Abstract
Background
Both tumor deposits (TD) and perineural invasion (PNI) have been identified as risk factors for poor survival in patients with non-metastatic colorectal adenocarcinoma (CRC). However, the adverse impacts of TD and PNI on the survival of patients with non-metastatic CRC have not been compared.
Method
Patients with non-metastatic CRC with known TD and PNI status were selected from the Surveillance, Epidemiology, and End Results (SEER) database. First, bivariate logistic regression analysis was utilized to identify the factors associated with TD and PNI status. Then, patients were divided into four groups, according to TD and PNI status. Propensity score matching (PSM) was performed to balance the baseline covariates. The impact of TD and PNI on survival was assessed by analyzing overall survival (OS) and cancer-specific mortality (CSM) rates. OS was calculated by the Kaplan–Meier method with log-rank analysis. CSM was estimated by competing risk analysis using the Fine and Gray model.
Results
A total of 70 689 patients with CRC met the inclusion and exclusion criteria. The positive rates of TD and PNI were 9.37% and 9.91%, respectively. For TD, the most important risk factor was N stage. With respect to PNI, the most significant factor was T stage. Tumor location, tumor size, differentiation grade, and serum CEA level were also correlated with TD and PNI status. After PSM, 1849 pairs were selected. Patients with TD+PNI+ status had the worst 5 year CSM and 5 year OS. In addition, the long-term survival outcomes of patients with TD+PNI− and TD−PNI+ status were comparable.
Conclusion
The adverse impacts of TD and PNI on the survival of patients with non-metastatic CRC were comparable. CRC patients with both TD and PNI positive had the worst survival outcome.
Keywords: colorectal adenocarcinoma, tumor deposits, perineural invasion, propensity score matching, competing risk analysis, surveillance epidemiology and end results database
Introduction
The International Duration Evaluation of Adjuvant Chemotherapy (IDEA) proposed that the choice and duration of chemotherapy regimen for patients with colon cancer should be personalized.1-3 So, it is of great importance to identify patients who are at higher risk of relapse or metastasis. The IDEA research stratified colon cancer patients into high risk group and low risk group, according to T stage and N stage only.
Tumor deposits (TD) are defined as isolated tumor foci found in the pericolic or perirectal fat or in the adjacent mesentery that are discontinuous with the primary lesion and with no evidence of residual lymph node tissue.4,5 TD have been reported to be a unique factor, different from lymph node metastasis, that predict poor prognosis in patients with colorectal adenocarcinoma (CRC).6,7
The generally accepted definition of perineural invasion (PNI) is the presence of tumor cells within any layer of the nerve sheath. Tumor cells surrounding at least 33% of the nerve circumference are also defined as PNI.8,9 Several studies have confirmed that PNI impacts the long-term survival of patients with CRC.10,11
TD and PNI are demonstrated to be risk factors for poor survival in patients with non-metastatic CRC. However, these two factors are not involved in the risk stratification model for personalized chemotherapy. Further data on the adverse impacts of TD and PNI on survival were lacking. Which factor has a greater impact on survival is not clear. There is no evidence that patients with CRC that is both TD and PNI positive have the worst outcome than those with CRC positive for either TD or PNI alone.
In this study, we analyzed clinical features associated with TD and PNI status and compared the survival of patients with non-metastatic CRC with positive TD and/or PNI status. Based on these analyses, we attempted to optimize the personalized chemotherapy regimen for CRC patients based on TNM stage system and TD, PNI status.
Patients and Methods
Patients
Patient data were retrieved from the following Surveillance, Epidemiology, and End Results (SEER) database: Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying). CRC was identified by three variables “Site recode ICD-O-3/WHO 2008,” “Behavior and Histology recode-broad grouping,” and “Behavior code ICD-O-3,” with the values of “Colon and rectum,” “8140-8389 adenomas and adenocarcinomas,” and “Malignant”, respectively.
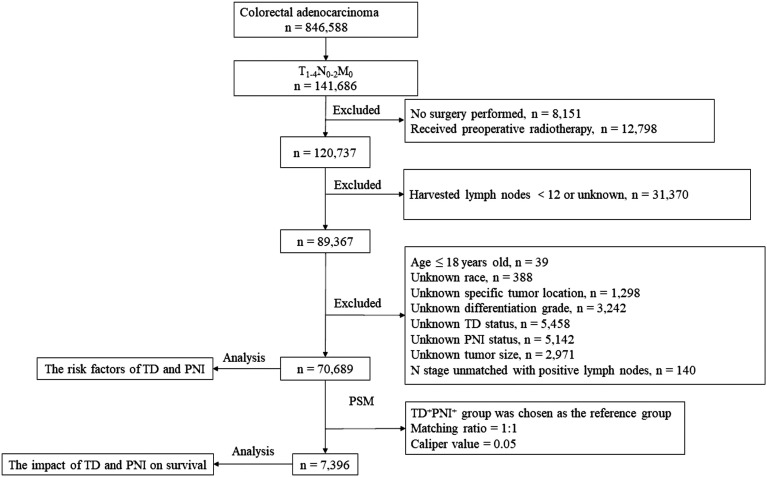
Patients with non-metastatic CRC who underwent radical surgery with no fewer than 12 harvested lymph nodes were enrolled in this study. Patients with missing values for race, specific tumor location, differentiation grade, TD status, PNI status, and tumor size were excluded from this study. In addition, patients who received radiotherapy before surgery were also excluded because tumor regression post-neoadjuvant therapy would interfere with the diagnosis of TD (Figure 1). 12
Figure 1.
The flow diagram of selection process for the study population.
Statistics Analysis
Bivariate logistic regression analysis was performed to identify factors associated with TD and PNI status. Then, all patients were divided into four groups according to TD and PNI status (TD−PNI- vs TD−PNI+ vs TD+PNI− vs TD+PNI+). The TD+PNI+ group was chosen as the reference group. The other three groups were matched with the reference group by propensity score analysis (PSM). The PSM was carried out using SPSS (https://sourceforge.net/projects/psmspss/files/psmatching3.04/). The matching ratio was 1:1, and the caliper value was set as .05.
The Wilcoxon rank-sum test was used for non-normally distributed data. The χ2 test was performed to compare the enumeration data. The overall survival rate was calculated by the Kaplan–Meier method with the log-rank test. The cause of mortality was classified into the following two subsets: death from CRC and death attributed to other diseases. The cumulative incidence of cause-specific mortality was calculated by competing risk analysis using “cpmrsk” package in R. All statistical analyses were performed using the SPSS 22.0 (SPSS Inc, Chicago, IL, USA) and R software (version 4.0.3; http://www.r-project.org/). Two-sided P < .05 was considered statistically significant.
