Highlights
-
•
Riboflavin demonstrates antioxidant and photosensitizing properties.
-
•
Riboflavin is able to induce ROS and modulate immune response.
-
•
Riboflavin possesses potent antimicrobial activity when used alone or combined with other anti-infectives.
-
•
The riboflavin biosynthesis pathway serves as an ideal drug target against microbes.
-
•
UVA combination with riboflavin exhibits remarkable antimicrobial effects.
Keywords: Riboflavin, Immune-modulation, Antimicrobial, Antibiofilm, Photosensitiser
Abstract
Riboflavin, or more commonly known as vitamin B2, forms part of the component of vitamin B complex. Riboflavin consisting of two important cofactors, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which are involved in multiple oxidative-reduction processes and energy metabolism. Besides maintaining human health, different sources reported that riboflavin can inhibit or inactivate the growth of different pathogens including bacteria, viruses, fungi and parasites, highlighting the possible role of riboflavin as an antimicrobial agent. Moreover, riboflavin and flavins could produce reactive oxygen species (ROS) when exposed to light, inducing oxidative damage in cells and tissues, and thus are excellent natural photosensitizers. Several studies have illustrated the therapeutic efficacy of photoactivated riboflavin against nosocomial infections and multidrug resistant bacterial infections as well as microbial associated biofilm infections, revealing the potential role of riboflavin as a promising antimicrobial candidate, which could serve as one of the alternatives in fighting the global crisis of the emergence of antimicrobial resistance seen in different pathogenic microbes. Riboflavin could also be involved in modulating host immune responses, which might increase the pathogen clearance from host cells and increase host defense against microbial infections. Thus, the dual effects of riboflavin on both pathogens and host immunity, reflected by its potent bactericidal effect and alleviation of inflammation in host cells further imply that riboflavin could be a potential candidate for therapeutic intervention in resolving microbial infections. Hence, this review aimed to provide some insights on the promising role of riboflavin as an antimicrobial candidate and also a host immune-modulator from a multi-perspective view as well as to discuss the application and challenges on using riboflavin in photodynamic therapy against various pathogens and microbial biofilm-associated infections.
Graphical Abstract
1. Introduction
The comprehensive identification and characterization of the biological-chemical-physical properties of vitamins since the beginning of the early twentieth century have unveiled the potential of applying vitamins in therapeutic settings besides being long recognized as essential micronutrients for human health and development (Uribe et al., 2017). In general, vitamins are organic micronutrients that are chiefly synthesized by microorganisms and plants. Humans or animals often acquire vitamins in a minute proportion through their daily dietary intakes (Bender, 2003; Mora et al., 2008). Some vitamins such as vitamin D and niacin can be produced endogenously by the human body, via exposure of skin under sunlight or from the amino acid tryptophan, respectively (Bender, 2003; Nair, 2012). Vitamins can be classified based on their solubility in different solvents, for instance, vitamin B complex and vitamin C are water- soluble vitamins whilst vitamins A, D, E and K are fat-soluble vitamins (Bender, 2003; Mora et al., 2008). In addition, vitamins can be grouped into five distinct groups in accordance to their biological roles, highlighting the specialty of different vitamins in regulating physiological processes in humans (Fidanza and Audisio, 1982; McCormick, 1999; Uribe et al., 2017). (Fig. 1)
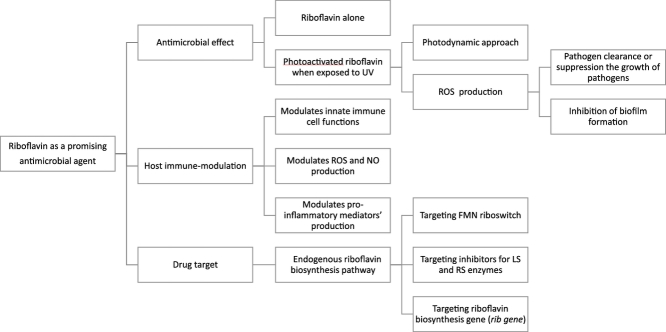
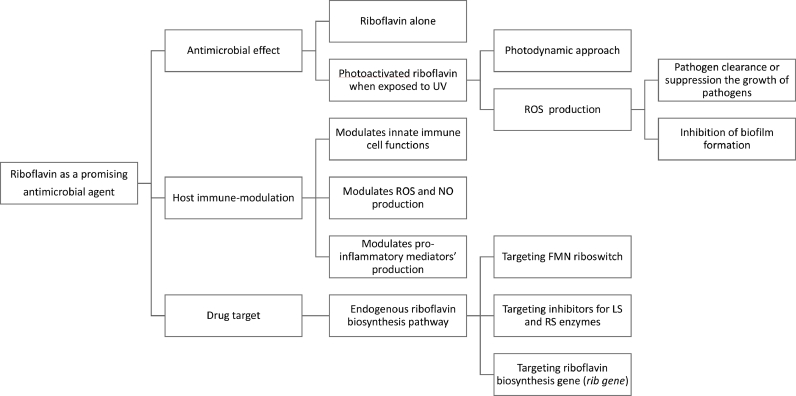
Fig. 1.
A summary of riboflavin as a promising antimicrobial agent
UV = ultraviolet; ROS= reactive oxygen species; NO= nitric oxide; FMN= Flavin mononucleotide; LS= lumazine synthase, RS= riboflavin synthase, rib gene= riboflavin biosynthesis gene
Miscellaneous studies with the emphasis on the biological roles or functionality of vitamins in various human diseases and/or the studies related to the consequences that arise from deficiency or excessive intake of vitamins have been actively conducted. Riboflavin, also known as vitamin B2, is part of the component of vitamin B complex. It can be found in dairy products (milk and cheese), meat, fish, fruit, dark-green vegetables, breads, cereals and grain product (Powers, 2003; Pinto, 2013). Riboflavin possesses a prosthetic group consisting of two important cofactors, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which are essential coenzymes for oxidases, reductases and dehydrogenases in multiple oxidative-reduction processes and have a major role in energy metabolism (Nathan and Cunningham-Bussel, 2013; Saedisomeolia and Ashoori, 2018). Riboflavin plays multiple roles in human health, including maintenance of the homeostasis of human body systems (Dey and Bishayi, 2016), regulation of metabolic pathways through redox reactions (Powers, 2003) and involved in the metabolism of various vitamins, such as niacin, pyridoxine, folic acid, and cobalamin through FMN and FAD (Nair and Maseeh, 2012; Pinto, 2013).
Aside from being critically important for maintaining human health, recent evidence reported that riboflavin can suppress or inactivate the growth of different microbes including bacteria, viruses, fungi and parasites, suggesting the possible role of riboflavin as an antimicrobial agent (Akompong et al., 2000; Kashiwabuchi et al.,2013; Keil et al., 2013; Tonnetti et al., 2013; Ahgilan et al., 2016;). Furthermore, riboflavin and flavins (building blocks or degradation products of riboflavin) are natural and effective sources of photosensitizers (PS), which are capable of producing reactive oxygen species (ROS) under light exposure thereby inducing oxidative damage in tissues resulting in cellular damage (Agostinis et al., 2011; Cardoso et al., 2012). This photosensitizing property of riboflavin is therefore exploited as photodynamic therapy, which is considered as non-invasive and safe therapeutic modality against various bacterial infections (Kornman, 1997). Two studies have illustrated the therapeutic efficacies of photoactivated riboflavin against nosocomial infections and multidrug resistant bacterial infections, highlighting the potential of riboflavin as an ideal antimicrobial candidate (Khan et al., 2019; Makdoumi et al., 2010), which could serve as one of the alternatives in tackling the global crisis of emergence of antimicrobial resistance (AMR) (Nathan and Cunningham- Bussel, 2013; Ahgilan et al., 2016; Dey and Bishayi, 2016; Saedisomeolia et al., 2018).
Host immunity comprising of both innate and adaptive immunity is a crucial player in microbial defense. Several reports claimed that riboflavin can manipulate host immune responses against invading microbes by several mechanisms, which might enhance pathogenic clearance from host cells and increase host resistance towards microbial infections (Araki et al., 1995; Toyosama et al., 2004; Nathan and Cunningham-Bussel, 2013). These mechanisms include stimulation of the multiplication of monocytes and neutrophils, activation and enhancement of macrophage functions, suppression of pro-inflammatory cytokines and nitric oxide production and alleviation of tissue damages due to excessive inflammation in host cells (Araki et al., 1995; Toyosama et al., 2004; Nathan and Cunningham-Bussel, 2013; Mazur-Bialy et al., 2013; Dey and Bishayi, 2016). Thus, this review aims to provide some up- to-date scientific results on the mechanistic aspects of antimicrobial activity and host immunomodulation of riboflavin from multi-perspective approach. Besides, the antimicrobial and antibiofilm formation properties of photodynamic therapy using a combination of ultraviolet ray A (UVA) and riboflavin in microbial infections together with some potential challenges and future directions on using this approach in tackling microbial infections were also highlighted.
2. Riboflavin biosynthesis and antimicrobial drug target
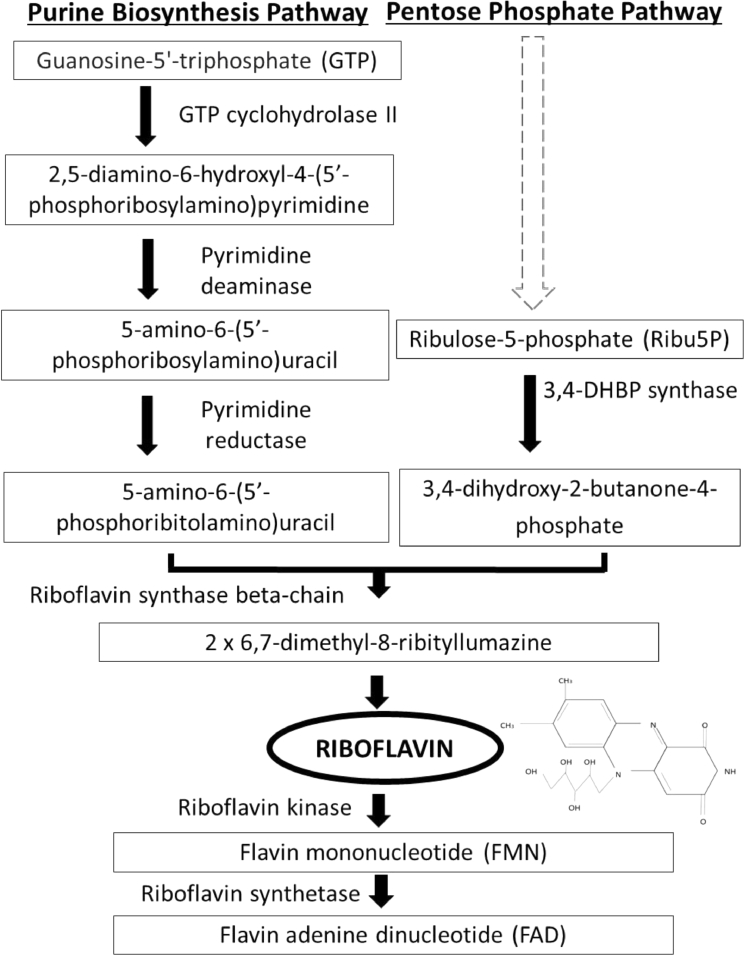
The biosynthesis pathway of riboflavin has been well elucidated in a number of previous studies (Bacher et al., 2000; Fischer and Bacher, 2005; Fisher and Bacher, 2006). As depicted in Fig. 2, presumably, riboflavin is generated from one molecule of guanosine 5-phosphate (GTP) and two molecules of ribulose 5-phosphate (Ribu5P) through a cascade of enzymatic reactions consisting of six enzymes (Rib1–Rib5p and Rib7p) (Bacher et al., 2000; Fischer and Bacher, 2005; Fisher and Bacher, 2006). Higher animals including humans usually obtain riboflavin from daily diets due to a lack of inherent system to produce riboflavin endogenously. Conversely, riboflavin can be synthesized de novo by certain bacteria, fungi and plants. Some pathogenic Gram- negative enterobacteria such as Escherichia coli, Mycobacterium tuberculosis, and Salmonella typhimurium are highly reliant on the endogenous source of riboflavin due to the absence of effective riboflavin uptake system (Oltmanns and Lingens, 1967; Neuberger and Bacher, 1985; Wang, 1992;). Thus, riboflavin biosynthesis pathways in microbes could be a promising drug target for pathogenic microbes that utilise the endogenous biosynthesis in alignment with the continuous efforts in the development of new antibiotics against multidrug resistance in various pathogens.
