Abstract
Introduction: Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors arising from cells that are part of the diffuse neuroendocrine system. Patients, Materials and Methods: We conducted a retrospective study in which we included a number of 91 cases diagnosed with neuroendocrine tumors (NETs). Descriptive statistics was performed: number of cases based on location, distribution by gender (male/female), distribution by age, and we also performed a morphological and immunohistochemical (IHC) study. Results: The highest number of cases was found in lungs (60 cases). Tumors located on the skin, breast or bladder have been discovered, locations considered rare for this type of tumor. Of all cases diagnosed in the lungs, 59 were diagnosed as small cell carcinomas (SCCs) and only one case as NET. All surgical specimens were positive for chromogranin A (CgA), with a different expression for the other immunomarkers. For the lung biopsies, the most frequently IHC staining was CgA and cluster of differentiation 56 (CD56), with an increased positivity for the latter. Conclusions: CgA remains the most sensitive immunomarker in the diagnosis of NETs. CD56 is the most widely used immunomarker for diagnosing small cell lung tumors. Positive expression of thyroid transcription factor 1 (TTF1) immunomarker does not confirm pulmonary origin of SCCs.
Keywords: neuroendocrine tumors, Merkel cell carcinoma, immunohistochemistry, NETs/NECs
⧉ Introduction
Neuroendocrine neoplasms (NENs) are a heterogeneous group of tumors arising from cells that are part of the diffuse neuroendocrine (NE) system [1]. The NE system is represented by endocrine glands like the pituitary gland, parathyroid glands, the NE part of the adrenal glands and also the endocrine islet tissue located at the level of glandular tissues (pancreatic, thyroid). This diffuse NE system also includes the endocrine cells that are located in the respiratory and digestive tracts [2,3]. In 1907, Kulchitsky identified the NE cells, and in the same year, Oberndorfer first described the carcinoid [4,5].
NENs were previously classified differently, based on location, with different terminology. The classification criteria according to organ systems created a lot of confusion. After the Conference held by World Health Organization (WHO) in November 2017, a new uniform classification for all neuroendocrine tumors (NETs) was published in 2018. Following this common classification, the distinction between well-differentiated NETs, formerly known as carcinoid tumors, and poorly differentiated neuroendocrine carcinomas (NECs) was made. Although both NETs and NECs express the same NE immunomarkers, these tumors are not related [6,7].
The association of two, low-grade and high-grade components in the same NET indicates that the high-grade component remains a well-differentiated tumor. On the contrary, NECs are not often associated with NETs, and they develop from precursor lesions [7].
An essential aspect from a clinical and treatment point of view is the functional and non-functional feature of NETs. The definition of endocrine tumors is given by their association with clinical syndromes that occur in the context of increased and abnormal production of hormones. Its presence can be proven by elevated serum levels or by immunohistochemical (IHC) reactions performed on the operative specimen [6,7,8].
Aim
The aim of this study was to analyze the epidemiological, morphological and IHC aspects of NETs in our Center and to study new perspectives in the literature in terms of molecular biology and targeted therapy.
⧉ Patients, Materials and Methods
We conducted a retrospective study which included patients admitted in Mureş Clinical County Hospital, Târgu Mureş, Romania, and diagnosed with NETs (primary or metastatic, with different locations), between January 1, 2016–December 31, 2019, based on the pathological reports released by the Department of Pathology in this facility.
We used the Department of Pathology database that includes a number of 24 000 cases from 2016 to 2019. Using keywords such as: ‘neuroendocrine’, ‘carcinoid’, ‘small cells’, ‘large cells’, we selected a number of 150 cases. Cases with diagnosis established without performing IHC reactions or incomplete data were excluded. Ninety-one cases were included in the study.
We performed descriptive statistics: number of cases based on location, distribution by gender (male/female), distribution by age, and we also conducted a morphological and IHC study.
All the surgical specimens were fixed in 10% neutral buffered formalin and the sampled fragments were embedded in paraffin blocks using standard pathological report and staining with Hematoxylin–Eosin (HE).
