Abstract
Background:
The effectiveness of ustekinumab in patients with refractory Crohn’s disease (CD) has been investigated in several real-world studies. However, very few data concerning the real-life experience in Italy have been reported. Therefore, this study assessed the effectiveness of ustekinumab in a large cohort of Italian patients with refractory CD.
Methods:
All patients who had started on ustekinumab after failure of or intolerance to antitumour necrosis factor-α (TNF-α) treatment at five tertiary centres between November 2018 and February 2020 were retrospectively enrolled. The coprimary outcome was corticosteroid-free clinical remission, defined as a Harvey–Bradshaw Index (HBI) score of ⩽4, at weeks 26 and 52. The secondary outcomes were changes in the HBI and C-reactive protein (CRP) values at weeks 8, 26, and 52 from baseline and the normalization of CRP in patients with initially abnormal values.
Results:
Totally, 140 patients who had previously received at least one anti-TNF-α agent were enrolled; 40.0% received two anti-TNF-α agents and 20.0% received vedolizumab. At baseline, 108 patients (77.1%) had HBI scores of >4; of these, 56.5% and 58.3% achieved corticosteroid-free clinical remission at weeks 26 and 52, respectively. Significant decreases in HBI and CRP values were observed at weeks 8, 26, and 52 in the entire study cohort (all p < 0.0001). The CRP values were normalized in 34.9%, 37.8%, and 49.3% of the patients by weeks 8, 26, and 52, respectively. The baseline HBI score of ⩾8 was a negative predictor of corticosteroid-free clinical remission at week 52 (odds ratio: 0.21, 95% confidence interval: 0.08–0.56, p = 0.002). The probability of remaining on ustekinumab after 52 weeks was 92.1%. Eleven (7.9%) patients discontinued ustekinumab (three for adverse events).
Conclusion:
Our study findings confirm the effectiveness and safety of ustekinumab in patients with CD after failure of or intolerance to anti-TNF-α therapy.
Keywords: Crohn’s disease, effectiveness, refractory, ustekinumab
Introduction
Crohn’s disease (CD) is a chronic relapsing disease resulting from uncontrolled inflammation of the gastrointestinal tract. Although several genetic, environmental, gut bacterial, and immune factors are thought to have a role in the development of CD, the aetiology and pathogenesis of this disease remain unknown. 1
The introduction of tumour necrosis factor-alpha (TNF-α)-targeting biological agents, such as infliximab and adalimumab, has transformed the management of patients with moderately to severely active CD.2 –4 Patients refractory to conventional treatments have been shown to achieve clinical remission with anti-TNF-α agents; however, a proportion of patients are either primary nonresponders, lose their response over time, or are intolerant to these agents,5,6 and are thus a challenging group to treat.
Ustekinumab is a fully human monoclonal antibody that targets the p40 subunit of interleukin-12 and interleukin-23, inhibiting their receptor binding on CD4+ T lymphocytes, antigen-presenting cells, and natural killer cells. 7 The efficacy of ustekinumab in inducing and maintaining remission in patients with CD was demonstrated in the randomized, placebo-controlled Phase II CERTIFI clinical study and in the Phase III UNITI-1, UNITI-2, and IM-UNITI clinical trials.8,9 Based on the results of these trials, ustekinumab has been approved by the European Medicines Agency and US Food and Drug Administration since 2016 for the treatment of adult patients with moderate-to-severe CD who have had an inadequate response, lost their response, or been found to be intolerant to conventional therapy or TNF-α inhibitors.10,11 The drug was approved by the Italian Medicines Agency in September 2018. 12
Several real-world studies have confirmed the effectiveness and safety of ustekinumab in patients with CD after failure of or intolerance to anti-TNF-α therapy.13 –31 However, very few data concerning the Italian real-life experience using ustekinumab for the treatment of CD have been reported.22,24 Moreover, because of the relatively recent regulatory approval of ustekinumab, some studies either did not include the intravenous (IV) induction regimen or evaluated patients for short periods of time.13 –17,22,26
Therefore, the aim of this study was to assess the effectiveness, persistence with treatment, and safety of ustekinumab in a real-life multicentre cohort of Italian patients with CD who had failed to respond or were intolerant to anti-TNF-α agents. We also evaluated the impact of ustekinumab on extraintestinal manifestations (EIMs) and active perianal disease and sought to identify potential predictors of its effectiveness.
Methods
Study design and patient population
This retrospective, observational multicentre study was designed to assess the effectiveness and safety of ustekinumab in a cohort of consecutive adult patients with a confirmed diagnosis of CD who had failed to respond or were intolerant to at least one anti-TNF-α agent and were treated in a routine clinical setting. The study was conducted at five tertiary centres in Italy that provide care for patients with inflammatory bowel disease (IBD).
The study inclusion criteria were as follows: age ⩾18 years; diagnosis of CD according to the criteria of the European Crohn’s and Colitis Organisation; 32 previous exposure to at least one anti-TNF-α agent; treatment with ustekinumab for active disease after failure of or intolerance to anti-TNF-α therapy; and a minimum follow-up duration of 8 weeks after the induction infusion.
All patients underwent standard induction with an IV infusion of ustekinumab at baseline at a dose based on weight ranges (<55 kg, 260 mg; 55–85 kg, 390 mg; >85 kg, 520 mg), followed by subcutaneous administration of 90 mg after 8 weeks and subsequent subcutaneous treatment with 90 mg every 8 or 12 weeks at the discretion of the treating physician, according to the approved product label. The dose could be escalated every 8 weeks in patients with a loss of response to ustekinumab maintenance therapy.
