Abstract
The antimalarial hydroxychloroquine (HCQ) has demonstrated several crucial properties for the treatment of systemic lupus erythematosus (SLE). Herein, we reviewed the main HCQ pharmacologic features, detailed its mechanism of action, and summarized the existing guidelines and recommendations for HCQ use in rheumatology with a systematic literature search for the randomized controlled trials focused on lupus. HCQ has been shown to decrease SLE activity, especially in mild and moderate disease, to prevent disease flare and to lower the long-term glucocorticoid need. The numerous benefits of HCQ are extended to pregnancy and breastfeeding period. Based on cohort studies, antithrombotic and metabolic HCQ’s effects were shown, including lipid-lowering properties, which might contribute to an improved cardiovascular risk. Moreover, early HCQ use in antinuclear antibodies positive individuals might delay the progression to SLE. Finally, HCQ has a significant favorable impact on long-term outcomes such as damage accrual and mortality in SLE. Based on these multiple benefits, HCQ is now the mainstay long-term treatment in SLE, recommended by current guidelines in all patients unless contraindications or side effects. The daily dose associated with the best compromise between efficacy and safety is matter of debate. The concern regarding retinal toxicity rather than proper efficacy data is the one that dictated the daily dosage of ⩽5 mg/kg/day actual body weight currently agreed upon.
Keywords: antimalarials, cutaneous lupus erythematosus, hydroxychloroquine, immunomodulatory, lupus nephritis, systemic lupus erythematosus
Background
Hydroxychloroquine (HCQ) is an antimalarial drug used initially for the treatment of Plasmodium parasitic infection, from where the name of the drug class came from. Beyond its initial indication as antimalarial, HCQ has been used in autoimmune and infectious diseases, as well as in metabolic or neoplastic disorders. 1 But, as recently reviewed, 2 clear benefits were reported mainly in systemic lupus erythematosus (SLE).
Thus, HCQ is now one of the most valuable therapies in SLE, showing multiple benefits over several outcomes associated with the disease itself, but also to its related comorbidities. HCQ is an inexpensive, generally available, well-tolerated immunomodulator. 3 For more than a decade, different authors emphasized that all patients with SLE should be given HCQ4–7 and the latest guidelines’ recommendations also stated the HCQ importance in SLE unless there are contraindications or side effects.8–11
The history of HCQ is supposed to start circa 1600 with the Incas in Chile, from whom the cinchona bark properties were learned by the Jesuits. The main alkaloids of quinine and cinchonine were isolated in 1820 and subsequently chloroquine (CQ) was obtained much later in 1934. 12 HCQ sulfate is the hydroxylated analogue of CQ, synthesized in 1946. Due to a better safety profile, HCQ was given since 1955 as an alternative to CQ.12,13
For SLE, the first report of the antimalarials use dates back to 1894, regarding the improvement of cutaneous lupus lesions with quinine.14,15 In the United States, HCQ was approved for SLE in 1955 for symptoms like fatigue, rashes, joint pain, and mouth sores 16 and, with specific approval and license characteristics for each country, is now among the main drugs used for SLE treatment worldwide.
Pharmacology of hydroxychloroquine
Molecular structure
The knowledge about the pharmacokinetics of antimalarials is not completely understood and still debated. These pharmacokinetic characteristics are complex17–19 due to the large volume of distribution,19,20 significant tissue binding,20–22 and long terminal elimination half-life.18,19,23,24 Indeed, important differences have been observed between HCQ pharmacokinetic parameters as evidenced recently by its use in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease (COVID-19). 25 Historically, terminal elimination half-lives were considered very long, 40–50 days for HCQ18,23 and up to 60 days for CQ.19,24 More recent studies suggest a shorter half-life of about 5 days.25,26 A long HCQ half-life can be attributed to extensive tissue uptake rather than to an intrinsic inability to clear the drug. The expected delay in the attainment of steady-state concentrations (3–4 months) may be in part responsible for the slow therapeutic response observed with HCQ. 27 Renal clearance is an important consideration for both drugs as reduced clearance increases the bioavailability 28 and subsequently the related side effects.19,20,24 Finally, dose–response relationships and toxicity thresholds have not yet been fully defined. The main pharmacodynamic properties of antimalarials are shown in Table 1.
Table 1.
Main pharmacodynamic properties of antimalarials.
| Hydroxychloroquine (HCQ) | Chloroquine (CQ) | Quinacrine | |
|---|---|---|---|
| Chemical structure |
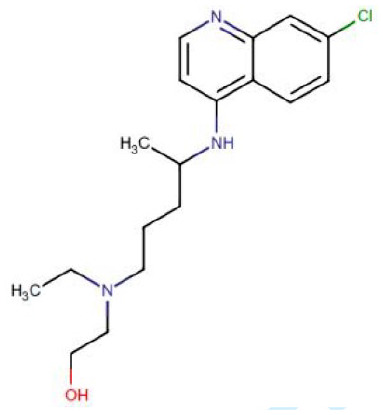
|
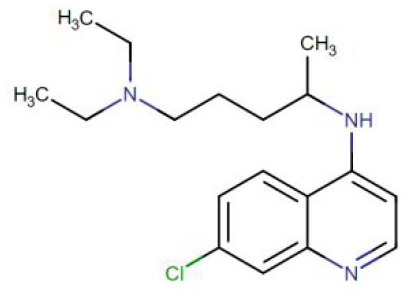
|
|
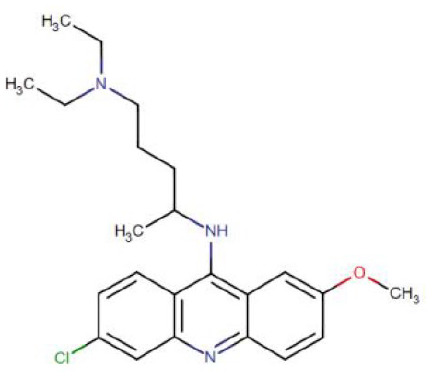
| Chemical formula | C18H26ClN3O | C18H26ClN3 | C23H30ClN3O |
| Way of administration | Oral intake | ||
| Absorption | In upper intestinal tract after a 200 mg oral dose, HCQ reached a Cmax of 129.6 ng/ml with a Tmax of 3.26 h in the blood 18 |
In upper intestinal tract oral CQ reaches a Cmax of 65–128 µg/L with a Tmax of 0.5 h 19 |
In upper intestinal tract more details not available |
| Bioavailability | 67–74% 20 | 67–100% 19 | Not available |
| Volume of distribution | 5522 liters from blood and 44,257 liters from plasma 20 | 200–800 L/kg 19 | Not available |
| Protein binding | 50% 20 | 46–74% 21 | 80–90% 22 |
| Metabolism | In the liver, N-dealkylated by CYP3A4 to the active metabolite desethylhydroxychloroquine, as well as the inactive metabolites desethylchloroquine and bidesethylchloroquine17,18 | In the liver, N-dealkylated primarily by CYP2 C8 and CYP3A4 to N-desethylchloroquine N-dealkylated to a lesser extent by CYP3A5, CYP2D6, and to an ever lesser extent by CYP1A119 |
Not available |
| Elimination | 40–50% of HCQ is excreted renally, while only 16–21% of a dose is excreted in the urine as unchanged drug 5% of a dose is sloughed off in skin and 24–25% is eliminated through the feces19,20 |
Predominantly eliminated in the urine, renal excretion: 65–70%.
24
50% of a dose is recovered in the urine as unchanged CQ, with 10% of the dose recovered in the urine as desethylchloroquine 19 |
Less than 11% is eliminated in the urine daily 28 |
| Elimination half-life | Historically, 40–50 days (chronic use) A 200 mg oral dose of HCQ: 537 h to 50 days (blood) or 32 days or 123 days in plasma18,23 Maybe shorter, about 5 days, according to more recent studies25,26 |
6–60 days (mean of 20 days)19,24 | 5–14 days |
Galenic and commercial presentations
HCQ is commercialized as 200 mg HCQ sulfate tablets corresponding to 155 mg HCQ base for each tablet. 29 The daily dosage of HCQ varies accordingly to its indication, 29 with the American Academy of Ophthalmology (2016-AAO) recommending no more than 5 mg/kg/day of real body weight in SLE to decrease retinopathy occurrence, 30 recommendation that has been recently reinforced by agreement of four medical societies. 31 The indication is based on an ophthalmological study by Melles and Marmor 32 of nearly 2500 patients in whom daily HCQ intake below 5 mg/kg/day of regular body weight was associated with a low risk of toxicity, <2% within the first 10 years of use. However, some authors highlighted that in that study, the dose of HCQ was based on pharmacy refill information and not on prescribed dose. 33
Dose adjustments with 50% reduction of posology are needed for patients with renal impairment and lower than 30 ml/min filtration rate. 34 For patients weighting more than 80 kg, a maximum daily dose of 400 mg is recommended in SLE. Doses for CQ were established only from extrapolation of HCQ and those lower than 2.3 mg/kg/day were considered safe.30,35
As the terminal elimination half time is not short, 36 dosing can be adjusted by alternate day regimens, such as 200 mg on the first day and 400 mg on the second day, yielding a mean dose equivalent to 300 mg per day. 32 Based on recent surveys, the most common daily dosage for HCQ is 400 mg daily.37,38
Mechanism of action
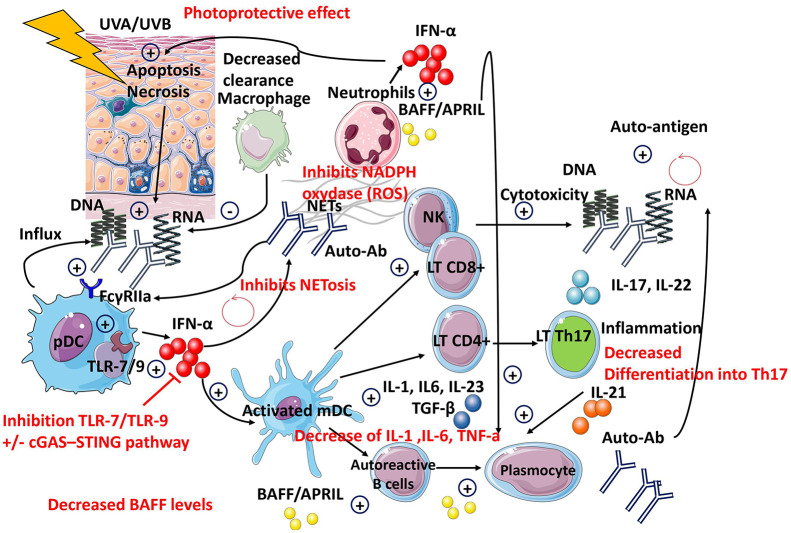
The mechanisms of action for HCQ are complex and still not completely understood (see Table 2 and Figure 1). Because of its high lipophilicity, lysosomotropism, and pH,39,40 HCQ can pass through cell membranes and accumulate into lysosomes 40 where it disrupts key important cellular functions via the inhibition of the Toll-like receptors (TLRs)41–43 and of the Cyclic GMP-AMP synthase–Stimulator of Interferon Genes (cGAS-STING) pathway. 44 The main effects include the inhibition of enzyme and cytokine release,45–47 receptor recycling, plasma membrane repair, cell signaling, apoptosis,48–50 autophagy,39,51 antigen presentation, 52 T-cell polarization,53–56 inhibition of the natural killer (NK) cells,57,58 energy metabolism, 40 and increases photoprotection against ultraviolet (UV)-A and B.59–65
Table 2.
Mechanisms of action of hydroxychloroquine.
| HCQ/CQ Mechanisms of action | Molecular mechanism(s) demonstrated | Potential consequence(s) in SLE pathogenesis | References |
|---|---|---|---|
| Inhibition of TLR-7 and TLR-9 | Suppression of endosomal TLR activation direct binding of antimalarials to nucleic acids rather than inhibition of endosomal acidification | Inhibition of IFN-I production by pDC | Lamphier et al.
41
Kužnik et al. 42 Gardet et al. 43 |
| Inhibition of cyclic GMP-AMP synthase (cGAS) activity | Inhibition of (cGAS)-STING pathway | Inhibition of IFN-I production | An et al. 44 |
| Inhibition of autophagy | Blockade of autophagosome fusion with the lysosome | Inhibition of MHC class II-mediated autoantigen presentation by antigen-presenting cells to CD4+ T cells | Levy et al.
51
Schrezenmeier and Dörner 39 |
| Inhibition of antigen presentation | CQ has been shown to inhibit presentation of antigen in vitro by affecting invariant chain dissociation from MHC class II | Inhibition of MHC class II-mediated autoantigen presentation by antigen-presenting cells to CD4+ T cells | Humbert et al. 52 |
| Inhibition of inflammatory cytokine production and angiogenesis | Decrease mRNA expression of IL-1β, IL-6, and TNF-α in CLE skin lesions Decrease VEGF expression in CLE skin lesion |
Decrease of local inflammation Decrease of mononuclear cellular infiltrate in the skin Inhibition of angiogenesis |
Wozniacka et al.
