Abstract
Introduction: Non-alcoholic steatohepatitis (NASH) is a progressive form of liver steatosis that involves a risk of progression towards fibrosis, cirrhosis, and end-stage liver disease. Low-grade inflammation is recognized to be involved in non-alcoholic fatty liver disease (NAFLD) pathogeny. Additionally, adipose tissue dysfunction plays an important role in the development of metabolic diseases. Patients, Materials and Methods: We conducted a study on 68 patients with liver steatosis confirmed through liver biopsy during the surgery. In all the patients, we recorded anthropometric parameters and we performed blood tests for systemic inflammation [high-sensitivity C-reactive protein (hs-CRP), fibrinogen] and serum adipokines related to adipose tissue inflammation (leptin, adiponectin). Additional to histopathological examination, we also performed the immunohistochemical study of inflammatory mononuclear cells. Results: The 68 patients had a mean age of 56.57±4.94 years old, had a mean value of hs-CRP of 2.30±0.91 mg/L, a mean value of leptin of 14.02±17.02 ng/mL and a mean value of adiponectin of 7.54±0.38 mg/L. In all the cases studied by liver biopsy, the steatosis exceeded 5% of hepatocytes, but the frequency of NASH was 26.47%. Cluster of differentiation (CD)45-positive, CD4-positive, and CD8-positive T-lymphocytes predominated in the studied cases. We obtained a statistically significant high association between definite NASH and the values of hs-CRP, serum adiponectin and leptin/adiponectin ratio (p<0.0001). Conclusions: Systemic and adipose tissue inflammation was statistically significant associated with histological lesions of steatosis and NASH, suggesting that the determination of hs-CRP and serum adipokines in dynamics in patients with NAFLD is predictive for the progression of the disease.
Keywords: non-alcoholic steatohepatitis, liver biopsy, high-sensitivity C-reactive protein, serum adipokines
⧉ Introduction
Non-alcoholic fatty liver disease (NAFDL) is a common liver disease, which is regarded as a variable aggregate of disorders related to obesity, insulin resistance, type 2 diabetes mellitus, arterial hypertension, and hyperlipidemia. Non-alcoholic steatohepatitis (NASH) is a progressive form of liver disease that involves a risk of progression towards fibrosis, cirrhosis, and end-stage liver disease [1]. Subjects with obesity have a higher prevalence of NASH (20%) compared to subjects without obesity (3%). Furthermore, studies showed that patients diagnosed with type 2 diabetes have a higher prevalence of NASH associated with cirrhosis and hepatocellular carcinoma (HCC), regardless of the presence of obesity [2].
The development of steatosis and the evolution to steatohepatitis, fibrosis, liver cirrhosis and even HCC are the consequence of multiple metabolic abnormalities, partially genetically conditioned, that appear or are favored by special environmental conditions [3].
Low-grade inflammation is recognized to be involved in metabolic syndrome, cardiovascular diseases, type 2 diabetes mellitus, arterial hypertension, some cancers, as well as NAFLD pathogeny [4]. An increase in the high-sensitivity C-reactive protein (hs-CRP), marker of chronic inflammation, can predict the development of many complications associated with obesity, including NAFLD [5]. Additionally, adipose tissue dysfunction was associated with the development of metabolic diseases, adipose tissue playing the role of an active endocrine organ, being able to secrete cytokines and adipokines [including leptin, adiponectin, resistin, interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α)] involved in inflammation modulation [5,6].
Studies published in the literature showed that patients with obesity and NAFLD present high leptin values, which lead to an increased susceptibility to hepatotoxicity, through the activation of T-cells and the regulation of cytokine production, acting as a pro-inflammatory catalyst [6]. Moreover, leptin can activate the peroxisome proliferator-activated receptor-alpha (PPAR-α), augmenting the oxidation of fatty acids at the level of the liver [7]. Studies linked the severity of NAFLD with elevated leptin levels. Furthermore, NAFLD was also associated with polymorphisms in the leptin receptor gene [8].
Adiponectin, an adipokine with protective effects, is involved in the oxidation of fatty acids and glucose, being associated with an increase in insulin sensitivity, the reduction of the plaque formation, as well as increased production of aldosterone. Regarding the beneficial hepatic effects of adiponectin, studies have described its protective roles against steatosis, inflammation, and fibrosis [8,9]. Circulating adiponectin levels are negatively correlated with the gravity of liver steatosis, inflammation and fibrosis, subjects with NAFLD presenting hypoadiponectinemia. What is more, hypoadiponectinemia was associated with the progression from NAFLD to NASH [8, 10].
Aim
The aim of this study was to identify the correlations between systemic and adipose tissue inflammation markers and the spectrum of histopathological lesions in patients diagnosed with NASH, assessed by liver biopsy.
⧉ Patients, Materials and Methods
Our study enrolled patients with a diagnosis of hepatic steatosis established by liver biopsy performed during surgical procedures for different pathologies, in patients that were hospitalized in the Emergency County Hospital of Craiova, Romania, between 2017–2020 and that were subsequent investigated in Filantropia Municipal Hospital of Craiova. Thus, we selected 68 patients with clinical and ultrasound criteria suggestive for fatty liver disease and that underwent abdominal surgical procedures for various diagnoses. In these patients, the following liver disorders were excluded: hemochromatosis, Wilson’s disease, alpha-1 antitrypsin deficiency, viral hepatitis, inflammatory conditions, neoplastic diseases, and alcohol consumption.
