Abstract
Cushing’s syndrome (CS) is an endocrine disease characterized by excessive adrenocortical steroid production. One of the mainstay pharmacological treatments for CS are steroidogenesis enzyme inhibitors, including the antifungal agent ketoconazole along with metyrapone, mitotane, and aminoglutethimide. Recently, osilodrostat was added to this drug class and approved by the US Food and Drug Administration (FDA) for the treatment of Cushing’s Disease. Steroidogenesis enzyme inhibitors inhibit various enzymes along the cortisol biosynthetic pathway and may be used preoperatively to lower cortisol levels and reduce surgical risk associated with tumor resection or postoperatively when surgery and/or radiation therapies are not curative. Because their selectivities for steroidogenic enzymes vary, they may even be administered in combination to achieve relatively rapid control of severe hypercortisolemia. Unfortunately, all currently available inhibitors are accompanied by serious adverse side effects that limit dosing and often result in treatment failures. Although more commonly known as a general anesthetic induction agent, etomidate is another member of the steroidogenesis enzyme inhibitor drug class. It suppresses cortisol production primarily by inhibiting 11β-hydroxylase and is the only inhibitor that may be given parenterally. However, the sedative-hypnotic actions of etomidate limit its use as an acute management option for CS. Thus, some have recommended that it be used only in intensive care settings. In this review, we discuss the initial development of etomidate as an anesthetic agent, its subsequent development as a treatment for CS, and the recent advances in dosing and drug development that dissociate sedative-hypnotic and adrenostatic drug actions to facilitate CS treatment in non-critical care settings.
Keywords: corticosterone, cortisol, Cushing’s syndrome, etomidate
Introduction
Cushing’s syndrome (CS) is a disease characterized by inappropriately high plasma concentrations of glucocorticoids with the predominate signs and symptoms reflecting an increase in cortisol levels.1–3 Most cases of CS are exogenously mediated, resulting from the administration of drugs with glucocorticoid actions. Endogenous CS is much rarer, having an annual incidence of 3 cases per 1,000,000 individuals according to a recent Danish Study. 4 Endogenous CS occurs via either adrenocorticotropin hormone (ACTH)-dependent or ACTH-independent mechanisms, accounting for about 80% and 20% of cases, respectively.5–7 ACTH-dependent CS occurs when circulating levels of ACTH are high, causing an increase in glucocorticoid production, and can result from pituitary corticotroph tumors (Cushing’s disease (CD); CS), ectopic ACTH-secreting tumors, and corticotropin-releasing hormone-secreting tumors. ACTH-independent CS occurs when adrenal glands overproduce cortisol even when ACTH levels are low and can result from adrenocortical adenomas, carcinomas, and macronodular hyperplasia.
The treatment goal for CS is to normalize cortisol levels or reduce its action on glucocorticoid receptors to resolve the signs, symptoms, and co-morbidities associated with hypercortisolism. Surgical resection of the pathological tissue is typically the first-line treatment. However, in cases of hypercortisolemia resistant to surgery and/or radiation, or when there are significant patient co-morbidities contraindicating surgery, pharmacotherapy is often indicated as primary or adjuvant therapy to reduce cortisol levels or actions. Currently, there are three classes of drugs used to medically treat hypercortisolemia in CS: inhibitors of steroidogenic enzyme activity, pituitary-directed drugs to inhibit ACTH secretion in CD, and glucocorticoid receptor antagonists to block end-organ cortisol activity. Therapy is typically initiated with inhibitors of steroidogenic enzyme activity such as ketoconazole, metyrapone, mitotane, or etomidate. Etomidate is among the most efficacious therapies to reduce cortisol levels but is not commonly utilized by endocrinologists, internists, or intensivists. Etomidate also has certain features that may be advantageous for the acute management of severe CS, including a rapid onset of action and the ability to administer parenterally.7–13 However, etomidate’s high efficacy in treating CS is generally thought to require administering doses which risk producing sedation and hypnosis.10,14,15 To overcome this limitation, efforts are being made to optimize etomidate dosing to allow reductions in cortisol levels without producing sedation or hypnosis and to develop etomidate analogs that retain the ability to inhibit cortisol production but lack sedative-hypnotic activity.16–18
Our purpose in writing this review is to provide readers with a synthesized overview of etomidate and the important role that it plays as one of the pharmacological treatments for severe CS, and to provide context for its use compared with other available treatments. We further sought to present the reader with information regarding the administration of non-sedating etomidate doses for the treatment of CS and discuss the development of novel etomidate analogs that lack sedative-hypnotic activity, but still act as adrenostatic agents and thus may someday facilitate CS treatment in non-ICU (intensive care unit) hospital settings. Pertinent published literature relevant to this task was identified by electronic literature searches using PubMed, Web of Science, Harvard Library catalog (HOLLIS), and Google Scholar with combinations of the search terms: adrenocortical, Cushing’s syndrome, Cushing’s disease, hypercortisolemia, and etomidate.
Treatment approaches for endogenous CS
Surgery and radiation therapy
Treatments for CS include surgery, radiation, and pharmacotherapy3,6 Their purpose is to reduce and normalize cortisol levels, reverse the clinical features of the disease, restore normal biochemical functions, and/or decrease the risk of recurrence or spread. Surgical tumor resection is the frontline treatment for endogenous CS regardless of its cause (ACTH-secreting pituitary tumor, ectopic ACTH-secreting tumor, or adrenal adenoma/carcinoma/nodular hyperplasia) and is associated with high remission rates for micro- and macroadenomas but not carcinomas. 6 When tumors locally reoccur in patients with CS, repeat surgery or stereotactic radiosurgery may be indicated.19–25 Radiotherapy may also be useful as a frontline or adjuvant treatment to slow the growth of tumors and reduce hormonal secretion, particularly in patients who are not surgical candidates or have failed surgery to normalize cortisol levels. 26 However, these beneficial actions may take months or even years to manifest, requiring concomitant pharmacotherapy to control cortisol levels while awaiting the therapeutic effects of radiation. Surgical and radiotherapeutic treatments can be accompanied by significant side effects including brain injury, hypopituitarism, or hypoadrenalism.6,27 Bilateral adrenalectomy provides absolute control of hypercortisolism and is indicated in CS patients who do not respond to more conservative therapies.6,27,28 However, it causes profound and irreversible adrenal insufficiency necessitating lifelong glucocorticoid and mineralocorticoid replacement therapy and increases the risk of Nelson’s syndrome.
