Abstract
Background
Chronic fatigue syndrome (CFS) is a complex and often disabling chronic condition emerging worldwide, with no curative or definitive therapy yet identified. Ginseng has been widely used to treat fatigue in other patient groups and conditions; however, a systematic review focusing solely on the impact of ginseng on fatigue in patients with CFS has not been performed.
Objective
This study aimed to assess the current state of evidence regarding ginseng for CFS.
Methods
Multiple databases were searched from inception to October 2020. All data was extracted independently and in duplicates. Outcomes of interest included the effectiveness and safety of ginseng in patients with CFS.
Results
2 studies enrolling 68 patients were deemed eligible, including one randomized clinical trial and one prospective observational study. The certainty of evidence in the effectiveness outcome was low and moderate from both studies, while the safety evidence was very low as reported from one study.
Conclusion
Study findings highlight a potential benefit of ginseng therapy in the treatment of CFS. However, we are not able to draw firm conclusions due to limited clinical studies. The paucity of data warrants limited confidence. There is a need for future rigorous studies to provide further evidence.
Keywords: ginseng, fatigue, chronic fatigue, chronic fatigue syndrome, systematic review
Introduction
Chronic fatigue syndrome (CFS), also known as myalgic encephalomyelitis (ME) or systemic exertion intolerance disease (SEID), is a complex and debilitating condition affecting almost 1 percent of the world’s population. 1 Although CFS has been reported in individuals younger than age 10 and older than age 70, the mean age of onset is 33. 2 Patients with CFS are characterized by persistent or relapsing chronic fatigue lasting at least 6 months, accompanied by complex and fluctuating symptoms of post-exertional malaise, autonomic dysfunction, cognitive impairment, unrefreshing sleep, and/or muscular or joint pain; these symptoms are not substantially alleviated by rest. 1 People with CFS often cannot maintain their daily work, social, or leisure activities; 25 to 29% of patients with CFS report being bedridden or house bound. 3 Only 19% work full time, 4 while over 50% are unemployed. 5 In the USA, about 836 000 to 2.5 million patients suffer with CFS, with resulting economic costs ranging between $17 to 24 billion per year. 6
The pathophysiology and etiology of CFS remain unclear. The diagnosis of CFS is mainly dependent on the absence of other exclusionary conditions and fulfilling established clinical criteria. Multiple case definitions have been used in clinical practice and research. In recent years, the most commonly used include the Fukuda criteria (CDC, 1994), 7 the 2003 Canadian Consensus Criteria (CCC, 2003), 8 the International Consensus Criteria (ICC, 2011), 9 and the Institute of Medicine (IOM, 2015) 10 diagnostic criteria. Compared to the 1994 CDC criteria, the CCC requires the presence of autonomic, neuroendocrine, and immune manifestations; the ICC includes post-exertional neuro-immune exhaustion and the removal of the 6-month duration; and the IOM focuses on the most specific features of the syndrome (chronic fatigue, post-exertional malaise, unrefreshed sleep, cognitive symptoms, and orthostatic intolerance).
Treatment of CFS is variable and of uncertain effectiveness. Pharmacological therapy (including antidepressants, anxiolytics, antimicrobials, immune modulators, analgesics, and muscle relaxants) and complementary and alternative medicine (CAM) are the most common approaches to treat CFS. 11 About 70% of the CFS population use at least one type of CAM therapy; of these modalities, herbal therapy, meditation, relaxation, homeopathy, acupuncture, massage therapy, and naturopathy are the most commonly used. 12
Traditionally known as the “King of Herbs,” ginseng is an important perennial herb derived from the family Araliaceae and the genus Panax. 13 Ginsenosides are known to have biological activity in maintaining homeostasis of the body and enhancing vital energy. 14 Being one of the most commonly used herbal dietary supplements and the most-studied herb for human physical performance, 15 ginseng has been used for centuries in traditional medicine to treat various diseases, such as cancer, 16 postmenopausal symptoms, 17 and erectile dysfunction. 18 The anti-fatigue effects of ginseng have already been documented in clinical practice and various animal-based experiments.19-22 Furthermore, a 2018 review evaluated whether Panax ginseng and American ginseng were safe and effective to treat patients with fatigue due to various chronic conditions; 10 trials were included in this study (4 using American ginseng (P. quinquefolius) and 6, Asian ginseng (P ginseng)). Results demonstrated that approximately 70% of participants showed significant improvements in fatigue scores, albeit with modest evidence due to small sample size and sample composition limitations. 21 At present, despite sparse reports of ginseng’s potential utility in CFS,23-26 there is no systematic review solely assessing the anti-fatigue effectiveness of ginseng in CFS. This systematic review was conducted to evaluate and present up-to-date evidence about the safety and effectiveness of ginseng in patients with CFS.
