Abstract
We report here the first analysis of Erysipelothrix spp. using pulsed-field gel electrophoresis (PFGE). Seventy strains of Erysipelothrix spp. were analyzed. SmaI, AscI, and NotI were tested for the ability to cleave the DNA extracted from those strains, and among them, SmaI was the most reliable enzyme. Sixty-three distinct PFGE patterns were produced, and no DNA degradation was observed, allowing the identification of all of the strains. Based on these results and on those of a previous analysis using randomly amplified polymorphic DNA and ribotyping, PFGE with SmaI might be considered to be more sensitive than those methods and to be the best method for epidemiological studies of strains of this genus.
Erysipelothrix rhusiopathiae is a gram-positive, slender, and straight or slightly curved rod that causes a wide spectrum of diseases in animals, birds, and humans (14, 49). This bacterium has been isolated in most parts of the world, not only from sick and healthy animals but even from pork, seafood, retail game meat, and chicken meat (11, 19, 26, 27, 38, 40, 44). Human infections with this bacterium are usually related to occupational exposure (33). However, infection after consumption of undercooked pork and infections of patients with no history of contact with animals or skin lesions have been reported, and in many cases, the source of infection has not been identified (6, 13, 20, 28, 37). Moreover, potential errors in the recognition of this organism isolated from human infections due to unusual clinical presentations and the possibility of underdiagnosed infections have been reported (3, 10, 34). PCR-based assays for the rapid diagnosis of Erysipelothrix species have been described (22, 39, 42). However, to proceed with an epidemiological study and identify the source of infection, it is necessary to be able to identify each strain isolated from a case or outbreak, as well as the relatedness among the strains isolated from the possible source.
During the last few years, molecular biological methods such as randomly amplified polymorphic DNA (RAPD), ribotyping, and pulsed-field gel electrophoresis (PFGE) have been demonstrated to be reliable tools for the differentiation of species and strains of one genus and for use in epidemiological studies of several pathogenic bacteria (9, 15, 16, 24, 32, 46–48). Although PFGE has been considered to be the “gold standard” among these methods (30), studies have shown that this method can be less sensitive than ribotyping and PCR-based methods with regard to the ability to differentiate between bacterial strains of some species (5, 36, 45). Moreover, there is no standard and universal PFGE protocol for all species of bacteria and it is necessary to adapt the procedures and choose a suitable enzyme for each genus or species. However, the use of this method for strains of the genus Erysipelothrix has not been reported. Therefore, we describe herein the first analysis of a large collection of Erysipelothrix species strains by PFGE.
MATERIALS AND METHODS
Bacterial strains.
Seventy strains, including 55 of E. rhusiopathiae and 12 of E. tonsillarum, as well as 2 strains of serovar 13 and 1 strain of serovar 18 that have been considered to be members of a possible new species (41), were chosen from our previous study (29). The sources for and details regarding each strain are shown in Table 1.
TABLE 1.
PFGE patterns of 70 strains of Erysipelothrix spp. produced by SmaI
| Species and serovar | Strain | Source | PFGE pattern | RAPD patternd | Ribo patterne |
|---|---|---|---|---|---|
| E. rhusiopathiae | |||||
| 1a | ME-7a | Unknown | P1 | a | B |
| E176 | Fish | P2 | b | ||
| E157 | Fish | P3 | a | ||
| 1b | 422/1E1a | Porcine spleen | P4 | a | A |
| E019 | Fish | P5 | g | ||
| K040 | Wild boar meat | P6 | a | ||
| K075 | Wild boar meat | P7 | a | ||
| 2 | ATCC 19414b | Pig with endocarditis | P8 | c | A |
| R32E11a | Unknown | P9 | d | ||
| NF4E1 | Porcine spleen | P10 | a | A | |
| 115 | Chicken | P11 | g | ||
| 17.2a | Chicken | P12 | a | ||
| 10.2a | Chicken | P12 | a | ||
| E037 | Fish | P13 | a | ||
| K003 | Wild boar meat | P14 | e | ||
| N026 | Chicken | P15 | d | ||
| 4 | Doggerscharbea | Fish | P16 | a | |
| 212 | Chicken meat | P17 | g | ||
| 213 | Chicken meat | P17 | g | ||
| E127 | Fish | P18 | f | ||
| 5 | Pécs 67a | Porcine tonsil | P19 | d | C |
| AKO | Chicken | P20 | a | ||
