated by xanthine and xanthine oxidase system. The absorbance was monitored at 550 nm. SOD activity was expressed in units per milligram of protein. One unit is defined as the amount of change in the absorbance by 0.1 hours−1 mg−1 proteins.
Determination of CAT Activity
CAT activity was determined as modified by Aebi. 24 The rate of H2O2 decomposition was spectrophotometrically monitored at 240 nm. Enzyme activities were calculated using 0.0394 mM−1 cm−1 as absorption coefficient at 240 nm. A unit of CAT activity is defined as the amount of enzyme required to decompose 1 mM of hydrogen peroxide in a minute. The CAT activity was expressed in units per milligram of protein.
Determination of POX Activity
POX activity was assayed according to the protocol described by Kumar and Khan. 25 The reaction mixture comprised 2.0 mL of 0.1 M potassium phosphate buffer (pH 6.8), 1.0 mL of 0.01 M Pyrogallol, 1 mL of 0.005 M H2O2, and 0.5 mL of enzyme extract prepared from whole insect homogenate. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm. The POX activity was expressed in units per milligram of protein. One unit is defined as a change in the absorbance by 0.1 minute−1 mg−1 protein.
PPO Activity
The method of Kumar and Khan 25 was used to estimate PPO activity in a reaction mixture containing 2 mL of 0.1 M potassium phosphate buffer (pH 6.0), 1 mL of 0.1 M catechol, and 0.5 mL of enzyme extract. The purpurogallin formed was read at 495 nm. PPO activity was expressed as units per milligram of protein (U = change in absorbance by 0.1 minute−1 mg−1 protein).
Statistical Analysis
All data obtained were subjected to one-way analysis of variance (ANOVA) using the SPSS version 17.0 statistical software (SPSS). The means were separated using Duncan's New Multiple Range Test (DNMRT) at 5% probability level where significant differences existed between them.
Results
Beetle Mortality
The result of percentage mortality of bioinsecticides and the synthetic insecticides used in this study on the adult C. maculatus after four days post-treatment is presented in Table 1. ANOVA revealed the significant difference between the mortality of CSB in the control and the treated samples. Among the synthetic insecticides, DDVP is the most potent with 100% mortality at 96 hours post-treatment. In all, 100% mortality of C. maculatus was observed in the group treated with T. diversifolia leaf extract at a concentration of 20,000 ppm, while H. suaveolens leaves have the least effect.
Table 1.
Percentage mortality of adult Callosobrochus maculatus in cowpea seeds treated with some bio-insecticides and insecticides.
| (A) BIO-INSECTICIDES | ||||
|---|---|---|---|---|
| CONCENTRATION (ppm) | MORTALITY OF C. Maculatus (%) |
|||
| C. Rotundus | T. Diversifolia | H. Suavolens | J. Curcas | |
| 0 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 5,000 | 75.00 ± 4.08a | 90.00 ± 1.83a | 16.25 ± 2.63a | 86.25 ± 1.71a |
| 10,000 | 85.00 ± 3.46b | 93.75 ± 0.96a | 17.50 ± 3.30b | 87.50 ± 3.70a |
| 15,000 | 88.75 ± 3.30b | 96.25 ± 0.96a | 21.25 ± 0.50c | 91.25 ± 1.71a |
| 20,000 | 91.25 ± 0.50c | 100.00 ± 0.00b | 22.50 ± 1.83d | 95.00 ± 1.41b |
Notes: The mean mortality values results were expressed as mean ± SEM (three replicates) determined by via one way ANOVA followed by Duncan's New Multiple Range Test (DNMRT). The mean values, in the same column with different superscripts are significantly different P ≤ 0.05 while means with the same superscripts indicate non-significant changes. Abbreviation: ppm, part per million.
Ascorbic Acid Estimation
Information on the ascorbic acid content of the bruchid is presented in Table 2. Ascorbic acid content of all C. maculatus-treated groups decreased significantly (P ≤ 0.05) compared to the control group. C. rotundus- and H. suaveolens-treated groups showed an increased ascorbic acid content after an initial decrease; however, the concentration of ascorbic acid decreased further in C. maculatus treated with T. diversifolia and J. curcas as the concentration of the extracts increased. The ascorbic acid content of the insecticide-treated groups decreased as the sample concentrations increased for DDVP and cypermethrin but increased for λ-cyhalothrin.
Table 2.