Data collection
The following baseline patient data were extracted from the clinical records and entered into a common database: sex, age at diagnosis, age at the start of ustekinumab therapy, body weight, duration of disease, smoking status, location, and behaviour of CD at the time of inclusion in the study according to the Montreal classification, 33 perianal disease, EIMs, history of surgery for CD, previous exposure to an anti-TNF-α agent, previous exposure to vedolizumab, previous use of immunomodulators (thiopurines or methotrexate), concomitant use of corticosteroids, concomitant use of immunomodulators, reason for suspension of anti-TNF-α therapy (primary failure, secondary failure, or intolerance), clinical disease activity indicated by the Harvey–Bradshaw Index (HBI) score, 34 and objective disease activity indicated by the C-reactive protein (CRP) level and endoscopic activity. Follow-up data were collected at weeks 8, 26, and 52 and included a change, if any, in the ustekinumab dosing interval, the use of concomitant corticosteroid and immunomodulator therapy, the HBI score, CRP level, and reason for discontinuation, if any. Information on EIMs and perianal disease was also collected at week 52. Data on persistence with ustekinumab therapy and adverse events (AEs) that appeared during follow-up were also recorded. We have de-identified all patient details so that their identity may not be ascertained in any way.
Outcomes and definitions
The co-primary outcome of the study was the corticosteroid-free clinical remission at weeks 26 and 52. The secondary outcomes were changes in the HBI score and CRP level and the normalization of the CRP level at weeks 8, 26, and 52 in patients with initially abnormal values. Additional outcomes included corticosteroid-free clinical remission at week 8 and the corticosteroid-free clinical response at weeks 8, 26, and 52. Persistence with treatment was also assessed. The impact of ustekinumab on EIMs and perianal disease was evaluated at week 52 in patients with these problems at baseline. Potential predictors of corticosteroid-free clinical remission at week 52 were also investigated.
Active disease at baseline was defined as an HBI score of >4. In patients with the baseline HBI score of ⩽4, active disease was defined as an increased CRP level (>0.5 mg/dl) and/or endoscopic activity at the start of ustekinumab therapy. Endoscopic activity was graded as mild, moderate, or severe according to the Simple Endoscopic Score for Crohn’s Disease. 35 Corticosteroid-free clinical remission was defined as an HBI score of ⩽4, and a corticosteroid-free clinical response was defined as a reduction in the HBI score of ⩾3 compared with that at baseline without the use of corticosteroids. Objective remission was defined as the normalization of the CRP level (to ⩽0.5 mg/dl) in patients with abnormal values at baseline (when available).
The EIMs evaluated were peripheral and/or axial spondyloarthropathy, erythema nodosum, pyoderma gangrenosum, iritis, and uveitis. Improvement or resolution of EIMs was assessed by the treating physician.16,18 Remission of perianal disease was defined as the resolution of all draining fistulas on physical examination. Improvement in perianal disease was defined as a reduction of at least 50% in the number of draining fistulas or a decrease in drainage from the fistula.18,36
The safety of ustekinumab was assessed, and all AEs in patients who received at least one dose of ustekinumab are reported. The evaluation of corticosteroid-free clinical remission and the response did not include patients with an HBI score of ⩽4 at baseline, who were analysed separately. All other outcomes were assessed in all patients.
Patients were deemed to have failed on ustekinumab if they experienced primary failure, defined as the lack of clinical improvement after the induction phase and subsequent discontinuation of therapy, or secondary failure, defined as a loss of response in an initially responding patient. Intolerance was defined as suspension of ustekinumab due to any drug-related AE.
Ethical considerations
The study protocol was approved by the Ethics Committee (Comitato Etico Lazio 1) of the coordinating centre (Gastroenterology Unit, A.O. San Camillo-Forlanini, Rome) on 5 May 2021 (protocol number 695/CE Lazio1). The need for informed consent was waived in view of the retrospective nature of the study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, as reflected by an a priori approval by the institutional human research committee.
Statistical analysis
Patients were analysed on an intention-to-treat basis. Descriptive statistics were obtained using the median and interquartile range (IQR) for continuous variables and proportions for dichotomous and categorical variables. The Kaplan–Meier method was used to plot the probability of persistence on ustekinumab therapy considering the time from the start of treatment up to 52 weeks or to discontinuation of therapy for any reason, or until loss to follow-up. The Wilcoxon signed-rank test was used to compare continuous variables at different time points during follow-up. A multivariable logistic regression model was built to identify independent predictors of corticosteroid-free clinical remission at week 52 considering patients who had an HBI score of >4 at baseline. Each variable was first examined by univariable analysis using Pearson’s chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Variables were then included in the model when the p value was <0.25 or when they were considered relevant to the outcome based on expert opinion. The final model was chosen using the Akaike information criterion. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All analyses were performed using STATA version 16.0 (StataCorp LLC, College Station, TX, USA). A two-sided p value of <0.05 was considered statistically significant.
The reporting of this study conforms to the STROBE statement. 37
Results
Study population
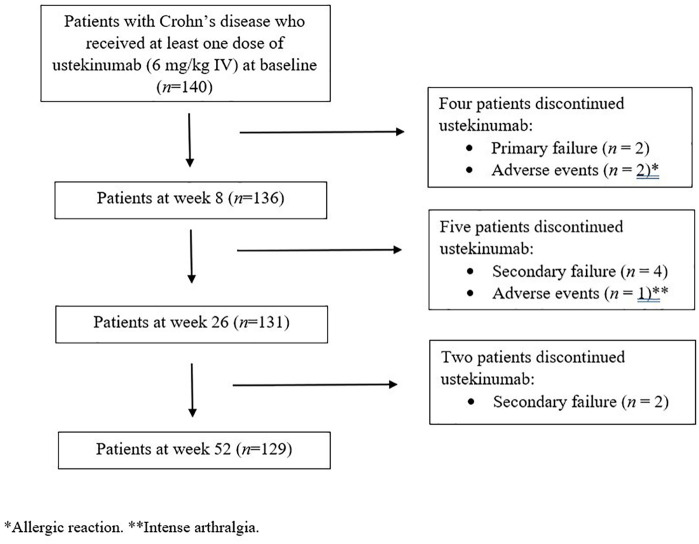
A database search identified 140 consecutive patients who had been started on ustekinumab for active refractory CD at any of the IBD tertiary centres in Italy between November 2018 and February 2020, and all of the patients were included in the study (Figure 1). The demographic and clinical characteristics of the overall population at baseline are presented in Table 1. All patients had previously been exposed to at least one anti-TNF-α agent; 40.0% received two anti-TNF-α agents and 20.0% received vedolizumab.