The study was conducted in accordance with good clinical practice, respecting the ethical principles described in the Helsinki Declaration, complying with the right to confidentiality and integrity, following approval granted by the Medical Ethics Committee of the Filantropia Municipal Hospital of Craiova (Approval No. 18085/7.11.2017). All patients signed the informed consent form and had the option to withdraw from the study at any time.
In all the patients, the following data were recorded: anthropometric parameters: weight, height, body mass index (BMI), common blood tests [total blood count, fasting plasma glucose, fasting insulinemia, lipid profile, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), serum protein electrophoresis], blood tests that evaluated systemic inflammation (hs-CRP, fibrinogen), adipokines related to adipose tissue inflammation (leptin, adiponectin). Venous blood samples were collected after 12 hours fasting.
To confirm liver steatosis, single specimen liver biopsies were performed during open or laparoscopic surgery. Biopsy samples of 3–5 mm were taken from the inferior margin of the left hepatic lobe using a 5 mm cold biopsy forceps. Low intensity local hemorrhage was controlled by monopolar electrocauterization. We did not record any procedure-related morbidity.
For the histopathological (HP) examination, the fragments of liver tissue were fixed in formalin (10%), then embedded in paraffin, sectioned at the microtome, and processed with Hematoxylin–Eosin (HE) staining.
For the immunohistochemical (IHC) study, 3 μm sections were performed. The sections were spread on poly-L-lysine slides and then processed by the IHC method Avidin–Biotin–Peroxidase complex (ABC) using specific antibodies: anti-cluster of differentiation (CD)45RO (monoclonal mouse anti-human CD45RO, clone MT310, 1/50 dilution, Dako), anti-CD4 (monoclonal mouse anti-human CD4, clone MT310, 1/50 dilution, Dako), anti-CD8 (monoclonal mouse anti-human CD8, clone C8/144B, 1/100 dilution, Dako), anti-CD20 (monoclonal mouse anti-human CD20cy, clone L26, 1/50 dilution, Dako) and anti-CD68 (monoclonal mouse anti-human CD68, clone KP1, 1/100 dilution, Dako).
The assessment of the HP grading and staging of the lesions was performed according to Kleiner & Brunt’s classification [11].
All the collected data were recorded and statistically analyzed using Microsoft Excel program. One-way analysis of variance (ANOVA) test was used to assess differences between multiple patient categories, while for correlations we used Kendell’s tau test. The limit of statistical significance was p<0.05.
⧉ Results
The 68 patients included in the study (25 males and 43 females) had a mean age of 56.57±4.94 years old, an average BMI of 31.19±3.62 kg/m2, mean triglyceride value of 192±31.81 mg/dL and mean value of 201.88±10.6 mg/dL for total cholesterol. Regarding liver enzymes, 39 (57.3%) patients had abnormal AST values with a mean value of 43.44±12.72 U/L and 48 (70.5%) patients had ALT values above the upper limits, with a mean value of 72.73±18.2 U/L. For systemic inflammation, we obtained a mean value of 2.30±0.91 mg/dL for hs-CRP and a mean value of 312.3±3.53 mg/dL for serum fibrinogen. By assessing the level of adipokines related to adipose tissue inflammation, we found a mean value of 14.02±17.02 ng/mL for leptin, 7.54±0.38 mg/L for adiponectin and 3.10±5.46 for leptin/adiponectin ratio, as it shows in Table 1.
Table 1.
The biological parameters of the patients
|
Parameter |
Mean ± SD |
|
AST [U/L] |
43.44±12.72 |
|
ALT [U/L] |
56.63±23.33 |
|
Total cholesterol [mg/dL] |
201.88±10.6 |
|
Triglycerides [mg/dL] |
192±31.81 |
|
hs-CRP [mg/dL] |
2.30±0.91 |
|
Fibrinogen [mg/dL] |
312.3±3.53 |
|
Leptin [ng/mL] |
14.02±17.02 |
|
Adiponectin [mg/L] |
7.54±0.38 |
|
Leptin/adiponectin ratio |
3.10±5.46 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; hs-CRP: High-sensitivity C-reactive protein; SD: Standard deviation
Regarding the histopathological aspects, steatosis was found in all the patients, 27 (39.7%) patients presenting mild steatosis (S1), with the accumulation of lipid drops in less of 33% of hepatic lobules and also 27 (39.7%) patients presenting moderate steatosis (S2) with lipid loading in 33% to 66% of hepatic lobules. Only 14 patients, representing 20.5% of study group, had severe steatosis (S3), with more than 66% of hepatic lobules affected. Inflammatory lesions were present in 18 (26.47%) patients, with eight (11.76%) patients presenting mild inflammation (I1), respectively moderate inflammation (I2). In a small number of cases, representing 2.65% of the analyzed patients, severe inflammation (I3) was described. Hepatocyte ballooning was seen in 32 (47.05%) patients, 23 (33.82%) patients presenting a few balloon cells (B1) and only nine (2.94%) patients showing important ballooning (B2) (Table 2).
Table 2.
The histopathological characteristics of the study patients
|
Histopathological lesion |
Staging |
Percentage (n) |
|
Steatosis |
S1 |
39.7% (27) |
|
S2 |
39.7% (27) |
|
|
S3 |
20.5% (14) |
|
|
Inflammation |
I0 |
53.52% (50) |
|
I1 |
11.76% (8) |
|
|
I2 |
11.76% (8) |
|
|
I3 |
2.94% (2) |
|
|
Hepatocyte ballooning |
B0 |
54.94% (36) |
|
B1 |
33.82% (23) |
|
|
B2 |
13.23% (9) |
n: No. of patients
In our study, the predominant type of steatosis was macrovesicular (large droplet), resulting in nuclear eccentricity, as the fat droplet occupies the entire cytoplasm of the cells and less frequently with multiple and small but well-defined droplets in the cytoplasm. We noticed a predominance of these lesions in zone 3 of hepatic lobule.
