Abstract
Precise detection of pathologically relevant biomolecules could provide essential information on important intercellular, cellular, and subcellular events for accurate disease diagnosis and staging, thus leading to appropriate treatment recommendation. Activatable nanoprobes are nanoscale objects that can be turned on through specific reactions or interactions with biomolecules of interest, and afford some advantageous properties for improved detection of biomolecules both in vitro and in vivo. In this brief review, we highlight several recent examples in the development of activatable nanoprobes for biomolecule detection.
Introduction
Recent advances in molecular biology reveal that the origin and progression of many human diseases such as cancer are associated with the abnormal activities of certain biomolecules [1]. Precise detection of these biomolecular activities could benefit early stage disease diagnosis and accurate evaluation of the disease progression, thus leading to appropriate medical treatments and successful clinical outcomes [2]. Conventional molecular probes (always ON probes) are best suited for in vitro analysis, as any non-specifically deposited probes can be removed easily and efficiently through extensive washing [3]. For in vivo applications, features such as improved targeting [4] (to increase signal) or enhanced clearance [5] (to reduce background noise) should be included in the probe design to obtain sufficient contrast for both imaging and quantitative evaluation. Only through these strategies can success be achieved in the use of conventional probes for biomedical research and applications, including fundamental study [6••], drug development [7], disease diagnosis [8–10], cancer staging [11], theranostics [12–14], and response monitoring [15]. However, in some cases it is the activity, not the expression level of the biomolecules (e.g. enzymatic activities) that inherently correlates with disease progression [1], and the inability to sense molecular activities limits the use of these always ON probes for disease diagnosis.
To detect the activities (not the expression level) of biomolecules of interest, activatable molecular probes are often needed to translate the specific enzymatic activity into measurable and quantifiable signals (enzymatic cleavage turns the probe from the ‘OFF’ state to the ‘ON’ state). For this reason, optical (fluorescent or bioluminescent) molecular probes have been extensively explored for the detection of proteases, hydrolases, peroxidases, telomerase, and kinases by monitoring the fluorescence change of pre-quenched probes. These probes are designed on the basis of the well-known Förster resonance energy transfer (FRET) effect [16], which is sensitive to molecular conformation, association, and separation in the 1–10 nm range. To create an activatable molecular probe, one popular strategy is the use of paired donor and acceptor molecules between which the energy can be transferred non-radiatively. The self-quenching effect as a result of short-range interactions among fluorophores linked to one scaffold molecule also allows for the rational design of molecular probes that can be activated by a change in local environment [17].
Activatable molecular probes for magnetic resonance imaging (MRI) have also been explored, on the basis that some biomolecular activities can modify the contrast agent’s chemical structures, molecular weights, or local surroundings in such a specific way that a corresponding change in relaxivity can be identified [18,19]. These changes, however, are fairly small in magnitude and as such only weak perturbations can be recorded. Nevertheless, their intrinsic property of low background noise makes the activatable molecular probes (both fluorescence-based and MRI-based ones) superior to conventional probes for in vivo application. This is despite potential drawbacks such as possible fast clearance and non-specific activation due to their small size and exposed linkage to non-specific biomolecules during transportation.
The development of activatable nanoprobes has opened up a new era of molecular imaging [20••], introducing unique features for biomolecular detection, especially in vivo (Figure 1). For instance, the use of inorganic nanoparticles (as either quencher or fluorophore) makes it possible for the design of multifunctional nanoprobes by supplying a universal FRET receptor for multiple fluorophores with different emission spectra [21]. Additional signals can also be recorded from those inorganic nanoparticles depending on alterations either to the nanoparticle itself or to its environment. Self-assembled nanoprobes offer an environment distinct from that of biological fluid for the fluorophores and also improve the apparent molecular weight of the probes, helping to establish a significant signal transition during the assembly or disassembly process [22]. Besides these unique features, the adoption of a ‘nano formulation’ also provides benefits such as cargo protection and targeted delivery [23].
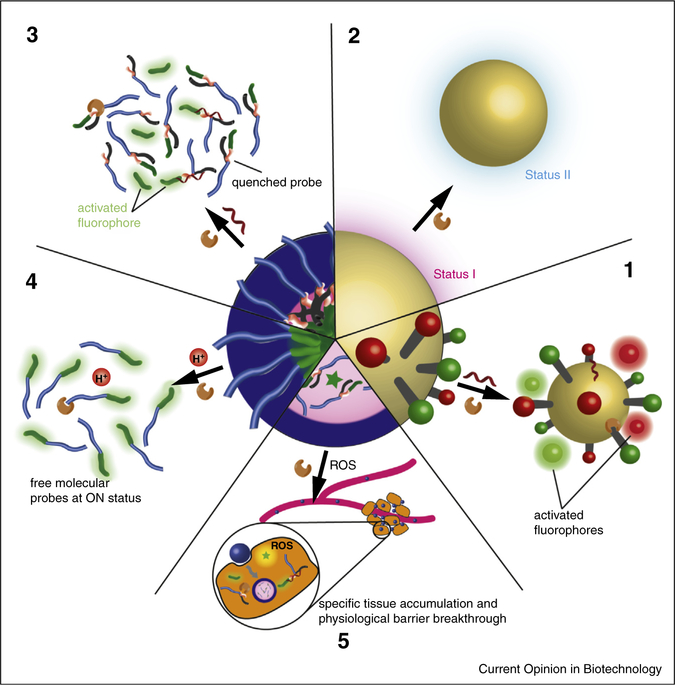
Figure 1.