Effect of insecticides and bio-insecticides on Ascorbic acid content of adult Callosobrochus maculatus.
| BIO-INSECTICIDES CONC. (ppm) | ASCORBIC ACID CONTENT (mg g−1 FRESH WEIGHT) |
|||
|---|---|---|---|---|
| C. Rotundus | T. Diversifolia | H. Suavolens | J. Curcas | |
| 0 | 29.71 ± 0.10a | 29.71 ± 0.10a | 29.71 ± 0.10a | 29.71 ± 0.10a |
| 5,000 | 5.013 ± 0.10e | 22.20 ± 0.60b | 4.54 ± 0.10e | 16.32 ± 0.50b |
| 10,000 | 5.82 ± 0.50d | 19.62 ± 0.60c | 10.66 ± 0.40d | 13.69 ± 0.50c |
| 15,000 | 11.23 ± 0.10c | 18.19 ± 0.90d | 12.55 ± 0.50c | 11.56 ± 0.40d |
| 20,000 | 12.65 ± 0.60b | 17.24 ± 0.10d | 15.08 ± 0.10b | 9.87 ± 0.20e |
Notes: The values were expressed as mean ± SEM (three replicates) determined by via one way ANOVA followed by Duncan's New Multiple Range Test (DNMRT). The mean values, in the same column, with different superscripts are significantly different P ≤ 0.05 while means with the same superscripts indicate non-significant changes.
Abbreviation: ppm, part per million.
Reduced GSH Content
The reduced GSH content of the cowpea seed beetle is shown in Table 3. There was a significant increase (P ≤ 0.05) in the GSH content of C. maculatus group treated with C. rotundus and H. suaveolens compared to the control. However, there was an initial decrease in the GSH content at 5,000 ppm in the group treated with T. diversifolia before the increase as the exposure concentration increases. The exogenous application of DDVP increased GSH content of the beetle.
Table 3.
Effect of insecticides and bio-insecticides on reduced Glutathione content of adult Callosobrochus maculatus.
| BIO-INSECTICIDES CONC. (ppm) | GSH CONTENT (μg g−1 FRESH WEIGHT) |
|||
|---|---|---|---|---|
| C. Rotundus | T. Diversifolia | H. Suavolens | J. Curcas | |
| 0 | 47.53 ± 0.50e | 47.53 ± 0.50d | 47.53 ± 0.50e | 47.53 ± 0.50d |
| 5,000 | 83.11 ± 1.00b | 13.27 ± 0.80e | 58.90 ± 0.50d | 120.67 ± 0.90a |
| 10,000 | 120.90 ± 0.20a | 64.73 ± 0.60b | 61.44 ± 0.60c | 107.83 ± 1.70b |
| 15,000 | 71.70 ± 2.10c | 56.30 ± 0.70c | 76.91 ± 2.30b | 25.67 ± 1.40e |
| 20,000 | 65.47 ± 1.20d | 83.30 ± 0.90a | 80.95 ± 0.60a | 56.34 ± 0.70c |
Notes: The values were expressed as mean ± SEM (three replicates) determined by via one way ANOVA followed by Duncan's Multiple Range Test (DNMRT). The mean values, in the same column, with different superscripts are significantly different P ≤ 0.05 while means with the same superscripts indicate non-significant changes.
Abbreviation: ppm, part per million.
α-Tocopherol Estimation
The result shown in Table 4 represents the effect of varied concentrations of bioinsecticides and synthetic insecticides on the α-tocopherol content of C. maculatus. C. maculatus group treated with 20,000 ppm C. rotundus had a 3% increase in α-tocopherol content compared with the control. This was after an initial decrease in the α-tocopherol content. C. maculatus group treated with H. suaveolens and T. diversifolia extracts had a significant increase (P ≤ 0.05) in α-tocopherol content at 5,000, 15,000, and 20,000 ppm when compared with the control value. α-Tocopherol values of the insects group treated with DDVP and cypermethrin decreased with the increase in insecticide concentrations compared with the values of the control group.
Table 4.
Effect of insecticides and bio-insecticides on α-Tocopherol content of adult Callosobrochus maculatus.
| BIO-INSECTICIDE CONC. (ppm) | α-TOC CONTENT (mg g−1 FRESH PROTEIN) |
|||
|---|---|---|---|---|
| C. Rotundus | T. Diversifolia | H. Suavolens | J. Curcas | |
| 0 | 33.37 ± 0.40b | 33.37 ± 0.40c | 33.37 ± 0.40c | 33.37 ± 0.40b |
| 5,000 | 19.93 ± 0.40c | 33.87 ± 0.30c | 39.20 ± 0.80b | 35.67 ± 0.50a |
| 10,000 | 13.37 ± 0.30d | 12.47 ± 0.30d | 13.93 ± 0.60d | 33.90 ± 0.20b |
| 15,000 | 20.50 ± 0.30c | 37.07 ± 0.60b | 40.97 ± 0.30a | 24.23 ± 0.70c |
| 20,000 | 34.36 ± 0.30a | 41.13 ± 1.00a | 41.83 ± 0.30a | 33.27 ± 0.40b |
Notes: The values were expressed as mean ± SEM (three replicates) determined by via one way ANOVA followed by Duncan's New Multiple Range Test (DNMRT). The mean values, in the same column, with different superscripts are significantly different P ≤ 0.05 while means with the same superscripts indicate non-significant changes.
Abbreviation: ppm, part per million.