Pharmacological management
Pharmacological management of CS is often instituted in patients who are awaiting the effect of radiosurgery, perioperatively to reduce surgical risk, or as an alternative therapy when other treatment methods have failed or surgery is contraindicated.5,6 These drugs target hypercortisolemia at various steps along the hypothalamic-pituitary-adrenal axis and include pituitary-directed agents, adrenostatic agents, and glucocorticoid receptor antagonists (Figure 1).5,6,29 Pituitary-directed agents such as pasireotide, cabergoline, and temozolomide inhibit ACTH secretion, thus preventing the signaling necessary to induce the adrenal cortex to synthesis and release glucocorticoids. Pasireotide and cabergoline have been shown to reduce urinary free cortisol (UFC) in CS patients while the alkylating agent temozolomide inhibits pituitary corticotroph tumor growth.3,7,29–34 The glucocorticoid and progesterone receptor antagonist mifepristone reduces hypercortisolemia by preventing cortisol from binding to its receptor and has been demonstrated to improve many of the clinical features of CS including hyperglycemia and hypotension. 35 The adrenostatic agents ketoconazole, metyrapone, mitotane, aminoglutethimide, and etomidate have long been the mainstay medical treatments for CS despite none being approved by the US Food and Drug Administration (FDA) to treat the disease.13,15 These drugs inhibit one or more steroidogenic enzymes to block cortisol biosynthesis, but cannot control the growth of corticotroph pituitary adenomas or restore normal hypothalamic-pituitary-adrenal axis function. 29 Mitotane has an additional antineoplastic action and has been approved by the FDA and European Medicines Agency (EMA) for the treatment of adrenocortical carcinoma. 36 New adrenostatic drugs are actively being developed. In 2020, osilodrostat received approval by the FDA and the EMA for the treatment of CD and CS, respectively. It is an adrenostatic agent that primarily acts by inhibiting the function of 11β-hydroxylase, the final enzyme in the pathway leading to cortisol biosynthesis. It also blocks the biosynthesis of aldosterone by inhibiting 18-hydroxylase. In an early, small phase II proof-of-concept study, osilodrostat normalized UFC after 10 weeks. 37 A subsequent phase II, open-label, prospective study showed that osilodrostat reduced UFC in 79% of patients after 22 weeks. 38 Osilodrostat was significantly superior to placebo in the pivitol phase III, multicenter, double-blind, randomized withdrawal study. 39 After 34 weeks, 86% of patients treated with osilodrostat had lower UFC without a dose increase after week 29 as compared with 29% of patients who received a placebo. After 48 weeks, 66% of treated patients continued to have lower UFC as compared with baseline.
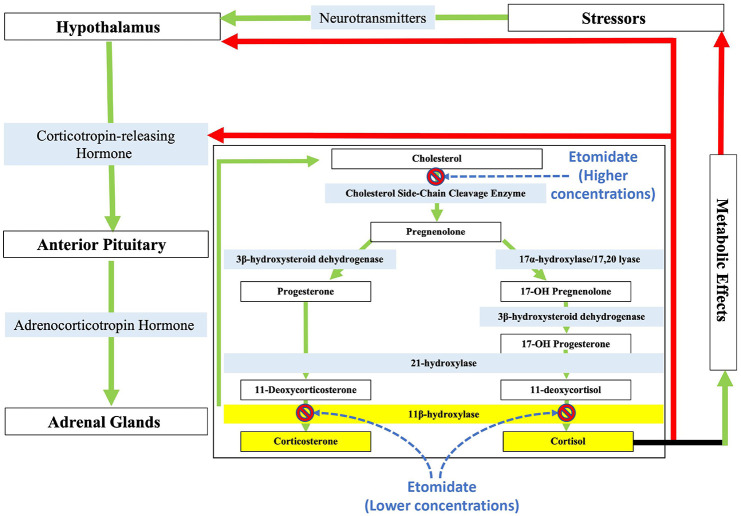
Figure 1.
Schematic of the hypothalamic-pituitary-adrenal axis with the cortisol and corticosterone biosynthetic pathways emphasized. Etomidate (and many etomidate-based analogs) potently inhibits the action of 11β-hydroxylase, thereby preventing steroidogenesis of cortisol. Etomidate also inhibits cholesterol side-chain cleavage enzyme, but this occurs at higher concentrations than those required to inhibit 11β-hydroxylase. The green arrows symbolize stimulating effects, while red arrows symbolize negative feedback.
Radiation and pharmacological therapies have been effective as adjuvant treatments with surgery, but each requires time to produce a therapeutic effect. An important role of medical therapy is in the acute treatment of severe cortisol elevation accompanied by life-threatening co-morbidities. Unfortunately, most adrenostatic agents reduce or normalize UFC or plasma cortisol levels with limited efficacy and are accompanied by significant side effects.3,6,15,29,40,41 Perhaps most notably, ketoconazole can produce hepatic injury. This led the FDA to issue a ‘black-box warning’ for the drug in 2013 and prompted the clinical development of levoketoconazole, a ketoconazole enantiomer that possess a higher potency than racemic ketoconazole and may have less hepatotoxicity.29,42,43
A clinical challenge has been to quickly reduce cortisol levels in severe CS to prepare for surgery or to reduce the life-threatening co-morbidities, including hyperglycemia, sepsis, and hypertension. The varying selectivities of these adrenostatic drugs for steroidogenic enzymes have been leveraged to achieve more rapid control of severe hypercortisolemia. For example, Corcuff et al. 44 demonstrated in a 14-patient study that combination therapy utilizing ketoconazole and metyrapone can reduce UFC by an order of magnitude in a week. Osilodrostat has also been shown to be capable of controlling severe CS relatively quickly. Haissaguerre et al. 45 reported their experience with three patients with severe CS due to nonpituitary cancers. Using starting osilodrostat doses of 2–7 mg/day with escalation as necessary every 2–5 days and reaching maximal doses ranging from 7–44 mg/day, plasma cortisol concentrations were controlled within 2 weeks. A subsequent case report by Bessiène et al. 46 described an approach to achieving even more rapid control by using a higher starting osilodrostat dose (20 mg/day) and employing a block and replace strategy. For patients who are unable to tolerate oral medications or require even faster control of severe CS, etomidate may be indicated. This is a drug that has not been optimally utilized in clinical practice in part because of the lack of familiarity of the drug by endocrinologists, internists, and intensivists. We will present the historical background, pharmacology, and clinical advances that make etomidate an important option to consider in the management of severe CS.