| 2.2a | Chicken | P21 | g | ||
| K059 | Wild boar meat | P22 | g | ||
| 6 | Tuzoka | Bustard | P23 | g | |
| 36.4a | Chicken | P24 | g | ||
| 136 | Chicken | P25 | f | ||
| 20.4a | Chicken | P25 | f | ||
| K002 | Wild boar meat | P26 | g | ||
| 8 | Godaa | Godwit | P27 | d | A |
| 47 | Chicken | P28 | g | ||
| N008 | Chicken | P28 | g | ||
| r4.1a | Chicken meat | P28 | g | ||
| r6.1a | Chicken meat | P28 | g | ||
| E024 | Fish | P29 | g | ||
| 9 | Kapareka | Fish | P30 | a | B |
| E112 | Fish | P31 | h | ||
| 280 | Chicken | P32 | g | ||
| K052 | Wild boar meat | P33 | f | ||
| 10 | K024f | Wild boar meat | P34 | g | |
| 11 | IV12/8a | Porcine tonsil | P35 | g | |
| K021 | Wild boar meat | P36 | a | ||
| 12 | Pécs 9a | Porcine tonsil | P37 | a | |
| 88 | Chicken meat | P38 | g | ||
| 97 | Chicken meat | P38 | g | ||
| E146 | Fish | P39 | g | ||
| 15 | Pécs 3597a | Porcine tonsil | P40 | d | A |
| 16 | Tanzaniaa | Parrot | P41 | d | A |
| 17 | 545a | Porcine spleen | P42 | d | A |
| 19 | 2017a | Porcine spleen | P43 | g | A |
| E053 | Fish | P44 | d | ||
| E051 | Fish | P45 | g | ||
| K031 | Deer meat | P46 | d | ||
| 21 | Bãno 36a | Sheep dip | P47 | a | C |
| N | MEW22a | Unknown | P48 | a | |
| E. tonsillarum | |||||
| 3 | Wittlinga | Fish | P49 | j | I |
| 7 | ATCC 43339c | Porcine tonsil | P50 | j | E |
| ATCC 43338 | Porcine tonsil | P51 | j | E | |
| P-43 | Fish | P52 | j | E | |
| K015 | Wild boar meat | P53 | j | ||
| 10 | Lengyel-Pa | Squirrel | P54 | j | E |
| 14 | Iszap-4a | Zoo pond mud | P55 | j | E |
| 15 | E073g | Fish | P56 | i | |
| 16 | K037g | Wild boar meat | P57 | j | |
| 20 | 2553a | Porcine spleen | P58 | k | E |
| 22 | Bãno 107a | Sheep dip | P59 | l | E |
| 23 | KS20Aa | Pig slurry | P60 | j | E |
| Erysipelothrix spp. | |||||
| 13 | Pécs 56a | Porcine tonsil | P61 | m | |
| 13 | Shiribeshi17 | Pigpen litter | P62 | m | |
| 18 | 715a | Porcine spleen | P63 | n | H |
Serovar reference strain.
E. rhusiopathiae type strain.
E. tonsillarum type strain.
RAPD pattern obtained by Okatani et al. (29).
Ribopattern obtained by Ahrné et al. (1).
Strain classified as E. rhusiopathiae by PCR-based methods used by Okatani et al. (29).
Strain classified as E. tonsillarum by PCR-based methods used by Okatani et al. (29).
DNA preparation.
Chromosomal DNAs from the strains were prepared by using the CHEF Bacterial Genomic DNA Plug Kit (Bio-Rad Laboratories, Richmond, Calif.) with some modifications of the manufacturer's instructions. All of the buffers and solutions, except those for which the manufacturer are identified, were supplied with the DNA Plug Kit. The strains were inoculated in 50 ml of tryptose phosphate broth (Difco Laboratories) and cultured overnight with shaking at 37°C. Bacterial cells were harvested by centrifugation, suspended in 1 ml of phosphate-buffered saline (8.45 mM NaH2PO4, 5.12 mM KH2PO4, 0.12 M NaCl [pH 7.2]), transferred to 2-ml Eppendorf tubes, washed once, resuspended, and then diluted with the same buffer to an optical density at 600 nm of 0.8 to 1.0. Chloramphenicol was added to a final concentration of 180 μg/ml, and the suspension was incubated at 37°C for 1 h. One milliliter of the suspension was centrifuged, and the harvested cells were resuspended in 50 μl of cell suspension buffer. The suspension was heated to 50°C in warm water and combined with the same amount of melted 2% CleanCut agarose, also equilibrated to 50°C. The mixture was transferred to disposable plug molds and allowed to solidify at 4°C for 10 min. Each plug was removed from the molds and placed in 250 μl of lysozyme solution, prepared by adding 200 μl of the lysozyme stock (25 mg/ml) and 100 μl of N-acetylmuramidase (1 mg/ml; Seikagaku Corp., Tokyo, Japan) to 2.5 ml of lysozyme buffer, and then incubated for 48 h at 37°C in a water bath. The lysozyme solution was removed, and the plugs were rinsed once with sterile deionized water, after which they were replaced in 250 μl of proteinase K reaction buffer containing 10 μl of proteinase K stock, yielding a final proteinase K concentration of 100 μg/ml. After further incubation for 24 h at 50°C, the plugs were washed four times in 1× wash buffer for 1 h each time at room temperature. Phenylmethylsulfonyl fluoride (Sigma) was added to a final concentration of 1 mM at the second wash. Plugs were stored in 1 ml of 1× wash buffer until enzyme treatment.