SOD Activity
Effect of varied concentrations of synthetic insecticides and bioinsecticides on CSB SOD activity is presented in Figure 1. The result revealed a significant decrease (P ≤ 0.05) in SOD activities of C. maculatus groups treated with the various bioinsecticides compared with the control. SOD activity of C. maculatus group treated with 20,000 ppm H. suaveolens (0.35 U mg−1 protein) was 32 fold lower than the value of the control. The enzyme activity for all the groups treated with synthetic insecticides increased with increasing concentrations of all the treatments. For the synthetic insecticides, the SOD activity was enhanced at higher concentration (100–200 ppm) of the insecticides. However, at a concentration of 10 ppm, the activity value was higher in the control than SOD activity value of the insects treated with the synthetic insecticides.
Figure 1.
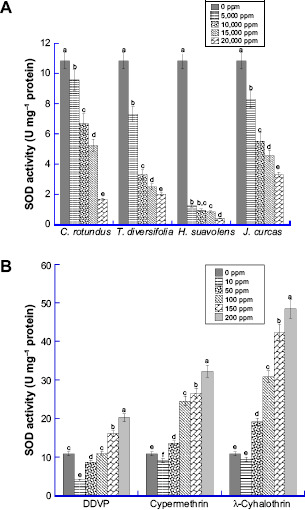
Level of Superoxide dis mutase (SOD) in the whole tissue homogenate of C. maculatus exposed to different concentrations of (A) bio-pesticides (Tithonia diversifolia, Cyperus rotundus, Hyptis suavolens leaves and Jatropha curcas seed) and (B) pesticides (DDVP, Cypermethrin and λ-cyhalothrin). The values are mean ± SD of triplicate determination of the results (U mg−1 proteins). Means within the same column followed by the same letter(s) are not statistically significant different using Duncan's Multiple Range Test (DMRT) at a 95% level of confidence (P ≤ 0.05). Different letters indicate significant differences.
CAT Activity
The effect of varied concentrations of synthetic insecticides and bioinsecticides on CSB CAT activity is presented in Figure 2. A significant increase (P ≤ 0.05) was observed for C. maculatus groups treated with T. diversifolia and J. curcas extracts at 5,000 and 10,000 ppm. At 20,000 ppm, CAT activities of C. maculatus groups treated with C. rotundus (0.85 U mg−1 protein), T. diversifolia (1.45 U mg−1 protein), H. suaveolens (0.14 U mg−1 protein), and J. curcas (0.75 U mg−1 protein) were 3.9, 2, 24, and 4.4 folds lower, respectively, compared with the values in the control group. There was increase in CAT activity in all the synthetic insecticide-treated groups compared to the value of the control. The enzyme showed increased activity with increasing concentration of DDVP.
Figure 2.
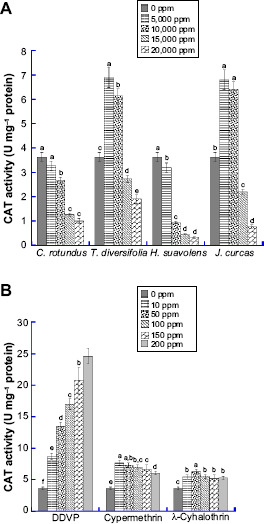
Level of Catalase (CAT) in the whole tissue homogenate of C. maculatus exposed to different concentrations of (A) bio-pesticides (Tithonia diversifolia, Cyperus rotundus, Hyptis suavolens leaves and Jatropha curcas seed) and (B) pesticides (DDVP, Cypermethrin and λ-cyhalothrin). The values are mean ± SD of triplicate determination of the results (U mg−1 proteins). Means within the same column followed by the same letter(s) are not statistically significant different using Duncan's Multiple Range Test (DMRT) at a 95% level of confidence (P ≤ 0.05).
POX Activity
Figure 3 shows the effect of varied concentrations of synthetic insecticides and bioinsecticides on CSB POX activity. C. maculatus group treated with 5,000 ppm T. diversifolia (3.65 U mg−1 protein) had a 28% increase in activity, whereas the group treated with 20,000 ppm J. curcas (2.99 U mg−1 protein) had 13% increase in POX activity compared with the control. The POX activity increased at higher concentrations of DDVP. Decrease in activity of the enzyme was obtained for cypermethrin-treated samples. The enzyme also exhibited increased activity in all concentrations of λ-cyhalothrin.
Figure 3.
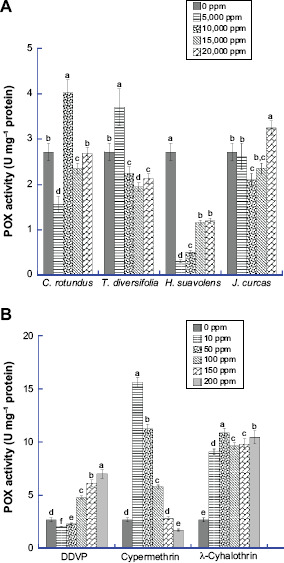
Level of Peroxidase (POX) in the whole tissue homogenate of C. maculatus exposed to different concentrations of (A) bio-pesticides (Tithonia diversifolia, Cyperus rotundus, Hyptis suavolens leaves and Jatropha curcas seed) and (B) pesticides (DDVP, Cypermethrin and λ-cyhalothrin). The values are mean ± SD of triplicate determination of the results (U mg−1 proteins). Means within the same column followed by the same letter(s) are not statistically significant different using Duncan's Multiple Range Test (DMRT) at 95% level of confidence (P ≤ 0.05).
