Abstract
Aims:
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), infection has been deemed as a global pandemic by the World Health Organisation. While diffuse alveolar damage (DAD) is recognised to be the primary manifestation of COVID-19 pneumonia, there has been little emphasis on the progression to the fibrosing phase of DAD. This topic is of great interest, due to growing concerns regarding the potential long-term complications in prolonged survivors.
Methods and results:
Here we report a detailed histopathological study of 30 autopsy cases with COVID-19 virus infection, based on minimally invasive autopsies performed between February and March, 2020. The mean age was 69 years, with 20 (67%) males and 10 (33%) females and frequent (70.0%) underlying comorbidities. The duration of illness ranged from 16 to 82 (median = 42) days. Histologically, the most common manifestation was diffuse alveolar damage (DAD) in 28 (93.3%) cases which showed predominantly acute (32%), organising (25%) and/or fibrosing (43%) patterns. Patients with fibrosing DAD were one decade younger (P = 0.034) and they had a longer duration of illness (P = 0.033), hospitalisation (P = 0.037) and mechanical ventilation (P = 0.014) compared to those with acute DAD. Patients with organising DAD had a longer duration of illness (P = 0.032) and hospitalisation (P = 0.023) compared to those with acute DAD.
Conclusions:
COVID-19 pneumonia patients who develop DAD can progress to the fibrosing pattern. While we observed fibrosing DAD in fatal cases, whether or not surviving patients are at risk for developing pulmonary fibrosis and the frequency of this complication will require further clinical and radiological follow-up studies.
Keywords: COVID-19 pneumonia, diffuse alveolar damage, fibrosis, lung pathology, SARS-CoV-2
Introduction
We are currently suffering an outbreak of a global coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was first found in Wuhan, China in December 20191 and was recognised as a global pandemic by the World Health Organisation in March 2020. As of August 16, 2020, there have been 21 294 845 cases and 761 779 deaths due to SARS-CoV-2 infection worldwide.2
While diffuse alveolar damage (DAD) is recognised as the primary manifestation of fatal COVID-19 pneumonia, most previous pathology studies have focused upon the acute and organising histological patterns, with little emphasis on the fibrosing pattern.3–14 Furthermore, pathological studies so far have not shown correlations between the pathological phases of DAD and age, duration of clinical course, hospitalisation and mechanical ventilation in relation to progression to the fibrosing DAD pattern. In most fatal cases, the paucity of literature concerning the fibrosing DAD pattern is largely explained by the relatively rapid progression to death. So far, much of the rapidly emerging data about the pulmonary pathological manifestations in patients with COVID-19 has been based on autopsies from patients with a short survival of less than 3 weeks. There is little known about the autopsy lung pathological findings of patients with a prolonged clinical course.
There is great concern that survivors of COVID-19 pneumonia may develop the long-term complication of fibrosing interstitial lung disease. Already there is clinical and computerised tomography (CT) evidence that patients with COVID-19 pneumonia can progress to pulmonary fibrosis.15–18 Given the many millions of infected patients worldwide, even if the long-term risk is small there could be significant numbers of patients who progress to pulmonary fibrosis. This has led to several proposals for consideration of antifibrotic therapies to prevent or to treat COVID-19 pneumonia.19–21 One CT study showed imaging features of interstitial fibrosis such as interstitial thickening, air bronchograms, irregular interface, coarse reticular pattern and parenchymal bands in 44% of patients discharged after treatment for COVID-19.17
The aim of this report is to document the evolution of DAD to the fibrosing pattern in a series of minimally invasive autopsies in 30 individuals who died from COVID-19 in Wuhan, China.
Materials and methods
PATIENTS AND CLINICAL DATA
All patients were from Union Hospital of Tongji Medical College, Huazhong University of Science and Technology and met the clinical diagnostic criteria provided by the National Health Commission of China,22 as well as a positive nucleic acid test on oral-pharyngeal swabs or a positive COVID-19 anti-body blood test. All our autopsies were consecutive cases. Ten of these cases were previously published with no emphasis on fibrosing DAD.23 Their electronic medical records were retrospectively reviewed to identify the patients’ clinical features and laboratory findings. Demographic data, medical history, CT scans or X-ray images of the chest, laboratory findings [including nucleic acid tests, complete blood count (CBC) and other biochemical parameters] and the duration of illness were all reviewed. This study conforms to the regulations issued by the National Health Commission of China and the Declaration of Helsinki and has been approved by the medical ethics committee of Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology (no. 2020–0043-1).
PROCUREMENT OF SPECIMENS AND PATHOLOGICAL EXAMINATION
With consent obtained from the patients’ families, minimally invasive autopsies were performed by performing ultrasound-guided multipoint post-mortem core biopsies on bilateral lungs within 2 h after death in a negative air isolation ward between February and March 2020. Ultrasound examinations were performed using an ultrasound scanner (EPIQ 7C; Philips Medical Systems, Andover, MA, USA, equipped with an L12–5/S5–1 probe, or a Mindray Portable Ultrasound M9; Guangzhou Medsinglong Medical Equipment Co., Ltd, Guangdong, China, equipped with an L10–3 probe). All autopsies were performed by pathologists, thoracic surgeons and ultrasound doctors. At least four to five pieces of tissue were taken from each patient, including samples from both lungs using a large-core (14-guage) needle biopsy. The tissues were fixed in 10% neutral buffered formalin for more than 24 h, and then routinely processed under standard biosafety measures. Haematoxylin and eosin (H&E)-stained sections were prepared, and slides were examined by three pathologists (Y.L., J.W. and W.D.T.). Histological slides were evaluated for diffuse alveolar damage, acute bronchopneumonia, acute pneumonia, bronchitis/bronchiolitis, thrombi and other histological lesions, summarised in Supporting information, Table S1. The patterns of DAD (acute, organising and fibrosing) were recorded and based on the most prominent component; the cases were categorised according to the predominant pattern.
HISTOCHEMISTRY
Masson trichrome stains were performed in five cases to confirm the presence of dense fibrosis in fibrosing DAD versus loose fibrosis in organising DAD. For the reverse transcription–polymerase chain reaction (RT–PCR) assay for SARS-CoV-2, see Supporting information.