Figure 1.
Flowchart of patient progress through the study.
*Allergic reaction. **Intense arthralgia.
Table 1.
Demographic and clinical characteristics of the study population.
| Baseline characteristics | Total patients (n = 140) |
|---|---|
| Male sex, n (%) | 72 (51.4) |
| Age (years), median (IQR) | 45.0 (36.3–54.0) |
| Age at diagnosis (years), median (IQR) | 26.0 (19.0–37.0) |
| Current smoker, n (%) | 50 (35.7) |
| Disease duration (years), median (IQR) | 16.0 (8.0–22.0) |
| Disease location, n (%) | |
| Ileal | 54 (38.6) |
| Colonic | 16 (11.4) |
| Ileocolonic | 70 (50.0) |
| Upper gastrointestinal involvement, n (%) | 10 (7.1) |
| Disease behaviour, n (%) | |
| Nonstricturing/nonpenetrating | 51 (50.7) |
| Stricturing | 56 (31.0) |
| Penetrating | 33 (18.3) |
| Perianal disease, n (%) | 47 (33.6) |
| Extraintestinal manifestations, n (%) | |
| Peripheral arthropathy | 40 (28.5) |
| Axial arthropathy | 4 (2.9) |
| Axial and peripheral arthropathy | 4 (2.9) |
| Psoriatic arthritis | 2 (1.4) |
| Psoriasis | 2 (1.4) |
| Erythema nodosum | 4 (2.9) |
| Ocular manifestations | 0 |
| Previous intestinal resection, n (%) | 86 (61.4) |
| Previous exposure to anti-TNF-α therapy, n (%) | |
| 1 | 140 (100) |
| 2 | 56 (40.0) |
| Reason for anti-TNF-α suspension, n (%) | |
| Primary failure | 17 (12.2) |
| Secondary failure | 92 (65.7) |
| Intolerance | 31 (22.1) |
| Previous exposure to vedolizumab, n (%) | 28 (20.0) |
| Previous immunomodulators (thiopurine/methotrexate), n (%) | 95 (67.9) |
| Concomitant medications, n (%) | |
| Corticosteroids | 22 (15.7) |
| Immunomodulators (thiopurine/methotrexate) | 12 (8.6) |
| Harvey–Bradshaw Index, median (IQR) | 6 (5.0–9.0) |
| C-reactive protein (mg/dl), median (IQR)* | 2.0 (1.0–4.9) |
IQR, interquartile range; TNF-α, tumour necrosis factor-α.
Available for 119 patients (85%).
All patients received the standard induction dose of ustekinumab (6 mg/kg IV); the weight-based doses were 260 mg in 34 patients, 390 mg in 92 patients, and 520 mg in 14 patients. A total of 126 patients (90%) received a maintenance subcutaneous dose of 90 mg every 8 weeks, and 14 patients (10%) received the maintenance dose every 12 weeks. During the 52 weeks of follow-up, the dose was escalated every 8 weeks in one patient (7.1%). At the discretion of the treating physician, the dosing interval was extended from every 8 weeks to every 12 weeks in 36 patients (28.6%) who were in corticosteroid-free clinical remission.
Clinical effectiveness of ustekinumab
All patients were treated for active disease according to the definition. In total, 108 patients (77.1%) had an HBI score of >4 at baseline. The remaining 32 patients (22.9%) had an HBI score of ⩽4 with elevated CRP and/or endoscopic activity when started on ustekinumab; the CRP level was elevated in 18 (56.3%) of these patients at baseline. Endoscopy was performed at the beginning of treatment in 20 (62.5%) of these 32 patients and showed severe activity in 14 and moderate activity in 6 patients.
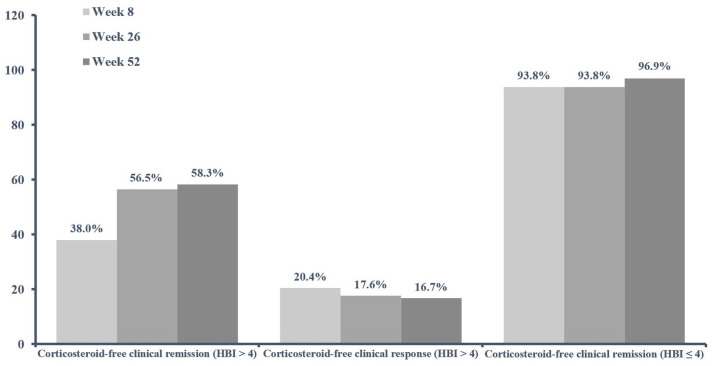
The corticosteroid-free clinical remission rate was 56.5% (61/108) and 58.3% (63/108) at weeks 26 and 52, respectively, in the patients with an HBI score of >4 at baseline (Figure 2). Furthermore, corticosteroid-free clinical remission was achieved by 38.0% (41/108) of patients at week 8 (Figure 2). A corticosteroid-free clinical response was obtained in 20.4% (22/108), 17.6% (19/108), 16.7% (18/108) of patients at weeks 8, 26, and 52, respectively (Figure 2). No difference concerning corticosteroid-free clinical remission and response was observed between the patients receiving a maintenance subcutaneous dose of 90 mg every 8 weeks than those treated with the maintenance dose every 12 weeks.
Figure 2.
Clinical effectiveness of ustekinumab at weeks 8, 26, and 52. Corticosteroid-free clinical remission is shown for the cohorts of patients with HBI scores of >4 (108/140, 77.1%) and ⩽4 (32/140, 22.9%) at baseline. A corticosteroid-free clinical response is only shown for the cohort of patients with an HBI score of >4 at baseline.
In the group of patients with an HBI score of ⩽4 at baseline, 93.8% (30/32), 93.8% (30/32), and 96.9% (31/32) were in corticosteroid-free clinical remission at weeks 8, 26, and 52, respectively (Figure 2). Significant decreases in the median HBI score were observed at weeks 8, 26, and 52 in the entire study cohort (all p < 0.0001; Table 2), which was more pronounced in the first weeks of treatment.
Table 2.