Etomidate
Development as an anesthetic agent
Etomidate is an anesthetic induction agent that was developed by Janssen Pharmaceuticals more than a half century ago. 47 At the time, the company was seeking to develop novel antifungal agents containing an imidazole pharmacophore to inhibit 14α-demethylase, an enzyme necessary for the synthesis of the fungal steroid ergosterol. However, several of their initial compounds unexpectedly produced sedation and hypnosis when tested in rodents. Etomidate was subsequently synthesized as a second-generation compound and found to have a high sedative-hypnotic potency and high therapeutic index compared with existing sedative-hypnotic agents.
GABAAR modulation by etomidate
The sedative-hypnotic activity of etomidate is mediated by the γ-aminobutyric acid type A receptor (GABAAR), the major inhibitory neurotransmitter receptor in the brain.48–50 This receptor, which is a member of the pentameric Cys-loop ligand-gated ion channel superfamily, is comprised of five homologous subunits that surround a chloride-selective ion pore. 50 Upon agonist binding to the receptor, the ion pore opens to allow an influx of chloride ions into neurons. This results in neuronal hyperpolarization and a reduction in neuronal excitability that manifests as sedation and hypnosis. Etomidate binds within the transmembrane domain of the GABAAR at the receptor’s two β-α subunit interfaces, enhancing the actions of agonists on the receptor at low concentrations and directly activating the receptor in the absence of agonist at high concentrations.49,51,52
Safety profile
Etomidate has a higher therapeutic index (>20 in rats) than other anesthetic agent (typically ~3–6) when given as a bolus dose for anesthetic induction, presumably reflecting its lower impact on cardiovascular and respiratory function.47,53,54 Janssen et al. 55 reported that as compared with the other intravenous anesthetics available at the time (e.g. thiopental, methohexital, and propanidid), etomidate also had a higher potency, a more rapid onset, and a longer duration of action. These features led to its initial embrace by anesthesiologists as an anesthetic induction and maintenance agent. 56 By the early 1980s, etomidate was even recommended as a sedative for critically ill patients receiving mechanical ventilation. 57
Adrenocortical suppression
Despite etomidate’s relatively high therapeutic index and rapid acceptance when first introduced into clinical practice in 1972, the drug was subsequently linked to an excessively high 30-day mortality in critically ill patients.58,59 Ledingham and Watt first published a letter in 1983 and then a full report the following year detailing a significant (threefold) increase in mortality in mechanically ventilated trauma patients who received etomidate infusions for sedation. The authors speculated that the mechanism responsible for the increased mortality was etomidate-induced suppression of adrenocortical function. 59
Binding to 11β-hydroxylase
Numerous in vitro studies have demonstrated etomidate’s adrenocortical inhibitory action.60–62 One of the first such studies was conducted by Wagner et al. 62 and documented the binding of etomidate to 11β-hydroxylase using rat adrenal mitochondrial fractions and rat whole adrenal cells. Wagner et al. specifically examined the ability of etomidate to inhibit the synthesis of corticosterone, a steroid that is structurally similar to cortisol, is the principal corticosteroid in rats, and also requires 11β-hydroxylase for biosynthesis (Figure 1). They found that corticosterone production was significantly inhibited even at the lowest concentration of etomidate studied (0.5 ug/ml; 2 µM) and completely inhibited by 5.0 ug/ml (20 µM). At higher concentrations, etomidate was also found to inhibit cholesterol side-chain-cleavage enzyme, but did not affect the activity of the non-mitochondrial enzymes 3β-hydroxysteroid dehydrogenase, Δ5-3 oxosteroid isomerase, or 21-hydroxylase, which catalyze the reactions that convert pregnenolone to the immediate corticosterone precursor deoxycorticosterone.
In silico docking studies have identified the molecular interactions responsible for the high-affinity reversible binding and potent inhibition of 11β-hydroxylase by etomidate. Using homology models of 11β-hydroxylase, Roumen et al. showed that etomidate forms a coordination bond between a nitrogen in its imidazole ring and the heme iron located at the active site of the enzyme. 63 Hydrogen bonding and ring stacking interactions further stabilize etomidate within the enzyme active site, thus enhancing its binding affinity. The resulting high-affinity binding allows etomidate to competitively inhibit 11-deoxycortisol (and deoxycorticosterone) binding with high potency, preventing its conversion into cortisol (and corticosterone).
By better understanding molecular interactions necessary for etomidate to bind to 11β-hydroxylase with high affinity, it may be possible to develop anesthetic etomidate analogs that retain the benign cardiorespiratory properties but do not inhibit the enzyme. One such potential drug is the pyrrole etomidate analog carboetomidate. It reduces 11β-hydroxylase binding affinity by replacing the imidazole ring nitrogen with a methylene group, thus abolishing its ability to form the necessary coordination bond with the enzyme. 64 Consequently, carboetomidate has an affinity for 11β-hydroxylase that is three orders of magnitude lower than etomidate and does not significantly affect adrenocortical function in vivo even when administered at sedative-hypnotic doses.64,65
Etomidate as a treatment for CS
In vitro studies
Coincident with a decline in the use of etomidate as an anesthetic agent was a growing interest in its potential application as a treatment for CS. A 1993 study using cultured human adrenocortical cells derived from two primary aldosteronism patients and one CS patient found an median inhibitory concentration (IC50) for etomidate inhibition of glucocorticoids (cortisol, aldosterone, corticosterone, 18-hydroxycorticosterone) synthesis to be in the range of 1–10 nM, with a greater than 90% block at 1 uM etomidate. 66 The latter concentration approximates its sedative-hypnotic concentration.67,68 Etomidate also had a biphasic effect on cortisol precursors, 11-deoxycortisol and deoxycorticosterone, concentrations in both the presence and absence of ACTH. At lower levels of etomidate, 11-deoxycortisol and deoxycorticosterone concentrations accumulated while at higher levels of etomidate these cortisol precursors decreased. 66 This likely reflects a lower etomidate affinity for side-chain cleavage enzyme (and perhaps other upstream enzymes) versus 11β-hydroxylase, resulting in the accumulation of cortisol precursors at low etomidate concentrations where only 11β-hydroxylase is significantly inhibited and reductions in precursor biosynthesis at high etomidate concentrations where upstream enzymes are also inhibited.