Fig. 2.
An overview of the riboflavin biosynthetic pathway producing riboflavin and the coenzyme FMN and FAD. One molecule of GTP and two molecules of ribulose-5-phosphate are required from the purine biosynthesis pathway and pentose phosphate pathway respectively to form one molecule of riboflavin in a series of enzyme- catalysed reactions. Two noticeable branches of the pathway are at the condensation between 3,4-dihydroxy-2-butanone-4-phosphate and 5-amino-6-(5’-phosphoribitylamino) uracil to yield the riboflavin precursor, 6,7-dimethyl-8-ribityllumazine. (Modified from: Crossley RA, Gaskin DJH, Holmes K, Mulholland F, Wells JM, Kelly DJ, et al. Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Appl Environ Microbiol. 2007)
In most of the Gram-positive bacteria, riboflavin biosynthesis or transport genes are regulated by a specific mRNA structure known as FMN riboswitch. Riboflavin is converted to FMN and FAD by riboflavin kinase and FAD synthetase in the cell, respectively. The recognition and binding of FMN to its cognate riboswitch receptor occurs when the FMN supply is enough in the cell. This binding will cause structural rearrangements leading to the formation of a terminator hairpin or sequestration structure, and consequently repression of adjacent gene expressions (Serganov and Patel, 2009; Ott et al., 2009). The highly conserved FMN riboswitch or other riboswitches in bacteria are promising therapeutic targets because of the absence of riboswitches counterpart in humans (Blount and Breaker, 2006; Lee et al., 2009). Additionally, majority of the riboswitches are regulated by small or simple metabolites which are easier to modify or deliver into the system. Furthermore, it is difficult for bacteria to develop resistance against antibiotics that targets riboswitches because it is impossible for bacteria to eliminate the antimicrobial effect exerted by a single type of riboswitches in multiple bacterial genes through single mutation (Machtel et al., 2016).
So far, Roseoflavin (RoF), the analog of riboflavin derived from Streptomyces davawensis is the sole example of naturally occurring antibiotic targeting the riboswitch (Ott et al., 2009; Lee et al., 2009). Roseoflavin has the capacity of inducing antibacterial effect in bacteria species containing FMN riboswitch. The underlying mechanism could be due to higher binding affinity of its cofactor, roseoflavin-5-monophosphate (RoFMN) to FMN riboswitch in comparison with native FMN, where the pattern of recognition by FMN riboswitch are similar for both ligands. Further investigation using both resistant and susceptible strains of Streptomyces davawensis showed that single nucleotide at position 61 in the aptamer could attribute to this ligand discrimination (Pedrolli et al., 2012). Ribocil-C, another inhibitor of FMN riboswitch binds to different Gram-negative bacteria including Pseudomonas aeruginosa, E. coli, and Acinetobacter baumannii to suppress ribB expression and riboflavin biosynthesis RF synthesis and consequently arrest bacterial growth (Howe et al., 2015; Howe et al., 2016).Besides, ribocil-C, together with roseoflavin specifically inhibit dual Staphylococcus aureus (MRSA) FMN riboswitches that coordinates the biosynthesis of riboflavin and uptake processes essential for pathogenesis and MRSA growth (Wang et al., 2017). The dual-target mechanism in Gram-positive bacteria provides a useful window in developing effective antibacterial agents targeting riboflavin metabolism.
Two studies suggested that riboflavin biosynthesis genes are associated with the virulence and pathogenesis of diseases caused by pathogenic microbes. For example, ribB is an essential precursor of the FMN and FAD cofactors in riboflavin biosynthesis. The crucial role of ribB has been expressed in different Salmonella disease experimental models (Becker et al., 2006; Rollenhagen and Bumann, 2006). The crucial roles of riboflavin biosynthesis genes have also been demonstrated in several pathogenic fungus species. Conditional repression of Rib2 leading to a loss of virulence has been observed in immunocompetent mice infected systemically with C. albicans (Becker et al., 2010). Similarly, disruption of Rib2 in H. capsulatum impedes fungal proliferation within macrophages in vitro. Moreover, its virulence is severely attenuated in intranasally infected immunocompetent mice, as exemplified by the inability of the fungi to replicate in the lungs or disseminate to other organs (Garfoot et al., 2014). However, Rib2 may not serve as an ideal antifungal target because it shares similar homology with an unidentified pseudouridylate synthase domain-containing protein (NP 689473) in humans (Meir and Osherov, 2018). Likewise, mutational inactivation or deletion of Rib1 (riboB) in A. nidulans and A. fumigatus, respectively has attenuated the virulence of pathogenic fungus species in different murine models (Purnell, 1973; Dietl et al., 2018). The ΔriboB strain exhibits higher sensitivity towards nitric oxide with less siderophores is being produced under riboflavin limitation, which reduces its ability to survive in hosts (Dietl et al., 2018).
Two sources have suggested that inhibitors which targets lumazine synthase (LS) and riboflavin synthase (RS) enzymes in riboflavin biosynthesis could serve as attractive antimicrobial drug targets (Fischer and Bacher, 2005; Fisher and Bacher, 2006). In general, several steps are involved in the identification of suitable inhibitors for LS and RS. These steps include elucidation of crystal structure of LS and RS, proposal of the mechanistic pathways of the enzymes, synthesis of possible intermediates and determination of the interaction sites through co-crystallizing the identified inhibitors with enzymes if inhibitory activity was demonstrated (Long et al., 2010). The designation usually focuses on modifications of central pyrimidinedione core and the extension of side chain from the central core, the ribitylamino fragment, active sites and reactive intermediates of LS and RS. Various analytical tools, such as rotational-echo, double resonance nuclear magnetic resonance (REDOR NMR), X-Ray crystallography and high-throughput screening (HTS) assays have been employed for high resolution analysis of the crystal structure of LS and RS, ligand-enzyme complexes elucidation and screening and identification of small molecules or HIT compounds that potentially inhibit RS and LS (Goetz et al., 1999; Gerhardt et al., 2002; Mehta et al., 2002; Cushman et al., 2005; Kaiser et al., 2007; Kundu et al., 2019) A recent review summarizes the concerted efforts of identification and designation of different classes of inhibitors that target LS and RS (Kundu et al., 2019). Indeed, riboflavin biosynthetic enzymes could be potential anti-tuberculosis drug targets. For instance, HTS hit compound and its analogues (trifluoromethylated pyrazoles were identified as inhibitors of RS which had shown potent antimicrobial activity against both Mycobacterium tuberculosis replicating and non-replicating persistent phenotypes (Zhao et al., 2009).Another recent study had identified ten molecules with 50% inhibitory concentrations from 44 000 highly diverse small drug-like molecules against RS through HTS Amongst these ten low molecular weight molecules, the authors describe that bactericidal compounds with a 2‐ Phenylamidazo (2,1‐ b) (1,3) benzothiazole chemical scaffold was effective against brucellosis, supported by Brucella culture and intramacrophagic replication experiments (Serer et al., 2019).
Analogs of biosynthetic enzymes LS and RS could be promising antifungal targets. The crystal structures of LS and RS have been illustrated in different fungal species including C. albicans, Schizosaccharomyces pombe (S. pombe) and Candida glabrata (Shankar et al., 2013). A high- throughput assay using fluorescence of riboflavin had successfully identified a few compounds that are able to suppress the LS of purified S. pombe. However, evidence on the antifungal property of these compounds were unavailable because of poor cell penetration (Chen et al., 2005). A study reported that the binding of four different inhibitors to the active site of LS from Candida albicans (Morgunova et al., 2007). Another study had designed inhibitors that target LS of pathogenic Candida glabrata, highlighting the potential of targeting LS for antifungal drug development
Nonetheless, the ability of LS to maintain copies of the catalytic products should be carefully considered in the designation of inhibitors and preparation of LS for inhibitors screening (Shankar et al., 2013). Similarly, a recent study also indicates that derivatives of C60 molecule exhibit high affinity towards the binding site of LS, suggesting the possibility of using fullerene C60 derivatives as theranostics agents against LS (Junaid et al., 2016). Through screening of 40,000 small drug-like molecules, a study had identified two compounds (G8-59 and D4-65) that lose their antifungal activities when there is an excess of riboflavin in the growth medium. This suggests that de novo synthesis of riboflavin is inhibited, and these compounds could have key roles in riboflavin biosynthesis. Nonetheless, this warrants future work to unravel their precise role in riboflavin biosynthesis (Ben et al., 2017).
Taken together, numerous studies supported the importance of de novo riboflavin biosynthesis in several pathogenic microbes and the promising benefits of targeting riboflavin biosynthesis regulatory mechanism or genes and enzymes as therapeutic targets for microbial infections due to the absence of its counterpart in humans, and hence, there will be minimal to low risk of toxicity on humans (Oltmanns and Lingens, 1967; Neuberger and Bacher, 1985; Wang, 1992, Fisher and Bacher 2000; Fisher and Bacher 2005; Fisher and Bacher 2006; Becker et al.,2006; Rollenhagen and Bumann, 2006). The quest for ideal riboflavin analogues for RS and LS biosynthetic enzymes is still on-going. Cell permeability and bioavailability of leads must be carefully assessed and improved for consistency between in vitro and in vivo efficacy. The structural modification of the leads is mostly empirically built. Therefore, radical amenable should be emphasised on those functional groups that are dispensable for target enzyme bindings (Long et al., 2010). More biological/functional studies in assessing the efficacy of the designed leads as antimicrobial targets are required to validate the hypothetical assumption built on predictive basis. On the other hand, when considering the appropriateness of analogues for FMN riboswitch, the identified analogue of riboflavin or FMN should exhibit good antibacterial activity that can suppress RS by catabolite repression, mimics pattern of recognition of FMN as well as displaying higher binding affinity to FMN riboswitch to repress downstream gene expressions. Moreover, the riboflavin inhibitor, if identified, should not interfere with the enzymes catalysing or binding riboflavin in human. Indeed, the riboflavin analogues should be recognised by RS and FMN riboswitch in bacteria and by binding or transporting proteins in humans via distinctive mechanisms (Long et al., 2010). Thus, it is rather challenging to identify or design highly specific riboflavin analogues. Nevertheless, with the advancement of high- throughput screening assays coupled with diverse chemical compounds libraries, it is hoped that more putative leads for controlling microbial infections and antimicrobial drug resistance are attainable.
3. Riboflavin biosynthesis pathway and host immunity
Mucosal-associated invariant T (MAIT) cells are lung-resident CD8+ T-cells found in abundance in humans. Not only these cells are capable of tissue repair (Hinks et al., 2019), they are also able to respond to a broad group of bacterial and fungal pathogens by recognizing conserved bacterial antigens derived from riboflavin precursors, presented by a non- polymorphic major histocompatibility complex class I–like molecule, MR1 (Chen et al., 2017). These bacterial antigens are small molecules derived from riboflavin metabolic pathway. Lymphocytes will then react to these riboflavin-synthesizing microbes in an MR1-restricted manner and infiltrate solid tissues to trigger an immune response (Kjer-Nielsen et al., 2018). MAIT cell types will then release Th0, Th1 and Th2 cytokines, and sCD40L in response to bacterial or fungal infection, showing cytotoxic capacity against infected cells and promote killing of intracellular bacteria (Chen et al., 2017; (Kjer-Nielsen et al., 2018). The rapid recognition of MAIT cells towards microbes is an important element in controlling the bacterium population in airway epithelium. Streptococcus pneumoniae, a common pathogen with different serotypes and commonly found in nasopharyngeal cavity, provide us some understanding on the importance of riboflavin in triggering host immune response. The expression of metabolic enzymes in riboflavin synthesizing pathways in these serotypes not only indicate a potential role for vaccination to enhance MAIT cell immunity (Wang et al., 2018) but are also very useful in determining the invasiveness of these pathogens towards the host (Hartmann et al., 2018).