PPO Activity
The result presented in Figure 4 shows the effect of varied concentrations of synthetic insecticides and bioinsecticides on CSB PPO activity. It revealed a significant decrease (P ≤ 0.05) in PPO activities of C. maculatus for all bioinsecticide-treated groups compared with the control. C. maculatus group treated with 20,000 ppm C. rotundus extract (0.30 U mg−1 protein) had a 15.2 fold decreased PPO activity compared with the control. The enzyme activity in the insecticide-treated groups increased with increasing concentrations in all the samples.
Figure 4.
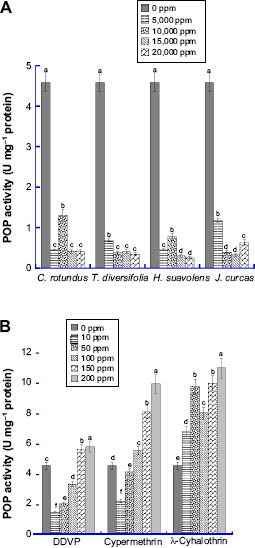
Level of Polyphenol oxidase (PPO) in the whole tissue homogenate of C. maculatus exposed to different concentrations of (A) bio-pesticides (Tithonia diversifolia, Cyperus rotundus, Hyptis suavolens leaves and Jatropha curcas seed) and (B) pesticides (DDVP, Cypermethrin and λ-cyhalothrin). The values are mean ± SD of triplicate determination of the results (U mg−1 proteins). Means within the same column followed by the same letter(s) are not statistically significant different using Duncan's Multiple Range Test (DMRT) at a 95% level of confidence (P ≤ 0.05).
Discussion
The results obtained in this study clearly demonstrate that bioinsecticides and synthetic insecticides have distinct effects on the survival/mortality of the beetles. While DDVP was the most potent among the synthetic insecticides with an LC50 of 13.43 ppm, the extract from T. diversifolia was the most potent among the bioinsecticides. Even though very high concentrations of bioinsecticides are required for them to be as effective as the synthetic insecticides in causing mortality, they are equally promising as the synthetic insecticides in insect control. The differences in the efficacy of the bioinsecticides might be a result of qualitative and quantitative composition of the secondary metabolites that characterized the ethanolic extract.26–28 Ascorbic acid is a water-soluble antioxidant that is capable of scavenging activated oxygen species such as O2−, OH•, and H2O2. 29 However, its efficiency in mopping up superoxide anions is an order of magnitude lower than that of SOD. The decrease in ascorbic acid concentration may be ascribed to the increased usage of this non-enzymatic antioxidant to prevent the build-up of peroxides, which could have been generated in the insect in response to the bioinsecticides' and the synthetic insecticides' stresses. This result agrees with the work of Barbehenn et al 30 who reported a decline in lumenal ascorbate levels in the posterior midguts of Malacosoma disstria larvae when fed diets containing tannic acid. A reduction in the ascorbic acid concentration was also observed in all the tested tissues of Aulocara ellioti after it was fed wheat plant. 31 Ascorbic acid is also a substrate for redox enzyme ascorbate POX, a function that is particularly important in stress-resistant insects. 32
GSH is a thiol group containing antioxidant that plays a very important role in the defense against ROS. GSH, together with ascorbate, participates in the protection against oxidative stress by its involvement in the ascorbate–GSH cycle, regulation of protein thiol-disulfide redox status, and reduction of H2O2 to water. 32 The exposure to the bioinsecticides might have brought about the induction of GSH synthesis. The inverse correlation between GSH and ascorbic acid observed in this study indicates that the increase in GSH content may be to compensate for the decline in the level of ascorbic acid. This inverse correlation also shows that intracellular GSH level appears to be a major determinant of the oxidative stress between the two indicators—GSH and ascorbic acid. All these suggest the possibility of cytotoxicity being mediated by oxidative stress. However, synthetic insecticides used do not appreciably alter the GSH content compared to bio-insecticides. The effect of bioinsecticides could be a result of the medley of allelochemicals that characterized the ethanolic extracts. 26 The response of the beetle GSH to the insecticides could demonstrate the insect sensitivity to xenobiotics and might be connected to enhance utilization of GSH as cosubstrate for GSH transferase or cofactor for GSH POX. 33
α-Tocopherols are lipid soluble antioxidants that play an important role in defense against oxidative stress. Their function is to interact with the polyunsaturated acyl groups of lipids, stabilize membranes, and scavenge and quench various ROS and lipid soluble by-products of oxidative stress. 34 Various protective antioxidant roles of α-tocopherols have been documented in many species of organisms. In this study, the effect of T. diversifolia and H. suaveolens bioinsecticides on CSB α-tocopherols indicates a greater degree of protection that can allow the insect survive oxidative damage. However, the protection might not be adequate. The increase in α-tocopherols result agrees with the report of Barbehenn 35 who also observed an elevated α-tocopherols concentration in the midgut tissues of Melanoplus sanguinipes after being fed on diet containing 15% tannic acid. In some cases, the decrease in protection against oxidative stress was directly linked to lipid peroxidation and free radical formation. 36