Generic illustration of the unique features offered by activatable nanoprobes for probing disease-relevant biomolecules. Inorganic nanoparticles can serve as universal quenchers for simultaneous detection of multiple biomolecules (1). Switchable signals from the inorganic nanoparticle itself can also serve as an indicator for the presence and activities of biomolecules of interest (2). Self-assembly of activatable molecular probes (3) or always-ON probes (4) can improve their chemical and structural stability, and also offer an additional activation mechanism. The use of additional nanoparticles as carriers could improve the accumulation of molecular probe in target tissues (5).
Since the principles of activatable probe design have been excellently reviewed recently [18,24–28], we will only touch upon these principles as needed. This review will only focus on recent advances in the design of activatable nanoprobes for the detection of biomolecules, highlighting their unique features of such probes and their associated benefits.
Inorganic nanoparticle-based activatable nanoprobes
The incorporation of inorganic nanoparticles into molecular probe designs, aided in part by well-established protocols for their preparation, has been well-developed in the past few decades. Conjugation of activatable fluorophores to inorganic nanoparticles offers a feasible way to convert molecular probes into nanoprobes. Due to the dipole-surface type energy transfer phenomenon [29], inorganic nanoparticles can serve as universal receptors of non-radiative energy from multiple excited fluorophores (Figure 2a). Many successful applications of this mechanism in the preparation of activatable nanoprobes have been reported, with the liberated fluorophores excited through external [30–32] or Cerenkov illumination (nuclear decay-derived irradiation) [33]. Taking advantage of dipole-surface type energy transfer, simultaneous detection of multiple biomolecules is also made possible. The ability to do so is not only important for high-throughput diagnosis, but also crucial for the evaluation of biological processes that involve different biomolecules.
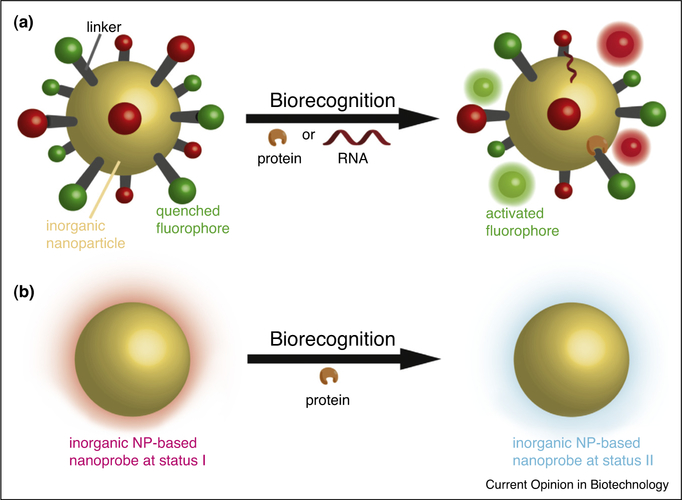
Figure 2.
Schematic illustration of using inorganic nanoparticles in the rational design of activatable nanoprobes. The inorganic nanoparticle can serve as a universal quencher for multiple fluorophores. Upon biorecognition (such as protein and/or RNA), the signals can be turned ON, allowing simultaneous detection of multiple biomolecules (a). The intrinsic signal from inorganic nanoparticles can also serve as an indicator for biomolecules based on biorecognition-induced perturbations
An excellent demonstration of this universal quenching phenomenon is provided by Mirkin and coworkers, who developed an activatable nanoprobe that allows the detection of two different mRNAs in cells [34•]. Their nanoprobes were composed of gold nanoparticles decorated with oligonucleotides taken from two target mRNA sequences (survivin and actin) that are hybridized to complementary reporter sequences, each possessing a distinct fluorophore for its respective target mRNA (Cy3 for actin and Cy5 for survivin). In the absence of target mRNA, the nanoprobes are in the OFF state due to energy transfer from the fluorophores to the gold nanoparticles. In the presence of the targeted mRNA, however, the fluorophores were liberated from the gold nanoparticles surface by pairing to the target mRNA. Red (survivin) or/and green (actin) fluorescence were then recorded, with the respective intensities representing represents the amount of individual mRNA in a single cell. The quantities of survivin mRNA could be normalized by the expression of actin allowing the elimination of the variance that results from uneven endocytosis between cell lines. Good correlation was observed between the results obtained using the nanoprobe and those determined by RT-PCR, suggesting its potential application in mRNA quantification in live systems. Tang and coworkers pushed the limit of this hybridization-induced fluorescence methodology further by incorporating four different molecular probes into a single nanoprobe [35]. They successfully demonstrated the simultaneous determination of the expression levels of TK1 mRNA (blue), survivin mRNA (green), c-myc mRNA (yellow), and GalNacT mRNA (red) in live cells.