45
Lesiak et al. 46 Zeidi et al. 47 |
| Photoprotection against UVA and UVB | Increase of c-Jun mRNA expression Decrease mRNA expression of IL-1β, IL-6, and TNF-α in CLE skin lesions Decrease UV-induced ICAM-1 expression in keratinocytes CQ inhibits lipid peroxidation and decrease UVB and induces phospholipase A2 activity in skin Decrease of the number of cutaneous HLA-DR+ and CD1a+ cells after UVB irradiation |
Decrease of local inflammation, apoptosis, and necrosis of keratinocytes Decrease of the release of skin nucleic acids Decrease of the mononuclear cellular infiltrate in the skin |
Nguyen et al.
65
Sjolin-Forsberg et al. 59 Wozniacka et al. 64 Wozniacka et al. 60 Bondeson and Sundler 61 el Tahir et al. 62 Segal-Eiras et al. 63 |
| Decrease NET formation and circulating DNA | HCQ inhibits NETs formation in vitro
Circulating DNA significantly decreases after CQ treatment |
Decrease of circulating nucleic acids Inhibition of IFN-I production Decrease of LL37 formation and inflammasome activation Decrease of MMP-9 and reduced endothelial cell death |
Smith et al.
48
Smith and Kaplan 49 Cepika et al. 50 |
| Change in T-cell polarization | HCQ decreases Th17-related cytokines HCQ decreases Th22-related cytokines HCQ blood concentrations correlate negatively with the percentage of CD45RO+ CD4+ cells |
Decrease of mononuclear cellular infiltrate in the skin Decrease of survival and proliferation of human B cells as well as the differentiation of B cells into antibody-producing cells Recruitment and activation of inflammatory cells with tissue damage Inhibition of angiogenesis |
Silva et al.
53
Zhao et al. 54 Shin et al. 55 Sailler et al. 56 |
| Inhibition of NK cells | Decrease proliferation, cytotoxicity, and cytokine production of NK cells | Possible deleterious effects of NK cells in SLE: tissue infiltration, proinflammatory cytokine production: IFNγ, IL-15 | Spada et al.
57
Fox 58 |
cGAS, cyclic GMP-AMP synthase; CLE, cutaneous lupus erythematosus; CQ, chloroquine; DC, dendritic cells; HCQ, hydroxychloroquine; ICAM, intercellular adhesion molecule-1; IFN, interferon; IL, interleukin; MHC, major histocompatibility complex; MMP, matrix metalloproteinase; NETs, neutrophil extracellular traps; NK, natural killer; SLE, systemic lupus erythematosus; STING, stimulator of interferon genes; Th, T helper; TLRs, Toll-like receptors; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Figure 1.
Hydroxychloroquine’s mechanisms of action.
Efficacy in systemic lupus erythematosus
Systematic search of randomized controlled trials regarding hydroxychloroquine in systemic lupus erythematosus
A systematic search for randomized controlled trials (RCTs) regarding HCQ treatment in SLE was performed using the medical subject headings (MeSH) terms ‘Hydroxychloroquine’ AND ‘Lupus Erythematosus, Systemic’ AND ‘Clinical Trials, Randomized’. The search was performed on Excerpta Medica/EMBASE, MEDLINE via PubMed, Cochrane Library, and Thomson Reuters’ Web of Science Core Collection using the same combination of relevant keywords (see Supplemental File 1). The four databases were systematically searched from inception to 1 February 2021, without any language, geographic, or type of article restrictions. The references and citations of the articles identified were also screened.
Reports not referring to HCQ or CQ use in SLE, not involving human subjects, not including adult cases, and presenting other types of studies than RCTs were excluded. A total of eight RCTs were identified in the initial search with one more identified after the references and citations screen (see Supplemental Figure 1–Flowchart Diagram, Supplemental Table 1). For each RCT included, the following information was extracted: study design, drug posology, time of follow-up, study’s endpoints, proven efficacy, and side effects noted (as presented in Supplemental Table 2a, 2b).
To the best of our knowledge, the first RCT involving antimalarial therapy in SLE was published in 1991 by Canadian Hydroxychloroquine Study Group 66 and reported a 2.5-fold increase in the risk of mild flare after HCQ withdrawal in the placebo group. 66 In 1998, Tsakonas et al. 67 presented an extension phase in 1991 and evaluated the risk of major flare after HCQ withdrawal. 67 The endpoint considered, namely flare, subtype of flare, and hospitalization, were all improved under long-term HCQ therapy; however, the results did not reach statistical significance most probably due to the small sample size. 67
Other RCTs have also demonstrated improvement of arthralgia 14 even if without a significant impact over arthritis, 68 prevention of SLE flares and reduction of the corticosteroids dose, 14 improvement of lipid metabolism 69 with decrease in total cholesterol and triglycerides, while increase in HDL-cholesterol, 70 and a safety profile of administration during pregnancy. 71 Also, the PLUS (Plaquenil LUpus Systemic) failed to demonstrate that adjusted HCQ dosing schedules targeting [HCQ] ⩾1000 ng/ml might reduce the occurrence of SLE flares. 72 Most recently, Zanetti et al. 73 tested the efficacy of lower HCQ doses (2–3 mg/kg/day) 30 and found similar 6- and 12-month flare rates between groups. 73
For cutaneous lupus erythematosus (CLE), the first RCT by Kraak et al. 74 in 1965 tested HCQ up to a maximum posology of 1200 mg daily. Furthermore, the efficacy of antimalarials has been tested in RCTs against placebo, 75 acitrecin, 76 or clofazimine 77 in RCTs showing proven efficacy in RCT with better safety profile than clofazimine or acitrecin.
Observational data for hydroxychloroquine in systemic lupus erythematosus
Currently published RCTs do not cover the whole spectrum of SLE features. Many of the data regarding HCQ benefits are from prospective SLE cohorts, such as the Hopkins Lupus Cohort,78–83 LUMINA (Lupus in Minorities: Nature versus Nurture) Cohort,84–89 Toronto Lupus Cohort,90,91 or GLADEL (multinational Latin American lupus) Cohort92–94 (see Table 3; Supplemental Table 3).
Table 3.
Research for antimalarials in systemic lupus erythematosus.
| Effects | Randomized controlled trials | Observational studies | Systematic reviews |
|---|---|---|---|
| Decrease of disease severity | Prospective study, 25 patients
95
Prospective LUMINA Cohort, 256 patients 84 Cross-sectional study, 57 patients 96 Longitudinal study, LUMINA cohort, 35 patients 85 Prospective study, 41 SLE patients 97 Observational study, 28 SLE pregnant women 98 Retrospective study, 165 SLE patients 99 Retrospective study, 101 SLE patients 100 Prospective Hopkins Lupus Cohort, 916 patients 101 |
Databases: Medline and Embase 13 | |
| Prevent of disease flare | RCT, NCT03122431: 73 stable LN patients
73
RCT, NCT00413361: 573 patients 72 RCT, 24 SLE patients stable disease 14 RCT, 47 clinically stable SLE patients 66 RCT, 20 patients lupus pregnancy 71 |
Retrospective, matched with themselves, 43/209 patients
102
Retrospective, matched with themselves, 43 patients, 76 matched years 103 Prospective, Padua Lupus Cohort, 319 SLE patients 104 Retrospective study, 101 SLE patients 100 Prospective, Hopkins Lupus Cohort, 2512 patients 78 Longitudinal, 143 SLE patients 105 |
Databases: Medline and Embase 13 |
| Cutaneous lupus | RCT, NCT01551069: 103 patients Cutaneous Lupus
75
RCT, 20 patients lupus pregnancy 71 |
Retrospective, matched with themselves, 43/209 patients
102
Prospective, 17/27 patients SLE 106 Prospective, 300 patients subacute or chronic CLE 107 Retrospective cohort, 200 patients DLE 108 Cross-sectional study, 1002 patients CLE 109 Prospective cohort, 218 CLE and SLE patients 110 Retrospective, 36 LE tumidus 111 Retrospective, 61 DLE and SCLE patients 112 Prospective, 34 CLE patients 113 |
Databases: Medline, Embase, Scopus, Cochrane 114 |
| Adjuvant for lupus nephritis remission | Prospective, Hopkins Lupus Cohort, 29 patients
79
Retrospective study, 35 patients 115 Retrospective study, 206 patients lupus nephritis 116 Prospective LUMINA Cohort, 256 patients 84 Retrospective study, 90 patients with lupus nephritis 117 |
Databases: Medline and Embase 13 | |
| Improvement of articular complaints | RCT, 71 SLE patients mild SLE
68
RCT, 24 SLE patients stable disease 14 |
Databases: Medline and Embase 13 | |
| Decrease disease activity/prevent flare during pregnancy | RCT, 20 patients lupus pregnancy 71 | Prospective study, 60 patients – 103 pregnancies
118
Prospective, Hopkins Lupus Pregnancy Cohort, 282 (163 + 56 + 68) pregnancies 80 Retrospective study, 176 patients – 396 pregnancies 119 Retrospective study, 179 pregnancies 120 |
Databases: Medline and Embase
3
Databases: Medline and Embase 13 |
| Protection against preeclampsia | Retrospective cohort, 151 pregnancies
121
Prospective cohort, 316 pregnancies 122 114 HCQ-exposed pregnancies 123 |
||
| Prevention of fetal growth restriction and prematurity | Observational study, 28 SLE pregnant women 98 | ||
| Reducing antiphospholipid antibodies persistence | Retrospective study, 90 patients – 17 patients with persistent LA 124 | ||
| Reduce the risk of thrombosis | Prospective cohort, 92 patients
125
Retrospective study, 272 patients 126 Prospective cohort, 232 patients 127 Prospective cohort, 67 SLE-aPL patients 128 Retrospective study, 206 patients lupus nephritis 116 Longitudinal, cross-sectional, 144 patients 129 Prospective, Tromso Lupus cohort, 158 patients 130 Retrospective study, 1930 patients 131 Nested case–control study, 54 SLE cases versus 108 controls 132 Prospective Hopkins Cohort, 1795 SLE patients, 193 thrombotic events, 10,508 person-years 133 Prospective study, 189 SLE patients 134 Prospective Hopkins Cohort, 739 patients 135 |
Databases: Medline and Embase
3
Databases: Medline and Embase 13 |
|
| Lower fasting glucose/diabetes mellitus protection | Cross-sectional study, 149 SLE patients
136
Population-based cohort study, 221 with diabetes mellitus out of 8628 SLE patients 137 |
||
| Improving lipidic profile | RCT, 72 SLE patients
69
RCT, 17/19 SLE female patients 70 |
Cross-sectional, 155 patients (SLE + AR)
138
Case-control, 18 SLE patients 139 Longitudinal Cohort – John Hopkins, 264 patients 81 Retrospective study, 382 patients 140 Cross-sectional study, 123 patients 141 Cross-sectional study, 90 subjects – 60 SLE patients 142 Cross-sectional study, 86 patients 143 Prospective study, 30 subjects – 20 SLE patients 144 Cross-sectional study, 185 outpatients 145 Prospective – Toronto Lupus Cohort – 1260 patients 90 Case-control, 100 lupus nephritis patients 146 Cross-sectional study, 24 patients 147 Prospective Hopkins Cohort, 51 patients, over 229 visits 82 Cross-sectional study, 48 patients 148 |
Databases: PubMed, Embase, Cochrane
149
Databases: PubMed, Embase, Web of Science, Medline/Ovid, Google Scholar, CINAHL, Cochrane 150 Databases: Medline and Embase 3 Databases: Medline and Embase 13 |
| Reduction of atherosclerosis | Pittsburgh Lupus Registry, 220 women
151
Prospective study, 41 SLE patients and 96 controls 152 |
Databases: Medline and Embase 153 | |
| Decrease the risk of infections | Retrospective study, 206 patients lupus nephritis
116
A nested case–control study, Lupus-Cruces cohort, 83/166 patients 154 Prospective cohort, Northern California, 3030 patients 155 Retrospective study, Spanish Rheumatology Society Lupus Registry (RELESSER), 3658 patients 156 Case–control study, 65 SLE patients versus 130 controls 157 Prospective RELES Cohort, 282 SLE patients 158 Retrospective study, 339 patients 159 Inception cohort study GLADEL, 1243 patients 92 Population-based study, 24343 SLE patients 160 |
Databases: PubMed, Embase, Cochrane 161 | |
| Improvement of bone mineral density | Prospective study, 92 patients
162
Prospective study, 34 SLE patients 163 |
Databases: Medline and Embase 13 | |
| Protection against osteonecrosis | Nested matched case–control study, LUMINA cohort 86 | Databases: Medline and Embase 13 | |
| Decrease the corticosteroids need | RCT, 20 patients lupus pregnancy 71 | Retrospective, matched with themselves, 43 patients, 76 matched years
103
Prospective LUMINA Cohort, 256 patients 84 Prospective study, 257 pregnancies 80 |
|
| Protection against accrual damage | Prospective Israeli Cohort, 151 patients
164
Prospective LUMINA Cohort, 632 patients 88 Prospective LUMINA Cohort, 256 lupus nephritis 84 Prospective LUMINA Cohort, 580 patients 89 Prospective Hopkins Cohort, 2054 patients 83 Nested case-control, Inception cohort – Toronto Lupus Cohort, 685 patients: 174/307 patients 3 SLICC Inception Cohort Study, 1722 patients 165 Retrospective inception cohort, 476 subjects, 26 years 166 Early Lupus Project, Prospective Inception Cohort, 230 patients 167 |
Databases: Medline and Embase 3 | |
| Protection against neoplasia | Prospective cohort, 235 patients 168 | ||
| Reducing SLE-related hospitalization | Retrospective study, 339 patients
159
Retrospective study, 526 patients 169 Retrospective registry-based, 40,381 patients 170 |
||
| Improvement of survival | Case-control, 76 matched pairs
171
Prospective cohort, 232 patients 127 Case-control study – LUMINA L cohort, 608 patients 87 Retrospective study, 206 patients lupus nephritis 116 Prospective University of Toronto Lupus Clinic, 1241 patients 91 Prospective GLADEL cohort, 1480 patients 93 Retrospective, 1956 SLE inpatients 172 Retrospective, 42 patients lupus nephritis 173 Retrospective study, 491 patients with lupus nephritis 174 Prospective cohort, 803 SLE patients 175 Longitudinal cohort, 345 lupus nephritis patients 176 Prospective cohort, 914 SLE patients 177 Retrospective study, 6241 patients 178 |
Databases: Medline and Embase
3
Databases: Medline and Embase 13 |
|
| Delays the evolution to SLE | Retrospective study, 130 military personal
179
Nested case–control study, GLADEL cohort, 265/530 patients 94 |
Databases: Medline and Embase 13 |
CC, case-control; CLE, cutaneous lupus erythematosus; CS, cross-sectional; DLE, discoid lupus erythematosus; DS, descriptive studies; GLADEL, Grupo Latino Americano de Estudio del Lupus; HCQ, hydroxychloroquine; LAC, lupus anticoagulant; LN, lupus nephritis; LUMINA, Lupus in Minorities: Nature vs Nurture; PC, prospective cohort; RA, retrospective analysis; RC, retrospective cohort; RCT, randomized controlled trial; SCLE, subacute cutaneous lupus erythematosus; SLICC, Systemic Lupus International Collaborating Clinics.
