Abstract
Because rotavirus diarrhea can be reduced through vaccination and because current vaccine candidates provide protection against only the most common G antigenic types (G1 to G4), detection of uncommon G types is one of the main goals of rotavirus surveillance. After a 2-year nationwide rotavirus surveillance study in Argentina concluded, surveillance was continued and an increase of G9 prevalence in several Argentine cities was detected. During this period G9 strains predominated in the south, and a gradient of decreasing G9 prevalence was observed from south to north (41 to 0%). Sequence analysis of gene 9, encoding the G antigen, showed that Argentine strains cluster with most G9 isolates from other countries, showing less than 2% nucleotide divergence among them, but are distinctive from them in that they present some unique amino acid changes. Our results agree with reports of increased G9 prevalence in other parts of the world, suggesting the need to incorporate G9 into candidate rotavirus vaccines.
Rotaviruses are the major cause of severe gastroenteritis in infants and young children worldwide (12, 26). Their genome includes 11 segments of double-stranded RNA that are located inside a triple-layered virus particle. Rotaviruses are classified into G and P types, according to the genetic and antigenic diversity of the two outer capsid proteins VP7 and VP4, respectively. At least 14 G types and 20 P types have been identified (9).
Because the severity of disease can be reduced through vaccination, several vaccines are under development to provide specific protection against the most prevalent rotavirus G types, G1 to G4 (2, 3, 7, 11). However, patterns of G type distribution appear to be changing, as less common rotavirus G types, such as G9, have been reported recently to be circulating in the United States (F. E. Campos, P. Azimi, M. A. Staat, T. Berke, L. J. Jackson, D. I. Bernstein, D. Ward, L. K. Pickering, and D. O. Matson, Abstr. 37th Infect. Dis. Soc. Am. [IDSA], abstr. 702, 1999), Malawi, Bangladesh, the United Kingdom, France, and Australia (6, 16, 17, 25, 27, 29). Although the tetravalent rhesus rotavirus vaccine (RRV) was recently withdrawn in the United States, the information available suggests that future vaccines might need to incorporate additional antigenic specificities.
In Argentina, G9 rotaviruses were reported only at a very low prevalence from October 1996 to September 1998 (0.4 and 0.8% each year, respectively), during a nationwide rotavirus surveillance conducted by our laboratory (4). After that study concluded, rotavirus surveillance continued at some locations in 1999 while a permanent surveillance system was being established. Surprisingly, we detected an increase of G9 prevalence in several Argentine cities during the 1998–1999 rotavirus season. Here, we report the high prevalence of G9 strains in our country and present results of epidemiological and virologic analysis of this emerging rotavirus type.
MATERIALS AND METHODS
Surveillance system.
After the previous surveillance study concluded (4), surveillance continued at seven sentinel units (SUs) from September 1998 through June 1999, together with a newly added SU in Ushuaia, mainly to continue monitoring of the prevalence of rotavirus G and P types. SUs participating during this period were placed in the following regions of Argentina (in the indicated large cities): South (Ushuaia), Greater Buenos Aires (La Plata and Buenos Aires), Center (Mendoza, Cordoba, and Rosario), and North (Tucuman and Resistencia). A reference laboratory at the Viral Gastroenteritis Laboratory (VGL) in the National Institute of Infectious Diseases provided core support, collected data from the SUs, and typed the rotavirus strains. Each SU consisted of a hospitalization site and a virology laboratory. A virologist and a pediatrician were responsible for each SU.
The study was conducted in hospitalized patients, under 3 years of age, who presented with diarrhea of less than 5 days' duration. The diagnostic stool samples were collected within 24 h of admission, and patients transferred from another hospital were not included. The patient form and the stool sample were sent to the virology laboratory of the SU.
Rotavirus diagnosis.