Interestingly, group A Streptococcus species, which does not encode riboflavin metabolic enzymes did not elicit MAIT-cell responses whereas group B Streptococcus species, which encodes riboflavin metabolic enzymes were found to elicit MAIT-cell responses. Besides riboflavin metabolic enzymes, MAIT cell activation also requires IL-23 as a co-stimulator. MAIT cells of mice infected with pulmonary Legionella or Salmonella require IL-23 for constant expansion and maintenance of MAIT-17/1-type responses (Wang et al., 2018). These findings reveal cellular and molecular targets for manipulating MAIT cell function under physiological and pathological conditions. MAIT cells respond aggressively towards riboflavin over expressive pathogens especially group A Streptococcus, causing Streptococcal toxic shock syndrome whereby pathological cytokine storm was induced in the process (Emgård et al., 2019). Hence, regulating the activity of riboflavin synthesis pathway is important to ensure MAIT cells act efficiently.
One of the drawbacks of chemotherapy is the unwanted side effects. 5- Fluorouracil (5-FU) is a common chemotherapy used in treatment of various cancers despite its unwanted side effects such as diarrhoea, nausea, mouth sores and low blood count. To make matters worse, 5-FU alters the architecture and integrity of the small intestine due to high cellular infiltration in the lamina propria and significantly increases pro-inflammatory cytokine concentrations (IL-17, TNF-α, INF-γ and IL-6 in serum and IL-17, TNF-α, IFN-γ, IL-6, IL-4 and IL-2 in intestinal contents) and decreased IL-10 levels. Certain microbes such as Lactobacillus plantarum CRL2130 is a riboflavin-overproducing strain that has previously been shown to display anti- inflammatory properties (Levit et al., 2018). Taking advantage of the anti-inflammatory properties, these riboflavin-overexpressing bacteria were demonstrated to be capable of preventing mucositis in animals treated with 5-FU, with amelioration of unwanted common side-effects such as diarrhoea and nausea induced by 5-FU without compromising the efficacy of chemotherapy. It is believed that high amount of riboflavin produced by microbes is responsible for easing the side effects.
4. Riboflavin and its antimicrobial properties
4.1. Overview
Antibiotics and/or antimicrobial agents are undoubtedly amongst the most prominent discoveries in medicine. The steadily increasing use of antimicrobial drugs enable the effective treatment of complicated and life-threatening microbial infections and has become fundamental in modern medicine, facilitating a broad range of medical procedures including cancer chemotherapy, orthopaedic surgeries and transplantation. This eventually reduces microbial-related infections and improve the quality of human life. However, currently, we are facing the emergence of antimicrobial resistance (AMR) and multidrug-resistant (MDR) to the clinically available antimicrobial drugs attributed by misuse and/or intensive use in agriculture and/or health sectors, which pose a serious challenge and threat to community and healthcare systems globally (Davies and Davies, 2010; Prestinaci et al. 2015; Aslam et al. 2018; Matos De Opitz and Sass, 2020). Indeed, World Health Organization (WHO) declares AMR as one of the most serious threats to animal and human health ((Mendelson and Matsoso, 2015). With fewer new antimicrobial agents that have resistance-breaking features in the developmental pipeline and/or even less leads are successfully marketed; it is unlikely that we would see significant improvement in AMR and MDR scenarios in the near future. These scenarios presumably will cause high fatality rate and huge economic burden on both individuals and countries (O'Neill, 2016 (a); O'Neill, 2016 (b)). Therefore, the current resistance warrants the investigations on the mechanism of resistance deployed by pathogenic microbes and also to actively explore novel antimicrobial agents to counteract these scenarios. The following section will discuss the potential of riboflavin or combination of riboflavin with UVA (photodynamic therapy) as a suitable antimicrobial agent based on its antimicrobial activity against various pathogenic microbes, host immune-modulation and anti-biofilm formation activity.
4.2. Riboflavin alone and antimicrobial activity
Studies on antimicrobial activity of riboflavin are in scarcity. A few studies have demonstrated that riboflavin, when used alone exhibits antimicrobial properties against pathogenic microbes including Staphylococcus aureus, Enterococcus faecalis, Salmonella typhi, Klebsiella pneumonia Pseudomonas aeruginosa, C. albicans and Plasmodium falciparum (Akompong et al., 2000a; Akompong et al., 2000b; Kashiwabuchi et al., 2013; Ahgilan et al., 2016;). Antibacterial effect of riboflavin is demonstrated through a study found that riboflavin alone at a concentration of 50 µL inhibits the growth of the laboratory cultured Gram-positive bacteria such as S. aureus, E. faecalis, S. typhi and P. aeruginosa whilst E. coli showed intermediate effect towards riboflavin and K. pneumonia was found to be resistant to the riboflavin treatment (Ahgilan et al., 2016). Riboflavin also exerts antifungal properties. An in vitro study reported an intermediate suppression of C. albicans growth (Ahgilan et al., 2016) while significant changes on the morphology of C. albicans treated with riboflavin alone (Kashiwabuchi et al., 2013). Also, riboflavin is effective in treating malarial infection caused by P. falciparum (Akompong et al., 2000a; Akompong et al., 2000b). The authors highlighted that riboflavin at a concentration of 10 to 100 µM could successfully inhibit the growth and function of asexual and sexual forms of P. falciparum in vitro (Akompong et al., 2000a; Akompong et al., 2000b). The group of researchers demonstrated that riboflavin synergistically interacts with standard antimalarial drugs including mefloquine, pyrimethamine and quinine whereas interaction between riboflavin with artemisinin and choloroquine are additive and mildly antagonistic, respectively (Akompong et al.,2000b).
4.3. Riboflavin and host immunomodulation in microbial infections
It is well documented that riboflavin deficiency significantly altered the immune system by delaying immune cell function, interfering nucleic acid synthesis and protein production, contributing to oxidative stress and disrupting with metabolic processes (Toyosawa et al., 2004 (a); Kodama et al., 2005). Two studies have illustrated the potent host immune-modulation of riboflavin in microbial infections. A study reported that administration of riboflavin increases host resistance to different pathogens such as E. coli (in dose-dependent manner), P. aeruginosa, K. pneumonia, S. aureus and Actinobacillus pleuropneumoniae (Araki et al., 1995). The protective mechanisms of riboflavin lie on the activation of phagocytic activity of macrophages and early elimination of pathogens by stimulating multiplication of monocytes and neutrophils (Araki et al., 1995). One study had demonstrated that riboflavin is undoubtedly imperative to maintain the viability and activity of macrophage RAW 264.7 cells. It is observed that proliferation of macrophage cells is disrupted with decreased riboflavin concentration (Mazur-Bialy et al., 2013). Nonetheless, supplementation of riboflavin might interfere with migration of neutrophils and suppresses the influx of infiltrations and activated granulocytes to the peripheral sites, which could result in poor inflammatory responses to the site of infection (Verdrengh and Tarkowski et al., 2005).
Sepsis is a consequence of overwhelming systemic inflammatory response to microbial infections that cause multiple organ-system damages in host. Numerous pro-inflammatory mediators such as inflammatory cytokines (IL1, IL-6 and TNF-α), chemokines, macrophage inflammatory protein (MIP-2) and monocyte chemo attractant protein 1 (MCP-1) and nitric oxide (NO) that consists of high-mobility group protein B1 (HMGB1) are expressed during septic shock. Various experimental animal models have revealed the promising effect of riboflavin, attributed by its anti-inflammation property in improving the mortality rates of exotoxin and exotoxin-induced shock, gram-positive and gram-negative bacterial infections and LPS-induced septic shock. The potent effects of riboflavin are achieved by reducing the high expression levels of IL-1β, IFN-γ, TNF-α, IL-1, IL-6, MIP-2, MCP-1 and NO (Toyosawa et al., 2004 (a); Toyosawa et al., 2004 (b); Kodama et al., 2005). Riboflavin is broken down into two cofactors, FMN and FAD by riboflavin kinase wherein they are considered as key cofactors for phagocytic nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 2 (Nox2). One study had shown that deficiency in riboflavin impairs Nox2 priming via conditional RFK knockout isolates leading to abruption of ROS production and subsequently impairs phagocyte defence against Listeria monocytogenes. The study highlighted that TNF-induced priming of Nox2 is RF-dependent whereby riboflavin is required for optimal ROS production for an effective phagocytic immune response (Schramm et al., 2014). Another study also demonstrated that RF-deficient mice are highly susceptible to experimental pneumococcus infection compared to control groups (Suwannasom et al., 2020). Furthermore, a study documented the efficacy of riboflavin alone and combination of riboflavin with antibiotics such as ciprofloxacin and azithromycin against S. aureus infection (Dey and Bishayi, 2016). This study indicates that riboflavin coupled with antibiotics controls S. aureus infection by activating phagocytosis of macrophages, enhancing the superoxide and hydrogen peroxide production and downregulating the production of pro-inflammatory cytokines including IFN-γ, IL-6, IL-1β, NO and TNF-α (Dey and Bishayi, 2016).
Although studies on riboflavin and its host immunomodulation activities are limited, there is a robust connection between riboflavin and host immune system. Riboflavin could modulate innate effector immune cells activity and mediates ROS and NO production to control microbial infections effectively. It is highly possible that riboflavin with its anti-inflammation property could have crucial role in balancing the pro-inflammatory responses to avoid destructive cell/tissue damages in host after the clearance of microbial cells. Additionally, riboflavin, being a proteasomes inhibitor could probably inhibit the release of NO and TNF-α to exert its anti-inflammatory activity by down regulation of NF-κβ activation triggered by ROS, whereby NF-κβ activation is associated with the production of pro-inflammatory mediators, such as TNF-α and IL-6. It has been shown that riboflavin confers protective roles in several age-associated diseases by lowering the production of NO, reduces the release of TNF-α, and inhibits the activation of NF- κβ, and degradation (Wooley and Sebrell, 1942). Such mechanism remains much to be explored in microbial infections since current evidence is still scarce.
4.4. Overview of antimicrobial photodynamic therapy (aPDT)
Antimicrobial photodynamic therapy (aPDT) is an approach adopting a photosensitizer (PS) that is initially applied topically or systemically to a confined area, followed by photoactivation using a specific range of light that can excite PS to generate cytotoxic ROS in the presence of oxygen molecule. The oxidative burst produced during photoactivation imposes lethal effects on both pathogenic microbes and/or cancerous cells. Based on its dual selectivity, aPDT can be tailored to be specific for cancers or microbial infections and the lights can be adjusted accordingly to the confined area for therapeutic application. A few studies have documented that aPDT is safe and effective to be used in vivo and ex vivo, respectively (Jori et al., 2006; Dai et al., 2009). aPDT utilizes specific wavelength in visible light to excite the PS from its lowest energy (ground singlet state, 1PS) to the short-lived excited singlet state (1PS*), and can be changed to the long-lived excited triplet state, 3PS*. The triplet PS then undergoes type I and type II chemical reactions in the presence of ambient oxygen. Type I reaction involves the transfer of electrons to form reactive oxygen species (H2O2, hydroxyl radicals, superoxide etc) while type II reaction produces highly reactive oxygen species (1O2) that arise from an energy transfer ground state triplet oxygen (Maisch et al., 2007; Alves et al., 2014; Baptista et al., 2017; Wainwright et al., 2017). PS are generally non-toxic, highly conjugated unsaturated organic compounds with a large absorption coefficient in the visible to infrared spectrum to ensure good tissue penetration (Wainwright et al., 1998; Wainwright et al., 2017; Hu et al., 2018;). A plentiful of PS have been identified and are well elucidated in different recently published reviews (Abrahamse and Hamblin, 2016; Hu et al., 2018; Cieplik et al., 2018;).