As a result of improved understandings of complex molecular mechanisms of diseases such as cancer, the multifunctional property of activatable nanoprobes allows them to play a even more important role in disease staging, therapeutic planning and response monitoring. However, the difficulty in probe design, quality control and signal interpretation increases with an increased number of parameters being monitored or modalities being utilized. Many factors could significantly affect the information readout, including spatial hindrance that could retard signal activation, differences in reaction kinetics between different modules, inter-particle or batch-to-batch variation between the ratio of different reaction modules, and crosstalk between fluorophores. These issues must first be addressed before these types of nanoprobes can be successfully translated into clinical applications.
Besides serving as universal quenchers, inorganic nanoparticles have their own characteristic signals such as absorbance [36] and localized surface plasmon resonance (LSPR), [37••] and therefore allow the detection of biomolecules that could induce a change in these signals (Figure 2b). Copper sulfide (CuS) nanoparticles, for example, have a characteristic absorbance at 930 nm. Chen and colleagues successfully detected the expression of matrix metalloproteinase (MMP) by conjugating black hole quencher 3 (BHQ-3, absorption maximum at 680 nm) to CuS nanoparticles through an MMP cleavable linker. Cleavage of this linker would result in a change in the 930/680 nm photoacoustic signal ratio in vivo, thereby providing an indication of the MMP expression level [36]. The LSPR signal of metal nanoparticles can shift in response to a biorecognition event, offering another modality with which to detect biomolecules. Stevens and coworkers designed a gold nanostar system that exhibited a major or minor LSPR shift depending on whether the silver nanocrystal growth, catalyzed by glucose oxidase (GOx), took place on the surface of the gold nanostar or in solution [37••]. Low concentrations of GOx generated silver nanocrystals at a slow rate that preferentially grew on the surface of the nanostar, while a high concentration of GOx led to crystal growth in the solution. For prostate specific antigen (PSA) detection, they first decorated the gold nanostar with polyclonal antibodies against PSA. Next, the nanostars were incubated with PSA-containing samples, monoclonal antibody against PSA, and secondary antibody linked with GOx. The purified complexes were then transferred into an Ag+-containing buffer for signal generation. This probe allows the detection of PSA as low as 10−18 g ml−1 (4 × 10−20 M) in whole serum, which is clearly very useful for the early diagnosis of prostate cancer.
Self-assembled nanoprobes
An alternative approach to design activatable nanoprobes is the use of self-assembling components. Through rational design, activatable molecular probes can be created such that they can be induced to form activatable nanoprobes (Figure 3a). In one example, Tan and colleagues introduced a phospholipid into their oligonucleotide-based molecular probe, and the resulting amphiphilic conjugate could self-assemble into micelles under physiological conditions [38]. The fluorescence signal was OFF when the fluorophore and quencher were brought together by a hairpin-forming oligonucleotide, but could be turned ON upon encountering the complementary mRNA in the cells. This hybridization event resulted in a significant conformational change that separated the fluorophore and quencher. Therefore, this activatable nanoprobe could potentially be used for the detection of intracellular mRNA both in vitro and in vivo. Cui and coworkers designed an activatable molecular probe that could assemble on its own into nanostructures named nanobeacons [39]. The molecule was composed of a Tat cell-penetrating peptide derived from HIV, BHQ 1 quencher, and a green fluorophore 5-carboxyfluoroscein that is linked to the peptide through a cathepsin B (CatB) sensitive linker GFLG (Gly-Phe-Leu-Gly). Under physiological conditions, the conjugates could self-assemble into core–shell micelles, hiding the GFLG linker inside and therefore preventing the premature activation of the nanobeacon. Upon accumulation in the tumor microenvironment or entering lysosomal compartments that are known to be acidic, the nanobeacons are expected to gradually dissociate into individual molecules due to dilution and/or acidification, thereby exposing the cleavable linker to the CatB protease. As a result, the nanobeacon offers improved signal-to-background contrast and is useful for CatB activity assays.