Restriction enzymes and PFGE.
DNA plugs were sliced, and DNAs were digested with SmaI (Takara Co. Ltd., Tokyo, Japan), AscI (New England BioLabs), and NotI (Takara). A slice of each plug was placed twice in 0.1× wash buffer and incubated for 1 h each time at room temperature. After removal of the wash buffer, the DNAs were digested with 10 U of each enzyme in the respective reaction mixture in accordance with the manufacturer's instructions. The DNA fragments were separated in 1% agarose NA gel (Amersham Pharmacia) that was prepared in 0.5× Tris-borate-EDTA buffer (50 mM Tris base, 50 mM boric acid, 2 mM EDTA) on a Gene Navigator (Pharmacia Biotech). Electrophoresis was carried out for 24 h at 12°C and 200 V with pulse times of 1 to 35s. The CHEF DNA Size Standard Lambda Ladder (Bio-Rad) was used as a DNA size marker. Thereafter, the gels were stained with ethidium bromide for 1 h, destained in distilled water, and photographed under UV light. PFGE patterns were inspected visually, each PFGE pattern that differed by one or more DNA fragment bands was identified, and the relatedness among the patterns was analyzed based on the guidelines described by Tenover et al. (43) and Maslow et al. (24).
RESULTS
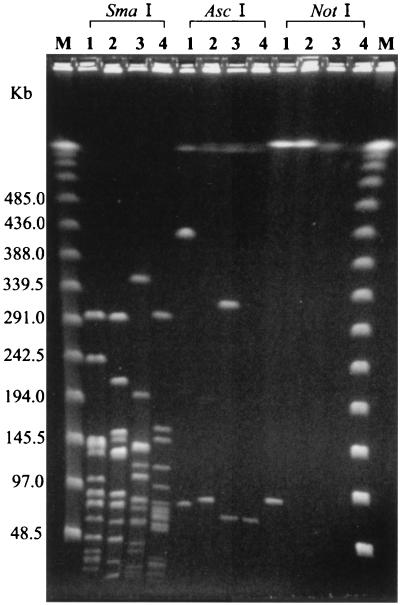
Of the three restriction enzymes tested, only SmaI produced several bands in repeated screening tests and was able to clearly differentiate between strains. Thus, SmaI was used for further analysis of all 70 strains. The PFGE patterns produced from four strains with the three enzymes are shown in Fig. 1. To determine the optimal electrophoresis protocol, a series of trials changing the pulse and electrophoresis times was carried out (data not shown). Of the protocols tested, the one described above was best able to discern the high- and even low-molecular-weight DNA fragment bands produced by SmaI.
FIG. 1.
PFGE patterns produced from four strains of Erysipelothrix spp. by SmaI, AscI, and NotI. Lanes: 1, E. rhusiopathiae strain ME-7, serovar 1a; 2, E. rhusiopathiae strain ATCC 19414, serovar 2; 3, E. tonsillarum strain ATCC 43339, serovar 7; 4, Erysipelothrix species strain 715, serovar 18; M, CHEF DNA Size Standard Lambda Ladder (Bio-Rad).