STATISTICAL ANALYSIS
Categorical variables were expressed as numbers and compared by t-test between groups with or without pathological findings. Continuous variables were analysed using the independent-samples t-test to compare means. A two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 26.0.
Results
CLINICAL CHARACTERISTICS
A total of 30 patients diagnosed as COVID-19 were included in this study, comprising 20 males and 10 females in Wuhan, China between February and March. The average age was 69 years, ranging from 39 to 91 years. Most cases (21 of 30, 70.0%) had at least one underlying comorbidity, the most common of which were chronic diseases such as hypertension (11 of 30, 36.7%), cancer (eight of 30, 26.7%) and coronary heart disease (five of 30, 16.7%). One patient each had chronic obstructive pulmonary disease (COPD) and asthma.
The most common symptoms at onset of illness were cough (22 of 30, 73.3%), fever (20 of 30, 66.7%) and myalgia or fatigue (13 of 30, 43.3%); less common symptoms were chest tightness (12 of 30, 40.0%), dyspnoea (11 of 30, 36.7%), sputum production (10 of 30, 33.3%), diarrhoea (five of 30, 16.7%) and chills (two of 30, 6.7%). For patients with DAD, the median duration of illness was 40 (16–82) days. The median time of hospitalisation was 33 (7–74) days, and the median time on mechanical ventilation was 23 (4–56) days. Most patients received multiple treatments, including antibiotic treatment (30 of 30, 100.0%), antiviral treatment (30 of 30, 100.0%), antifungal treatment (17 of 30, 56.7%) and glucocorticoid treatment (23 of 30, 76.7%). The clinical characteristics are listed in Table 1. Radiology is reviewed in the Supporting information.
Table 1.
Presenting symptoms and signs of the 30 autopsy cases confirmed SARS–CoV-2 infection
| Case no. |
Age/ sex |
Underlying disease |
Duration of illness (days) | Hospitalisation (days) |
Clinical symptoms |
Antibiotic Treatment |
Antiviral treatment |
Antifungal treatment |
Glucocorticoid treatment |
Bacteria/fungus isolation (sputum) |
Oxygen support on ventilation (days) | Number of core tissues |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 77/M | None | 16 | 10 | Fever, anorexia, fatigue | + | + | + | + | ND | IMV (8) | 5 |
| 2 | 60/F | Hypertension | 28 | 16 | Fever, cough, expectoration, chest tightness, dyspnoea, chills | + | + | - | + | ND | IMV (9) | 6 |
| 3 | 51/M | Pancreatic cancer, pancreatic portal hypertension | 24 | 3 | Fatigue | + | + | - | + | ND | NIV (3) | 8 |
| 4 | 87/M | Hypertension, coronary heart disease, chronic renal disease, Alzheimer’s disease | 23 | 20 | Fever, cough, itchy throat | + | + | - | - | ND | NIV (6) | 5 |
| 5 | 39/ M | Gastric cancer | 30 | 27 | Fever, chest tightness, myalgia, anorexia, bloating | + | + | + | + | ND | IMV (27) | 4 |
| 6 | 66/ M | Liver cancer | 36 | 20 | Fever, cough, dyspnoea, fatigue, myalgias | + | + | + | + | ND | IMV (20) | 6 |
| 7 | 77/ M | Skin cancer | 27 | 20 | Fever, cough, expectoration | + | + | - | + | AB | IMV (17) | 6 |
| 8 | 87/F | Coronary heart disease, Type 2 diabetes | 35 | 25 | Fever, cough, expectoration, myalgias, vomiting, diarrhoea | + | + | - | - | ND | NIV (22) | 4 |
| 9 | 70/ M | Lung cancer | 20 | 11 | Cough, chest tightness | + | + | + | + | ND | IMV (5) | 5 |
| 10 | 84/F | None | 34 | 20 | Cough, expectoration | + | + | + | + | AB, CA | IMV (20) | 6 |
| 11 | 83/F | Coronary heart disease, cerebral infarction, myocardial infarction | 32 | 7 | Anorexia | + | + | - | + | ND | NIV (7) | 6 |