Bulk stool specimens (at least 1 g) were received in the virology laboratory at each SU, where they were kept at 4°C. They were tested for rotavirus within a week of collection. Pathfinder (Kallestad, Austin, Tex.) or Rotazyme II (Abbott Laboratories, Abbott Park, Ill.) kits were used following the manufacturers' recommendations. Each of these assays is defined as a confirmatory assay by the U.S. Food and Drug Administration, meaning that they have >95% sensitivity and >95% specificity. After rotavirus diagnosis was completed, positive samples were kept at −20°C until they were shipped to the VGL for further strain characterization.
Characterization of rotavirus strains.
Rotavirus prototype strains Wa, DS1, ST3, K8, 69M, Ito (kindly provided by Roger Glass, Centers for Disease Control and Prevention, Atlanta, Ga.), OSU (Fernando Fernandez, INTA, Buenos Aires, Argentina) and F45 were cultivated in MA104 cells and used as controls for typing assays. Genotyping was carried out using a nested reverse transcription (RT)-PCR method, as previously described, with some modifications (8, 14). Rotavirus RNA was extracted from 10% fecal suspensions using TRIzol (Life Technologies, Inc., Frederick, Md.), following the manufacturer's instructions. Gene 9 was amplified using a pair of generic primers (Beg and End), and then a pool of internal primers for G1, G2, G3, G4, G5, and G9, with consensus primer 9C1, was used. P genotypes were determined by a similar RT-PCR strategy (10). Agarose gel electrophoresis and ethidium bromide staining were performed to visualize resulting bands.
Sequence analysis.
Partial gene 9 (encoding VP7) DNA sequence was obtained from amplicons generated in the first round of the G-typing RT-PCR (28). cDNA was purified with a commercial kit (MicroSpin S-400 HR; Amersham Pharmacia Biotech; or Wizard PCR Preps; Promega) and then sequenced (Thermo Sequenase Cy 5 Dye Terminator Kit, ALFexpress automated sequencer; Amersham Pharmacia Biotech; or ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit; Perkin-Elmer, Applied Biosystems).
Phylogenetic analysis was performed on VP7 sequences (nucleotides 82 to 781) and on partial deduced amino acid sequences of the VP7 gene (amino acids 12 to 244). Alignments were obtained with Clustal X and analyzed using DNAdist or Protdist and Kitsch of the PHYLIP software package. The statistical significance of phylogenies constructed was estimated using the Seqboot program by bootstrap analysis with 100 pseudoreplicate data sets. The tree was displayed with Treeview program. The sequences used for comparison are available in the GenBank/EMBL database.
Nucleotide sequence accession numbers.
The Argentine VP7 gene sequences described in this study have been deposited in the GenBank sequence data base, and the strains and their gene sequence accession numbers respectively, are as follows: Ush1754, AF323707; Ush1755, AF323708; Ush2029, AF323709; Ush1574, AF323710; Ush1575, AF323711; Ush1490, AF323712; Ush1753, AF323713; BA1977, AF323714; BA1939, AF323715; LP1552, AF323716; LP1550, AF323717M; Men 1742, AF323718; Men1740, AF323719.
RESULTS
Characterized samples.
A total of 88 rotavirus-positive fecal samples were characterized at the VGL from September 1998 through June 1999. Unexpectedly, we found a significant increase in the percentage of G9 strains at some SUs, with G9 becoming the third most prevalent type, as shown by the results given in Table 1: G1 was the most prevalent (47%), followed by G4 (28%) and G9 (18%). G9 was most common in the South (Ushuaia), causing 41% of cases, less common in Greater Buenos Aires (27%) and the Center (18%), and least common in the North, where only G1 and G4 strains were present. This gradient of decreasing G9 prevalence was statistically significant (chi-square test for trend, 15.8; P < 0.001). Only two (2%) samples could not be typed, and four samples (5%) were mixed infections.
TABLE 1.