For the IHC analysis, 4 μm thick sections made of paraffin blocks and an immunostainer (BenchMark GX, Ventana Medical Systems, Inc., Tucson, AZ, USA) were used. Staining of IHC tests was performed automatically using the automatic staining tool from Ventana BenchMark GX according to the manufacturer’s instructions. The deparaffinizing of the slides was performed at 90°C using the EZ Prep solution (Ventana Medical Systems, Inc.) and the reactants and incubation times recommended on the antibody leaflet. Slides were developed using the OmniMap 3,3’-Diaminobenzidine (DAB) detection kit (Ventana Medical Systems, Inc.) and counterstained with Mayer’s Hematoxylin. The antibodies used are shown in Table 1.
Table 1.
Antibodies used for immunohistochemistry
|
Antibody |
Clone |
Manufacturer |
Reactivity |
Dilution |
|
CD56 |
123C3 |
Ventana Medical Systems, Inc. |
Neuroendocrine immunomarker |
RTU |
|
CgA |
LK2H10 |
Ventana Medical Systems, Inc. |
Neuroendocrine immunomarker |
|
|
CK20 |
SP33 |
Ventana Medical Systems, Inc. |
Epithelial immunomarker |
|
|
CK AE1/AE3 |
PCK26 |
Ventana Medical Systems, Inc. |
Epithelial immunomarker |
|
|
EMA |
E29 |
Ventana Medical Systems, Inc. |
Epithelial immunomarker |
|
|
NSE |
MRQ-55 |
Cell Marque, Inc. |
Neuroendocrine immunomarker |
|
|
p63 |
4A4 |
Ventana Medical Systems, Inc. |
Myoepithelial immunomarker |
|
|
Synaptophysin |
MRQ-40 |
Cell Marque, Inc. |
Neuroendocrine immunomarker |
|
|
TTF1 |
SP141 |
Ventana Medical Systems, Inc. |
Transcription factor |
CD56: Cluster of differentiation 56; CgA: Chromogranin A; CK: Cytokeratin; EMA: Epithelial membrane antigen; NSE: Neuron-specific enolase; RTU: Ready-to-use; TTF1: Thyroid transcription factor 1
⧉ Results
Out of a total of 91 cases, 17 (18.68%) of them were diagnosed based on the surgical specimens containing the primary tumor, 63 (69.23%) were diagnosed by biopsy, while the remaining 11 (12.08%) cases were secondary/metastatic tumors located in the liver, lymph nodes and skin, with unknown site of the primary tumor.
The mean age of the selected cases was 63.84 years (ranges between 17 and 84 years), with a mean age of 61.22 years in females, and 65.2 years in males. Gender distribution: 34% (n=31) females and 66% (n=60) males (Table 2).
Table 2.
Gender, age distribution, and localization of primary cancer
|
All known |
||||||
|
N |
Col% |
Age [years] |
||||
|
Primary cancer |
||||||
|
All known |
80 |
100% |
63.84 |
|||
|
Skin MCC |
1 |
1.25% |
77 |
|||
|
Bladder |
2 |
2.5% |
64.5 |
|||
|
Breast |
2 |
2.5% |
71 |
|||
|
Stomach |
4 |
5% |
63 |
|||
|
Small bowel |
5 |
6% |
72.5 |
|||
|
Appendix |
3 |
3.75% |
25.3 |
|||
|
Colon |
1 |
1.25% |
80 |
|||
|
Rectum |
2 |
2.5% |
63 |
|||
|
Lung |
60 |
75% |
66 |
|||
|
Men |
Women |
|||||
|
N |
Col% |
Mean age [years] |
N |
Col% |
Mean age [years] |
|
|
Primary cancer |
||||||
|
All known |
54 |
100% |
64.5 |
26 |
100% |
59.85 |
|
Skin MCC |
0 |
0% |
0 |
1 |
3.84% |
77 |
|
Bladder |
2 |
3.7% |
64.5 |
0 |
0% |
0 |
|
Breast |
0 |
0% |
0 |
2 |
7.69% |
71 |
|
Stomach |
0 |
0% |
0 |
4 |
15.38% |
63 |
|
Small bowel |
3 |
5.5% |
80.5 |
2 |
7.69% |
64.5 |
|
Appendix |
1 |
1.85% |
57 |
2 |
7.69% |
19 |
|
Colon |
1 |
1.85% |
80 |
0 |
0% |
0 |
|
Rectum |
1 |
1.85% |
68 |
1 |
3.84% |
58 |
|
Lung |
46 |
85.18% |
66 |
14 |
53.84% |
66.5 |
Col%: Percent distribution of patients (column percentage); MCC: Merkel cell carcinoma; N: No. of cases
Pathological features: tumor site – the primary tumor was located as follows: five cases with a rare location: skin one case (1.25%), bladder two (2.5%) cases, breast two (2.5%) cases, 15 (18%) cases in the gastrointestinal tract, with different sites: four (5%) cases in the stomach, five (6.25%) cases in the small bowel, three (3.75%) cases in the appendix, one case (1.25%) in the right colon, two (2.5%) cases in the rectum, and 60 (75%) cases in the lung (Figure 1).