| 12 | 63/ M | Hypertension | 44 | 24 | Fever, fatigue, anorexia | + | + | - | + | AB | IMV (24) | 5 |
| 13 | 52/ M | Asthma | 45 | 32 | Fever, cough | + | + | + | + | AB, KP | IMV (32) | 5 |
| 14 | 61/ M | None | 36 | 28 | Fever, cough, expectoration, chest tightness, wheezing, dyspnoea, sore throat, fatigue | + | + | + | + | AB, KP | IMV (28) | 4 |
| 15 | 70/F | Lung cancer | 45 | 31 | Cough, expectoration, chest tightness, wheezing, myalgia | + | + | - | + | ND | IMV (31) | 6 |
| 16 | 64/M | Hypertension, coronary heart disease, myocardial infarction | 42 | 37 | Fever, cough, dyspnoea | + | + | + | + | KP | IMV (36) | 5 |
| 17 | 66/M | Hypertension | 30 | 19 | Cough, chest tightness, dyspnoea, fatigue | + | + | + | + | AB | IMV (18) | 5 |
| 18 | 62/F | None | 37 | 30 | Fever, dizziness, chest tightness, diarrhoea | + | + | + | + | AB | IMV (22) | 6 |
| 19 | 55/M | None | 37 | 29 | Fever, chills, cough, expectoration, dyspnoea, fatigue, diarrhoea | + | + | + | + | AB, KP, EC | IMV (29) | 5 |
| 20 | 73/M | Hypertension, gastric cancer | 49 | 35 | Fever, cough, expectoration | + | + | + | + | AB, SA | IMV (35) | 6 |
| 21 | 91/F | Hypertension, coronary heart disease | 31 | 31 | Chest tightness | + | + | + | — | AB, CA | IMV (18) | 5 |
| 22 | 42/ M | Hypertension | 52 | 45 | Fever, cough, dyspnoea, dizziness, palpitation, fatigue, diarrhoea | + | + | + | + | AB, KP, SM | IMV (43) | 6 |
| 23 | 64/M | None | 75 | 45 | Cough, chest tightness | + | + | + | + | AB, CA | IMV (28) | 5 |
| 24 | 78/F | None | 63 | 53 | Cough, chest tightness, dyspnoea | + | + | - | + | AB, KP | IMV (4) | 10 |
| 25 | 57/M | None | 63 | 54 | Fever, cough, expectoration, chest tightness, dyspnoea | + | + | + | + | AB, KP, PA, Cl | IMV (42) | 5 |
| 26 | 70/M | Cerebral infarction, COPD, Type 2 diabetes | 71 | 57 | Fever, diarrhoea, fatigue | + | + | ND | IMV (56) | 10 | ||
| 27 | 67/M | Chronic renal insufficiency | 68 | 65 | Fever, fatigue | + | + | - | - | ND | IMV (13) | 5 |
| 28 | 80/ M | Hypertension, Alzheimer’s disease | 50 | 49 | Cough, expectoration, dyspnoea | + | + | - | + | ND | IMV (28) | 5 |
| 29 | 89/F | None | 45 | 44 | Chest tightness | + | + | + | - | CT, EF, SM | IMV (42) | 5 |
| 30 | 70/F | Hypertension, breast cancer | 82 | 74 | Fever, cough, dyspnoea | + | + | - | - | ND | IMV (39) | 5 |
AB, Acinetobacter baumannii; CA, Candida albicans; CI, Candida lusitaniae; COPD, chronic obstructive pulmonary disease; +, positive; –, negative; SARS-COV-2, severe acute respiratory syndrome coronavirus-2; CT, Candida tropicalis; EC, Escherichia coli; EF, Enterococcus faecium; F, female; IMV, invasive mechanical ventilation; KP, Klebsiella pneumoniae; M, male; ND, not done; NIV, non-invasive ventilation; SA, Staphylococcus aureus; SM, Stenotrophomonas maltophilia; PA, Pseudomonas aeruginosa.
LIGHT MICROSCOPY FINDING
The most common histological manifestation was DAD in 28 (93.3%) cases, which manifested acute (n = 12, 40.0%), organising (n = 21, 70.0%) and/or fibrosing (n = 12, 40.0%) patterns, often in combination. After excluding two cases that lacked DAD and only showed bronchopneumonia, when grouped according to the predominant pattern there were nine acute (32%), seven organising (25%) and 12 twelve fibrosing (43%) patterns.
