Abstract
Postharvest losses of cut flowers is one of the considerable challenges restricting their efficient marketability. Consequently, such challenges have triggered a constant hunt for developing compatible postharvest treatments to mitigate postharvest losses. Interestingly, recent studies entrench extensive role of salicylic acid (SA) in mitigating postharvest losses in various flower systems. The current investigation focusses on role of SA in augmenting physiological and biochemical responses to mitigate postharvest senescence in cut spikes of Consolida ajacis. The cut spikes of C. ajacis were supplemented with various SA treatments viz, 2 mM, 4 mM, 6 mM. The effects of these treatments were evaluated against control set of spikes placed in distilled water. Our study indicates considerable increment in postharvest longevity of cut spikes, besides an increase in solution uptake, sugar and protein content of tepal tissues.SA augmented antioxidant system via upsurge in phenolic content and antioxidant enzymes viz, superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) to forfend reactive oxygen species (ROS) related oxidative damage. SA profoundly reduced lipoxygenase (LOX) activity to preserve the membrane integrity and thus prevented seepage of solutes from tepal tissues. These results authenticate SA particularly 4 mM concentration as effective postharvest treatment to preserve the postharvest quality of C. ajacis cut spikes.
Keywords: Salicylic acid, Superoxide dismutase, Catalase, Ascorbate peroxidase, Sugars
1. Introduction
Postharvest senescence is a crucial factor afflicting the marketability of cut flowers (Babarabie, 2018). Consequently, quality preservation of cut flowers is arguably an ongoing challenge for florists (Schroeder and Stimart, 2005). The key factors provoking postharvest senescence include depletion of carbohydrates, increase in temperature and respiration rates, microbial interference, water stress and ethylene sensitivity (Gupta and Dubey, 2018). Instigation of postharvest senescence adversely affects various physiological and biochemical attributes resulting in water loss from the senescing tissue, ion leakage, upsurge in ROS, alteration in membrane fluidity, nucleic acids breakdown, decline in carbohydrates and proteins (Lone et al., 2021). Flower longevity is an important quality attribute determining the customer satisfaction and market value of cut flowers (Vehniwal and Abbey, 2019). Therefore, optimization of suitable postharvest treatments to improve flower longevity demands a critical attention (Nguyen et al., 2020). C. ajacis is an exquisite ornamental plant producing entrancing spikes of different hues and colors. It is highly sensitive to ethylene which provokes senescence of its cut spikes (Shahri et al., 2011). Visible signs of postharvest senescence in C. ajacis flowers include wilting followed by abscission of tepals. Earlier studies indicate the use of several anti-ethylene treatments in combating postharvest senescence in cut spikes of C. ajacis (Shahri and Tahir, 2010), yet the efficacy of salicylic acid (SA) has not been reconnoitered in improving postharvest longevity in cut spikes of C. ajacis. SA is a phenolic compound occurring ubiquitously in plants (Singh et al., 2018). It is widely used plant growth regulator which is also effective in other forms in plants such as acetyl salicylic acid and methyl salicylate (Heidarnezhadian et al., 2017). It is synthesized in plants via shikimate pathway by two metabolic routes viz, phenylalanine route and isochorismate route. Phenylalanine pathway operates in cytolplasm while as isochorismate pathway operates in chloroplast (Sharma et al., 2020). It mediates defense response in plants and regulates various physiological processes like seed germination, crop yield, plant growth, photosynthetic efficiency, senescence and flowering (Handa et al., 2017, Hernández et al., 2017, Ahmad et al., 2018). SA not only confers resistance against biotic stress but also provides tolerance against various types of abiotic stresses through osmolite accumulation, synthesis of secondary metabolites, increasing activity of ROS scavenging enzymes, regulation of other hormonal pathways and mineral uptake (Kohli et al., 2018a, Kohli et al., 2018b, Kohli et al., 2019, Kaya et al., 2020a, Kaya et al., 2020b, Koo et al., 2020). Over the recent years, SA has gained increasing interest due to its role in postharvest quality maintenance of fruits and vegetables (Shabanian et al., 2019). Owing to its potential role as postharvest preservative, SA has also been reported to improve longevity in flowers like, Rosa hybrida, Dianthus caryophyllus, Lilium pumilum and Chrysanthemum (Phi et al., 2021). SA has been implicated in suppression of ethylene biosynthesis by impeding conversion of ACC to ethylene (Hassan and Ali, 2014) which otherwise trigger surge in ROS and provoke senescence. It also counteracts oxidative stress through upsurge in antioxidant enzyme activity viz., CAT, SOD, POD and APX and thus curtail lipid peroxidation to prevent membrane outpouring of petal tissues (Tareen et al., 2012, Ahmad et al., 2011, Ahanger et al., 2020, Kaya et al., 2020a, Kaya et al., 2020b). Moreover, SA has been found to modulate senescence in Nicotiana plumbaginifolia by improving sugar and protein content of petal tissues (Nisar et al., 2021). In view of multifaceted implications of SA in senescence regulation, the current investigation was undertaken to explicate the efficacy of SA in alleviating postharvest losses in cut spikes of C. ajacis to ameliorate their display life.
2. Material methods
2.1. Plant material
In our study C. ajacis spikes were obtained from experimental plots at commercial maturity and transported to laboratory in water filled bucket. Each spike was defoliated and excised to 30 cm length under water to prevent vascular occlusion. The processed spikes were put in 100 ml Erlenmeyer flasks containing SA test solutions with different concentrations viz., 2 mM, 4 mM and 6 mM. The effect of these treatments was evaluated against control set of spikes held in distilled water. All the treatments as well as control comprised of 10 flasks (replicates). After standardization, 4 mM SA, was found to be optimum concentration in improving various postharvest attributes. SA concentrations above 6 mM were found to be toxic provoking early senescence. The different biochemical assays were performed by randomly selecting open flowers from each treatment at different time intervals (day 2nd and day 5th). The day of transfer of C. ajacis spikes to test solutions was designated as day zero. The treatment effects were observed by keeping the spikes in laboratory under 12 h light period per day, at a temperature of 25 ± 2 °C and RH of 60 ± 10 %.