The severity of steatosis was statistically significant associated with the value of ALT (mean ALT value for mild steatosis was 47.33±0.7 U/L, 54.07±28.28 U/L for moderate steatosis and 79.5±21.21 U/L for severe steatosis), leptin (mean leptin value was 10.52±3.39 ng/mL for mild steatosis, 16.10±8.62 ng/mL for moderate steatosis and 16.75±2.98 ng/mL for severe steatosis, adiponectin (mean adiponectin value for mild steatosis was 8.88±2.26 mg/L, 7.37±4.66 mg/L for moderate steatosis and 5.29±3.39 mg/L for severe steatosis) and the leptin/adiponectin ratio (mean ratio value for mild steatosis was 1.39±0.07, 3.39±2.69 for moderate steatosis and 5.85±5.95 for severe steatosis) (Table 3). The highest value of leptin/adiponectin ratio was found in a 56-year-old woman with BMI 36.60 kg/m2, and the lowest value was found in a 32-year-old woman with BMI 26.60 kg/m2. However, we did not find statistically significant differences when we analyzed the relationship between steatosis and the markers of systemic inflammation (mean hs-CRP value for mild steatosis was 1.95±0.42 mg/L, 2.54±3.74 mg/L for moderate steatosis and 3.30±1.69 mg/L for severe steatosis). The highest value of hs-CRP was found in the same case of the woman with the lowest leptin/adiponectin ratio value.
Table 3.
The relationships between the degree of hepatic steatosis and the clinical and biological parameters
|
Parameter |
Steatosis S1 (n=27) |
Steatosis S2 (n=27) |
Steatosis S3 (n=14) |
F ANOVA |
p ANOVA |
|
Gender (M/F) [n] |
9/18 |
14/13 |
2/12 |
– |
– |
|
Age [years] |
53.18±5.65 |
59.44±8.48 |
57.57±6.36 |
5.508 |
0.006 |
|
BMI [kg/m2] |
29.34±5.79 |
32.23±16.75 |
32.75±2.96 |
0.611 |
0.546 |
|
AST [U/L] |
37.92±2.12 |
45.66±8.48 |
49.78±31.81 |
3.259 |
0.045 |
|
ALT [U/L] |
47.33±0.7 |
54.07±28.28 |
79.5±21.21 |
11.991 |
<0.0001 |
|
Total cholesterol [mg/dL] |
198.14±31.81 |
198.55±9.89 |
215.5±26.87 |
2.783 |
0.069 |
|
Triglycerides [mg/dL] |
172±57.98 |
208±100.4 |
198±28.28 |
1.644 |
0.201 |
|
hs-CRP [mg/L] |
1.95±0.42 |
2.54±3.74 |
3.30±1.69 |
1.369 |
0.262 |
|
Fibrinogen [mg/dL] |
320.9±16.26 |
309.74±199.17 |
300.64±16.97 |
0.127 |
0.881 |
|
Leptin [ng/mL] |
10.52±3.39 |
16.10±8.62 |
16.75±2.98 |
7.64 |
0.001 |
|
Adiponectin [mg/L] |
8.88±2.26 |
7.37±4.66 |
5.29±3.39 |
4.611 |
0.013 |
|
Leptin/adiponectin ratio |
1.39±0.07 |
3.39±2.69 |
5.85±5.95 |
9.376 |
<0.001 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; F: Female; hs-CRP: High-sensitivity C-reactive protein; M: Male; n: No. of patients
Hepatocyte ballooning, a HP finding that expresses cell damage, was documented in 32 (47.05%) patients with affected cells often mixed in areas of steatosis. Ballooning degeneration has been most commonly seen in regions of perisinusoidal fibrosis.
Lobular inflammation is an integral part of the diagnosis of steatohepatitis. Of the 68 patients, 18 had steatohepatitis lesions, with mild lobular inflammation in 11.76% of cases, moderate in 11.76% of cases and severe in 2.94% of cases (Figure 1).
Figure 1.
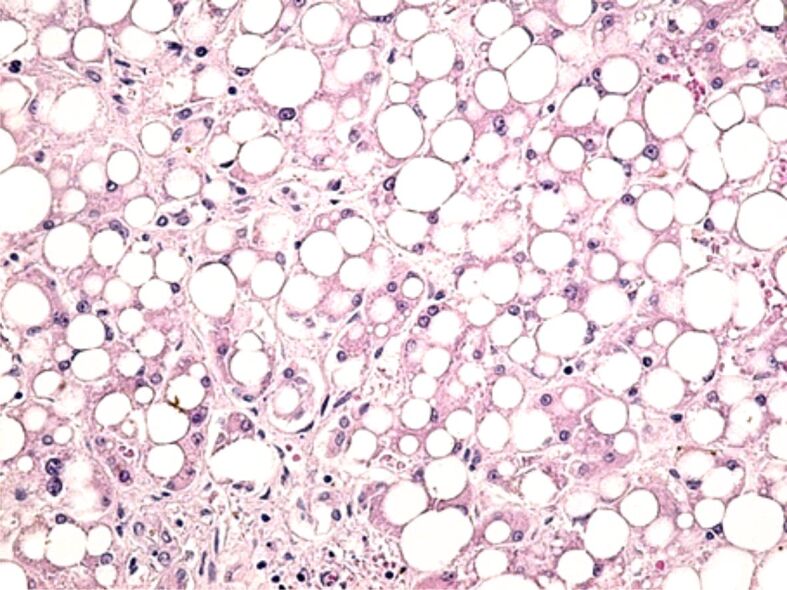
Macrovesicular steatosis, reduced intralobular inflammatory infiltrate, hepatocyte ballooning degeneration. HE staining, ×200. HE: Hematoxylin–Eosin
The expression of cluster of differentiation (CD)45RO, CD4, CD8, CD20 and CD68 markers was evaluated by immunohistochemistry, to assess the role of inflammatory mononuclear cells (T- and B-lymphocytes and Kupffer cells) in the 18 liver fragments in which inflammatory lesions were identified. In our cases, we observed that CD20-positive B-lymphocytes were reduced in number in both portal and intralobular spaces (Figure 2). Regardless of the Brunt’s grading, T-cells were the predominant cell type, but the intensity of the T-cell infiltrate presented differences. Portal lymphocytic infiltrate (CD45RO-positive cells) was reduced in four cases, moderate in six cases and severe in two cases, while intralobular lymphocytic infiltrate was reduced in two cases, moderate in three cases and severe in one case (Figure 3).
