Abstract
Background and Aims:
Exacerbations of chronic obstructive pulmonary disease (COPD) drive disease progression and can lead to an accelerated decline in lung function and a burden on healthcare systems. The retrospective, observational cohort Study on HEalthcare Resource utiLization related to exacerbatiOns in patients with COPD (SHERLOCK; D5980R00014) evaluated the associations between exacerbation history and rates of subsequent COPD exacerbations in primary care patients from the National Health Service in Greater Glasgow and Clyde, United Kingdom.
Methods:
Patients were stratified into four groups according to exacerbation history in the year before the index date: Group A (no exacerbations), Group B (1 moderate exacerbation only), Group C (1 severe exacerbation only), and Group D (⩾2 moderate or severe exacerbations). The frequencies of moderate and/or severe exacerbations were recorded over 36 months of follow-up and compared with reference Group A, using generalized linear models.
Results:
Over 36 months of follow-up, the adjusted rate ratios (RRs, 95% confidence interval) of moderate or severe exacerbations relative to Group A were 1.60 (1.53, 1.67), 1.75 (1.50, 2.04), 1.61 (1.54, 1.68), and 3.61 (3.48, 3.74) for Groups B, C, B + C, and D, respectively. Compared with Group A, patients in Group C exhibited an increased rate of moderate (RR, 1.58 (1.35, 1.85)) and severe exacerbations (RR, 3.13 (2.20, 4.46)).
Conclusion:
SHERLOCK highlights that even one moderate exacerbation increases the risk for subsequent exacerbations compared with having no recent prior exacerbations. Reviewing recent exacerbation history to ascertain future exacerbation risk and inform COPD management may reduce hospitalizations and improve patient outcomes.
Keywords: chronic obstructive pulmonary disease, exacerbations, risk
Introduction
In patients with chronic obstructive pulmonary disease (COPD), exacerbations drive disease progression and can lead to a decline in lung function and a higher risk of mortality.1–3 The combined total direct and indirect costs of COPD in the European Union (EU) have been estimated to be approximately €48 billion. 4 Exacerbations of COPD are key drivers for the costs of COPD, with a study of UK primarycare patients reporting that a history of exacerbations increases COPD treatment costs. 5 Although inhaled therapies are used to manage symptoms, improve quality of life, and reduce exacerbation frequency, 1 patients who have experienced one or more moderate acute (requiring additional medication) or severe acute (requiring inpatient care) exacerbations are at an increased risk of future exacerbations. 6 Also, patients experiencing severe COPD exacerbations have poorer prognoses than patients having no prior exacerbations or those who have experienced moderate exacerbations. 7
Longitudinal and observational studies have examined patients’ prior exacerbation history and COPD severity, the impact of early or late COPD diagnosis, and the relationship with future exacerbation risk.2,6–8 For example, an analysis of 2138 patients enrolled in the ECLIPSE observational study indicated that prior exacerbation history and disease severity were key predictors of future exacerbation risk over 3 years of follow-up. 2 Furthermore, in an analysis of data from the UK Clinical Practice Research Datalink over 10 years, Rothnie et al. stratified patients by frequency and severity of exacerbations in the previous year. They demonstrated that the risk of future exacerbations increased as a function of prior exacerbation history. 7
Patients who experience frequent exacerbations are generally the focus of clinical practice,9,10 with severe exacerbations being an area of concern due to their association with increased mortality. 3 However, focusing solely on patients with severe and/or frequent exacerbations is inadequate, based on emerging evidence indicating that patients who have experienced infrequent or moderate exacerbations may be at increased risk of future exacerbations.2,7 Thus, it is important that all patients who are at risk of any exacerbation be identified as early as possible to optimize any necessary interventions. 11
At present, there is limited research on the association between the exacerbation history of patients in primary care who experience infrequent and less-severe exacerbations and the gradual impact of these events on future exacerbations. Furthermore, there remains a gap in the literature regarding the association between a history of a single exacerbation event and future exacerbations. The retrospective, observational cohort Study on HEalthcare Resource utiLization related to exacerbatiOns in patients with COPD (SHERLOCK; D5980R00014) was conducted to address this gap. The primary objective of SHERLOCK was to evaluate the associations between exacerbation history—based on both the frequency and severity of prior exacerbations—and the patterns of subsequent COPD exacerbations in terms of frequency and severity in primary care patients in the NHS Greater Glasgow and Clyde (NHSGGC) Health Board. By examining patterns in both the frequency and severity of exacerbations for 3 years, SHERLOCK expands the current understanding of the burden associated with exacerbations of COPD.