Clinical case studies
A growing number of case studies have been published describing the off-label use of etomidate to treat severe hypercortisolism. Applicable in emergency settings and sometimes as a long-term treatment, etomidate can be used to rapidly treat patients with CS who have concurrent significant metabolic derangements, severe hypertension, or psychosis.5,69–74 One of the earliest published clinical investigations demonstrating the adrenostatic potential of etomidate was a 1990 study using low-dose etomidate infusions administered to six patients with severe CS and 15 controls. 75 Both CS and control patients demonstrated a dose-dependent reduction in cortisol levels with etomidate infusion, although cortisol levels increased to pretreatment levels after infusion termination consistent with reversible binding to steroidogenic enzymes. Consistent with the findings of in vitro studies, 66 Schulte et al. 75 also reported an increase in the levels of the precursor steroids 11-deoxycotisol and 11-deoxycorticosterone with such low-dose etomidate administration.
In addition to managing hypercortisolemia, etomidate may provide specific advantages over other pharmacologic management options for certain severe CS cases. Most notably, etomidate is the only steroidogenic inhibitor that may be administered parenterally. Perforation of the gastrointestinal (GI) tract is a well-documented complication of CS and precludes management with ketoconazole and metyrapone as they must be administered orally. In cases of medullary thyroid carcinoma with ectopic ACTH production, GI perforation accounts for 30% of all mortality. 76 A 2016 case study of a 67-year-old male with ectopic ACTH production and CS due to metastatic medullary thyroid carcinoma showed normalized cortisol levels following initiation of an etomidate infusion after the patient’s diverticulum perforated. 77 Similarly, a 35-year-old male with ectopic CS was treated with etomidate to reduce cortisol levels below detection after developing peritonitis. 69
Etomidate may also be useful in cases of psychosis and non-compliance related to CS. A 2010 case study of a 14-year-old patient with a nearly 3-year history of CS symptoms showed successful management and significant patient improvement following etomidate administration in the ICU after she became catatonic following an acute psychotic episode that left her unable to take oral medications. 78 In addition, a 66-year-old patient with ectopic CS who had discontinued all medication (including ketoconazole and antipsychotic drugs) demonstrated clinical and biochemical improvement with an etomidate infusion after previously presenting with severe hypercortisolemia and hypokalemia, decompensation of diabetes mellitus, and mood disorders. 74 It should be noted that this patient’s cortisol levels initially remained unstable despite multidrug therapy due to frequent hospital complications, but after 2 months of etomidate treatment, she was able to undergo a complete adrenalectomy without further complications.
In addition to its use in managing CS patients with altered mentation or an inability to take oral medications, etomidate is an important but not frequently utilized option for the acute treatment of patients with severe hypercortisolism who are not immediate candidates for surgery and who fail or cannot tolerate multidrug therapy. For example, a 1-week etomidate infusion was administered to a 31-year-old patient with a history of breast cancer treated with chemotherapy, bilateral mastectomy, and radiation who presented with psychotic, and paranoid behavior and ectopic ACTH-associated CS. 79 The etomidate infusion successfully controlled the patient’s hypercortisolemia and hypertension. She was later transitioned to a combination of oral metyrapone and spironolactone, and received chemotherapy. Intolerance to multidrug therapy was also described in the hospital course of a 23-year-old patient who presented with rapidly progressing CS due to a metastatic adrenocortical carcinoma 2 years after having undergone a right-side adrenalectomy. 74 The patient presented with uncontrolled diabetes mellitus, hypertension, hypokalemia, hypocalcemia, and a possible upper respiratory infection and was initially treated with palliative surgery and a combination of ketoconazole, mitotane, and metyrapone. Nevertheless, her cortisol levels remained high. Therefore, her oral medications were replaced with a continuous intravenous infusion of etomidate. As a result, her cortisol levels decreased, and symptoms improved sufficiently after 1 week of etomidate treatment to permit chemotherapy. Unfortunately, her cortisol levels rebounded after the etomidate infusion was discontinued and she died shortly thereafter. This patient’s death highlights the shortcomings of etomidate and most other pharmacological treatments: they reduce plasma cortisol concentrations and improve CS signs and symptoms, but are not curative.
Despite its shortcomings (e.g. poor oral bioavailability and sedative-hypnotic actions), etomidate remains a potentially useful treatment option in the acute management of severe CS especially when accompanied by life-threatening co-morbidities due to its rapid onset. Titration with etomidate can occur within days, or even hours when a block and replace strategy is used. For example, Greening et al. 71 described the case of a 6-year-old with severe Cushingoid features and hepatic steatosis in whom ketoconazole and metyrapone proved intolerable. Forty-eight hours after initiating an etomidate infusion, the child’s condition improved sufficiently to allow successful bilateral adrenalectomy. Similarly, etomidate reduced serum cortisol levels by a factor of four within 48 h in a 65-year-old patient with severe CS due to an adrenocortical carcinoma, enabling him to receive surgical treatment 1 week later. 80
Etomidate infusion protocol for the treatment of CS
A standard protocol for administering etomidate in the ICU to patients with severe and life-threatening CS has been developed and evaluated. 16 Pursuant to that protocol, patients receive an initial 5 mg bolus dose of etomidate over 2–3 min. Such an etomidate dose is approximately 25% of that required to produce unconsciousness. 50 This is immediately followed by a continuous infusion starting at a rate of 0.2 mg/kg/h that may be titrated no more frequently than every 6 h based on measured serum cortisol concentrations. Provided that plasma cortisol concentrations are trending toward the desired endpoint, no adjustments in the infusion rate are made. However, if the cortisol concentrations are not trending toward the goal, then the infusion is adjusted in increments of 0.1–0.2 mg/kg/h.