Results
Patient Characteristics
As shown in Table 1, a total of 70 689 patients with CRC were enrolled in this study. Most patients were white (79.9%), with a median age of 68 (58–78) years. The majority of lesions arose from the right hemicolon (46.0%), followed by the left hemicolon (29.5%), rectum (15.6%), and transverse colon (8.9%). The most common histological differentiation grade was moderately differentiated (Grade II, 75.5%). Lymph node metastasis was observed in 37.3% patients. TD was identified in approximately 9.4% of patients, and the positivity rate of PNI was approximately 9.9%. Approximately 30.8% patients received chemotherapy.
Table 1.
Characteristics of patients enrolled in risk factor analysis for TD and PNI.
| Characteristic | Total (N = 70,689) | Training Set (N = 49,482) | Validation Set (N = 21,207) | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |||
| Age | 68 (58,78) | 69 (58,78) | 68 (58,78) | |||||
| Sex | ||||||||
| Male | 35,271 | 49.9 | 24,754 | 50.0 | 10,517 | 49.6 | ||
| Female | 35,418 | 50.1 | 24,728 | 50.0 | 10,690 | 50.4 | ||
| Race | ||||||||
| White | 56,460 | 79.9 | 39,513 | 79.9 | 16,947 | 79.9 | ||
| Black | 7,927 | 11.2 | 5,589 | 11.3 | 2,338 | 11.0 | ||
| Others | 6,302 | 8.9 | 4,380 | 8.8 | 1,922 | 9.1 | ||
| Serum CEA level | ||||||||
| Normal | 27,737 | 39.3 | 19,304 | 39.0 | 8,433 | 39.8 | ||
| Elevated | 14,766 | 20.9 | 10,356 | 20.9 | 4,410 | 20.8 | ||
| Unknown a | 28,186 | 39.8 | 19,822 | 40.1 | 8,364 | 39.4 | ||
| Tumor location | ||||||||
| Right hemicolon b | 32,503 | 46.0 | 22,728 | 45.9 | 9,775 | 46.1 | ||
| Transverse Colon | 6,306 | 8.9 | 4,488 | 9.1 | 1,818 | 8.6 | ||
| Left hemicolon c | 20,821 | 29.5 | 14,512 | 29.3 | 6,309 | 29.7 | ||
| Rectum d | 11,059 | 15.6 | 7,754 | 15.7 | 3,305 | 15.6 | ||
| Differentiation | ||||||||
| Grade I | 5,277 | 7.5 | 3,708 | 7.5 | 1,569 | 7.4 | ||
| Grade II | 53,106 | 75.1 | 37,123 | 75.0 | 15,983 | 75.4 | ||
| Grade III | 10,225 | 14.5 | 7,190 | 14.5 | 3,035 | 14.3 | ||
| Grade IV | 2,081 | 2.9 | 1,461 | 3.0 | 620 | 2.9 | ||
| T stage | ||||||||
| T1 | 8,403 | 11.9 | 5,888 | 11.9 | 2,515 | 11.8 | ||
| T2 | 13,247 | 18.7 | 9,260 | 18.7 | 3,987 | 18.8 | ||
| T3 | 40,000 | 56.6 | 27,981 | 56.6 | 12,019 | 56.7 | ||
| T4 | 9,039 | 12.8 | 6,353 | 12.8 | 2,686 | 12.7 | ||
| N stage | ||||||||
| N0 | 44,313 | 62.7 | 31,072 | 62.8 | 13,241 | 62.4 | ||
| N1 | 17,445 | 24.7 | 12,205 | 24.7 | 5,240 | 24.7 | ||
| N2 | 8,931 | 12.6 | 6,205 | 12.5 | 2,726 | 12.9 | ||
| Tumor size | ||||||||
| < 5.0 cm | 43,515 | 61.6 | 30,340 | 61.3 | 13,175 | 62.1 | ||
| ≥ 5.0 cm | 27,174 | 38.4 | 19,142 | 38.7 | 8,032 | 37.9 | ||
| Harvested lymph nodes | 19 (15,25) | 19 (15,25) | 19 (15,25) | |||||
| Tumor deposits | ||||||||
| Negative | 64,062 | 90.6 | 44,889 | 90.7 | 19,173 | 90.4 | ||
| Positive | 6,627 | 9.4 | 4,593 | 9.3 | 2,034 | 9.6 | ||
| Perineural invasion | ||||||||
| Negative | 63,658 | 90.1 | 44,549 | 90.0 | 19,109 | 90.1 | ||
| Positive | 7,031 | 9.9 | 4,933 | 10.0 | 2,098 | 9.9 | ||
| Radiotherapy | ||||||||
| No | 68,079 | 96.3 | 47,672 | 96.3 | 20,407 | 96.2 | ||
| Yes | 2,610 | 3.7 | 1,810 | 3.7 | 800 | 3.8 | ||
| Chemotherapy | ||||||||
| No | 48,921 | 69.2 | 34,276 | 69.3 | 14,645 | 69.1 | ||
| Yes | 21,768 | 30.8 | 15,206 | 30.7 | 6,562 | 30.9 | ||
TD, tumor deposit; PNI, perineural invasion; CEA, carcinoma embryonic antigen.
aIncluding borderline and untested.
bIncluding cecum, ascending colon, and hepatic flexure.
cIncluding splenic flexure, descending colon, and sigmoid colon.
dIncluding rectosigmoid junction and rectum.