Figure 1.
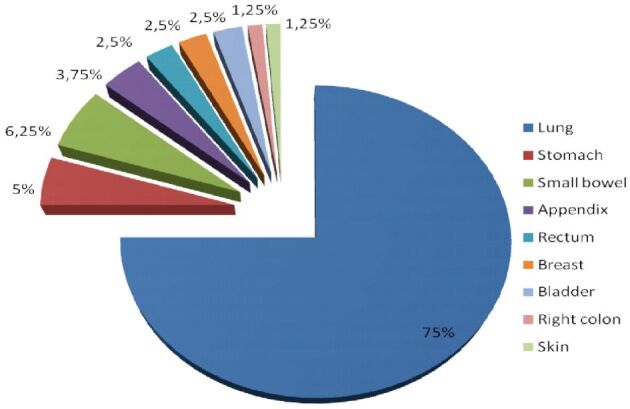
Tumors distribution: primary site
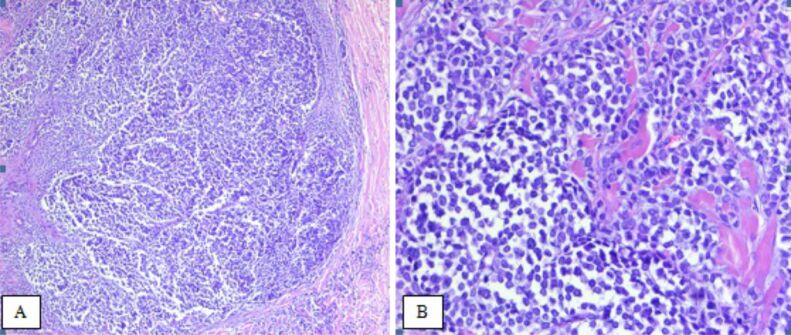
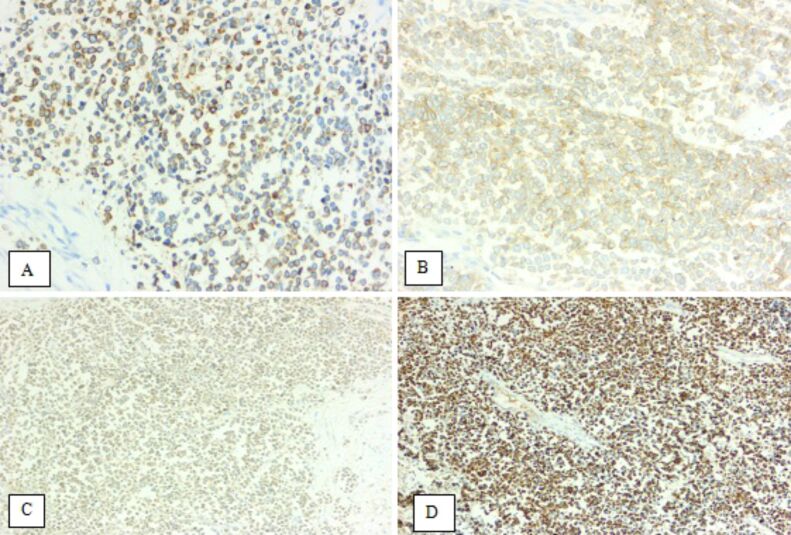
The tumor found in the skin was diagnosed as Merkel cell carcinoma (MCC) based on morphological features: tumor cells with scant eosinophilic cytoplasm and round nuclei with finely granular and dusty chromatin, and IHC profile: positivity for NE immunomarkers [chromogranin A (CgA), synaptophysin and cluster of differentiation 56 (CD56)], and also for the epithelial immunomarkers cytokeratin (CK) AE1/AE3, CK20 and epithelial membrane antigen (EMA) (Figures 2 and 3).