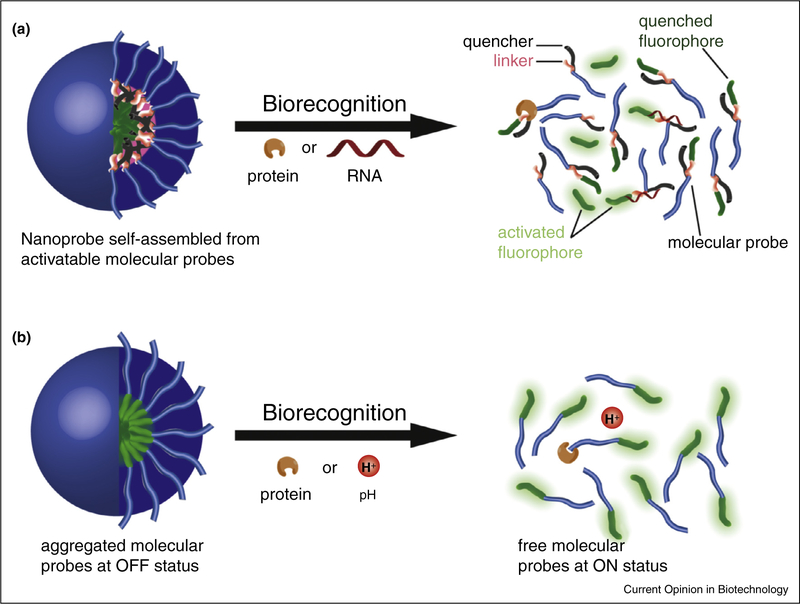
Figure 3.
Schematic illustration of creating self-assembled supramolecular activatable nanoprobes. The nanoprobe could be formed by self-assembly of rationally designed molecular probes that are activatable upon their interaction with biomolecules (a). The nanoprobe could also be created using molecular probes without a quencher, and the signal can be turned ON by the dissociation process (b).
In the two examples discussed above, the self-assembly or disassembly process itself does not turn the signal ON or OFF, but it could do so if the nanoprobe is built using a fluorophore and quencher that are conjugated to separate building blocks [40,41] or using self-quenchable fluorophores [42,43•] (Figure 3b). In the first case, the fluorophore and quencher could be conjugated to different polymers [40] or subunits of a protein [41] separately, with the co-assembly of the two components leading to the effective quenching of the fluorescence. The second method relies on the observation that many fluorophores can also be quenched when they are closely packed under some specific conditions [17], negating the need for a separate quenching moiety. Activatable nanoprobes can therefore be designed based on this mechanism. Zheng and colleagues, taking advantage of the self-quenching property of porphyrins, prepared an activatable nanoprobe called porphysome that was self-assembled from phospholipid-porphyrin conjugates [44••]. The resulting porphysome vesicles (~100 nm in size) contained closely-packed porphyrin units in the bilayer, and accordingly exhibited limited fluorescence signal. Upon cellular entry, the porphyrin was liberated due to lipase-induced degradation and subsequent disassociation of porphy-some, generating a strong fluorescent signal in the tumor. More interestingly, the formation of porphysome also introduced unique photoacoustic properties that enabled the visualization of the lymphatic system through photoacoustic tomography.
Recently, Gao and co-workers developed a homoFRET-based activatable nanoprobe that was ultra-sensitive to small changes in pH (<0.4 pH unit difference) [43•]. They conjugated self-quenchable dye Cy5.5 to copolymers with different pKa values. The copolymers were amphiphilic under neutral pH, and can self-assemble into micelles with poor emissive properties. At lower pH, the polymers became hydrophilic and could dissociate from the micelles, inducing a 100-fold increase in fluorescence intensity. In tumor-bearing animal models, the nanoprobes could be specifically turned ON extracellularly using extracellular pH-activatable nanoprobe (UPSe, transition pH 6.9) or intracellularly using cRGDfK-modified intracellular pH-activatable nanoprobe (UPSi, transition pH 6.2) [43•]. The cRGD-UPSe-Cy5.5 nanoprobe (iUPS), which was designed to bind to tumor blood vessels and get activated extracellularly, showed its universal potential to detect the tumors in different tumor models [43•]. In contrast to these disassembly activated nanoprobes, Xu and colleagues found that the fluorescence of 4-nitro-2,1,3-benzoxadiazole (NBD) could be turned ON due to the phosphoesterase-mediated self-assembly [45], which could be an alternative strategy to design activatable nanoprobes.
In addition to fluorescence-based activatable nanoprobes, MRI probes that are sensitive to biorecognition events can also be designed. However, conventional 1H NMR probes were unable to generate very strong transition of the signal due to their signal switching mechanism and also strong background noise from the abundant hydrogen atoms in biological tissues and fluids [18,46,47]. Recently, 19F-based MRI probes have received great attention for their apparent molecular weight-dependent signal due to chemical shift anisotropy (CSA) [19], which is ideal for the development of self-assembled activatable nanoprobes (Figure 3b). Hamachi and colleagues first reported 19F-based MRI probes for the detection of multiple proteins [48••]. In their design, 3,5-bis(trifluoromethyl)benzene (FB) was conjugated to a specific ligand or inhibitor of the target proteins. The resulting conjugates self-assembled into micelles that could be triggered to dissociate through the specific recognition of the target protein. This transition in the aggregation status induces a huge change in the apparent molecular weight (supramolecules to small molecules) that translates into a significant enhancement of the 19F NMR signal. Later, the groups of Hamachi and Gao reported other 19F-based MRI probes for MMP [49] and pH detection [50], resulting in a 10,000-fold decrease in the detection limitation of MMP and an up to 27-fold increase in the signal upon encountering the acidic environment of a tumor, respectively.