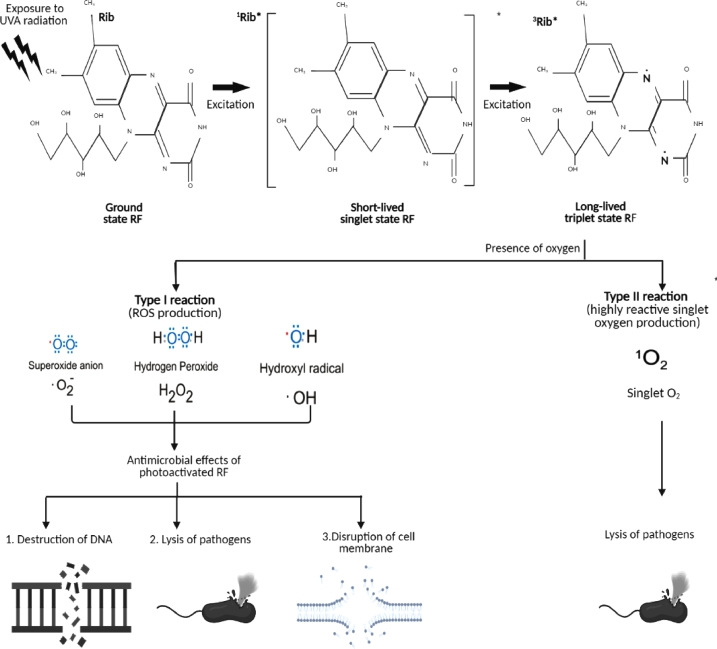
Tsugita and his collaborators (1965), are among other researchers, who firstly demonstrated that riboflavin, when exposed to visible or UVA light may cause inactivation of RNA containing tobacco mosaic virus (Tsugita et al., 1965). The excitation of riboflavin by UVA had been studied extensively. Riboflavin is a planar molecule that is able to intercalate DNA and RNA base pairs of pathogens and absorbs the visible light or UVA (Corbin, 2002). Riboflavin, once photoactivated by UVA, could result in destruction of nucleic acids by generating ROS inclusive of superoxide anion (O2-), hydrogen peroxide (H2O2) and formation of hydroxyl radical (OH), which aids in preventing the replication and toxicity of pathogens (Mohania et al., 2017). A schematic diagram on the UVA irradiated oxidation mediated by riboflavin is shown in Fig. 3. Norval (2006) reported that the antimicrobial activity of UVA irradiation includes sporicidal and virucidal effects (Norval, 2006). The drawbacks of UVA include its penetration is fully dependent on the distance from the UV source, of which uneven penetration will result in nonhomogeneous microbial inactivation (Mohania et al., 2017). Exposure to ultraviolet light in the presence of riboflavin on white blood cell, on the other hand prevents these cells from proliferation, antigen presentation, cytokine production and T- cell activation. Inactivation of white blood cells in fresh whole blood using both riboflavin and ultraviolet light provides the transfusion medicine community with a better alternative to gamma irradiation given that the combination produced white blood cells with better prevention of antigen presentation, cytokine production and T- cell activation (Fast et al., 2013). For instance, Mirasol™ pathogen-reduction technology (PRT) system is a commercially available device that applies UVA/riboflavin mechanism for pathogen inactivation in platelets and plasma during blood transfusions. In this system, UVA/riboflavin inhibits the replication of pathogens such as bacteria, fungi, parasite, virus and donor leukocytes, while maintaining homeostasis of the erythrocytes, platelets and plasma proteins (Hornsey et al., 2009; Smith and Rock, 2010; Balint et al., 2013).
Fig. 3.
Upon exposure to UV radiation, riboflavin gets excited and undergoes intersystem conversion from singlet state into triplet state. With the presence of oxygen atom, there are two types of reaction that will take place, Type 1 reaction produced reactive oxygen species (ROS). These radicals cause destruction of DNA, lysis of pathogens and disruption of cell membrane. Type 2 reaction generates highly reactive singlet oxygen species leading to lysis of pathogens.
4.4.1. Antibacterial effects and riboflavin/UVA
Extensive research has been carried out to assess the antibacterial effect of riboflavin/UVA. A study reported that extensive eradication of the colony forming unit (CFU) of S. aureus, P. aeruginosa and S. epidermis cultured on blood/hematin-agar plates when exposed to UVA/riboflavin (Makdoumi et al., 2010). The team also concluded that doubling of the UVA dose and in combination with riboflavin is more potent in decreasing bacterial number than UVA alone (Makdoumi et al., 2010). Similarly, an in vitro study reported that UVA/riboflavin treatment has antibacterial activity to a broad spectrum of bacteria including S. aureus, methicillin-resistant S. aureus (MRSA) and P. aeruginosa (Schrier et al., 2009). A study showed that UVA/riboflavin was effective against S. aureus, P. aeruginosa and S. epidermis, MRSA, multidrug-resistant P. aeruginosa (MDRPA), and drug-resistant Streptococcus pneumonia (DRSP) by using Kirby-Bauer method (Martins et al., 2008). The UVA/riboflavin irradiation procedure not only inactivated the bacterial pathogen but also will destroy leukocytes DNA that are impossible to be repaired by normal pathways (Martins et al., 2008) A study using riboflavin as photosensitizer in PDT demonstrated that combination of riboflavin and PDT have the ability to exert bactericidal activities towards MRSA, P. aeruginosa, enterohemorrhagic E. coli (EHEC) and A. baumannii (Maisch et al., 2014). Also, an in vitro study demonstrated that Mirasol™ PRT system is capable of reducing both Gram-positive and Gram-negative bacterial pathogen in donor platelet products including S. aureus, S. epidermidis, B. cereus, E. coli, P.aeruginosa, and Serratia marcescens (Martins et al., 2008)
4.4.2. Antifungal effects and riboflavin/UVA
UVA/riboflavin combination has demonstrated inhibitory activities against fungal pathogens. Fusaium, Bipolaris, Candida, Curvularia and Aspergillus are the common genera that cause fungal keratitis (Gower et al., 2010; Revankar and Sutton, 2010; Garg, 2012). Due to the minimal number of antifungal drugs, surface toxicity after prolonged treatment, limited ocular penetration, poor bioavailability interaction with other drugs and emergence of resistant strains, UVA/riboflavin light-mediated collagen crosslinking (CXL) has been proposed to inhibit the fungal pathogens growth (Thomas, 2003; Ganegoda and Rao, 2004; Kashiwabuchi et al., 2013, Maharana et al., 2016). A study of UVA/riboflavin light-mediated CXL on the microbiologically proven fungal keratitis of patients (Adams, 2004). The case series showed that no intraoperative complications lead to the recovery of corneal epithelium and ulcer. On the contrary, a study reported that UVA/ riboflavin combination was ineffective on C. albicans (Martins et al., 2008). The finding was supported that the long-wave UVA combined with riboflavin showed no antifungal effect on C. albicans and Fusarium solani (Kashiwabuchi et al., 2013). They hypothesized that the total energy delivered and wavelength of the UVA/riboflavin system conducted are not sufficient enough for complete inactivation of both filamentous fungi and yeast (Kashiwabuchi et al., 2013). However, the same study reported that slight decrease in the cell structure of C. albicans after the UVA irradiation alone treatment was observed (Kashiwabuchi et al., 2013). This showed that C. albicans cell wall may be more susceptible to UVA light irradiation which can be explained by the difference in the components of cell wall including polysaccharides, glucan, cellulose chitin and enzymes between the blank control and experimental groups (Bartnicki-Garcia, 1968; Adams, 2004) Recently, a study has reported that significant reduction in the number of Aspergillus and Fusarium species on agar plates when the fungal suspensions were treated with UVA/riboflavin (Kunt et al., 2020). Furthermore, no fungal growth was seen when UVA/riboflavin were combined with antifungal drugs including amphotericin B, variconazole and chlorhexidine in vitro (Kunt et al., 2020).
4.4.3. Antiparasitic effects and riboflavin/UVA
Riboflavin activated by UVA has also been reported to intensify antiparasitic properties. A study demonstrated that UVA/riboflavin has the ability to reduce Babesia microti of hamster whole blood (Tonnetti et al., 2013). In addition, UVA/riboflavin was effective in partial reduction of Leishmania donovani amastigotes in human monocytes (Tonnetti et al., 2015). This result was supported with finding that reported that, L. donovani and other pathogens was not found in human plasma and platelets after illuminated using Mirasol™ PRT system (Cardo et al., 2006). Besides, the Mirasol™ PRT system reduced viable P. falciparum parasitemia in vitro and P. yoelii in an in vivo murine model for malaria (Keil et al., 2013). Another study) showed that Mirasol™ PRT system is able to reduce viable Trypanosoma cruzi, the protozoan parasitic agent of Chagas disease in the whole blood (Tonnetti et al., 2015).
4.4.4. Antiviral effects and riboflavin/UVA
UVA/riboflavin has been reported to be capable of inactivating several viruses including vesicular stomatitis virus (VSV), herpes simplex virus (HSV), and polio virus in platelets with little effects on the platelets quality (Yuksel et al., 2011; Mirshafiee et al., 2015; Al-Qarni et al., 2015). The treatment of UVA/riboflavin had moderate effect to decrease dengue viruses (DENV 1-4) titres (Faddy et al., 2016). Recently, the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) were inactivated effectively in both plasma and platelet products to below the limit of detection in tissue culture following the treatment with UVA/riboflavin (Keil et al., 2020).
4.5. Antibiofilm formation and riboflavin/UVA
Biofilm is a complex structure comprises of microbial populations embedded by extracellular polymeric substances (EPS), consisting of polysaccharides, extracellular DNA, proteins, membrane vesicles, etc (Davey and O'toole, 2000; Jamal et al. 2018; Fulaz et al. 2019). Biofilm growth is critical for microbial survival in hostile environments such as on living tissues inside humans or animals, wound surfaces and medical devices (Donlan, 2002; Hall-Stoodley et al., 2004;). Microbial biofilms constitute for up to 80% of all fungal and bacterial infections in humans (Høiby, 2017) and remain difficult-to-eradicate infections because it protects pathogenic microbes against host immunity by increasing microbial resistance to various biocides and antibiotics (Stewart, 2003; Tascini et al. 2017). Comparing with their free-floating planktonic counterparts, microbial biofilm communities exhibit altered metabolic and physiological states including elevated EPS production, activation and/or suppression of genes associated with biofilm formation, reduction in growth rate (Flemming et al., 2007; Singh et al. 2017) and confer protection against antibiotics 10- 1000 times higher than their planktonic cells (Ceri et al., 1999). The need of the hour is to explore novel therapeutic approaches against highly resistant microbial biofilms. Numerous sources have proposed that antimicrobial photodynamic therapy (aPDT) could be an efficient alternative, among others in tackling recurrent and chronic microbial biofilm infections (Orlandi et al., 2014).
