Abstract
The presence of various amines in vaginal fluid from women with malodorous vaginal discharge has been reported before. The investigations have used several techniques to identify the amines. However, an optimized quantification, together with a sensitive analysis method in connection with a diagnostic procedure for vaginal discharge, including the syndrome of bacterial vaginosis, as defined by the accepted “gold standard,” has not been done before. We now report a sensitive gas chromatographic and mass spectrometric method for identifying the amines isobutylamine, phenethylamine, putrescine, cadaverine, and tyramine in vaginal fluid. We used weighted samples of vaginal fluid to obtain a correct quantification. In addition, a proper diagnosis was obtained using Gram-stained smears of the vaginal fluid that were Nugent scored according to the method of Nugent et al. (R. P. Nugent et al., J. Clin. Microbiol., 29:297–301, 1991). We found that putrescine, cadaverine, and tyramine occurred in high concentrations in vaginal fluid from 24 women with Nugent scores between 7 and 10. These amines either were not found or were found only in very low concentrations in vaginal fluid from women with Nugent scores of 0 to 3. There is a strong correlation between bacterial vaginosis and the presence of putrescine, cadaverine, and tyramine in high concentrations in vaginal fluid.
Bacterial vaginosis (BV) commonly occurs in women of childbearing age. Prevalences of 10 to 31% have been reported in various populations (7). Several investigations have been performed to identify one or several bacteria comprising the decisive pathogenic factor in the syndrome, but so far no specific bacteria have been implicated in causing BV. Rather, it seems that BV is accompanied by a shift in the normal lactobacillus flora to a mixed vaginal anaerobic flora including Gardnerella vaginalis, Bacteroides spp., and Mobiluncus spp. (for a review, see reference 18). The importance of BV with respect to women's health is emphasized by the association between BV and pelvic inflammatory diseases, adverse outcome of pregnancy, postpartum endometritis, and cuff cellulitis (8, 12, 14, 15).
Amsel et al. (1) proposed a set of practical diagnostic criteria for the clinical diagnosis of BV that is now often accepted as the “gold standard.” Three of the four criteria, i.e., a vaginal pH of >4.5, the presence of an adherent white discharge, a fishy amine odor after addition of KOH, and clue cells, must be met for the diagnosis of BV. A scoring system based on microscopically detectable changes in vaginal fluid is now also commonly used; Gram-stained vaginal smears and wet-mount smears in particular are well documented for use in BV diagnostics (11, 13). Several other alternative methods have also been used to develop easy, inexpensive, and reproducible diagnostic methods such as the rapid nucleic acid hybridization test (5), proline aminopeptidase activity (20), and the amine test (17).
The syndrome of BV as defined by Amsel's clinical criteria seems to be a well-defined polythetic concept of major importance to women's health. Consequently, it is important to describe the most important dimensions that can be of use in women's health care. One dimension is the increased numbers of anaerobic bacteria in the vagina that lead to the characteristic amine production and odor. Amine production is thus an important property of the concept of BV and is clearly related to scoring with Amsel's criteria or Nugent scores. Production of amines is a property of several anaerobic bacterial species occurring in the human vagina, and these amines are released into the vaginal fluid in BV (4). In a study by Chen et al. (3), methylamine, isobutylamine, putrescine, cadaverine, histamine, tyramine, and phenethylamine were found together in nonspecific vaginitis (NSV). However, investigations up until now have not used a standardized method to obtain samples, and thus the amine concentrations in the different investigations are not comparable. In two studies (2, 10) amines were found in some samples from women without NSV or BV, while in other NSV or BV samples no amines were found. In another study (9), amines were found in the same amounts in all samples from both women with BV and healthy women.
The aim of our investigation was to study whether the amine content in the vaginal fluid and BV as scored according to the Nugent method are quantitatively related to BV. We developed a sensitive and specific method for analysis of the amines isobutylamine, phenethylamine, putrescine, cadaverine, and tyramine with gas chromatography and mass spectrometry (GC-MS) and determined the concentrations of the amines in the vaginal fluid using weighted samples. Our results indicate that the production of putrecine, cadaverine, and tyramine is a property of BV and that samples from healthy women do not contain these amines.