G types from four regions of Argentina during 1998–1999
| Region | No. of strains tested | G typea
|
No. of mixed infections | No. of nontypeable strains | ||
|---|---|---|---|---|---|---|
| G1 | G4 | G9 | ||||
| South | 22 | 8 | 4 | 9 | –b | 1 |
| Greater Buenos Aires | 15 | 1 | 9 | 4 | 1 | – |
| Center | 17 | 10 | 4 | 3 | – | – |
| North | 34 | 22 | 8 | – | 3 | 1 |
| Total (%)c | 88 | 41 (47) | 25 (28) | 16 (18) | 4 (5) | 2 (2) |
Bold numbers indicate predominant type by site.
–, no samples were identified as this type.
Percentage based on tested specimens.
All 16 G9 strains were associated to P[6], and 13 of them, which presented a polyacrylamide gel electrophoresis-positive result, had identical short electropherotypes (Ush1490, LP1550, LP1552, Ush1574, Ush1575, Men1740, Men1742, Ush1753, Ush1754, Ush1755, BA1939, BA1977, Ush2029) (data not shown). G1 and G4 were associated with P[8].
Phylogenetic relationships.
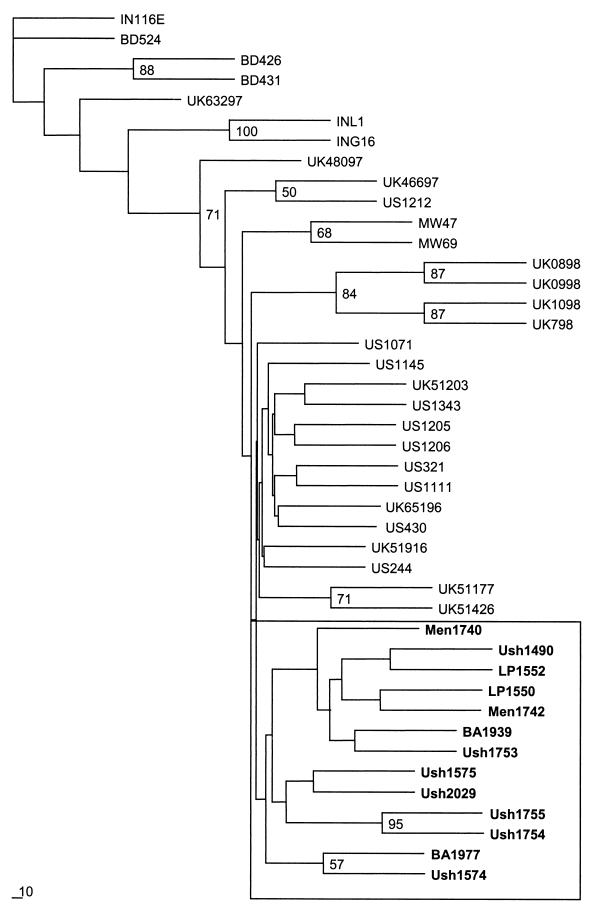
We obtained partial gene 9 sequences for 13 G9 strains, including 7 from Ushuaia (Ush1490, Ush1574, Ush1575, Ush1753, Ush1754, Ush1755, and Ush2029), 4 from Greater Buenos Aires (BA1939, BA1977, LP1550, and LP1552), and 2 from the Center (Men1740 and Men1742). Figure 1 shows the phylogenetic tree obtained from the analysis of these and homologous published sequences. A tree was also obtained from the analysis of nucleotide sequences, but because of the very low divergence among strains, relationships were more distinct in the protein analysis. Most worldwide G9 strains grouped together with Argentine strains into one cluster, with less than 2% nucleotide divergence among them, and just two pairs (Ush1754 and Ush1755; BA1977 and Ush1574) of Argentine strains grouped together, with significant bootstrap values. Only three strains from Bangladesh (BD426, BD431, and BD524), two strains from India (INL1 and ING16), and one strain from Reading, United Kingdom (UK63297), clustered separately, but these presented only 2 to 4% nucleotide divergence compared with Argentine strains. The high sequence conservation among the G9 VP7 sequences is similar to that observed for other G types, which exhibit >91% VP7 amino acid identity (20). The low degree of divergence found among G9 strains agrees with the degree of divergence found among G1, G2, G6, or G8 types in other studies (18, 23, 24, 30). All the G9 strains analyzed in this study were distantly related to the prototype Indian strain 116E, with 12 to 14% nucleotide divergence, demonstrating that this reference strain is quite distinct from most circulating G9 strains. Similar results were found when comparing these strains with prototype G9 strain W161 (data not shown).