The change in (SOD activity at all concentrations of the bioinsecticides tested against CSB suggests that the bioinsecticides may possibly induce O2− in vivo. Synthetic insecticides also enhanced SOD activity. The increase might not be unconnected to chemical structure of the synthetic insecticides. However, multivarious chemical components of bioinsecticides might have suppressed the SOD induction. SODs are the first line of defense against oxygen free radicals. This enzyme removes superoxide radicals (O2−) through the process of dismutation to oxygen and hydrogen peroxide (H2O2).37,38 The ability of SOD to scavenge O2− is temporary and limited. 39 SOD is an antioxidant enzyme that can protect normal cells from ROS. The ability of this enzyme to overcome the toxic effects of ROS in insects has been documented. 40 It is also plausible to suggest that at higher concentration of the bioinsecticides, more O2− are generated, which may accumulate to an extent that may overwhelm the scavenging ability of the SOD enzyme, hence decreasing the activity at higher concentrations of the plant extract and synthetic chemicals. Decrease in activity of SOD could induce the accumulation of ROS, which in turn causes oxidative damage in the insecticide-stressed beetles. Decrease in SOD activity could also impair the O2− scavenging ability of the cells, thus favoring the accumulation of O2− and H2O2. 41 SOD activity for all the insecticide-treated groups increases with increased concentration. The increased insecticides concentration in all the treated samples stimulated the synthesis of SOD, resulting in higher dismutation of superoxide anion (O2−), thus preventing the production of hydroxyl radical (OH−)—a highly reactive specie.
The possible reasons for the initial increase in the CAT activity of C. maculatus groups treated with T. diversifolia and J. curcas at the initial low concentration of 5,000 and 10,000 ppm compared with the control and later reduction in the activity at higher concentration are not clear. CAT has high turnover rate for hydrogen peroxide, and its activity is up-regulated by the H2O2 concentration; 42 it catalyzes the conversion of hydrogen peroxide to water and molecular oxygen. A higher CAT activity after exposure to bioinsecticides may signify an enhanced H2O2 removal and, hence, prevention of oxidative damage. This consequently lowers the risk of hydroxyl radicals formation via Fenton reaction. 43 CAT in the midgut of Lymantria dispar larvae fed on an unfavorable plant showed similar results. 44 Decreased activity of CAT was also detected because of high level of superoxide radical generation during oxidative stress in the acute stage of bacteriosis in Galleria mellonella. 45 CAT exhibited higher activity in all concentrations of DDVP, having activity as high as 91.7 U mg−1 protein at 200 ppm. This implied that beetles induce and utilize CAT as a major antioxidant enzyme against ROS generation. High CAT activity in DDVP-treated samples may explain the increase in SOD activity in DDVP. Reduction in CAT activity obtained for the pyrethroids (cypermethrin and λ-cyhalothrin) may be because of inhibition of the synthesis of CAT and impairment of its activity. It is fairly well documented that insects possess POX activities 46 in which POX breaks down H2O2 to oxygen and water. 47 The significant increase (P ≤ 0.05) in the POX activity obtained for C. maculatus groups treated with T. diversifolia, J. curcas, and C. rotundus extracts at various concentrations compared with the control (Fig. 3) might indicate increased chemical stress tolerance by the insects. Corona and Robinson 48 had earlier observed a significant increase in POX activity of honey bee Apis mellifera L., after exposure to thermal stress. The majority of the other treatment groups, however, showed a decrease in POX activity. This is similar to the report of Fu-Xian et al 49 after assessing the effect of thermal oxidation on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis. Activity of POX decreased with increase in the concentration of cypermethrin. This may imply that cypermethrin exhibits inhibitory effects on the synthesis of POX. Unlike what was obtained for cypermethrin, POX has higher activity in samples treated with DDVP and λ-cyhalothrin, which may explain the higher activity values obtained for SOD in DDVP and λ-cyhalothrin, because POX catalyzes the decomposition of hydrogen peroxide. 50
Measurable amount of PPO was found in stressed CSB in this study. PPO is an oxidoreductase that catalyzes the oxidation of a wide range of phenolic compounds using molecular oxygen. 51 PPO activity has been reported in insects, where they were found to be responsible for exoskeleton formation. 52 A decrease in PPO activities was observed for all the C. maculatus groups treated with all the bioinsecticides compared with the control. This suggests that the bioinsecticides inhibit PPO activity in C. maculatus. This is not unusual because all the allelochemicals that characterized both bioinsecticides and insecticides are made of phenolic rings. This result agrees with various reports of inhibition of PPO by substances from natural and non-natural sources. Isao et al 53 reported the inhibition of the tyrosinase enzyme of the aphid, Melaphis chinensis by extract from the leaves of Rhus javanica plant. Isao and Ikuyo 54 observed the inhibition of mushroom tyrosinase by anisaldehyde extracted from the seed of anise oil. Exoskeletons on the other hand serve to protect insects against desiccation and external injury. As this enzyme was significantly inhibited in this study, it may be postulated that body injury because of inhibition of exoskeleton production could be another possible explanation for the efficacy of these bioinsecticides against C. maculatus. Increase in the activity was also obtained for PPO in all the insecticide-treated samples. PPO functions in the oxidation of phenoxyl-end of pyrethroid insecticides. 55 This explains the higher activity values obtained for λ-cyhalothrin and cypermethrin (both pyrethroid), and it had been reported that oxidase provides resistance for pyrethroid. 56 PPO activity at 50 ppm λ-cyhalothrin was observed to be higher than activity at 100 ppm λ-cyhalothrin. Reason for this usual behavior is conjectural, but it can be assumed that the 50 ppm concentration favored the synthesis of the enzyme than situation obtained in the 100 ppm concentration.