MATERIALS AND METHODS
Sampling.
Menstruating women more than 18 years of age being seen for various gynecological disorders, such as infertility, PAP smear screening, myoma, and bleeding disorders, at an planed outpatient gynecology clinic in Drammen, Norway, were asked to participate in this study and agreed to do so. Twenty-four women, including both cases of BV and cases without BV according to the Amsel criteria, were selected for the study. None of the women were seeking advice because of overt sexually transmitted disease (STD) symptoms, nor were they diagnosed as having STDs. Vaginal samples from the posterior fornix were taken with a plastic loop, which was then placed in a vial on a scale. The weight of the vaginal fluid from each woman was measured by placing the plastic loop in a glass vial that was tared on a 0.000-g scale. The weight of the samples was determined, 2 ml of 1 M HCl was added to the sample, and the vial was closed and then frozen at −20°C until analysis.
The physician caring for the patients screened a fresh wet mount for Trichomonas vaginalis, Candida albicans, and bacterial morphotypes (11). In addition, a sample of vaginal fluid was taken with a plastic loop from the posterior fomix and smeared onto a glass slide, air dried, and saved for batchwise staining later by heat fixing and Gram staining according to standard procedure. The Gram-stained smears were scored according to the Nugent protocol (13) in a fully blinded manner by another member of the research group after the clinical visit and amine determinations.
Reagents and standards.
Isobutylamine, phenethylamine hydrochloride, 1,4-diaminobutane dihydrochloride (putrescine), 1,5-diaminopentane dihydrochloride (cadaverine), and tyramine hydrochloride were obtained from the Aldrich Chemical Co. (Milwaukee, Wis.). Benzylamine was obtained from Merck-Schuchardt (Hohenbrunn, Germany).
Stock standard solutions of 0.2 g and 20 mg of each of the amines isobutylamine, phenethylamine, putrescine, cadaverine, and tyramine per liter were prepared in 0.2 M HCl. The stock standard solutions were stored at 4°C. Calibration solutions containing 0 to 3,200 μg of the amines were prepared by diluting the stock standard solution in 0.2 M HCl. A stock solution of the internal standard of 10 mg/liter was prepared in 0.2 M HCl and stored at 4°C.
Analytical procedure.
The frozen samples were thawed, 10 μl of a solution of benzylamine (100 ng/μl) was added as an internal standard, and the samples were evaporated to dryness. For calibration, the calibration solutions and the internal standard were mixed in water at pH 4 before evaporation. The samples were dissolved in 50 μl of pyridine, and 50 μl of pentafluoropropionic anhydride was added for derivatization at room temperature for 2 min. The derivatized samples were cooled on ice, and 1 M NaOH was added until the samples were slightly alkaline. Then, 200 μl of dichloromethane was added, and the samples were shaken. The dichloromethane solution was transferred to a new tube, and 2 M HCl was added until the samples were acidified. The samples were again shaken, and the organic phase was kept in a freezer until analysis.
GC parameters and MS conditions.
The GC-MS analyses were done in selected ion monitoring (SIM) mode on a Hewlett-Packard 6890 GC system combined with an HP 5973 mass-selective detector and equipped with an HP-5MS column (cross-linked 5% phenylmethyl siloxane, 30 m by 0.25 mm, 0.25-μm phase thickness). The mass numbers used are given in Fig. 1 and 2. Helium was used as carrier gas at a flow rate of 1.2 ml/min. Split injection (split ratio of 20.1, split flow at 24.1 ml/min, an injector temperature of 200°C, and a temperature program of 40°C for 3 min [8°C/min until 200°C] was used.