Methods
This systematic review followed the standards of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 27
Study Selection Criteria
Our specific selection criteria included the following: (1) patients with a diagnosis of CFS, regardless of age, gender, disease course, treatment session, and severity; (2) any type of ginseng use (alone or in combination with the other non-medication treatments) was included; and (3) the controls could be conventional medicine, placebo, no treatment, or other CAM therapies. We used Review Manager (RevMan) version 5.3 for reference management and study data assessment. 28 Exclusion criteria consisted of the following: (1) it did not include ginseng or CFS, (2) did not report fatigue as an outcome, (3) ginseng was just one of several combined/utilized herbs (formula), (4) ginseng was in the control group, and (5) the trial was an animal or cellular study.
Literature Search
We conducted a comprehensive search of several databases from each database’s inception date through October 2020. The databases included Ovid MEDLINE(R), APA PsycInfo, Embase, In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) Daily, EBM Reviews—Cochrane Central Register of Controlled Trials, and EBM Reviews—Cochrane Database of Systematic Reviews. We limited the search to English language. The search terms (MeSH (Medical Subject Heading)) with free terms included: fatigue syndrome, chronic fatigue syndrome, fatigue, myalgic encephalomyelitis, systemic exertion intolerance, epidemic neuromyasthenia, ginseng, panax, quinquefolius, and ginsenoside. Publications on the reference lists of the articles were also cross referenced.
Data Extraction and Synthesis
Using the predefined inclusion and exclusion criteria, 2 assessors (JY and KMS) independently screened abstracts and full texts, and reviewed each article at the appropriate phase. Data of interest were extracted on demographic and outcome-related data, including first author, publication year, country, condition, population, sample size, intervention type, control or comparator, dosage, treatment duration, follow-up duration, and effectiveness and/or safety-related measures.
Quality of Study/Risk of Bias Assessment
For methodological quality of the included studies, we used the Cochrane risk of bias assessment tool for randomized trials, 29 while the risk of bias for the observational case series study was assessed using the Joanna Briggs case series tool. 30 Low (L), high (H), or unclear (U) quality levels were determined for each of the included studies. Any disagreement in opinions between the 2 investigators was settled through consensus or resort to a senior team member. The certainty of evidence followed the general framework reported by the GRADE group. 31
Results
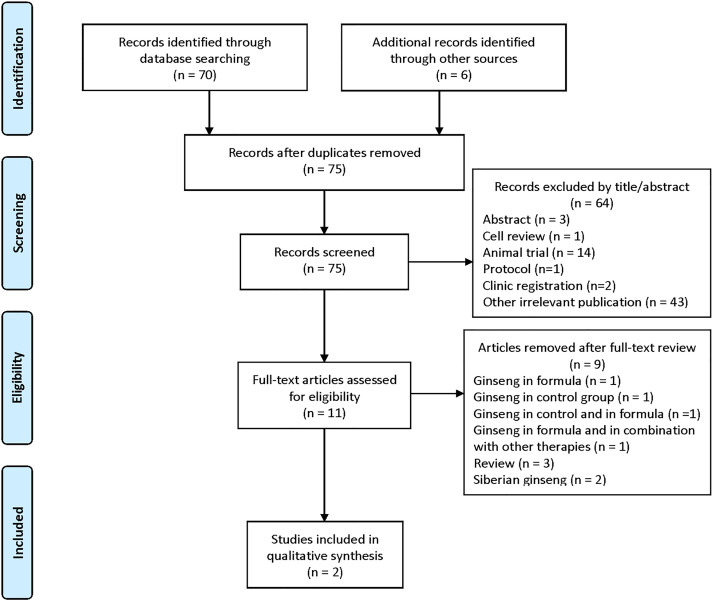
The search terms initially yielded a total of 76 publications from the databases and only 2 studies enrolling 68 patients were deemed eligible as shown in Figure 1. The eligible 2 studies (N = 68 patients) included one prospective observational pre-post study by Bentler et al 32 in 2005 and one randomized controlled trial (RCT) by Sung et al 33 in 2020. The baseline characteristics of the 2 included articles are summarized in Table 1. Other important studies that were excluded for various reasons are summarized in Table 2. Because of the insufficient reporting from the small number of included studies, we did not pursue a meta-analysis. Bentler et al 32 included 155 adult patients over the age of 17; participant enrollment was based on the 1994 CDC criteria. 7 The authors did not provide details of the type of ginseng used by the participants who were followed for symptomatic improvement of fatigue at multiple time points (baseline, 6 months, and 2 years) via self-reported survey questionnaires. Linear regression was conducted to evaluate for any association between each utilized therapy and reduction in fatigue. Overall, of the 18 participants taking ginseng, 56% reported symptomatic improvement in their fatigue. Also, ginseng was more often used in combination with acupuncture (OR = 6.3, P = .0005). Data of adverse events associated with ginseng were not reported.