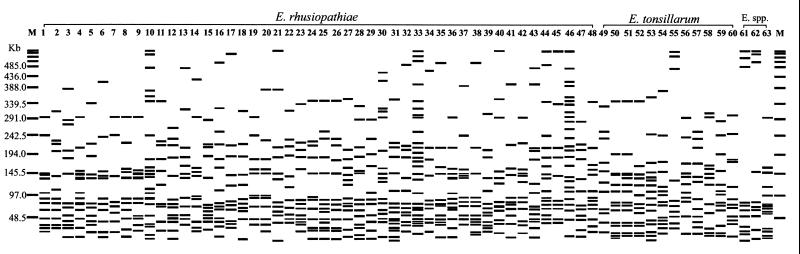
From the 70 strains analyzed, 63 distinct PFGE patterns with 8 to 27 DNA fragment bands were produced (Fig. 2; Table 1). Twelve strains showed five distinct PFGE patterns with no DNA fragment band difference observed among strains sharing the same PFGE pattern. These were strains 47, N008, r4.1a, and r6.1a, which shared pattern P28 and strains 17.2a and 10.2a, 212 and 213, 136 and 20.4a, and 88 and 97, which shared, respectively, patterns P12, P17, P25, and P38 (Table 1). Single and distinct PFGE patterns were produced for 58 strains. Among them, patterns P50 and P51 of strains ATCC 43339 and ATCC 43338 differed by two bands, patterns P4 and P36 of strains 422/1E1 and K021 differed by three bands, patterns P32 and P39 of strains 280 and E146 differed by four bands, and patterns P19 and P20 of strains Pécs 67 and AKO differed by five bands. Patterns P24 of strain 36.4a and P25 of strains 136 and 20.4a differed by six bands from pattern P26 of strain K002, as well as patterns P34 and P45 of strains K024 and E051. The remaining 45 patterns differed by at least seven bands (Fig. 2). Although some similar bands were observed among many strains, no characteristic PFGE patterns related to the species or even serovar could be differentiated (Fig. 2). The PFGE analysis was repeated twice, and the same results were obtained.
FIG. 2.
Schematic representation of the 63 PFGE patterns produced from the 70 Erysipelothrix spp. strains studied by using SmaI. E. spp., Erysipelothrix species. The serovar and PFGE pattern designation of each strain are given, respectively, in parentheses. Lanes: 1, ME-7 (1a, P1); 2, E176 (1a, P2); 3, E157 (1a, P3); 4, 422/1E1 (1b, P4); 5, E019 (1b, P5); 6, K040 (1b, P6); 7, K075 (1b, P7); 8, ATCC 19414 (2, P8); 9, R32E11 (2, P9); 10, NF4E1 (2, P10); 11, 115 (2, P11); 12, 17.2a (2, P12); 13, E037 (2, P13); 14, K003 (2, P14); 15, N026 (2, P15); 16, Doggerscharbe (4, P16); 17, 212 (4, P17); 18, E127 (4, P18); 19, Pécs 67 (5, P19); 20, AKO (5, P20); 21, 2.2a (5, P21); 22, K059 (5, P22); 23, Tuzok (6, P23); 24, 36.4a (6, P24); 25, 136 (6, P25); 26, K002 (6, P26); 27, Goda (8, P27); 28, 47 (8, P28); 29, E024 (8, P29); 30, Kaparek (9, P30), 31, E112 (9, P31); 32, 280 (9, P32); 33, K052 (9, P33); 34, K024 (10, P34); 35, IV 12/8 (11, P35); 36, K021 (11, P36); 37, Pécs 9 (12, P37); 38, 88 (12, P38); 39, E146 (12, P39); 40, Pécs 3597 (15, P40); 41, Tanzania (16, P41); 42, 545 (17, P42); 43, 2017 (19, P43); 44, E053 (19, P44); 45, E051 (19, P45); 46, K031 (19, P46); 47, Bãno 36 (21, P47); 48, MEW22 (N, P48); 49, Wittling (3, P49); 50, ATCC 43339 (7, P50); 51, ATCC 43338 (7, P51); 52, P-43 (7, P52); 53, K015 (7, P53); 54, Lengyel-P (10, P54); 55, Iszap-4 (14, P55); 56, E073 (15, P56); 57, K037 (16, P57); 58, 2553 (20, P58); 59, Bãno 107 (22, P59); 60, KS20A (23, P60); 61, Pécs 56 (13, P61); 62, Shiribeshi 17 (13, P62); 63, 715 (18, P63); M, CHEF DNA Size Standard Lambda Ladder (Bio-Rad).
DISCUSSION
Distinct and reproducible PFGE patterns were produced by using SmaI, and this allowed us to differentiate 63 PFGE patterns among the 70 strains analyzed. Studies have shown that for some bacterial species, the PFGE method is less sensitive than ribotyping and PCR-based methods. In addition, a high incidence of DNA degradation, which leads to a decrease in typeability by this method, has been cited (17, 23). Although DNA degradation should be avoided by addition of thiourea to the gel buffer (8, 35), no DNA degradation was observed with the strains used in this study and PFGE patterns were obtained for all of the strains analyzed. In a previous study using the same strains, we determined that the RAPD method is able to differentiate between species and to distinguish strains of this genus (29). By that method, 14 distinct amplification profiles were obtained (Table 1). Arhné et al. (1) have analyzed 39 strains by ribotyping and identified nine different patterns. Of those 39 strains, 23 producing six different patterns by ribotyping were also analyzed in our study and unique PFGE patterns were produced for each strain (Table 1). Although analysis including more detailed epidemiological data and comparing the PFGE patterns of more strains of one species and one serovar and the inclusion of human isolates may be desirable, based on those previous results and on the results of this study, in which all of the strains studied were typed by PFGE and distinct PFGE patterns were obtained for 90% of the strains analyzed, it might be concluded that this method is better than RAPD analysis and ribotyping for epidemiological studies of strains of this genus.