Methods
Study design
SHERLOCK was a retrospective cohort study using data stored within the Safe Haven database of the NHSGGC Health Board, an extensive research resource linking health information data sets at the patient level, including routinely collected data. This database holds information on each patient living within the NHSGGC Health Board area (n = 1.25 million), covering the west of Scotland. As part of routine clinical practice, disease codes are entered into electronic patient records, with Read codes generally being used in primary care and International Classification of Diseases, Tenth Revision (ICD-10) codes in secondary care. These codes, and associated notes, comments, or expansions are entered by clinicians on an ongoing basis at each patient consultation and can be subsequently retrieved electronically. The Safe Haven database also holds, inter alia, records of prescribed and dispensed medications; hospital admissions, and dates and places of other healthcare contacts; and laboratory results and medical imaging records. All information is held and linked by a unique identifier, the Community Health Index number.
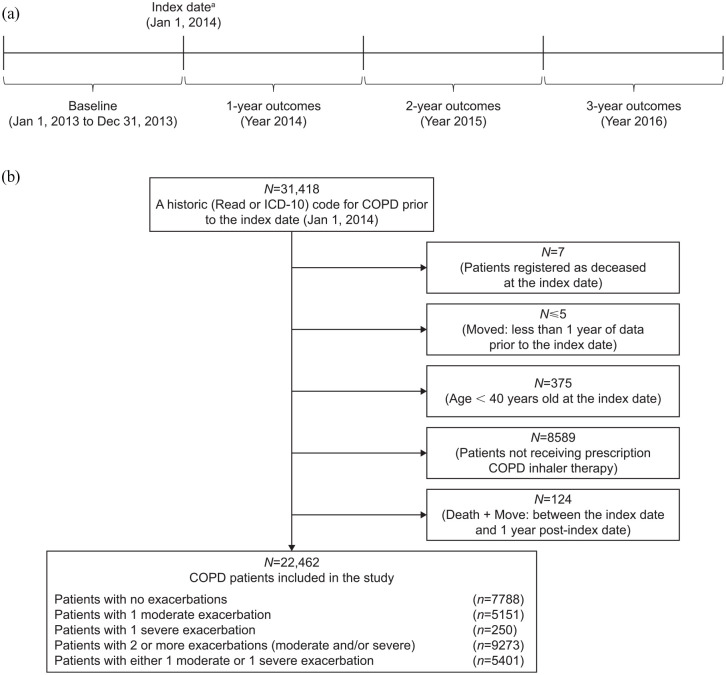
The study included a 1-year baseline period and a study period of up to 3 years (Figure 1(a)).
Figure 1.
(a) Study design and (b) patient disposition in the SHERLOCK study. COPD, chronic obstructive pulmonary disease; ICD-10, International Classification of Diseases, Tenth Revision; SHERLOCK, Study on HEalthcare Resource utiLization related to exacerbatiOns in patients with COPD.
aPatients were required to have a historic (Read or ICD-10) code for COPD before the index date and not be registered as deceased to be included in the study.
The 1-year baseline period (January 1 to December 31, 2013) was used to characterize patients’ exacerbation history. The index date was January 1, 2014. All patients were followed for a minimum of 12 months and a maximum of 3 years (until December 31, 2016, death, or migration outside of the geographical NHSGGC Health Board area).
As SHERLOCK was a retrospective, secondary database cohort study, patient informed consent was not required. Ethics review and approvals were obtained (NHSGGC Project Number: GSH/19/RM/002). Patient identifiable data were not released to investigators; data sets were linked in-house prior to releasing the full research data set in an anonymized format. The full data set was accessed remotely via NHSGGC Safe Haven by designated members of the Medical Statistics Team at the University of Aberdeen.