The ability of this protocol to rapidly treat hypercortisolemia was demonstrated by a retrospective analysis of seven patients with CS whose serum cortisol concentrations reached a median nadir of 15.8 µg/dl (range, 6.9–27 µg/dl) at a median time of 38 h (range, 26–134 h) after beginning etomidate administration. 16 During etomidate infusion, side effects were relatively mild as only one patient experienced any level of sedation (which was mild) and none had electrolyte abnormalities or substantial changes in renal function. There were two episodes of nausea and vomiting that were tentatively attributed to the rapid lowering of cortisol concentrations; however, etomidate itself is known to be pro-emetogenic and may have been the cause. 81 The safety of the protocol suggested by this study has led to the proposition that low-dose etomidate infusions can be administered outside of an intensive care setting and without the need for hydrocortisone supplementation. 82
Side effects of etomidate
Adrenocortical insufficiency
Similar to the other adrenostatic or adrenolytic therapies, administration of etomidate may be accompanied by serious side effects including the risk of adrenocortical insufficiency when not co-administered with glucocorticoid replacement. Such adrenal insufficiency can persist long after etomidate administration has been terminated, as was reported in a 2001 case study of a 39-year-old man with ectopic CS who developed transient renal failure. 70 In this patient, adrenocortical insufficiency persisted for at least 14 days after the end of etomidate treatment and may have lasted as long as 6 weeks. Such prolonged action likely reflects etomidate accumulation and subsequent slow release from adipose tissue after etomidate infusion has been discontinued. 83
Propylene glycol toxicity
Because of its hydrophobicity, etomidate is typically formulated using 35% propylene glycol in water. At the high total doses sometimes reached with prolonged etomidate infusion, propylene glycol may be toxic, particularly in patients with renal failure. Manifestations of propylene glycol toxicity include an increased plasma hyperosmolarity, lactic acidosis, central nervous system depression, and cardiac arrythmias. Bedichek and Kirschbaum reported, for example, the development of high anion gap metabolic acidosis in a neurosurgical patient who received a total of 479 g of propylene glycol over a 24-h period. 84 More than 90% of the propylene glycol dose was attributed to an etomidate infusion used to control intracranial hypertension. In another report, Levy et al. 85 reported propylene glycol-induced nephrotoxicity in three patients who similarly received etomidate with the goal of achieving burst suppression to manage intracranial hypertension. Although all of these patients received etomidate doses that are likely beyond those required to treat CS, any potential risk of propylene glycol toxicity can be completely eliminated by using etomidate compounded in either a lipid emulsion (i.e. etomidate-lipuro) or cyclodextrin.10,86
Sedation
Perhaps etomidate’s most notable and undesirable side effect when used as an adrenostatic agent is its ability to produce sedation. Schulte et al.’s 75 aforementioned case study reported side effects of tiredness in two of its control volunteers receiving the highest dose of etomidate (0.03 mg/kg bolus followed by an infusion at 0.3 mg/kg/h). Similarly, Gärtner, Albrecht, and Müller reported that at an infusion at 15–30 mg/h, etomidate had an acute sedative effect on a 53-year-old patient who was being treated for ectopic CS. 87 In contrast, none of the seven CS patients described in a retrospective review experienced sedation or other mental status changes with etomidate administration at doses ranging from 0.033–0.15 mg/kg/h. 16 Although etomidate’s sedative-hypnotic effects typically occur at doses that are higher than necessary to suppress adrenocortical function, patients with CD and those with low sensitivity to steroidogenic inhibitors due to genetic polymorphisms may require sedating etomidate doses to fully correct their hypercortisolemia.10,88 This has led some to recommend that etomidate only be administered to treat CS in an ICU setting.10,14,15
Non-sedating etomidate analogs
In order to avoid the sedative-hypnotic action of etomidate, etomidate analogs (e.g. dimethoxy-etomidate) have recently been developed which retain the potent and efficacious adrenocortical suppressive actions of etomidate but lack its potentiating effect on GABAARs and thus its ability to produce sedation or hypnosis.17,18 McGrath, Ma, and Raines demonstrated that although both etomidate and dimethoxy-etomidate enhance peak GABAAR currents in in vitro electrophysiological recordings, dimethoxy-etomidate’s receptor potentiating actions were neither potent nor efficacious. 17 In vivo studies in rats showed that although dimethoxy-etomidate inhibited corticosterone synthesis with a potency that was similar to that of etomidate, it did not produce loss-of-righting reflexes or myoclonus even after the administration of doses as high as 50 mg/kg. At this very high dose, dimethoxy-etomidate also reduced plasma 11-deoxycorticosterone levels, suggesting that this etomidate analog binds with relatively low affinity to 21α-hydroxylase compared with etomidate. Subsequent studies identified three additional phenyl-ring substituted analogs of etomidate that inhibit steroidogenesis but lack sedative-hypnotic activity. 18 These compounds inhibited the production of adrenocortical steroids (corticosterone and aldosterone) in rats while variably affecting that of androgenic steroids (testosterone, dihydrotestosterone dehydroepiandrosterone, and androstenedione).
Conclusion
Etomidate is a valuable pharmacological tool for the acute treatment of critically ill patients with severe CS because it is highly efficacious, has a rapid onset of action, can be administered parenterally, and has a reduced risk of hepatic injury compared with ketoconazole. It reduces plasma cortisol levels by potently inhibiting the adrenocortical enzyme 11β-hydroxylase, a mechanism that is distinct from the GABAergic one responsible for its sedative-hypnotic activity. The etomidate doses required to treat CS are typically – but not always – below those which produce sedation or hypnosis. This potential for producing sedation or hypnosis has led some to recommend that it only be administered in an intensive care setting where such side effects can be closely monitored. However, recent low-dose etomidate infusion protocols have been developed which challenge that assumption. In addition, etomidate analogs are under development that retain the potent adrenostatic action of etomidate without its sedative-hypnotic activity, offering another potential future strategy for treating patients with severe CS in non-ICU hospital settings.
Footnotes
Author contributions: Andrea Pence: Literature research; Writing – original draft
Megan McGrath: Writing – review & editing
Stephanie L. Lee: Writing – review & editing
Douglas E. Raines: Literature resreach; Conceptualization; Writing – review & editing
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Raines is the inventor of patented technologies involving the design of etomidate analogs that potently inhibit cortisol production but lack sedative-hypnotic activity.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Health, Bethesda, MA [grant number GM122806] and the Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts.
ORCID iD: Douglas E. Raines  https://orcid.org/0000-0003-3790-978X
https://orcid.org/0000-0003-3790-978X
Contributor Information
Andrea Pence, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA, USA.
Megan McGrath, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, MA, USA.