Figure 2.
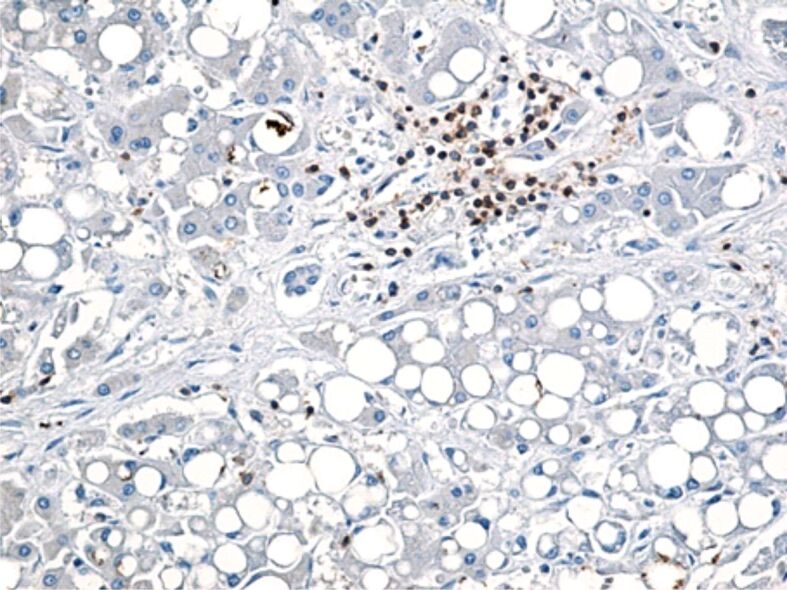
Few CD20-positive B-lymphocytes present in interlobular conjunctival septa. Immunolabeling with anti-CD20 antibody, ×200. CD20: Cluster of differentiation 20
Figure 3.
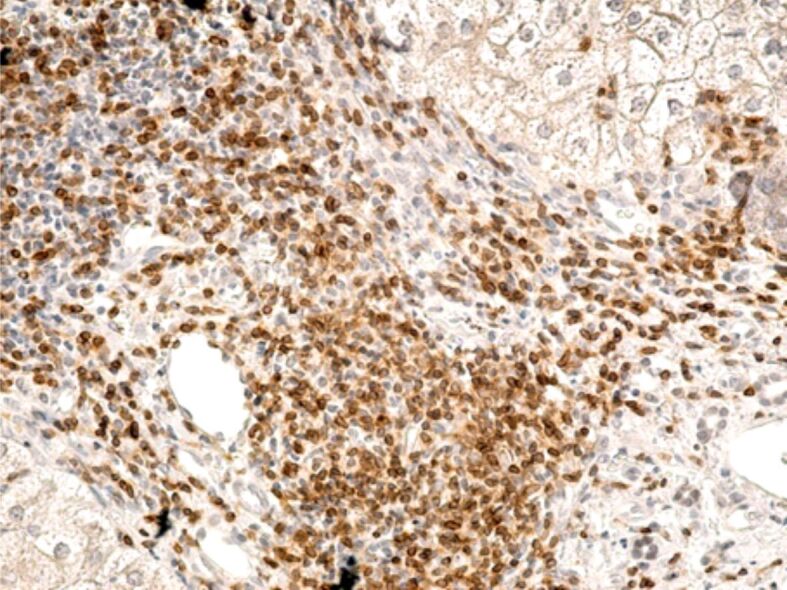
Intense inflammatory infiltrate present in the portal space, rich in CD45RO-positive T-cells. Immunolabeling with anti-CD45RO, antibody, ×200. CD45RO: Cluster of differentiation 45RO
Anti-CD4 and anti-CD8 antibodies were used to identify T-helper (CD4-positive) and T-suppressor (CD8-positive) lymphocytes. CD4-positive cells in the portal space were rare in four cases, moderately present in five cases and numerous in one case, while their intralobular expression was rare in three cases, moderate in three cases and numerous in two cases (Figure 4).
Figure 4.