Figure 1.
Flowchart of the literature search.
Table 1.
Characteristics of Included Studies of Ginseng for the Treatment of Chronic Fatigue Syndrome.
| First Author/Year | Country | Type | N | Population | Intervention | Control | Dosage | Period | Outcome | Effect | Adverse Event |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bentler, 2005 32 | USA | Self-controlled case series | 18 | > 17 y | Multiple treatments (ginseng) | / | Not reported | 6 months | 5-question fatigue score | Helpful | Not reported |
| Sung, 2020 33 | South Korea | RCT | 50 | 1965 y | Korean red ginseng | Placebo | 3 g Korean red ginseng or placebo twice daily | 6 weeks | VAS; FSS, CFSQ, SRI, BDI; ISI; and EQ-5D 5 L, biochemical test, and blinding assessment | Potentially effective | None |
Note: RCT = randomized controlled trial; VAS = Visual Analog Scale; FSS = fatigue severity scale; CFSQ = Chalder fatigue severity questionnaire; SRI = stress response inventory; BDI = The Beck depression inventory; ISI = insomnia severity index; EQ-5D 5 L= five-level EuroQol-5 dimension.
Table 2.
Characteristics of Excluded Studies of Ginseng for the Treatment of Chronic Fatigue Syndrome.
| First author/Year | Type | Excluded Reason |
|---|---|---|
| Shin, 2004 34 | Prospective study | Ginseng in formula |
| Hartz, 2004 35 | RCT | Siberian ginseng |
| Guo, 2007 36 | RCT | Ginseng in control and in formula |
| Adams, 2009 37 | Systematic review | Simply mentioned ginseng |
| Chen, 2010 23 | Review | Simply mentioned CFS and/or ginseng |
| Alraek, 2011 26 | Systematic review | Siberian ginseng |
| Semalty, 2012 24 | Review | Simply mentioned CFS and/or ginseng |
| Son, 2013 38 | Case report | Ginseng in formula and in combination with other therapies |
| Wu, 2020 39 | RCT | Ginseng in control group |
Note: RCT = randomized controlled trial.
Sung et al 33 included 50 adult patients, aged 19-65, with enrollment based on the 1994 CDC criteria. 7 Participants were randomized to either Korean red ginseng (KRG) or placebo for 6 weeks with an additional 4 weeks of follow-up. Fatigue, measured by visual analog scale (VAS), declined significantly in both groups. However, patients > 50 years with an initial fatigue VAS below 80 mm had a significant improvement in fatigue VAS scores in the KRG group as compared to placebo. No adverse events associated with KRG were reported.
Risk of Bias and Certainty of Evidence
The risk of bias of this RCT was deemed low, while that of the prospective observational trial was deemed high as shown in Table 3. The certainty for each outcome was low to moderate for efficacy (mainly due to relevant inconsistency, serious imprecision, and moderate risk of bias) and very low for safety (mainly due to serious imprecision, high risk of bias, serious indirectness, and very serious limitations).
Table 3.
Risk of Bias Assessment of the Included Studies.