2.2. Postharvest longevity and floral diameter
Postharvest longevity of C. ajacis spikes was calculated from day 1 of transfer to test solutions till senescence of 70% florets on each spike. During the study, the floral diameter was estimated as mean of two perpendicular distances across a flower at different time intervals.
2.3. Membrane stability index (MSI)
The membrane stability index was measured by Sairam’s method 1994. It was estimated as leakage of electrolytes from the sample tissues. The formula for calculation of MSI is given as;
Where C1 & C2 represent the tissue samples incubated at 25 °C and 100 °C respectively in deionized water.
2.4. Bacterial density and solution uptake
The bacterial contamination of holding solutions was estimated by Naing et al. (2017), using UV–VIS spectrophotometer. The bacterial density was expressed as cfu ml−1. Solution uptake (ml) was measured as the difference between the total volume of vase solution and solution left in the vases after the senescence of spikes.
2.5. Protein quantification method
Protein content of the sample tissue was quantified according to Lowry et al. (1951) method using bovine serum albumin as a standard.
2.6. Sugar and phenol quantification method
For estimation of sugars and phenols 1 g of tepal tissue was taken from each treatment and fixed in 70% boiling ethanol. This tissue was later mashed and centrifuged. After centrifugation suitable volume of supernatant was taken for estimation of sugars and phenols. The amount of reducing sugars in sample tissues was quantified by following a procedure elaborated by Nelson (1944). Total sugars were estimated by converting non-reducing sugars to reducing sugars using invertase. The quantity of non-reducing sugars was obtained as difference between total sugars and reducing sugars. Phenols were quantified by a method described by Swain and Hillis (1959) using gallic acid as standard.
2.7. Quantification of antioxidant enzymes
2.7.1. Superoxide dismutase
Superoxide dismutase activity was determined by Dhindsa et al. (1981) method and the activity of enzyme was expressed as units min−1 mg−1 protein.
2.7.2. Ascorbate peroxidase
Ascorbate peroxidase activity of tepal tissues was estimated by following Chen and Asada (1989) protocol. The units of APX activity were expressed as units min−1 mg−1 protein.
2.7.3. Catalase
The activity of catalase enzyme was quantified as consumption of H2O2 at 240 nm for 3 min as described by Aebi (1984) method. The units of enzyme activity were expressed as units min−1 mg−1 protein.
2.7.4. Lipoxygenase
Lipoxygenase activity of the tepal tissues was estimated by Axelrod et al. (1981) method and was expressed as μmol. min−1 mg−1 protein.
2.8. Layout of the experiment and statistical analysis of the data
Complete randomized design was followed during the experiment and ANOVA was used to compare the treatment means (analysis of variance). Each value is obtained as mean of three biological replicates. Treatments were considered significant at (p < 0.05) according to Duncan’s multiple range test. SPSS software was used for statistical analysis of the data.
3. Results
3.1. Postharvest longevity and floral diameter
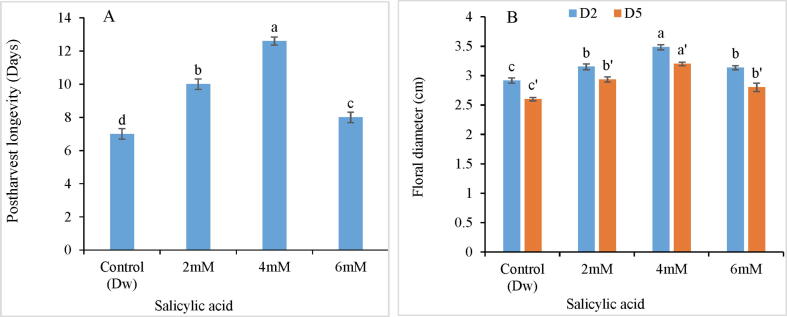
Postharvest longevity was profoundly accentuated in all SA treatments as compared to control Fig. 1. The maximum increase in postharvest longevity (80%) was observed in 4 mM SA treatment Fig. 2A. In addition to postharvest longevity the flower diameter also exhibited maximum increase (17.24 %) at 4 mM SA. However with the progression of time, a marginal decrease was recorded in flower diameter as revealed by day 5th analysis. Fig. 2B.
Fig. 1.
(A-D). Postharvest longevity C. ajacis cut spikes as affected by application of different SA treatments on day 0th(a), day 2th (b), day 5th (c) and day 11th (d) of experiment.
Fig. 2.
(A-B). Variation in postharvest longevity (A) and floral diameter (B) of C. ajacis cut spikes at different time intervals in response to application of different SA treatments. Data are represented as mean of 3 replicates ± S.E. Different letters above the error bars indicate significant differences between different treatments as determined by Duncan’s test.
3.2. Bacterial density and solution uptake
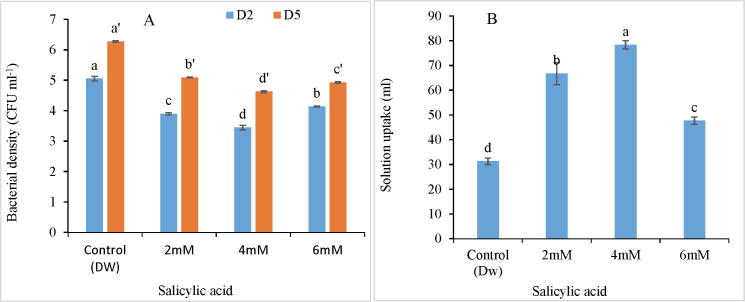
SA treatments improved the solution uptake in C. ajacis cut spikes by reducing the bacterial density in holding solutions as depicted in Fig. 3A-B.Minimum bacterial density was recorded in 4 mM SA treatments. Bacterial density decreased by 32.6% in 4 mM SA solutions while as, solution uptake of spikes held in this solution was increased by 150.15%. Highest bacterial density was observed in control treatments while as lowest bacterial density was recorded in SA treatments. However, at day 5, the bacterial density was found to increase both in control and SA treatments but increase in bacterial density was found to be much higher in control as compared to SA treatments.