It is clear that the adoption of the self-assembly methodology in nanoprobe development opens a new area for biomolecule detection, and allows the design of FRET-based, photoacoustic-based, and CSA-based activatable nanoprobes for the detection of various biomolecules from small targets (such as H+) to large targets (such as enzymes and mRNA). However, special attention should always be paid to the undesired signals generated by the disassembly of the nanoprobes. Since those supramolecular nanostructures are constructed by the self-assembly of molecular building units via non-covalent interactions, the monomers and nanostructures are always in dynamic equilibrium. In dilute condition or during their interaction with other non-specific biomolecules such as serum proteins [51], dissociation is inevitable and could give rise to false positive signals. Therefore, analysis methods that can differentiate non-specific activation from specific activation are crucial, especially for the in vivo detection of biomolecules with ultra-low concentrations.
Other activatable nanoprobes
Many activatable molecular probes, especially peptide-based or oligonucleotide-based examples, may suffer from premature activation and an inability to penetrate physiological barriers, limiting their in vivo application. Fortunately, there have already been a number of nano-carriers developed for drug delivery that have had some success in differentiated cargo delivery and cell membrane breakthrough (Figure 4) [23]. For instance, Hub and colleagues designed an activatable molecular probe for intracellular miR-34a detection that could be turned ON after hybridization with the complementary microRNA [52]. However, its chemical nature as an oligonucleotide renders it unstable in the bloodstream and unable to penetrate the cell membrane. To address this, they encapsulated the miR-34a beacon into liposomes that were decorated with polyethylene glycol (PEG) for long circulation and hyaluronic acid for CD44 receptor targeting. After intravenous injection into tumor-bearing mice, fluorescent signals were detected specifically from tumors initialized by MDA-MB-231 cells, which express both CD44 and miR-34a. For the same reason, Chen and co-workers delivered their peptide-based molecular probes for caspase-8 or caspase-3 detection with the commercially available transfection reagent PULSin® [53]. After cellular entry, these two probes could successfully detect the sequential activation of caspase-8 and caspase-3 upon induction of apoptosis by the TRAIL cytokine (TNF-related apoptosis-inducing ligand).
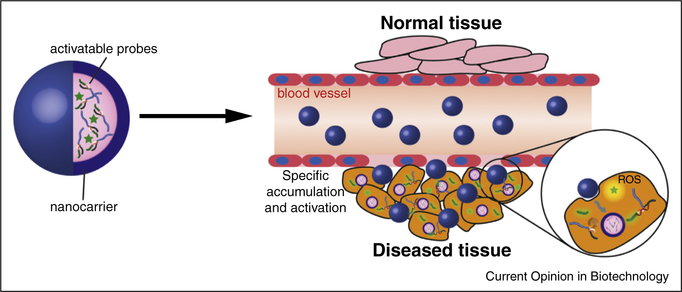
Figure 4.
Schematic illustration of using nanostructures to deliver activatable nanoprobes. The nanocarrier could improve the pharmacokinetic profiles of the loaded probes, by both increasing their accumulation in targeted tissue and enhancing their ability to overcome physiological barriers.
A further issue that other activatable molecular probes may suffer from is that of non-specific tissue distribution. Although the probes are supposed to be activated only in targeted tissues, non-specific activation is inevitable and consequently may result in weak contrast due to low accumulation in the targeted tissues. Nanoparticles usually have better selectivity toward tissues with porous blood vessels such as tumors, and could be used as vehicles to deliver corresponding molecular probes. Work from Tsien’s group on MMP activatable nanoprobes clearly demonstrates the advantages of PAMAM dendrimer-conjugated probes, exhibiting a decreased accumulation in normal tissues [54] and an up to 15-fold increase in the tumor [55,56•]. Benefiting from the improved contrast, residual tumorous tissues or metastases as small as 200 μm could be detected, and with further improvements these nanoprobes could prove useful in clinical staging, presurgical planning, and intra-operative fluorescence-guided surgeries.
Interestingly, for activatable nanoprobes that enable reactive oxygen species (ROS) detection, the peroxalate ester-based materials that are used to deliver the pentacene-based fluorophore could also serve as fuel for pentacene excitation through their reaction with hydrogen peroxide [57–60]. These types of activatable nanoprobes thus offer a new way to develop probes for biomolecule detection that do not require any external source of excitation.