Antimalarials: chloroquine diphosphate (CDP) or hydroxychloroquine sulfate (HCQ).The most significant HCQ effect is the control of SLE disease activity itself, which implies amelioration of active clinical involvements, decrease in serum markers, decrease in activity scores, prevention of disease flares, and sustained remission on long-term use.
Therefore, decrease in disease activity,84,85,95–101 prevention of disease flares,78,100,103–105 and improvement of proinflammatory cytokine profiles85,95,97,104,180,181 have been highlighted with HCQ.
Moreover, delay of the immune clinical spectrum to overt SLE was described in antinuclear antibodies (ANA)-positive patients.94,179 A recent study showed that HCQ might suppress early mediators like the B cell activating factor (BAFF) and interferon (IFN), lowering the IFN-γ-induced protein 10 (IP-10) levels in incomplete or new-onset SLE, supporting the hypothesis that HCQ could influence disease progression. 182
In observational studies, HCQ has been shown beneficial for cutaneous lupus,95,102,106–112 musculoskeletal involvement, 99 and various other key manifestations of SLE. The management of lupus nephritis (LN) remains suboptimal 183 and HCQ is adjuvant therapy to the immunosuppressive regimens in obtaining remission.79,84,115–117
HCQ decreases disease activity and prevents SLE flare during pregnancy,80,118,119,122 and furthermore, there are reports sustaining a possible protective role for preeclampsia,120–123 fetal growth restriction, and prematurity. 98 Current data regarding HCQ efficacy during pregnancy are conclusive, however for other outcomes the results are contradictory. Thus, there are reports that did not found the impact of HCQ on pregnancy loss, preterm delivery or intrauterine growth retardation, 119 or upon miscarriage, stillbirth, pregnancy loss, or congenital abnormality rates. 80
For neonatal lupus, one retrospective study that analyzed data of a historical cohort counting more than 200 pregnancies in SLE patients with positive anti-Ro/SS-A antibodies found HCQ benefits over recurrence and outcome of the neonatal lupus. 184 In another research, HCQ was not identified as independent protective factor for neonatal lupus after adjusting for confounders like age, race, antibodies status, corticosteroids, and prior cardiac-neonatal lupus risk, even if the neonatal lupus cases were less frequent in pregnancies treated by HCQ (14% versus 37%). 185
Despite potential benefits of HCQ during pregnancy, adherence seems to be low. A population-based registry identified 376 pregnancies in which discontinuation of antimalarials occurred in 16.7% of cases in the year prior to pregnancy, 29.8% in the first trimester, 9.7% in the second, and 26.0% in the third. 186
Importantly, HCQ passes the placenta and has fetal serum concentrations equal to those measured in the maternal blood. However, HCQ use during pregnancy80,119,120,123,187–189 and breastfeeding is considered safe.5,190 During lactation, HCQ passes in the maternal milk, but with lower concentrations than in maternal blood, estimated to be 0.2 mg/kg/day. 5
There are reports of CQ overdose in children and, by parallel, cautions are related to HCQ. Antimalarials might be toxic in children in relatively small doses and patients should be counseled to keep these drugs out of children. 5
SLE disease itself is a risk factor for thrombosis. Also, about 20% of patients with SLE have antiphospholipid syndrome (APS). 191 Antimalarials might reduce the antiphospholipid antibodies titers 124 and the risk of thrombosis,116,125–135 but not all published studies reported a protective effect over thrombosis.192–194
HCQ has also some metabolic effects by lowering fasting glucose, 136 yielding protection against diabetes, 137 and improvement of the lipids profile in most81,90,138–147 but not all195,196 studies. However, the efficacy of HCQ upon atherosclerosis is more controversial.151,152,197,198
It is to remember that smoking might inhibit HCQ effects7,109,110,112 and determine a twofold lower response of cutaneous involvement under HCQ; 199 counseling for smoking cessation is therefore important. Possible anti-neoplastic properties of HCQ have been poorly assessed in SLE. 168
HCQ might inhibit the conversion of 25-(OH)-vitamin D to 1,25-(OH)2-vitamin D. 200 However, data regarding the impact of HCQ on bone metabolism in SLE remain controversial.86,162,163,201,202 Many data suggest that HCQ has a protective role against infections92,116,154–160 and severe events included92,154–156 in SLE.
Corticosteroids are widely prescribed, but also important determinants of cardiovascular, gastrointestinal, and metabolic comorbidities as well as of accrual damage and impaired quality of life in SLE. Thus, another important role for HCQ in SLE is that of corticosteroid-sparing agent.80,84,103 However, as for other outcomes, there are also studies with negative results. 102
SLE is a severe disease with survival rates at 5 years of only 50% in early studies, which now exceed 90%. 203 While mortality in early stages is usually related to severe organ involvement and SLE disease activity itself, in late, long-standing SLE, accrual damage, and cardiovascular risk are the main determinants. In spite of some contrary results, 204 many studies reported HCQ protective effects for accrual damage3,83,84,87–89,164–167 and HCQ has also been associated with shorter SLE-related hospitalization length.159,169,170 And last, but not least, HCQ is one of the few treatments that has been shown to improve survival rates in SLE.87,91,93,116,127,171–178
Therefore, based on its wide spectrum of effects, HCQ should probably be considered a possible confounder in all research involving patients with SLE.
Systematic reviews and meta-analyses on hydroxychloroquine use in systemic lupus erythematosus
The first systematic review regarding HCQ in SLE included a total of 95 studies published between 1982 and 2007. 13 All studies which considered disease activity as the main outcome (11 articles) found positive results, with more than 50% reduction in disease activity in most reports and a decrease in corticosteroid needs in three studies; 13 however, the risk of severe SLE flare was reduced only with borderline significance. 13 Also, the HCQ benefits as adjuvant therapy for LN was also confirmed. 13 The potential benefits upon accrual damage and survival were reported in a limited number of studies. 13 This systematic review was continued by another one using a similar methodology for the 2007–2012 period. 3 The authors reported further evidence thrombosis prevention, increased survival, control of disease activity, lipid profile improvement, and prevention of damage accrual 3 (see Supplemental Table 4).
The protective effect of HCQ against infections was further confirmed in two systematic reviews and meta-analysis.153,161 Also, two meta-analyses reported improvement of the lipid profile under HCQ in SLE.149,150 For cutaneous involvement, Fairley et al. 114 reported in one systematic review only moderate HCQ efficacy.
A 2018 meta-analysis of observational data failed to identify any significant beneficial effect of HCQ over fetal growth restriction and prematurity. However, the authors mentioned that these results should be regarded with caution due to lack of RCTs, high heterogeneity among reported data, and of numerous missing data like those on the antiphospholipid antibodies status. 205
Overview of guidelines
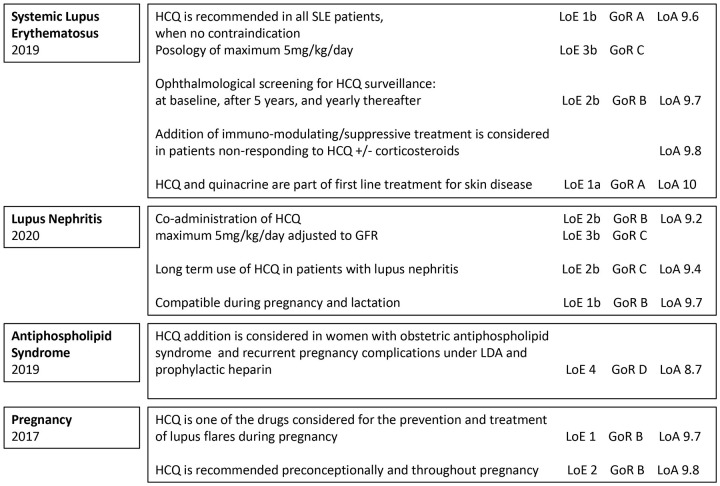
We reviewed here systematically the European League against Rheumatism (EULAR) recommendations referring to the use of HCQ. We identified all EULAR guidelines (www.eular.org) for the last 5 years and searched for HCQ-related paragraphs using the terms ‘Hydroxychloroquine’ and the respective abbreviation ‘HCQ’. All paragraphs found were extracted (see Supplemental Table 5) and data were further analyzed and summarized (see Supplemental Figure 2).
From the total 30 EULAR management guidelines published since 2016, 10 referring to HCQ were identified, and main indications were noted (see Supplemental Table 6). Recommendations addressing specifically to HCQ were found in seven guidelines8,34,206–210 while in others, HCQ was included as part of Disease Modifying AntiRheumatic Drugs (DMARDs).211–213 The EULAR Guidelines recommendations referring mainly to SLE and related conditions are summarized in Figure 2.
Figure 2.
Recommendations for hydroxychloroquine (HCQ) use according to the European League against Rheumatism (EULAR) guidelines.
Tunnincliffe et al. 214 and Tamirou et al. 215 reviewed SLE recommendations published up to 2014 and between 2004 and 2017, respectively, and identified not least than 14 and 23, respectively, original clinical guidelines or original statements with focus on SLE.
The 2020 American College of Rheumatology (ACR) Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases 190 advise for HCQ use during pregnancy and breastfeeding, in cases with positive anti-Ro/SS-A and anti-La/SS-B antibodies as well as additional or alternative therapy in SLE women with refractory obstetric APS. HCQ continuation is strongly recommended in men who are planning to father a pregnancy. 190 The 2012 ACR Guidelines for Screening, Treatment, and Management of Lupus Nephritis specifies that all SLE patients with nephritis should be treated with HCQ as background therapy. 9
The 2018 British Society for Rheumatology guideline for the management of SLE in adults 10 identified 45 studies to sustain the recommendation of antimalarial use (<6.5 mg/kg/day) for mild disease, prevention of flare in all patients, prevention of damage, and as steroid-sparing agent (overall SIGN level of evidence 1+++ and grade A of recommendation). 10
Finally, the Latin American Group for the Study of Lupus (GLADEL, Grupo Latino Americano de Estudio del Lupus)–Pan-American League of Associations of Rheumatology (PANLAR) stated also that antimalarials should be used in all SLE patients with exception of those who refuse or who have absolute contraindications, as first line for musculoskeletal or cutaneous involvement as well as associated with immunosuppressive treatments for other SLE organ involvements. 11
Hydroxychloroquine safety profile
A wide range of side effects such as cardiovascular, dermatological, digestive, hematological, metabolic, ophthalmologic, as well as other rare side effects were reported to be associated with HCQ use.4,13,30,31,32,35,216–238 The main side effects of HCQ are summarized in Table 4.
Table 4.