The acute DAD pattern (Figure 1A) was characterised by hyaline membranes, intra-alveolar fibrin exudation, prominent reactive pneumocytes and pulmonary oedema. In six (50%) of these cases, alveolar walls showed focal interstitial infiltration by neutrophils that were not associated with acute bronchopneumonia (Figure 1B). Organising DAD demonstrated loose organising fibroblastic tissue consisting of polypoid plugs within distal air spaces (Figure 1C,D) or rounded nodular foci of loose myxoid fibrosis centred on alveolar ducts, often with cystic central air spaces (Figure 1E,F). Masson trichrome stains showed predominantly pale blue staining of the loose, myxoid fibrous connective tissue with very little or no dark blue staining of dense collagen (Figure 1D,F). Focal interstitial chronic inflammation was seen in 16 (76%) of these cases. The fibrosing DAD pattern contrasted with the loose organising connective tissue of organising DAD by the presence of dense interstitial fibrous tissue (Figure 2A–F) where, on high power, an eosinophilic dense appearance and/or collagen fibres could be seen highlighted by dark blue staining with Masson trichrome stains (Figure 2B,D, F). The fibrosing DAD pattern either showed alveolar duct fibrosis (Figures 2A–D) or diffuse thickening of alveolar walls (Figures 2E,F) In all 12 cases with pre- dominantly fibrosing DAD, alveolar walls thickened by dense collagen were identified, and in six of these cases alveolar duct fibrosis was also seen. Alveolar duct fibrosis in fibrosing DAD was sometimes associated with cystic spaces surrounded by dense collagen- rich fibrosis (Figure 2C,D).
Figure 1.
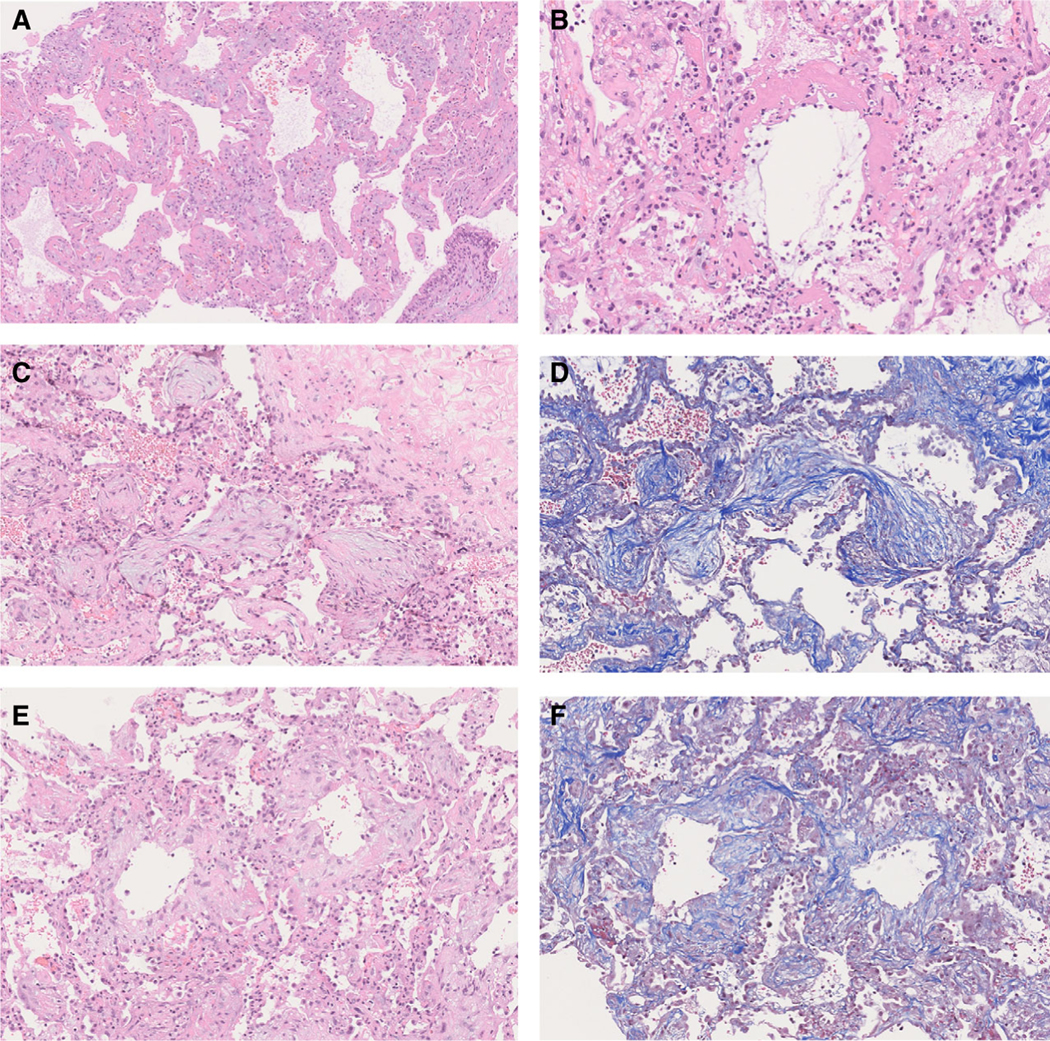
Acute and organising diffuse alveolar damage (DAD) patterns in coronavirus disease 2019 (COVID-19) patients. A, Acute phase of DAD, with hyaline membranes; B, acute DAD hyaline membranes accompanied by interstitial neutrophil infiltrates; C, organising DAD with polypoid plugs of loose connective tissue within distal alveolar spaces. The myxoid appearance of the loose connective tissue contrasts with the dense eosinophilic appearance of the collagen in perivascular interstitial fibrous connective tissue (top right); D, Masson trichrome stain shows mainly pale blue staining of the myxoid connective tissue of organising DAD; E, loose myxoid connective tissue of organising DAD situated on alveolar ducts surrounds empty air space forming small cysts; F, Masson trichrome stains show mainly pale blue staining of the myxoid connective tissue of the alveolar duct fibrosis in organising DAD.
Figure 2.
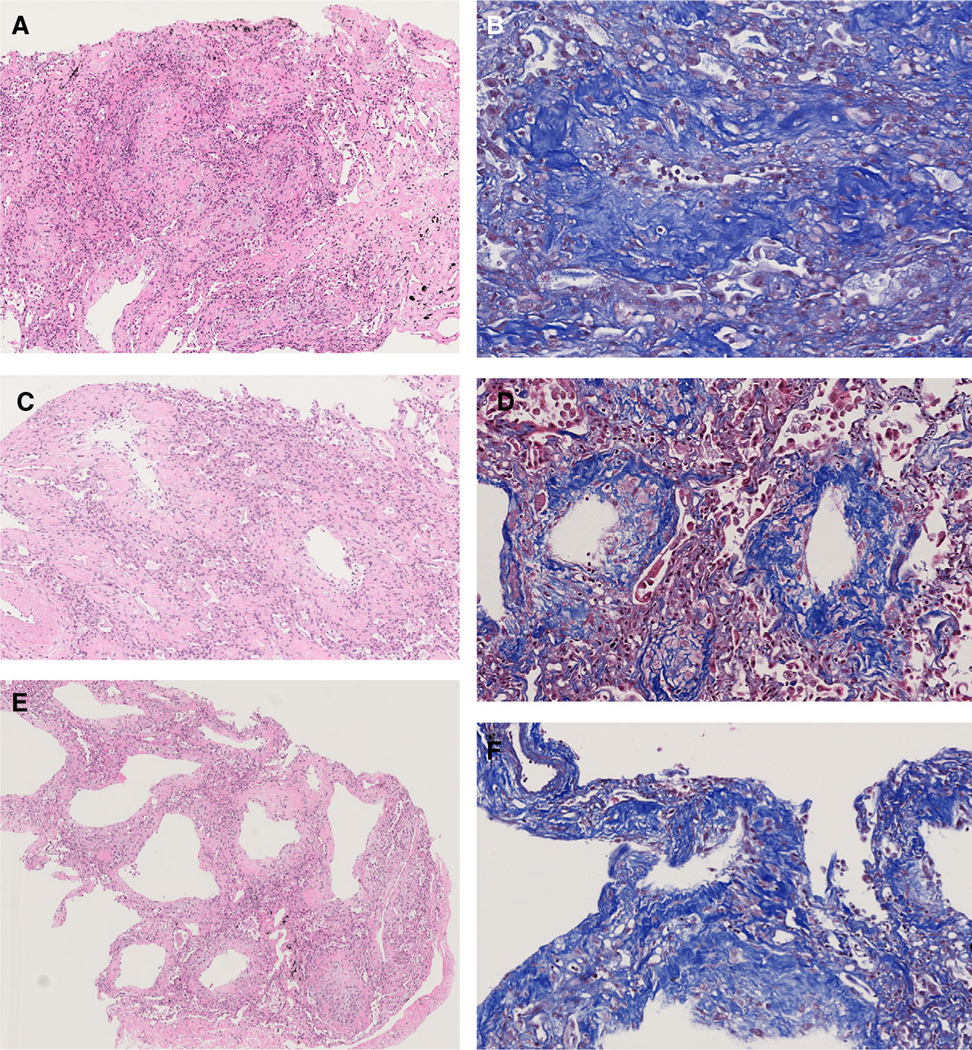
Fibrosing diffuse alveolar damage (DAD) patterns in coronavirus disease 2019 (COVID-19) patients. A, Abundant fibrosis with mainly dense eosinophilic collagen expands the interstitium. Mild interstitial chronic inflammation is also present; B, the dense collagen is highlighted by the Masson trichrome stain. In this image the rounded fibrotic area is centred on an alveolar duct; C, dense eosinophilic fibrosis surrounds three alveolar ducts and forms small cystic spaces; D, the abundant dense collagen in the alveolar duct fibrosis is highlighted by the dark blue staining with the Masson trichrome stain; E, these alveolar walls are markedly thickened by dense fibrosis and interstitial chronic inflammation. While the alveolar architecture is preserved, small cysts are present retaining some features of alveolar duct fibrosis. F, Masson trichrome stain shows dark blue staining of the dense collagen, causing thickening of the alveolar walls.