Of the 70 strains, 12 were clustered in five PFGE patterns and no band differences were observed among strains classified into the same cluster. Since this study did not include an epidemiological study, only the sources from which the strains were isolated are shown in Table 1. However, although those strains that shared the same PFGE patterns were isolated in our laboratory at different times and from different samples, all of the strains were isolated from chickens or chicken meat from the same abattoir or processing plant, and each group was composed of strains sharing the same serovar. Moreover, by the guidelines for the interpretation of PFGE patterns of bacterial isolates in an epidemiological study proposed by Tenover et al. (43), isolates might be considered genetically indistinguishable when their PFGE patterns have the same numbers of bands and the corresponding bands are the same apparent size. Therefore, it might be considered that these strains are possibly members of the same clonal line. According to the same guidelines, isolates might be considered closely related when PFGE patterns differ by one to three bands, which is consistent with a single genetic event (e.g., a point mutation resulting in the loss or gain of a restriction site, an insertion, a deletion, or a chromosomal inversion); possibly related when PFGE patterns differ by four to six bands; and different when they differ by seven or more bands. Among the PFGE patterns obtained in this study, two pairs of strains showed PFGE patterns differing by three or fewer DNA fragment bands. The PFGE analysis of strains ATCC 43339 and ATCC 43338 showed, respectively, patterns P50 and P51, which differed by two bands, and that of strains 422/1E1 and K021 showed, respectively, patterns P4 and P36, which differed by three bands. The difference between the PFGE patterns of the first pair would be explained by a single genetic event, as described by Tenover et al. (43). Therefore, it might be that one strain is a subtype of the other and corroborate the results obtained by RAPD analysis and ribotyping since both of the strains showed, respectively, the same RAPD pattern (j) and ribopattern (E) (Table 1). However, the differences between the patterns of the second pair do not match any pattern differences proposed in those guidelines. Maslow et al. (24) reported that when there are a very limited number of band differences that are not explained by a single genetic event, the isolates may be closely related but distinct. Thus, we believe that although these two strains are closely related, they should be classified as distinct strains. Similarly, and as described elsewhere (12), the other strains that differed by fewer than seven bands but by four or more were classified as distinct strains. In an epidemiological study using PFGE, a common pattern must be identified and the comparisons among PFGE patterns must be done based on that pattern (43). Some common bands could be identified among the strains analyzed in this study. However, few strains showed nearly identical patterns. Based on results of previous studies, in which many distinct PFGE patterns were produced from strains of only one species (2, 21, 23), the high variability of PFGE patterns observed in this study is not surprising since we included not only the two species of the genus Erysipelothrix but also numerous strains from many sources, strains representing the 23 serovars and type N, as well as serovars 13 and 18, which are considered possible members of new and separate species (41).
SmaI, AscI, and NotI have been described as effective enzymes in producing a clear and reliable number of DNA fragments producing distinct PFGE patterns for several bacterial species (4, 7, 18, 25, 31). However, with DNAs of strains of this genus, few fragments, such as two or three, were produced with AscI and NotI (Fig. 1). In the same set of guidelines for the interpretation of DNA restriction patterns generated by PFGE described above, Tenover et al. (43) emphasized the need for at least 10 distinct DNA fragment bands for reliable analysis of the relationship among strains. Thus, AscI and NotI were considered to be inappropriate for analysis of strains of this genus.
To avoid the labor necessary to prepare the buffers and solutions required to obtain DNA from the strains studied, a commercial kit was used. Although DNA could not be obtained by using the protocol described by the manufacturer, reliable results were obtained with small modifications such as an increase in the lysozyme stock amount supplied with the kit and the addition of N-acetylmuramidase, which has been used for DNA extraction from strains of this genus (22, 29), to the lysis solution, as well as an increase in the incubation time.