| Case Series | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study ID | Clear Inclusion Criteria | Validity of Identifying Participants | Reliability Assessment and Measurements of the Studied Condition | Consecutive Sampling of Participants | Clearly Reported Study Demographics | Clearly Reported outcome(s) of Interest | Appropriate Statistical Analysis | Attrition Risk | Overall Risk |
| Bentler 2005 32 | No (high risk) | No (high risk) | No (high risk) | Unclear (high risk) | Yes (low risk) | Yes (low risk) | No (high risk) | 4/159 (2%) (low risk) | High |
| Answers: Yes (high risk); no (low risk); unclear; not applicable (NA) | |||||||||
| Randomized controlled trial | |||||||||
| Study ID | Randomization | Deviation from protocol | Missing outcome data | Outcomes measurement | Selection (blinding) bias | Attrition | Other reporting bias | Overall risk | |
| Sung 2020 33 | Yes 1:1 block; concealed (low risk) | No (low risk) | No (low risk) | Reported appropriately (low risk) | Yes investigators and patients (low risk) | 3/50 (6%) (low risk) | No (low risk) | Low | |
| Answers: High risk; low; unclear | |||||||||
Discussion
In this systematic review, we found limited evidence from 2 studies (one observational and one RCT), suggesting ginseng as an effective and safe treatment option for patients suffering from CFS. Though ginseng has been previously utilized to treat fatigue in many different patient groups and conditions, paucity in robust supporting evidence in the setting of CFS remains.
There is an existing common misconception regarding the use of Siberian ginseng for CFS. Siberian ginseng, also named Eleutherococcus senticosus, is not true ginseng as it contains eleutherosides rather than ginsenosides. The name Siberian ginseng became popular because it shares some similar properties with the ginseng plant, and has been previously used to alleviate both physical and mental fatigue. 40 A previous RCT compared Siberian ginseng with placebo for CFS and demonstrated that Siberian ginseng might demonstrate possible effectiveness for patients with moderate fatigue. Unfortunately, many subsequent publications have confused this trial (utilizing Siberian ginseng) as evidence for the use of true ginseng in CFS.23,25,26,41 We did not include any trials utilizing Siberian ginseng in our systematic review, given the lack of ginsenosides.
In terms of safety, among the included studies, one study reported no adverse events, 33 while the other one did not report on adverse events. 32 Therefore, the evaluation of the safety of ginseng in CFS is limited.
It should be noted that there is significant variability in the definition and diagnostic criteria for CFS. Given the numerous diagnostic criteria (CDC, CCC, ICC, IOM, etc.), this inherently creates significant variability in clinical practice and research in terms of inclusion and exclusion criteria and case definitions. This in turn limits the generalizability of the studies. In our review, we did not initially limit the case definition in the inclusion criteria to include as many publications as possible. Both included trials used the 1994 CDC criteria for defining the patient population. Though this may not be the most effective tool for clinical or research purposes, it is still the most used diagnostic criteria in clinical practice. Future research should utilize a single standardized and validated diagnostic criterion, which can help enhance study generalizability and streamline future research in the field of CFS.
Limitations and Strengths
A noteworthy limitation is that this present systematic review highlights the paucity of applicable clinical trials in the setting of CFS. This could in part be due to limited CFS-based studies, but also could be because we confined our literature search to studies published in English databases only. This could increase the potential risk of bias and minimize the generalizability of the findings. In the future, we will consider synthesizing more articles across multiple language databases to see if we are able to acquire more robust data.
The strength of this evidence-based report is that this is the first systematic review that solely collected and appraised available literature relating to clinical studies investigating ginseng in CFS. Again, though ginseng has been widely utilized and studied in various other fatigue-associated conditions, with demonstration of promising results, we identified only 2 applicable studies in CFS specifically. Our study results are driven from a rigorous methodological approach that included a comprehensive literature search of multiple databases and duplicate study selection and appraisal. Our data synthesis objectively highlighted safety and effectiveness findings to provide current recommendations about the potential use of ginseng in CFS.
Our review highlights some of the issues surrounding CFS and ginseng research in general. Firstly, despite the high prevalence of ginseng usage among patients with fatigue across many different conditions, studies solely dedicated to CFS remain very limited. Though we did identify several other potential articles in our search strategy, most of the these were excluded due to various reasons (utilization of Siberian ginseng,26,35 simply mentioning ginseng in the study,23,24,37 ginseng in the control group,37,39 or ginseng used in complex/combined formulations34,36,38); this information is summarized in Table 2. These factors prevented us from including these studies in our analysis, since it would have been near impossible to decipher the sole impact of ginseng on fatigue due to various potential confounders.
Conclusion
CFS is a complex and debilitating condition causing significant disability, with no curative or definitive therapy yet identified. Two identified studies suggest ginseng as an effective and safe treatment option for patients suffering from CFS. However, given the paucity of data coupled with the generally low quality of published evidence, it is difficult to draw more generalizable conclusions. More rigorous studies are required to re-examine this subject in the future.
Acknowledgments
We would like to thank The HEAD Foundation for their support for Dr. Yang and Dr. Bauer.