Risk factors for TD- and PNI-positive status
We randomized the 70 689 patients into a training cohort and a validation cohort at a ratio of 7:3. The baseline characteristics of the patients in the two cohorts are shown in Table 1. Logistical regression analysis was performed on the training cohort to identify risk factors associated with TD and PNI. Predictive models for TD/PNI status were constructed based on the logistical regression analysis. The performance of the predictive models was assessed in the validation cohort by area under the curve (AUC) and calibration curve. For TD, the most important risk factor was N stage (N1: OR = 11.650, P < .001; N2: OR = 16.764, P < .001). Differentiation grade, T stage, and serum CEA level were also correlated with positive TD status. Tumor location also correlated with TD status. Tumors in the transverse colon (OR = 1.199, P = .001), left hemicolon (OR = 1.356, P < .001), and rectum (OR = 1.718, P< .001) were at higher risk for positive TD status than those in the right hemicolon (Table 2). External validation was performed in the validation cohort, and the area under the curve (AUC) was .844 (Supplementary Figure S1). The most significant factor associated with PNI status was T stage (T2: OR = 1.943, P < .001; T3: OR = 6.020, P < .001; T4: OR = 12.921, P < .001). Race, tumor location, differentiation grade, N stage, and serum CEA level were also significantly correlated with PNI status. Interestingly, tumor size was an independent risk factor for PNI. Compared with patients with <5.0 cm tumors, those with tumors ≥5.0 cm were at lower risk for positive PNI status (OR = .757, P < .001) (Table 2). The AUC of PNI in the validation cohort was .798 (Supplementary Figure S2).
Table 2.
Risk factors associated with TD and PNI status according to the logistical regression model.
| Characteristics | TD | PNI | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Age | 1.000 | 0.998–1.002 | 0.783 | 0.998 | 0.993–1.003 | 0.578 | |
| Sex | 0.411 | 0.229 | |||||
| Male | Reference | Reference | |||||
| Female | 0.977 | 0.924–1.033 | 0.411 | 0.968 | 0.919–1.020 | 0.229 | |
| Race | 0.386 | < 0.001 | |||||
| White | Reference | Reference | |||||
| Black | 1.023 | 0.938–1.115 | 0.606 | 1.201 | 1.109–1.300 | < 0.001 | |
| Other | 0.944 | 0.860–1.037 | 0.229 | 0.924 | 0.844–1.012 | 0.090 | |
| Tumor location | < 0.001 | < 0.001 | |||||
| Right hemicolona | Reference | Reference | |||||
| Transverse colon | 1.199 | 1.078–1.332 | 0.001 | 1.010 | 0.914–1.117 | 0.841 | |
| Left hemicolonb | 1.356 | 1.268–1.451 | < 0.001 | 1.221 | 1.146–1.301 | < 0.001 | |
| Rectumc | 1.718 | 1.586–1.860 | < 0.001 | 1.590 | 1.475–1.714 | < 0.001 | |
| Tumor size | 0.947 | < 0.001 | |||||
| < 5.0 cm | Reference | Reference | |||||
| ≥ 5.0 cm | 0.998 | 0.943–1.057 | 0.947 | 0.757 | 0.717–0.800 | < 0.001 | |
| Differentiation | < 0.001 | < 0.001 | |||||
| Grade I | Reference | Reference | |||||
| Grade II | 1.096 | 0.955–1.258 | 0.192 | 1.256 | 1.100–1.433 | 0.001 | |
| Grade III | 1.271 | 1.096–1.473 | 0.001 | 1.944 | 1.688–2.238 | < 0.001 | |
| Grade IV | 1.478 | 1.227–1.779 | < 0.001 | 1.890 | 1.583–2.258 | < 0.001 | |
| T stage | < 0.001 | < 0.001 | |||||
| T1 | Reference | Reference | |||||
| T2 | 1.723 | 1.358–2.186 | < 0.001 | 1.943 | 1.578–2.392 | < 0.001 | |
| T3 | 4.351 | 3.508–5.396 | < 0.001 | 6.020 | 4.991–7.260 | < 0.001 | |
| T4 | 7.740 | 6.204–9.658 | < 0.001 | 12.921 | 10.646–15.682 | < 0.001 | |
| N stage | < 0.001 | < 0.001 | |||||
| N0 | Reference | Reference | |||||
| N1 | 11.650 | 10.697–12.688 | < 0.001 | 2.274 | 2.135–2.421 | < 0.001 | |
| N2 | 16.764 | 15.301–18.367 | < 0.001 | 4.047 | 3.781–4.332 | < 0.001 | |
| Serum CEA level | < 0.001 | < 0.001 | |||||
| Normal | Reference | Reference | |||||
| Elevated | 1.194 | 1.112–1.282 | < 0.001 | 1.184 | 1.107–1.267 | < 0.001 | |
| Unknownd | 1.117 | 1.046–1.193 | 0.001 | 1.072 | 1.009–1.140 | 0.025 | |
TD, tumor deposit; PNI, perineural invasion; OR, odds ratio; CI, confidence interval; CEA, carcinoma embryonic antigen.
The impact of TD and PNI on oncological outcome
The above analysis demonstrated that patients with either TD- or PNI-positive status had higher TNM stage and worse histological differentiation. To eliminate the impact of these variables on OS and cancer-specific mortality, we performed PSM to balance the baseline characteristics. After PSM, 1849 pairs of balanced patients were selected. The baseline characteristics of the selected patients are shown in Table 3.
Table 3.
Baseline characteristics of patients with different TD/PNI status before and after PSM.