To date, a plethora of pathogenic microbes have been isolated from diverse biofilm-associated infections such as urinary tract infections, endocarditis, oral cavity and medical implants. Different photosensitizers through aPDT approach have been shown to exhibit wide spectrum of antibiofilm activity against various susceptible bacterial and fungal species, including Gram-positive bacteria (MRSA Enterococcus faecalis, Streptococcus spp., MSSA, Actinomyces naeslundii), Gram-negative bacteria (Fusobacterium nucleatum, P. aeruginosa, Moraxella catarrhalis, E. coli, Aggregatibacter actinomycetemcomitans) and fungi (C. albicans). The ROS-mediated aPDT inactivation of biofilm involves multiple targets and is thought to act on three separate fronts: biofilm matrix, cell surface and cytoplasm, where successful elimination of biofilm relies on the synergistic destruction on these three fronts (Hu et al., 2018; Cieplik et al., 2018). Briefly, the antibiofilm mechanism of distinctive photosensitizers (PS) is initiated by binding of PS to biofilm matrix. Under this circumstance, some PS will be totally sequestered by EPS whilst some PS partially penetrate through EPS and make a contact with the cell surface of microbial cells. Several types of PS will remain bound to cell surface whereas other types of PS can travel across cytoplasmic membrane into cellular cytoplasm and/or even penetrate organelles (Hu et al., 2018). Abundance of ROS are produced after illumination of PS, leading to multitarget oxidative damage in various adjacent molecules including destruction and detachment of polysaccharides from biofilm matrix, damage to the cell membrane (Ceri et al., 1999;Orlandi et al., 2014; Shrestha and Kishen, 2014), oxidation of lipids on the cell surface (Hu et al., 2018) inactivation of proteins by targeting sulphur containing and aromatic amino acids (Gracanin et al., 2009; Dosselli et al., 2012) and/or inducing intracellular DNA damage, in particular DNA cleavage at guanine base (Bertoloni et al., 2000; Hirakawa et al., 2014). Other organelles such as nucleus and mitochondria in fungal cells could be damaged during oxidative burst (Lam et al., 2011). The oxidative damages in multiple targets eventually result in the disintegration of biofilm matrix and cellular damage in microbial cells. The generation of large amounts of ROS is also able to surpass the antioxidant defence mechanism by microbial cells in the biofilm matrix. Typically, hydroxide ion and singlet oxygen molecules are two most common and damaging oxidative species produced by ROS during oxidative burst. The actual targets of oxidative species depend on the PS localization and its amounts, since most ROS are not able to travel for a long distance before disappearing (Hu et al., 2018).
Recently, two reviews have summarized and discuss properties of antibiofilm and efficacies of different types of photosensitizers in aPDT. These reviews also offer some insights on the mechanistic aspects on ROS-mediated aPDT inactivation of biofilm (Hu et al., 2018; Cieplik et al., 2018). However, studies using riboflavin as photosensitizer in aPDT for antibiofilm is extremely limited. A study reported that riboflavin/UV combination suppresses C. albicans biofilm by 24.5% but it is not able to inhibit biofilm formation in C. parapsilosis. The authors also showed that riboflavin/UV combination has minimal effect on biofilm formation of S. epidermidis and S. aureus (Güzel Tunccan et al., 2018). A recent study demonstrated that photo-activated riboflavin increases intracellular ROS production and inhibits mono- and mixed- species biofilms produced by antibiotic resistant Staphylococcus aureus and Escherichia coli using crystal violet assay. Nevertheless, the authors observed that mixed species biofilms are more resistant to photo-activated riboflavin than mono species biofilms. The disparity could be due to the interaction between different EPS production in mixed species biofilms (Banerjee et al., 2020). In another study, the researchers observed an antibiofilm activity against biofilm produced by Enterococcus faecalis using riboflavin with 405nm diode laser (Avianti et al., 2020). Overall, these studies highlight the potential of riboflavin as photosensitizer in aPDT against biofilm infections caused by various pathogens. However, the antibiofilm mechanisms exerted by photoilluminated riboflavin remain poorly understood. Further studies are needed to elucidate the mechanistic aspects of antibiofilm induced by photosensitized riboflavin in different pathogens.
4.5.1. Challenges and future directions of antimicrobial photodynamic therapy (aPDT) as antimicrobial and antibiofilm agent
Growing evidence have illustrated the promising effect of aPDT coupling with different photosensitizers in controlling microbial infections and biofilm formation in various bacterial and fungal infections (Tsugita et al., 1965; Wainwright et al., 1998; Corbin, 2002; Norval, 2006; Schrier et al., 2009; Makdoumi et al.,2010; Maisch et al.,2014; Mohania et al., 2017 Hu et al., 2018; Cieplik et al., 2018; Güzel Tunccan et al., 2018; Kunt et al., 2019; Khan et al.,2019). As mentioned previously in the “Overview of antimicrobial photodynamic therapy (aPDT)” on how aPDT played pivotal roles in antibacterial, antifungal, antiparasitic, antiviral effects and antibiofilm formation, it is therefore crystal clear that much more to be explored in this interesting approach to provide us with another angle of view in our quest to understand further on antimicrobial and antibiofilm agents. The journey towards the discovery and application of aPDT in the field of medical microbiology is indeed crucial and warrants necessary attention to complete the ever-challenging puzzle of tackling microbial and biofilm resistant pathogens. However, there are several aspects that deserve attention before we can effectively translate this approach into future clinical application. Firstly, the selection, binding and attachment efficiency of PS to the microbial cell surface, in particular cytoplasmic membrane needs to be carefully assessed. The architecture of cell surface structure varies among different microbes where different microbial cells will exhibit different levels of susceptibility to aPDT depending on the chemical structure of PS (Avianti et al., 2020). For example, yeasts and Gram-positive bacteria are more vulnerable to anionic and neutral PS whilst Gram-negative bacteria are more resistant because of the existence of additional asymmetric outer membrane which are negatively charged. Several methods to overcome the disadvantages have been carried out, such as conjugating PS to positively charged compounds including polyethylenimine, producing positively charged PS and treating the bacterial cells with polymyxin B nonapeptide or Tris-EDTA before the experiment (Nitzan et al., 1992).
The systemic effects of multiple targets of ROS-mediated aPDT using riboflavin or other PS against microbial and biofilm infections have not been investigated. A profound understanding on the actual mechanism behind this approach and identification of susceptible targets in ROS-mediated aPDT allow precise and accurate application of this approach in controlling microbial and biofilm infections. Hence, a comprehensive investigation of the intracellular events by means of “multiomics” approach could provide a holistic view on the underlying mechanisms of antimicrobial and antibiofilm properties of different photosensitizers. The fabrication of PS into an appropriate drug delivery type is crucial for effective control of microbial infections and biofilm inhibition. Several nanoparticles such as silver nanoparticles (Nitzan et al., 1992), chitosan nanoparticles (Darabpour et al., 2016) and graphene oxide (Xie et al., 2017) have been demonstrated to enhance the microbial killing and antibiofilm activity. Additionally, factors such as incubation period and particle size should also be considered, since these factors are important in controlling C. albicans and S. mutans biofilm (Chen et al., 2012). Moreover, optimization of PS concentration is required since high concentration of PS might hinder the light from penetrating deep ER, which affects the efficacy of photoilluminated PS (Orlandi et al., 2014; Araújo et al., 2014). Usage of complementary compounds, such as quorum sensing (Chung and Toh, 2014) or efflux pump inhibitors (De Aguiar Coletti et al., 2017) could boost the aPDT efficacy of antibiofilm activity.
Variables that influence the susceptibility of different microbial isolates to aPDT should be carefully examined. For example, the capability of S. aureus clinical strains to form biofilm has increased after sub-lethal treatment of aPDT (Kashef et al., 2013), which renders its susceptibility to another application of aPDT. Other virulence determinants such as increased resistance to antibiotics after aPDT (Kashef et al., 2013) should be identified to ensure the efficacy of aPDT in microbial and biofilm controls. The potential hazards or side effects arise from aPDT should be investigated more deeply although no toxicity of the light sources has been documented so far.
5. Conclusion
In summary, riboflavin exhibits both antimicrobial and anti-biofilm activities both when used alone or in combination with other anti-infectives. Further, its capability of modulating host immune responses could enable riboflavin to serve as an adjuvant or immunotherapeutic agent against pathogenic microbes. Nevertheless, the precise mechanism underscores these activities remain to be explored. It is also imperative to have more data that conclude precise evidence and dosage information for effective treatment or prophylaxis against microbial infections. Targeting riboflavin biosynthesis pathway, in particular riboflavin biosynthesis regulatory mechanism or enzymes/genes involved in the biosynthesis deem to be a promising therapeutic target against selected pathogenic microbes. However, identification of highly specific riboflavin analogues is rather challenging, and this quest is continuing.
The use of riboflavin in combination with UVA in combating pathogens seems to be a motivating approach as it has shown remarkable antiparasitic effects as well as promising antibacterial, antifungal and antiviral activities. Overall, this area holds promise for providing an additional arsenal against the AMR crisis and should be explored further.
6. Funding
This work was supported by Fundamental Research Grant Scheme (FRGS/1/2019/SKK11/PERDANA/03/1) from Ministry of Higher Education, Malaysia and Malaysian Medical Association Foundation Research Funding (MMA/2020/PUScLST/LTY/019) from Malaysian Medical Association (MMA) Foundation, both awarded to Tze Yan Lee.
7. Authors’ contribution
NF: writing original draft, manuscript review and editing; VKC: conceptualization, manuscript draft, manuscript review and editing; PPC: manuscript review and editing; WFL: manuscript review and editing; CWL: manuscript review and editing; RB: manuscript review and editing; SKC: manuscript review and editing; TYL: grant recipient, manuscript draft, supervision, manuscript review and editing; NF, VKC, PPC, WFL, CWL, RB, SKC, TYL: checked and approved final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All figures were created with BioRender.com.