Conclusion and perspectives
Advances have been achieved in understanding of the molecular basis of many diseases over the past few decades. However, it still remains a challenge to monitor these important biomolecules dynamically in vivo, especially in clinical settings to help the prevention, diagnosis, and treatment of these diseases. Imaging is one of the few technologies that can generate the longitudinal data sets from intact host environments necessary to improve our knowledge of disease pathologies. The development of activatable nanoprobes allows multiple biomolecules to be detected sensitively and specifically by different techniques owing to their unique features, although it can suffer from complexity in probe preparation, quality control and data analysis. The major challenges remaining in the imaging of multiple biomolecules are their low and transient expression, limited probe accessibility, and complex mechanisms of action that are highly dependent on their location, quantity and activity. Activatable nanoprobes with tunable physiological barrier permeability, that can be specifically activated by single or multiple target molecules via sensitive amplification mechanisms to give absolute quantitative information at high spatiotemporal resolution are highly desired. The heterogeneity of diseases such as cancer poses a further challenge for imaging because of ill-defined areas that remain completely or partially ‘dark’. The incorporation of multiple imaging agents into one nanoprobe is a feasible way to light up more dark areas. However, the combination of these agents and their associated activation mechanisms means that the imaging protocols and data processing methodologies need to be carefully optimized to maximize the benefits of multi-functional and multi-modality imaging. The last and maybe the most important is the safety concern of activatable nanoprobes, especially for those that are inorganic nanoparticle-based.
Acknowledgement
The authors acknowledge the financial support from the W.W. Smith Charitable Trust.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Weinberg RA: The Biology of Cancer. Garland Science; 2007. [Google Scholar]
- 2.Clayton TA, Lindon JC, Cloarec O, Antti H, Charuel C, Hanton G, Provost JP, Le Net JL, Baker D, Walley RJ et al. : Pharmaco-metabonomic phenotyping and personalized drug treatment. Nature 2006, 440:1073–1077. [DOI] [PubMed] [Google Scholar]
- 3.Aebersold R, Mann M: Mass spectrometry-based proteomics. Nature 2003, 422:198–207. [DOI] [PubMed] [Google Scholar]
- 4.Yu MK, Park J, Jon S: Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2:3–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi HS, Liu WH, Liu FB, Nasr K, Misra P, Bawendi MG, Frangioni JV: Design considerations for tumour-targeted nanoparticles. Nat Nanotechnol 2010, 5:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weissleder R, Pittet MJ: Imaging in the era of molecular oncology. Nature 2008, 452:580–589.18385732 •• Very comprehensive and informative review on the imaging technologies used in cancer biology and oncology.
- 7.Willmann JK, van Bruggen N, Dinkelborg LM, Gambhir SS: Molecular imaging in drug development. Nat Rev Drug Discov 2008, 7:591–607. [DOI] [PubMed] [Google Scholar]
- 8.Hussain T, Nguyen QT: Molecular imaging for cancer diagnosis and surgery. Adv Drug Deliver Rev 2014, 66:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dam GM, Themelis G, Crane LMA, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJG, van der Zee AGJ et al. : Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med 2011, 17 1315–1319. [DOI] [PubMed] [Google Scholar]
- 10.Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie SM: Semiconductor quantum dots for bioimaging and biodiagnostic applications. Annu Rev Anal Chem 2013, 6:143–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoeks CMA, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SWTPJ, Scheenen TWJ, Vos PC, Huisman H, van Oort IM et al. : Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 2011, 261:46–66. [DOI] [PubMed] [Google Scholar]
- 12.Janib SM, Moses AS, MacKay JA: Imaging and drug delivery using theranostic nanoparticles. Adv Drug Deliver Rev 2010, 62:1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi KY, Liu G, Lee S, Chen XY: Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale 2012, 4:330–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lammers T, Aime S, Hennink WE, Storm G, Kiessling F: Theranostic nanomedicine. Accounts Chem Res 2011, 44:1029–1038. [DOI] [PubMed] [Google Scholar]
- 15.Brindle K: New approaches for imaging tumour responses to treatment. Nat Rev Cancer 2008, 8:94–107. [DOI] [PubMed] [Google Scholar]
- 16.Jares-Erijman EA, Jovin TM: FRET imaging. Nat Biotechnol 2003, 21:1387–1395. [DOI] [PubMed] [Google Scholar]
- 17.Zhou KJ, Liu HM, Zhang SR, Huang XN, Wang YG, Huang G, Sumer BD, Gao JM: Multicolored pH-tunable and activatable fluorescence nanoplatform responsive to physiologic pH stimuli. J Am Chem Soc 2012, 134:7803–7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu CQ, Osborne EA, Louie AY: Activatable T (1) and T (2) magnetic resonance imaging contrast agents. Ann Biomed Eng 2011, 39:1335–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Pan J, Hogen-Esch TE: Synthesis and characterization of one-ended perfluorocarbon-functionalized derivatives of poly(ethylene glycol)s. Macromolecules 1998, 31:2815–2821. [Google Scholar]
- 20. Nie SM, Xing Y, Kim GJ, Simons JW: Nanotechnology applications in cancer. Annu Rev Biomed Eng 2007, 9:257–288.17439359 • Overview of the use of nanotechnology in cancer diagnosis, therapy and their combination.