In this report, we have described a protocol for the performance of PFGE with strains of the genus Erysipelothrix, demonstrating that PFGE performed with SmaI might be more sensitive than RAPD and ribotyping and also that this method might be a useful and reliable tool for epidemiological studies of strains of this genus. Moreover, as this was the first study to apply this method to strains of this genus, without disregarding the need to screen other enzymes, we expect that the procedure will be of value as a basis for new approaches using this method with strains of Erysipelothrix species.
ACKNOWLEDGMENT
We thank Toshio Takahashi (National Veterinary Assay Laboratory, Tokyo, Japan) for kindly providing us with Erysipelothrix strains.
REFERENCES
- 1.Ahrné S, Stenströn I, Jensen N E, Pettersson B, Uhlén M, Molin G. Classification of Erysipelothrix strains on the basis of restriction fragment length polymorphisms. Int J Syst Bacteriol. 1995;45:382–385. doi: 10.1099/00207713-45-2-382. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman T L, Hancock G A, Tenover F C, Miller J M. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;33:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooke C J, Riley T V. Erysipelothrix rhusiopathiae: bacteriology, epidemiology and clinical manifestations of an occupational pathogen. J Med Microbiol. 1999;48:789–799. doi: 10.1099/00222615-48-9-789. [DOI] [PubMed] [Google Scholar]
- 4.Brosh R, Chen J, Luchansky J B. Pulsed-field fingerprinting of listeriae: identification of genomic divisions for Listeria monocytogenes and their correlation with serovar. Appl Environ Microbiol. 1994;60:2584–2592. doi: 10.1128/aem.60.7.2584-2592.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burucoa C, Lhomme V, Fauchere J L. Performance criteria of DNA fingerprinting methods for typing of Helicobacter pylori isolates: experimental results and meta-analysis. J Clin Microbiol. 1999;37:4071–4080. doi: 10.1128/jcm.37.12.4071-4080.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callon R A, Jr, Brady P G. Toothpick perforation of the sigmoid colon: an unusual case report associated with Erysipelothrix rhusiopathiae septicemia. Gastrointest Endosc. 1990;36:141–143. doi: 10.1016/s0016-5107(90)70970-4. [DOI] [PubMed] [Google Scholar]
- 7.Chiou C-S, Hsu W-B, Wei H-L, Chen J-H. Molecular epidemiology of a Shigella flexneri outbreak in a mountainous township in Taiwan, Republic of China. J Clin Microbiol. 2001;39:1048–1056. doi: 10.1128/JCM.39.3.1048-1056.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corkill J E, Graham R, Hart C A. Pulsed-field gel electrophoresis of degradation-sensitive DNAs from Clostridium difficile PCR ribotype 1 strains. J Clin Microbiol. 2000;38:2791–2792. doi: 10.1128/jcm.38.7.2791-2792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Destro M T, Leitão M F F, Farber J M. Use of molecular typing methods to trace the dissemination of Listeria monocytogenes in a shrimp processing plant. Appl Environ Microbiol. 1996;62:705–711. doi: 10.1128/aem.62.2.705-711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunbar S A, Clarridge J E., III Potential errors in recognition of Erysipelothrix rhusiopathiae. J Clin Microbiol. 2000;38:1302–1304. doi: 10.1128/jcm.38.3.1302-1304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidalgo S G, Wang Q, Riley T V. Comparison of methods for detection of Erysipelothrix spp. and their distribution in some Australasian seafoods. Appl Environ Microbiol. 2000;66:2066–2070. doi: 10.1128/aem.66.5.2066-2070.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galbart J-O, Morvan A, El Solh N. Phenotypic and molecular typing of nosocomial methicillin-resistant Staphylococcus aureus strains susceptible to gentamicin isolated in France from 1995 to 1997. J Clin Microbiol. 2000;38:185–190. doi: 10.1128/jcm.38.1.185-190.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giménez M, Fernández P, Padilla E, Matas L, Ausina V. Endocarditis and acute renal failure due to Erysipelothrix rhusiopathiae. Eur J Clin Microbiol Infect Dis Lett. 1996;15:347–348. doi: 10.1007/BF01695671. [DOI] [PubMed] [Google Scholar]