Changes in the Harvey–Bradshaw Index scores and C-reactive protein levels over time.
| Variable | Median (IQR) | Mean (SD) | p value versus baseline* |
|---|---|---|---|
| Harvey–Bradshaw Index score | |||
| Baseline, n = 140 | 6.0 (5.0–9.0) | 6.9 (3.4) | |
| Week 8, n = 136 | 4.0 (2.0–6.0) | 4.2 (3.3) | <0.0001 |
| Week 26, n = 131 | 3.0 (2.0–5.0) | 3.5 (2.9) | <0.0001 |
| Week 52, n = 129 | 3.0 (2.0–5.0) | 3.4 (2.3) | <0.0001 |
| C-reactive protein (mg/dl) | |||
| Baseline, n = 119 | 2.0 (1.0–4.9) | 4.1 (6.0) | |
| Week 8, n = 110 | 0.5 (0.3–2.2) | 2.3 (5.2) | <0.0001 |
| Week 26, n = 95 | 0.5 (0.3–1.5) | 1.7 (3.2) | <0.0001 |
| Week 52, n = 87 | 0.4 (0.2–1.0) | 1.2 (2.3) | <0.0001 |
IQR, interquartile range; SD, standard deviation.
Wilcoxon matched-pairs signed-rank test.
Of the 22 patients on corticosteroid therapy at baseline, only 1 of the remaining 20 patients (5%) was still on corticosteroids at weeks 8 and 26, and none of the patients was on corticosteroids at week 52. Of the 118 patients who were not receiving corticosteroids at baseline, 1.7% (2/116), 3.7% (4/109), and 3.7% (4/109) of the remaining patients required corticosteroid therapy at weeks 8, 26, and 52, respectively, to restore or achieve clinical remission.
Biochemical effectiveness of ustekinumab
Significant decreases in the median CRP level were reported between baseline and weeks 8, 26, and 52 in the group of 119 patients (85%) in whom the CRP level was available at baseline (p < 0.0001; Table 2). The decrease was more pronounced between baseline and week 26 than between weeks 26 and 52. At baseline, the majority of patients (95/119, 79.8%) had a high CRP level, which was normalized in 34.9%, 37.8%, and 49.3% of the patients at weeks 8, 26, and 52, respectively. A high CRP level was observed in 56.3% of the patients with an HBI score of ⩽4 at baseline (median, 1.3 mg/dl, IQR 0.4–4.0). C-reactive protein values decreased from baseline to 0.9 mg/dl at week 8 (IQR 0.3–2.5, p = 0.0013), 1.0 mg/dl at week 26 (IQR 0.2–0.5, p = 0.0140), and 1.0 mg/dl at week 52 (IQR 0.2–0.9, p = 0.0259).
Persistence with treatment and predictors of corticosteroid-free clinical remission
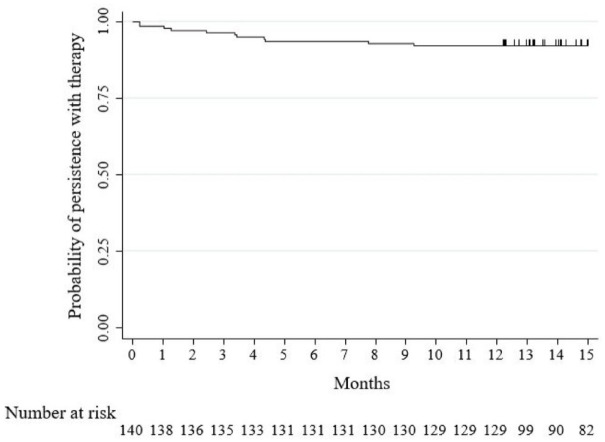
The cumulative probability of persistence with ustekinumab was 97.1% (95% CI: 92.6–98.9) at 8 weeks, 93.6% (95% CI: 88.0–96.6) at 26 weeks, and 92.1% (95% CI: 86.3–95.6) at 52 weeks (Figure 3). Ustekinumab was stopped in 11 patients (7.9%) within 52 weeks. The reasons for discontinuation were primary failure in two (18.2%), secondary failure in six (54.5%), and AEs in three (27.3%) patients (Figure 1).
Figure 3.
Kaplan–Meier survival curve showing persistence with ustekinumab therapy in the study population.
Univariable and multivariable analyses of the group of patients with an HBI score of >4 at baseline identified only a score of ⩾8 to be an independent predictor of a lower likelihood of corticosteroid-free clinical remission at week 52 (adjusted OR: 0.21, 95% CI: 0.08–0.56, p = 0.002). The patient’s age of >40 years at baseline showed a numerical trend, but the data did not reach statistical significance (adjusted OR: 0.39, 95% CI: 0.14–1.10, p = 0.07; Table 3 and Supplementary Table 1).
Table 3.
Multivariable analysis for potential predictors of corticosteroid-free clinical remission at week 52.
| Variable | Odds ratio | 95% Confidence interval | p value |
|---|---|---|---|
| Sex | |||
| Female | Ref. | ||
| Male | 1.20 | 0.46–3.09 | 0.71 |
| Age at baseline, years | |||
| ⩽40 | Ref. | ||
| >40 | 0.39 | 0.14–1.10 | 0.07 |
| Disease location | |||
| Ileal | Ref. | ||
| Colonic | 1.73 | 0.31–9.69 | 0.53 |
| Ileocolonic | 0.82 | 0.26–2.62 | 0.74 |
| Disease behaviour | |||
| Nonstricturing/nonpenetrating | Ref. | ||
| Stricturing | 0.65 | 0.19–2.20 | 0.49 |
| Penetrating | 0.44 | 1.12–1.68 | 0.23 |
| Clinical disease activity at baseline | |||
| Mild (HBI score 5–7) | Ref. | ||
| Moderate–severe (HBI score ⩾8) | 0.21 | 0.08–0.56 | 0.002 |
HBI, Harvey–Bradshaw Index.
Bold indicates a significant value.
Effectiveness of ustekinumab for extraintestinal manifestations and perianal disease
All patients were treated with ustekinumab for luminal disease activity and not for EIMs or perianal disease. At baseline, 56 patients (40.0%) had concomitant EIMs (Table 1). No ocular manifestations were observed.