Results of this study strongly support the hypothesis that antioxidant systems are modulated as a response to cytotoxicity mediated by oxidative stress from bioinsecticides and insecticides. There is a possibility of the bruchid developing specific adaptive response mechanism to combat the stresses. However, the present and respective efficacy is based on the chemical constituent, structure, and composition of either the bioinsecticides or the insecticides.
Author Contributions
Conceived and designed the experiments: AOK, JOA, COA. Analyzed the data: AOK, COA. Wrote the first draft of the manuscript: AOK, FMO, JOA, COA. Contributed to the writing of the manuscript: AOK, FMO, JOA, COA. Agree with manuscript results and conclusions: AOK, FMO, JOA, COA. Jointly developed the structure and arguments for the paper: AOK, FMO, JOA, COA. Made critical revisions and approved final version: AOK, FMO, JOA, COA. All authors reviewed and approved of the final manuscript.
References
- 1.Oparaeke A.M., Dike M.C., Onu I. Evaluation of Seed and Leaf Powders of Neem (Azadirachta indica A. Juss) and Pirimiphos-methyl for the Control of Callosobruchus maculatus (F.) in stored cowpea. Vol 31. ESN Occasional Publication Maiduguri, Nigeria; 1998: 237–242. [Google Scholar]
- 2.Steele W.M., Allen D.J., Summerfield R.J. Cowpea (Vigna unguiculata (L.) Walp.), in Grain Legume Crops, ed. by Summerfield R.J., Roberts E.H. Collins, London, UK. 1985: 520–583. [Google Scholar]
- 3.Jackai L.E.N., Adalla C.B. Pest management practices in cowpea: a review, in Advances in Cowpea Research, ed. by Singh B.B., Mohan Raj D.R., Dashiell K.E., Jackai L.E.N. IITA/JIRCAS, Ibadan, Nigeria. 1997: 240–258. [Google Scholar]
- 4.Zhu-Salzman K., Murdock L.L. Cowpea: insects, ecology and control. An invited article for the Encyclopedia of Pest Management, published by Marcel Dekker; 2006: 1–3. [Google Scholar]
- 5.Boeke S.J., Baumgart I.R., Van loon J.J.A., Van huis A., Dicke M., Kossou D.K. Toxicity and repellence of African plants traditionally used for the protection of stored cowpea against Callosobruchus maculatus. J Stored Products Res. 2004; 40: 423–438. [Google Scholar]
- 6.Appleby J.H., Credland P.F. Variation in responses to susceptible and resistant cowpea among West African populations of Callosobruchus maculates (Coleoptera: Bruchidae). J Econ Entomol. 2003; 96: 489–502. [DOI] [PubMed] [Google Scholar]
- 7.Ohiagu C.C. Storage of food grains in the savannah zones of Northern Nigeria. In: Menyonga J.M., Bezunah T., Youdeowei A., eds. Food Grain Production in Semi-arid Africa. Ouagadougou: Safgra; 1987: 361–368. [Google Scholar]
- 8.Guo F., Lei J., Sun Y. et al. Antagonistic regulation, yet synergistic defense: effect of bergapten and protease inhibitor on the development of cowpea bruchid Callosobrochus maculatus. PlosOne. 2012; 7(8): e41877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalequzzaman M., Chowdhury F.D. Evaluation of mixtures of plant oils as synergists for pirimiphos-methyl in mixed formulation against Tribolium castaneum (Herbst). Online J Biol Sci. 2003; 3: 347–359. [Google Scholar]
- 10.Govindan K., Jeyarajan N.S., David P.M.M. Fly ash—excellent filler for black pepper, piper nigrum dust formulation against Callosobruchus maculatus (F). J Biopesticides. 2010; 3(1): 320–324. [Google Scholar]
- 11.Ileke K.D., Oni M.O. Toxicity of some plant powders to maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae) on stored wheat grains. African J Agric Res. 2011; 6: 3043–3048. [Google Scholar]
- 12.War A.R., Paulraj M.G., Hussain B., Ahmad T., War M.Y., Ignacimuthu S. Efficacy of a combined treatment of neem oil formulation and endosulfan against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Int J Insect Sci. 2014; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeke S.J., Barnaud C., Van Loon J.J.A., Kossou D.K., Van Huis A., Dicke M. Efficacy of plant extracts against the cowpea beetle, Callosobruchus maculatus. Int J Pest Manage. 2004; 50: 251–258. [Google Scholar]