Study population
Participants in this study were ⩾40 years of age at the index date and identified by the presence of a Read or ICD-10 code for COPD (Supplemental Table 1). This method for identifying COPD has been validated as an accurate approach to patient identification from electronic medical records. 12 We also excluded participants without a prescription for COPD medication in the index year to minimize the risk of historically incorrect labeling. Thus, those included had a coded diagnosis of COPD and a prescription for COPD medication in the year before study entry.
Exacerbation history during the baseline period was recorded. Patients were stratified into four groups based on exacerbation history, in terms of both severity and frequency, during the baseline period: Group A (no exacerbations), Group B (1 moderate exacerbation only), Group C (1 severe exacerbation only), and Group D (⩾2 moderate or severe exacerbations). To provide an additional summary of these groups, we also combined patients of Groups B and C to represent patients who had a history of exactly one moderate or severe exacerbation, denoted here as Group B + C. Based on patient records, moderate COPD exacerbations were identified as those records indicating a course of oral corticosteroids and/or a prescription of a respiratory-related antibiotic (amoxicillin, clarithromycin, doxycycline, co-amoxiclav, etc.), and/or a visit to the emergency department with a primary diagnosis of any COPD-related event. Severe exacerbations were those associated with admission to hospital (general ward and/or inpatient care) in a patient with a primary diagnosis of any COPD-related event, as identified by Read codes (Supplemental Table 2).
Study outcomes and statistical analysis
The primary study outcomes assessed were moderate and/or severe exacerbations during follow-up.
Sample size calculations were based on estimates from two previous UK-based COPD cohorts.7,13 Based on these studies, it was estimated that in a total baseline population of approximately 25,000 patients, 19% of patients would have had no exacerbations (Group A), and 19.5% of patients would have had one moderate exacerbation within 12 months of follow-up (Group B). Under this assumption, the study had 90% power at the two-sided 5% significance level to detect a mean difference of at least 0.13 in the number of exacerbations in the first year of follow-up.
Patient demographics (sociodemographic and clinical characteristics, inhaler use, comorbid conditions, etc.) were extracted from records during the 1-year baseline period and summarized by exacerbation history using descriptive statistics.
The frequencies and rates of moderate or severe exacerbations were assessed at 12, 24, and 36 months of follow-up. A 14-day window was used to address the frequency of individual exacerbations, based on information suggesting that starting a new treatment with an antibiotic or systemic corticosteroid more than 14 days after the start of an exacerbation constitutes a new exacerbation. 14 If a moderate exacerbation (reference event) was identified, all subsequent moderate exacerbation events within a 14-day window of the reference event were considered part of the same moderate exacerbation event. If a severe exacerbation was identified, all moderate and/or severe events within a 14-day window were considered a single severe event.
Censored patients, defined as those who died during the follow-up period or moved out of the area covered by the NHSGGC Health Board, were included in the statistical models until the date they were censored. All unadjusted outcomes were tabulated by the exacerbationhistory group.
Adjusted rates of moderate and/or severe exacerbations were compared using a generalized linear model (GLM) with a negative binomial distribution and logarithmic link function. The single variable GLM was evaluated for each baseline covariate (sociodemographic and clinical characteristics and comorbidities), and baseline covariates that were significant at p < 0.10 were incorporated in the adjusted GLM. The final adjusted model included the following baseline sociodemographic and clinical characteristics, and comorbidities: sex, age, smoking status, cardiovascular disease, cerebrovascular disease, depression, and Medical Research Council dyspnea score; the model did not include any interaction terms. For both unadjusted and adjusted analyses, differences in exacerbation rates for Groups B, C, D, and B + C versus Group A (the reference group with no history of exacerbations) are presented as rate ratios (RRs) with 95% confidence intervals (CIs) at each year of follow-up. Missing data were not imputed for any of the analyses. For smoking status, a missing value category (or unknown category) was created to allow for its inclusion in the statistical models.