Fig. 3.
Variation in Bacterial density (A) and solution uptake (B) of C. ajacis cut spikes at different time intervals in response to application of different SA treatments. Data are represented as mean of 3 replicates ± S.E. Different letters above the error bars indicate significant differences between different treatments as determined by Duncan’s test.
3.3. Membrane stability index and lipoxygenase activity
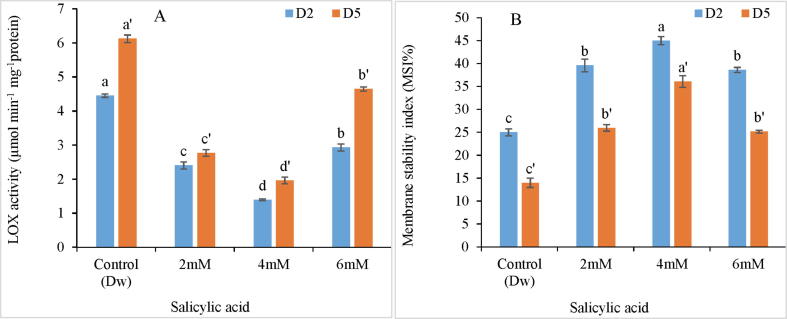
During the current study, SA maintained the membrane stability of tepal tissues as compared to control by preventing membrane lipid peroxidation through attenuation of LOX activity. Among the range of treatments, MSI values increased by 80.32% in 4 mM SA treatment while as, LOX activity decreased by 70.4 % in these tepal tissues. However, MSI values exhibited a declining trend, while as LOX activity exhibited an increasing trend towards the advanced phase of flower development as evident from the second analysis carried on day 5th of the experiment Fig. 4A-B.
Fig. 4.
Variation in LOX activity (A) and membrane stability index (B) of C. ajacis cut spikes at different time intervals in response to application of different SA treatments. Data are represented as mean of 3 replicates ± S.E. Different letters above the error bars indicate significant differences between different treatments as determined by Duncan’s test.
3.4. Soluble proteins
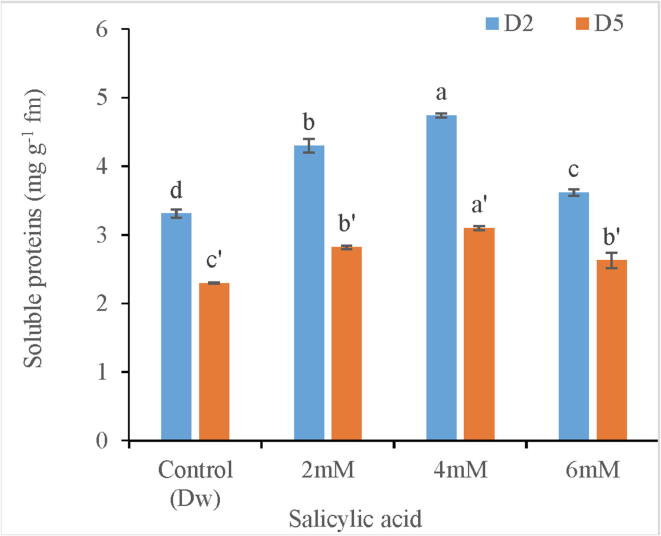
Decrease in soluble protein content of tepal tissues due to upregulation of protease activity marks the key feature of petal senescence. However, during the present investigation soluble proteins were found to be in higher concentration in SA treated tepal tissues as compared to control. Among the different SA treatments, highest increase in soluble proteins (42.4%) was found in tepal tissues treated with 4 mM SA. Moreover, Protein content of tepal tissues gradually declined towards the end stage of experiment Fig. 5.
Fig. 5.
Variation in soluble protein content of C. ajacis cut spikes at different time intervals in response to application of different SA treatments. Data are represented as mean of 3 replicates ± S.E. Different letters above the error bars indicate significant differences between different treatments as determined by Duncan’s test.
3.5. Sugar and phenolic content
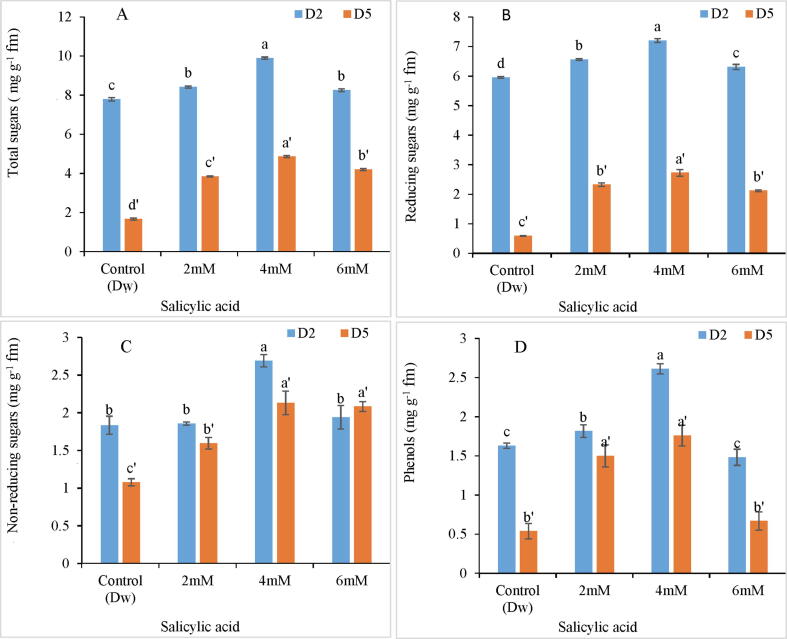
Exogenous inclusion of SA resulted in significant upsurge in sugar and phenolic content of tepal tissues in comparison to control. Maximum enrichment of sugars (27.27%) and phenols (62.5%) was stimulated by 4 mM SA as signposted in Fig. 6A-D. The phenolic content increased by two fold as compared to sugars. On the contrary, both sugar and phenolic content declined with the passage of time from day 2 to day 5.