Side effects of hydroxychloroquine.
| System | HCQ’s side effects | ||
|---|---|---|---|
| Short term | Long term | References | |
| Cardiovascular | Hours-days: prolonged QT
a
(attention to the association with other drugs that affect the QT interval) Overdose: cardiovascular shock, collapse |
Weeks-months: Conduction troubles, cardiomyopathy, vacuolar myopathy, valvular disorders a | Costedoat-Chalumeau et al.; 4 Doyno et al.; 216 Nishiyama et al.; 217 Ruiz-Irastorza et al.; 13 Chatre et al.; 218 Zhao et al.; 219 Fiehn et al. 35 |
| Dermatologic | Days-weeks: pruritus, rashes, urticaria, exanthematous pustulosis, toxic epidermal necrolysis, Stevens–Johnson syndrome a | Years: hyperpigmentation | Costedoat-Chalumeau et al.; 4 Ruiz-Irastorza et al.; 13 Chatre et al.; 218 Fiehn et al. 35 |
| Digestive intolerance | Days: nausea, vomiting, diarrhea, bloating | Costedoat-Chalumeau et al.; 4 Ruiz-Irastorza et al.; 13 Chatre et al.; 218 Fiehn et al. 35 | |
| Hematological | Days to weeks: bone marrow toxicity, cytopenia (neutropenia) a | Weeks-months: bone marrow toxicity, cytopenia (neutropenia) a | Sames et al.; 220 Chatre et al.; 218 Fiehn et al. 35 |
| Metabolic | Days: hypoglycemia a | El-Solia et al.; 221 Cansu and Korkmaz; 222 Ruiz-Irastorza et al.; 13 Chatre et al.; 218 Fiehn et al. 35 | |
| Neuropsychiatric | One-two days: confusion, disorientation, hallucination Overdose: psychosis, seizurea,b |
Weeks-months: agitation, bradyphrenia, delirium, disorientation, drowsiness, confusion, pseudo-parkinsonisma,b | Mascolo et al.; 225 Chatre et al.; 218 Fiehn et al. 35 |
| Neuromuscular | Days: increase of creatine kinase a | Months: myositis, muscle weakness a | Ruiz-Irastorza et al.; 13 Chatre et al.; 218 Stein et al.; 223 Fiehn et al.; 35 Siddiqui et al. 224 |
| Ophthalmologic | Days-weeks: eye accommodation troubles | Months–years (5–20 years): retinopathy (maculopathy) | Marmor et al.; 30 Rosenbaum et al.; 31 Fiehn et al.; 35 Petri et al.; 226 Xie and Zhang; 227 Marmor et al.; 30 Melles and Marmor; 32 Wolfe and Marmor; 228 Ruiz-Irastorza et al. 13 |
| Otorhinolaryngology | Days-weeks: ototoxicity, tinnitus a | Chatre et al.; 218 Fiehn et al. 35 | |
| Only case reports | Fulminant hepatic failure; toxic myopathy with respiratory failure; podocytopathy mimicking Fabry disease; rare cutaneous side effects (erythroderma, dark rash, gray skin, erythema multiforme) | Chatre et al.; 218 Makin et al.; 229 Abou Assalie et al.; 239 Koumaki et al.; 230 Pai et al.; 231 Pelechas and Drosos; 232 Ivo et al.; 233 Serre et al.; 234 Wu et al. 235 | |
HCQ, hydroxychloroquine.
The HCQ-related side effects, in terms of frequency and severity, are related to daily posology, treatment duration, concomitant therapies, and associated comorbidities.
Only rare reported.
Association not confirmed yet.
Reviewing the antimalarials’ safety profile in SLE, Ruiz-Irastorza et al. 13 noted low prevalence of antimalarials’ toxicity, mainly mild gastrointestinal and cutaneous side effects. These were significantly more frequent under CQ when compared with HCQ, results parallel by higher discontinuation rates for CQ. Overall, the HCQ global safety was rated as high. 13 Eljaaly et al. 236 published recently a meta-analysis for the HCQ safety when administrated for different pathologies (chronic urticaria, RA, SLE, osteoarthritis, IgA nephropathy, asymptomatic HIV infection, Alzheimer disease, cutaneous lupus) in daily doses of 200–400 mg and presented also encouraging results. Besides significant more frequent occurrence of skin pigmentation under HCQ, no other side effect reached a significant difference (rash, gastrointestinal complaints, headache, fatigue, visual troubles) and also no cardiac toxicity was reported. 236
Thus, for long-term HCQ use, medium uptake duration of 32 months, 35 the skin hyperpigmentation is not rarely reported and might be favored by factors like ecchymosis, bruising, platelet antiaggregant, and oral anticoagulants. Beside hyperpigmentation, all other HCQ-related side effects are only rarely encountered.
On short-term use, the digestive intolerance is the most frequently encountered side effect, with occurrence possible since first HCQ administration.237,238
A wide range of mild neuropsychiatric manifestations, but also psychosis, was reported in relation to HCQ use, especially in elderly. However, this relation remains controversial as other concomitant factors like concomitant drugs, alcohol intake, use of glucocorticoids, or background disease itself could originate the neuropsychiatric manifestations occurrence in patients with SLE under HCQ. 225
Retinopathy occurrence remains the most discussed and studied HCQ’s side effect in SLE. The main risk factors for HCQ-related retinopathy are the treatment duration, daily and cumulative dose, chronic kidney disease, as well as pre-existent retinal disease. 34 Ophthalmologic screening is mandatory, yearly from baseline if there are known risk factors or at baseline, after 5 years on HCQ, and yearly therefore in patients without retinopathy risk factors.8,30,31,34 The current 2020 Joint Statement on HCQ 31 reinforced the old recommendations8,30,34,32 of the need of sensitive testing modalities such as optical coherence tomography (OCT) and automated visual fields that could detect early toxicity. 31 When available, quinacrine (mepacrine) might be considered as an alternative in SLE patients with HCQ-related ocular or cutaneous side effects.
As the eye side effects are dose-related, not only the duration of use but also the blood levels are predictors of retinopathy development with a statistical association in patients with [HCQ] blood levels >1200 ng/ml.226,227 However, association between HCQ blood concentration and retinopathy has not been confirmed in another study. 240 For non-rheumatic diseases, doses of up to 1000 mg daily (up to 20 mg/kg daily) showed eye toxicity within 2 years in 25–40% of the patients exposed, 30 while for the doses up to 5 mg/kg of real body weight, the risk of retinopathy within 10 years was 2%. 32 For lifetime HCQ users, definite or probable toxicity was documented in only 0.65% even if 6.5% patients discontinued therapy because of eye-related side effects. 228 One longitudinal study showed ophthalmological alterations confirmed by ophthalmological examination in 5.5% of cases. 241
When compared with HCQ, the risk of retinopathy related to CQ seems to be much higher, hence CQ is not recommended as the first-line antimalarial for the SLE treatment. One systematic review including four studies for CQ versus six studies for HCQ found definite retinal toxicity in 2.5% versus 0.1% and probable retinopathy in 2.6% versus 0.3% patients. 13 A recent report from the Hopkins cohort showed a higher overall frequency of retinopathy of 4.3%, but the risk increased significantly after 15 years of HCQ use, 226 namely 1% in the first 5 years, 1.8% for 6–10 years, 3.3% for 11–15 years, and 11.5% for 16–20 years. 226
For antimalarials cardiac toxicity, the results of 86 articles were systematically reviewed and a total of 127 patients (65.4% female) were identified, of which about 60% had taken CQ, while the rest HCQ. 218 The most frequent cardiac side effects reported were conduction disorders (85%), followed by cardiac hypertrophy (22%), hypokinesia (9.4%), cardiac failure (26.8%), pulmonary arterial hypertension (3.9%), and valvular dysfunction (7.1%). Less than half of the patients (44.9%) recovered normal heart function after the antimalarial drug withdrawal. 218
Disparate cases of HCQ-related neuromyopathy, particularly manifested as insidious onset of proximal myopathy that may be later associated with peripheral neuropathy and cardiac myotoxicity, are reported. The frequency of HCQ-related myopathies is not known, but is probably extremely rare. 35 Early recognition is important as the recovery after the drug withdrawal might be incomplete. 223
Different case reports presented rare and very rare sides effects attributable to HCQ in the absence of other identifiable causes, like early fulminant hepatic failure, 229 toxic myopathy with respiratory failure, 224 and rare cutaneous lesions.230–235,239
Hydroxychloroquine blood level monitoring and withdrawal
Even if the HCQ role in SLE is acknowledged, less than half of the patients are taking HCQ as prescribed. 242 Measurement of HCQ in whole blood was proposed to monitor both response and adherence to treatment, but an appropriate cut-off for defining efficient HCQ’s blood levels remains under debate. For CLE, one prospective multicenter study found significantly higher median blood [HCQ] levels in patients with complete remission (910 ng/ml in remission versus 692 ng/ml when partial remission and 569 ng/ml in treatment failure, p = 0.007). 107 In a prospective study, improvement of cutaneous lesions was observed when [HCQ] blood levels higher than 750 ng/ml were reached. 113 Also, one study defined subtherapeutic [HCQ] levels, associated with trend of more disease flares, as less than 500 ng/ml. 243 A recent report showed that low [HCQ] blood levels are associated with thrombotic events (720 ng/ml versus 935 ng/ml; p = 0.025). 135
On one hand, a decrease in the flare rate was not observed when [HCQ] level was maintained over 1000 ng/ml. 72 On the other hand, decrease to 2–3 mg/kg/day did not modify serum [HCQ] levels significantly at 3 and 6 months, but only at 12 months. 73
One of the main reasons for using [HCQ] blood levels in daily practice is the great interindividual variability, of which determinants are not completely characterized. 5 [HCQ] levels were found to be related to its major metabolite, N-desethylhydroxychloroquine (DHCQ), to HCQ weight-adjusted oral dose and also to the time since last dose taken.243,244
Analyzing a longitudinal cohort, Mok et al. 243 found that the majority of SLE patients screened had mainly [HCQ] subtherapeutic levels: <10 ng/ml (defined as total non-adherence) in 11%, 10–500 ng/ml (subtherapeutic levels) in 77%, and >500 ng/ml (therapeutic levels) in only 12% patients. Levels correlated with the dose prescribed 243 and, importantly, higher [HCQ] levels were associated with less SLE flare occurrence over time. 243
Monitoring HCQ levels might allow identification of early nonadherence 243 and improve nonadherence. 72 HCQ levels measurement might help in counseling before the treatment change in regard to lack of adherence versus lack of treatment efficacy. 5
Finally, considering the HCQ’s side effects related to long-term use, one important question is how to identify the appropriate moment for stopping the treatment. The first RCT designed for HCQ66,67 showed efficacy of long-term HCQ use in sustaining remission. In this RCT, the average HCQ total treatment duration before withdrawal was about 3 years.66,67 A more recent retrospective study showed that HCQ discontinuation in patients older than 55 years with quiescent SLE and more than 5 years treatment, due to retinal toxicity, patient’s preference, cardiac toxicity, or other suspected adverse effects, did not result in significant increase in flare occurrence. 245 Finally, a recent survey across large international sample of physicians has shown that in case of sustained remission, 49.7% maintained the same dose indefinitely, 48.3% reduced the dose, while only 2.0% discontinued antimalarials. 37
Conclusion
In summary, HCQ is indicated in all patients with SLE in the absence of any contraindications or side effects, with high grade evidence in case of LN, cutaneous involvement, or during pregnancy and breastfeeding. However, there is a relatively small effect size for the prevention of severe flares in SLE. Monitoring HCQ blood levels might help to overcome adherence issues, which are quite common in SLE and adjust the daily dosage based on individual pharmacokinetic variability. Still, there is a need for additional research focused on defining the optimal conditions for HCQ withdrawal.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X211073001 for Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge by Alina Dima, Ciprian Jurcut, François Chasset, Renaud Felten and Laurent Arnaud in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Author contributions: Alina Dima: Conceptualization; Methodology; Writing – original draft.
Ciprian Jurcut: Conceptualization; Writing – original draft.
Francois Chasset: Conceptualization; Writing – original draft.
Renaud Felten: Conceptualization; Writing – original draft.
Laurent Arnaud: Conceptualization; Methodology; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs: Alina Dima  https://orcid.org/0000-0001-8743-3236
https://orcid.org/0000-0001-8743-3236
Renaud Felten  https://orcid.org/0000-0002-4951-4032
https://orcid.org/0000-0002-4951-4032
Laurent Arnaud  https://orcid.org/0000-0002-8077-8394
https://orcid.org/0000-0002-8077-8394
Contributor Information
Alina Dima, Department of Rheumatology, Colentina Clinical Hospital, Bucharest, Romania.
Ciprian Jurcut, Department of Internal Medicine, Dr. Carol Davila Central Military Emergency University Hospital, Bucharest, Romania.
François Chasset, Department of Dermatology and Allergology, Hôpital Tenon, Paris, France; Faculté de Médecine, Sorbonne Université, Paris, France.
Renaud Felten, National Reference Center for Rare Auto-immune and Systemic Diseases Est Sud-Est (RESO), Strasbourg, France; Department of Rheumatology, Les Hôpitaux Universitaires de Strasbourg, Strasbourg, France.
Laurent Arnaud, National Reference Center for Rare Auto-immune and Systemic Diseases Est Sud-Est (RESO), Strasbourg, France; Department of Rheumatology, Les Hôpitaux Universitaires de Strasbourg, Strasbourg, France; Université de Strasbourg, Inserm UMR-S 1109, Strasbourg, France; Service de Rhumatologie, Hôpital de Hautepierre, 1, avenue Molière BP 83049, 67098 Strasbourg Cedex, France.