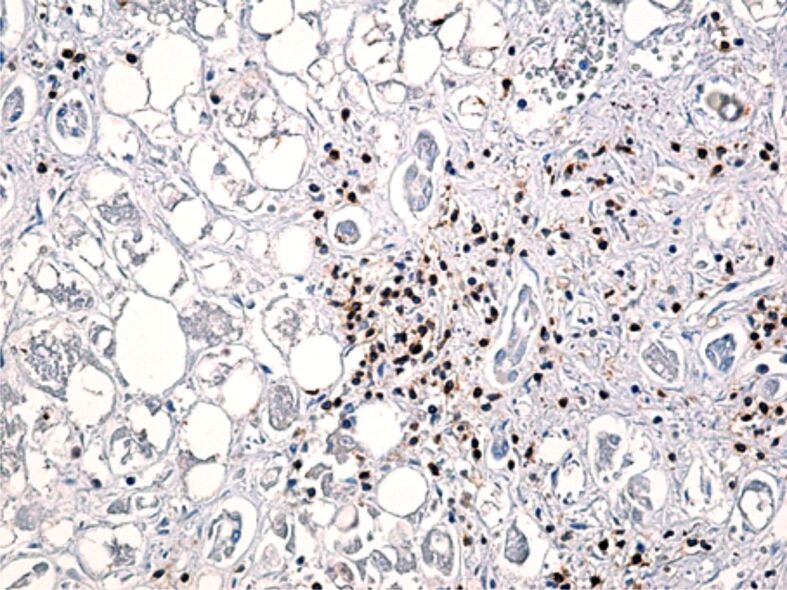
CD4-positive lymphocytes present both in the portal and intralobular space, among hepatocytes. Immunolabeling with anti-CD4 antibody, ×200. CD4: Cluster of differentiation 4
CD8-positive lymphocytes had approximately the same distribution as CD45RO-positive lymphocytes, respectively in the port space the lymphocyte infiltrate was reduced in three cases, moderate in five cases and severe in two cases, whereas intralobular lymphocyte infiltrate was reduced in three cases, moderate in three cases and severe in two cases (Figure 5).
Figure 5.
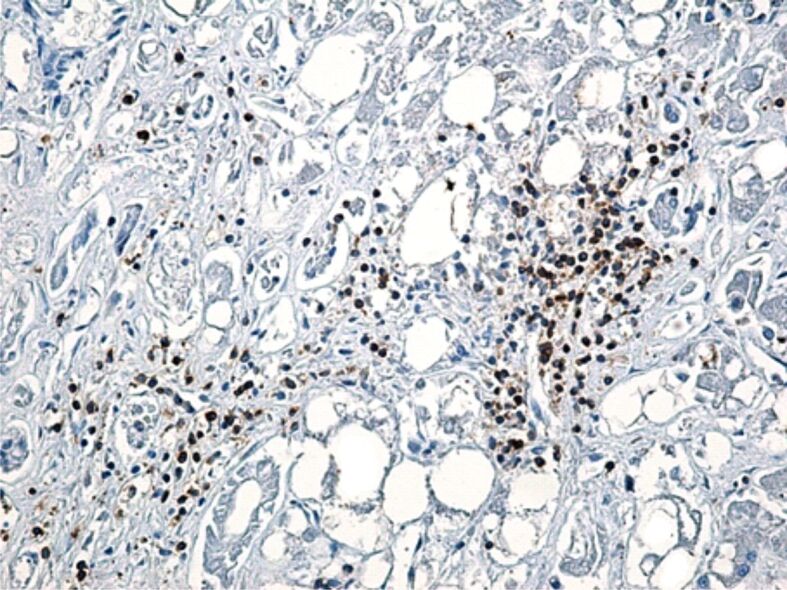
CD8-positive lymphocytes present in the portal space, in the interlobular septa, but also intralobular. Immunolabeling with anti-CD8 antibody, ×200. CD8: Cluster of differentiation 8
Cells in the CD68-positive monocyte lineage were present in all the analyzed samples. These cells were described mainly intralobular, but we found them also in the portal space. CD68-positive Kupffer cells in the portal space were rare in three cases, had a moderate representation in three cases and were numerous in one case, whilst intralobular these cells were rare in two cases, moderately represented in six cases and numerous in three cases (Figure 6).
Figure 6.
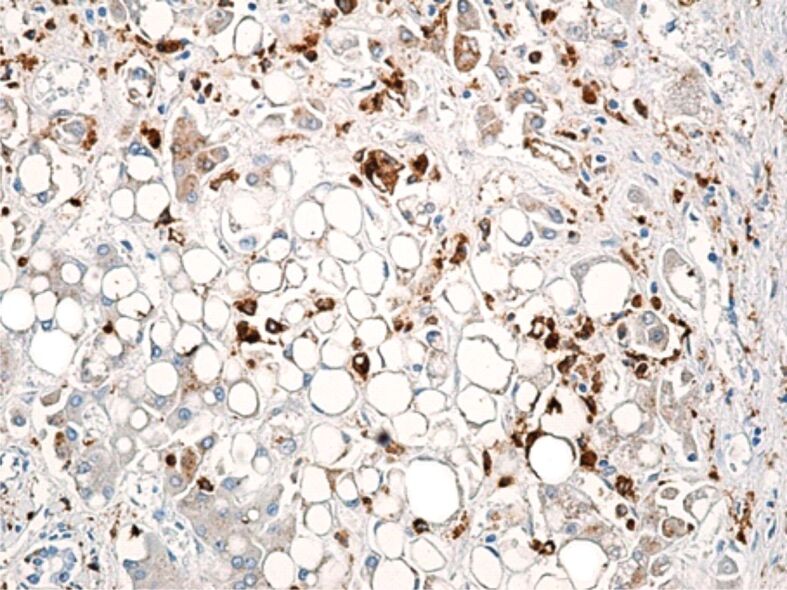
Increased number of large CD68-positive Kupffer cells, with inhomogeneous and vacuolar cytoplasm, present in the wall of sinusoidal capillaries. Immunolabeling with anti-CD68 antibody, ×200. CD68: Cluster of differentiation 68
According to the Brunt’s classification, the definite diagnosis of NASH requires the presence of the three types of histological lesions, steatosis, inflammation, and ballooning, with a NAFLD activity score (NAS) greater than or equal to 5. In accordance with this score, 26.47% of the cases were diagnosed with definite NASH, 52.94% of cases had a score less than or equal to 2, corresponding to the absence of NASH, and 20.58% had uncertain results for the presence of NASH. There was a strong statistically significant correlation between the NAS score and the steatosis and inflammation grading, with Kendall’s correlation coefficient being 0.698 and 0.646, respectively, indicating a strong direct correlation – the higher the NAS score, the higher the degree of steatosis or inflammation (p<0.0001), presented in Table 4.