Fig. 6.
Variation in total sugar (A), reducing sugar (B), non-reducing sugars (C) and phenolic content (D) of C. ajacis cut spikes at different time intervals in response to application of different SA treatments. Data are represented as mean of 3 replicates ± S.E. Different letters above the error bars indicate significant differences between different treatments as determined by Duncan’s test.
3.6. Antioxidant enzymes
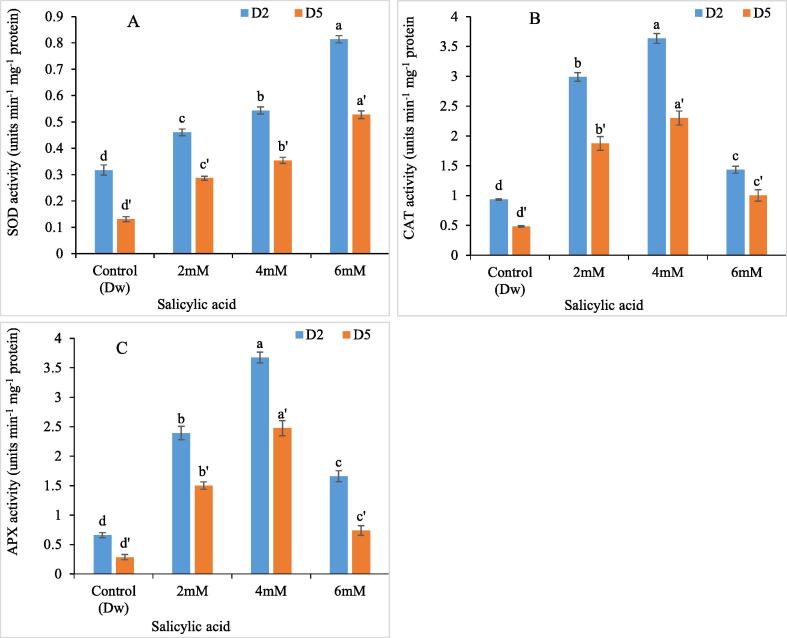
The activity of antioxidant enzymes like CAT, APX and SOD was profoundly upregulated in SA treated tepal tissues as compared to control. The peak in antioxidant enzyme activity was recorded in flower tissues held in 4 mM SA. The increase in SOD, CAT and APX enzymes was about 74.19%, 287%, 464% respectively. However the enzymatic activity decreased with onset of senescence in tepal tissues towards the end phase of experiment. Fig. 7A-C.
Fig.7.
Variation in SOD (A) CAT (B) APX (C) activity of C. ajacis cut spikes in response to application of different SA treatments. Data are represented as mean of 3 replicates ± S.E. Different letters above the error bars indicate significant differences between different treatments as determined by Duncan’s test.
4. Discussion
Postharvest longevity of cut flowers is one of the key issues challenging the progress and development of floriculture industry (Hussen and Yassin, 2013). Moreover, cut flowers act as an expedient and realistic experimental system for understanding flower senescence. Clipping of cut flowers from their mother plant deprives them from incessant supply of water and nutrients (Patel et al., 2018). Hence, inclusion of postharvest treatments is essential for their prolonged survival (Nair et al., 2006). In our study, exogenous inclusion of SA considerably delayed the postharvest senescence in cut spikes of C. ajacis. Increase in postharvest longevity due to SA application has also been reported in Alstromeria peruviana, Gerbera jamesonii, Lillium asiaticum, Rosa hybrida and Polianthes tuberose (Bayat and Aminifard, 2017). Postharvest senescence is triggered by water loss and reduced water uptake owing to bacterial xylem occlusion (van Meeteren, 1981). Our results indicate maximum solution uptake and reduced bacterial count in SA containing solutions as compared to control. The increased solution uptake may be attributed to germicidal activity of SA, which can prevent the vascular blockage by suppressing bacterial proliferation thereby, maintaining continuous flux through xylem vessels (Singh et al., 2018). Moreover, SA fosters stomatal closure to slow down respiratory rate and to prevent water loss through transpiration (Hatamzadeh et al., 2012). Besides postharvest longevity, floral diameter also exhibited a marginal increase by SA application. The increase in floral diameter may be ascribed to increased solution uptake which maintains the turgidity of the petal tissues and preserves display quality of cut flowers (Bayat and Aminifard, 2017). Furthermore, SA has been implicated in suppression of ethylene production which might prevent onset of senescence as observed in Companula and Rosa (Bahrami et al., 2013).