Other histological findings are summarised in the Supporting information results and Supporting information, Table S1.
CLINICOPATHOLOGICAL CORRELATIONS
Comparison of clinical features between predominant DAD patterns
Correlations between the phases of DAD and duration of illness, length of hospitalisation and mechanical ventilation are summarised in Table 2. Patients with fibrosing DAD were approximately one decade younger than those with acute DAD (P = 0.034). In addition, patients with fibrosing DAD had a longer duration of illness (P = 0.033), hospitalisation (P = 0.037) and ventilation (P = 0.014) compared to patients with acute DAD. Patients with organising DAD had a longer duration of illness (P = 0.032) and hospitalisation (P = 0.023) compared to those with acute DAD.
Table 2.
Clinical correlations with patterns of DAD in fatal COVID-19 pneumonia
| Acute predominant | Organising predominant | Fibrosing predominant | ||
|---|---|---|---|---|
| DAD | DAD | DAD | No DAD | |
| n = 9 | n = 7 | n = 12 | n = 2 | |
| Age, years: median (range) | 77 (62–87) | 77 (42–89) | 64 (55–91) ††P = 0.034 |
45 (39– 51) |
| Sex: female/male | 4/5 | 3/ 4 | 3/9 | 0/2 |
| Days of illness, median (range) | 34 (16–50) *P = 0.005 †P = 0.032 |
45 (27–68) | 42 (28–82) ††P = 0.033 |
27 (24– 30) |
| Days of hospitalisation, median (range) | 20 (7–49) *P = 0.002 †P = 0.023 |
38 (20–65) | 31 (16–74) ††P = 0.037 |
15 (3–27) |
| Days of ventilation, median (range) | 20 (5–31) *P = 0.014 |
20 (4–42) | 28 (9–56) **P = 0.045 ††P = 0.014 |
15 (3–27) |
DAD = diffuse alveolar damage.
Acute versus other DAD patterns;
fibrosing versus other DAD patterns;
acute versus organising DAD;
acute versus fibrosing DAD.
Comparison of clinical features in predominant DAD pattern versus other patterns
Patients with fibrosing DAD were on ventilation longer than patients without fibrosing DAD (30 versus 20 days, respectively, P = 0.045). Patients with, compared to those without, acute DAD had a shorter mean duration of illness (34 versus 51, P = 0.0005), shorter mean hospitalisation (22 versus 42, P = 0.002) and fewer days on ventilation (17 versus 30, P = 0.014).
CAUSE OF DEATH
DAD was the primary cause of death for all 28 affected patients, while acute pneumonia and gastric cancer contributed to death in two patients without DAD findings.
REVERSE TRANSCRIPTION–POLYMERASE CHAIN REACTION (RT–PCR) ASSAY FOR SARS-COV-2
Details concerning the results of the RT–PCR assay are shown in the Supporting information results.
Discussion
Our study documents pathological evidence of DAD that progressed to the fibrosing pattern in patients who died in the COVID-19 outbreak in Wuhan, China. The prolonged clinical course (≥ 4 weeks) and duration of mechanical ventilation (median 20 days) in the patients who progressed to fibrosing DAD in our cohort differed from the clinical course in previous autopsy studies, where the patients died more rapidly. We also demonstrated that the patients with fibrosing DAD, compared to those with acute DAD, were one decade younger and had significantly longer duration of illness, hospitalisation and mechanical ventilation.
In prior viral pandemics, the paucity of pathology studies describing the fibrosing DAD pattern is remarkable.24–34 In contrast, while we are still relatively early in the COVID-19 pandemic, fibrosis is already being described in the CT literature35–38 and we observed it pathologically in more than 40% of our cases. In addition, early pathology studies are emerging describing the development of interstitial fibrosis in COVID-19.39,40 This raises the question of whether differences in the pathogenesis of COVID-19 pneumonia or in the management of our patients compared to other cohorts of viral epidemics could explain this difference. The frequency of fibrosing DAD in our study is probably skewed due to the high percentage of patients with a prolonged clinical course in our cohort compared to previous autopsy studies, together with the relatively long duration of illness, hospitalisation and mechanical ventilation. However, pulmonary fibrosis was seen by CT in 41% of symptomatic COVID-19 patients suspected to have interstitial pneumonia.35 Although this was significantly associated with ‘late phase’ disease, defined as greater than only 7 days, it was a component of a scoring system that predicted mortality.35
In contrast to our observation of fibrosing DAD in fatal COVID-19 pneumonia in patients with a prolonged clinical course, there was a relatively rapid progression to death in previous autopsy studies of patients who died with SARS-CoV, Middle East respiratory syndrome (MERS) and influenza infections. Reports of fatal SARS-CoV infection showed a median duration of 11–23.5 days.28,30,41 Although CT studies of SARS-CoV pneumonia showed signs of interstitial fibrosis in 62% of patients after an average of 37 days42 and interstitial abnormalities in 5% of patients after 15 years’ follow-up,43 in fatal cases we could only find one autopsy study documenting fibrosing DAD in three patients who survived into the fourth week of disease or longer.28–30,41 In addition, the median time from onset of symptoms to death of influenza A/H1N1 virus infection was 7–8 days (range = 1–44).32,33 The estimated illness interval of MERS-CoV infection was even shorter, with a median time to death of 11 days (range = 5–27).44
It is known that after the inciting injury DAD progresses through three phases, beginning with the acute phase within the first 7–10 days, the organising phase after the first 7–10 days and the fibrosing phase after approximately 3 weeks.45–48 In this study we were able to describe the histological features of the fibrosing pattern of DAD that have not been well documented in previous autopsy studies of COVID-19 pneumonia. The fibrosing pattern differs from the organising DAD pattern by the presence of dense fibrosis with visible collagen fibres and/or hyalinisation, unlike the loose proliferative connective tissue of the organising pattern, which frequently shows a myxoid stroma and lacks dense collagen. In cases where there is a question concerning H&E as to whether or not dense collagen is present, Masson trichrome stains may be helpful. The dark blue staining of dense fibrosis in fibrosing DAD contrasts with the light blue staining seen with the loose connective tissue of organising DAD. As we observed, the fibrosing DAD pattern showed diffuse thickening of alveolar walls in all cases, and in addition a mixed pattern in half these cases that also showed nodular foci of fibrosis centred on alveolar ducts or alveolar duct fibrosis.46–48 Pratt et al. observed that the early organising fibrosis of organising DAD tended to be localised along the alveolar ducts.46 This distribution was evident in our case material, where in the fibrosing DAD cases the alveolar duct connective tissue was replaced by dense collagen. A similar progression to dense collagen-rich fibrosis from organising connective tissue has also been described in organising pneumonia under a variety of names, such as fibrosing or cicatricial organising pneumonia.49–51 The fibrosing lesions were sometimes associated with small cystic spaces. The overall histological features of DAD in COVID-19 pneumonia appear similar to those seen in DAD due to other causes or idiopathic cases.5,14