Stephanie L. Lee, Section of Endocrinology, Diabetes and Nutrition, Department of Medicine, Boston Medical Center, Boston, MA, USA
Douglas E. Raines, Edward Mallinckrodt Jr. Professor of Anaesthesiology in the Field of Pharmacology and Innovation, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA.
References
- 1. Fernandez-Rodriguez E, Stewart PM, Cooper MS. The pituitary-adrenal axis and body composition. Pituitary 2009; 12: 105–115. [DOI] [PubMed] [Google Scholar]
- 2. Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in Cushing’s syndrome. Neuroendocrinology 2010; 92(Suppl. 1): 65–70. [DOI] [PubMed] [Google Scholar]
- 3. Hinojosa-Amaya JM, Cuevas-Ramos D, Fleseriu M. Medical management of Cushing’s syndrome: current and emerging treatments. Drugs 2019; 79: 935–956. [DOI] [PubMed] [Google Scholar]
- 4. Wengander S, Trimpou P, Papakokkinou E, et al. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol 2019; 91: 263–270. [DOI] [PubMed] [Google Scholar]
- 5. Feelders RA, Hofland LJ, de Herder WW. Medical treatment of Cushing’s syndrome: adrenal-blocking drugs and ketaconazole. Neuroendocrinology 2010; 92(Suppl. 1): 111–115. [DOI] [PubMed] [Google Scholar]
- 6. Mancini T, Porcelli T, Giustina A. Treatment of Cushing disease: overview and recent findings. Ther Clin Risk Manag 2010; 6: 505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nishioka H, Yamada S. Cushing’s disease. J Clin Med 2019; 8: 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lambert A, Frost J, Mitchell R, et al. On the assessment of the in vitro biopotency and site(s) of action of drugs affecting adrenal steroidogenesis. Ann Clin Biochem 1986; 23: 225–229. [DOI] [PubMed] [Google Scholar]
- 9. Lamberts SW, Bons EG, Bruining HA, et al. Differential effects of the imidazole derivatives etomidate, ketoconazole and miconazole and of metyrapone on the secretion of cortisol and its precursors by human adrenocortical cells. J Pharmacol Exp Ther 1987; 240: 259–264. [PubMed] [Google Scholar]
- 10. Preda VA, Sen J, Karavitaki N, et al. Etomidate in the management of hypercortisolaemia in Cushing’s syndrome: a review. Eur J Endocrinol 2012; 167: 137–143. [DOI] [PubMed] [Google Scholar]
- 11. Cuevas-Ramos D, Fleseriu M. Treatment of Cushing’s disease: a mechanistic update. J Endocrinol 2014; 223: R19–R39. [DOI] [PubMed] [Google Scholar]
- 12. Gadelha MR, Vieira Neto L. Efficacy of medical treatment in Cushing’s disease: a systematic review. Clin Endocrinol 2014; 80: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Ambrogio AG, Cavagnini F. Role of ‘old’ pharmacological agents in the treatment of Cushing’s syndrome. J Endocrinol Invest 2016; 39: 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tritos NA, Biller BM. Medical management of Cushing’s disease. J Neurooncol 2014; 117: 407–414. [DOI] [PubMed] [Google Scholar]
- 15. Daniel E, Newell-Price JD. Therapy of endocrine disease: steroidogenesis enzyme inhibitors in Cushing’s syndrome. Eur J Endocrinol 2015; 172: R263–R280. [DOI] [PubMed] [Google Scholar]
- 16. Carroll TB, Peppard WJ, Herrmann DJ, et al. Continuous etomidate infusion for the management of severe Cushing syndrome: validation of a standard protocol. J Endocr Soc 2019; 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGrath M, Ma C, Raines DE. Dimethoxy-etomidate: a nonhypnotic etomidate analog that potently inhibits steroidogenesis. J Pharmacol Exp Ther 2018; 364: 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGrath M, Hofmann A, Raines DE. Behavioral and steroidogenic pharmacology of phenyl ring substituted etomidate analogs in rats. BMC Pharmacol Toxicol 2019; 20: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Littley MD, Shalet SM, Beardwell CG, et al. Long-term follow-up of low-dose external pituitary irradiation for Cushing’s disease. Clin Endocrinol 1990; 33: 445–455. [DOI] [PubMed] [Google Scholar]
- 20. Devin JK, Allen GS, Cmelak AJ, et al. The efficacy of linear accelerator radiosurgery in the management of patients with Cushing’s disease. Stereotact Funct Neurosurg 2004; 82: 254–262. [DOI] [PubMed] [Google Scholar]
- 21. Hammer GD, Tyrrell JB, Lamborn KR, et al. Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab 2004; 89: 6348–6357. [DOI] [PubMed] [Google Scholar]
- 22. Atkinson AB, Kennedy A, Wiggam MI, et al. Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol 2005; 63: 549–559. [DOI] [PubMed] [Google Scholar]
- 23. Biller BM, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 2008; 93: 2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vance ML. Cushing’s disease: radiation therapy. Pituitary 2009; 12: 11–14. [DOI] [PubMed] [Google Scholar]
- 25. Pivonello R, De Leo M, Cozzolino A, et al. The treatment of Cushing’s disease. Endocr Rev 2015; 36: 385–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gheorghiu ML, Fleseriu M. Stereotactic radiation therapy in pituitary adenomas, is it better than conventional radiation therapy. Acta Endocrinol 2017; 13: 476–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katznelson L. Bilateral adrenalectomy for Cushing’s disease. Pituitary 2015; 18: 269–273. [DOI] [PubMed] [Google Scholar]
- 28. Pozza C, Graziadio C, Giannetta E, et al. Management strategies for aggressive Cushing’s syndrome: from macroadenomas to ectopics. J Oncol 2012; 2012: 685213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tritos NA, Biller BMK. Medical management of Cushing disease. Neurosurg Clin N Am 2019; 30: 499–508. [DOI] [PubMed] [Google Scholar]
- 30. Pivonello R, De Martino MC, Cappabianca P, et al. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 2009; 94: 223–230. [DOI] [PubMed] [Google Scholar]
- 31. Dillard TH, Gultekin SH, Delashaw JB, Jr, et al. Temozolomide for corticotroph pituitary adenomas refractory to standard therapy. Pituitary 2011; 14: 80–91. [DOI] [PubMed] [Google Scholar]