Figure 2.
(A and B) Merkel cell carcinoma. HE staining: (A) ×50; (B) ×200. HE: Hematoxylin–Eosin
Figure 3.
Merkel cell carcinoma: immunoexpressions (×200) of CgA (A), CD56 (B), synaptophysin (C), and CK20 (D). CD56: Cluster of differentiation 56; CgA: Chromogranin A; CK20: Cytokeratin 20
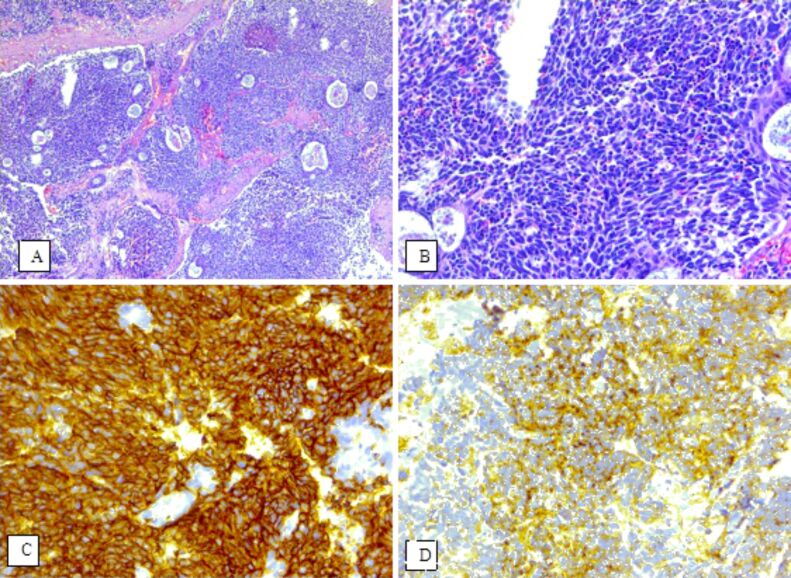
Both bladder cases were diagnosed with SCC. Tumor cells were positive for the following NE immunomarkers: the first case for synaptophysin and CgA, and the second for CD56 and neuron-specific enolase (NSE) (Figure 4). The tumor cells were negative for p63 immunostaining, with positive internal control in the normal urothelium. One of the cases was positive for thyroid transcription factor 1 (TTF1).
Figure 4.
(A–D) Small cell carcinoma of the bladder; immunoexpressions (×200) of CD56 (C) and NSE (D). HE staining: (A) ×50; (B) ×200. CD56: Cluster of differentiation 56; HE: Hematoxylin–Eosin; NSE: Neuron-specific enolase
All lung cases were diagnosed from tissue fragments collected by biopsy. Out of the 60 cases, 59 were classified as SCCs and only one case was diagnosed as a NET.
Tumor grade: only patients diagnosed with primary NETs were included in this evaluation.
Most of the cases were classified as G1 – seven (46.6%) cases, followed by G3 – six (40%) cases, and two (13.3%) cases as G2. Small cell NEC is by definition classified as a tumor with a high grade of malignancy.
Five (0.54%) patients diagnosed with primary NETs on surgical pieces presented lymph node metastases. Lymph node metastases were also identified in all the patients presenting lymphovascular invasion. None of the tumors showed perineural invasions.
In patients where diagnosis was established on metastases, in seven (63.63%) cases the tumor was identified in the liver, in three (27.27%) cases in the lymph nodes and one case (9.09%) was located in the skin.
The IHC profile of the analyzed cases is highlighted in Table 3.
Table 3.