Table 4.
The correlations between NAS and steatosis/inflammation
|
Statistical parameter |
NAS–steatosis correlation |
NAS–inflammation correlation |
|
Kendall’s tau |
0.698 |
0.646 |
|
p Kendall |
<0.0001 (HS) |
<0.0001 (HS) |
HS: Highly significant; NAS: Non-alcoholic fatty liver disease (NAFLD) activity score
Table 5 presents the relationships between the three NAS categories and the clinical and paraclinical parameters studied, including the markers of systemic and adipose tissue inflammation. These results prove a statistically significant high association between definite NASH and the values of hs-CRP, serum adiponectin and leptin/adiponectin ratio (p<0.0001).
Table 5.
The relationships between NAS categories and the clinical and paraclinical parameters
|
Parameter |
NAS ≤2 (n=36) |
NAS 3, 4 (n=14) |
NAS ≥5 (n=18) |
F ANOVA |
p ANOVA |
|
Gender (M/F) [n] |
16/20 |
4/10 |
5/13 |
– |
– |
|
Age [years] |
56.22±2.12 |
56±7.77 |
57.72±16.26 |
0.196 |
0.823 |
|
BMI [kg/m2] |
30.47±1.07 |
31.64±3.16 |
32.27±4.10 |
3.031 |
0.055 |
|
AST [U/L] |
35.63±18.38 |
49.21±19.09 |
54.55±22.62 |
6.125 |
0.004 |
|
ALT [U/L] |
44.55±23.33 |
68.54±20.5 |
71.55±32.52 |
8.591 |
<0.0001 |
|
Total cholesterol [mg/dL] |
199.19±30.4 |
206.55±27.57 |
204.05±9.19 |
0.484 |
0.619 |
|
Triglycerides [mg/dL] |
179±38.8 |
177±83.43 |
228±7.77 |
7.336 |
0.001 |
|
hs-CRP [mg/dL] |
1.98±0.14 |
2.14±0.21 |
3.68±0.70 |
123.769 |
<0.0001 |
|
Fibrinogen [mg/dL] |
322.88±2.12 |
287.11±129.4 |
310±33.23 |
1.786 |
0.176 |
|
Leptin [ng/mL] |
12.64±0.61 |
15.31±8.69 |
15.76±2.13 |
1.838 |
0.167 |
|
Adiponectin [mg/L] |
9±1.65 |
6.89±3.88 |
5.14±3.11 |
13.297 |
<0.0001 |
|
Leptin/adiponectin |
2.01±1.3 |
3.58±3.30 |
4.91±1.78 |
13.400 |
<0.0001 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; BMI: Body mass index; F: Female; hs-CRP: High-sensitivity C-reactive protein; M: Male; n: No. of patients; NAS: Non-alcoholic fatty liver disease (NAFLD) activity score
⧉ Discussions
Although hepatic steatosis is often harmless, the lobular inflammation that characterizes NASH is considered the key element in the progression of NAFLD. In NASH, the histological lesions are multiple and complex. In Ludwig’s original description, NASH was characterized by the presence of diffuse or perivenular macrovesicular steatosis (around the central vein) associated with mixed inflammatory infiltrate (neutrophils and lymphocytes) and focal necrosis [12]. At present, the diagnosis of NASH is established by liver biopsy in the presence of steatosis, inflammation, and hepatocyte ballooning, in the absence of significant alcohol consumption [11, 13].
In our study, even though in all the cases studied by liver biopsy, the steatosis exceeded 5% of hepatocytes, the frequency of NASH was 26.47%, the lobular inflammatory lesions being associated with hepatic steatosis and hepatocyte ballooning, obtaining a disease activity score greater than or equal to 5. We proved a statistically significant link between the NAS score and the degree of steatosis and inflammation, which indicates a strong direct correlation – the higher the NAS score, the higher the degree of steatosis/inflammation (p<0.0001).
NASH involves an innate immune response, represented by the recruitment to the liver of leukocytes [natural killer (NK) and NK T-cells, monocytes, neutrophils], as well as the activation of local Kupffer cells, leading to the release of chemokines, cytokines, eicosanoids, reactive oxygen species and nitric oxide, resulting in inflammation [14,15]. The originality of our study consisted in the IHC characterization of the inflammatory infiltrate in the cases diagnosed with NASH. CD45-positive, CD4-positive, and CD8-positive T-lymphocytes predominated in the studied cases. CD2-positive B-lymphocytes were present in small numbers in both the portal and intralobular spaces. The medical literature is scarce in IHC studies conducted in patients with NAFLD. In a study that included 34 children with a NAFLD-positive liver biopsy, an IHC examination found a significant association between CD45-positive, CD3-positive, and CD163-positive cells and the severity of steatosis, ballooning, and fibrosis [16]. Other studies have shown that CD3-positive and CD56-positive T-cells are present in large numbers in patients with NAFLD, in direct proportion to the NAS score. These cells can increase the disease activity by promoting the production of cytokines [17].