- 14.Gorby G L, Peacock J E., Jr Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis. 1988;10:317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- 15.Gori A, Espinasse F, Deplano A, Nonhoff C, Nicolas M H, Struelens M J. Comparison of pulsed-field gel electrophoresis and randomly amplified DNA polymorphism analysis for typing extended-spectrum-β-lactamase-producing Klebsiella pneumoniae. J Clin Microbiol. 1996;34:2448–2453. doi: 10.1128/jcm.34.10.2448-2453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayford A E, Petersen A, Vogensen F K, Jakobsen M. Use of conserved randomly amplified polymorphic DNA (RAPD) fragments and RAPD pattern for characterization of Lactobacillus fermentum in Ghanaian fermented maize dough. Appl Environ Microbiol. 1999;65:3213–3221. doi: 10.1128/aem.65.7.3213-3221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollis R J, Bruce J L, Fritschel S J, Pfaller M A. Comparative evaluation of an automated ribotyping instrument versus pulsed-field gel electrophoresis for epidemiological investigation of clinical isolates of bacteria. Diagn Microbiol Infect Dis. 1999;34:263–268. doi: 10.1016/s0732-8893(99)00033-4. [DOI] [PubMed] [Google Scholar]
- 18.Howard P J, Harsono K D, Luchansky J B. Differentiation of Listeria monocytogenes, Listeria innocua, Listeria ivanovii, and Listeria seeligeri by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1992;58:709–712. doi: 10.1128/aem.58.2.709-712.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanai Y, Hayashidani H, Kaneko K, Ogawa M, Takahashi T, Nakamura M. Occurrence of zoonotic bacteria in retail game meat in Japan with special reference to Erysipelothrix. J Food Prot. 1997;60:328–331. doi: 10.4315/0362-028X-60.3.328. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix J, Delage G, Mitchell G. Erysipeloid in an infant. J Pediatr. 1981;99:745–746. doi: 10.1016/s0022-3476(81)80400-3. [DOI] [PubMed] [Google Scholar]
- 21.Ling J M, Lo N W S, Ho Y M, Kam K M, Hoa N T T, Phi L T, Cheng A F. Molecular methods for the epidemiological typing of Salmonella enterica serotype typhi from Hong Kong and Vietnam. J Clin Microbiol. 2000;38:292–300. doi: 10.1128/jcm.38.1.292-300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makino S, Okada Y, Maruyama T, Ishikawa K, Takahashi T, Nakamura M, Ezaki T, Morita H. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J Clin Microbiol. 1994;32:1526–1531. doi: 10.1128/jcm.32.6.1526-1531.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall S, Clark C G, Wang G, Mulvey M, Kelly M T, Johnson W M. Comparison of molecular methods for typing Vibrio parahaemolyticus. J Clin Microbiol. 1999;37:2473–2478. doi: 10.1128/jcm.37.8.2473-2478.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maslow J N, Slutsky A M, Arbeit R D. Application of pulsed-field gel electrophoresis to molecular epidemiology. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 563–572. [Google Scholar]
- 25.Najdenski H, Iteman I, Carniel E. Efficient subtyping of pathogenic Yersinia enterocolitica strains by pulsed-field gel electrophoresis. J Clin Microbiol. 1994;32:2913–2920. doi: 10.1128/jcm.32.12.2913-2920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakazawa H, Hayashidani H, Higashi J, Kaneko K, Takahashi T, Ogawa M. Occurrence of Erysipelothrix spp. in broiler chickens at an abattoir. J Food Prot. 1998;61:907–909. doi: 10.4315/0362-028x-61.7.907. [DOI] [PubMed] [Google Scholar]
- 27.Nakazawa H, Hayashidani H, Higashi J, Kaneko K, Takahashi T, Ogawa M. Occurrence of Erysipelothrix spp. in chicken meat parts from a processing plant. J Food Prot. 1998;61:1207–1209. doi: 10.4315/0362-028x-61.9.1207. [DOI] [PubMed] [Google Scholar]
- 28.Nandish S, Khardori N. Valvular and myocardial abscess due to Erysipelothrix rhusiopathiae. Clin Infect Dis. 1999;29:1351–1352. doi: 10.1086/313457. [DOI] [PubMed] [Google Scholar]