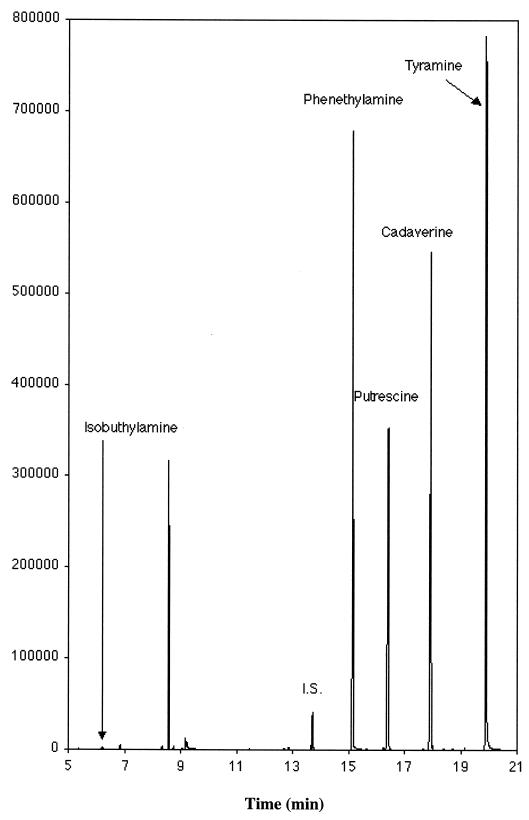
FIG. 1.
GC-MS SIM chromatogram for the ions 176, 177, and 204 between 4 and 12 min That are characteristic for isobutylamine; ions 91, 134, and 253 between 12 and 14.7 min characteristic for benzylamin (I.S.); ions 91, 104, 176, and 267 between 14.7 and 16.2 min characteristic for phenethylamine; ions 176, 204, 217, and 261 between 16.2 and 17.6 min characteristic for putrescine; ions 176, 218, 230, and 394 between 17.6 and 19.6 min characteristic for cadaverine; and ions 107, 120, and 283 between 19.6 and 21 min characteristic for tyramine from a sample with Nugent scores of 7 to 10 (BV).
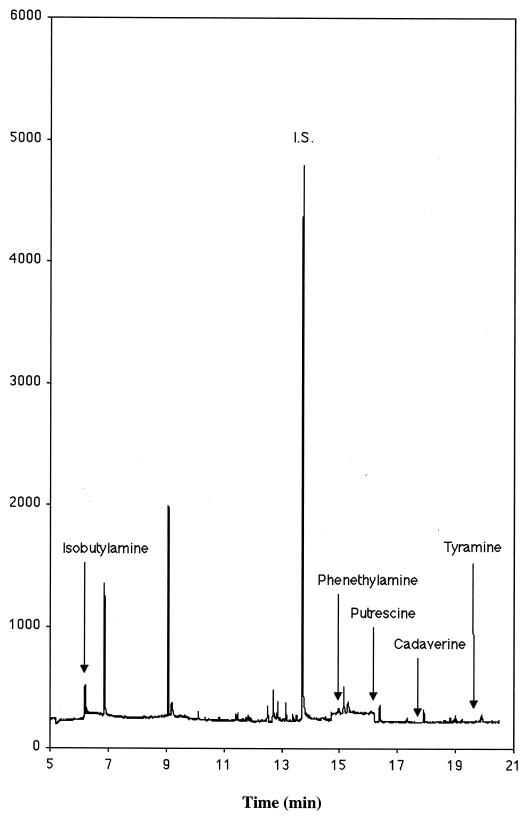
FIG. 2.
GC-MS SIM chromatogram for the ions 176, 177, and 204 between 4 and 12 min characteristic for isobutylamine; ions 91, 134, and 253 between 12 and 14.7 min characteristic for benzylamin (I.S.); ions 91, 104, 176, and 267 between 14.7 and 16.2 min characteristic for phenethylamine; ions 176, 204, 217, and 261 between 16.2 and 17.6 min characteristic for putrescine; ions 176, 218, 230, and 394 between 17.6 and 19.6 min characteristic for cadaverine; and ions 107, 120, and 283 between 19.6 and 21 min characteristic for tyramine from a sample with Nugent scores of 0 to 3 (normal).
Statistical methods.
The Wilcoxon rank sum test was used.