Results
Study population
Patient disposition is described in Figure 1(b). In total, 22,462 patients with a previous diagnosis code of COPD and a recent COPD-related prescription from a routine medical care database were included in the study and were followed up for 36 months; all included patients were assessed at 12, 24, and 36 months. Patient demographic and clinical characteristics by exacerbation history group are summarized in Table 1.
Table 1.
Sociodemographic and clinical characteristics by exacerbation history. a
| Group A (n = 7788) |
Group B (n = 5151) |
Group C (n = 250) |
Groups B + C (n = 5401) |
Group D (n = 9273) |
|
|---|---|---|---|---|---|
| Female, n (%) | 3947 (50.7) | 3023 (58.7) | 141 (56.4) | 3164 (58.6) | 6221(67.1) |
| Age, mean (SD), years | 66.0 (10.8) | 65.3 (10.6) | 70.9 (10.6) | 65.6 (10.7) | 65.5 (10.6) |
| Smoking status, n (%) | |||||
| Current | 3890 (49.9) | 2702 (52.5) | 127 (50.8) | 2829 (52.4) | 4737 (51.1) |
| Ex | 2620 (33.6) | 1771 (34.4) | 84 (33.6) | 1855 (34.3) | 3366 (36.3) |
| Never | 257 (3.3) | 134 (2.6) | 5 (2.0) | 139 (2.6) | 244 (2.6) |
| Missing | 1021 (13.1) | 544 (10.6) | 34 (13.6) | 578 (10.7) | 926 (10.0) |
| BMI, n
mean (SD), kg/m2 |
5639 27.3 (6.2) |
3734 27.5 (6.2) |
182 25.9 (7.0) |
3916 27.4 (6.2) |
6777 27.5 (6.2) |
| Primary-care visits | |||||
| Any, median number | 12 | 12 | 15 | 12 | 15 |
| (Q1, Q3) | (7, 17) | (8, 18) | (9, 20) | (8, 18) | (10, 21) |
| COPD-related, median number | 9 | 10 | 11 | 10 | 12 |
| (Q1, Q3) | (6, 13) | (6, 14) | (7, 15) | (6, 14) | (8, 17) |
| Oxygen therapy, b n (%) | |||||
| Yes | 22 (0.3) | 11 (0.2) | 1 (0.4) | 12 (0.2) | 62 (0.7) |
| MRC dyspnea score, n | 5051 | 3322 | 149 | 3471 | 6164 |
| Median | 2 | 2 | 3 | 2 | 3 |
| (Q1, Q3) | (2, 3) | (2, 3) | (2, 3) | (2, 3) | (2, 3) |
| Comorbidities, n (%) | |||||
| Cardiovascular disease c | 1917 (24.6) | 1294 (25.1) | 94 (37.6) | 1388 (25.7) | 2522 (27.2) |
| Cerebrovascular disease | 929 (11.9) | 660 (12.8) | 45 (18.0) | 705 (13.1) | 1194 (12.9) |
| Diabetes mellitus | 1218 (15.6) | 826 (16.0) | 54 (21.6) | 880 (16.3) | 1505 (16.2) |
| Depression | 1739 (22.3) | 1255 (24.4) | 49 (19.6) | 1304 (24.1) | 2697 (29.1) |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; MRC, Medical Research Council; Q, quartile; SD, standard deviation.
Exacerbation history group: A, no exacerbations; B, 1 moderate exacerbation only; C, 1 severe exacerbation only; D, ⩾2 moderate or severe exacerbations.
Based on information from the physician and pharmacy (prescribed oxygen cylinders); excludes data on concentrator from the nurse/home visit database.
Combined with heart failure.
In the overall population, mean (standard deviation [SD]) age was 65.7 (10.7) years old, and 59.4% of patients were female. The median (quartile 1, quartile 3) number of COPD-related physician consultations, which captured primary care visits only, was 11 (7, 15).
Exacerbation rates over the follow-up period
Rates of severe exacerbations (0.04 at 12 months, 0.05 at 24 months, and 0.07 at 36 months) were substantially lower than rates of moderate exacerbations (0.65 at 12 months, 0.70 at 24 months, and 0.76 at 36 months). The exacerbation rates and unadjusted RRs for each exacerbation history group versus Group A at 12, 24, and 36 months of follow-up are summarized in Supplemental Table 3.