Immunohistochemical aspects of neuroendocrine tumors
|
Antibodies |
Synaptophysin |
CgA |
CD56 |
NSE |
|
Primary site |
Analyzed cases/positive cases |
|||
|
Skin |
1/1 |
1/1 |
1/1 |
0 |
|
Bladder |
2/1 |
2/1 |
1/1 |
1/1 |
|
Breast |
2/2 |
1/1 |
1/0 |
1/0 |
|
Lung |
29/13 |
59/18 |
60/54 |
10/8 |
|
Stomach |
3/2 |
4/4 |
1/0 |
1/0 |
|
Small bowel |
4/0 |
4/4 |
4/0 |
5/1 |
|
Appendix |
3/1 |
3/3 |
2/1 |
2/1 |
|
Right colon |
1/0 |
1/0 |
1/1 |
1/1 |
|
Rectum |
1/0 |
2/1 |
1/1 |
1/1 |
|
Metastases |
Analyzed cases/positive cases |
|||
|
Liver |
7/5 |
7/6 |
7/2 |
7/4 |
|
Lymph nodes |
3/3 |
3/3 |
3/2 |
3/2 |
|
Skin |
1/0 |
1/1 |
1/0 |
1/1 |
CD56: Cluster of differentiation 56; CgA: Chromogranin A; NSE: Neuron-specific enolase
⧉ Discussions
This study presented the experience of a single Center – Department of Pathology, Mureş Clinical County Hospital – on NETs. NETs are rare neoplasms whose clinical and pathological features have been extensively studied in recent decades to understand the behavior of these heterogeneous tumors. To highlight the importance of studying these tumors we just have to pay attention to figures reported by various studies or health organizations.
According to the Surveillance, Epidemiology and End Results (SEER) Program, NETs have shown an alarming incidence growth. A study conducted in Beijing, China, highlighted that the incidence of NETs in digestive tract increased from 0.51 cases per 100 000 people in 1973 to 6.20 cases per 100 000 people in 2015 [4].
The skin, breast, and bladder are included among the rare places of NETs occurrence.
MCC (primary cutaneous NET) is a tumor with a very low incidence, both worldwide and in Europe, where the incidence is estimated to be 0.13 cases/100 000 people per year [9]. Given its low incidence, this diagnosis should be exclusionary, and the tumor must be differentiated primarily from metastasis of a NET of extracutaneous origin [10]. The first IHC marker used to diagnose MCC was CK20 [11]. MCC is positive for both epithelial immunomarkers (CK20, CK AE1/AE3 and EMA) and for NE immunomarkers (CgA, synaptophysin and CD56) [12]. The characteristic ‘dot-like’ perinuclear immunostaining of CK20 helps clarifying the diagnosis [13]. In our case, MCC was diagnosed in a 77-year-old woman. The tumor was positive in both epithelial immunomarkers (CK20, EMA, CK AE1/AE3) and the NE ones (CgA, synaptophysin and CD56). After melanoma, MCC represents the second cause of skin cancer death [14,15]. This type of carcinoma is closely related to ultraviolet radiations exposure, the presence of Merkel cell polyomavirus and immunosuppression [10].
In the literature, the percentage of NETs occurring in the breast is between 1–5% [16]. Both breast NETs evaluated in this study were classified as G1 tumors and were positive for synaptophysin and CgA labeling.
Bladder NETs represent less than 1% of all tumors concerning this site. Small cell bladder carcinoma generally affects men over the age of 60. In general, patients have an advanced stage of cancer when they are first diagnosed. The cases analyzed in our study are in accordance with the literature, both being diagnosed in two men with a mean age of 64.5 years. At the time of diagnosis, in both cases, the tumor infiltrated the muscular layer of the bladder [17,18].
An analysis of the most common primary location among NETs shows that the lung is the most affected organ, followed by the stomach [19]; our study results are consistent with these findings, 75% of the NETs being located in the lung. This is in contradiction with many studies that showed a higher prevalence in the gastrointestinal tract, especially pancreas [20]. The main reason for this is that WHO included SCC in the group of NETs [21]. If we excluded this group from our study, we would only have one NET case diagnosed in lungs. The NE properties of SCCs are thought to be mediated by key transcription factor achaete-scute family basic helix-loop-helix (BHLH) transcription factor 1 (ASCL1), possibly using neurogenic differentiation 1 (NeuroD1) factor [22,23]. Association with other large histological types of lung tumors (squamous cell carcinoma, adenocarcinoma) suggests that SCC cells have the same endodermal origin as the respiratory epithelium [21].