In the subgroup of patients with peripheral arthropathy, the rates of complete symptom resolution and improvement were 20.0% (7/35) and 51.4% (18/35), respectively, at week 52. Only one patient reported an exacerbation of peripheral arthropathy, which resulted in discontinuation of ustekinumab after 8 weeks. At 52 weeks, 50% of patients with axial arthropathy, axial and peripheral arthropathy, or psoriatic arthritis showed symptomatic improvement. Furthermore, complete resolution was observed in one patient with axial arthropathy, all patients with psoriasis, and all patients with erythema nodosum. No de novo EIMs were observed.
Overall, 47 patients (33.6%) had concomitant perianal disease at baseline (Table 1). The percentage of patients with one or more active perianal fistulas was 12.1% (17/140). At week 52, complete clinical resolution of all perianal fistulas was reported by 35.3% (6/17) of the remaining patients, while 23.5% (4/17) achieved improvement. One patient underwent surgery (fistulectomy) owing to an exacerbation of perianal disease during follow-up. No other patients experienced worsening or de novo development of perianal disease.
Safety profile of ustekinumab
The entire cohort of 140 patients was included in the safety analysis. Ten AEs were reported in nine patients (6.4%), three of which led to treatment suspension (Table 4). Two patients discontinued treatment because of an allergic reaction to the IV ustekinumab infusion, and one discontinued treatment because of an exacerbation of peripheral arthropathy. Six patients (4.3%) underwent surgery for CD during the study period (definitive ileostomy, n = 3; intestinal resection, n = 2; and perianal fistula, n = 1. Patients who received a maintenance subcutaneous dose of 90 mg every 8 weeks (90%) did not experience more AEs than those treated with the maintenance dose every 12 weeks (10%). No infection was observed.
Table 4.
Adverse events during ustekinumab therapy.
| Adverse event | n |
|---|---|
| Exacerbation of peripheral arthropathy | 1 |
| Allergic reaction to intravenous ustekinumab infusion | 2 |
| Skin reactions | 3 |
| Renal colic | 2 |
| Intestinal obstruction | 1 |
| Headache | 1 |
Discussion
This study evaluated the effectiveness and safety of ustekinumab in a cohort of Italian patients with refractory CD and is one of the largest studies of its type to date in Italy. The proportions of patients with active disease at baseline (HBI score >4) who achieved corticosteroid-free clinical remission at weeks 26 and 52 were 56.5% and 58.3%, respectively. In the entire cohort, there were significant decreases in the mean HBI score and mean CRP level between baseline and weeks 8, 26, and 52. At week 52, objective remission was recorded in half of the patients with a high CRP level at baseline. The low proportion of patients (22.9%) without symptomatic disease activity at the time of starting ustekinumab can be explained by the concomitant use of corticosteroids and by the known discrepancy between biomarker/endoscopic activity and clinical symptoms in some patients.
The effectiveness of ustekinumab in patients with refractory CD has also been demonstrated in other real-world studies, even though the results of different studies are sometimes difficult to compare. Our clinical effectiveness rates were similar to those reported in the Spanish multicentre retrospective ENEIDA registry study, 19 which evaluated a cohort of 407 patients with refractory CD and found that the corticosteroid-free clinical remission rates in patients with HBI scores of >4 at the start of treatment with ustekinumab were 57.3% and 64.4% at weeks 26 and 52, respectively. Progressive decreases in HBI and CRP values between baseline and week 52 were observed in all treated patients. Furthermore, the reductions were more pronounced between weeks 0 and 26 than between weeks 26 and 52, which is similar to the findings of our study.
A recent retrospective multicentre Italian study that assessed the effectiveness of ustekinumab in 194 patients with CD reported corticosteroid-free remission (HBI score <4) in 59.3% of the patients after a mean follow-up of 6 months. 22 A significant decrease in the CRP level from the baseline up to 12 months was also reported. The proportions of patients who had been exposed to anti-TNF-α therapy or to both anti-TNF-α therapy and vedolizumab prior to ustekinumab treatment were 75.8% and 24.2%, respectively. The findings of this large cohort study are in line with those in our study.
The results of other real-world cohort studies are in contrast with our findings. Lower effectiveness percentages were reported in the Dutch nationwide prospective ICC Registry study, which included 221 patients with CD who had failed on at least one anti-TNF-α agent (98.6% of the study population). 18 In that study, the corticosteroid-free clinical remission rates in patients with clinical disease activity at baseline (HBI score >4) were 24.2%, 38.2%, and 37.1% at weeks 12, 24, and 52, respectively. Likewise, in a multicentre retrospective Belgian study that included 152 patients with active CD, of whom 99.4% had had previous exposure to anti-TNF-α therapy, the respective corticosteroid-free clinical remission rates were 19.7%, 26.9%, and 24.3% after 8, 16, and 52 weeks of follow-up. 16
There are several potential explanations for the differences between our findings and those of the Dutch and Belgian studies. Compared with our study, the Dutch ICC Registry study included higher proportions of patients who had previously failed on more than one anti-TNF-α agent (40.0% versus 73.3%, respectively) and those who had received vedolizumab (20.0% versus 46.6%, respectively). Similarly, in the Belgian study, more patients had had previous exposure to two anti-TNF-α agents (82.2%) or two anti-TNF-α agents plus vedolizumab (69.7%). Furthermore, compared with our cohort, corticosteroid therapy was used in a higher proportion of patients in the Belgian cohort (15.7% versus 44.7%, respectively), and the median HBI score at baseline was worse in that study (6 versus 10, respectively).