| Characteristics | Before Matching | After Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TD-PNI- | TD-PNI+ | TD+PNI- | TD+PNI+ | p-value | TD-PNI- | TD-PNI+ | TD+PNI- | TD+PNI+ | p-value | ||
| Age | 69 (59,79) | 67 (56,78) | 68 (57,78) | 64 (54,76) | < 0.001 | 65 (54,75) | 65 (54,75) | 65 (55,76) | 65 (54,76) | 0.442 | |
| Sex | |||||||||||
| Male | 29,329 | 2,588 | 2,394 | 960 | < 0.001 | 907 | 928 | 896 | 921 | 0.722 | |
| Female | 29,626 | 2,519 | 2,309 | 964 | 942 | 921 | 953 | 928 | |||
| Race | |||||||||||
| White | 47,300 | 3,981 | 3,690 | 1,489 | < 0.001 | 1,452 | 1,412 | 1,439 | 1,427 | 0.828 | |
| Black | 6,475 | 661 | 539 | 252 | 232 | 250 | 238 | 245 | |||
| Others | 5,180 | 465 | 474 | 183 | 165 | 187 | 172 | 177 | |||
| Serum CEA level | |||||||||||
| Normal | 23,746 | 1,812 | 1,604 | 575 | < 0.001 | 595 | 579 | 548 | 571 | 0.483 | |
| Elevated | 11,474 | 1,353 | 1,297 | 642 | 603 | 583 | 587 | 601 | |||
| Unknown a | 23,735 | 1,942 | 1,802 | 707 | 651 | 687 | 714 | 677 | |||
| Tumor location | |||||||||||
| Right hemicolon b | 27,766 | 2,193 | 1,874 | 670 | < 0.001 | 652 | 652 | 651 | 666 | 0.999 | |
| Transverse Colon | 5,358 | 413 | 403 | 132 | 127 | 132 | 130 | 130 | |||
| Left hemicolon c | 16,961 | 1,620 | 1,572 | 668 | 651 | 652 | 661 | 637 | |||
| Rectum d | 8,870 | 881 | 854 | 454 | 419 | 413 | 407 | 416 | |||
| Differentiation | |||||||||||
| Grade I | 4,803 | 209 | 209 | 56 | < 0.001 | 51 | 58 | 63 | 56 | 0.909 | |
| Grade II | 45,139 | 3,451 | 3,303 | 1,213 | 1,189 | 1,189 | 1,172 | 1,191 | |||
| Grade III | 7,510 | 1,215 | 971 | 529 | 503 | 495 | 488 | 491 | |||
| Grade IV | 1,503 | 232 | 220 | 126 | 106 | 107 | 126 | 111 | |||
| T stage | |||||||||||
| T1 | 8,203 | 109 | 81 | 10 | < 0.001 | 17 | 12 | 6 | 10 | 0.609 | |
| T2 | 12,562 | 351 | 290 | 44 | 45 | 47 | 44 | 44 | |||
| T3 | 32,479 | 3,288 | 3,158 | 1,075 | 1,098 | 1,074 | 1,073 | 1,074 | |||
| T4 | 5,711 | 1,359 | 1,174 | 795 | 689 | 716 | 726 | 721 | |||
| N stage | |||||||||||
| N0 | 41,564 | 2,056 | 582 | 111 | < 0.001 | 106 | 111 | 112 | 111 | 0.993 | |
| N1 | 12,409 | 1,675 | 2,555 | 806 | 826 | 824 | 817 | 805 | |||
| N2 | 4,982 | 1,376 | 1,566 | 1,007 | 917 | 914 | 920 | 933 | |||
| Tumor size | |||||||||||
| < 5.0 cm | 37,047 | 2,960 | 2,474 | 1,034 | < 0.001 | 995 | 1,042 | 987 | 998 | 0.260 | |
| ≥ 5.0 cm | 21,908 | 2,147 | 2,229 | 890 | 854 | 807 | 862 | 851 | |||
| Harvested lymph nodes | < 0.001 | ||||||||||
| 19 (15,25) | 19 (15,25) | 19 (15,25) | 19 (15,25) | 19 (15,26) | 20 (16,26) | 19 (15,25) | 19 (15,25) | 0.001 | |||
| Radiotherapy | |||||||||||
| No | 57,165 | 4,790 | 4,372 | 1,752 | < 0.001 | 1,703 | 1,681 | 1,694 | 1,688 | 0.612 | |
| Yes | 1,790 | 317 | 331 | 172 | 146 | 168 | 155 | 161 | |||
| Chemotherapy | |||||||||||
| No | 43,714 | 2,639 | 1,901 | 667 | < 0.001 | 685 | 655 | 662 | 649 | 0.626 | |
| Yes | 15,241 | 2,468 | 2,802 | 1,257 | 1,164 | 1,194 | 1,187 | 1,200 | |||
PSM, propensity score matching; TD, tumor deposit; PNI, perineural invasion; CEA, carcinoma embryonic antigen.
aIncluding borderline and untested
bIncluding cecum, ascending colon, and hepatic flexure
cIncluding splenic flexure, descending colon, and sigmoid colon
dIncluding rectosigmoid junction and rectum
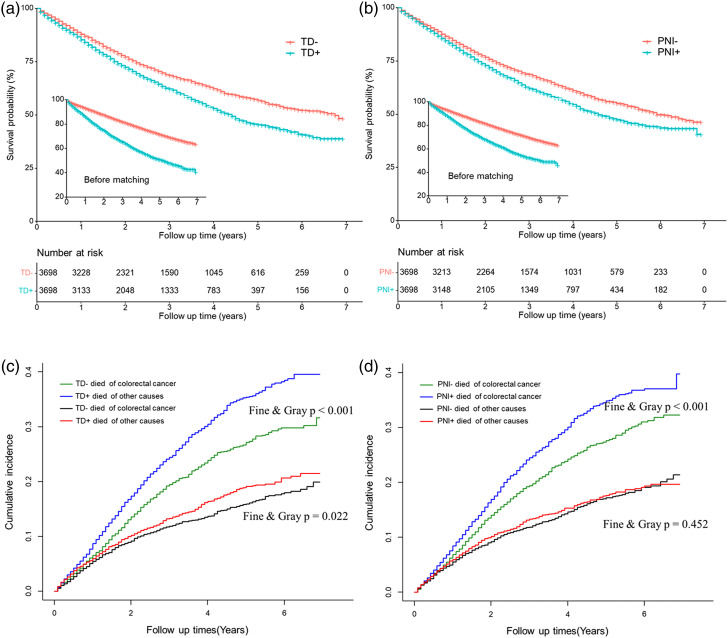
The median OS times were 81 months and 53 months for patients with TD-negative and TD-positive status, respectively. Patients with TD-positive status had a significantly worse OS rate (P < .001) and higher cancer-specific mortality rate (P < .001) than those with TD-negative status. The 1-, 3-, and 5-year OS rates were 85.3%, 62.2%, and 45.4% in the TD-positive group, and 88.0%, 68.6%, and 56.9% in the TD-negative group, respectively (Figure 2(A)). The corresponding cancer-specific mortality rates for the TD-positive group at 1-, 3-, and 5- years were 8.7%, 24.5%, and 35.4%, respectively. In contrast, the cancer-specific mortality rates for the TD-negative group were 6.6%, 19.5%, and 27.2% at 1, 3, and 5 years, respectively. The TD-positive group had a higher rate of death attributed to other causes, such as heart diseases and diabetes (P = .022) (Figure 2(C)).
Figure 2.
Overall survival (A, B) and cause-specific mortality (C, D) of patients with different TD or PNI status after propensity score matching.