- 32. Ji Y, Vogel RI, Lou E. Temozolomide treatment of pituitary carcinomas and atypical adenomas: systematic review of case reports. Neurooncol Pract 2016; 3: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ferriere A, Cortet C, Chanson P, et al. Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur J Endocrinol 2017; 176: 305–314. [DOI] [PubMed] [Google Scholar]
- 34. Lacroix A, Gu F, Gallardo W, et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol 2018; 6: 17–26. [DOI] [PubMed] [Google Scholar]
- 35. Fleseriu M. Medical management of persistent and recurrent Cushing disease. Neurosurg Clin N Am 2012; 23: 653–668. [DOI] [PubMed] [Google Scholar]
- 36. Paragliola RM, Torino F, Papi G, et al. Role of mitotane in adrenocortical carcinoma – review and state of the art. Eur Endocrinol 2018; 14: 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bertagna X, Pivonello R, Fleseriu M, et al. LCI699, a potent 11beta-hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing’s disease: results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab 2014; 99: 1375–1383. [DOI] [PubMed] [Google Scholar]
- 38. Fleseriu M, Pivonello R, Young J, et al. Osilodrostat, a potent oral 11beta-hydroxylase inhibitor: 22-week, prospective, phase II study in Cushing’s disease. Pituitary 2016; 19: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pivonello R, Fleseriu M, Newell-Price J, et al. Efficacy and safety of osilodrostat in patients with Cushing’s disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol 2020; 8: 748–761. [DOI] [PubMed] [Google Scholar]
- 40. Misbin RI, Canary J, Willard D. Aminoglutethimide in the treatment of Cushing’s syndrome. J Clin Pharmacol 1976; 16: 645–651. [DOI] [PubMed] [Google Scholar]
- 41. Baudry C, Coste J, Bou Khalil R, et al. Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol 2012; 167: 473–481. [DOI] [PubMed] [Google Scholar]
- 42. Administration FDA. FDA Drug Safety Communication: FDA limits usage of Nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems, https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-limits-usage-nizoral-ketoconazole-oral-tablets-due-potentially (2013, accessed 31 March 2021). [PubMed]
- 43. Geer EB, Salvatori R, Elenkova A, et al. Levoketoconazole improves clinical signs and symptoms and patient-reported outcomes in patients with Cushing’s syndrome. Pituitary 2021; 24: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Corcuff JB, Young J, Masquefa-Giraud P, et al. Rapid control of severe neoplastic hypercortisolism with metyrapone and ketoconazole. Eur J Endocrinol 2015; 172: 473–481. [DOI] [PubMed] [Google Scholar]
- 45. Haissaguerre M, Puerto M, Nunes ML, et al. Efficacy and tolerance of osilodrostat in patients with severe Cushing’s syndrome due to non-pituitary cancers. Eur J Endocrinol 2020; 183: L7–L9. [DOI] [PubMed] [Google Scholar]
- 46. Bessiène L, Bonnet F, Tenenbaum F, et al. Rapid control of severe ectopic Cushing’s syndrome by oral osilodrostat monotherapy. Eur J Endocrinol 2021; 184: L13–L15. [DOI] [PubMed] [Google Scholar]
- 47. Godefroi EF, Janssen PA, Vandereycken CA, et al. Dl-1-(1-arylalkyl)imidazole-5-carboxylate esters. A novel type of hypnotic agents. J Med Chem 1965; 8: 220–223. [DOI] [PubMed] [Google Scholar]
- 48. Jurd R, Arras M, Lambert S, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J 2003; 17: 250–252. [DOI] [PubMed] [Google Scholar]
- 49. Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol 2006; 147(Suppl. 1): S72–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Forman SA. Clinical and molecular pharmacology of etomidate. Anesthesiology 2011; 114: 695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiara DC, Jayakar SS, Zhou X, et al. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human alpha1beta3gamma2 gamma-aminobutyric acid type A (GABAA) receptor. J Biol Chem 2013; 288: 19343–19357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maldifassi MC, Baur R, Sigel E. Functional sites involved in modulation of the GABAA receptor channel by the intravenous anesthetics propofol, etomidate and pentobarbital. Neuropharmacology 2016; 105: 207–214. [DOI] [PubMed] [Google Scholar]
- 53. Glen JB. Animal studies of the anaesthetic activity of ICI 35 868. Br J Anaesth 1980; 52: 731–742. [DOI] [PubMed] [Google Scholar]
- 54. Marietta MP, WAY WL, Castagnoli N, Jr, et al. On the pharmacology of the ketamine enantiomorphs in the rat. J Pharmacol Exp Ther 1977; 202: 157–165. [PubMed] [Google Scholar]
- 55. Janssen PA, Niemegeers CJ, Schellekens KH, et al. Etomidate, R-(+)-ethyl-1-(-methyl-benzyl)imidazole-5-carboxylate (R 16659), a potent, short-acting and relatively atoxic intravenous hypnotic agent in rats. Arzneimittelforschung 1971; 21: 1234–1243. [PubMed] [Google Scholar]
- 56. Janssen PA, Niemegeers CJ, Marsboom RP. Etomidate, a potent non-barbiturate hypnotic. Intravenous etomidate in mice, rats, guinea-pigs, rabbits and dogs. Arch Int Pharmacodyn Ther 1975; 214: 92–132. [PubMed] [Google Scholar]
- 57. Edbrooke DL, Newby DM, Mather SJ, et al. Safer sedation for ventilated patients. A new application for etomidate. Anaesthesia 1982; 37: 765–771. [DOI] [PubMed] [Google Scholar]
- 58. Ledingham IM, Watt I. Influence of sedation on mortality in critically ill multiple trauma patients. Lancet 1983; 1: 1270. [DOI] [PubMed] [Google Scholar]
- 59. Watt I, Ledingham IM. Mortality amongst multiple trauma patients admitted to an intensive therapy unit. Anaesthesia 1984; 39: 973–981. [DOI] [PubMed] [Google Scholar]
- 60. Vanden Bossche H, Willemsens G, Cools W, et al. Effects of etomidate on steroid biosynthesis in subcellular fractions of bovine adrenals. Biochem Pharmacol 1984; 33: 3861–3868. [DOI] [PubMed] [Google Scholar]