In non-COVID-19 patients with acute respiratory distress syndrome (ARDS), CT findings that suggest progression to the fibrosing pattern of DAD consist of reticulation, traction bronchiectasis and cystic changes or honeycombing.52,53 When DAD progresses to fibrosis, it is known to evolve into a pattern that bears some resemblance to non-specific interstitial pneumonia.47,54–56 CT studies have shown progression to fibrosis in COVID-19,17,57 SARS43,58,59 and in one follow-up study of ARDS.53 In the few reported cases of SARS where a fibrosing DAD pattern was described pathologically, the figure provided shows a pattern of alveolar duct fibrosis similar to that seen in our cases.29
Interestingly, similar to our finding of a clinical association with the fibrosing DAD pattern histologically, Desai et al. found that the presence of a reticular pattern by high resolution CT in non-COVID ARDS patients was independently related to the total duration of mechanical ventilation.53 It is known that ventilation can contribute to the causation of DAD, while paradoxically it can be essential for prolonging survival.45,47,48,60,61 It is not clear in our cases whether the progression to fibrosing DAD was directly related to the viral pneumonia, or if the mechanical ventilation or other factors may have also played a role.
The description of a ‘fibrotic’ pattern in the lung of 22% of lung samples in a meta-analysis of articles published on COVID-19 pathology needs to be reviewed carefully.62 The lung findings were not characterised according to phases of DAD, but rather epithelial, vascular and fibrotic patterns.62 Furthermore, the review summarised cases as a ‘fibrotic’ pattern if the word ‘fibrosis’ was mentioned in the article under the histological description. In fact, the authors of the study that contributed the largest number of ‘fibrotic’ cases (n = 12) to this meta-analysis specifically stated that the focality of the findings suggested: ‘that none of the patients had progressed to the fibrotic phase (of DAD) possibly due to the short duration of disease’.63 This raises the challenge of distinguishing the fibrosing pattern of DAD from the loose connective tissue of the organising pattern, an issue that is not consistently addressed in the literature on COVID-19 pneumonia.63–65
While the minimally invasive approach to autopsies using core biopsies provides more limited sampling than would have been available with complete autopsies, it is of interest that a recent study in patients with yellow fever compared minimally invasive biopsies with complete autopsies in the same cases and found perfect agreement.66 In addition, the results of several minimally invasive autopsy studies of COVID-19 pneumonia have shown similar findings to those published in studies of complete autopsies, although the limited sampling compromises the ability to evaluate lesions in major central structures such as the trachea, bronchi and major blood vessels.7,8 While is it appreciated that assessment of patterns of interstitial lung disease are best made in larger samples of lung tissue, the multiple lobe and bilateral sampling in our minimally invasive autopsies, along with the compatible clinical findings, seem sufficient to support the concept that DAD in COVID-19 pneumonia can progress a fibrosing pattern.16,19,67 We cannot exclude the possibility that a fibrosing pattern of DAD, in addition to other pathological lesions, may have been missed due to the limited sampling of the core biopsies, so such findings may have been underestimated in our study. The burden of fibrosis is also difficult to assess based on core biopsies. As we have pointed out above, one limitation of our study is the skewed data with a large percentage of patients with long hospitalisations. Based on autopsy studies, it is difficult to determine the actual frequency of evolution of COVID-19 pneumonia to a fibrosing pattern of DAD because of the many potential variables that might contribute to developing fibrosis. It is not clear why our cohort had a larger percentage of patients with a prolonged clinical course compared to other autopsy studies, but this could be due to a variety of factors, including differences in management such as prolonged mechanical ventilation, varying pathogenicity based on differing strains of the virus, or differing host genetic or epidemiological factors. We also cannot exclude the possibility that the patients with fibrosing DAD in our study recovered from COVID-19 but failed after a prolonged clinical course. As stated above, we also cannot rule out the possibility that the prolonged mechanical ventilation contributed to the development of fibrosis. Due to the uniqueness of our cohort, the frequency of fibrosis we observed probably does not reflect that expected for the entire COVID-19 patient population.
In conclusion, our study provides pathological evidence of progression of DAD to a fibrosing pattern in a cohort of patients from Wuhan, China. Compared to patients with acute DAD, this was associated with younger age as well as prolonged duration of disease, hospitalisation and ventilation. This has significant implications for long-term sequelae of COVID-19 disease. Already lung transplantation has been used to treat the severe interstitial fibrosis that develops after initial recovery from COVID-19 infection.68,69 In analysing autopsy studies that provide important information advancing our knowledge of the pathology and pathogenesis of COVID-19, it is important to keep in mind that only a small percentage of these patients will develop serious respiratory failure requiring intensive care admission and invasive mechanical ventilation. Therefore, it is not possible to extrapolate our autopsy findings to the clinical course of surviving patients.
In a study of 1099 patients from China with laboratory-confirmed COVID-19 only 5% were admitted to the intensive care unit, 2.3% underwent invasive mechanical ventilation and 1.4% died.70 While these percentages seem small, given the millions of COVID-19-infected patients worldwide, a sizeable number of patients who develop serious respiratory disease will be at risk for long-term complications.