The median OS times were 71 months and 55 months for patients with PNI-negative and PNI-positive status, respectively. The PNI-positive group had a significantly worse OS rate (P < .001) and higher cancer-specific mortality rate (P < .001) than the PNI-negative group. The 1-, 3-, and 5-year OS rates were 85.6%, 62.2%, and 47.5% for the PNI-positive group and 87.6%, 68.7%, and 55.1% for the PNI-negative group, respectively (Figure 2(B)). The corresponding cancer-specific mortality rates for the PNI-positive group at 1, 3, and 5 years were 8.4%, 24.5%, and 34.8%, respectively. In contrast, the cancer-specific mortality rates for the PNI-negative group were 6.8%, 19.4%, and 27.6% at 1, 3, and 5 years, respectively. There was no significant difference in the number of patients who died due to other causes between these two groups (P = .452) (Figure 2(D)).
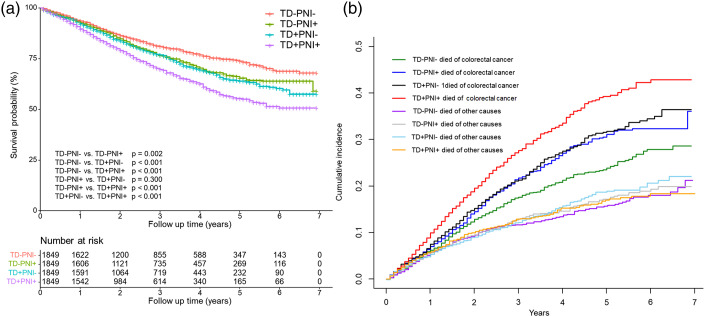
We also compared the adverse influence of TD and PNI on survival. As shown in Figure 3, patients with CRC simultaneously positive for TD and PNI had a worse 5 year OS rate than the other three groups (73.8% vs 65.5% vs 64.0% vs 55.3%, P < .001). Patients who were positive for TD or PNI had similar 5-year OS rates (P = .300) (Figure 3(A)). A similar pattern was observed with respect to cancer-specific mortality (Figure 3(B)). We further quantitatively analyzed the impact of TD and PNI on survival through Cox regression analysis. As shown in Table 4, the HR values of TD and PNI for OS were 1.316 and 1.262, respectively (P < .05). For cancer-specific survival, the HR values of TD and PNI were 1.403 and 1.349, respectively (P < .05).
Figure 3.
Overall survival (A) and cause-specific mortality (B) for patients with different TD and PNI status after propensity score matching.
Table 4.
Multivariate Cox regression analysis of overall survival and cancer-specific survival after PSM.
| Characteristics | Multivariate Cox of OS | Multivariate Cox of CSS | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Tumor location | < 0.001 | < 0.001 | |||||
| Right hemicolon | Reference | Reference | |||||
| Transverse colon | 0.941 | 0.813–1.089 | 0.415 | 0.809 | 0.665–0.985 | 0.035 | |
| Left hemicolon | 0.759 | 0.691–0.834 | < 0.001 | 0.746 | 0.664–0.838 | < 0.001 | |
| Rectum | 0.834 | 0.734–0.937 | 0.002 | 0.773 | 0.667–0.897 | 0.001 | |
| Differentiation | < 0.001 | < 0.001 | |||||
| Grade I | Reference | Reference | |||||
| Grade II | 1.078 | 0.850–1.366 | 0.537 | 1.147 | 0.838–1.570 | 0.391 | |
| Grade III | 1.465 | 1.151–1.865 | 0.002 | 1.682 | 1.224–2.312 | 0.001 | |
| Grade IV | 1.539 | 1.176–2.013 | 0.002 | 1.725 | 1.216–2.449 | 0.002 | |
| Serum CEA level | < 0.001 | < 0.001 | |||||
| Normal | Reference | Reference | |||||
| Elevated | 1.368 | 1.239–1.510 | < 0.001 | 1.348 | 1.192–1.524 | < 0.001 | |
| T stage | < 0.001 | < 0.001 | |||||
| T1 | Reference | Reference | |||||
| T2 | 1.493 | 0.627–3.552 | 0.365 | 0.914 | 0.303–2.756 | 0.873 | |
| T3 | 2.210 | 0.989–4.937 | 0.051 | 1.877 | 0.701–5.026 | 0.210 | |
| T4 | 3.618 | 1.618–8.090 | 0.002 | 3.319 | 1.238–8.897 | 0.017 | |
| N stage | < 0.001 | < 0.001 | |||||
| N0 | Reference | Reference | |||||
| N1 | 1.261 | 1.057–1.505 | 0.010 | 1.222 | 0.966–1.545 | 0.095 | |
| N2 | 2.118 | 1.779–2.521 | < 0.001 | 2.33 | 1.852–2.931 | < 0.001 | |
| Tumor size | 0.007 | 0.001 | |||||
| < 5.0 cm | Reference | Reference | |||||
| ≥ 5.0 cm | 1.112 | 1.029–1.201 | 0.007 | 1.175 | 1.066–1.295 | 0.001 | |
| Tumor deposit | < 0.001 | < 0.001 | |||||
| Negative | Reference | Reference | |||||
| Positive | 1.316 | 1.239–1.441 | < 0.001 | 1.403 | 1.276–1.543 | < 0.001 | |
| Perineural invasion | < 0.001 | < 0.001 | |||||
| Negative | Reference | Reference | |||||
| Positive | 1.262 | 1.171–1.361 | < 0.001 | 1.349 | 1.218–1.472 | < 0.001 | |
HR, hazard ratio; CI confidence interval; OS, overall survival; CSS, cancer-specific survival.
Subgroup Analysis
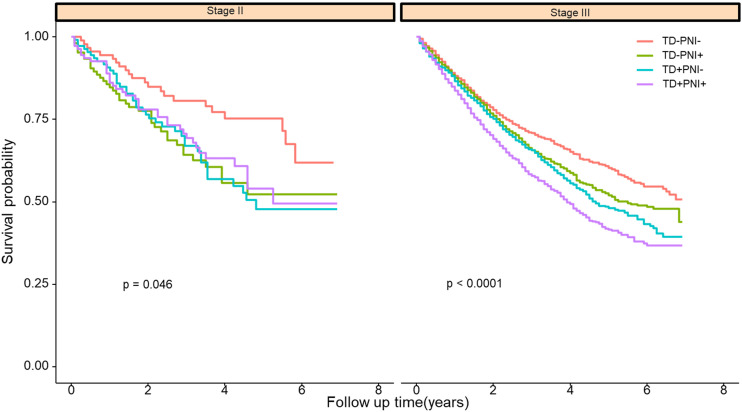
TD and PNI were significantly associated with N stage and T stage. However, it is not clear whether the impact of TD and PNI on patient survival changes with different TNM stages. Hence, we stratified matched patients into subgroups with respect to T stage and N stage. As shown in Figure 4 and Supplementary Figure S3, patients who were simultaneously positive for TD and PNI had the worst 5 year cancer-specific survival, and the survival curves of patients with stage III CRC in the TD−PNI+ group overlapped that of those in the TD+PNI− group.