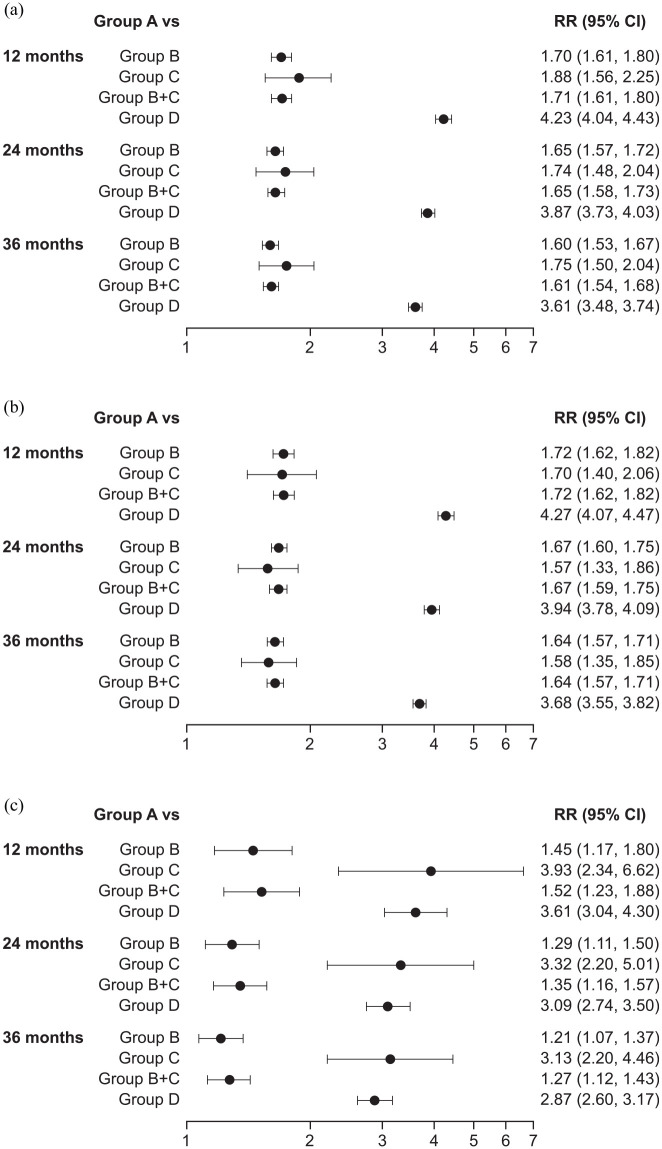
Adjusted RRs for each exacerbation history group versus Group A at 12, 24, and 36 months of follow-up are summarized in Figure 2. At 12 months of follow-up, adjusted RRs (95% CI) for moderate and/or severe exacerbations in Groups B, C, B + C, and D, versus Group A, respectively, were 1.70 (1.61, 1.80), 1.88 (1.56, 2.25), 1.71 (1.61, 1.80), and 4.23 (4.04, 4.43) (Figure 2(a)).
Figure 2.
Forest plot showing adjusteda RRs of (a) moderate and/or severe, (b) moderate, and (c) severe exacerbations by exacerbation historyb group at 12, 24, and 36 months of follow-up. RRs >1 indicate that patients with prior exacerbations have a greater event rate than patients with no prior exacerbations (Group A).
CI, confidence interval; MRC, Medical Research Council; RR, rate ratio.
aAdjusted for sex, age, smoking status, cardiovascular disease, cerebrovascular disease, depression, and MRC dyspnea score.
bExacerbation history group: A, no exacerbations; B, 1 moderate exacerbation only; C, 1 severe exacerbation only; D, ⩾2 moderate or severe exacerbations.
The relative rate of moderate exacerbations was highest in Group D versus Group A (RR 4.27 (4.07, 4.47); Figure 2(b)) and the relative rate of severe exacerbations was highest in Group C versus Group A (RR 3.93 (2.34, 6.62); Figure 2(c)).