According to other published studies that included patients diagnosed in different Centers, the average age of onset is in the 6–7th decade of life. This is consistent with data analyzed in our center [19, 24], with small exceptions concerning the appendix, where the youngest patient was 19 years old. In our survey, in accordance with the literature, we observed that if we ruled out lung tumors, women present a slightly higher prevalence in the development of these tumors compared to men [19, 25], but after including SCC of the lung, the most affected by NETs are men.
Most metastases with unidentified primary tumors were in liver, an aspect also supported by literature [20, 26]. Diagnosis of NETs should be established in the presence of at least two NE immunomarkers positivity, preferably the panel should contain CgA and synaptophysin, considered to be general NE immunomarkers [27,28]. All the cases analyzed on surgical specimens were positive for CgA, with a different expression for the other immunomarkers. As for lung biopsies, the most frequently IHC staining was CgA and CD56, with an increased positivity for the last. Although CD56 appears to be the most sensitive NE immunomarker for lung NETs diagnosis, especially SCC, it is not specific. The negative reaction in all NE immunomarkers can be found in up to 10% of cases [21]. MCC was positive for all three NE immunomarkers and also for epithelial immunomarkers CK AE1/AE3, CK20 and EMA.
The first series of cases in which TTF1 immunoexpression was analyzed in SCCs of extrapulmonary origin was reported in the 2000s [29]. This study supports the use of TTF1 as an immunomarker of differentiation between SCC of pulmonary origin compared to those of extrapulmonary origin. In 2007, two other studies ruled out this hypothesis and reported an increased number of extrapulmonary cases that are positive for TTF1 [30]. TTF1 is currently used for differential diagnosis of MCC from other NE skin metastases of other origins [31].
A very important aspect is the division of NETs into functional and non-functional tumors [32]. ‘Functional NET’ term refers to the fact that these tumors can secrete biologically active amines or peptides. As a result of this secretory activity, patients may develop certain symptoms, which are known as the carcinoid syndrome that often help raising the suspicion of a NET [33]. The classical carcinoid syndrome is characterized by diarrhea, flushing, hypotension, right heart disease. These correlate with the effects of serotonin hypersecretion [2].
Even if they have a common origin and express neuronal and NE immunomarkers, diversity and heterogeneity are traits characterizing NETs. They differ in their malignant potential, presence or absence of a clinical syndrome, biological behavior, and molecular abnormalities [6, 34]. This is also noticeable in tumors having the same location [35,36]. In the last 10 years, many genetic and epigenetic changes have been published. These reports confirmed a radical difference between well-differentiated NETs, including those with a high Ki67 proliferation index, and NECs. The reports showed frequent inactivation of retinoblastoma 1 (RB1) gene and tumor protein P53 (TP53) gene, a rare aspect in NETs [37]. This finding is included in the new 2017 WHO Classification of pancreatic NETs but is expected to be extended to other levels in the coming years [38].
⧉ Conclusions
CgA remains the most sensitive immunomarker in diagnosis of NETs. CD56 is the most widely used immunomarker for diagnosing small cell lung tumors. Positive expression of TTF1 immunomarker does not confirm pulmonary origin of SCCs. Although the diagnosis of NETs has increased greatly in recent decades, they are still relatively rare among pathological tumor diagnosis. Given the heterogeneity of these tumors, the expertise of each Center must be shared to help manage these cases.
Conflict of interest
The authors declare that they have no conflict of interests.