FIG. 1.
Phylogenetic analysis of the VP7 deduced amino acid sequences of serotype G9 strains. A phylogenetic tree was constructed based on the Kimura method of the PHYLIP package. Percentage bootstrap values above 50% are shown at the branch nodes. Bold letters indicate Argentine strains. IN, India; UK, United Kingdom; BD, Bangladesh; MW, Malawi; US, United States; Men, Mendoza; Ush, Ushuaia; LP, La Plata; BA, Buenos Aires.
Variation among G9 strains.
Although variation occurred at low frequency, deduced VP7 amino acid sequences differed at several sites among Argentine strains and also when compared with the reference prototype strain IN116E (Fig. 2). In comparison with IN116E, a total of 20 amino acid changes were identified in the region of VP7 analyzed, of which 16 were present in all Argentine samples. Moreover, 11 of these changes occurred in regions of the VP7 protein (variable regions) that are normally highly divergent among members of different G types. Some changes were unique among the characterized strains such as at positions 42 and 51 in Ush1754 and Ush1755 and at positions 217 and 230 in a strain from Buenos Aires (BA 1977). One amino acid substitution unique and consistent among the Argentine strains was found: at position 171, threonine present in every isolate but strain IN116E (which has an alanine at this position) was replaced by an isoleucine.
FIG. 2.
Deduced amino acid sequences of Argentine strains compared to reference strain 116E. Amino acids underlined and in bold letters indicate regions that are highly variable among G types.
DISCUSSION
In a countrywide surveillance network operating between 1996 and 1998, human rotavirus G9 strains was detected at a low prevalence in Argentina (0.4% during 1996–1997 and 0.8% during 1997–1998) (4, 5). Continuation of the surveillance into 1999 revealed a sharp increase in G9 prevalence (18% overall), not equally distributed across the country, being highest in the south (41%) and lowest in the north (0%). Human rotavirus G9 strains had not been previously reported at high frequencies in Latin American countries, except for a case report from Brazil during a vaccine trial (21). The increased prevalence in Argentina is consistent with what appears to be an extending global distribution of this type (6, 16, 17, 25, 27).
The increased prevalence of G9 strains during the 10-month period made it the third most prevalent G type during that period. This was especially notable in Ushuaia, where the G9 prevalence at 41% represents the highest G9 prevalence ever reported. It is also interesting to note the gradient of decreasing G9 prevalence from south to north. This gradient may be attributable to several reasons, including the extremely different climate conditions in Ushuaia, because it is the most southern city in our country or because it attracts tourists not only from elsewhere in Argentina but also from other countries, potentially enabling the introduction and emergence of a new strain there.
G9 strains may not have been detected in previous studies in our country due to the unavailability of monoclonal antibodies directed against this type. A previous study conducted in Argentina from 1983 to 1985, in which rotavirus-positive samples were characterized using monoclonal antibodies directed against G1 to G4 serotypes only, detected 5.5% nontypeable strains (13). Moreover, there are studies reporting that some G4-specific monoclonal antibodies reacted with rotavirus G9 strains (29). Although another study recently conducted in the southern region of Buenos Aires, based on RT-PCR characterization, did not detect any G9 strains, this may be due to the restricted geographic area analyzed (1). Despite that, we confirmed the emergence of this type during 1998–1999 by continuing multisite surveillance and utilizing genotyping assays capable of detecting G9 strains.