The Sicilian Network for IBD has recently published prospectively collected data for 131 patients with CD who were treated with ustekinumab. 24 Almost all patients (99%) had been previously treated with biologic therapy. At week 8, corticosteroid-free clinical remission was achieved in 35% of the patients, which was similar to the rate obtained in our cohort. Corticosteroid-free clinical remission rates were reported to be 40% for 117 patients who were followed up for 24 weeks and 43% for 76 patients who were followed up for 52 weeks. Significant decreases in the HBI score were reported between baseline and weeks 8, 24, and 52; however, the decrease in the CRP level did not reach statistical significance during follow-up. The lower corticosteroid-free clinical remission rates at weeks 24 and 52 in that study might be explained by a more common corticosteroid use in their cohort than in our cohort (43% versus 15.7%) at baseline, higher rates of previous exposure to vedolizumab (7%) or vedolizumab plus anti-TNF-α therapy (35%), and fewer patients available for analysis at weeks 24 (n = 117) and 52 (n = 76).
Our patients showed a very high treatment persistence rate, with 92.1% remaining on ustekinumab after 52 weeks. Similarly high rates (79.7%, 89%, and 81.9%) were reported at 1 year in the FINUSTE2, Sicilian Network for IBD, and Hungarian multicentre cohort studies, respectively.23,24,31 By contrast, in the prospective ICC Registry study, the cumulative probability for patients remaining on ustekinumab therapy after 52 weeks was 62.9% in the survival analysis, and a similar rate of drug sustainability (61.2% at 12 months) was reported by the Belgian study.16,18 The differences between studies might be explained by a number of reasons, as discussed above.
In our patients, both the HBI and CRP values showed more pronounced decreases between baseline and week 26 than between weeks 26 and 52. Similar reductions were observed over time in other studies,16,19,22 indicating that the effect of ustekinumab therapy is greater in the first 26 weeks. Furthermore, in our study, the corticosteroid-free remission rate at week 52 was similar to that at week 26. All these findings indicate that few patients achieve a late benefit from ustekinumab use.
Moreover, we found that treatment with ustekinumab had positive effects on rheumatologic and cutaneous EIMs, which were present in a high proportion of patients at baseline. The benefit of this treatment was mainly observed in patients with peripheral arthropathy, 71.4% of who achieved complete symptom resolution or improvement by week 52. These data are consistent with those of other real-world reports on ustekinumab-treated patients with refractory CD and arthralgia.16,18,22,25 However, unlike in several other CD cohorts on ustekinumab,16,18,19,22,31 no de novo cases of arthralgia were recorded in our cohort; yet, the patients in our study were not routinely evaluated by a rheumatologist.
A post hoc analysis of pooled data from the CERTIFI, UNITI-1, and UNITI-2 trials suggested that ustekinumab might be effective against perianal fistulas in patients with CD. 38 However, those studies were not designed to primarily assess the response or remission of fistulas in patients treated with ustekinumab. The effectiveness of ustekinumab in patients with perianal CD has been assessed in several real-world studies.18,19,26,30 In the ICC Registry study, a clinical response rate of 14.3% and a complete resolution rate of 35.7% were reported for all fistulas after 24 weeks of treatment in 28 patients (12.7%) with active perianal disease at baseline. 18 A real-life multicentre retrospective study of 207 patients with CD, in which the effect of ustekinumab on perianal disease was the primary outcome, has been recently published. 39 In 148 patients with active perianal disease at the start of ustekinumab therapy, the clinical success rate was assessed by the investigators to be 38.5% at 6 months. In our study, a small proportion of patients (12.1%) had concomitant active perianal disease at baseline; at week 52, 23.5% of these patients reported improvement, and 35.3% reported complete clinical resolution of their perianal fistulas. Our present results are encouraging and similar to the aforementioned data; however, only very few patients were assessed. In our cohort, only one patient experienced worsening of perianal disease, which required a surgical procedure during follow-up. None of the patients developed de novo perianal disease.
In our analysis, the only factor associated with a lower corticosteroid-free clinical remission rate at week 52 was an HBI score of ⩾8 at baseline. There was a trend for the patient’s age >40 years; however, the data did not reach statistical significance, which may have been due to the small sample size. The identification of predictive factors for ustekinumab efficacy has been considered in other real-world studies, although some of them did not include the IV induction regimen.13 –16,18,19,24,28,29,40 In other cohorts, patients with a higher HBI score at induction were less likely to have a clinical response to ustekinumab maintenance therapy, and an older age was associated with a reduced clinical response, at least in the short term.14,24,28,40 However, more robust data are needed, as no predictive factors of response have currently been identified to help in clinical practice.
The safety profile of ustekinumab was confirmed to be good in the entire cohort of 140 patients, as has also been reported by the pivotal UNITI trials and several real-world studies.9,13 –17,19 –24,26,28 –31 The patients receiving a maintenance subcutaneous dose of 90 mg every 8 weeks (90%) did not report more AEs than those treated with the maintenance dose every 12 weeks (10%). Only three patients discontinued ustekinumab therapy because of intolerance. No infections occurred in our cohort.
This multicentre real-life study has several strengths. First, all patients in our cohort had previously been exposed to at least one anti-TNF agent; 40.0% received two anti-TNF-α agents and 20.0% were treated with vedolizumab. Furthermore, most patients had long-standing disease (median duration, 16.0 years); 35.7% were smokers, almost 50% had stricturing or penetrating disease, and 61.4% had previously undergone surgery for luminal CD. Therefore, they were a challenging group to treat. Second, we focused on a robust primary outcome, namely the corticosteroid-free clinical remission rates at weeks 26 and 52. Therefore, the effectiveness and safety of ustekinumab were evaluated in all patients at week 52, unlike in other real-life studies, which evaluated patients for shorter periods of time.17,22,26 Third, we also evaluated the effectiveness of ustekinumab in patients with EIMs and those with active perianal disease.
The study also has some limitations that should be considered when interpreting the findings. First, similar to other real-world studies that have investigated the effectiveness and safety of ustekinumab in patients with refractory CD,13 –17,19,20,22,23,25,28,30 our study had a retrospective design, which could have led to overestimation of the positive response rate and underestimation of AEs, especially of the mild intensity. Second, all patients were treated at tertiary referral centres providing care for patients with IBD. Therefore, our findings may have limited external validity. Third, there were no endoscopic data available for the assessment of mucosal healing or improvement. Only few patients underwent endoscopy at week 52, and therefore, endoscopic findings were excluded from the analysis. However, it should be noted that most of the patients completed their 52 weeks of therapy during the first months of the COVID-19 pandemic, when nonemergency colonoscopies were cancelled or delayed because of healthcare restrictions. Furthermore, faecal calprotectin data were only available for a small number of patients. This laboratory test is not covered by the national health system in Italy and thus, is not routinely performed. Therefore, data on faecal calprotectin were not included in our analysis.