- 61. de Jong FH, Mallios C, Jansen C, et al. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab 1984; 59: 1143–1147. [DOI] [PubMed] [Google Scholar]
- 62. Wagner RL, White PF, Kan PB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med 1984; 310: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 63. Roumen L, Sanders MP, Pieterse K, et al. Construction of 3D models of the CYP11B family as a tool to predict ligand binding characteristics. J Comput Aided Mol Des 2007; 21: 455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cotten JF, Forman SA, Laha JK, et al. Carboetomidate: a pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology 2010; 112: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pejo E, Zhou X, Husain SS, et al. Sedative-hypnotic binding to 11beta-hydroxylase. Anesthesiology 2016; 125: 943–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Varga I, Racz K, Kiss R, et al. Direct inhibitory effect of etomidate on corticosteroid secretion in human pathologic adrenocortical cells. Steroids 1993; 58: 64–68. [DOI] [PubMed] [Google Scholar]
- 67. Hebron BS. Plasma concentrations of etomidate during an intravenous infusion over 48 hours. Anaesthesia 1983; 38(Suppl.): 39–43. [DOI] [PubMed] [Google Scholar]
- 68. Husain SS, Ziebell MR, Ruesch D, et al. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem 2003; 46: 1257–1265. [DOI] [PubMed] [Google Scholar]
- 69. Drake WM, Perry LA, Hinds CJ, et al. Emergency and prolonged use of intravenous etomidate to control hypercortisolemia in a patient with Cushing’s syndrome and peritonitis. J Clin Endocrinol Metab 1998; 83: 3542–3544. [DOI] [PubMed] [Google Scholar]
- 70. Krakoff J, Koch CA, Calis KA, et al. Use of a parenteral propylene glycol-containing etomidate preparation for the long-term management of ectopic Cushing’s syndrome. J Clin Endocrinol Metab 2001; 86: 4104–4108. [DOI] [PubMed] [Google Scholar]
- 71. Greening JE, Brain CE, Perry LA, et al. Efficient short-term control of hypercortisolaemia by low-dose etomidate in severe paediatric Cushing’s disease. Horm Res 2005; 64: 140–143. [DOI] [PubMed] [Google Scholar]
- 72. Dabbagh A, Sa’adat N, Heidari Z. Etomidate infusion in the critical care setting for suppressing the acute phase of Cushing’s syndrome. Anesth Analg 2009; 108: 238–239. [DOI] [PubMed] [Google Scholar]
- 73. Lutgers HL, Vergragt J, Dong PV, et al. Severe hypercortisolism: a medical emergency requiring urgent intervention. Crit Care Med 2010; 38: 1598–1601. [DOI] [PubMed] [Google Scholar]
- 74. Lebek-Szatanska A, Nowak KM, Zgliczynski W, et al. Low-dose etomidate for the management of severe hypercortisolaemia in different clinical scenarios: a case series and review of the literature. Ther Adv Endocrinol Metab 2019; 10: 2042018819825541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schulte HM, Benker G, Reinwein D, et al. Infusion of low dose etomidate: correction of hypercortisolemia in patients with Cushing’s syndrome and dose-response relationship in normal subjects. J Clin Endocrinol Metab 1990; 70: 1426–1430. [DOI] [PubMed] [Google Scholar]
- 76. Barbosa SL, Rodien P, Leboulleux S, et al. Ectopic adrenocorticotropic hormone-syndrome in medullary carcinoma of the thyroid: a retrospective analysis and review of the literature. Thyroid 2005; 15: 618–623. [DOI] [PubMed] [Google Scholar]
- 77. Matheny LN, Wilson JR, Baum HB. Ectopic ACTH production leading to diagnosis of underlying medullary thyroid carcinoma. J Investig Med High Impact Case Rep 2016; 4: 2324709616643989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chan LF, Vaidya M, Westphal B, et al. Use of intravenous etomidate to control acute psychosis induced by the hypercortisolaemia in severe paediatric Cushing’s disease. Horm Res Paediatr 2011; 75: 441–446. [DOI] [PubMed] [Google Scholar]
- 79. Bucciarelli M, Lee YY, Magaji V. Cushing’s storm secondary to a rare case of ectopic ACTH secreting metastatic breast cancer. Endocrinol Diabetes Metab Case Rep 2015; 2015: 150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Huang CJ, Wang TH, Lo YH, et al. Adrenocortical carcinoma initially presenting with hypokalemia and hypertension mimicking hyperaldosteronism: a case report. BMC Res Notes 2013; 6: 405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Clarke RS. Nausea and vomiting. Br J Anaesth 1984; 56: 19–27. [DOI] [PubMed] [Google Scholar]
- 82. Constantinescu SM, Driessens N, Lefebvre A, et al. Etomidate infusion at low doses is an effective and safe treatment for severe Cushing’s syndrome outside intensive care. Eur J Endocrinol 2020; 183: 161–167. [DOI] [PubMed] [Google Scholar]
- 83. Campagna JA, Pojasek K, Grayzel D, et al. Advancing novel anesthetics: pharmacodynamic and pharmacokinetic studies of cyclopropyl-methoxycarbonyl metomidate in dogs. Anesthesiology 2014; 121: 1203–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bedichek E, Kirschbaum B. A case of propylene glycol toxic reaction associated with etomidate infusion. Arch Intern Med 1991; 151: 2297–2298. [PubMed] [Google Scholar]
- 85. Levy ML, Aranda M, Zelman V, et al. Propylene glycol toxicity following continuous etomidate infusion for the control of refractory cerebral edema. Neurosurgery 1995; 37: 363–369; discussion 369–371. [DOI] [PubMed] [Google Scholar]
- 86. Soh LM, Gunganah K, Akker SA, et al. Etomidate in the emergency management of hypercortisolemia. Eur J Endocrinol 2012; 167: 727–728; author reply 729. [DOI] [PubMed] [Google Scholar]
- 87. Gartner R, Albrecht M, Muller OA. Effect of etomidate on hypercortisolism due to ectopic ACTH production. Lancet 1986; 1: 275. [DOI] [PubMed] [Google Scholar]
- 88. Valassi E, Aulinas A, Glad CA, et al. A polymorphism in the CYP17A1 gene influences the therapeutic response to steroidogenesis inhibitors in Cushing’s syndrome. Clin Endocrinol 2017; 87: 433–439. [DOI] [PubMed] [Google Scholar]