References
- 1. Olsen NJ, Schleich MA, Karp DR. Multifaceted effects of hydroxychloroquine in human disease. Semin Arthritis Rheum 2013; 43: 264–272. [DOI] [PubMed] [Google Scholar]
- 2. Dima A, Jurcut C, Arnaud L. Hydroxychloroquine in systemic and auto-immune diseases: where are we now? Jt Bone Spine 2021; 88: 105143. [DOI] [PubMed] [Google Scholar]
- 3. Akhavan PS, Su J, Lou W, et al. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol 2013; 40: 831–841. [DOI] [PubMed] [Google Scholar]
- 4. Costedoat-Chalumeau N, Leroux G, Piette JC, et al. Why all systemic lupus erythematosus patients should be given hydroxychloroquine treatment. Joint Bone Spine 2010; 77: 4–5. [DOI] [PubMed] [Google Scholar]
- 5. Costedoat-Chalumeau N, Dunogué B, Morel N, et al. Hydroxychloroquine: a multifaceted treatment in lupus. Press Médicale 2014; 43: e167–e180. [DOI] [PubMed] [Google Scholar]
- 6. Petri M. Hydroxychloroquine: past, present, future. Lupus 1998; 7: 65–67. [DOI] [PubMed] [Google Scholar]
- 7. Stojan G, Petri M. Atherosclerosis in systemic lupus erythematosus. J Cardiovasc Pharmacol 2013; 62: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 9. Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res 2012; 64: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon C, Amissah-Arthur M-B, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 2017; 57: e1–45. [DOI] [PubMed] [Google Scholar]
- 11. Pons-Estel BA, Bonfa E, Soriano ER, et al. First Latin American clinical practice guidelines for the treatment of systemic lupus erythematosus: Latin American Group for the Study of Lupus (GLADEL, Grupo Latino Americano de Estudio del Lupus)-Pan-American League of Associations of Rheumatology (PANLAR). Ann Rheum Dis 2018; 77: 1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felten R, Lipsker D, Sibilia J, et al. The history of lupus throughout the ages. J Am Acad Dermatol. Epub ahead of print 4 May 2020. DOI: 10.1016/j.jaad.2020.04.150. [DOI] [PubMed] [Google Scholar]
- 13. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69: 20–28. [DOI] [PubMed] [Google Scholar]
- 14. Meinao IM, Sato EI, Andrade LE, et al. Controlled trial with chloroquine diphosphate in systemic lupus erythematosus. Lupus 1996; 5: 237–241. [DOI] [PubMed] [Google Scholar]
- 15. Dubois EL. Antimalarials in the management of discoid and systemic lupus erythematosus. Semin Arthritis Rheum 1978; 8: 33–51. [DOI] [PubMed] [Google Scholar]
- 16. Lupus therapies continue to evolve. FDA, https://www.fda.gov/consumers/consumer-updates/lupus-therapies-continue-evolve (accessed 27 February 2021).
- 17. Lim HS, Im JS, Cho JY, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother 2009; 53: 1468–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Browning DJ. Hydroxychloroquine and chloroquine retinopathy. New York: Springer, 2014. [Google Scholar]
- 19. Ducharme J, Farinotti R. Clinical pharmacokinetics and metabolism of chloroquine. Clin Pharmacokinet 1996; 31: 257–274. [DOI] [PubMed] [Google Scholar]
- 20. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus 1996; 5: S11–S15, https://pubmed.ncbi.nlm.nih.gov/8803904/ (accessed 4 March 2021). [PubMed] [Google Scholar]
- 21. Walker O, Birkett D, Alvan G, et al. Characterization of chloroquine plasma protein binding in man. Br J Clin Pharmacol 1983; 15: 375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shannon JA, Earle DP, Brodie BB, et al. The pharmacological basis for the rational use of Abatrine in the treatment of malaria. J Pharmacol Exp Ther 1944; 81: 304–330. [Google Scholar]
- 23. Tett S, Cutler D, Day R, et al. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol 1989; 27: 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frisk-Holmberg M, Bergqvist Y, Termond E, et al. The single dose kinetics of chloroquine and its major metabolite desethylchloroquine in healthy subjects. Eur J Clin Pharmacol 1984; 26: 521–530. [DOI] [PubMed] [Google Scholar]
- 25. Zahr N, Urien S, Llopis B, et al. Pharmacokinetics and pharmacodynamics of hydroxychloroquine in hospitalized patients with COVID-19. Therapie 2021; 76: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Munster T, Gibbs JP, Shen D, et al. Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis. Arthritis Rheum 2002; 46: 1460–1469. [DOI] [PubMed] [Google Scholar]
- 27. Tett S, Cutler D, Day R, et al. A dose-ranging study of the pharmacokinetics of hydroxyl-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 1988; 26: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plasma quinacrine concentration as a function of dosage and environment: joint report of the Armored Medical Research Laboratory, Fort Knox, Ky. and the Commission on Tropical Diseases, Army Epidemiological Board, Preventive Medicine Service, Office of the Surgeon General, United States Army. Arch Intern Med 1946; 78: 64–107. [DOI] [PubMed] [Google Scholar]
- 29. Plaquenil (hydroxychloroquine sulfate) dosing, indications, interactions, adverse effects, and more, https://reference.medscape.com/drug/plaquenil-hydroxychloroquine-sulfate-343205 (accessed 17 June 2020).
- 30. Marmor MF, Kellner U, Lai TYY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology 2016; 123: 1386–1394. [DOI] [PubMed] [Google Scholar]
- 31. Rosenbaum JT, Costenbader KH, Desmarais J, et al. ACR, AAD, RDS, and AAO 2020 joint statement on hydroxychloroquine use with respect to retinal toxicity. Arthritis Rheumatol 2021; 73: 908–911. [DOI] [PubMed] [Google Scholar]
- 32. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol 2014; 132: 1453–1460. [DOI] [PubMed] [Google Scholar]
- 33. Costedoat-Chalumeau N, Isenberg D, Petri M. Comment on the 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus by Fanouriakis et al. Ann Rheum Dis 2020; 79: e90. [DOI] [PubMed] [Google Scholar]
- 34. Fanouriakis A, Kostopoulou M, Cheema K, et al. 2019 Update of the Joint European League against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2020; 79: 713–723. [DOI] [PubMed] [Google Scholar]
- 35. Fiehn C, Ness T, Weseloh C, et al. Safety management in treatment with antimalarials in rheumatology. Interdisciplinary recommendations on the basis of a systematic literature review. Z Rheumatol 2021; 80: 1–9. [DOI] [PubMed] [Google Scholar]
- 36. Rainsford KD, Parke AL, Clifford-Rashotte M, et al. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology 2015; 23: 231–269. [DOI] [PubMed] [Google Scholar]
- 37. Petitdemange A, Felten R, Sibilia J, et al. Prescription strategy of antimalarials in cutaneous and systemic lupus erythematosus: an international survey. Ther Adv Musculoskelet Dis 2021; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wallace DJ, Tse K, Hanrahan L, et al. Hydroxychloroquine usage in US patients, their experiences of tolerability and adherence, and implications for treatment: survey results from 3127 patients with SLE conducted by the Lupus Foundation of America. Lupus Sci Med 2019; 6: e000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol 2020; 16: 155–166. [DOI] [PubMed] [Google Scholar]
- 40. Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf 2017; 16: 411–419. [DOI] [PubMed] [Google Scholar]
- 41. Lamphier M, Zheng W, Latz E, et al. Novel small molecule inhibitors of tlr7 and tlr9: mechanism of action and efficacy in vivo. Mol Pharmacol 2014; 85: 429–440. [DOI] [PubMed] [Google Scholar]
- 42. Kužnik A, Bencˇina M, Švajger U, et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol 2011; 186: 4794–4804. [DOI] [PubMed] [Google Scholar]
- 43. Gardet A, Pellerin A, McCarl C-A, et al. Effect of in vivo Hydroxychloroquine and ex vivo Anti-BDCA2 mAb treatment on pDC IFNα production from patients affected with cutaneous lupus erythematosus. Front Immunol 2019; 10: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. An J, Woodward JJ, Lai W, et al. Inhibition of cyclic GMP-AMP synthase using a novel antimalarial drug derivative in trex1-deficient mice. Arthritis Rheumatol 2018; 70: 1807–1819. [DOI] [PubMed] [Google Scholar]
- 45. Wozniacka A, Lesiak A, Boncela J, et al. The influence of antimalarial treatment on IL-1β, IL-6 and TNF-α mRNA expression on UVB-irradiated skin in systemic lupus erythematosus. Br J Dermatol 2008; 159: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 46. Lesiak A, Narbutt J, Kobos J, et al. Systematic administration of chloroquine in discoid lupus erythematosus reduces skin lesions via inhibition of angiogenesis. Clin Exp Dermatol 2009; 34: 570–575. [DOI] [PubMed] [Google Scholar]
- 47. Zeidi M, Kim HJ, Werth VP. Increased myeloid dendritic cells and TNF-α expression predicts poor response to hydroxychloroquine in cutaneous lupus erythematosus. J Invest Dermatol 2019; 139: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smith CK, Vivekanandan-Giri A, Tang C, et al. Neutrophil extracellular trap-derived enzymes oxidize high-density lipoprotein: an additional proatherogenic mechanism in systemic lupus erythematosus. Arthritis Rheumatol 2014; 66: 2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smith CK, Kaplan MJ. The role of neutrophils in the pathogenesis of systemic lupus erythematosus. Curr Opin Rheumatol 2015; 27: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cepika AM, Soldo Jureša D, Morovic Vergles J, et al. Decrease in circulating DNA, IL-10 and BAFF levels in newly-diagnosed SLE patients after corticosteroid and chloroquine treatment. Cell Immunol 2012; 276: 196–203. [DOI] [PubMed] [Google Scholar]
- 51. Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer 2017; 17: 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Humbert M, Bertolino P, Forquet F, et al. Major histocompatibility complex class II-restricted presentation of secreted and endoplasmic reticulum resident antigens requires the invariant chains and is sensitive to lysosomotropic agents. Eur J Immunol 1993; 23: 3167–3172. [DOI] [PubMed] [Google Scholar]
- 53. Silva JC, Mariz HA, Rocha LF, Jr, et al. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics (Sao Paulo) 2013; 68: 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao L, Ma H, Jiang Z, et al. Immunoregulation therapy changes the frequency of interleukin (IL)-22 + CD4 + T cells in systemic lupus erythematosus patients. Clin Exp Immunol 2014; 177: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shin MS, Lee N, Kang I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol 2011; 23: 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sailler L, Puissant B, Méliani P, et al. Blood concentrations of hydroxychloroquine and its desethyl derivative correlate negatively with the percentage of CD45RO+ cells among CD4+ lymphocytes in hydroxychloroquine-treated lupus patients. Ann NY Acad Sci 2007; 1108: 41–50. [DOI] [PubMed] [Google Scholar]
- 57. Spada R, Rojas JM, Barber DF. Recent findings on the role of natural killer cells in the pathogenesis of systemic lupus erythematosus. J Leukoc Biol 2015; 98: 479–487. [DOI] [PubMed] [Google Scholar]
- 58. Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum 1993; 23: 82–91. [DOI] [PubMed] [Google Scholar]
- 59. Sjolin-Forsberg G, Berne B, Eggelte TA, et al. In situ localization of chloroquine and immunohistological studies in UVB-irradiated skin of photosensitive patients. Acta Derm Venereol 1995; 75: 228–231. [DOI] [PubMed] [Google Scholar]
- 60. Wozniacka A, Lesiak A, Narbutt J, et al. Chloroquine treatment reduces the number of cutaneous HLA-DR+ and CD1a+ cells in patients with systemic lupus erythematosus. Lupus 2007; 16: 89–94. [DOI] [PubMed] [Google Scholar]
- 61. Bondeson J, Sundler R. Antimalarial drugs inhibit phospholipase A2 activation and induction of interleukin 1β and tumor necrosis factor α in macrophages: implications for their mode of action in rheumatoid arthritis. Gen Pharmacol 1998; 30: 357–366. [DOI] [PubMed] [Google Scholar]
- 62. el Tahir KE. Influence of niridazole and chloroquine on arterial and myometrial prostacyclin synthesis. Br J Pharmacol 1987; 92: 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Segal-Eiras A, Segura GM, Babini JC, et al. Effect of antimalarial treatment on circulating immune complexes in rheumatoid arthritis. J Rheumatol 1985; 12: 87–89. [PubMed] [Google Scholar]
- 64. Wozniacka A, Carter A, McCauliffe DP. Antimalarials in cutaneous lupus erythematosus: mechanisms of therapeutic benefit. Lupus 2002; 11: 71–81. [DOI] [PubMed] [Google Scholar]
- 65. Nguyen TQ, Capra JD, Sontheimer RD. 4-Aminoquinoline antimalarials enhance UV-B induced c-jun transcriptional activation. Lupus 1998; 7: 148–153. [DOI] [PubMed] [Google Scholar]
- 66. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N Engl J Med 1991; 324: 150–154. [DOI] [PubMed] [Google Scholar]