References
- 1.Cives M, Strosberg JR. Gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2018;68(6):471–487. doi: 10.3322/caac.21493. [DOI] [PubMed] [Google Scholar]
- 2.Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19(12):991–1002. doi: 10.1016/j.neo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaltsas GA, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev. 2004;25(3):458–511. doi: 10.1210/er.2003-0014. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Z, Chen C, Jiang L, Zhou X, Dai X, Song Y, Li Y. Incidence and risk factors of gastrointestinal neuroendocrine neoplasm metastasis in liver, lung, bone, and brain: a population-based study. Cancer Med. 2019;8(17):7288–7298. doi: 10.1002/cam4.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Modlin IM, Shapiro MD, Kidd M. Siegfried Oberndorfer: origins and perspectives of carcinoid tumors. Hum Pathol. 2004;35(12):1440–1451. doi: 10.1016/j.humpath.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. World Health Organization (WHO) Classification of tumours of the digestive system. 4. Vol. 3. Lyon, France: International Agency for Research on Cancer (IARC) Press; 2010. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-The-Digestive-System-2010 [Google Scholar]
- 7.Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA, WHO Classification of Tumours Editorial Board The 2019 WHO Classification of tumours of the digestive system. Histopathology. 2020;76(2):182–188. doi: 10.1111/his.13975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19(10):1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Zwan JM, Trama A, Otter R, Larrañaga N, Tavilla A, Marcos-Gragera R, Dei Tos AP, Baudin E, Poston G, Links T, RARECARE WG Rare neuroendocrine tumours: results of the surveillance of rare cancers in Europe project. Eur J Cancer. 2013;49(11):2565–2578. doi: 10.1016/j.ejca.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Elder DE, Massi D, Scolyer RA, Willemze R, editors. WHO Classification of skin tumors. 4 Vol. 11. , Lyon, France: IARC Press; pp. 48–50.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Skin-Tumours-2018 [Google Scholar]
- 11.Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78(3):433–442. doi: 10.1016/j.jaad.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Daoud MA, Mete O, Al Habeeb A, Ghazarian D. Neuroendocrine carcinoma of the skin – an updated review. Semin Diagn Pathol. 2013;30(3):234–244. doi: 10.1053/j.semdp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 13.McCalmont TH. Paranuclear dots of neurofilament reliably identify Merkel cell carcinoma. J Cutan Pathol. 2010;37(8):821–823. doi: 10.1111/j.1600-0560.2010.01567_1.x. [DOI] [PubMed] [Google Scholar]
- 14.Schadendorf D, Lebbé C, Zur Hausen A, Avril MF, Hariharan S, Bharmal M, Becker JC. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Agelli M, Clegg LX, Becker JC, Rollison DE. The etiology and epidemiology of Merkel cell carcinoma. Curr Probl Cancer. 2010;34(1):14–37. doi: 10.1016/j.currproblcancer.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Miremadi A, Pinder SE, Lee AHS, Bell JA, Paish EC, Wencyk P, Elston CW, Nicholson RI, Blamey RW, Robertson JF, Ellis IO. Neuroendocrine differentiation and prognosis in breast adenocarcinoma. Histopathology. 2002;40(3):215–222. doi: 10.1046/j.1365-2559.2002.01336.x. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L, Pan CX, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H, Kuzel TM, Papavero V, Tretiakova M, Nigro K, Koch MO, Eble JN. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer. 2004;101(5):957–962. doi: 10.1002/cncr.20456. [DOI] [PubMed] [Google Scholar]
- 18.Shatagopam K, Kaimakliotis HZ, Cheng L, Koch MO. Genito-urinary small cell malignancies: prostate and bladder. Future Oncol. 2015;11(3):479–488. doi: 10.2217/fon.14.277. [DOI] [PubMed] [Google Scholar]
- 19.Alsina M, Marcos-Gragera R, Capdevila J, Buxó M, Ortiz RM, Barretina P, Vilardell L, Brunet J, Beltran M, Izquierdo Á. Neuroendocrine tumors: a population-based study of incidence and survival in Girona Province, 1994–2004. Cancer Epidemiol. 2011;35(6):e49–e54. doi: 10.1016/j.canep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Silveira F, Basile ML, Kuga FS, Próspero JD, Paes RAP, Bernardi FDC. Neuroendocrine tumors: an epidemiological study of 250 cases at a tertiary hospital. Rev Assoc Med Bras (1992) 2017;63(10):856–861. doi: 10.1590/1806-9282.63.10.856. [DOI] [PubMed] [Google Scholar]