Proteolysis is the hallmark of petal senescence as recognized in various flower systems such as Petunia, Rosa, Iris, Dianthus, Hemerocallis, Sandersonia and Gladiolus (Tripathi et al., 2009). During the current investigation SA treated spikes maintained higher concentration of soluble proteins in tepal tissues as compared to control. Reportedly, SA functions as protease inhibitor to attenuate proteolysis and maintains higher concentration of proteins in petal tissues (Kant and Arora, 2012). Higher protein content was correlated with the prolonged vaselife of various flowers like Rosa and Calendula (Farshid et al., 2012, Lone et al., 2021). Proteins are also metabolized as alternate respiratory substrates to circumvent sugar starvation (Hirota et al., 2018). Furthermore, protein accumulation activates stress-related defense mechanisms by augmenting antioxidant enzyme activity and stimulating stress-specific protein synthesis, to improve postharvest longevity (Doganlar et al., 2010, Promyou et al., 2012).
Membrane outpouring is inversely related to flower longevity. It is a key indicator signaling the onset of senescence (Khandan-Mirkohi et al., 2021). Our study revealed, higher values of MSI in SA treated tepal tissues as compared to control. These values corresponded to lower LOX activity. SA preserves membrane consistency by impairing LOX activity through conversion of LOX-Fe+3 to LOX-Fe+2 (Lapenna et al., 2009). Increase in lipoxygenase activity prompts breakdown of membrane lipids and alters membrane integrity (Shabanian et al., 2019). SA has been found to extend the vase life in various flowers like Rosa, Lisianthus and Chrysanthemum by preserving membrane integrity (Gerailoo and Ghasemnezhad, 2011, Bahrami et al., 2013, Balieiro et al., 2018). Moreover, SA masks free radical production which otherwise trigger peroxidation of lipids and impairs synthesis of cell membrane macromolecules and cytoplasm (Rao et al., 1997, El-Tayeb et al., 2006).
SA treated spikes exhibited profound phenolic enrichment in the tepal tissues as compared to untreated ones. SA augments PAL (phenyl ammonia lyase) activity coupled with decrease in PPO activity (Polyphenol oxidase), which causes phenolic accumulation and preserves quality of cut products by preventing oxidation of phenolic compounds which otherwise cause browning of fruits, cut flowers and vegetables (Wei et al., 2011, Siddiqui et al., 2016, Shabanian et al., 2019). Phenolic enrichment reinforces antioxidant defense mechanisms and prevents flowers from oxidative stress through scavenging of free radicals (Ahmad and Tahir, 2017). Moreover, SA treatments can be suggested as relevant approach to improve floral vase life by reinforcing antioxidant system as observed in various flower systems such as Lisianthus, Petunia and Rosa (Ghadimian and Danaei, 2016, Nisar et al., 2018, Pourzarnegar et al., 2020). Thus, in the light of our findings decrease in phenolic content can be suggested as a driving force for senescence instigation in cut spikes of C. ajacis due to impaired antioxidant activity.
Excision of flowers from the mother plant causes a decrease in concentration of sugars due to interruption of nutrient supply, which negatively affects flower longevity (Gómez-Merino et al., 2020). Pertinently, inclusion of SA in the holding solutions reduced the carbohydrate starvation and improved the sugar content in tepal tissues of C. ajacis. Maintenance of higher sugar content in C. ajacis tepal tissues can be correlated with their prolonged vase life as observed in Petunia hybrida and Gerbera jasmonii (Nisar et al., 2018, Hemati et al., 2019). Moreover, higher sugar content in C. ajacis tepal tissues may be ascribed to increase in solution uptake and sugar translocation by SA which foster accumulation of more resources thereby, maintaining petal turgidity due to optimum water content and delay senescence as observed in Gladiolus flowers (Ezhilmathi et al., 2007, Saeed et al., 2016). Sugars serve as osmoticum to regulate osmotic changes, maintain membrane integrity and counteract ROS against different types of stresses (Keunen et al., 2013, Singh et al., 2015). Also, sugars induce synthesis of anthocyanins responsible for petal coloration and hence improve flower quality. Decrease in carbohydrates lead to undesirable color changes and ultimately increases susceptibility to microorganisms (Hemati et al., 2019).
Oxidative stress due to ROS accumulation is another key factor affecting postharvest quality of flowers. Oxidative stress impairs vital physiological functions and provokes flower senescence (Arora et al., 2007). Petal tissues with attenuated antioxidant enzyme activity (APX) exhibit early signs of senescence due to ROS accumulation (Mittler et al., 2004, Saeed et al., 2014). Consequently, elicitation of antioxidant machinery is a prerequisite condition for mitigation of oxidative stress. In current investigation, application of SA in holding solution augmented the activity of various antioxidant enzymes such as SOD, CAT and APX which act as effective defense system against oxidative stress (Kohli et al., 2018a, Kohli et al., 2018b, Kohli et al., 2019, Kaya et al., 2020a, Kaya et al., 2020b, Ahanger et al., 2020). These findings suggest the conspicuous role of SA in orchestrating antioxidant system and regulation of ROS levels in the cell as reported by Khan et al. (2015). SA mediated surge in antioxidant enzymes (SOD, CAT and APX) has also been reported in gerbera cultivars (Shabanian et al., 2019). Moreover, upregulation in antioxidant enzymes positively improve the postharvest longevity and preserve floral quality as observed in Calendula flowers (Lone et al., 2021).
5. Conclusion and future prospects
In conclusion, our research authenticates SA as effective postharvest treatment in alleviating postharvest senescence. Inclusion of SA ameliorated postharvest longevity of C. ajacis spikes by modulating physiological and biochemical attributes such as proteins, sugars and antioxidant system. Moreover, the current research offers an immense scope for molecular investigation to dig out the expression patterns of various senescence associated genes underlying the senescence of C. ajacis flowers. In addition, understanding crosstalk of SA with other phytoharmones especially ethylene will unravel vast vistas of signaling networks involved in regulation of senescence process. Together, these studies will bridge the knowledge gaps and enable us to formulate cost effective and ecofriendly postharvest treatments for efficient marketing of these cut spikes, which can have huge economic implications.
Declarations
Conflicts of interest/Competing interests: Authors declare that no conflict of interest exists.