In summary, the findings of this study confirmed the effectiveness, a high persistence rate, and a good safety profile of ustekinumab in Italian patients with CD who had failed to respond or were intolerant to other biologic agents. Further prospective studies are needed to determine the place of ustekinumab in the treatment algorithms for CD and to evaluate trough levels of ustekinumab and antidrug antibodies.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848211072412 for Effectiveness of ustekinumab in patients with refractory Crohn’s disease: a multicentre real-life study in Italy by Maria Lia Scribano, Annalisa Aratari, Benedetto Neri, Cristina Bezzio, Paola Balestrieri, Valentina Baccolini, Giuliano Falasco, Caterina Camastra, Paolo Pantanella, Rita Monterubbianesi, Alessandro Tullio, Simone Saibeni, Claudio Papi, Livia Biancone, Rocco Cosintino and Roberto Faggiani in Therapeutic Advances in Gastroenterology
Acknowledgments
We would like to thank Editage (www.editage.com) for its support in proofreading the manuscript.
Footnotes
Author contributions: Maria Lia Scribano: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Visualization; Writing – original draft.
Annalisa Aratari: Investigation; Writing – review & editing.
Benedetto Neri: Investigation; Writing – review & editing.
Cristina Bezzio: Investigation; Writing – review & editing.
Paola Balestrieri: Investigation; Writing – review & editing.
Valentina Baccolini: Formal analysis; Writing – review & editing.
Giuliano Falasco: Investigation; Writing – review & editing.
Caterina Camastra: Investigation; Writing – review & editing.
Paolo Pantanella: Investigation; Writing – review & editing.
Rita Monterubbianesi: Investigation; Writing – review & editing.
Alessandro Tullio: Investigation; Writing – review & editing.
Simone Saibeni: Investigation; Writing – review & editing.
Claudio Papi: Investigation; Writing – review & editing.
Livia Biancone: Investigation; Writing – review & editing.
Rocco Cosintino: Investigation; Writing – review & editing.
Roberto Faggiani: Investigation; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MLS: advisory board and/or lecture fees from AbbVie, Celltrion, Janssen, Pfizer, and Takeda. CB: lecture fees from Takeda, AbbVie, and Janssen. SS: advisory board and lecture fees from Arena, Gilead, Janssen, Takeda, and AbbVie. CP: consultancy fees and/or educational grants from AbbVie, MSD, Takeda, Pfizer, Janssen-Cilag, Chiesi, Sofar, Ferring, and Zambon. LB: speaker fees from Ferring, AbbVie, Janssen, and Zambon. The other authors declare no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Maria Lia Scribano  https://orcid.org/0000-0003-1839-8136
https://orcid.org/0000-0003-1839-8136
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Maria Lia Scribano, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Circonvallazione Gianicolense, 87, 00152 Rome, Italy.
Annalisa Aratari, IBD Unit, San Filippo Neri Hospital, Rome, Italy.
Benedetto Neri, Gastroenterology Unit, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Cristina Bezzio, Gastroenterology Unit, Rho Hospital, ASST Rhodense, Rho, Italy.
Paola Balestrieri, Gastroenterology Unit, Campus Bio-Medico University, Rome, Italy.
Valentina Baccolini, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy.
Giuliano Falasco, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy.
Caterina Camastra, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy.
Paolo Pantanella, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy.
Rita Monterubbianesi, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy.
Alessandro Tullio, Gastroenterology Unit, Campus Bio-Medico University, Rome, Italy.
Simone Saibeni, Gastroenterology Unit, Rho Hospital, ASST Rhodense, Rho, Italy.
Claudio Papi, IBD Unit, San Filippo Neri Hospital, Rome, Italy.
Livia Biancone, Gastroenterology Unit, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy.
Rocco Cosintino, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy.
Roberto Faggiani, Gastroenterology Unit, Azienda Ospedaliera San Camillo Forlanini, Rome, Italy.
References
- 1. Torres J, Mehandru S, Colombel JF, et al. Crohn’s disease. Lancet 2017; 389: 1741–1755. [DOI] [PubMed] [Google Scholar]
- 2. Orlando A, Armuzzi A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43: 1–20. [DOI] [PubMed] [Google Scholar]
- 3. Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med 2013; 369: 754–762. [DOI] [PubMed] [Google Scholar]
- 4. Lichtenstein GR, Feagan BG, Cohen RD, et al. Infliximab for Crohn’s disease: more than 13 years of real-world experience. Inflamm Bowel Dis 2018; 24: 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease – algorithm for practical management. Aliment Pharmacol Ther 2016; 43: 30–51. [DOI] [PubMed] [Google Scholar]
- 6. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther 2011; 33: 987–995. [DOI] [PubMed] [Google Scholar]
- 7. Benson JM, Peritt D, Scallon BJ, et al. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs 2011; 3: 535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012; 367: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 9. Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 10. European Commission Approves Stelara® (Ustekinumab) for treatment of adults with moderately to severely active Crohn’s disease. Beerse: Johnson & Johnson Media Center, 2016. [Google Scholar]
- 11. FDA approves STELARA® (Ustekinumab) for treatment of moderate to severe Crohn’s disease. New York: Crohn’s & Colitis Foundation of America, 2016. [Google Scholar]
- 12. Regime di rimborsabilita’ e prezzo, a seguito di nuove indicazioni terapeutiche, del medicinale per uso umano «Stelara». (Determina n. DG/1320/2018). (18A05714) (GU Serie Generale n.204 del 03-09-2018). [Google Scholar]
- 13. Khorrami S, Ginard D, Marín-Jiménez I, et al. Ustekinumab for the treatment of refractory Crohn’s disease: the Spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis 2016; 22: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 14. Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis 2017; 23: 833–839. [DOI] [PubMed] [Google Scholar]