At 24 months of follow-up, adjusted RRs for moderate and/or severe exacerbations for Groups B, C, B + C, and D, versus Group A, respectively, were 1.65 (1.57, 1.72), 1.74 (1.48, 2.04), 1.65 (1.58, 1.73), and 3.87 (3.73, 4.03) (Figure 2(a)). The relative rate of moderate exacerbations was highest in Group D versus Group A (RR 3.94 (3.78, 4.09); Figure 2(b)) and the relative rate of severe exacerbations was greatest in Group C versus Group A (3.32 (2.20, 5.01); Figure 2(c)).
At 36 months of follow-up, an increased rate of exacerbations relative to Group A continued to be observed. Adjusted RRs at 36 months of follow-up for moderate and/or severe exacerbations for Groups B, C, B + C, and D, respectively, versus Group A were 1.60 (1.53, 1.67), 1.75 (1.50, 2.04), 1.61 (1.54, 1.68), and 3.61 (3.48, 3.74) (Figure 2(a)). The adjusted RR for moderate exacerbations was highest for Group D versus Group A (3.68 (3.55, 3.82): Figure 2(b)) and the adjusted RR for severe exacerbations was highest for Group C versus Group A (3.13 (2.20, 4.46): Figure 2(c)).
Discussion
The SHERLOCK study provides insight into the relationship between a recent history of exacerbations, in terms of both frequency and severity, and patterns of future exacerbations over 36 months in patients with COPD. The key findings demonstrate that a recent history of any exacerbation was associated with an increased rate of future exacerbations at 12, 24, and up to 36 months of follow-up, relative to having no prior exacerbations within 1 year of the index date. Having multiple prior exacerbations (Group D) was associated with the greatest increased rate of future exacerbations, with adjusted RRs ranging from 3.61 to 4.23 for moderate and/or severe exacerbations over 12–36 months of follow-up (3.68–4.27 for moderate exacerbations; 2.87–3.61 for severe exacerbations) relative to having no exacerbations. Perhaps most importantly, even a single moderate exacerbation in the previous year was associated with an increased rate of both future moderate and severe exacerbations over 36 months of follow-up, compared with no prior exacerbations.
The data from 36 months of follow-up in this study support previously reported data and extend our understanding of the impact of recent prior exacerbations on future exacerbation risk. Previous observational, longitudinal, and post hoc studies have shown that exacerbation history and disease severity are key predictors of future exacerbation events over varying time periods over 3 years or 10 years of follow-up.2,6,7 For example, Rothnie et al. 7 reported adjusted hazard ratios (relative to patients with no prior exacerbations) for future moderate exacerbations of 2.35 in patients with two prior moderate exacerbations to 5.50 in patients with ⩾5 prior moderate exacerbations. Furthermore, hazard ratios (relative to patients with no prior exacerbations) were 3.27 and 3.69, respectively, for future moderate exacerbations and future severe exacerbations among patients with ⩾1 prior severe exacerbation. 7 However, the severe exacerbation history group included patients who had experienced ⩾1 severe exacerbations and any number of moderate exacerbations. With the observation of increased rates of future exacerbations among those who had experienced a single exacerbation, either moderate or severe, SHERLOCK expands upon the existing literature.
The rate per person per year of moderate and/or severe exacerbations ranged from 0.69 at the 12-month follow-up to 0.83 at the 36-month follow-up among patients with no prior exacerbations in this study, with moderate exacerbations occurring at a higher rate than severe exacerbations, as expected. These findings are generally consistent with other published data that have shown a relatively high frequency of exacerbations after 3 years of follow-up. For example, in a subgroup analysis of the SPIROMICS study, 48.7% of patients experienced ⩾1 exacerbation during the 3-year follow-up. 6 The SHERLOCK study emphasizes the progression of COPD with time and the high percentage of patients with COPD who experience exacerbations over time.
It is known that COPD exacerbations are associated with a worse prognosis, including a decline in lung function, increased mortality, and increased healthcare resource utilization and costs.5,7,15–17 Therefore, early diagnosis and effective treatment of COPD and management of exacerbations are key to limiting future exacerbation events to improve patient outcomes and reduce healthcare resource utilization and associated costs.5,7,8 The risk of death from COPD has been shown to increase with the frequency of moderate exacerbations. 7 The SHERLOCK study has shown that having a history of a single moderate or severe exacerbation increases the rate for subsequent exacerbations. Future studies should further explore the risk of mortality among patients who have only experienced a single moderate or severe COPD exacerbation.