References
- Abrahamse H., Hamblin M.R. New photosensitizers for photodynamic therapy. Biochemical Journal. 2016;473(4):347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D.J. Fungal cell wall chitinases and glucanases. Microbiology. 2004;150(7):2029–2035. doi: 10.1099/mic.0.26980-0. [DOI] [PubMed] [Google Scholar]
- Ahgilan A., Sabaratnam V., Periasamy V. Antimicrobial properties of vitamin B2. International Journal of Food Properties. 2016;19(5):1173–1181. [Google Scholar]
- Akompong T., Ghori N., Haldar K. In vitro activity of riboflavin against the human malaria parasite Plasmodium falciparum. Antimicrobial agents and chemotherapy. 2000;44(1):88–96. doi: 10.1128/aac.44.1.88-96.2000. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akompong T., Eksi S., Williamson K., Haldar K. Gametocytocidal activity and synergistic interactions of riboflavin with standard antimalarial drugs against growth of Plasmodium falciparum in vitro. Antimicrobial agents and chemotherapy. 2000;44(11):3107–3111. doi: 10.1128/aac.44.11.3107-3111.2000. (b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qarni A., AlHarbi M. Herpetic keratitis after corneal collagen cross-linking with riboflavin and ultraviolet-A for keratoconus. Middle East African journal of ophthalmology. 2015;22(3):389. doi: 10.4103/0974-9233.159777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis P., Berg K., Cengel K.A., Foster T.H., Girotti A.W., Gollnick S.O., Golab J. Photodynamic therapy of cancer: an update. CA: a cancer journal for clinicians. 2011;61(4):250–281. doi: 10.3322/caac.20114. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Suzuki M., Fujimoto M., Kimura M. Enhancement of resistance to bacterial infection in mice by vitamin B2. Journal of Veterinary Medical Science. 1995;57(4):599–602. doi: 10.1292/jvms.57.599. [DOI] [PubMed] [Google Scholar]
- Araújo N.C., Fontana C.R., Bagnato V.S., Gerbi M.E.M. Photodynamic antimicrobial therapy of curcumin in biofilms and carious dentine. Lasers in medical science. 2014;29(2):629–635. doi: 10.1007/s10103-013-1369-3. [DOI] [PubMed] [Google Scholar]
- Alves E., Faustino M.A., Neves M.G., Cunha A., Tome J., Almeida A. An insight on bacterial cellular targets of photodynamic inactivation. Future medicinal chemistry. 2014;6(2):141–164. doi: 10.4155/fmc.13.211. [DOI] [PubMed] [Google Scholar]
- Avianti R.S., Kunarti S., Subiyanto A. A comparative study of the E. faecalis antibiofilm efficacy of photoactivated curcumin, chlorophyll and riboflavin. Dental Journal (Majalah Kedokteran Gigi) 2020;53(2):62–66. [Google Scholar]
- Aslam B., Wang W., Arshad M.I., Khurshid M., Muzammil S., Rasool M.H., Baloch Z. Antibiotic resistance: a rundown of a global crisis. Infection and drug resistance. 2018;11:1645. doi: 10.2147/IDR.S173867. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher A., Eberhardt S., Fischer M., Kis K., Richter G. Biosynthesis of vitamin b2 (riboflavin) Annual review of nutrition. 2000;20(1):153–167. doi: 10.1146/annurev.nutr.20.1.153. [DOI] [PubMed] [Google Scholar]
- Balint B., Jovicic-Gojkov D., Todorovic-Balint M., Subota V., Pavlovic M., Goodrich R. Plasma constituent integrity in pre-storage vs. post-storage riboflavin and UV-light treatment–A comparative study. Transfusion and Apheresis Science. 2013;49(3):434–439. doi: 10.1016/j.transci.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Banerjee S., Ghosh D., Vishakha K., Das S., Mondal S., Ganguli A. Photodynamic antimicrobial chemotherapy (PACT) using riboflavin inhibits the mono and dual species biofilm produced by antibiotic resistant Staphylococcus aureus and Escherichia coli. Photodiagnosis and Photodynamic Therapy. 2020;32 doi: 10.1016/j.pdpdt.2020.102002. [DOI] [PubMed] [Google Scholar]
- Baptista M.S., Cadet J., Di Mascio P., Ghogare A.A., Greer A., Hamblin M.R., Yoshimura T.M. Type I and type II photosensitized oxidation reactions: guidelines and mechanistic pathways. Photochemistry and photobiology. 2017;93(4):912–919. doi: 10.1111/php.12716. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annual Reviews in Microbiology. 1968;22(1):87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Becker J.M., Kauffman S.J., Hauser M., Huang L., Lin M., Sillaots S., Roemer T. Pathway analysis of Candida albicans survival and virulence determinants in a murine infection model. Proceedings of the National Academy of Sciences. 2010;107(51):22044–22049. doi: 10.1073/pnas.1009845107. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Selbach M., Rollenhagen C., Ballmaier M., Meyer T.F., Mann M., Bumann D. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature. 2006;440(7082):303–307. doi: 10.1038/nature04616. [DOI] [PubMed] [Google Scholar]
- Bender D.A. Nutritional biochemistry of the vitamins. Cambridge university press. 2003 [Google Scholar]
- Bertoloni G., Lauro F.M., Cortella G., Merchat M. Photosensitizing activity of hematoporphyrin on Staphylococcus aureus cells. Biochimica et Biophysica Acta (BBA)-General Subjects. 2000;1475(2):169–174. doi: 10.1016/s0304-4165(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Blount K.F., Breaker R.R. Riboswitches as antibacterial drug targets. Nature biotechnology. 2006;24(12):1558–1564. doi: 10.1038/nbt1268. [DOI] [PubMed] [Google Scholar]
- Ben Yaakov D., Shadkchan Y., Albert N., Kontoyiannis D.P., Osherov N. The quinoline bromoquinol exhibits broad-spectrum antifungal activity and induces oxidative stress and apoptosis in Aspergillus fumigatus. Journal of Antimicrobial Chemotherapy. 2017;72(8):2263–2272. doi: 10.1093/jac/dkx117. [DOI] [PubMed] [Google Scholar]
- Cardo L.J., Rentas F.J., Ketchum L., Salata J., Harman R., Melvin W., Goodrich R. Pathogen inactivation of Leishmania donovani infantum in plasma and platelet concentrates using riboflavin and ultraviolet light. Vox sanguinis. 2006;90(2):85–91. doi: 10.1111/j.1423-0410.2005.00736.x. ... &. [DOI] [PubMed] [Google Scholar]
- Cardoso D.R., Libardi S.H., Skibsted L.H. Riboflavin as a photosensitizer. Effects on human health and food quality. Food & Function. 2012;3(5):487–502. doi: 10.1039/c2fo10246c. [DOI] [PubMed] [Google Scholar]
- Ceri H., Olson M.E., Stremick C., Read R.R., Morck D., Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. Journal of clinical microbiology. 1999;37(6):1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.P., Chen C.T., Tsai T. Chitosan nanoparticles for antimicrobial photodynamic inactivation: characterization and in vitro investigation. Photochemistry and photobiology. 2012;88(3):570–576. doi: 10.1111/j.1751-1097.2012.01101.x. [DOI] [PubMed] [Google Scholar]
- Chen Z., Wang H., D'souza C., Sun S., Kostenko L., Eckle S.B., Corbett A.J. Mucosal-associated invariant T-cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal immunology. 2017;10(1):58–68. doi: 10.1038/mi.2016.39. ... &. [DOI] [PubMed] [Google Scholar]
- Chen J., Illarionov B., Bacher A., Fischer M., Haase I., Georg G., Cushman M. A high-throughput screen utilizing the fluorescence of riboflavin for identification of lumazine synthase inhibitors. Analytical biochemistry. 2005;338(1):124–130. doi: 10.1016/j.ab.2004.11.033. ... &. [DOI] [PubMed] [Google Scholar]
- Chung P.Y., Toh Y.S. Anti-biofilm agents: recent breakthrough against multi-drug resistant Staphylococcus aureus. Pathogens and disease. 2014;70(3):231–239. doi: 10.1111/2049-632X.12141. [DOI] [PubMed] [Google Scholar]
- Cieplik F., Deng D., Crielaard W., Buchalla W., Hellwig E., Al-Ahmad A., Maisch T. Antimicrobial photodynamic therapy–what we know and what we don't. Critical Reviews in Microbiology. 2018;44(5):571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- Corbin F. Pathogen inactivation of blood components: current status and introduction of an approach using riboflavin as a photosensitizer. International journal of hematology. 2002;76(2):253–257. doi: 10.1007/BF03165125. [DOI] [PubMed] [Google Scholar]
- Crossley R.A., Gaskin D.J., Holmes K., Mulholland F., Wells J.M., Kelly D.J., Walton N.J. Riboflavin biosynthesis is associated with assimilatory ferric reduction and iron acquisition by Campylobacter jejuni. Applied and environmental microbiology. 2007;73(24):7819–7825. doi: 10.1128/AEM.01919-07. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman M., Jin G., Illarionov B., Fischer M., Ladenstein R., Bacher A. Design, synthesis, and biochemical evaluation of 1, 5, 6, 7-tetrahydro-6, 7-dioxo-9-D-ribitylaminolumazines bearing alkyl phosphate substituents as inhibitors of lumazine synthase and riboflavin synthase. The Journal of organic chemistry. 2005;70(20):8162–8170. doi: 10.1021/jo051332v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai T., Huang Y.Y., Hamblin M.R. Photodynamic therapy for localized infections—state of the art. Photodiagnosis and photodynamic therapy. 2009;6(3-4):170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabpour E., Kashef N., Mashayekhan S. Chitosan nanoparticles enhance the efficiency of methylene blue-mediated antimicrobial photodynamic inactivation of bacterial biofilms: An in vitro study. Photodiagnosis and photodynamic therapy. 2016;14:211–217. doi: 10.1016/j.pdpdt.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Davey M.E., O'toole G.A. Microbial biofilms: from ecology to molecular genetics. Microbiology and molecular biology reviews. 2000;64(4):847–867. doi: 10.1128/mmbr.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Aguiar Coletti T.M.S.F., De Freitas L.M., Almeida A.M.F., Fontana C.R. Optimization of antimicrobial photodynamic therapy in biofilms by inhibiting efflux pump. Photomedicine and laser surgery. 2017;35(7):378–385. doi: 10.1089/pho.2016.4246. [DOI] [PubMed] [Google Scholar]
- Dey S., Bishayi B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. Journal of Inflammation. 2016;13(1):1–21. doi: 10.1186/s12950-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietl A.M., Meir Z., Shadkchan Y., Osherov N., Haas H. Riboflavin and pantothenic acid biosynthesis are crucial for iron homeostasis and virulence in the pathogenic mold Aspergillus fumigatus. Virulence. 2018;9(1):1036–1049. doi: 10.1080/21505594.2018.1482181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlan R.M. Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases. 2002 doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosselli R., Millioni R., Puricelli L., Tessari P., Arrigoni G., Franchin C., Reddi E. Molecular targets of antimicrobial photodynamic therapy identified by a proteomic approach. Journal of proteomics. 2012;77:329–343. doi: 10.1016/j.jprot.2012.09.007. ... &. [DOI] [PubMed] [Google Scholar]
- Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiology and molecular biology reviews. 2010;74(3):417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emgård J., Bergsten H., McCormick J.K., Barrantes I., Skrede S., Sandberg J.K., Norrby-Teglund A. MAIT cells are major contributors to the cytokine response in group A streptococcal toxic shock syndrome. Proceedings of the National Academy of Sciences. 2019;116(51):25923–25931. doi: 10.1073/pnas.1910883116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faddy H.M., Fryk J.J., Watterson D., Young P.R., Modhiran N., Muller D.A., Marks D.C. Riboflavin and ultraviolet light: impact on dengue virus infectivity. Vox sanguinis. 2016;111(3):235–241. doi: 10.1111/vox.12414. ... &. [DOI] [PubMed] [Google Scholar]
- Fast L.D., Nevola M., Tavares J., Reddy H.L., Goodrich R.P., Marschner S. Treatment of whole blood with riboflavin plus ultraviolet light, an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion. 2013;53(2):373–381. doi: 10.1111/j.1537-2995.2012.03715.x. [DOI] [PubMed] [Google Scholar]
- Fidanza A., Audisio M. Vitamins and lipid metabolism. Acta vitaminologica et enzymologica. 1982;4(1-2):105–114. [PubMed] [Google Scholar]
- Fischer M., Bacher A. Biosynthesis of flavocoenzymes. Natural product reports. 2005;22(3):324–350. doi: 10.1039/b210142b. [DOI] [PubMed] [Google Scholar]