Ethics approval: Not Applicable.
Consent to participate: All authors consent to participate in this manuscript.
Consent for publication: All authors consent to publish this manuscript in Saudi Journal of Biological Science.
Availability of data and material: Data will be available on request to corresponding or first author.
Code availability: Not Applicable.
Author contributions
AuH and IT designed the experimental setup and AuH performed the experiments. MLL, SP, FA and SF helped in data collection and initial draft of the manuscript. PK and HAES analysed the data and helped in revision of this manuscript. All authors read the manuscript before communication.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/19), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Ahanger M.A., Aziz U., Alsahli A.A., Alyemeni M.N., Ahmad P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vigna angularis. Biomolecules. 2020;10(1):42. doi: 10.3390/biom10010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P., Alyemeni M.N., Ahanger M.A., Egamberdieva D., Wijaya L., Alam P. Salicylic Acid (SA) Induced Alterations in Growth, Biochemical attributes and Antioxidant Enzyme activity in Faba Bean (Vicia faba L.) Seedlings under NaCl Toxicity. Russian. J. Plant Physiol. 2018;65(1):104–114. [Google Scholar]
- Ahmad P., Nabi G., Ashraf M. Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. South African J Bot. 77: 36–44. Ahmad, S.S., Tahir, I., 2017. Regulatory role of phenols in flower development and senescence in the genus Iris. Indian J. Plant Physiol. 2011;22:135–140. [Google Scholar]
- Arora A., Singh V., Sindhu S., Rao D., Voleti S. Oxidative stress mechanisms during flower senescence. Plant Stress. 2007;1:157–172. [Google Scholar]
- Axelrod, B., Cheesbrough, T.M., Laakso, S., 1981. [53] Lipoxygenase from soybeans: EC 1.13. 11.12 Linoleate: oxygen oxidoreductase. In: Methods in enzymology, Elsevier, pp: 441–451.
- Babarabie M. An investigation into the potential enhancement of vase life and 1 physiological characteristics of gerbera cut flowers by apple 2 fruit extract and rosemary essential oils 3. J. Anim. Plant Sci. 2018;28:527–532. [Google Scholar]
- Bahrami S.N., Zakizadeh H., Hamidoghli Y., Ghasemnezhad M. Salicylic acid retards petal senescence in cut lisianthus (Eustoma grandiflorum ‘Miarichi Grand White’) flowers. Hortic. Environ. Biotechnol. 2013;54(6):519–523. [Google Scholar]
- Balieiro B.T.S., Júnior M.A., Vieira M.R.d.S., de Souza A.V., de O. Moreira S.M.C., do Nascimento A.H.C., Júnior W.S.E., de Souza G.R.B. Postharvest life of cut chrysanthemum flowers as affected by citric acid, boric acid and salicylic acid. Amazonian J. Plant Res. 2018;2(1):127–144. [Google Scholar]
- Bayat H., Aminifard M. Salicylic acid treatment extends the vase life of five commercial cut flowers. Electron. J. Biol. 2017;13:67–72. [Google Scholar]
- Chen G.-X., Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Dhindsa R.S., Plumb-dhindsa P., Thorpe T.A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981;32(1):93–101. [Google Scholar]
- Doganlar Z.B., Demir K., Basak H., Gul I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agric. Res. 2010;5:2056–2065. [Google Scholar]
- El-Tayeb M.A., El-Enany A.E., Ahmed N.L. Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.) Plant Growth Regul. 2006;50(2-3):191–199. [Google Scholar]
- Ezhilmathi K., Singh V.P., Arora A., Sairam R.K. Effect of 5-sulfosalicylic acid on antioxidant activity in relation to vase life of Gladiolus cut flowers. Plant Growth Regul. 2007;51(2):99–108. [Google Scholar]
- Farshid F., Hadavi E., Hekmati J. In: Abdullah H., Latifah M. N., editors. Vol. 1012. VII International Postharvest Symposium; 2012. Glutamin and malic acid increased the vase life of cut rose flowers (’Avalanch’) pp. 461–465. [DOI] [Google Scholar]
- Gerailoo S., Ghasemnezhad M. Effect of salicylic acid on antioxidant enzyme activity and petal senescence in ‘Yellow Island’cut rose flowers. J. Fruit Ornamental Plant Res. 2011;19:183–193. [Google Scholar]
- Ghadimian S., Danaei E. Rosa hybrid acv; Black Magic: 2016. The Effect of Salicylic Acid Function on Vase life and Activity of the Phenylalanine Ammonia Lyase Enzyme of Cut Flowers. [Google Scholar]
- Gómez-Merino F.C., Ramírez-Martínez M., Castillo-González A.M., Trejo-Téllez L.I. Lanthanum prolongs vase life of cut tulip flowers by increasing water consumption and concentrations of sugars, proteins and chlorophylls. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-61200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta J., Dubey R.K. Factors Affecting Post-Harvest Life of Flower Crops. Int. J. Curr. Microbiol. Appl. 2018;7(1):548–557. [Google Scholar]
- Handa N., Kohli S.K., Kaur R., Khanna K., Bakshi P., Thukral A.K., Arora S., Ohri P., Mir B.A., Bhardwaj R. Salicylic Acid: A Multifaceted Hormone. Springer Singapore; Singapore: 2017. pp. 47–75. [DOI] [Google Scholar]
- Hassan F.A.S., Ali E.F. Protective effects of 1-methylcyclopropene and salicylic acid on senescence regulation of gladiolus cut spikes. Sci. Hortic. 2014;179:146–152. [Google Scholar]
- Hatamzadeh A., Hatami M., Ghasemnezhad M. Efficiency of salicylic acid delay petal senescence and extended quality of cut spikes of Gladiolus grandiflora cv wings sensation. Afr. J. Agric. Res. 2012;7:540–545. [Google Scholar]
- Heidarnezhadian H., Eghbali B., Kazemi M. Postharvest life of cut Gerbera flowers as affected by salicylic acid and citric acid. Trakia J. Sci. 2017;15(1):27–29. [Google Scholar]