- 14.Gbaye O.A., Holloway G.J., Callaghan A. Variation in the sensitivity of Callosobruchus (Coleoptera: Bruchidae) acetylcholinesterase to the organophosphate insecticide malaoxon: effect of species, geographical strain and food type. Pest Manage Sci. 2012; 68: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 15.Kolawole A.O., Ajele J.O., Sirdeshmuhk R. Studies on glutathione transferase of cowpea storage bruchid, Callosobrochus maculatus F. Pesticide Biochem Physiol. 2011; 100: 212–220. [Google Scholar]
- 16.Amin K., Hashem K.S. Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): antioxidant defense and role of alpha-tocopherol. BMC Veterinary Res. 2012; 2012(8): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mostafalou S., Abdollahi M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol Appl Pharmacol. 2013; 268: 157–177. [DOI] [PubMed] [Google Scholar]
- 18.Adedire C.O., Akinneye J.O. Biological activity of tree marigold, Tithonia diversifolia, on cowpea seed, Callosobruchus maculatus (Coleoptera: Bruchidae). Ann Appl Biol. 2003; 144: 185–189. [Google Scholar]
- 19.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 20.Omaye S.T., Turnbull J.D., Sauberilich H.E. Selected methods for the determination of ascorbic acid in animal cells tissues and fluids. Methods Enzym. 1979; 62: 3–11. [DOI] [PubMed] [Google Scholar]
- 21.Ellman G.L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959; 82: 70–77. [DOI] [PubMed] [Google Scholar]
- 22.Backer H., Frank O., De Angel B., Feingold S. Plasma tocopherol in man at various times after ingesting free or ocetylaned tocopherol. Nutr Report Intern. 1980; 21: 531–536. [Google Scholar]
- 23.Fridovich I. Superoxide dismutase. Adv Enzymol. 1974; 41: 35–97. [DOI] [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105: 121–126. [DOI] [PubMed] [Google Scholar]
- 25.Kumar K.B., Khan P.A. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Indian J Exp Botany. 1982; 20: 412–416. [PubMed] [Google Scholar]
- 26.Kolawole A.O., Okonji R.E., Ajele J.O. Tithonia diversifolia, Cyperus rotundus and Hyptis suaveloensis ethanol extracts combinatorially and competitively inhibit affinity purified cowpea storage bruchid (Callosobrochus maculatus) glutathione S-transferase. Arthropod–Plant Interactions. 2011; 5: 175–184. [Google Scholar]
- 27.War A.R., Paulraj M.G., Hussain B., Buhroo A.A., Ignacimuthu S., Sharma H.C. Effect of plant secondary metabolites on Helicoverpa armigera. J Pest Sci. 2013; 86: 399–408. [Google Scholar]
- 28.Isman M.B., Miresmailli S., Machial C.M. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem Rev. 2010; 10: 197–204. [Google Scholar]
- 29.Sies H. Role of reactive oxygen species in biological processes. Klin Wochenschr. 1991; 69: 965–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbehenn R.V., Bumgarner S.L., Roosen E.F., Martin M.M. Antioxidant defenses in caterpillars: role of the ascorbate-recycling system in the midgut lumen. J Insect Physiol. 2001; 47: 1095–1095. [DOI] [PubMed] [Google Scholar]
- 31.Barbehenn R. Antioxidants in Grasshoppers: higher levels defend the midgut tissues of a polyphagous species than a graminivorous species. J Chemi Ecol. 2003; 29: 683–702. [DOI] [PubMed] [Google Scholar]
- 32.Shigeoka S., Ishikawa T., Tamoi M. et al. Regulation and function of ascorbate peroxidase isoenzymes. J Exp Botany. 2002; 53: 1305–1319. [PubMed] [Google Scholar]
- 33.Supratim R. Evaluation of protective role of ascorbic acid on flutamide-induced lipid peroxidation. Int J PharmTech Res. 2012; 4: 135–140. [Google Scholar]
- 34.Kamal-Eldin A., Appelqvist L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996; 31: 671–701. [DOI] [PubMed] [Google Scholar]
- 35.Barbehenn R.V. Antioxidants in grasshoppers: higher levels defend the midgut tissues of a polyphagous species than a graminivorous species. J Chem Ecol. 2003; 29: 683–702. [DOI] [PubMed] [Google Scholar]