- Fischer M., Bacher A. Biosynthesis of vitamin B2 in plants. Physiologia Plantarum. 2006;126(3):304–318. [Google Scholar]
- Flemming H.C., Neu T.R., Wozniak D.J. The EPS matrix: the “house of biofilm cells. Journal of bacteriology. 2007;189(22):7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulaz S., Vitale S., Quinn L., Casey E. Nanoparticle–biofilm interactions: the role of the EPS matrix. Trends in microbiology. 2019;27(11):915–926. doi: 10.1016/j.tim.2019.07.004. [DOI] [PubMed] [Google Scholar]
- Garfoot A.L., Zemska O., Rappleye C.A. Histoplasma capsulatum depends on de novo vitamin biosynthesis for intraphagosomal proliferation. Infection and immunity. 2014;82(1):393–404. doi: 10.1128/IAI.00824-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P. Fungal, mycobacterial, and nocardia infections and the eye: an update. Eye. 2012;26(2):245–251. doi: 10.1038/eye.2011.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganegoda N., Rao S.K. Antifungal therapy for keratomycoses. Expert opinion on pharmacotherapy. 2004;5(4):865–874. doi: 10.1517/14656566.5.4.865. [DOI] [PubMed] [Google Scholar]
- Gerhardt S., Haase I., Steinbacher S., Kaiser J.T., Cushman M., Bacher A., Fischer M. The structural basis of riboflavin binding to Schizosaccharomyces pombe 6, 7-dimethyl-8-ribityllumazine synthase. Journal of molecular biology. 2002;318(5):1317–1329. doi: 10.1016/s0022-2836(02)00116-x. ... &. [DOI] [PubMed] [Google Scholar]
- Goetz J.M., Poliks B., Studelska D.R., Fischer M., Kugelbrey K., Bacher A., Schaefer J. Investigation of the binding of fluorolumazines to the 1-MDa capsid of lumazine synthase by 15N {19F} REDOR NMR. Journal of the American Chemical Society. 1999;121(33):7500–7508. ... &. [Google Scholar]
- Gower E.W., Keay L.J., Oechsler R.A., Iovieno A., Alfonso E.C., Jones D.B., Schein O.D. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117(12):2263–2267. doi: 10.1016/j.ophtha.2010.03.048. ... &. [DOI] [PubMed] [Google Scholar]
- Gracanin M., Hawkins C.L., Pattison D.I., Davies M.J. Singlet-oxygen-mediated amino acid and protein oxidation: formation of tryptophan peroxides and decomposition products. Free radical biology and medicine. 2009;47(1):92–102. doi: 10.1016/j.freeradbiomed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews microbiology. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Hartmann N., McMurtrey C., Sorensen M.L., Huber M.E., Kurapova R., Coleman F.T., Harriff M.J. Riboflavin metabolism variation among clinical isolates of Streptococcus pneumoniae results in differential activation of mucosal-associated invariant T cells. American journal of respiratory cell and molecular biology. 2018;58(6):767–776. doi: 10.1165/rcmb.2017-0290OC. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks T.S., Marchi E., Jabeen M., Olshansky M., Kurioka A., Pediongco T.J., McCluskey J. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell reports. 2019;28(12):3249–3262. doi: 10.1016/j.celrep.2019.07.039. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa K., Ota K., Hirayama J., Oikawa S., Kawanishi S. Nile blue can photosensitize DNA damage through electron transfer. Chemical research in toxicology. 2014;27(4):649–655. doi: 10.1021/tx400475c. [DOI] [PubMed] [Google Scholar]
- Høiby N. A short history of microbial biofilms and biofilm infections. Apmis. 2017;125(4):272–275. doi: 10.1111/apm.12686. [DOI] [PubMed] [Google Scholar]
- Hornsey V.S., Drummond O., Morrison A., McMillan L., MacGregor I.R., Prowse C.V. Pathogen reduction of fresh plasma using riboflavin and ultraviolet light: effects on plasma coagulation proteins. Transfusion. 2009;49(10):2167–2172. doi: 10.1111/j.1537-2995.2009.02272.x. [DOI] [PubMed] [Google Scholar]
- Howe J.A., Wang H., Fischmann T.O., Balibar C.J., Xiao L., Galgoci A.M., Roemer T. Selective small-molecule inhibition of an RNA structural element. Nature. 2015;526(7575):672–677. doi: 10.1038/nature15542. ... &. [DOI] [PubMed] [Google Scholar]
- Howe J.A., Xiao L., Fischmann T.O., Wang H., Tang H., Villafania A., Roemer T. Atomic resolution mechanistic studies of ribocil: a highly selective unnatural ligand mimic of the E. coli FMN riboswitch. RNA biology. 2016;13(10):946–954. doi: 10.1080/15476286.2016.1216304. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Huang Y.Y., Wang Y., Wang X., Hamblin M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Frontiers in microbiology. 2018;9:1299. doi: 10.3389/fmicb.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G., Fabris C., Soncin M., Ferro S., Coppellotti O., Dei D., Roncucci G. Photodynamic therapy in the treatment of microbial infections: basic principles and perspective applications. Lasers in Surgery and Medicine: The Official Journal of the American Society for Laser Medicine and Surgery. 2006;38(5):468–481. doi: 10.1002/lsm.20361. ... &. [DOI] [PubMed] [Google Scholar]
- Junaid M., Almuqri E.A., Liu J., Zhang H. Analyses of the binding between water soluble C60 derivatives and potential drug targets through a molecular docking approach. PloS one. 2016;11(2) doi: 10.1371/journal.pone.0147761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal M., Ahmad W., Andleeb S., Jalil F., Imran M., Nawaz M.A., Kamil M.A. Bacterial biofilm and associated infections. Journal of the Chinese Medical Association. 2018;81(1):7–11. doi: 10.1016/j.jcma.2017.07.012. ... &. [DOI] [PubMed] [Google Scholar]
- Kashef N., Akbarizare M., Kamrava S.K. Effect of sub-lethal photodynamic inactivation on the antibiotic susceptibility and biofilm formation of clinical Staphylococcus aureus isolates. Photodiagnosis and photodynamic therapy. 2013;10(4):368–373. doi: 10.1016/j.pdpdt.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Kaiser J., Illarionov B., Rohdich F., Eisenreich W., Saller S., Van den Brulle J., Fischer M. A high-throughput screening platform for inhibitors of the riboflavin biosynthesis pathway. Analytical biochemistry. 2007;365(1):52–61. doi: 10.1016/j.ab.2007.02.033. ... &. [DOI] [PubMed] [Google Scholar]
- Kashiwabuchi R.T., Carvalho F.R., Khan Y.A., Hirai F., Campos M.S., McDonnell P.J. Assessment of fungal viability after long-wave ultraviolet light irradiation combined with riboflavin administration. Graefe's Archive for. Clinical and Experimental Ophthalmology. 2013;251(2):521–527. doi: 10.1007/s00417-012-2209-z. [DOI] [PubMed] [Google Scholar]
- Keil S.D., Ragan I., Yonemura S., Hartson L., Dart N.K., Bowen R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light-based photochemical treatment. Vox sanguinis. 2020;115(6):495–501. doi: 10.1111/vox.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil S.D., Kiser P., Sullivan J.J., Kong A.S., Reddy H.L., Avery A., Goodrich R.P. Inactivation of P lasmodium spp. in plasma and platelet concentrates using riboflavin and ultraviolet light. Transfusion. 2013;53(10):2278–2286. doi: 10.1111/trf.12079. [DOI] [PubMed] [Google Scholar]
- Khan S., Rayis M., Rizvi A., Alam M.M., Rizvi M., Naseem I. ROS mediated antibacterial activity of photoilluminated riboflavin: a photodynamic mechanism against nosocomial infections. Toxicology reports. 2019;6:136–142. doi: 10.1016/j.toxrep.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L., Corbett A.J., Chen Z., Liu L., Mak J.Y., Godfrey D.I., Eckle S.B. An overview on the identification of MAIT cell antigens. Immunology and cell biology. 2018;96(6):573–587. doi: 10.1111/imcb.12057. ... &. [DOI] [PubMed] [Google Scholar]
- Kodama K., Suzuki M., Toyosawa T., Araki S. Inhibitory mechanisms of highly purified vitamin B2 on the productions of proinflammatory cytokine and NO in endotoxin-induced shock in mice. Life sciences. 2005;78(2):134–139. doi: 10.1016/j.lfs.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Kornman K.S., Page R.C., Tonetti M.S. The host response to the microbial challenge in periodontitis: assembling the players. Periodontology 2000. 1997;14(1):33–53. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Kundu B., Sarkar D., Ray N., Talukdar A. Understanding the riboflavin biosynthesis pathway for the development of antimicrobial agents. Medicinal research reviews. 2019;39(4):1338–1371. doi: 10.1002/med.21576. [DOI] [PubMed] [Google Scholar]
- Kunt Z., Yağmur M., Kandemir H., Harbiyeli I., Erdem E., Kalkancı A., Ilkit M. In vitro efficacy of chlorhexidine and a riboflavin/UVA combination on fungal agents of keratitis. Current eye research. 2020;45(1):7–11. doi: 10.1080/02713683.2019.1652916. ... &. [DOI] [PubMed] [Google Scholar]
- Lam M., Jou P.C., Lattif A.A., Lee Y., Malbasa C.L., Mukherjee P.K., Baron E.D. Photodynamic therapy with Pc 4 induces apoptosis of Candida albicans. Photochemistry and photobiology. 2011;87(4):904–909. doi: 10.1111/j.1751-1097.2011.00938.x. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.R., Blount K.F., Breaker R.R. Roseoflavin is a natural antibacterial compound that binds to FMN riboswitches and regulates gene expression. RNA biology. 2009;6(2):187–194. doi: 10.4161/rna.6.2.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levit R., de Giori G.S., de LeBlanc A.D.M., LeBlanc J.G. Protective effect of the riboflavin-overproducing strain Lactobacillus plantarum CRL2130 on intestinal mucositis in mice. Nutrition. 2018;54:165–172. doi: 10.1016/j.nut.2018.03.056. [DOI] [PubMed] [Google Scholar]
- Long Q., Ji L., Wang H., Xie J. Riboflavin biosynthetic and regulatory factors as potential novel anti-infective drug targets. Chemical biology & drug design. 2010;75(4):339–347. doi: 10.1111/j.1747-0285.2010.00946.x. [DOI] [PubMed] [Google Scholar]
- Machtel P., Bąkowska-Żywicka K., Żywicki M. Emerging applications of riboswitches–from antibacterial targets to molecular tools. Journal of applied genetics. 2016;57(4):531–541. doi: 10.1007/s13353-016-0341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharana P.K., Sharma N., Nagpal R., Jhanji V., Das S., Vajpayee R.B. Recent advances in diagnosis and management of Mycotic Keratitis. Indian journal of ophthalmology. 2016;64(5):346. doi: 10.4103/0301-4738.185592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch T., Eichner A., Späth A., Gollmer A., König B., Regensburger J., Bäumler W. Fast and effective photodynamic inactivation of multiresistant bacteria by cationic riboflavin derivatives. PloS one. 2014;9(12) doi: 10.1371/journal.pone.0111792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch T., Baier J., Franz B., Maier M., Landthaler M., Szeimies R.M., Bäumler W. The role of singlet oxygen and oxygen concentration in photodynamic inactivation of bacteria. Proceedings of the National Academy of Sciences. 2007;104(17):7223–7228. doi: 10.1073/pnas.0611328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins S.A.R., Combs J.C., Noguera G., Camacho W., Wittmann P., Walther R., Behrens A. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Investigative ophthalmology & visual science. 2008;49(8):3402–3408. doi: 10.1167/iovs.07-1592. ... &. [DOI] [PubMed] [Google Scholar]
- Matos de Opitz C.L., Sass P. Tackling antimicrobial resistance by exploring new mechanisms of antibiotic action. Future Microbiology. 2020;15(9):703–708. doi: 10.2217/fmb-2020-0048. [DOI] [PubMed] [Google Scholar]
- Mazur-Bialy A.I., Buchala B., Plytycz B. Riboflavin deprivation inhibits macrophage viability and activity–a study on the RAW 264.7 cell line. British journal of nutrition. 2013;110(3):509–514. doi: 10.1017/S0007114512005351. [DOI] [PubMed] [Google Scholar]
- McCormick D.B. The Vitamins: Fundamental Aspects in Nutrition and Health, by Gerald F Combs Jr. American journal of clinical nutrition. 1999;70 426-426. [Google Scholar]
- Mirshafiee H., Sharifi Z., Hosseini S.M., Yari F., Nikbakht H., Latifi H. The effects of ultraviolet light and riboflavin on inactivation of viruses and the quality of platelet concentrates at laboratory scale. Avicenna journal of medical biotechnology. 2015;7(2):57. [PMC free article] [PubMed] [Google Scholar]