While we observed fibrosing DAD in fatal cases, further studies are needed to determine the risk of COVID-19 survivors developing pulmonary fibrosis. Hopefully, our autopsy findings will encourage long-term clinical and CT follow-up studies of these patients. In addition, further investigations with complete autopsies and detailed clinical and radiological correlation are needed to understand the pathogenesis of the pulmonary interstitial fibrosis that develops in COVID-19 pneumonia.
Supplementary Material
Fig S1. High resolution chest CT scan of patient with fibrosing diffuse alveolar damage (DAD) shows patchy bilateral reticulation, traction bronchiectasis and cystic changes consistent with fibrosis in addition to ground glass opacities.
Table S1. Pulmonary histology findings of the 30 autopsy cases
Appendix S1. Supplemental methods
Acknowledgements
This study was supported by grant from National Natural Science Foundation of China (X.N., no. 81773022), Key Special Project of Ministry of Science and Technology, China (X.N., no.2020YFC0845700). We would like to thank all the patients and relatives involved in the present study for their great efforts and acknowledge all the healthcare professionals who helped and took care of the patients with COVID-19 from Union Hospital, Tongji Medical College, Huazhong University of Science and Technology in Hebei Province for their selfless dedication in the medical relief operation against SAR-CoV-2.
Footnotes
Conflicts of interest
None declared.
References
- 1.Li Q, Guan X, Wu P et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020; 382; 1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Coronavirus disease (COVID-2019) Situation Report 209. Geneva, Switzerland: World Health Organization; 2020. [accessed 2020 August 16, 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Google Scholar]
- 3.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am. J. Clin. Pathol. 2020; 153; 725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. Cardiovasc. Pathol. 2020; 8; 681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konopka KE, Nguyen T, Jentzen JM et al. Diffuse alveolar damage (DAD) from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology 2020; 77; 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martines RB, Ritter JM, Matkovic E et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus dis- ease, United States. Emerg. Infect. Dis. 2020; 26; 2005–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nunes Duarte-Neto A, de Almeida Monteiro RA, da Silva LFF et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology 2020. 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian S, Xiong Y, Liu H et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod. Pathol. 2020; 33; 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D, Sperhake JP, Lutgehetmann M et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020; 173; 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Shi L, Wang Y et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020; 8; 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Zhou P, Wei Y et al. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann. Intern. Med. 2020; 172; 629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adachi T, Chong JM, Nakajima N et al. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19. Japan. Emerg. Infect. Dis. 2020; 26; 2157–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lax SF, Skok K, Zechner P et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: results from a prospective, single-center, clinicopathologic case series. Ann. Intern. Med. 2020; 173; 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauter JL, Baine MK, Butnor KJ et al. Insights into pathogene- sis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology 2020. 10.1111/his.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentile F, Aimo A, Forfori F et al. COVID-19 and risk of pulmonary fibrosis: the importance of planning ahead. Eur. J. Prev. Cardiol. 2020; 27; 1442–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spagnolo P, Balestro E, Aliberti S et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir. Med. 2020; 8; 750–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu M, Liu Y, Xu D, Zhang R, Lan L, Xu H. Prediction of the development of pulmonary fibrosis using serial thin-section CT and clinical features in patients discharged after treatment for COVID-19 pneumonia. Korean J. Radiol. 2020; 21; 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayhan S, Kocakoc E. Pulmonary fibrosis due to COVID-19 pneumonia. Korean J. Radiol. 2020; 21; 1273–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir. Med. 2020; 8; 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechowicz K, Drozdzal S, Machaj F et al. COVID-19: the potential treatment of pulmonary fibrosis associated with SARS- CoV-2 infection. J. Clin. Med. 2020; 9; 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifirad S. Pirfenidone: a novel hypothetical treatment for COVID-19. Med. Hypoth. 2020; 144; 110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Commission of the People’s Republic of China. 2020. Available at: http://www.nhc.gov.cn/yzygj/s7652m/202002/e84bd30142ab4d8982326326e4db22ea.shtml Accessed August 16, 2020. [DOI] [PMC free article] [PubMed]
- 23.Wu JH, Li X, Huang B et al. [Pathological changes of fatal coronavirus disease 2019 (COVID-19) in the lungs: report of 10 cases by postmortem needle autopsy]. Zhonghua bing li xue za zh/Chin. J. Pathol. 2020; 49; 568–575. [DOI] [PubMed] [Google Scholar]
- 24.Goodpasture EW. The significance of certain pulmonary lesions in relation to the etiology of influenza. Am. J. Med. Sci. 1919; 158; 863–870. [DOI] [PubMed] [Google Scholar]
- 25.LeCount ER. The pathological anatomy of influenzal bronchopneumonia. JAMA 1919; 72; 227–233. [Google Scholar]
- 26.Opie EL. The pathologic anatomy of influenza. Arch. Pathol. Lab. Med. 1928; 5; 285–303. [Google Scholar]
- 27.Taubenberger JK, Morens DM. The pathology of influenza virus infections. Ann. Rev. Pathol. 2008; 3; 499–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franks TJ, Chong PY, Chui P et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum. Pathol. 2003; 34; 743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung OY, Chan JW, Ng CK, Koo CK. The spectrum of pathological changes in severe acute respiratory syndrome (SARS). Histopathology 2004; 45; 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse GM, To KF, Chan PK et al. Pulmonary pathological features in coronavirus associated severe acute respiratory syndrome (SARS). J. Clin. Pathol. 2004; 57; 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007; 170; 1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill JR, Sheng ZM, Ely SF et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch. Pathol. Lab. Med. 2010; 134; 235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shieh WJ, Blau DM, Denison AM et al. 2009 pandemic influenza A (H1N1): pathology and pathogenesis of 100 fatal cases in the United States. Am. J. Pathol. 