Studies performed in patients with NASH confirmed experimental findings in models of steatohepatitis, proving the recruitment of CD4-positive T-helper lymphocytes to the liver [14, 18]. Furthermore, the study of inflammatory infiltrates in subjects diagnosed with NASH showed the presence of CD8-positive effector T-lymphocytes and B-lymphocytes, but their roles are still not fully described [19].
Molecular studies have provided important insights into the pathogenesis of this relatively common condition. One of the most important advances has been the identification of the links between obesity, insulin resistance, inflammation and NAFLD [20,21]. Data from the literature have shown that insulin resistance and its hepatic consequence, NAFLD, could be the result of widespread inflammation [22]. Clinical studies have shown correlations of NASH histological features with markers of systemic and adipose tissue inflammation [23].
This study demonstrated a direct link between definite NASH, assessed by liver biopsy, and both hs-CRP as a marker of systemic inflammation and adiponectin and the leptin/adiponectin ratio as markers of adipose tissue inflammation. The mean value of hs-CRP in our study group was 2.3 mg/L, showing a statistically significant association with the presence of NASH (p<0.0001), although the relationship between this marker and the presence of liver steatosis did not reach statistical significance (p=0.262).
The results of our study are confirmed by other studies published in the literature that have shown a direct relationship between elevated hs-CRP levels and varying degrees of hepatic steatosis. Thus, high normal serum hs-CRP may be a predictive factor for the development of NASH, as it was shown by a cohort study which included only male subjects [24]. In another study that enrolled 100 adult subjects, the relationship between elevated serum hs-CRP and the severity of NAFLD was also demonstrated [25].
Studies published till present report conflicting results regarding the link between circulating leptin levels and the presence of NASH [26]. Studies showed an association between increased NAFLD activity and higher leptin levels in patients with obesity [27,28]. Interestingly, in patients with NAFLD and lipodystrophy presenting low leptin levels, leptin therapy proved to be highly effective [29]. In our study, although leptin was statistically significant associated with the presence of liver steatosis (p=0.001), when we analyzed the relationship between this adipokine and definite NASH we did not find a statistically significant association (p=0.167), even though the patients diagnosed with definite NASH presented the highest leptin values.
In contrast, all the studies have reported low circulating adiponectin levels in patients with NASH, as well as a negative correlation of this adipokine with the severity of hepatic steatosis and the degree of inflammation and fibrosis [30]. Similarly, in our study, by comparing adiponectin values in patients by steatosis degree and by the presence of steatohepatitis lesions, we observed a statistically significant inverse association between adiponectin level and steatosis degree, patients with severe steatosis included in our study having a mean adiponectin of 5.29±3.39 mg/L (p=0.013). Also, when we compared the mean adiponectin values according to the presence of NASH, we obtained a highly statistically significant inverse association (p<0.0001).
In the present study, the best parameter that was associated with both the degree of steatosis and the presence of NASH was the leptin/adiponectin ratio (p<0.0001). Though no standard value is currently set for this parameter, data from the literature report that an increased leptin/adiponectin ratio is suggestive of visceral obesity, being statistically significant higher in patients with NAFLD [31].
⧉ Conclusions
In our study, systemic and adipose tissue inflammation was statistically significant associated with histological lesions of steatosis and NASH, suggesting that the determination of hs-CRP in dynamics in patients with NAFLD is predictive for the progression of the disease. Another important marker for the progression from steatosis to NASH is the leptin/adiponectin ratio, which in our study was statistically significant associated with both types of lesions. Regarding HP changes, we obtained a statistically significant correlation between the disease activity score and the severity of steatosis and inflammation. The IHC characterization of the inflammatory infiltrates showed a predominance of CD45-positive, CD4-positive, and CD8-positive T-cells in the portal and intralobular spaces. Nevertheless, the small number of patients in whom this technique was used prevents us to draw pertinent conclusions. The characterization of histological inflammation lesions and the correlations with systemic and adipose tissue inflammation markers remains a challenge for future studies.
Conflict of interest
The authors declare no conflict of interests.
Authors’ contribution
Elena Simona Micu and Anca Maria Amzolini equally contributed to the manuscript and share main authorship.
Funding
This research received no external funding.