- 15. Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther 2018; 47: 588–595. [DOI] [PubMed] [Google Scholar]
- 16. Liefferinckx C, Verstockt B, Gils A, et al. Long-term clinical effectiveness of ustekinumab in patients with Crohn’s disease who failed biological therapies: a national cohort study. J Crohns Colitis 2019; 13: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 17. Eberl A, Hallinen T, Af Björkesten CG, et al. Ustekinumab for Crohn’s disease: a nationwide real-life cohort study from Finland (FINUSTE). Scand J Gastroenterol 2019; 54: 718–725. [DOI] [PubMed] [Google Scholar]
- 18. Biemans VBC, Van Der Meulen-De Jong AE, Van Der Woude CJ, et al. Ustekinumab for Crohn’s disease: results of the ICC Registry, a nationwide prospective observational cohort study. J Crohns Colitis 2020; 14: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iborra M, Beltrán B, Fernández-Clotet A, et al. Real-world long-term effectiveness of ustekinumab in Crohn’s disease: results from the ENEIDA registry. Aliment Pharmacol Ther 2020; 52: 1017–1030. [DOI] [PubMed] [Google Scholar]
- 20. Monin L, Dubois S, Reenaers C, et al. Ustekinumab in bio-naïve and bio-failure Crohn’s disease patients: results from a ‘real-life’ monocentric cohort. Dig Liver Dis 2021; 53: 72–78. [DOI] [PubMed] [Google Scholar]
- 21. Macaluso FS, Maida M, Ventimiglia M, et al. Effectiveness and safety of ustekinumab for the treatment of Crohn’s disease in real-life experiences: a meta-analysis of observational studies. Expert Opin Biol Ther 2020; 20: 193–203. [DOI] [PubMed] [Google Scholar]
- 22. Tursi A, Mocci G, Cuomo A, et al. Real-life efficacy and safety of Ustekinumab as second- or third-line therapy in Crohn’s disease: results from a large Italian cohort study. Eur Rev Med Pharmacol Sci 2021; 25: 2099–2108. [DOI] [PubMed] [Google Scholar]
- 23. Af Björkesten CG, Ilus T, Hallinen T, et al. Objectively assessed disease activity and drug persistence during ustekinumab treatment in a nationwide real-world Crohn’s disease cohort. Eur J Gastroenterol Hepatol 2020; 32: 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Viola A, Muscianisi M, Macaluso FS, et al. P717 Ustekinumab in Crohn’s disease: real-world outcomes from the Sicilian network for inflammatory bowel diseases. JGH Open 2021; 5: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bennett A, Evers Carlini L, Duley C, et al. A single center experience with long-term ustekinumab use and reinduction in patients with refractory Crohn disease. Crohns Colitis 360 2020; 2: otaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bar-Gil Shitrit A, Ben-Ya’acov A, Siterman M, et al. Safety and effectiveness of ustekinumab for induction of remission in patients with Crohn’s disease: a multicenter Israeli study. United European Gastroenterol J 2020; 8: 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haider SA, Yadav A, Perry C, et al. Ustekinumab dose escalation improves clinical responses in refractory Crohn’s disease. Therap Adv Gastroenterol 2020; 13: 1756284820959245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Casas Deza D, Garcia Lopez S, Lafuente Blasco M, et al. Efficacy and safety of ustekinumab in real clinical practice. Retrospective multicentre study. ARAINF cohort. Gastroenterol Hepatol 2020; 43: 126–132. [DOI] [PubMed] [Google Scholar]
- 29. Straatmijer T, Biemans VBC, Hoentjen F, et al. Ustekinumab for Crohn’s disease: two-year results of the initiative on Crohn and Colitis (ICC) registry, a nationwide prospective observational cohort study. J Crohns Colitis 2021; 15: 1920–1930. [DOI] [PubMed] [Google Scholar]
- 30. Plevris N, Fulforth J, Siakavellas S, et al. Real-world effectiveness and safety of ustekinumab for the treatment of Crohn’s disease: the Scottish ustekinumab cohort. J Gastroenterol Hepatol 2021; 36: 2067–2075. [DOI] [PubMed] [Google Scholar]
- 31. Gonczi L, Szanto K, Farkas K, et al. Clinical efficacy, drug sustainability and serum drug levels in Crohn’s disease patients treated with ustekinumab – a prospective, multicenter cohort from Hungary. Dig Liver Dis. Epub ahead of print 31 July 2021. DOI: 10.1016/j.dld.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 32. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019; 13: 144–164. [DOI] [PubMed] [Google Scholar]
- 33. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl. A): 5A–36A. [DOI] [PubMed] [Google Scholar]
- 34. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet 1980; 1: 514. [DOI] [PubMed] [Google Scholar]
- 35. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 36. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350: 876–885. [DOI] [PubMed] [Google Scholar]
- 37. Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Attauabi M, Burisch J, Seidelin JB. Efficacy of ustekinumab for active perianal fistulizing Crohn’s disease: a double-center cohort study. Inflamm Bowel Dis 2021; 27: e37–e38. [DOI] [PubMed] [Google Scholar]
- 39. Chapuis-Biron C, Kirchgesner J, Pariente B, et al. Ustekinumab for perianal Crohn’s disease: the BioLAP multicenter study from the GETAID. Am J Gastroenterol 2020; 115: 1812–1820. [DOI] [PubMed] [Google Scholar]
- 40. Gutierrez A, Rodriguez-Lago I. How to optimize treatment with ustekinumab in inflammatory bowel disease: lessons learned from clinical trials and real-world data. Front Med 2021; 8: 640813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848211072412 for Effectiveness of ustekinumab in patients with refractory Crohn’s disease: a multicentre real-life study in Italy by Maria Lia Scribano, Annalisa Aratari, Benedetto Neri, Cristina Bezzio, Paola Balestrieri, Valentina Baccolini, Giuliano Falasco, Caterina Camastra, Paolo Pantanella, Rita Monterubbianesi, Alessandro Tullio, Simone Saibeni, Claudio Papi, Livia Biancone, Rocco Cosintino and Roberto Faggiani in Therapeutic Advances in Gastroenterology