- 21.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. WHO Classification of Tumours. 3. Vol. 10. Lyon, France: IARC Press; Pathology & genetics of tumors of the lung, pleura, thymus and heart; pp. 63–69.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Pathology-And-Genetics-Of-Tumours-Of-The-Lung-Pleura-Thymus-And-Heart-2004 [Google Scholar]
- 22.Miki M, Ball DW, Linnoila RI. Insights into the achaete-scute homolog-1 gene (hASH1) in normal and neoplastic human lung. Lung Cancer. 2012;75(1):58–65. doi: 10.1016/j.lungcan.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Osborne JK, Larsen JE, Gonzales JX, Shames DS, Sato M, Wistuba II, Girard L, Minna JD, Cobb MH. NeuroD1 regulation of migration accompanies the differential sensitivity of neuroendocrine carcinomas to TrkB inhibition. Oncogenesis. 2013;2(8):e63–e63. doi: 10.1038/oncsis.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3–7. doi: 10.1159/000080731. [DOI] [PubMed] [Google Scholar]
- 25.Caldarella A, Crocetti E, Paci E. Distribution, incidence, and prognosis in neuroendocrine tumors: a population based study from a cancer registry. Pathol Oncol Res. 2011;17(3):759–763. doi: 10.1007/s12253-011-9382-y. [DOI] [PubMed] [Google Scholar]
- 26.Faggiano A, Mansueto G, Ferolla P, Milone F, del Basso de Caro ML, Lombardi G, Colao A, De Rosa G. Diagnostic and prognostic implications of the World Health Organization Classification of neuroendocrine tumors. J Endocrinol Invest. 2008;31(3):216–223. doi: 10.1007/BF03345593. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence B, Gustafsson BI, Kidd M, Pavel M, Svejda B, Modlin IM. The clinical relevance of chromogranin A as a biomarker for gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40(1):111–134 viii. doi: 10.1016/j.ecl.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Oberg K, Stridsberg M. Chromogranins as diagnostic and prognostic markers in neuroendocrine tumours. Adv Exp Med Biol. 2000;482:329–337. doi: 10.1007/0-306-46837-9_26. [DOI] [PubMed] [Google Scholar]
- 29.Ordóñez NG. Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol. 2000;24(9):1217–1223. doi: 10.1097/00000478-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Siami K, McCluggage WG, Ordonez NG, Euscher ED, Malpica A, Sneige N, Silva EG, Deavers MT. Thyroid transcription factor-1 expression in endometrial and endocervical adenocarcinomas. Am J Surg Pathol. 2007;31(11):1759–1763. doi: 10.1097/PAS.0b013e3181131e21. [DOI] [PubMed] [Google Scholar]
- 31.Byrd-Gloster AL, Khoor A, Glass LF, Messina JL, Whitsett JA, Livingston SK, Cagle PT. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol. 2000;31(1):58–62. doi: 10.1016/s0046-8177(00)80199-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Ma L, Bao H, Zhang J, Wang Z, Gong P. Clinical, pathological and prognostic characteristics of gastroenteropancreatic neuroendocrine neoplasms in China: a retrospective study. BMC Endocr Disord. 2014;14:54–54. doi: 10.1186/1472-6823-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melosky B. Low grade neuroendocrine tumors of the lung. Front Oncol. 2017;7:119–119. doi: 10.3389/fonc.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lloyd RV, Osamura RY, Klöppel G, Rosai J, editors. WHO Classification of tumours of endocrine organs. 4. Vol. 10. Lyon, France: IARC Press; 2017. pp. 215–222.https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/WHO-Classification-Of-Tumours-Of-Endocrine-Organs-2017 [Google Scholar]
- 35.Oberg K, Casanovas O, Castaño JP, Chung D, Delle Fave G, Denèfle P, Harris P, Khan MS, Kulke MH, Scarpa A, Tang LH, Wiedenmann B. Molecular pathogenesis of neuroendocrine tumors: implications for current and future therapeutic approaches. Clin Cancer Res. 2013;19(11):2842–2849. doi: 10.1158/1078-0432.CCR-12-3458. [DOI] [PubMed] [Google Scholar]
- 36.Meeker A, Heaphy C. Gastroenteropancreatic endocrine tumors. Mol Cell Endocrinol. 2014;386(1–2):101–120. doi: 10.1016/j.mce.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36(2):173–184. doi: 10.1097/PAS.0b013e3182417d36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec JY, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–1786. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]