- Hemati E., Daneshvar M.H., Heidari M. The roles of sodium nitroprusside, salicylic acid and methyl jasmonate as hold solutions on vase life of Gerbera jamesonii ‘Sun Spot’. Adv. Hortic. Sci. 2019;33:187–195. [Google Scholar]
- Hernández J.A., Diaz-Vivancos P., Barba-Espín G., Clemente-Moreno M.J. Salicylic Acid: A Multifaceted Hormone. Springer Singapore; Singapore: 2017. pp. 17–34. [DOI] [Google Scholar]
- Hirota T., Izumi M., Wada S., Makino A., Ishida H. Vacuolar protein degradation via autophagy provides substrates to amino acid catabolic pathways as an adaptive response to sugar starvation in Arabidopsis thaliana. Plant Cell Physiol. 2018;59:1363–1376. doi: 10.1093/pcp/pcy005. [DOI] [PubMed] [Google Scholar]
- Hussen S., Yassin H. Review on the impact of different vase solutions on the postharvest life of rose flower. Int. J. Agric. Res. Rev. 2013;1:13–17. [Google Scholar]
- Kaya C., Ashraf M., Alyemeni M.N., Ahmad P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020;147:10–20. doi: 10.1016/j.plaphy.2019.11.040. [DOI] [PubMed] [Google Scholar]
- Kaya C., Ashraf M., Alyemeni M.N., Corpas F.J., Ahmad P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020;399:123020. doi: 10.1016/j.jhazmat.2020.123020. [DOI] [PubMed] [Google Scholar]
- Kant K., Arora A. Characterization of proteases during flower senescence in gladiolus (Gladiolus grandifloraHort.) Indian J. Plant Physiol. 2012;17:444–451. [Google Scholar]
- Keunen E., Peshev D., Vangronsveld J., Van den ende W., Cuypers A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: extending the traditional concept. Plant, Cell Environ. 2013;36(7):1242–1255. doi: 10.1111/pce.12061. [DOI] [PubMed] [Google Scholar]
- Khan M.I.R., Fatma M., Per T.S., Anjum N.A., Khan N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015;6:462. doi: 10.3389/fpls.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandan-Mirkohi A., Pirgazi R., Taheri M.R., Ajdanian L., Babaei M., Jozay M., Hesari M. Effects of salicylic acid and humic material preharvest treatments on postharvest physiological properties of statice cut flowers. Sci. Hortic. 2021;283:110009. doi: 10.1016/j.scienta.2021.110009. [DOI] [Google Scholar]
- Kohli S.K., Bali S., Tejpal R., Bhalla V., Verma V., Bhardwaj R., Alqarawi A.A., Abd_Allah E.F., Ahmad P. In-situ localization and biochemical analysis of bio-molecules reveals Pb-stress amelioration in Brassica juncea L. by co-application of 24-Epibrassinolide and Salicylic Acid. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-39712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S.K., Handa N., Sharma A., Gautam V., Arora S., Bhardwaj R., Wijaya L., Alyemeni M.N., Ahmad P. Interaction of 24-epibrassinolide and Salicylic Acid regulates Pigment Contents, Antioxidative Defense Responses and Gene Expression in Brassica juncea L. Seedlings under Pb Stress. Environ. Sci. Pollut. Res. 2018;25(15):15159–15173. doi: 10.1007/s11356-018-1742-7. [DOI] [PubMed] [Google Scholar]
- Kohli S.K., Handa N., Sharma A., Gautam V., Arora S., Bhardwaj R., Wijaya L., Alyemeni M.N., Wijaya L., Ahmad P. Combined effect of 24-Epibrassinolide and Salicylic Acid Mitigates Lead (Pb) Toxicity by Modulating Various Metabolites in Brassica juncea L. Seedlings. Protoplasma. 2018;255:11–24. doi: 10.1007/s00709-017-1124-x. [DOI] [PubMed] [Google Scholar]
- Koo Y.M., Heo A.Y., Choi H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020;36(1):1–10. doi: 10.5423/PPJ.RW.12.2019.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapenna D., Ciofani G., Pierdomenico S.D., Neri M., Cuccurullo C., Giamberardino M.A., Cuccurullo F. Inhibitory activity of salicylic acid on lipoxygenase-dependent lipid peroxidation. Biochimica et Biophysica Acta (BBA) - General Subjects. 2009;1790(1):25–30. doi: 10.1016/j.bbagen.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Lone M.L., Farooq S., ul haq A., Parveen S., Tahir I. 6-Benzylamino purine outperforms Kinetin and Thidiazuron in ameliorating flower longevity in Calendula officinalis L. by orchestrating physiological and biochemical responses. Ornamental Hortic. 2021;27(2):183–195. [Google Scholar]
- Lowry OliverH., Rosebrough NiraJ., Farr A.L., Randall RoseJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Gollery M., Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–498. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Nair S.A., Singh V., Sharma T. Effect of chemical preservatives on enhancing vase-life of gerbera flowers. J. Trop. Agric. 2006;41:56–58. [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944;153(2):375–380. [Google Scholar]
- Nguyen T.K., Jung Y.O., Lim J.H. Tools for Cut Flower for Export: Is It a Genuine Challenge from Growers to Customers? 화훼연구. 2020;28(4):241–249. [Google Scholar]
- Naing A.H., Lee K., Kim K.O., Ai T.N., Kim C.K. Involvement of sodium nitroprusside (SNP)in the mechanism that delays stem bending of different gerbera cultivars. Front. Plant Sci. 2017;8:2045. doi: 10.3389/fpls.2017.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar S., Dar R.A., Tahir I. Salicylic acid extends flower longevity in isolated flowers of Petunia hybrida Vilm. Int. J. Bot. Stud. 2018;3:71–77. [Google Scholar]
- Nisar S., Dar R.A., Tahir I. Salicylic acid retards senescence and makes flowers last longer in Nicotiana plumbaginifolia (Viv) Plant Physiol. Reports. 2021;26(1):128–136. [Google Scholar]
- Patel, D.K., SL, C., GN, V., 2018. Effect of botanicals on vase life of cut flowers: a review. Bull. Environ., Pharmacol. Life Sci. 8, 01–08.