The management and prevention of exacerbations generally focus on patients who experience frequent or severe exacerbations.9,10 but there is an increasing awareness that patients who experience infrequent or moderate exacerbations may have an increased risk of future exacerbations and should be monitored.2,7 Although not specifically explored in this study, it should be recognized that strategies to prevent exacerbations should include all patients, particularly those identified through increasing use of a short-acting bronchodilator (‘mild exacerbations’) because these patients may also encounter disease progression. The importance of mild exacerbations as a proxy for symptomatic breakthrough and as a factor influencing disease progression should be recognized and considered to expand on this study’s findings through future randomized controlled trials and observational studies.
Strengths and limitations
The main strengths of the SHERLOCK study include its large patient population, which was entirely inclusive of the geographical area covered by the NHSGGC Health Board, and the use of relatively few exclusion criteria, making the findings generalizable to the broader real-world population of patients with COPD in both primary and specialty care. The study’s primary limitations are those inherent to retrospective studies and observational studies that utilize a routine clinical practice database rather than academic research use. These include being reliant on a large and disparate group of clinicians to diagnose and record data accurately, incomplete or missing data (particularly for home visits and out-of-hours care), and the inability to extract data in an electronic form, such as spirometry data. For the spirometry data, only a small proportion of individual-level data was electronically retrievable. The lack of standardized, accurate, and electronically retrievable spirometry data within the electronic health records setting has been previously reported. 18 Nevertheless, using diagnostic codes to identify COPD patients from electronic medical records is a validated approach, and the addition of COPD medications and spirometry data improves accuracy marginally. 12 It should also be noted that this study did not control for lung function because spirometry data could be sparse.
In this study, Group C (i.e. those experiencing 1 severe exacerbation) was slightly older and a greater percentage had cardiovascular disease, cerebrovascular disease, and diabetes than the other treatment groups. However, due to the small sample size of patients in this group (n = 250), baseline characteristics may be skewed. The percentage of never smokers was lower than might be expected in a population of patients with COPD (ranging from approximately 2%–3%) and may include some mislabeling and/or misreporting.
Conclusion
The SHERLOCK study demonstrates that a history of even 1 recent moderate or severe exacerbation within the previous year increases the rate of subsequent exacerbations, compared with not having a recent prior exacerbation at 12 months and for up to 36 months, emphasizing the importance of early identification and management of COPD to minimize the impact of exacerbations. These findings indicate that having a history of multiple exacerbations was associated with the greatest increases in the rate of future exacerbations. When considered together, these data further emphasize the importance of early identification of those at risk for exacerbations to prevent further exacerbations and their associated negative clinical and socioeconomic consequences. An appropriate management approach can be identified to improve patient outcomes and reduce hospitalizations by reviewing recent exacerbation history to ascertain future exacerbation risk.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666211070139 for The long-term clinical impact of COPD exacerbations: a 3-year observational study (SHERLOCK) by John Haughney, Amanda J. Lee, Mintu Nath, Hana Müllerová, Ulf Holmgren, Enrico de Nigris and Bo Ding in Therapeutic Advances in Respiratory Disease
Acknowledgments
Medical writing support, under the direction of the authors, was provided by Sara Cameron, M. Phil., of CMC Connect, McCann Health Medical Communications, funded by AstraZeneca in accordance with Good Publication Practice (GPP3) guidelines. 19
Footnotes
Author contributions: All authors were involved in the design and conduct of the study, and in the interpretation of the data. All authors were involved in the writing of the manuscript and the final decision to submit to Therapeutic Advances in Respiratory Disease. The authors CRediT (Contributor Roles Taxonomy) statements are as follows:
John Haughney: Taxonomy: Conceptualization, Methodology, Writing – review & editing.
Amanda J Lee: Methodology, Formal Analysis, Investigation, Writing – review & editing.