- 67. Tsakonas E, Joseph L, Esdaile JM, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. Lupus 1998; 7: 80–85. [DOI] [PubMed] [Google Scholar]
- 68. Williams HJ, Egger MJ, Singer JZ, et al. Comparison of hydroxychloroquine and placebo in the treatment of the arthropathy of mild systemic lupus erythematosus. J Rheumatol 1994; 21: 1457–1462. [PubMed] [Google Scholar]
- 69. Meng J, Lu Y, Dong X, et al. Long-term effects of hydroxychloroquine on metabolism of serum lipids and left ventricular structure and function in patients of systemic lupus erythematosus. Natl Med J China 2014; 94: 965–968. [PubMed] [Google Scholar]
- 70. Kavanaugh A, Adams-Huet B, Jain R, et al. Hydroxychloroquine effects on lipoprotein profiles (the HELP trial): a double-blind, randomized, placebo-controlled, pilot study in patients with systemic lupus erythematosus. J Clin Rheumatol 1997; 3: 3–8. [PubMed] [Google Scholar]
- 71. Levy RA, Vilela VS, Cataldo MJ, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus 2001; 10: 401–404. [DOI] [PubMed] [Google Scholar]
- 72. Costedoat-Chalumeau N, Galicier L, Aumaître O, et al. Hydroxychloroquine in systemic lupus erythematosus: results of a French multicentre controlled trial (PLUS Study). Ann Rheum Dis 2013; 72: 1786–1792. [DOI] [PubMed] [Google Scholar]
- 73. Zanetti CB, Pedrosa T, Kupa L, et al. Hydroxychloroquine blood levels in stable lupus nephritis under low dose (2–3 mg/kg/day): 12-month prospective randomized controlled trial. Clin Rheumatol 2021; 40: 2745–2751. [DOI] [PubMed] [Google Scholar]
- 74. Kraak JH, Van Ketel W, Prakken JR, et al. The value of hydroxychloroquine (Plaquenil) for the treatment of chronic discoid lupus erythematosus; a double blind trial. Dermatologica 1965; 130: 293–305. [DOI] [PubMed] [Google Scholar]
- 75. Yokogawa N, Eto H, Tanikawa A, et al. Effects of hydroxychloroquine in patients with cutaneous lupus erythematosus: a multicenter, double-blind, randomized, parallel-group trial. Arthritis Rheumatol 2017; 69: 791–799. [DOI] [PubMed] [Google Scholar]
- 76. Ruzicka T, Sommerburg C, Goerz G, et al. Treatment of cutaneous lupus erythematosus with acitretin and hydroxychloroquine. Br J Dermatol 1992; 127: 513–518. [DOI] [PubMed] [Google Scholar]
- 77. Bezerra EL, Vilar MJ, da Trindade Neto PB, et al. Double-blind, randomized, controlled clinical trial of clofazimine compared with chloroquine in patients with systemic lupus erythematosus. Arthritis Rheum 2005; 52: 3073–3078. [DOI] [PubMed] [Google Scholar]
- 78. Babaoglu H, Li J, Goldman D, et al. Time to lupus low disease activity state in the Hopkins lupus cohort: role of African American ethnicity. Arthritis Care Res (Hoboken) 2020; 72: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kasitanon N, Fine DM, Haas M, et al. Hydroxychloroquine use predicts complete renal remission within 12 months among patients treated with mycophenolate mofetil therapy for membranous lupus nephritis. Lupus 2006; 15: 366–370. [DOI] [PubMed] [Google Scholar]
- 80. Clowse MEB, Magder L, Witter F, et al. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006; 54: 3640–3647. [DOI] [PubMed] [Google Scholar]
- 81. Petri M, Lakatta C, Magder L, et al. Effect of prednisone and hydroxychloroquine on coronary artery disease risk factors in systemic lupus erythematosus: a longitudinal data analysis. Am J Med 1994; 96: 254–259. [DOI] [PubMed] [Google Scholar]
- 82. Durcan L, Winegar DA, Connelly MA, et al. Longitudinal evaluation of lipoprotein variables in Systemic Lupus Erythematosus reveals adverse changes with disease activity and prednisone and more favorable profiles with hydroxychloroquine therapy. J Rheumatol 2016; 43: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Petri MMPH, Purvey S, Fang H, et al. Predictors of organ damage in Systemic Lupus Erythematosus: the Hopkins’ Lupus cohort. Arthritis Rheum 2012; 64: 4021–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pons-Estel GJ, Alarcón GS, Mcgwin G, et al. Protective effect of hydroxychloroquine on renal damage in patients with lupus nephritis: data from LUMINA, a multiethnic U.S. Cohort NIH Public Access. Arthritis Rheum 2009; 61: 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Willis R, Seif AM, McGwin G, Jr, et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus 2012; 21: 830–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Calvo-Alén J, McGwin G, Toloza S, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA): XXIV. Cytotoxic treatment is an additional risk factor for the development of symptomatic osteonecrosis in lupus patients: results of a nested matched case-control study. Ann Rheum Dis 2006; 65: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Alarcón GS, McGwin G, Bertoli AM, et al. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: data from LUMINA, a multiethnic US cohort (LUMINA L). Ann Rheum Dis 2007; 66: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. González LA, Pons-Estel GJ, Zhang J, et al. Time to neuropsychiatric damage occurrence in LUMINA (LXVI): a multi-ethnic lupus cohort. Lupus 2009; 18: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pons-Estel GJ, Alarcón GS, González LA, et al. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res 2010; 62: 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nikpour M, Gladman DD, Ibanez D, et al. Variability over time and correlates of cholesterol and blood pressure in systemic lupus erythematosus: a longitudinal cohort study. Arthritis Res Ther 2010; 12: R125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Urowitz MB, Gladman DD, Tom BDM, et al. Changing patterns in mortality and disease outcomes for patients with systemic lupus erythematosus. J Rheumatol 2008; 35: 2152–2158. [DOI] [PubMed] [Google Scholar]
- 92. Pimentel-Quiroz VR, Ugarte-Gil MF, Harvey GB, et al. Factors predictive of serious infections over time in systemic lupus erythematosus patients: data from a multi-ethnic, multi-national, Latin American lupus cohort. Lupus 2019; 28: 1101–1110. [DOI] [PubMed] [Google Scholar]
- 93. Shinjo SK, Bonfá E, Wojdyla D, et al. Antimalarial treatment may have a time-dependent effect on lupus survival: data from a multinational Latin American inception cohort. Arthritis Rheum 2010; 62: 855–862. [DOI] [PubMed] [Google Scholar]
- 94. Pons-Estel GJ, Alarcón GS, Hachuel L, et al. Anti-malarials exert a protective effect while mestizo patients are at increased risk of developing SLE renal disease: data from a Latin-American cohort. Rheumatology 2012; 51: 1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wozniacka A, Lesiak A, Narbutt J, et al. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 2006; 15: 268–275. [DOI] [PubMed] [Google Scholar]
- 96. Shinjo SK. Systemic lupus erythematosus in the elderly: antimalarials in disease remission. Rheumatol Int 2009; 29: 1087–1090. [DOI] [PubMed] [Google Scholar]
- 97. Monzavi SM, Alirezaei A, Shariati-Sarabi Z, et al. Efficacy analysis of hydroxychloroquine therapy in systemic lupus erythematosus: a study on disease activity and immunological biomarkers. Inflammopharmacology 2018; 26: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 98. Balevic SJ, Cohen-Wolkowiez M, Eudy AM, et al. Hydroxychloroquine levels throughout pregnancies complicated by rheumatic disease: implications for maternal and neonatal outcomes. J Rheumatol 2019; 46: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hanaoka H, Iida H, Kiyokawa T, et al. Hydroxychloroquine improves the disease activity and allows the reduction of the corticosteroid dose regardless of background treatment in Japanese patients with systemic lupus erythematosus. Intern Med 2019; 58: 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Miyagawa I, Nakano K, Nakayamada S, et al. The additive effects of hydroxychloroquine to maintenance therapy with standard of care in patients with systemic lupus erythematosus. Int J Rheum Dis 2020; 23: 549–558. [DOI] [PubMed] [Google Scholar]
- 101. Giannakou I, Chatzidionysiou K, Magder LS, et al. Predictors of persistent disease activity and long quiescence in systemic lupus erythematosus: results from the Hopkins Lupus Cohort. Lupus Sci Med 2018; 5: e000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rudnicki GEG. The efficacy of antimalarials in systemic lupus erythematosus. J Rheumatol 1975; 2: 323–330. [PubMed] [Google Scholar]
- 103. Rothfield N. Efficacy of antimalarials in systemic lupus erythematosus. Am J Med 1988; 85: 53–56. [DOI] [PubMed] [Google Scholar]
- 104. Zen M, Saccon F, Gatto M, et al. Prevalence and predictors of flare after immunosuppressant discontinuation in patients with systemic lupus erythematosus in remission. Rheumatology 2020; 59: 1591–1598. [DOI] [PubMed] [Google Scholar]
- 105. Costedoat-Chalumeau N, Amoura Z, Hulot J-S, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with Systemic Lupus Erythematosus. Arthritis Rheum 2006; 54: 3284–3290. [DOI] [PubMed] [Google Scholar]
- 106. Yokogawa N, Tanikawa A, Amagai M, et al. Response to hydroxychloroquine in Japanese patients with lupus-related skin disease using the cutaneous lupus erythematosus disease area and severity index (CLASI). Mod Rheumatol 2013; 23: 318–322. [DOI] [PubMed] [Google Scholar]
- 107. Francès C, Cosnes A, Duhaut P, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol 2012; 148: 479–484. [DOI] [PubMed] [Google Scholar]
- 108. Wahie S, Daly AK, Cordell HJ, et al. Clinical and pharmacogenetic influences on response to hydroxychloroquine in discoid lupus erythematosus: a retrospective cohort study. J Invest Dermatol 2011; 131: 1981–1986. [DOI] [PubMed] [Google Scholar]
- 109. Kuhn A, Sigges J, Biazar C, et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br J Dermatol 2014; 171: 571–579. [DOI] [PubMed] [Google Scholar]
- 110. Piette EW, Foering KP, Chang AY, et al. Impact of smoking in cutaneous lupus erythematosus. Arch Dermatol 2012; 148: 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kreuter A, Gaifullina R, Tigges C, et al. Lupus erythematosus tumidus response to antimalarial treatment in 36 patients with emphasis on smoking. Arch Dermatol 2009; 145: 244–248. [DOI] [PubMed] [Google Scholar]
- 112. Jewell ML, McCauliffe DE. Patients with cutaneous lupus erythematosus who smoke are less responsive to antimalarial treatment. J Am Acad Dermatol 2000; 42: 983–987. [PubMed] [Google Scholar]
- 113. Chasset F, Arnaud L, Costedoat-Chalumeau N, et al. The effect of increasing the dose of hydroxychloroquine (HCQ) in patients with refractory cutaneous lupus erythematosus (CLE): an open-label prospective pilot study. J Am Acad Dermatol 2016; 74: 693–699. [DOI] [PubMed] [Google Scholar]
- 114. Fairley JL, Oon S, Saracino AM, et al. Management of cutaneous manifestations of lupus erythematosus: a systematic review. Semin Arthritis Rheum 2020; 50: 95–127. [DOI] [PubMed] [Google Scholar]
- 115. Barber CEH, Geldenhuys L, Hanly JG. Sustained remission of lupus nephritis. Lupus 2006; 15: 94–101. [DOI] [PubMed] [Google Scholar]
- 116. Sisó A, Ramos-Casals M, Bové A, et al. Previous antimalarial therapy in patients diagnosed with lupus nephritis: influence on outcomes and survival. Lupus 2008; 17: 281–288. [DOI] [PubMed] [Google Scholar]
- 117. Lee JS, Oh JS, Kim YG, et al. Recovery of renal function in patients with lupus nephritis and reduced renal function: the beneficial effect of hydroxychloroquine. Lupus 2020; 29: 52–57. [DOI] [PubMed] [Google Scholar]
- 118. Cortés-Hernández J, Ordi-Ros J, Paredes F, et al. Clinical predictors of fetal and maternal outcome in systemic lupus erythematosus: a prospective study of 103 pregnancies. Rheumatology 2002; 41: 643–650. [DOI] [PubMed] [Google Scholar]
- 119. Al Arfaj AS, Khalil N. Pregnancy outcome in 396 pregnancies in patients with SLE in Saudi Arabia. Lupus 2010; 19: 1665–1673. [DOI] [PubMed] [Google Scholar]
- 120. Koh JH, Ko HS, Kwok SK, et al. Hydroxychloroquine and pregnancy on lupus flares in Korean patients with systemic lupus erythematosus. Lupus 2015; 24: 210–217. [DOI] [PubMed] [Google Scholar]
- 121. Seo MR, Chae J, Kim YM, et al. Hydroxychloroquine treatment during pregnancy in lupus patients is associated with lower risk of preeclampsia. Lupus 2019; 28: 722–730. [DOI] [PubMed] [Google Scholar]
- 122. Saavedra MÁ, Miranda-Hernández D, Lara-Mejía A, et al. Use of antimalarial drugs is associated with a lower risk of preeclampsia in lupus pregnancy: a prospective cohort study. Int J Rheum Dis 2020; 23: 633–640. [DOI] [PubMed] [Google Scholar]
- 123. Diav-Citrin O, Blyakhman S, Shechtman S, et al. Pregnancy outcome following in utero exposure to hydroxychloroquine: a prospective comparative observational study. Reprod Toxicol 2013; 39: 58–62. [DOI] [PubMed] [Google Scholar]