- 29.Okatani A T, Hayashidani H, Takahashi T, Taniguchi T, Ogawa M, Kaneko K. Randomly amplified polymorphic DNA analysis of Erysipelothrix spp. J Clin Microbiol. 2000;38:4332–4336. doi: 10.1128/jcm.38.12.4332-4336.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive D M, Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37:1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor M E, Brosh R, Mellen J W, Garret L A, Kaspar C W, Luchansky J B. Use of pulsed-field gel electrophoresis to link sporadic cases of invasive listeriosis with recalled chocolate milk. Appl Environ Microbiol. 1995;61:3177–3179. doi: 10.1128/aem.61.8.3177-3179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasmussen H N, Olsen J E, Rasmussen O F. RAPD analysis of Yersinia enterocolitica. Lett Appl Microbiol. 1994;19:359–362. doi: 10.1111/j.1472-765x.1994.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 33.Reboli A C, Farrar W E. Erysipelothrix rhusiopathiae: an occupational pathogen. Clin Microbiol Rev. 1989;2:354–359. doi: 10.1128/cmr.2.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robson J M, McDougall R, Van Der Valk S, Waite S D, Sullivan J J. Erysipelothrix rhusiopathiae: an uncommon but ever present zoonosis. Pathology. 1998;30:391–394. doi: 10.1080/00313029800169686. [DOI] [PubMed] [Google Scholar]
- 35.Römling U, Tümmler B. Achieving 100% typeability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J Clin Microbiol. 2000;38:464–465. doi: 10.1128/jcm.38.1.464-465.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sechi L A, Spanu T, Sanguinetti M, Dupre I, Masucci L, Siddu A, Tortorolo G, Vento G, Maggio L, Cambieri A, Zanetti S, Fadda G. Molecular analysis of Klebsiella pneumoniae strains isolated in pediatric wards by ribotyping, pulsed field gel electrophoresis and antimicrobial susceptibilities. New Microbiol. 2001;24:35–45. [PubMed] [Google Scholar]
- 37.Sheng W-H, Hsueh P-R, Hung C-C, Fang C-T, Chang S-C, Luh K-T. Fatal outcome of Erysipelothrix rhusiopathiae bacteremia in a patient with oropharyngeal cancer. J Formosan Med Assoc. 2000;99:431–434. [PubMed] [Google Scholar]
- 38.Shewan J M. The microbiology of fish and fishery products—a progress report. J Appl Bacteriol. 1971;34:299–315. doi: 10.1111/j.1365-2672.1971.tb02291.x. [DOI] [PubMed] [Google Scholar]
- 39.Shimoji Y, Mori Y, Hyakutake K, Sekizaki T, Yokomizo Y. Use of an enrichment broth cultivation-PCR combination assay for rapid diagnosis of swine erysipelas. J Clin Microbiol. 1998;36:86–89. doi: 10.1128/jcm.36.1.86-89.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiono H, Hayashidani H, Kaneko K, Ogawa M, Muramatsu M. Occurrence of Erysipelothrix rhusiopathiae in retail raw pork. J Food Prot. 1990;53:856–858. doi: 10.4315/0362-028X-53.10.856. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Fujisawa T, Tamura Y, Suzuki S, Muramatsu M, Sawada T, Benno Y, Mitsuoka T. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int J Syst Bacteriol. 1992;42:469–473. doi: 10.1099/00207713-42-3-469. [DOI] [PubMed] [Google Scholar]
- 42.Takeshi K, Makino S, Ikeda T, Takada N, Nakashiro A, Nakanishi K, Oguma K, Katoh Y, Sunagawa H, Ohyama T. Direct and rapid detection by PCR of Erysipelothrix sp. DNAs prepared from bacterial strains and animal tissues. J Clin Microbiol. 1999;37:4093–4098. doi: 10.1128/jcm.37.12.4093-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ternström A, Molin G. Incidence of potential pathogens on raw pork, beef and chicken in Sweden, with special reference to Erysipelothrix rhusiopathiae. J Food Prot. 1987;50:141–146. doi: 10.4315/0362-028X-50.2.141. [DOI] [PubMed] [Google Scholar]
- 45.Thong K-L, Ngeow Y-F, Altwegg M, Navaratnam P, Pang T. Molecular analysis of Salmonella enteritidis by pulsed-field gel electrophoresis and ribotyping. J Clin Microbiol. 1995;33:1070–1074. doi: 10.1128/jcm.33.5.1070-1074.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towner K J, Cockayne A. Molecular methods for microbial identification and typing. 1st ed. London, England: Chapman & Hall, Ltd.; 1993. Analysis of nucleic acid profiles; pp. 28–63. [Google Scholar]
- 47.Towner K J, Cockayne A. Molecular methods for microbial identification and typing. 1st ed. London, England: Chapman & Hall, Ltd.; 1993. Identification and typing by nucleic acid hybridization techniques; pp. 64–92. [Google Scholar]
- 48.Tynkkynen S, Satokari R, Saarela M, Mattila-Sandholm T, Saxelin M. Comparison of ribotyping, randomly amplified polymorphic DNA analysis, and pulsed-field gel electrophoresis in typing of Lactobacillus rhamnosus and L. casei strains. Appl Environ Microbiol. 1999;65:3908–3914. doi: 10.1128/aem.65.9.3908-3914.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood R L. Erysipelas. In: Straw B E, D'Allaire S, Mengeling W L, Taylor D J, editors. Diseases of swine. 8th ed. Ames: Iowa State University Press; 1999. pp. 419–430. [Google Scholar]