Mintu Nath: Methodology, Formal Analysis, Investigation, Writing – review & editing.
Hana Müllerová: Conceptualization, Methodology, Writing – review & editing.
Ulf Holmgren: Conceptualization, Methodology, Writing – review & editing.
Enrico de Nigris: Conceptualization, Methodology, Writing – review & editing.
Bo Ding: Conceptualization, Methodology, Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JH reports personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, Circassia, and Teva, unrelated to the conduct of the study. AJL and MN have no conflicts to disclose. HM and UH are employees of AstraZeneca and hold stock and/or stock options in the company. BD is an employee of AstraZeneca. EDN is a former employee of AstraZeneca.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by AstraZeneca. Employees of AstraZeneca were involved in the design of the study, interpretation of the data (but not the data collection), in the writing of the report, and in the decision to submit the article for publication.
Previous presentation: These data were presented at the 2020 European Respiratory Society International VirtualCongress.
ORCID iD: John Haughney  https://orcid.org/0000-0002-6809-6964
https://orcid.org/0000-0002-6809-6964
Data availability: Remote access to this data set was provided to the study statisticians via Safe Haven. Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data are not available.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
John Haughney, Glasgow Clinical Research Facility, Queen Elizabeth University Hospital, 1345 Govan Road, Glasgow G51 4TF, UK.
Amanda J. Lee, Medical Statistics Team, University of Aberdeen, Aberdeen, UK
Mintu Nath, Medical Statistics Team, University of Aberdeen, Aberdeen, UK.
Hana Müllerová, AstraZeneca, Cambridge, UK.
Ulf Holmgren, AstraZeneca, Gothenburg, Sweden.
Enrico de Nigris, Formerly of AstraZeneca, Cambridge, UK.
Bo Ding, AstraZeneca, Gothenburg, Sweden.
References
- 1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2021 report. Portland, OR: Global Initiative for Chronic Obstructive Lung Disease, 2021. [Google Scholar]
- 2. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363: 1128–1138. [DOI] [PubMed] [Google Scholar]
- 3. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012; 67: 957–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Respiratory Society. European lung white book. Lausanne: European Respiratory Society, 2013. [Google Scholar]
- 5. Punekar YS, Shukla A, Müllerova H. COPD management costs according to the frequency of COPD exacerbations in UK primary care. Int J Chron Obstruct Pulmon Dis 2014; 9: 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rothnie KJ, Müllerová H, Smeeth L, et al. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2018; 198: 464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis 2020; 15: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone PW, Hickman K, Steiner MC, et al. Predictors of referral to pulmonary rehabilitation from UK primary care. Int J Chron Obstruct Pulmon Dis 2020; 15: 2941–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schermer T, Smeenk F, van Weel C. Referral and consultation in asthma and COPD: an exploration of pulmonologists’ views. Neth J Med 2003; 61: 71–81. [PubMed] [Google Scholar]
- 11. Wilkinson TMA, Donaldson GC, Hurst JR, et al. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169: 1298–1303. [DOI] [PubMed] [Google Scholar]
- 12. Quint JK, Müllerová H, DiSantostefano RL, et al. . Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open 2014; 4: e005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vestbo J, Leather D, Diar Bakerly N, et al. Effectiveness of fluticasone furoate–vilanterol for COPD in clinical practice. N Engl J Med 2016; 375: 1253–1260. [DOI] [PubMed] [Google Scholar]
- 14. Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J 2003; 21 (41 suppl): 46s–53s. [DOI] [PubMed] [Google Scholar]
- 15. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerkhof M, Voorham J, Dorinsky P, et al. Association between COPD exacerbations and lung function decline during maintenance therapy. Thorax 2020; 75: 744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; 195: 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harries TH, White P. Spotlight on primary care management of COPD: electronic health records. London: SAGE, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015; 163: 461–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666211070139 for The long-term clinical impact of COPD exacerbations: a 3-year observational study (SHERLOCK) by John Haughney, Amanda J. Lee, Mintu Nath, Hana Müllerová, Ulf Holmgren, Enrico de Nigris and Bo Ding in Therapeutic Advances in Respiratory Disease