Many studies report differences among strains of the same genotype, which often describe sublineages of the same G type (19, 22). Therefore, it is notable that several G9 strains from distant parts of the world, such as the United Kingdom, the United States, Malawi, and Argentina, clustered together in the phylogenetic analysis. The high degree of identity found among this group and the fact that they are more closely related to each other than to reference strain 116E suggest that the same strains are emerging all around the world and that they are a recent introduction in the population.
Most strains from Ushuaia share distinctive changes located in highly variable regions that may define a common antigenic pattern. A singular amino acid substitution at position 171 was present only in all Argentine strains. This pattern of substitution agrees with the graphical representation of amino acid exchangeability according to Argyle's method (15). This method assumes that depending on the protein, 60 to 90% of the observed amino acid replacements involve the nearest or second-nearest neighbors of the amino acid ring, where alanine is followed by threonine and then by isoleucine. This succession of amino acid changes is clearly shown in Fig. 2. The oldest strain analyzed (IN116E) has at this position an alanine, which is then replaced by a threonine (present in other non-Argentine strains) and eventually by isoleucine, an amino acid present only in Argentine strains, which are the newest isolates in this analysis. However, the replacement of a less hydrophobic residue like threonine (−0.7 according to the Kyte and Doolittle scale) or alanine (1.8) by a highly hydrophobic amino acid like isoleucine (4.5) shows that a generally constrained property of proteins is being modified. Although this change is not present in a highly variable region, where the major neutralization epitopes have been identified (amino acids 87 to 99, 145 to 150, and 211 to 223), the substitution of amino acids with very different properties may affect viral structure and protein properties. Considering the low degree of diversity among G9 strains, a single substitution that is found only in Argentine strains appears to identify our strains, because no other similar substitution was shared by every strain from another country. The pattern of amino acid exchangeability shown by Argyle's method is also valid for most changes observed among strains analyzed (Fig. 2).
Finally, this study has limitations. Relatively few samples were analyzed in some locations during a short study period, and so the results may not accurately reflect the prevalence of G9 strains in the studied population. The absence of G9 strains in the northern area of the country may also be attributed to the same fact. Although we are showing the increased prevalence of G9 strains in several locations during 1999, it is possible that the emergence of this G type in Ushuaia occurred prior to 1999, because this location was not included in the first surveillance period (4). Despite that, our results agree with the increasing reports of G9 strains in other parts of the world, suggesting that the incorporation of this type into candidate rotavirus vaccines should be considered. In addition, these results emphasize the need to install a continual surveillance system for rotavirus infections at several locations, in order to monitor the prevalence of this or other emerging strains properly. This will facilitate determination of the appropriate rotavirus vaccine constituents at launch and subsequently.
ACKNOWLEDGMENTS
We thank all the professionals and nonprofessionals from the Sentinel Units who kindly contributed to this study.
This work was partially supported by a grant from John Wyeth Laboratories and a grant from the National Agency for the Advancement of Science (PICT' 98 no. 0503533 to J.G.).