- 21.Howes PD, Chandrawati R, Stevens MM: Colloidal nanoparticles as advanced biological sensors. Science 2014, 346:1247390. [DOI] [PubMed] [Google Scholar]
- 22.Ren C, Zhang J, Chen M, Yang Z: Self-assembling small molecules for the detection of important analytes. Chem Soc Rev 2014, 43:7257–7266. [DOI] [PubMed] [Google Scholar]
- 23.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R: Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2007, 2:751–760. [DOI] [PubMed] [Google Scholar]
- 24.Hilderbrand SA, Weissleder R: Near-infrared fluorescence: application to in vivo molecular imaging. Curr Opin Chem Biol 2010, 14:71–79. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Longmire MR, Ogawa M, Choyke PL: Rational chemical design of the next generation of molecular imaging probes based on physics and biology: mixing modalities, colors and signals. Chem Soc Rev 2011, 40:4626–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Xie J, Chen XY: Activatable molecular probes for cancer imaging. Curr Top Med Chem 2010, 10:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pysz MA, Gambhir SS, Willmann JK: Molecular imaging: current status and emerging strategies. Clin Radiol 2010, 65:500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razgulin A, Ma N, Rao J: Strategies for in vivo imaging of enzyme activity: an overview and recent advances. Chem Soc Rev 2011, 40(7):4186–4216 [DOI] [PubMed] [Google Scholar]
- 29.Yun CS, Javier A, Jennings T, Fisher M, Hira S, Peterson S, Hopkins B, Reich NO, Strouse GF: Nanometal surface energy transfer in optical rulers, breaking the FRET barrier. J Am Chem Soc 2005, 127:3115–3119. [DOI] [PubMed] [Google Scholar]
- 30.Mu CJ, Lavan DA, Langer RS, Zetter BR: Self-assembled gold nanoparticle molecular probes for detecting proteolytic activity in vivo. Acs Nano 2010, 4:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian RC, Ding L, Yan LW, Lin MF, Ju HX: A robust probe for lighting up intracellular telomerase via primer extension to open a nicked molecular beacon. J Am Chem Soc 2014, 136:8205–8208. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Lee K, Kim IK, Park TG: Fluorescent gold nanoprobe sensitive to intracellular reactive oxygen species. Adv Funct Mater 2009, 19:1884–1890. [Google Scholar]
- 33.Thorek DLJ, Ogirala A, Beattie BJ, Grimm J: Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med 2013, 19:1345–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prigodich AE, Randeria PS, Briley WE, Kim NJ, Daniel WL, Giljohann DA, Mirkin CA: Multiplexed nanoflares: mRNA detection in live cells. Anal Chem 2012, 84:2062–2066.22288418 • Excellent and very elaborate research article describing the development of an activatable nanoprobe for simultaneous detection of two intracellular mRNA strands.
- 35.Pan W, Zhang TT, Yang HJ, Diao W, Li N, Tang B: Multiplexed detection and imaging of intracellular mRNAs using a four-color nanoprobe. Anal Chem 2013, 85:10581–10588. [DOI] [PubMed] [Google Scholar]
- 36.Yang K, Zhu L, Nie LM, Sun XL, Cheng L, Wu CX, Niu G, Chen XY, Liu Z: Visualization of protease activity in vivo using an activatable photo-acoustic imaging probe based on CuS nanoparticles. Theranostics 2014, 4:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rodriguez-Lorenzo L, de la Rica R, Alvarez-Puebla RA, Liz-Marzan LM, Stevens MM: Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat Mater 2012, 11:604–607.22635043 •• Pioneering proof-of-principle trial demonstrating the potential of plasmonic nanosensors with inverse sensitivity for the detection of ultra-low concentration biomolecules.
- 38.Chen T, Wu CS, Jimenez E, Zhu Z, Dajac JG, You MX, Han D, Zhang XB, Tan WH: DNA micelle flares for intracellular mRNA imaging and gene therapy. Angew Chem Int Ed 2013, 52:2012–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lock LL, Cheetham AG, Zhang PC, Cui HG: Design and construction of supramolecular nanobeacons for enzyme detection. Acs Nano 2013, 7:4924–4932. [DOI] [PubMed] [Google Scholar]
- 40.Ma XP, Wang YG, Zhao T, Li Y, Su LC, Wang ZH, Huang G, Sumer BD, Gao JM: Ultra-pH-sensitive nanoprobe library with broad pH tunability and fluorescence emissions. J Am Chem Soc 2014, 136:11085–11092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X, Xie J, Zhu L, Lee S, Niu G, Ma Y, Kim K, Chen XY: Hybrid ferritin nanoparticles as activatable probes for tumor imaging. Angew Chem Int Ed 2011, 50:1569–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mok H, Jeong H, Kim SJ, Chung BH: Indocyanine green encapsulated nanogels for hyaluronidase activatable and selective near infrared imaging of tumors and lymph nodes. Chem Commun (Camb) 2012, 48:8628–8630. [DOI] [PubMed] [Google Scholar]
- 43. Wang YG, Zhou KJ, Huang G, Hensley C, Huang XN, Ma XP, Zhao T, Sumer BD, DeBerardinis RJ, Gao JM: A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater 2014, 13:204–212.24317187 • Excellent research article describing ultra-pH sensitive nanoprobes for the detection of tumors.