- Meir Z., Osherov N. Vitamin biosynthesis as an antifungal target. Journal of Fungi. 2018;4(2):72. doi: 10.3390/jof4020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A.K., Studelska D.R., Fischer M., Giessauf A., Kemter K., Bacher A., Schaefer J. Investigation of the Binding of Epimer A of the Covalent Hydrate of 6, 7-Bis (trifluoromethyl)-8-D-ribityllumazine to a Recombinant F22W Bacillus subtilis Lumazine Synthase Mutant by 15N {19F} REDOR NMR. The Journal of organic chemistry. 2002;67(7):2087–2092. doi: 10.1021/jo010920f. ... &. [DOI] [PubMed] [Google Scholar]
- Mendelson M., Matsoso M.P. The World Health Organization global action plan for antimicrobial resistance. SAMJ: South African Medical Journal. 2015;105(5) doi: 10.7196/samj.9644. 325-325. [DOI] [PubMed] [Google Scholar]
- Mohania D., Chandel S., Kumar P., Verma V., Digvijay K., Tripathi D., Shah D. Ultraviolet radiations: Skin defense-damage mechanism. Ultraviolet Light in Human Health. Diseases and Environment. 2017:71–87. doi: 10.1007/978-3-319-56017-5_7. ... &. [DOI] [PubMed] [Google Scholar]
- Morgunova E., Saller S., Haase I., Cushman M., Bacher A., Fischer M., Ladenstein R. Lumazine synthase from Candida albicans as an anti-fungal target enzyme: structural and biochemical basis for drug design. Journal of Biological Chemistry. 2007;282(23):17231–17241. doi: 10.1074/jbc.M701724200. [DOI] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Von Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature Reviews Immunology. 2008;8(9):685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair R., Maseeh A. Vitamin D: The “sunshine” vitamin. Journal of pharmacology & pharmacotherapeutics. 2012;3(2):118. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nature Reviews Immunology. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger G., Bacher A. Biosynthesis of riboflavin. An aliphatic intermediate in the formation of 6, 7-dimethyl-8-ribityllumazine from pentose phosphate. Biochemical and biophysical research communications. 1985;127(1):175–181. doi: 10.1016/s0006-291x(85)80141-8. [DOI] [PubMed] [Google Scholar]
- Nitzan Y., Gutterman M., Malik Z., Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochemistry and photobiology. 1992;55(1):89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- Norval M. The effect of ultraviolet radiation on human viral infections. Photochemistry and photobiology. 2006;82(6):1495–1504. doi: 10.1562/2006-07-28-IR-987. [DOI] [PubMed] [Google Scholar]
- Oltmanns O., Lingens F. Isolierung von Riboflavin-Mangelmutanten von Saccharomyces cerevisiae. Zeitschrift für Naturforschung B. 1967;22(7):751–754. [PubMed] [Google Scholar]
- O'Neill J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations (HM Government and Wellcome Trust) 2014.(a) 2016 [Google Scholar]
- O'Neill J. Review on antimicrobial resistance: tackling drug-resistant infections globally: final report and recommendations. Review on antimicrobial resistance: tackling drug-resistant infections globally: final report and recommendations.(b) 2016 [Google Scholar]
- Ott E., Stolz J., Mack M. The RFN riboswitch of Bacillus subtilis is a target for the antibiotic roseoflavin produced by Streptomyces davawensis. RNA biology. 2009;6(3):276–280. doi: 10.4161/rna.6.3.8342. [DOI] [PubMed] [Google Scholar]
- Orlandi V.T., Rybtke M., Caruso E., Banfi S., Tolker-Nielsen T., Barbieri P. Antimicrobial and anti-biofilm effect of a novel BODIPY photosensitizer against Pseudomonas aeruginosa PAO1. Biofouling. 2014;30(8):883–891. doi: 10.1080/08927014.2014.940921. [DOI] [PubMed] [Google Scholar]
- Pedrolli D.B., Matern A., Wang J., Ester M., Siedler K., Breaker R., Mack M. A highly specialized flavin mononucleotide riboswitch responds differently to similar ligands and confers roseoflavin resistance to Streptomyces davawensis. Nucleic acids research. 2012;40(17):8662–8673. doi: 10.1093/nar/gks616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto J., Rivlin R. Riboflavin (vitamin B2) Handbook of Vitamins. 2013:191–266. [Google Scholar]
- Powers H.J. Riboflavin (vitamin B-2) and health. The American journal of clinical nutrition. 2003;77(6):1352–1360. doi: 10.1093/ajcn/77.6.1352. [DOI] [PubMed] [Google Scholar]
- Purnell D.M. The effects of specific auxotrophic mutations on the virulence of Aspergillus nidulans for mice. Mycopathologia et mycologia applicata. 1973;50(3):195–203. doi: 10.1007/BF02053368. [DOI] [PubMed] [Google Scholar]
- Prestinaci F., Pezzotti P., Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathogens and global health. 2015;109(7):309–318. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar S.G., Sutton D.A. Melanized fungi in human disease. Clinical Microbiology Reviews. 2010;23(4):884–928. doi: 10.1128/CMR.00019-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen C., Bumann D. Salmonella enterica highly expressed genes are disease specific. Infection and immunity. 2006;74(3):1649–1660. doi: 10.1128/IAI.74.3.1649-1660.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saedisomeolia A., Ashoori M. Riboflavin in human health: a review of current evidences. Advances in food and nutrition research. 2018;83:57–81. doi: 10.1016/bs.afnr.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Schramm M., Wiegmann K., Schramm S., Gluschko A., Herb M., Utermöhlen O., Krönke M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. European journal of immunology. 2014;44(3):728–741. doi: 10.1002/eji.201343940. [DOI] [PubMed] [Google Scholar]
- Schrier A., Greebel G., Attia H., Trokel S., Smith E.F. In vitro antimicrobial efficacy of riboflavin and ultraviolet light on Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa. Journal of refractive surgery. 2009;25(9) doi: 10.3928/1081597X-20090813-07. [DOI] [PubMed] [Google Scholar]
- Serer M.I., Carrica M.D.C., Trappe J., López Romero S., Bonomi H.R., Klinke S., Goldbaum F.A. A high-throughput screening for inhibitors of riboflavin synthase identifies novel antimicrobial compounds to treat brucellosis. The FEBS journal. 2019;286(13):2522–2535. doi: 10.1111/febs.14829. ... &. [DOI] [PubMed] [Google Scholar]
- Serganov A., Patel D.J. Amino acid recognition and gene regulation by riboswitches. Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2009;1789(9-10):592–611. doi: 10.1016/j.bbagrm.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar M., Wilbanks S.M., Nakatani Y., Monk B.C., Tyndall J.D. Catalysis product captured in lumazine synthase from the fungal pathogen Candida glabrata. Acta Crystallographica Section D: Biological Crystallography. 2013;69(8):1580–1586. doi: 10.1107/S0907444913010949. [DOI] [PubMed] [Google Scholar]
- Shrestha A., Kishen A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. Journal of Endodontics, 2014;40(10):1604–1610. doi: 10.1016/j.joen.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Stewart P.S. New ways to stop biofilm infections. The Lancet. 2003;361(9352):97. doi: 10.1016/S0140-6736(03)12245-3. [DOI] [PubMed] [Google Scholar]
- Smith J., Rock G. Protein quality in Mirasol pathogen reduction technology–treated, apheresis-derived fresh-frozen plasma. Transfusion. 2010;50(4):926–931. doi: 10.1111/j.1537-2995.2009.02517.x. [DOI] [PubMed] [Google Scholar]
- Suwannasom N., Kao I., Pruß A., Georgieva R., Bäumler H. Riboflavin: The health benefits of a forgotten natural vitamin. International Journal of Molecular Sciences. 2020;21(3):950. doi: 10.3390/ijms21030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Singh S.K., Chowdhury I., Singh R. Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. The open microbiology journal. 2017;11:53. doi: 10.2174/1874285801711010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P.A. Fungal infections of the cornea. Eye. 2003;17(8):852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- Tonnetti L., Thorp A.M., Reddy H.L., Keil S.D., Goodrich R.P., Leiby D.A. Riboflavin and ultraviolet light reduce the infectivity of Babesia microti in whole blood. Transfusion. 2013;53(4):860–867. doi: 10.1111/j.1537-2995.2012.03791.x. [DOI] [PubMed] [Google Scholar]
- Tonnetti L., Thorp A.M., Reddy H.L., Keil S.D., Doane S.K., Goodrich R.P., Leiby D.A. Reduction of L eishmania donovani infectivity in whole blood using riboflavin and ultraviolet light. Transfusion. 2015;55(2):326–329. doi: 10.1111/trf.12820. [DOI] [PubMed] [Google Scholar]
- Toyosawa T., Suzuki M., Kodama K., Araki S. Highly purified vitamin B2 presents a promising therapeutic strategy for sepsis and septic shock. Infection and immunity. 2004;72(3):1820–1823. doi: 10.1128/IAI.72.3.1820-1823.2004. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyosawa T., Suzuki M., Kodama K., Araki S. Effects of intravenous infusion of highly purified vitamin B2 on lipopolysaccharide-induced shock and bacterial infection in mice. European journal of pharmacology. 2004;492(2-3):273–280. doi: 10.1016/j.ejphar.2004.04.004. (b) [DOI] [PubMed] [Google Scholar]
- Tsugita A., Okada Y., Uehara K. Photosensitized inactivation of ribonucleic acids in the presence of riboflavin. Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis. 1965;103(2):360–363. doi: 10.1016/0005-2787(65)90182-6. [DOI] [PubMed] [Google Scholar]
- Uribe N.G., García-Galbis M.R., Espinosa R.M.M. New advances about the effect of vitamins on human health: vitamins supplements and nutritional aspects. Functional food—improve health through adequate food. 2017:57–75. [Google Scholar]
- Verdrengh M., Tarkowski A. Riboflavin in innate and acquired immune responses. Inflammation Research. 2005;54(9):390–393. doi: 10.1007/s00011-005-1372-7. [DOI] [PubMed] [Google Scholar]
- Wainwright M., Maisch T., Nonell S., Plaetzer K., Almeida A., Tegos G.P., Hamblin M.R. Photoantimicrobials—are we afraid of the light? The Lancet Infectious Diseases. 2017;17(2):e49–e55. doi: 10.1016/S1473-3099(16)30268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright M., Phoenix D.A., Laycock S.L., Wareing D.R.A., Wright P.A. Photobactericidal activity of phenothiazinium dyes against methicillin-resistant strains of Staphylococcus aureus. FEMS microbiology letters. 1998;160(2):177–181. doi: 10.1111/j.1574-6968.1998.tb12908.x. [DOI] [PubMed] [Google Scholar]
- Wang A.O.Q.U.A.N. Isolation of vitamin B2 auxotroph and preliminary genetic mapping in Salmonella typhimurium. Yi chuan xue bao= Acta genetica Sinica. 1992;19(4):362–368. [PubMed] [Google Scholar]
- Wang H., Mann P.A., Xiao L., Gill C., Galgoci A.M., Howe J.A., Roemer T. Dual-targeting small-molecule inhibitors of the Staphylococcus aureus FMN riboswitch disrupt riboflavin homeostasis in an infectious setting. Cell chemical biology. 2017;24(5):576–588. doi: 10.1016/j.chembiol.2017.03.014. ... &. [DOI] [PubMed] [Google Scholar]
- Wang H., D'Souza C., Lim X.Y., Kostenko L., Pediongco T.J., Eckle S.B., Chen Z. MAIT cells protect against pulmonary Legionella longbeachae infection. Nature communications. 2018;9(1):1–15. doi: 10.1038/s41467-018-05202-8. ... &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley J.G., Sebrell W.H. Influence of riboflavin or thiamine deficiency on fatal experimental pneumococcal infection in white mice. J. Bact. 1942;44:148. [Google Scholar]
- Xie X., Mao C., Liu X., Zhang Y., Cui Z., Yang X., Wu S. Synergistic bacteria killing through photodynamic and physical actions of graphene oxide/Ag/collagen coating. ACS applied materials & interfaces. 2017;9(31):26417–26428. doi: 10.1021/acsami.7b06702. ... &. [DOI] [PubMed] [Google Scholar]
- Yuksel N., Bilgihan K., Hondur A.M. Herpetic keratitis after corneal collagen cross-linking with riboflavin and ultraviolet-A for progressive keratoconus. International ophthalmology. 2011;31(6):513–515. doi: 10.1007/s10792-011-9489-x. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Bacher A., Illarionov B., Fischer M., Georg G., Ye Q.Z., Cushman M. Discovery and development of the covalent hydrates of trifluoromethylated pyrazoles as riboflavin synthase inhibitors with antibiotic activity against Mycobacterium tuberculosis. The Journal of organic chemistry. 2009;74(15):5297–5303. doi: 10.1021/jo900768c. ... &. [DOI] [PubMed] [Google Scholar]