REFERENCES
- 1.Arguelles M H, Villegas G A, Castello A, Abrami A, Ghiringhelli P D, Semorile L, Glikmann G. VP7 and VP4 genotyping of human group A rotavirus in Buenos Aires, Argentina. J Clin Microbiol. 2000;38:252–259. doi: 10.1128/jcm.38.1.252-259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein D I, Sack D A, Rothstein E, Reisinger K, Smith V E, O'Sullivan D, Spriggs D R, Ward R L. Efficacy of live, attenuated, human rotavirus vaccine 89-12 in infants: a randomised placebo-controlled trial. Lancet. 1999;354:287–290. doi: 10.1016/S0140-6736(98)12106-2. [DOI] [PubMed] [Google Scholar]
- 3.Bishop R F. Development of candidate rotavirus vaccines. Vaccine. 1993;11:247–254. doi: 10.1016/0264-410x(93)90025-s. [DOI] [PubMed] [Google Scholar]
- 4.Bok K, Castagnaro N, Borsa A, Nates S, Espul C, Fay O, Fabri A, Grinstein S, Miceli I, Matson D, Gomez J. Surveillance for rotavirus in Argentina. J Med Virol. 2001;65:190–198. [PubMed] [Google Scholar]
- 5.Bok K, Castagnaro N C, Diaz N E, Borsa A, Cagnoli M R, Nates S, Yudowsky S, Espul C, Cuello H, Fay O, Brunet B, Ues O C, Santoro R, Grinstein S, Gonzalez F, Miceli I, Gomez J A. [Rotavirus laboratory network: results after one year of observation] Rev Argent Microbiol. 1999;31:1–12. . (In Spanish.) [PubMed] [Google Scholar]
- 6.Bon F, Fromantin C, Aho S, Pothier P, Kohli E The Azay Group. G and P genotyping of rotavirus strains circulating in France over a three-year period: detection of G9 and P[6] strains at low frequencies. J Clin Microbiol. 2000;38:1681–1683. doi: 10.1128/jcm.38.4.1681-1683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements-Mann M L, Makhene M K, Mrukowicz J, Wright P F, Hoshino Y, Midthun K, Sperber E, Karron R, Kapikian A Z. Safety and immunogenicity of live attenuated human-bovine (UK) reassortant rotavirus vaccines with VP7-specificity for serotypes 1, 2, 3 or 4 in adults, children and infants. Vaccine. 1999;17:2715–2725. doi: 10.1016/s0264-410x(98)00497-6. [DOI] [PubMed] [Google Scholar]
- 8.Das B K, Gentsch J R, Hoshino Y, Ishida S, Nakagomi O, Bhan M K, Kumar R, Glass R I. Characterization of the G serotype and genogroup of New Delhi newborn rotavirus strain 116E. Virology. 1993;197:99–107. doi: 10.1006/viro.1993.1570. [DOI] [PubMed] [Google Scholar]
- 9.Estes M. Rotaviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1625–1655. [Google Scholar]
- 10.Gentsch J R, Glass R I, Woods P, Gouvea V, Gorziglia M, Flores J, Das B K, Bhan M K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentsch J R, Woods P A, Ramachandran M, Das B K, Leite J P, Alfieri A, Kumar R, Bhan M K, Glass R I. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J Infect Dis. 1996;174:S30–S36. doi: 10.1093/infdis/174.supplement_1.s30. [DOI] [PubMed] [Google Scholar]
- 12.Glass R I, Kilgore P E, Holman R C, Jin S, Smith J C, Woods P A, Clarke M J, Ho M S, Gentsch J R. The epidemiology of rotavirus diarrhea in the United States: surveillance and estimates of disease burden. J Infect Dis. 1996;174:S5–S11. doi: 10.1093/infdis/174.supplement_1.s5. [DOI] [PubMed] [Google Scholar]
- 13.Gomez J, Estes M K, Matson D O, Bellinzoni R, Alvarez A, Grinstein S. Serotyping of human rotaviruses in Argentina by ELISA with monoclonal antibodies. Arch Virol. 1990;112:249–259. doi: 10.1007/BF01323169. [DOI] [PubMed] [Google Scholar]
- 14.Gouvea V, Glass R I, Woods P, Taniguchi K, Clark H F, Forrester B, Fang Z Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graur D, Li W. Fundamentals of molecular evolution. 2nd ed. Sunderland, Mass: Sinauer Associates, Inc.; 1999. [Google Scholar]