Figure 4.
Cancer-specific survival of patients with different TNM stage.
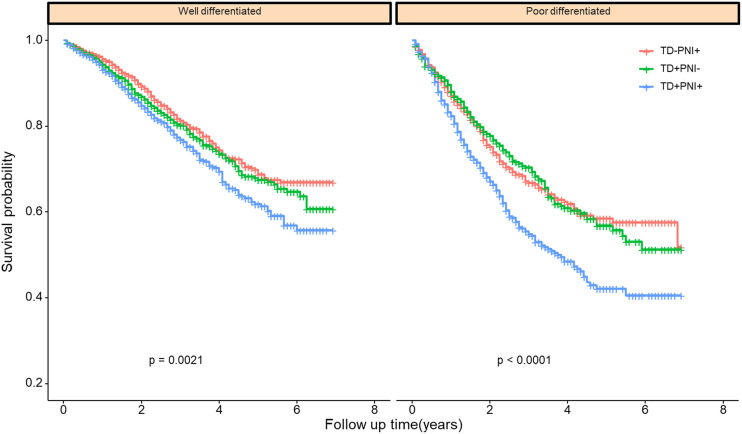
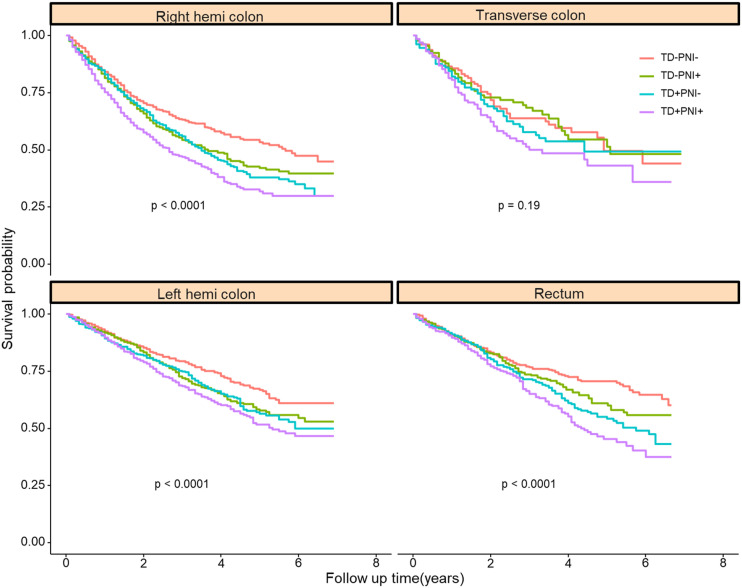
Because TD and PNI status were also correlated with histological differentiation, patients who had a poor differentiation grade were more likely to be TD-and PNI-positive. We also investigated whether the impact of TD and PNI on patient survival would change with different histological differentiation. Grade I (well differentiated) and Grade II (moderately differentiated) were grouped as “well differentiated”. Grade III (poorly differentiated) and Grade IV (undifferentiated) were classified into the “poorly differentiated” group. Patients in the TD+PNI+ group had the worst prognosis. Patients in the TD-positive or PNI-positive groups had comparable outcomes (Figure 5). The same pattern was observed with respect to different tumor locations (Figure 6).
Figure 5.
Cancer-specific survival of patients with different differentiation grade.
Figure 6.
Cancer-specific survival of patients with different tumor location.
From the above subgroup analysis, we found that the adverse impact of TD and PNI did not change with TNM stage, histological differentiation, or tumor location. Hence, TD and PNI status were independent prognostic factors associated with worse survival.
Discussion
In this study, we compared the impact of TD and PNI on the survival of patients with non-metastatic CRC in 1849 pairs of matched patients by using PSM to balance the baseline covariates. We found that the long-term survival outcomes of patients in the TD+PNI− and TD−PNI+ groups were comparable, and that those in the TD+PNI+ group had the worst 5-year OS and 5-year cancer-specific mortality rates. To the best of our knowledge, this is the first study comparing the survival impact of TD and PNI with such a large population.
The former largest population study investigating the prognostic value of TD and PNI enrolled approximately 60 495 cases. 12 However, approximately 30% of cases in that study lacked information on TD status or PNI status, and the baseline covariates were not balanced. Thus, that study did not compare the prognostic impact of TD and PNI. In our study, we enrolled 70 689 CRC patients with complete data, and the baseline covariates were well balanced through PSM with a standardized difference of less than 5%.
In addition, we utilized competing risk analysis to estimate the cancer-specific mortality associated with TD and PNI. Competing risk analysis has been used in the analysis of survival data in recent years. The primary event of interest is often precluded by competing events. For example, if the primary event of a study is death attributed to CRC, death due to non-CRC diseases, such as cardiovascular diseases, is a competing event. The occurrence of competing events leads to the overestimation of CRC-specific survival. Competing risk analysis can reduce the overestimation of cancer-specific mortality.13,14 Our use of the largest population to date in combination with the aforementioned statistical methods increases the reliability of our research.
Several studies have investigated risk factors associated with TD and PNI. These studies identified age, T stage, N stage, and differentiation grade as risk factors.15-17 Our result is consistent with those studies, except for age. This difference may result from population size and different demarcation of age.
Interestingly, our study found that TD and PNI status differed by tumor location. The positive rate of TD and PNI increased from the right hemicolon to the rectum (for TD, right hemicolon: reference, transverse colon: OR = 1.199, left hemicolon: OR = 1.356, rectum: OR = 1.718). This phenomenon has only been reported in one other study. Kim CW et al reported that the extra nodal extension rates differed significantly among patients with right colon (36.9%), left colon (42.6%), and rectal (48.7%) cancers. 18 The mesentery becomes thinner from the right hemicolon to the left hemicolon and ends at the rectum. Thus, rectal cancer is more likely to be TD- and PNI-positive. Another interesting finding of our study is the relationship between tumor size and PNI status. We found that patients with tumor sizes less than 5.0 cm were more likely to be PNI-positive. This may be caused by the aggressive feature of small size tumor. Several studies suggested that small size tumor had worse survival compared with large size tumor, if the TNM stage of CRC patients were similar.19-23
Our study also quantitatively analyzed risk factors related to TD and PNI status through bivariate logistic regression analysis. For TD, the most important risk factor was N stage. With respect to PNI, the most significant factor was T stage. The relationship between TD and N stage has been reported. 12 However, the most significant risk factor for PNI has never been reported.