- 124. Broder A, Putterman C. Hydroxychloroquine use is associated with lower odds of persistently positive antiphospholipid antibodies and/or lupus anticoagulant in SLE. J Rheumatol 2013; 40: 30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wallace DJ. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus. Arthritis Rheum 1987; 30: 1435–1436. [DOI] [PubMed] [Google Scholar]
- 126. Mok MY, Chan EY, Fong DY, et al. Antiphospholipid antibody profiles and their clinical associations in Chinese patients with systemic lupus erythematosus. J Rheumatol 2005; 32: 622–628, https://europepmc.org/article/med/15801016 (accessed 22 February 2021). [PubMed] [Google Scholar]
- 127. Ruiz-Irastorza G, Egurbide MV, Pijoan JI, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus 2006; 15: 577–583. [DOI] [PubMed] [Google Scholar]
- 128. Choojitarom K, Verasertniyom O, Totemchokchyakarn K, et al. Lupus nephritis and Raynaud’s phenomenon are significant risk factors for vascular thrombosis in SLE patients with positive antiphospholipid antibodies. Clin Rheumatol 2008; 27: 345–351. [DOI] [PubMed] [Google Scholar]
- 129. Tektonidou MG, Laskari K, Panagiotakos DB, et al. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Care Res 2009; 61: 29–36. [DOI] [PubMed] [Google Scholar]
- 130. Becker-Merok A, Nossent JC. Prevalence, predictors and outcome of vascular damage in systemic lupus erythematosus. Lupus 2009; 18: 508–515. [DOI] [PubMed] [Google Scholar]
- 131. Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis 2009; 68: 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jung H, Bobba R, Su J, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum 2010; 62: 863–868. [DOI] [PubMed] [Google Scholar]
- 133. Law G, Magder L, Fang H, et al. SAT0222 hydroxychloroquine reduces thrombosis (both arterial and venous) in systemic lupus erythematosus, particularly in antiphospholipid positive patients. Ann Rheum Dis 2013; 71: 5471–5547. [Google Scholar]
- 134. Fasano S, Pierro L, Pantano I, et al. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J Rheumatol 2017; 44: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 135. Petri M, Konig MF, Li J, et al. Higher hydroxychloroquine blood levels are associated with reduced thrombosis risk in systemic lupus erythematosus. Arthritis Rheumatol 2021; 73: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kaprove Penn S, Kao AH, Schott LL, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus NIH public access. J Rheumatol 2010; 37: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chen Y-M, Lin C-H, Lan T-H, et al. Hydroxychloroquine reduces risk of incident diabetes mellitus in lupus patients in a dose-dependent manner: a population-based cohort study. Rheumatology 2015; 54: 1244–1249. [DOI] [PubMed] [Google Scholar]
- 138. Wallace DJ, Metzger AL, Stecher VJ, et al. Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. Am J Med 1990; 89: 322–326. [DOI] [PubMed] [Google Scholar]
- 139. Hodis HN, Quismorio FP, Jr, Wickham E, et al. The lipid, lipoprotein, and apolipoprotein effects of hydroxychloroquine in patients with systemic lupus erythematosus. J Rheumatol 1993; 20: 661–665, http://europepmc.org/article/med/8496861 (accessed 15 February 2021). [PubMed] [Google Scholar]
- 140. Rahman P, Gladman DD, Urowitz MB, et al. The cholesterol lowering effect of antimalarial drugs is enhanced in patients with lupus taking corticosteroid drugs. J Rheumatol 1999; 26: 325–330, http://europepmc.org/article/med/9972966 (accessed 15 February 2021). [PubMed] [Google Scholar]
- 141. Effect of antimalarial agents on the fasting lipid profile in systemic lupus erythematosus. J Rheumatol 2000; 27: 2142–2145. https://pubmed.ncbi.nlm.nih.gov/10990225/ (accessed 15 February 2021). [PubMed] [Google Scholar]
- 142. Borba EF, Bonfá E. Longterm beneficial effect of chloroquine diphosphate on lipoprotein profile in lupus patients with and without steroid therapy. J Rheumatol 2001; 28: 780–785. [PubMed] [Google Scholar]
- 143. Karimifar M, Gharibdoost F, Akbarian M, et al. Triglyceride and high-density lipoprotein levels as the markers of disease activity and their association with TNF-? and TNF receptor system in systemic lupus erythematosus. APLAR J Rheumatol 2007; 10: 221–226. [Google Scholar]
- 144. Sachet JC, Borba EF, Bonfá E, et al. Chloroquine increases low-density lipoprotein removal from plasma in systemic lupus patients. Lupus 2007; 16: 273–278. [DOI] [PubMed] [Google Scholar]
- 145. Cardoso CRL, Signorelli FV, Papi JA, et al. Prevalence and factors associated with dyslipoproteinemias in Brazilian systemic lupus erythematosus patients. Rheumatol Int 2008; 28: 323–327. [DOI] [PubMed] [Google Scholar]
- 146. Chong YB, Yap DY, Tang CS, et al. Dyslipidaemia in patients with lupus nephritis. Nephrology 2011; 16: 511–517. [DOI] [PubMed] [Google Scholar]
- 147. Cairoli E, Rebella M, Danese N, et al. Hydroxychloroquine reduces low-density lipoprotein cholesterol levels in systemic lupus erythematosus: a longitudinal evaluation of the lipid-lowering effect. Lupus 2012; 21: 1178–1182. [DOI] [PubMed] [Google Scholar]
- 148. Ali Abdalla M, Mostafa El Desouky S, Sayed Ahmed A. Clinical significance of lipid profile in systemic lupus erythematosus patients: relation to disease activity and therapeutic potential of drugs. Egypt Rheumatol 2017; 39: 93–98. [Google Scholar]
- 149. Tao CY, Shang J, Chen T, et al. Impact of antimalarial (AM) on serum lipids in systemic lupus erythematosus (SLE) patients: a systematic review and meta-analysis. Medicine 2019; 98: e15030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Babary H, Liu X, Ayatollahi Y, et al. Favorable effects of hydroxychloroquine on serum low density lipid in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Int J Rheum Dis 2018; 21: 84–92. [DOI] [PubMed] [Google Scholar]
- 151. Selzer F, Sutton-Tyrrell K, Fitzgerald S, et al. Vascular stiffness in women with systemic lupus erythematosus. Hypertension 2001; 37: 1075–1082, http://www.hypertensionaha.org [DOI] [PubMed] [Google Scholar]
- 152. Tanay A, Leibovitz E, Frayman A, et al. Vascular elasticity of systemic lupus erythematosus patients is associated with steroids and hydroxychloroquine treatment. Ann NY Acad Sci 2007; 1108: 24–34. [DOI] [PubMed] [Google Scholar]
- 153. Pego-Reigosa JM, Nicholson L, Pooley N, et al. The risk of infections in adult patients with systemic lupus erythematosus: systematic review and meta-analysis. Rheumatology 2021; 60: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Ruiz-Irastorza G, Olivares N, Ruiz-Arruza I, et al. Open Access Predictors of major infections in systemic lupus erythematosus. Arthritis Res Ther 2009; 11: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Herrinton LJ, Liu L, Goldfien R, et al. Risk of serious infection for patients with systemic lupus erythematosus starting glucocorticoids with or without antimalarials. J Rheumatol 2016; 43: 1503–1509. [DOI] [PubMed] [Google Scholar]
- 156. Rúa-Figueroa Í, López-Longo J, Galindo-Izquierdo M, et al. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum 2017; 47: 38–45. [DOI] [PubMed] [Google Scholar]
- 157. Zamora LD, Collante MTM, Navarra S. Risk factors for herpes zoster infection among Filipinos with systemic lupus erythematosus. Int J Rheum Dis 2020; 23: 197–202. [DOI] [PubMed] [Google Scholar]
- 158. González-Echavarri C, Capdevila O, Espinosa G, et al. Infections in newly diagnosed Spanish patients with systemic lupus erythematosus: data from the RELES cohort. Lupus 2018; 27: 2253–2261. [DOI] [PubMed] [Google Scholar]
- 159. Rosa GPD, Ortega MF, Teixeira A, et al. Causes and factors related to hospitalizations in patients with systemic lupus erythematosus: analysis of a 20-year period (1995–2015) from a single referral centre in Catalonia. Lupus 2019; 28: 1158–1166. [DOI] [PubMed] [Google Scholar]
- 160. Yeo KJ, Chen HH, Chen YM, et al. Hydroxychloroquine may reduce risk of pneumocystis pneumonia in lupus patients: a nationwide, population-based case-control study. BMC Infect Dis 2020; 20: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yuan Q, Xing X, Lu Z, et al. Clinical characteristics and risk factors of infection in patients with systemic lupus erythematosus: a systematic review and meta-analysis of observational studies. Semin Arthritis Rheum 2020; 50: 1022–1039. [DOI] [PubMed] [Google Scholar]
- 162. Lakshminarayanan S, Walsh S, Mohanraj M, et al. Factors associated with low bone mineral density in female patients with Systemic Lupus Erythematosus. J Rheumatol 2001; 28: 102–108, www.jrheum.org (accessed 15 February 2021). [PubMed] [Google Scholar]
- 163. Mok CC, Mak A, Ma KM. Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 2005; 14: 106–112. [DOI] [PubMed] [Google Scholar]
- 164. Molad Y, Gorshtein A, Wysenbeek AJ, et al. Protective effect of hydroxychloroquine in systemic lupus erythematosus. Prospective long-term study of an Israeli cohort. Lupus 2002; 11: 356–361. [DOI] [PubMed] [Google Scholar]
- 165. Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015; 74: 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lim LSH, Pullenayegum E, Lim L, et al. From childhood to adulthood: the trajectory of damage in patients with juvenile-onset Systemic Lupus Erythematosus. Arthritis Care Res 2017; 69: 1627–1635. [DOI] [PubMed] [Google Scholar]
- 167. Piga M, Floris A, Sebastiani GD, et al. Risk factors of damage in early diagnosed systemic lupus erythematosus: results of the Italian multicentre Early Lupus Project inception cohort. Rheumatology 2020; 59: 2272–2281. [DOI] [PubMed] [Google Scholar]
- 168. Ruiz-Irastorza G, Ugarte A, Egurbide MV, et al. Antimalarials may influence the risk of malignancy in systemic lupus erythematosus. Ann Rheum Dis 2007; 66: 815–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Liang H, Pan HF, Tao JH, et al. Causes and factors associated with frequent hospitalization in Chinese patients with systemic lupus erythematosus: an ambispective cohort study. Med Sci Monit 2019; 25: 8061–8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Feldman CH, Xu C, Williams J, et al. Patterns and predictors of recurrent acute care use among Medicaid beneficiaries with systemic lupus erythematosus. Semin Arthritis Rheum 2020; 50: 1428–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hernández-Cruz B, Tapia N, Villa-Romero AR, et al. Risk factors associated with mortality in systemic lupus erythematosus. Clin Exp Rheumatol 2001; 19: 395–401. [PubMed] [Google Scholar]
- 172. Feng X, Zou Y, Pan W, et al. Prognostic indicators of hospitalized patients with systemic lupus erythematosus: a large retrospective multicenter study in China. J Rheumatol 2011; 38: 1289–1295. [DOI] [PubMed] [Google Scholar]
- 173. Okpechi IG, Ayodele OE, Jones ESW, et al. Outcome of patients with membranous lupus nephritis in Cape Town South Africa. Nephrol Dial Transplant 2012; 27: 3509–3515. [DOI] [PubMed] [Google Scholar]
- 174. Zheng ZH, Zhang LJ, Liu WX, et al. Predictors of survival in Chinese patients with lupus nephritis. Lupus 2012; 21: 1049–1056. [DOI] [PubMed] [Google Scholar]
- 175. Mok CC, Tse SM, Chan KL, et al. Effect of immunosuppressive therapies on survival of systemic lupus erythematosus: a propensity score analysis of a longitudinal cohort. Lupus 2018; 27: 722–727. [DOI] [PubMed] [Google Scholar]
- 176. Pakchotanon R, Gladman DD, Su J, et al. Sustained complete renal remission is a predictor of reduced mortality, chronic kidney disease and end-stage renal disease in lupus nephritis. Lupus 2018; 27: 468–474. [DOI] [PubMed] [Google Scholar]
- 177. Mok CC, Ho LY, Chan KL, et al. Trend of survival of a cohort of Chinese patients with Systemic Lupus Erythematosus over 25 years. Front Med 2020; 7: 552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Jorge A, McCormick N, Lu N, et al. Hydroxychloroquine and mortality among patients with Systemic Lupus Erythematosus in the general population. Arthritis Care Res 2021; 73: 1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. James JA, Kim-Howard XR, Bruner BF, et al. Hydroxychloroquine sulfate treatment is associated with later onset of systemic lupus erythematosus. Lupus 2007; 16: 401–409. [DOI] [PubMed] [Google Scholar]
- 180. López P, Gómez J, Mozo L, et al. Cytokine polymorphisms influence treatment outcomes in SLE patients treated with antimalarial drugs. Arthritis Res Ther 2006; 8: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Dima A, Jurcut C, Balanescu P, et al. Clinical significance of serum and urinary interleukin-6 in systemic lupus erythematosus patients. Egypt Rheumatol 2016; 39: 1–6. [Google Scholar]