- 16.Griffin D D, Kirkwood C D, Parashar U D, Woods P A, Bresee J S, Glass R I, Gentsch J R. Surveillance of rotavirus strains in the United States: identification of unusual strains. The National Rotavirus Strain Surveillance System collaborating laboratories. J Clin Microbiol. 2000;38:2784–2787. doi: 10.1128/jcm.38.7.2784-2787.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iturriza-Gomara M, Cubitt D, Steele D, Green J, Brown D, Kang G, Desselberger U, Gray J. Characterisation of rotavirus G9 strains isolated in the UK between 1995 and 1998. J Med Virol. 2000;61:510–517. doi: 10.1002/1096-9071(200008)61:4<510::aid-jmv15>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Jayasinghe S M, Palombo E A. Genetic homogeneity of human serotype G1 rotaviruses isolated during a single epidemic season: implications for vaccine strategies. Acta Virol. 1999;43:53–55. [PubMed] [Google Scholar]
- 19.Jin Q, Ward R L, Knowlton D R, Gabbay Y B, Linhares A C, Rappaport R, Woods P A, Glass R I, Gentsch J R. Divergence of VP7 genes of G1 rotaviruses isolated from infants vaccinated with reassortant rhesus rotaviruses. Arch Virol. 1996;141:2057–2076. doi: 10.1007/BF01718215. [DOI] [PubMed] [Google Scholar]
- 20.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1657–1708. [Google Scholar]
- 21.Linhares A C, Gabbay Y B, Mascarenhas J D, de Freitas R B, Oliveira C S, Bellesi N, Monteiro T A, Lins-Lainson Z, Ramos F L, Valente S A. Immunogenicity, safety and efficacy of tetravalent rhesus-human, reassortant rotavirus vaccine in Belem, Brazil. Bull W H O. 1996;74:491–500. [PMC free article] [PubMed] [Google Scholar]
- 22.Maunula L, von Bonsdorff C H. Short sequences define genetic lineages: phylogenetic analysis of group A rotaviruses based on partial sequences of genome segments 4 and 9. J Gen Virol. 1998;79:321–332. doi: 10.1099/0022-1317-79-2-321. [DOI] [PubMed] [Google Scholar]
- 23.Palombo E A, Bishop R F. Genetic and antigenic characterization of a serotype G6 human rotavirus isolated in Melbourne, Australia. J Med Virol. 1995;47:348–354. doi: 10.1002/jmv.1890470410. [DOI] [PubMed] [Google Scholar]
- 24.Palombo E A, Clark R, Bishop R F. Characterisation of a “European-like” serotype G8 human rotavirus isolated in Australia. J Med Virol. 2000;60:56–62. [PubMed] [Google Scholar]
- 25.Palombo E A, Masendycz P J, Bugg H C, Bogdanovic-Sakran N, Barnes G L, Bishop R F. Emergence of serotype G9 human rotaviruses in Australia. J Clin Microbiol. 2000;38:1305–1306. doi: 10.1128/jcm.38.3.1305-1306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parashar U D, Bresee J S, Gentsch J R, Glass R I. Rotavirus. Emerg Infect Dis. 1998;4:561–570. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramachandran M, Gentsch J R, Parashar U D, Jin S, Woods P A, Holmes J L, Kirkwood C D, Bishop R F, Greenberg H B, Urasawa S, Gerna G, Coulson B S, Taniguchi K, Bresee J S, Glass R I. Detection and characterization of novel rotavirus strains in the United States. J Clin Microbiol. 1998;36:3223–3229. doi: 10.1128/jcm.36.11.3223-3229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanger F, Nicklen S, Coulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Unicomb L E, Podder G, Gentsch J R, Woods P A, Hasan K Z, Faruque A S, Albert M J, Glass R I. Evidence of high-frequency genomic reassortment of group A rotavirus strains in Bangladesh: emergence of type G9 in 1995. J Clin Microbiol. 1999;37:1885–1891. doi: 10.1128/jcm.37.6.1885-1891.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zao C L, Yu W N, Kao C L, Taniguchi K, Lee C Y, Lee C N. Sequence analysis of VP1 and VP7 genes suggests occurrence of a reassortant of G2 rotavirus responsible for an epidemic of gastroenteritis. J Gen Virol. 1999;80:1407–1415. doi: 10.1099/0022-1317-80-6-1407. [DOI] [PubMed] [Google Scholar]