- 44. Lovell JF, Jin CS, Huynh E, Jin HL, Kim C, Rubinstein JL, Chan WCW, Cao WG, Wang LV, Zheng G: Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat Mater 2011, 10:324–332.21423187 •• Pioneering research article demonstrating the potential of porphysomes, a self-assembled nanoprobe, for both photoacoustic and NIR imaging in vivo.
- 45.Gao Y, Shi JF, Yuan D, Xu B: Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nat Commun 2012, 3:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen JW, Pham W, Weissleder R, Bogdanov A Jr: Human myeloperoxidase: a potential target for molecular MR imaging in atherosclerosis. Magn Resonance Med/Soc Magn Resonance Med 2004, 52:1021–1028. [DOI] [PubMed] [Google Scholar]
- 47.Kim T, Cho EJ, Chae Y, Kim M, Oh A, Jin J, Lee ES, Baik H, Haam S, Suh JS et al. : Urchin-shaped manganese oxide nanoparticles as pH-responsive activatable T-1 contrast agents for magnetic resonance imaging. Angew Chem Int Ed 2011, 50:10589–10593. [DOI] [PubMed] [Google Scholar]
- 48. Takaoka Y, Sakamoto T, Tsukiji S, Narazaki M, Matsuda T, Tochio H, Shirakawa M, Hamachi I: Self-assembling nanoprobes that display off/on 19F nuclear magnetic resonance signals for protein detection and imaging. Nat Chem 2009, 1:557–561.21378937 •• Pioneering research article demonstrating the utility of 19F chemical shift anisotropy for the detection of biomolecules.
- 49.Matsuo K, Kamada R, Mizusawa K, Imai H, Takayama Y, Narazaki M, Matsuda T, Takaoka Y, Hamachi I: Specific detection and imaging of enzyme activity by signal-amplifiable self-assembling 19F MRI probes. Chem-Eur J 2013, 19:12875–12883. [DOI] [PubMed] [Google Scholar]
- 50.Huang XN, Huang G, Zhang SR, Sagiyama K, Togao O, Ma XP, Wang YG, Li Y, Soesbe TC, Sumer BD et al. : Multi-chromatic pH-activatable 19F-MRI nanoprobes with binary ON/OFF pH transitions and chemical-shift barcodes. Angew Chem Int Ed 2013, 52:8074–8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kastantin M, Missirlis D, Black M, Ananthanarayanan B, Peters D, Tirrell M: Thermodynamic and kinetic stability of DSPE-PEG(2000) micelles in the presence of bovine serum albumin. J Phys Chem B 2010, 114:12632–12640. [DOI] [PubMed] [Google Scholar]
- 52.Kim E, Yang J, Park J, Kim S, Kim NH, Yook JI, Suh JS, Haam S, Huh YM: Consecutive targetable smart nanoprobe for molecular recognition of cytoplasmic microRNA in metastatic breast cancer. Acs Nano 2012, 6:8525–8535. [DOI] [PubMed] [Google Scholar]
- 53.Zhu L, Huang X, Choi KY, Ma Y, Zhang F, Liu G, Lee S, Chen X: Real-time monitoring of caspase cascade activation in living cells. J Control Release 2012, 163:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY: In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol 2009, 1:382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nguyen QT, Olson ES, Aguilera TA, Jiang T, Scadeng M, Ellies LG, Tsien RY: Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA 2010, 107:4317–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY: Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc Natl Acad Sci USA 2010, 107:4311–4316.20160077 • Elaborate research article clearly demonstrating the potential of activatable nanoprobes in small tumor metastases imaging, taking advantage of the differentiated tissue accumulation of nano-objects and their specific activation.
- 57.Viger ML, Sankaranarayanan J, Lux CD, Chan M, Almutairi A: Collective activation of MRI agents via encapsulation and disease-triggered release. J Am Chem Soc 2013, 135:7847–7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee D, Khaja S, Velasquez-Castano JC, Dasari M, Sun C, Petros J, Taylor WR, Murthy N: In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat Mater 2007, 6:765–769. [DOI] [PubMed] [Google Scholar]
- 59.Lim CK, Lee YD, Na J, Oh JM, Her S, Kim K, Choi K, Kim S, Kwon 1C: Chemiluminescence-generating nanoreactor formulation for near-infrared imaging of hydrogen peroxide and glucose level in vivo. Adv Fund Mater 2010, 20:2644–2648. [Google Scholar]
- 60.Lee D, Bae S, Ke QE, Lee J, Song B, Karumanchi SA, Khang G, Choi HS, Kang PM: Hydrogen peroxide-responsive copolyoxaiate nanoparticles for detection and therapy of ischemia-reperfusion injury. J Control Release 2013, 172:1102–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]