RESULTS
The study included one sample from each of 19 patients and two samples from 5 patients. The results from the five patients providing two samples are presented as the average of the patient since the samples were similar.
A sample of vaginal fluid was used to prepare an air-dried smear that was stained and scored according to the Nugent system. The scoring resulted in 12 samples diagnosed as BV, with Nugent scores of 7 to 10; 6 samples diagnosed as intermediate, with Nugent scores of 4 to 6; and 6 samples diagnosed as normal, with Nugent scores of 0 to 3. The 12 patients diagnosed as having BV by Nugent scores of 7 to 10 met also at least three of four Amsel criteria for BV.
In order to determine the concentrations of the amines, the weight of the vaginal fluid in each sample was measured and found to be in the range of 0.01 to 0.12 g.
The calibration functions for the amines were linear (R2 = 0.9996 for isobutylamine, 0.9995 for phenethylamine, 0.997 for putrescine, 0.998 for cadaverine, and 0.993 for tyramine) in the concentration range from 0 to 3,200 μg. The detection limits (five times the blank level) for the five amines were set at 6 to 58 ng per sample.
Figures 1 and 2 depict examples of GC-MS chromatograms. Figure 1 is derived from a patient with a Nugent score of 7 (BV). The concentrations of the amines isobutylamine, phenethylamine, putrescine, cadaverine, and tyramine were 1.09, 132.6, 158.4, 241.5, and 573.1 mg of amine/g of vaginal fluid, respectively. This can be compared with Fig. 2, which is a chromatogram of a sample from a patient with Nugent score of 0. All of the amines can be identified, although in much lower concentrations: 0.97 to 3.00 mg of amine/g of vaginal fluid.
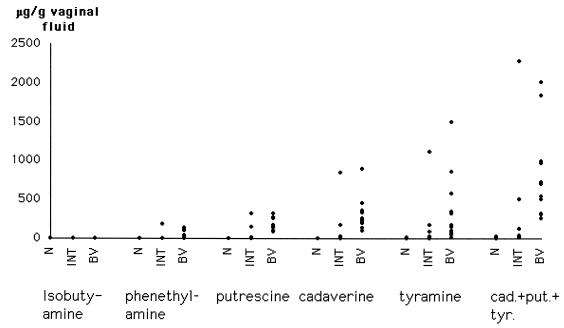
The concentrations of the amines isobutylamine, phenethylamine, putrescine, cadaverine, and tyramine in the vaginal fluid are summarized in Table 1 and in Fig. 3. Putrescine, cadaverine, and tyramine were all found in high concentrations in the vaginal fluids from all women with a Nugent score indicative of BV. Putrescine was found in the vaginal fluid from women with Nugent scores of 0 to 3, indicating the absence of BV, although in low concentrations (P < 0.001). Cadaverine and tyramine were both found in four of six women with Nugent scores of 0 to 3, although in low concentrations (P < 0.001). Phenethylamine was found in vaginal fluid from all but one woman with Nugent scores indicative of BV. Phenethylamine was also found in five of six women with intermediate BV (Nugent scores of 4 to 6) and in almost the same amounts as in those with BV. In the women diagnosed as healthy, with Nugent scores of 0 to 3, phenethylamine was found in very low concentrations in three of six samples (P < 0.001 for normal versus BV). Isobutylamine, in low concentrations, was only found in 6 of 12 women with Nugent scores indicative of BV, and isobutylamine was also found in 2 of 6 women with intermediate BV (Nugent scores of 4 to 6) in the same low concentrations. In women diagnosed as healthy, with Nugent scores of 0 to 3, isobutylamine was found in one of six samples (not significant).
TABLE 1.