References
- 1.Povsic M, Wong OY, Perry R, Bottomley J. A structured literature review of the epidemiology and disease burden of non-alcoholic steatohepatitis (NASH) Adv Ther. 2019;36(7):1574–1594. doi: 10.1007/s12325-019-00960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiri Dash Atan N, Koushki M, Motedayen M, Dousti M, Sayehmiri F, Vafaee R, Norouzinia M, Gholami R. Type 2 diabetes mellitus and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2017;10(Suppl 1):S1–S7. [PMC free article] [PubMed] [Google Scholar]
- 3.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 4.Rodríguez-Hernández H, Simental-Mendía LE, Rodríguez-Ramírez G, Reyes-Romero MA. Obesity and inflammation: epidemiology, risk factors, and markers of inflammation. Int J Endocrinol. 2013;2013:678159–678159. doi: 10.1155/2013/678159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeniova AO, Küçükazman M, Ata N, Dal K, Kefeli A, Başyiğit S, Aktaş B, Ağladioğlu K, Akin KO, Ertugrul DT, Nazligül Y, Beyan E. High-sensitivity C-reactive protein is a strong predictor of non-alcoholic fatty liver disease. Hepatogastroenterology. 2014;61(130):422–425. [PubMed] [Google Scholar]
- 6.Boutari C, Perakakis N, Mantzoros CS. Association of adipokines with development and progression of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul) 2018;33(1):33–43. doi: 10.3803/EnM.2018.33.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giby VG, Ajith TA. Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J Hepatol. 2014;6(8):570–579. doi: 10.4254/wjh.v6.i8.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang ML, Yang Z, Yang SS. Roles of adipokines in digestive diseases: markers of inflammation, metabolic alteration and disease progression. Int J Mol Sci. 2020;21(21):8308–8308. doi: 10.3390/ijms21218308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva TE, Colombo G, Schiavon LL. Adiponectin: a multitasking player in the field of liver diseases. Diabetes Metab. 2014;40(2):95–107. doi: 10.1016/j.diabet.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434–438. [PubMed] [Google Scholar]
- 13.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17(2):81–92. doi: 10.1038/s41575-019-0210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Vito R, Alisi A, Masotti A, Ceccarelli S, Panera N, Citti A, Salata M, Valenti L, Feldstein AE, Nobili V. Markers of activated inflammatory cells correlate with severity of liver damage in children with nonalcoholic fatty liver disease. Int J Mol Med. 2012;30(1):49–56. doi: 10.3892/ijmm.2012.965. [DOI] [PubMed] [Google Scholar]
- 17.Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. Role of liver-infiltrating CD3+CD56+ natural killer T cells in the pathogenesis of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21(6):673–680. doi: 10.1097/MEG.0b013e32831bc3d6. [DOI] [PubMed] [Google Scholar]
- 18.Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C, Albano E. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59(3):886–897. doi: 10.1002/hep.26749. [DOI] [PubMed] [Google Scholar]
- 19.Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, Ringelhan M, Simonavicius N, Egger M, Wohlleber D, Lorentzen A, Einer C, Schulz S, Clavel T, Protzer U, Thiele C, Zischka H, Moch H, Tschöp M, Tumanov AV, Haller D, Unger K, Karin M, Kopf M, Knolle P, Weber A, Heikenwalder M. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Ayuob NN, Abdel-Hamid AAHM, Helal GMM, Mubarak WA. Thymoquinone reverses nonalcoholic fatty liver disease (NAFLD) associated with experimental hypothyroidism. Rom J Morphol Embryol. 2019;60(2):479–486. [PubMed] [Google Scholar]
- 21.Neagoe CD, Farmazon AS, Amzolini AM, Singer CE, Ianoşi SL, Tutunaru CV, Genunche-Dumitrescu AV, Ianoşi NG, Păun I, Leru PM, Tica OS, Popescu M. The role of non-invasive scores in determining the liver fibrosis in NAFLD and psoriatic patients. Rom J Morphol Embryol. 2020;61(2):503–511. doi: 10.47162/RJME.61.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruzzì S, Sutti S, Giudici G, Burlone ME, Ramavath NN, Toscani A, Bozzola C, Schneider P, Morello E, Parola M, Pirisi M, Albano E. B2-lymphocyte responses to oxidative stress-derived antigens contribute to the evolution of nonalcoholic fatty liver disease (NAFLD) Free Radic Biol Med. 2018;124:249–259. doi: 10.1016/j.freeradbiomed.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Ndumele CE, Nasir K, Conceiçao RD, Carvalho JAM, Blumenthal RS, Santos RD. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31(8):1927–1932. doi: 10.1161/ATVBAHA.111.228262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Yoon K, Ryu S, Chang Y, Kim HR. High-normal levels of hs-CRP predict the development of non-alcoholic fatty liver in healthy men. PLoS One, 2017, 12(2):e0172666. https://doi.org/10.1371/journal.pone.0172666. Erratum in: PLoS One. 2018;13(10):e0206834–e0206834. doi: 10.1371/journal.pone.0172666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar R, Porwal YC, Dev N, Kumar P, Chakravarthy S, Kumawat A. Association of high-sensitivity C-reactive protein (hs-CRP) with non-alcoholic fatty liver disease (NAFLD) in Asian Indians: a cross-sectional study. J Family Med Prim Care. 2020;9(1):390–394. doi: 10.4103/jfmpc.jfmpc_887_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polyzos SA, Aronis KN, Kountouras J, Raptis DD, Vasiloglou MF, Mantzoros CS. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia. 2016;59(1):30–43. doi: 10.1007/s00125-015-3769-3. [DOI] [PubMed] [Google Scholar]
- 28.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87(5):2395–2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 29.Safar Zadeh E, Lungu AO, Cochran EK, Brown RJ, Ghany MG, Heller T, Kleiner DE, Gorden P. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131–137. doi: 10.1016/j.jhep.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter R, Wanninger J, Bauer S, Eisinger K, Neumeier M, Weiss TS, Amann T, Hellerbrand C, Schäffler A, Schölmerich J, Buechler C. Adiponectin reduces connective tissue growth factor in human hepatocytes which is already induced in non-fibrotic non-alcoholic steatohepatitis. Exp Mol Pathol. 2011;91(3):740–744. doi: 10.1016/j.yexmp.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Nier A, Huber Y, Labenz C, Michel M, Bergheim I, Schattenberg JM. Adipokines and endotoxemia correlate with hepatic steatosis in non-alcoholic fatty liver disease (NAFLD) Nutrients. 2020;12(3):699–699. doi: 10.3390/nu12030699. [DOI] [PMC free article] [PubMed] [Google Scholar]