- 36.Gopi R., Jalee C.A., Lakshmanan G.M.A., Gomathinayagam M., Panneerselvam R. Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids Surf. B: Biointerfaces. 2007; 60: 180–186. [DOI] [PubMed] [Google Scholar]
- 37.Henry W.L., Chao-Lin K., Wen-Hui Y., Chia-Hsien L., Hong-Zin L. Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol. 2006; 534: 2123–2130. [DOI] [PubMed] [Google Scholar]
- 38.Olawale O., Ikechukwu N.E., Grace T.O., Chidiebere E.U. Oxidative stress and superoxide dismutase activity in brain of rats fed with diet containing permethrin. Biochemistry. 2008; 20: 93–98. [Google Scholar]
- 39.Wang H., Wen C., Chang X., Duan C. Some detoxication mechanisms of different wheat varieties under cadmium treatment. Acta Sci Circum. 2002; 22: 523–528. [Google Scholar]
- 40.Suh H.J., Kim S.R., Lee K.S., Park S., Kang S.C. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. J Photochem Photobiol B: Biology. 2010; 99: 67–73. [DOI] [PubMed] [Google Scholar]
- 41.Abdul Jaleel C., Lakshmanan G.M.A., Gomathinayagam M., Panneerselvam R. Triadimefon induced salt stress tolerance in Withania somnifera and its relationship to antioxidant defense system. South Africa J Botany. 2008; 74: 126–132. [Google Scholar]
- 42.Fornazier R.F., Ferreira R.R., Pereira G.J.G. et al. Cadmium stress in sugar cane callus cultures: effect on antioxidant enzymes. Plant Cell Tissue Organ Cult. 2002; 71: 125–131. [Google Scholar]
- 43.Fridovich I. Fundamental aspects of reactive oxygen species, or what's the matter with oxygen. Annu N Y Acad Sci. 1999; 893: 13–18. [DOI] [PubMed] [Google Scholar]
- 44.Peric-Mataruga V., Blagojevic D., Spasic M.B., Ivanovic J., Jankovic-Hladni M. Effect of the host plant on the antioxidative defence in the midgut of Lymantria dispar L. caterpillars of different population origins. J Insect Physiol. 1997; 43: 101–106. [DOI] [PubMed] [Google Scholar]
- 45.Dubovskiy I.M., Martemyanow V.V., Vorontsova Y.L., Rantala M.J., Gryzanova E.V., Glupov V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae). Comp Biochem Physiol C. 2008; 148: 1–5. [DOI] [PubMed] [Google Scholar]
- 46.Mathews M.C., Summers C.B., Felton G.W. Ascorbate peroxidase: a novel antioxidant enzyme in insects. Arch Insect Biochem Physiol. 1997; 34: 57–68. [Google Scholar]
- 47.Lee I.M., Cook N.R., Gaziano J.M. et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005; 294: 56–65. [DOI] [PubMed] [Google Scholar]
- 48.Corona M., Robinson G.E. Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect Mole Biol. 2006; 15: 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu-Xian J., Wei D., Fei H., Jin-Jun W. Effects of thermal stress on lipid peroxidation and antioxidant enzyme activities of oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae). Florida Entomol. 2011; 94: 956–963. [Google Scholar]
- 50.Karthikeyan M. Induction of resistance in host against the infection of leaf blight pathogen (Alternaria palandui) in onion (Allium cepa var aggregatum). Indian J Biochem Biophys. 2005; 42: 371–377. [PubMed] [Google Scholar]
- 51.Mayer A.M. Polyphenol oxidase in plants-recent progress. Phytochemistry. 1987; 26: 11–20. [Google Scholar]
- 52.Halaouli S., Asther M., Sigoillot J.C., Hamdi M., Lomascolo A. Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications. J Appl Microb. 2006; 100: 219–232. [DOI] [PubMed] [Google Scholar]
- 53.Isao K., Kinst-Hor I., Nihei K.I. et al. Tyrosinase inhibitors from Galls of Rhus javanica leaves and their effects on insects. Zei fuer Natur C J. Biosci. 2003; 58: 719–725. [DOI] [PubMed] [Google Scholar]
- 54.Isao K., Ikuyo K. Tyrosinase Inhibitors from Cumin. J Agric Food Chem. 1998; 46(12): 5338–5341. [Google Scholar]
- 55.Friedman M. Chemistry, biochemistry, and dietary role of potato polyphenols. J Agric Food Chem. 1997; 45: 1523–1540. [Google Scholar]
- 56.Karunaratne S.H. Insecticide cross-resistance in insects: review. Ceylon J Sci. 1998; 26: 73–98. [Google Scholar]