2010; 177; 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsaad KO, Hajeer AH, Al Balwi M et al. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection – clinicopathological and ultrastructural study. Histopathology 2018; 72; 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Francone M, Iafrate F, Masci GM et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur. Radiol. 2020; 4; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen C, Yu N, Cai S et al. Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019. J. Pharmaceut. Anal. 2020; 10; 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du S, Gao S, Huang G et al. Chest lesion CT radiological features and quantitative analysis in RT–PCR turned negative and clinical symptoms resolved COVID-19 patients. Quant. Imaging Med. Surg. 2020; 10; 1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Pan J, Teng D, Xu X, Feng J, Chen YC. Interpretation of CT signs of 2019 novel coronavirus (COVID-19) pneumonia. Eur. Radiol 2020; 4; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grillo F, Barisione E, Ball L, Mastracci L, Fiocca R. Lung fibrosis: an undervalued finding in COVID-19 pathological series. Lancet Infect. Dis. 2020. 10.1016/s1473-3099(20)30582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwensen HF, Borreschmidt LK, Storgaard M, Redsted S, Christensen S, Madsen LB. Fatal pulmonary fibrosis: a post-COVID-19 autopsy case. J. Clin. Pathol. 2020; 28; S1473–3099. 10.1136/jclinpath-2020-206879. [DOI] [PubMed] [Google Scholar]
- 41.Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005; 18; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antonio GE, Wong KT, Hui DS et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology 2003; 228; 810–815. [DOI] [PubMed] [Google Scholar]
- 43.Zhang P, Li J, Liu H et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020; 8; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Assiri A, McGeer A, Perl TM et al. Hospital outbreak of Middle East respiratory syndrome coronavirus. N. Engl. J. Med. 2013; 369; 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage – the role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976; 85; 209–228. [PMC free article] [PubMed] [Google Scholar]
- 46.Pratt PC. Pathology of adult respiratory distress syndrome. Monogr. Pathol. 1978; 19; 43–57. [PubMed] [Google Scholar]
- 47.Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome Clin. Chest Med. 2000; 21; 435–466. [DOI] [PubMed] [Google Scholar]
- 48.Travis W, Colby T, Koss M et al. Diffuse alveolar damage and acute interstitial pneumonia. In King D ed. Nonneoplastic disorders of the lower respiratory tract. Washington, DC: American Registry of Pathology, 2002; 89–106. [Google Scholar]
- 49.Beardsley B, Rassl D. Fibrosing organising pneumonia. J. Clin. Pathol. 2013; 66; 875–881. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Kitaichi M, Tachibana K et al. A cryptogenic case of fulminant fibrosing organizing pneumonia. Intern. Med. 2017; 56; 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Churg A, Wright JL, Bilawich A. Cicatricial organising pneumonia mimicking a fibrosing interstitial pneumonia. Histopathology 2018; 72; 846–854. [DOI] [PubMed] [Google Scholar]
- 52.Sverzellati N, Lynch DA, Hansell DM. American Thoracic Society-European Respiratory Society classification of the idiopathic interstitial pneumonias: advances in knowledge since 2002. Radiographics 2015; 35; 1849–1871. [DOI] [PubMed] [Google Scholar]
- 53.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology 1999; 210; 29–35. [DOI] [PubMed] [Google Scholar]
- 54.Kambouchner M, Levy P, Nicholson AG et al. Prognostic relevance of histological variants in nonspecific interstitial pneumonia. Histopathology 2014; 65; 549–560. [DOI] [PubMed] [Google Scholar]
- 55.Travis WD, Colby TV, Koss MN, Rosado-de-Christenson M, Müller NL, King TE Jr. Nonspecific interstitial pneumonia. In King DW ed. Nonneoplastic disorders of the lower respiratory tract. Washington, DC: American Registry of Pathology, 2002; 73–82. [Google Scholar]
- 56.Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/fibrosis. Histologic features and clinical significance. Am. J. Surg. Pathol. 1994; 18; 136–147. [PubMed] [Google Scholar]
- 57.Wei J, Yang H, Lei P et al. Analysis of thin-section CT in patients with coronavirus disease (COVID-19) after hospital discharge. J. X-ray Sci. Technol. 2020; 28; 383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Müller NL, Ooi GC, Khong PL, Zhou LJ, Tsang KW, Nicolaou S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. Am. J. Roentgenol. 2004; 182; 39–44. [DOI] [PubMed] [Google Scholar]
- 59.Ooi GC, Khong PL, Müller NL et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology 2004; 230; 836–844. [DOI] [PubMed] [Google Scholar]
- 60.Pratt DS, Schwarz MI, May JJ, Dreisin RB. Rapidly fatal pulmonary fibrosis: the accelerated variant of interstitial pneumonitis. Thorax 1979; 34; 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres A, Fabregas N, Arce Y, Lopez-Boado MA. Histopathology of ventilator-associated pneumonia (VAP) and its clinical implications. Infection 1999; 27; 71–76. [DOI] [PubMed] [Google Scholar]
- 62.Polak SB, Van Gool IC, Cohen D, von der Thusen JH, van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020; 22; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carsana L, Sonzogni A, Nasr A et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect. Dis. 2020; 20; 1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wichmann D, Sperhake JP, Lutgehetmann M et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann. Intern. Med. 2020; 173; 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Copin MC, Parmentier E, Duburcq T et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intens. Care Med 2020; 46; 1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duarte-Neto AN, Monteiro RAA, Johnsson J et al. Ultrasound-guided minimally invasive autopsy as a tool for rapid post-mortem diagnosis in the 2018 Sao Paulo yellow fever epidemic: correlation with conventional autopsy. PLOS Negl. Trop. Dis. 2019; 13; e0007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schaller T, Hirschbuhl K, Burkhardt K et al. Postmortem examination of patients with COVID-19. JAMA 2020; 323; 2518–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen JY, Qiao K, Liu F et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin. Med. J. (Engl.) 2020; 133; 1390–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han W, Zhu M, Chen J et al. Lung transplantation for elderly patients with end-stage COVID-19 pneumonia. Ann. Surg. 2020; 272; e33–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382; 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. High resolution chest CT scan of patient with fibrosing diffuse alveolar damage (DAD) shows patchy bilateral reticulation, traction bronchiectasis and cystic changes consistent with fibrosis in addition to ground glass opacities.
Table S1. Pulmonary histology findings of the 30 autopsy cases
Appendix S1. Supplemental methods