Amine concentration in vaginal fluid from 24 women scored according to the Nugent system as normal, intermediate, or BV
| Analyzed amine | Amine concna (μ/g)
|
|||||
|---|---|---|---|---|---|---|
| Normal (n = 6)
|
Intermediate (n = 6)
|
BV (n = 12)
|
||||
| Range | Mean | Range | Mean | Range | Mean | |
| Isobutylamine | 0–2.1 | 0.4 | 0–1.3 | 0.3 | 0–3.0 | 0.6 |
| Phenetylamine | 0–3.4 | 0.7 | 0–184 | 32 | 0–136 | 61 |
| Putrescine | 0.8–5.7 | 2.8 | 4.0–320 | 83 | 84–324 | 171 |
| Cadaverine | 0–4.0 | 1.6 | 3.6–842 | 178 | 92–892 | 301 |
| Tyramine | 0–9.9 | 3.2 | 0–1,113 | 237 | 66–1,495 | 351 |
Range, concentrations of the lowest and highest sample; mean, mean concentration for the whole group.
FIG. 3.
Concentration of amines in vaginal fluid in all 24 patients studied: 6 normal, with Nugent scores of 0 to 3 (N); 6 intermediate, with Nugent scores of 4 to 6 (INT); and 12 BV, with Nugent scores of 7 of 10 (BV).
DISCUSSION
Many studies have been performed to investigate the amine content in vaginal fluid with the intended purpose of relating the amine content to syndromes first recognized as NSV (6). Progress in understanding the clinical manifestations of the syndrome was rapid after Amsel et al. (1) published their clinical criteria, and at the first BV meeting in Stockholm in 1984 the use of the term BV to denote the syndrome was accepted by several active researchers (21). Later, Spiegel et al. (19) defined criteria that made it easy to diagnose BV by scoring Gram-stained vaginal secretion smears, and this procedure was refined by Nugent et al. (13).
Many of the studies on the amine content in vaginal fluid were conducted at the same time as the defining criteria for the clinical syndrome were redefined and made more precise. Thus, the clinical diagnostic procedures have evolved during the same time period when the studies on amine content were performed, and this calls for a close perusal of older data in relation to our findings.
In 1979 Chen et al. (3) examined 10 women with symptoms and signs of NSV. Chen et al. (2) studied 520 women in 1982, and in 1983 Sanderson et al. (16) examined 10 women for amines in the vaginal fluid. In 1995 Kubota et al. (10) performed a study to determine whether the fishy odor was due to the presence of amines in the vaginal fluid and whether detection of amines can be a useful biochemical indicator of BV. In all four studies vaginal washing was done, and the samples were analyzed as dansyl derivates on thin-layer chromatography plates.
Chen et al. (3) found putrescine, cadaverine, methylamine, isobutylamine, phenethylamine, histamine, and tyramine in the 10 women with symptoms of NSV. Putrescine and cadaverine were the two most abundant amines and were identified in all vaginal washings from the women with untreated NSV. In the study by Chen et al. from 1982 (2), test results were classified as positive for diamines in 8% of 280 women with normal results from vaginal examinations, in 88% of 151 women with NSV only, in 79% of 29 women with BV plus yeast infection, in 17% of 63 women with vaginitis due to yeast, and in 100% of 17 women with vaginitis due to T. vaginalis. Sanderson et al. (16) showed that putrescine and cadaverine were present in vaginal washings from patients with BV, in some cases together with tyramine. Kubota et al. (10) found that 29 of 32 patients with BV had amines in the vaginal fluid. However, 4 of 14 healthy controls also had amines in their vaginal fluid. Trimethylamine and spermidine were found in 21.4 and 7.1%, respectively, of the healthy controls. Spermidine was not found in the vaginal fluid from women with BV. In the vaginal fluid from women with BV, it was found that 31.3% of the samples had methylamine, 12.5% had ethylamine, 6.3% had isobutylamine, 43.8% had trimethylamine, 15.6% had cadaverine, 15.6% had tyramine, and 21.9% had histamine. Kubota et al. concluded that the danzyl method was not suitable for detecting a volatile form of amine but rather that it could be used to detect a nonvolatile salt form dissolved in a water solution.