- Phi, C., Van, N., Thi, L., Thi, P., Xuan, V., Thi, C., 2021. Influence of Salicylic Acid on Some Physiological Responses of Chrysanthemum “Mai Vang”. Asian J. Plant Sci., 20, 44–44.
- Pourzarnegar F., Hashemabadi D., Kaviani B. Cerium nitrate and salicylic acid on vase life, lipid peroxidation, and antioxidant enzymes activity in cut lisianthus flowers. Ornamental Hortic. 2020;26(4):658–669. [Google Scholar]
- Promyou S., Ketsa S., van Doorn W.G. Salicylic acid alleviates chilling injury in anthurium (Anthurium andraeanum L.) flowers. Postharvest Biol. Technol. 2012;64(1):104–110. [Google Scholar]
- Rao, M.V., Paliyath, G., Ormrod, D.P., Murr, D.P., Watkins, C.B., 1997. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes (salicylic acid-mediated oxidative damage requires H2O2). Plant Physiol., 115, 137–149. [DOI] [PMC free article] [PubMed]
- Saeed T., Hassan I., Abbasi N.A., Jilani G. Effect of gibberellic acid on the vase life and oxidative activities in senescing cut gladiolus flowers. Plant Growth Regul. 2014;72(1):89–95. [Google Scholar]
- Saeed T., Hassan I., Abbasi N.A., Jilani G. Antioxidative activities and qualitative changes in gladiolus cut flowers in response to salicylic acid application. Sci. Hortic. 2016;210:236–241. [Google Scholar]
- Sairam R., K Effect of moisture-stress on physiological activities of two contrasting wheat genotypes. Indian J. Exp. Biol. 1994;32:594. [Google Scholar]
- Schroeder K.R., Stimart D.P. Comparison of stomatal density and postharvest transpiration between long-and short-lived cut flower genotypes of Antirrhinum majus l. J. Am. Soc. Hortic. Sci. 2005;130(5):742–746. [Google Scholar]
- Shabanian S., Nasr Esfahani M., Karamian R., Tran L.-S. Salicylic acid modulates cutting-induced physiological and biochemical responses to delay senescence in two gerbera cultivars. Plant Growth Regul. 2019;87(2):245–256. [Google Scholar]
- Shahri W., Tahir I. Comparative effect of ethylene antagonists: silver thiosulphate (STS) and amino-oxy acetic acid (AOA) on postharvest performance of cut spikes of Consolida ajacis cv Violet Blue. Int. J. Agric. Food Sci. Technol. 2010;1:103–113. [Google Scholar]
- Shahri W., Tahir I., Islam S.T., Bhat M.A. Synergistic effect of STS and cool storage on postharvest performance of cut spikes of Consolida ajacis cv. violet blue. Hortic. Environ. Biotechnol. 2011;52(5):466–470. [Google Scholar]
- Sharma A., Sidhu G.P.S., Araniti F., Bali A.S., Shahzad B., Tripathi D.K., Brestic M., Skalicky M., Landi M. The role of salicylic acid in plants exposed to heavy metals. Molecules. 2020;25(3):540. doi: 10.3390/molecules25030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M.W., Ayala Zavala J.F., Hwang C.-A., editors. Postharvest Management Approaches for Maintaining Quality of Fresh Produce. Springer International Publishing; Switzerland: 2016. pp. 1–222. [Google Scholar]
- Singh A.K., Barman K., Sisodia A., Pal A., Padhi M., Saurabh V. Effect of salicylic acid and nitric oxide on postharvest quality and senescence of cut gerbera flowers. J. Pharmacognosy Phytochem. 2018;7:715–719. [Google Scholar]
- Singh M., Kumar J., Singh S., Singh V.P., Prasad S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: a review. Rev. Environ. Sci. Bio/Technol. 2015;14(3):407–426. [Google Scholar]
- Swain T., Hillis W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959;10(1):63–68. [Google Scholar]
- Tareen M.J., Abbasi N.A., Hafiz I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’fruit during storage. Sci. Hortic. 2012;142:221–228. [Google Scholar]
- Tripathi S.K., Singh A.P., Sane A.P., Nath P. Transcriptional activation of a 37 kDa ethylene responsive cysteine protease gene, RbCP1, is associated with protein degradation during petal abscission in rose. J. Exp. Bot. 2009;60(7):2035–2044. doi: 10.1093/jxb/erp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren U. Vol. 113. 1981. Role of pressure potential in keeping quality of cut gerbera inflorescences; pp. 143–150. (II International Symposium on Post-harvest Physiology of Cut Flowers). [DOI] [Google Scholar]
- Vehniwal S., Abbey L. Cut flower vase life-influential factors, metabolism and organic formulation. Hortic. Int. J. 2019;3:275–281. [Google Scholar]
- Wei Y., Liu Z., Su Y., Liu D., Ye X. Effect of salicylic acid treatment on postharvest quality, antioxidant activities, and free polyamines of asparagus. J. Food Sci. 2011;76:S126–S132. doi: 10.1111/j.1750-3841.2010.01987.x. [DOI] [PubMed] [Google Scholar]