Footnotes
Author contribution: Both ABM and DMB initially conceived this review; JY and KMS screened and selected the data; JY drafted the preliminary manuscript; all authors reviewed and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Juan Yang, MD, PhD https://orcid.org/0000-0002-0850-679X
Abd Moain Abu Dabrh, MB, BCh, MS https://orcid.org/0000-0002-2481-483X
Brent A. Bauer, MD https://orcid.org/0000-0003-3453-6906
References
- 1.Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Journal of translational medicine. 2020;18(1):100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clayton EW. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Jama. 2015;313(11):1101–1102. [DOI] [PubMed] [Google Scholar]
- 3.Pendergrast T, Brown A, Sunnquist M, et al. Housebound versus nonhousebound patients with myalgic encephalomyelitis and chronic fatigue syndrome. Chronic Illness. 2016;12(4):292–307. doi: 10.1177/1742395316644770. [Medline:PMC5464362]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falk Hvidberg M, Brinth LS, Olesen AV, Petersen KD, Ehlers L. The Health-Related Quality of Life for Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS One. 2015;10(7):e0132421. doi: 10.1371/journal.pone.0132421. [Medline:PMC4492975 The Danish ME/CFS association has supplied manpower in the survey process as well as printing supplies etc. The collaboration of the authors and the Danish ME/CFS association has not involved any economic transactions in any way between the parties]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castro-Marrero J, Faro M, Zaragozá MC, Aliste L, de Sevilla TF, Alegre J. Unemployment and work disability in individuals with chronic fatigue syndrome/myalgic encephalomyelitis: a community-based cross-sectional study from Spain. BMC Public Health. 2019;19(1):840. doi: 10.1186/s12889-019-7225-z. [Medline:PMC6599355]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komaroff AL. Myalgic encephalomyelitis/chronic fatigue syndrome: A real illness. Annals of internal medicine. 2015;162(12):871–872. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The Chronic Fatigue Syndrome: A Comprehensive Approach to Its Definition and Study. Ann Intern Med. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 8.Carruthers BM, Jain AK, De Meirleir KL, et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J Chronic Fatigue Syndrome. 2003;11(1):7–115. [Google Scholar]
- 9.Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270(4):327-338. doi: 10.1111/j.1365-2796.2011.02428.x. [Medline:PMC3427890]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maksoud R, du Preez S, Eaton-Fitch N, Thapaliya K, Barnden L, Cabanas H, et al. A systematic review of neurological impairments in myalgic encephalomyelitis/chronic fatigue syndrome using neuroimaging techniques. PLoS One. 2020;15(4):e0232475. doi: 10.1371/journal.pone.0232475. [Medline:PMC7192498]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Marrero J, Sáez-Francàs N, Santillo D, Alegre J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br J Pharmacol. 2017;174(5):345–369. doi: 10.1111/bph.13702. [Medline: PMC5301046]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewith G, Stuart B, Chalder T, McDermott C, White PD. Complementary and alternative healthcare use by participants in the PACE trial of treatments for chronic fatigue syndrome. J Psychosom Res. 2016;87:37–42. [DOI] [PubMed] [Google Scholar]
- 13.Park H-J, Kim D-H, Park S-J, Kim J-M, Ryu J-H. Ginseng in traditional herbal prescriptions. Journal of Ginseng Research. 2012;36(3):225-241. doi: 10.5142/jgr.2012.36.3.225. [Medline:PMC3659587]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong SK, Oberholzer VG. Ginseng--is there a use in clinical medicine? Postgrad Med. 1988;64(757):841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellami M, Slimeni O, Pokrywka A, et al. Herbal medicine for sports: a review. Sports Nutr Rev J. 2018;15(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unlu A, Nayir E, Kirca O, Ay H, Ozdogan M. Ginseng and cancer. Journal of B.U.ON. : Official Journal of the Balkan Union of Oncology. 2016;21:1383–1387. [PubMed] [Google Scholar]