Jones et al. (9) used GC with a flame ionization detector to analyze the amines in vaginal fluid in two different studies with two different columns. Vaginal secretion was obtained on swabs. In both studies, the putrescine levels were broadly comparable, whereas methylamine and isobutylamine concentrations were found to be much higher in the second study than in the first. Both studies showed that putrescine was present at the highest level and that cadaverine, phenethylamine, isobutylamine, and methylamine were also present both in healthy women and in women with BV, in descending order of concentration. The only slight deviation from the overall similarity of results occurred in the first study, where women with either BV or candidiasis had higher putrescine levels than did healthy women.
In our investigation we decided to use split injection, since the original samples contained nonvolatile compounds. However, to enhance the sensitivity, the detection was done in SIM mode. We thereby obtained high enough sensitivity to detect amines in samples from healthy women as well.
In accord with the findings of Chen et al. (3) and of Sanderson et al. (16) in their studies of NSV, we found high amounts of putrescine and cadaverine in all our samples from women with BV, but we also found tyramine in all our BV samples, which they did not. We could not analyze histamine by our method, but Chen et al. (3) found it in high amounts in some of their NSV samples. Trimethylamine cannot be analyzed by our method.
In our investigation we found no overlap in the concentrations of the three amines putrescine, cadaverine, and tyramine, added together, between the normal controls and the samples with BV. The intermediate samples contained slightly more amines than the controls, with the exception of two samples. One of the intermediate samples contained high concentrations of phenethylamine, putrescine, cadaverine, and tyramine. When the concentrations of putrescine, cadaverine, and tyramine were added together, the result was an even higher amine concentration than in any of the samples with BV. This intermediate sample also contained T. vaginalis and, since amines have been reported to occur in the vaginal fluid of women with T. vaginalis (16), the high amine concentration was probably due to this. The second-strongest intermediate sample was obtained from a woman who had recently been treated for BV but did not fulfill the diagnostic criteria for BV according to Nugent.
We calculated the amine concentrations in the vaginal fluid, which are expressed as micrograms per gram of vaginal fluid. Our results are thereby independent of the amount of vaginal fluid obtained from the patient. Vaginal washing has been used in previous investigations, i.e., sterile water or saline was instilled into the vagina (2, 3, 10, 16). By using a plastic loop or a cotton-tipped applicator, adherent vaginal secretion was collected into the pooled fluid, which then was removed with a sterile syringe. However, with this method the actual amount of vaginal fluid remains unknown. In another method that was used previously, vaginal fluid was collected with swabs (9). This methodology does not reveal the absolute concentration of amines in vaginal fluid.
We think that this study makes an important contribution in defining all properties of the BV syndrome. The BV syndrome defined by the Amsel criteria does not necessarily encompass the complete natural syndrome. The change in vaginal flora, thought by many investigators to constitute the pathophysiological background of BV, can be regarded as one dimension in a polythetic description of BV, with many more dimensions to be discovered. As a major property of BV, the amine production that is related to BV must be defined and also delimited from amine production caused by other diseases such as trichomoniasis.
Nugent scoring, which is the most well-accepted scoring system for diagnosing BV in smears from vaginal discharge, was used here as a reference diagnostic framework. The close association found by us between amine production and the Nugent score intervals of 0 to 3, 4 to 6, and 7 to 10 indicates that amine production can indeed be regarded as one of the major dimensions of the BV syndrome.
In conclusion, this study shows that the amounts of amines produced in BV seem to be in good accordance with the Nugent scores assigned to the patients, and thus the production of amines is a useful dimension for developing various diagnostic tests for the syndrome of BV.
ACKNOWLEDGMENTS
We thank Bodil Carlsson for Gram staining all smears and for scoring of the smears.
This research was supported by the Foundation for Strategic Research (graduate student position for Helen Wolrath via Forum Scientum).