- 182. Lambers WM, Westra J, Bootsma H, et al. Hydroxychloroquine suppresses interferon-inducible genes and B cell activating factor in patients with incomplete and new-onset Systemic Lupus Erythematosus. J Rheumatol 2021; 48: 847–851. [DOI] [PubMed] [Google Scholar]
- 183. Fanouriakis A, Bertsias G. Changing paradigms in the treatment of systemic lupus erythematosus. Lupus Sci Med 2019; 6: e000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Izmirly PM, Costedoat-Chalumeau N, Pisoni C, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro associated cardiac manifestations of neonatal lupus. Circ July 2012; 3: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Izmirly PM, Kim MY, Llanos C, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis 2010; 69: 1827–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Zusman EZ, Sayre EC, Aviña-Zubieta JA, et al. Patterns of medication use before, during and after pregnancy in women with systemic lupus erythematosus: a population-based cohort study. Lupus 2019; 28: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 187. Smyth A, Oliveira GHM, Lahr BD, et al. A systematic review and meta-analysis of pregnancy outcomes in patients with Systemic Lupus Erythematosus and Lupus Nephritis. Clin J Am Soc Nephrol 2010; 5: 2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Buchanan NMM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis 1996; 55: 486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Costedoat-Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases a study of one hundred thirty-three cases compared with a control group. Arthritis Rheum 2003; 48: 3207–3211. [DOI] [PubMed] [Google Scholar]
- 190. Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of Rheumatology Guideline for the management of reproductive health in rheumatic and musculoskeletal diseases. Arthritis Rheumatol 2020; 72: 529–556. [DOI] [PubMed] [Google Scholar]
- 191. Mok CC, Tong KH, To CH, et al. Risk and predictors of arterial thrombosis in lupus and non-lupus primary glomerulonephritis. Medicine 2007; 86: 203–209. [DOI] [PubMed] [Google Scholar]
- 192. Toloza SMA, Uribe AG, McGwin G, Jr, et al. Systemic Lupus Erythematosus in a multiethnic US Cohort (LUMINA) XXIII. Baseline predictors of vascular events. Arthritis Rheum 2004; 50: 3947–3957. [DOI] [PubMed] [Google Scholar]
- 193. Mok CC, Tang SS, To CH, et al. Incidence and risk factors of thromboembolism in Systemic Lupus Erythematosus a comparison of three ethnic groups. Arthritis Rheum 2005; 52: 2774–2782. [DOI] [PubMed] [Google Scholar]
- 194. Ho KT, Ahn CW, Alarcon GS, et al. Systemic lupus erythematosus in a multiethnic cohort (LUMINA): XXVIII. Factors predictive of thrombotic events. Rheumatology 2005; 44: 1303–1307. [DOI] [PubMed] [Google Scholar]
- 195. Tam LS, Li EK, Lam CWK, et al. Hydroxychloroquine has no significant effect on lipids and apolipoproteins in Chinese systemic lupus erythematosus patients with mild or inactive disease. Lupus 2000; 9: 413–416. [DOI] [PubMed] [Google Scholar]
- 196. Antimalarials cholesterol profile of patients with systemic lupus erythematosus. Rev Bras Reumatol 2011; 51: 383–384, 386–387, https://pubmed.ncbi.nlm.nih.gov/21779713/ (accessed 25 February 2021). [PubMed] [Google Scholar]
- 197. Manzi S, Selzer F, Sutton-Tyrrell K, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum 1999; 42: 51–60. [DOI] [PubMed] [Google Scholar]
- 198. Roman MJ, Shanker B-A, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in Systemic Lupus Erythematosus. N Engl J Med 2003; 349: 2399–2406. [DOI] [PubMed] [Google Scholar]
- 199. Chasset F, Francès C, Barete S, et al. Influence of smoking on the efficacy of antimalarials in cutaneous lupus: a meta-analysis of the literature. J Am Acad Dermatol 2015; 72: 634–639. [DOI] [PubMed] [Google Scholar]
- 200. Huisman A, White KP, Algra A, et al. Vitamin D levels in women with Systemic Lupus Erythematosus and fibromyalgia. J Rheumatol 2001; 28: 2535–2539. [PubMed] [Google Scholar]
- 201. Jacobs J, Korswagen LA, Schilder AM, et al. Six-year follow-up study of bone mineral density in patients with systemic lupus erythematosus. Osteoporos Int 2013; 24: 1827–1833. [DOI] [PubMed] [Google Scholar]
- 202. Prasad R, Ibanez D, Gladman D, et al. The role of non-corticosteroid related factors in osteonecrosis (ON) in systemic lupus erythematosus: a nested case-control study of inception patients. Lupus 2007; 16: 157–162. [DOI] [PubMed] [Google Scholar]
- 203. Durcan L, Petri M. Why targeted therapies are necessary for systemic lupus erythematosus. Lupus 2016; 25: 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lopez R, Davidson JE, Beeby MD, et al. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology 2012; 51: 491–498. [DOI] [PubMed] [Google Scholar]
- 205. Guillotin V, Bouhet A, Barnetche T, et al. Hydroxychloroquine for the prevention of fetal growth restriction and prematurity in lupus pregnancy: a systematic review and meta-analysis. Joint Bone Spine 2018; 85: 663–668. [DOI] [PubMed] [Google Scholar]
- 206. Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017; 76: 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tektonidou MG, Andreoli L, Limper M, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019; 78: 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. EULAR recommendations for the management of Sjögren’s syndrome with topical and systemic therapies. Ann Rheum Dis 2020; 79: 3–18. [DOI] [PubMed] [Google Scholar]
- 209. Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis 2020; 79: 851–858. [DOI] [PubMed] [Google Scholar]
- 210. Kostine M, Finckh A, Bingham CO, et al. EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors. Ann Rheum Dis 2021; 80: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kloppenburg M, Kroon FPB, Blanco FJ, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis 2019; 78: 16–24. [DOI] [PubMed] [Google Scholar]
- 212. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020; 79: 685–699. [DOI] [PubMed] [Google Scholar]
- 213. Combe B, Landewe R, Daien CI, et al. 2016 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis 2017; 76: 948–959. [DOI] [PubMed] [Google Scholar]
- 214. Tunnicliffe DJ, Singh-Grewal D, Kim S, et al. Diagnosis, monitoring, and treatment of systemic lupus erythematosus: a systematic review of clinical practice guidelines. Arthritis Care Res 2015; 67: 1440–1452. [DOI] [PubMed] [Google Scholar]
- 215. Tamirou F, Arnaud L, Talarico R, et al. Systemic lupus erythematosus: state of the art on clinical practice guidelines. RMD Open 2018; 4: e000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Doyno C, Sobieraj DM, Baker WL. Toxicity of chloroquine and hydroxychloroquine following therapeutic use or overdose. Clin Toxicol 2020; 0: 1–12. [DOI] [PubMed] [Google Scholar]
- 217. Nishiyama T, Kondo Y, Tsuboi H, et al. QTc interval prolongation in patients with systemic lupus erythematosus treated with hydroxychloroquine. Mod Rheumatol 2021; 31: 1107–1112. [DOI] [PubMed] [Google Scholar]
- 218. Chatre C, Roubille F, Vernhet H, et al. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf 2018; 41: 919–931. [DOI] [PubMed] [Google Scholar]
- 219. Zhao H, Wald J, Palmer M, et al. Hydroxychloroquine-induced cardiomyopathy and heart failure in twins. J Thorac Dis 2018; 10: E70–E73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Sames E, Paterson H, Li C. Hydroxychloroquine-induced agranulocytosis in a patient with long-term rheumatoid arthritis. Eur J Rheumatol 2016; 3: 91–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. El-Solia A, Al-Otaibi K, Ai-Hwiesh AK. Hydroxychloroquine-induced hypoglycaemia in non-diabetic renal patient on peritoneal dialysis. BMJ Case Rep 2018; 2018: 2017–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Cansu DÜ, Korkmaz C. Hypoglycaemia induced by hydroxychloroquine in a non-diabetic patient treated for RA. Rheumatology 2008; 47: 378–379. [DOI] [PubMed] [Google Scholar]
- 223. Stein M, Bell MJ, Ang LC. Hydroxychloroquine neuromyotoxicity. J Rheumatol 2000; 27: 2927–2931. [PubMed] [Google Scholar]
- 224. Siddiqui AK, Huberfeld SI, Weidenheim KM, et al. Hydroxychloroquine-induced toxic myopathy causing respiratory failure. Chest 2007; 131: 588–590. [DOI] [PubMed] [Google Scholar]
- 225. Mascolo A, Berrino PM, Gareri P, et al. Neuropsychiatric clinical manifestations in elderly patients treated with hydroxychloroquine: a review article. Inflammopharmacology 2018; 26: 1141–1149. [DOI] [PubMed] [Google Scholar]
- 226. Petri M, Elkhalifa M, Li J, et al. Hydroxychloroquine blood levels predict hydroxychloroquine retinopathy. Arthritis Rheumatol 2020; 72: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Xie W, Zhang ZL. Additional analyses to confirm relationship of hydroxychloroquine blood levels to retinopathy: comment on the article by Petri et al. Arthritis Rheumatol 2020; 72: 694. [DOI] [PubMed] [Google Scholar]
- 228. Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res 2010; 62: 775–784. [DOI] [PubMed] [Google Scholar]
- 229. Makin AJ, Wendon J, Fitt S, et al. Fulminant hepatic failure secondary to hydroxychloroquine. Gut 1994; 35: 569–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Koumaki D, Koumaki V, Bertsias G, et al. Hydroxychloroquine-induced erythema multiforme. Clin Case Reports 2020; 8: 578–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Pai SB, Sudershan B, Kuruvilla M, et al. Hydroxychloroquine-induced erythroderma. Indian J Pharmacol 2017; 49: 132–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Pelechas E, Drosos AA. Hydroxychloroquine-induced dark butterfly rash in a rheumatoid arthritis patient. Rheumatology 2018; 57: 849. [DOI] [PubMed] [Google Scholar]
- 233. Ivo R, Lopes CA, Reis R. Woman in grey: hydroxychloroquine-induced hyperpigmentation. BMJ Case Rep 2018; 11: 2017–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Serre J, Buob D, Boffa JJ. Hydroxychloroquine-induced podocytopathy mimicking Fabry disease. BMJ Case Rep 2019; 12: 2018–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wu SZ, Liang X, Geng J, et al. Hydroxychloroquine-induced renal phospholipidosis resembling fabry disease in undifferentiated connective tissue disease: a case report. World J Clin Cases 2019; 7: 4377–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Eljaaly K, Alireza KH, Alshehri S, et al. Hydroxychloroquine safety: a meta-analysis of randomized controlled trials. Travel Med Infect Dis 2020; 36: 101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Bahloul E, Jallouli M, Garbaa S, et al. Hydroxychloroquine-induced hyperpigmentation in systemic diseases: prevalence, clinical features and risk factors: a cross-sectional study of 41 cases. Lupus 2017; 26: 1304–1308. [DOI] [PubMed] [Google Scholar]
- 238. Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus a case-control study. JAMA Dermatol 2013; 149: 935–940. [DOI] [PubMed] [Google Scholar]
- 239. Abou Assalie N, Durcan R, Durcan L, et al. Hydroxychloroquine-induced erythema multiforme. J Clin Rheumatol 2017; 23: 127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lenfant T, Salah S, Leroux G, et al. Risk factors for hydroxychloroquine retinopathy in systemic lupus erythematosus: a case-control study with hydroxychloroquine blood-level analysis. Revmatol 2020; 59: 3807–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Spinelli FR, Moscarelli E, Ceccarelli F, et al. Treating lupus patients with antimalarials: analysis of safety profile in a single-center cohort. Lupus 2018; 27: 1616–1623. [DOI] [PubMed] [Google Scholar]
- 242. Garg S, Unnithan R, Hansen KE, et al. The clinical significance of monitoring hydroxychloroquine levels in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Arthritis Care Res 2021; 73: 707–716. [DOI] [PubMed] [Google Scholar]
- 243. Mok CC, Penn HJ, Chan KL, et al. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res 2016; 68: 1295–1302. [DOI] [PubMed] [Google Scholar]
- 244. Lee JY, Lee J, Kwok SK, et al. Factors related to blood hydroxychloroquine concentration in patients with systemic lupus erythematosus. Arthritis Care Res 2017; 69: 536–542. [DOI] [PubMed] [Google Scholar]
- 245. Fernandez-Ruiz R, Bornkamp N, Kim MY, et al. Discontinuation of hydroxychloroquine in older patients with systemic lupus erythematosus: a multicenter retrospective study. Arthritis Res Ther 2020; 22: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X211073001 for Hydroxychloroquine in systemic lupus erythematosus: overview of current knowledge by Alina Dima, Ciprian Jurcut, François Chasset, Renaud Felten and Laurent Arnaud in Therapeutic Advances in Musculoskeletal Disease