TD and PNI are associated with poor disease-free survival and OS. As two different types of locoregional spread pathway, TD and PNI have their own characteristics. It was reported that TD in combination with lymph node metastasis was a strong predictor for liver (odds ratio [OR] = 5.5), lung (OR = 4.3), and peritoneal metastases (OR = 5.5). 15 As for PNI, a meta-analysis involving 22 900 patients demonstrated that PNI was significantly correlated with increased local recurrence (risk ration [RR] = 3.2, 95% CI: 2.33–4.44). 24 Nozawa H et al retrospectively reviewed 496 patients with pathological T3 or T4 colon cancer who did not receive preoperative treatment, and found that obstruction was more frequent in PNI-positive group than PNI-negative group (39 % vs 24%, P < .05). 25 He also reported that colitis-associated CRC was more likely to be PNI-positive, compared with sporadic CRC without obstruction (90% vs 45%, P = .007). 26 Some research investigated the onset of TD and PNI from the view of genetic mutation. A high BRAF mutation rate was observed in TD-positive patients. 27 Compared with PNI-positive patients, the expression of FLT1, FBXW7, FGFR1, SLC20A2, and SERPINI1 was significantly up-regulated in PNI-negative group. 28 However, detailed molecular mechanism of TD and PNI still remains unclear.
In the 8th edition of the AJCC TNM staging system for CRC, TD is considered only if lymph node metastasis is absent and is classified as N1c. Nagtegaal ID et al found that allocating TD into the nodal category N1c and only considering TD in the absence of lymph node metastasis resulted in the loss of valuable prognostic information. 15 Delattre JF et al proposed that TD should be added to the TNM staging system to better define the duration of adjuvant chemotherapy for patients with stage III CRC. 29 For CRC patients with T3–4 stage, positive TD status, and none lymph node metastasis, combined chemotherapy regimen is recommended. Our study demonstrated that the adverse impacts of TD and PNI on the survival were comparable. Hence, we proposed that CRC patients of T3-4N0M0PNI+ should be also treated as stage III. Combined chemotherapy regimen is recommended. We also found that patients in the TD+PNI+ group had the worst outcome. Based on the IDEA research, we proposed that 6 months of adjuvant chemotherapy regimen would be rational for CRC patients with both TD and PNI positive.
This study has several limitations that should be noted. First, the detailed information about surgery was not recorded in the SEER database. The extent of lymph node resection was not clear. Patients with CRC who received D3/D2 lymphadenectomy have superior OS.30-32 To avoid this limitation, we only enrolled patients with at least twelve harvested lymph nodes. Second, detailed information about chemotherapy was not recorded in the SEER database. We do not know whether the patients’ adjuvant chemotherapy was complete and standard. Third, our study was retrospectively designed, and some bias existed. To avoid this limitation, we utilized the PSM method. However, the limitation associated with PSM is inevitable. It is possible that residual confounders between the groups could have been omitted in the analysis.33,34 In addition, RAS gene status and MSI/MMR status, which influences the survival of patients with CRC,35-37 were not recorded in the SEER database, so these baseline factors were not analyzed in this study.
Conclusion
The adverse impacts of TD and PNI on the survival of patients with non-metastatic CRC were comparable. CRC patients with both TD and PNI positive had the worst survival outcome.
Supplemental Material
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211051533 for Tumor Deposits and Perineural Invasion had Comparable Impacts on the Survival of Patients With Non-metastatic Colorectal Adenocarcinoma: A Population-Based Propensity Score Matching and Competing Risk Analysis by Bin Luo, Xianzhe Chen, Guanfu Cai, Weixian Hu, Yong Li1, and Junjiang Wang in Cancer Control
Acknowledgment
We acknowledged Miss Zhan for contribution to figure editing.
Author Contributions: BL and XZC collected the data. BL analyzed the data, reviewed the literature, and contributed to the manuscript drafting. JJW and WXH revised the manuscript. GFC is responsible for quality control. YL is responsible for research design and revision of the manuscript. All authors issued final approval for the version to be submitted.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This research was supported by Science and Technology Program of Guangzhou (NO.: 201904010020), and Funding for Outstanding Young Medical Talents of Guangdong Province (KJ012019439)
Ethics Approval: The approval for use of all the data was obtained through a request submitted to the SEER database. There was no need to get approval from the institutional review board.