REFERENCES
- 1.Amsel R, Totten P A, Spiegel C A, Chen K C, Eschenbach D, Holmes K K. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen K C S, Amsel R, Escenbach D A, Holmes K K. Biochemical diagnosis of vaginitis: detection of diamines in vaginal fluid. J Infect Dis. 1982;145:337–345. doi: 10.1093/infdis/145.3.337. [DOI] [PubMed] [Google Scholar]
- 3.Chen K C S, Forsyth P S, Buchanan T M, Holmes K K. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginits. J Clin Investig. 1979;68:828–835. doi: 10.1172/JCI109382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruden D L, Galask R P. Reduction of trimethylamine oxide to trimethylamine by Mobiluncus strains isolated from patients with bacterial vaginosis. Microb Ecol Health Dis. 1988;1:95–100. [Google Scholar]
- 5.Ferris D G, Hendrich J, Payne P M, Getts A, Rassekh R, Mathis D, Litaker M S. Office laboratory diagnosis of vaginitis: clinician-performed tests compared with a rapid nucleic acid hybridization test. J Family Pract. 1995;41:575–581. [PubMed] [Google Scholar]
- 6.Gardner H L, Dukes C D. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified “nonspecific” vaginitis. Am J Obstet Gynecol. 1955;69:962–976. [PubMed] [Google Scholar]
- 7.Hart G. Factors associated with trichomoniasis, candidiasis, and bacterial vaginosis. Int J Study AIDS. 1993;1:21–25. doi: 10.1177/095646249300400105. [DOI] [PubMed] [Google Scholar]
- 8.Hay P E, Lamont R F, Taylor-Robinson D, Morgan D J, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ. 1994;308:295–298. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones B M, al F M, Gooch H. The determination of amines in the vaginal secretions of women in health and disease. Int J Study AIDS. 1994;5:52–55. doi: 10.1177/095646249400500112. [DOI] [PubMed] [Google Scholar]
- 10.Kubota T, Sakae U, Takeuchi H, Usui M. Detection and identification of amines in bacterial vaginosis. J Obstet Gynacol. 1995;21:51–55. doi: 10.1111/j.1447-0756.1995.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsson P-G, Platz-Christensen J J. Enumeration of clue cells in rehydrated air-dried vaginal wet smears for the diagnosis of bacterial vaginosis. Obstet Gynecol. 1990;76:727–30. [PubMed] [Google Scholar]
- 12.Larsson P G, Platz-Christensen J J, Forsum U, Påhlson C. Clue cells in predicting infections after abdominal hysterectomy. Obstet Gynecol. 1991;77:450–452. [PubMed] [Google Scholar]
- 13.Nugent R P, Krohn M A, Hillier S L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paige D M, Augustyn M, Adih W K, Witter F, Chang J. Bacterial vaginosis and preterm birth: a comprehensive review of the literature. J Nurse-Midwifery. 1998;43:83–89. doi: 10.1016/s0091-2182(97)00161-4. [DOI] [PubMed] [Google Scholar]
- 15.Peipert J F, Montagno A B, Cooper A S, Sung C J. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–1187. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson B E, White E, Balsdon M J. Amine content of vaginal fluid from patients with trichomoniasis and gardnerella associated nonspecific vaginitis. Br J Vener Dis. 1983;59:302–305. doi: 10.1136/sti.59.5.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnex C. The amine test: a simple, rapid, inexpensive method for diagnosing bacterial vaginosis. Br J Obstet Gynaecol. 1995;102:160–161. doi: 10.1111/j.1471-0528.1995.tb09071.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel C A. Bacterial vaginosis. Clin Microbiol Rev. 1991;4:485–502. doi: 10.1128/cmr.4.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel C A, Amsel R, Holmes K K. Diagnosis of bacterial vaginosis by direct Gram stain of vaginal fluid. J Clin Microbiol. 1983;18:170–177. doi: 10.1128/jcm.18.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomason J L, Gelbrat S M, Wilcoski L M, Peterson A K, Jilly B J, Hamilton P R. Proline aminopeptidase activity as a rapid diagnostic test to confirm bacterial vaginosis. Obstet Gynecol. 1988;71:607–611. [PubMed] [Google Scholar]
- 21.Weström L, Evaldsson G, Holmes K K, van der Meijden W I, Rylander E, Fredricsson B. Bacterial vaginosis—a definition. Scand J Urol Nephrol. 1985;86:255–256. [Google Scholar]