- 17.Lee HW, Choi J, Lee Y, Kil KJ, Lee MS. Ginseng for managing menopausal woman's health: a systematic review of double-blind, randomized, placebo-controlled trials. Medicine. 2016;95(38):e4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying A, Yu Q-T, Guo L, et al. Structural-Activity Relationship of Ginsenosides from Steamed Ginseng in the Treatment of Erectile Dysfunction. Am J Chin Med. 2018;46(01):137–155. [DOI] [PubMed] [Google Scholar]
- 19.Shin I-S, Kim D-H, Jang EY, Kim HY, Yoo H-S. Anti-Fatigue Properties of Cultivated Wild Ginseng Distilled Extract and Its Active Component Panaxydol in Rats. J Pharmacopuncture. 2019;22(2):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim H-G, Cho J-H, Yoo S-R, et al. Antifatigue Effects of Panax ginseng C.A. Meyer: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS One. 2013;8(4):e61271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arring NM, Millstine D, Marks LA, Nail LM. Ginseng as a treatment for fatigue: a systematic review. J Alternative Compl Med. 2018;24(7):624–633. [DOI] [PubMed] [Google Scholar]
- 22.Lee HW, Kil K-J, Lee MS. Ginseng for improving semen quality parameters: A systematic review. The world journal of men's health. 2020;38(3):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Moriya J, Yamakawa J-i., Takahashi T, Kanda T. Traditional Chinese medicine for chronic fatigue syndrome. Evid base Compl Alternative Med. 2010;7(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semalty A, Semalty M, Panda VS, Asrani KH, Ashar HD. Herbal drugs in chronic fatigue syndrome: an overview. Schweizerische Zeitschrift für Ganzheitsmedizin/Swiss Journal of Integrative Medicine. 2012;24(3):155–168. [Google Scholar]
- 25.Rimes KA, Chalder T. Treatments for chronic fatigue syndrome. Occup Med (Oxf). 2005;55(1):32–39. [DOI] [PubMed] [Google Scholar]
- 26.Alraek T, Lee MS, Choi TY, Cao H, Liu J. Complementary and alternative medicine for patients with chronic fatigue syndrome: a systematic review. BMC Complementary and Alternative Medicine. 2011;11(1):87–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. [DOI] [PubMed] [Google Scholar]
- 28.RevMan 5 download. https://trainingcochraneorg/online-learning/core-software-cochrane-reviews/revman/revman-5-download (accessed on 24 Jananuary 2021).
- 29.Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evidence Synthesis. 2019;18:2127-2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 31.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Bentler SE, Hartz AJ, Kuhn EM. Prospective observational study of treatments for unexplained chronic fatigue. J Clin Psychiatr. 2005;66(5):625–632. [DOI] [PubMed] [Google Scholar]
- 33.Sung W-S, Kang H-R, Jung C-Y, Park S-S, Lee S-H, Kim E-J. Efficacy of Korean red ginseng (Panax ginseng) for middle-aged and moderate level of chronic fatigue patients: A randomized, double-blind, placebo-controlled trial. Compl Ther Med. 2020;48:102246. [DOI] [PubMed] [Google Scholar]
- 34.Shin HY, An NH, Cha YJ, et al. Effect of Kuibitang on lipopolysaccharide-induced cytokine production in peripheral blood mononuclear cells of chronic fatigue syndrome patients. J Ethnopharmacol. 2004;90(2-3):253–259. [DOI] [PubMed] [Google Scholar]
- 35.Hartz AJ, Bentler S, Noyes R, et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med. 2004;34(1):51-61. [DOI] [PubMed] [Google Scholar]
- 36.Guo J. Chronic fatigue syndrome treated by acupuncture and moxibustion in combination with psychological approaches in 310 cases. Journal of traditional Chinese medicine = Chung i tsa chih ying wen pan. 2007;27(2):92–95. [PubMed] [Google Scholar]
- 37.Adams D, Wu T, Yang X, Tai S, Vohra S. Traditional Chinese medicinal herbs for the treatment of idiopathic chronic fatigue and chronic fatigue syndrome. Cochrane Database Syst Rev. 2009(4):CD006348. [DOI] [PubMed] [Google Scholar]
- 38.Son C-G. A case of chronic fatigue syndrome improved by traditional Korean medicine. Integrative medicine research. 2013;2(1):32–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Liu L-l. Observation on therapeutic efficacy of tuina plus cupping for chronic fatigue syndrome. Journal of Acupuncture and Tuina Science. 2020;18(1):53-58. doi: 10.1007/s11726-020-1157-0. [DOI] [Google Scholar]
- 40.Majid A. Panax ginseng–a review. University of Thi-Qar Journal of Science. 2019;7(1):96–102. [Google Scholar]
- 41.Elam JL, Carpenter JS, Shu X-O, Boyapati S, Friedmann-Gilchrist J. Methodological issues in the investigation of ginseng as an intervention for fatigue. Clin Nurse Spec. 2006;20(4):183–189. [DOI] [PubMed] [Google Scholar]