Data Availability Statement: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Bin Luo https://orcid.org/0000-0003-1061-1019
Junjiang Wang https://orcid.org/0000-0003-0097-9346
References
- 1.André T, Vernerey D, Mineur L, et al. Three versus 6 months of oxaliplatin-based adjuvant chemotherapy for patients with stage III colon cancer: disease-free survival results from a randomized, open-label, international duration evaluation of adjuvant (IDEA) france, phase III trial. J Clin Oncol. 2018;36(15):1469-1477. [DOI] [PubMed] [Google Scholar]
- 2.André T, Meyerhardt J, Iveson T, et al. Effect of duration of adjuvant chemotherapy for patients with stage III colon cancer (IDEA collaboration): final results from a prospective, pooled analysis of six randomised, phase 3 trials. Lancet Oncol. 2020;21(12):1620-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill S, Meyerhardt JA, Arun M, Veenstra CM. Translating IDEA to practice and beyond: managing stage II and III colon cancer. American Society of Clinical Oncology Educational Book. 2019;39:226-235. [DOI] [PubMed] [Google Scholar]
- 4.Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454-1455. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, Zhao J, Li C, et al. The unique prognostic characteristics of tumor deposits in colorectal cancer patients. Ann Transl Med. 2019;7:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basnet S, Lou Q-f., Liu N, et al. Tumor deposit is an independent prognostic indicator in patients who underwent radical resection for colorectal cancer. J Cancer. 2018;9:3979-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer. Cancer. 2009;115:3379-3391. [DOI] [PubMed] [Google Scholar]
- 9.Fagan JJ, Collins B, Barnes L, D'Amico F, Myers EN, Johnson JT. Perineural invasion in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1998;124:637-640. [DOI] [PubMed] [Google Scholar]
- 10.Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural Invasion Is a Strong Prognostic Factor in Colorectal Cancer. Am J Surg Pathol. 2016;40:103-112. [DOI] [PubMed] [Google Scholar]
- 11.Alotaibi AM, Lee JL, Kim J, et al. Prognostic and oncologic significance of perineural invasion in sporadic colorectal cancer. Ann Surg Oncol. 2017;24:1626-1634. [DOI] [PubMed] [Google Scholar]
- 12.Mayo E, Llanos AAM, Yi X, Duan S-Z, Zhang L. Prognostic value of tumour deposit and perineural invasion status in colorectal cancer patients: a SEER-based population study. Histopathology. 2016;69:230-238. [DOI] [PubMed] [Google Scholar]
- 13.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381-387. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagtegaal ID, Knijn N, Hugen N, et al. Tumor deposits in colorectal cancer: improving the value of modern staging-a systematic review and meta-analysis. J Clin Oncol. 2017;35:1119-1127. [DOI] [PubMed] [Google Scholar]
- 16.Poeschl EM, Pollheimer MJ, Kornprat P, et al. Perineural invasion: correlation with aggressive phenotype and independent prognostic variable in both colon and rectum cancer. J Clin Oncol. 2010;28:e358-e360. reply e361-2. [DOI] [PubMed] [Google Scholar]
- 17.Leijssen LGJ, Dinaux AM, Taylor MS, et al. Perineural invasion is a prognostic but not a predictive factor in nonmetastatic colon cancer. Dis Colon Rectum. 2019;62:1212-1221. [DOI] [PubMed] [Google Scholar]
- 18.Kim CW, Kim J, Park Y, et al. Prognostic implications of extranodal extension in relation to colorectal cancer location. Cancer Research and Treatment. 2019;51:1135-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muralidhar V, Nipp RD, Ryan DP, Hong TS, Nguyen PL, Wo JY. Association between very small tumor size and increased cancer-specific mortality in node-positive colon cancer. Dis Colon Rectum. 2016;59(3):187-193. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhuo C, Shi D, et al. Unfavorable effect of small tumor size on cause-specific survival in stage IIA colon cancer, a SEER-based study. Int J Colorectal Dis. 2015;30(1):131-137. [DOI] [PubMed] [Google Scholar]
- 21.Huang B, Feng Y, Zhu L, Xu T, Huang L, Cai G. Smaller tumor size is associated with poor survival in stage II colon cancer: an analysis of 7,719 patients in the SEER database. Int J Surg. 2016;33:157-163. A: [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Kim CH, Kim YJ, Kim HR. Macroscopic serosal invasion and small tumor size as independent prognostic factors in stage IIA colon cancer. Int J Colorectal Dis. 2018;33(8):1139-1142. [DOI] [PubMed] [Google Scholar]
- 23.Pan H, Cui J, Cai K, Zhou Y. Increased cancer-specific mortality of very small size in carcinoembryonic antigen-elevated rectal cancer. Ann Transl Med. 2019;7(18):447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID. Perineural invasion is a strong prognostic factor in colorectal cancer. Am J Surg Pathol. 2016;40(1):103-112. [DOI] [PubMed] [Google Scholar]
- 25.Nozawa H, Morikawa T, Kawai K, et al. Obstruction is associated with perineural invasion in T3/T4 colon cancer. Colorectal Dis. 2019;21(8):917-924. [DOI] [PubMed] [Google Scholar]
- 26.Nozawa H, Hata K, Ushiku T,et al. Accelerated perineural invasion in colitis-associated cancer. Medicine. 2019;98(42):e17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo TA, Wu YC, Tan C, et al. Clinicopathologic features and prognostic value of KRAS, NRAS and BRAF mutations and DNA mismatch repair status: a single‐center retrospective study of 1,834 Chinese patients with stage I-IV colorectal cancer. Int J Cancer. 2019;145(6):1625-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su H, Chang C, Hao J, et al. Identification of genomic alterations of perineural invasion in patients with stage II colorectal cancer. OncoTargets Ther. 2020;13:11571-11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delattre J-F, Cohen R, Henriques J, et al. Prognostic value of tumor deposits for disease-free survival in patients with stage III colon cancer: a post hoc analysis of the IDEA france phase III trial (PRODIGE-GERCOR). J Clin Oncol. 2020;38(15):1702-1710. [DOI] [PubMed] [Google Scholar]
- 30.Havenga K, Enker WE, Norstein J, et al. Improved survival and local control after total mesorectal excision or D3 lymphadenectomy in the treatment of primary rectal cancer: an international analysis of 1411 patients. Eur J Surg Oncol. 1999;25:368-374. [DOI] [PubMed] [Google Scholar]
- 31.Karachun A, Panaiotti L, Chernikovskiy I, et al. Short-term outcomes of a multicentre randomized clinical trial comparing D2 versus D3 lymph node dissection for colonic cancer (COLD trial). Br J Surg. 2020;107:499-508. [DOI] [PubMed] [Google Scholar]
- 32.Tsar'Kov PV, Efetov SK, Tulina IA, Kravchenko AY, Fedorov DN, Efetov SV. [Survival rate after D3-lymphadenectomy for right-sided colic cancer: case-match study]. Khirurgiia. 2015:72-79. [DOI] [PubMed] [Google Scholar]
- 33.Reiffel JA. Propensity score matching: the ‘devil is in the details’ where more may be hidden than you know. Am J Med. 2020;133:178-181. [DOI] [PubMed] [Google Scholar]
- 34.Reiffel JA. Propensity-score matching: optimal, adequate, or incomplete? J Atr Fibrillation. 2018;11:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinicrope FA. Evaluating the combination of microsatellite instability and mutation in BRAF as prognostic factors for patients with colorectal cancer. Clin Gastroenterol Hepatol. 2019;17:391-394. [DOI] [PubMed] [Google Scholar]
- 36.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal J, Muinelo L, Dalmases A, et al. Plasma ctDNA RAS mutation analysis for the diagnosis and treatment monitoring of metastatic colorectal cancer patients. Ann Oncol. 2017;28:1325-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-ccx-10.1177_10732748211051533 for Tumor Deposits and Perineural Invasion had Comparable Impacts on the Survival of Patients With Non-metastatic Colorectal Adenocarcinoma: A Population-Based Propensity Score Matching and Competing Risk Analysis by Bin Luo, Xianzhe Chen, Guanfu Cai, Weixian Hu, Yong Li1, and Junjiang Wang in Cancer Control