Abstract
Transparent antimicrobial coatings can maintain the aesthetic appeal of surfaces or the functionality of a touch screen while adding the benefit of reducing disease transmission. We fabricated an antimicrobial coating of silver oxide particles in a silicate matrix on glass. The matrix was grown by a modified Stöber sol-gel process with vapor-phase water and ammonia. A coating on glass with 2.4 mg of per mm2 caused a reduction of 99.3% of SARS-CoV-2, >99.5% of Pseudomonas aeruginosa, Staphylococcus aureus and methicillin-resistant Staphylococcus aureus compared to the uncoated glass after one hour. We envisage that screen protectors with transparent antimicrobial coatings will find particular application to communal touch screens, such as in supermarkets and other check-out or check-in facilities where a number of individuals utilize the same touch screen in a short interval.
Keywords: SARS-CoV-2, antibacterial, antiviral, antimicrobial, silver oxide, Ag2O, transparent, coating, COVID-19
Graphical Abstract

1. Introduction
Pathogenic microbes are responsible for a wide variety of diseases. The routes of transmission vary among microbes and depend on a variety of variables1 such as temperature and climate. Viruses and microorganisms are known to be transmitted through one or a combination of five main routes:2 1) direct contact, 2) airborne, 3) droplet, 4) vehicle-borne including via fomites, and 5) vector-borne. Strategies to reduce pathogen transmission can be used to reduce the prevalence of disease in the community and to reduce healthcare-associated infections (HAIs).
Staphylococcus aureus (S. aureus), Methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa (P. aeruginosa), three common infectious bacteria3, 4 in healthcare settings, cause mild to life-threatening infections that set the stage for maladies such as bloodstream, urinary tract and surgical site infections, sepsis and pneumonia3, 5 These bacteria are considered a major threat to public health6, 7 and can be transmitted through contaminated fomites.5, 8, 9
The Coronavirus disease 2019 (COVID-19) pandemic has dramatically increased the need to control pathogen transfer in different settings. By December 2021, COVID-19 had been responsible for the death of almost more than 5 million people10 and is known to spread more easily than other corona virus diseases.11 Although the main transmission route of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is the inhalation of contaminated respiratory droplets,12 the transmission modes of this virus are believed to be direct contact, airborne and contaminated fomites.13 One modelling study suggested that 25% of transmission is via fomites14 and SARS-CoV-2 is known to be stable on a skin model up to 96 hours at 22 °C.15
Hand hygiene is believed to be an effective measure to prevent the microbe transfer through contaminated surface,16 but in a fomite-rich environment, cleaning of hands would need to be very frequent.17 Therefore, health professionals suggest a combination of hand hygiene18 and surface disinfection19, 20 to mitigate the risk of these microbe transfers.
A parallel approach to the reduction of infection from fomites is to implement coatings on common-use surfaces21 that quickly inactivate microbes between users. SARS-CoV-2 can remain viable on solid surfaces up to 7 days,22, 23 and the above mentioned bacteria are stable on surfaces for months,24, 25 depending on the type of solid and environmental conditions. If these periods were reduced by antimicrobial coatings on common-touch surfaces, then the window of transmission could be shortened and the spread of COVID-19 and other microbial diseases could be reduced.
To this end, coatings have been developed to kill bacteria,8, 26, 27 or viruses28 and more recently to inactivate SARS-CoV-2.29–32 The speed of their action is of great importance; clearly one would like the viability of the surface-adherent microorganisms to be eliminated within minutes or even a shorter time after deposition of the microbe on the solid surface.
Another practical criterion for an antimicrobial coating is retention of the function of the surface after it has been coated. This is our motivation for creating transparent antimicrobial coatings. Transparency is necessary for phone screens, touch screens at supermarkets and check-in facilities, tablets, windows, etc. and touch screens are a known pathway for the spread of pathogens. For example, mobile phones are a major pathway for bacteria spread in hospitals.33, 34 Touch screens at supermarkets etc., have a series of users in a short time, so it is reasonable that pathogens may be spread by contact at these locations.
The antimicrobial coatings have attracted many researchers in the past few years.35–38 To date all published work on coatings that inactivate SARS-CoV-2 describe opaque coatings.29–32 Here we describe a novel transparent silver oxide coating capable of accelerating the inactivation of the virus, SARS-CoV-2, as well as rapidly killing the bacteria S. aureus, P. aeruginosa. The coating killed both methicillin-tolerant S. aureus and methicillin-resistant S. aureus. The latter, known as MRSA, is an important public health issue. The antibacterial properties of silver oxide have been previously reported in non-transparent coatings for medical implants39 and on catheters.40 No damage, change in morphology or cytotoxic effect has been observed against L929 fibroblast cells41 or G292 osteoblastic cells,42 which are mammalian cells. Because of such low cytotoxicity, can be used in wide variety of applications from tooth paste against dental pathogens43 to its use in wound healing injections44, anticancer carrier drugs for skin cancer45, orthopaedic42 and dental tissue regeneration.46
In order to fabricate the transparent coatings we employed a novel binding method based on the Stöber sol-gel process,47–49 that utilized vapor-phase reactants so that menisci could form and react.50 Our results indicated that the silver oxide transparent coating caused at least a 99.8% decrease in SARS-CoV-2, S. aureus and P. aeruginosa in one hour, and that the resulting coating was transparent and allowed operation of an iPhone touch-screen.
2. Materials and Methods
2.1. Materials
100% Ethanol (EtOH ACS grade), 70% ethanol (Reagent Grade), sodium hydroxide beads ( ACS grade), nitric acid (70%, ACS grade) and glass slides (25 × 75 × 1 mm) were purchased from VWR. Silver nitrate 99.9% and ammonium hydroxide (Certified ACS Plus) were obtained from Fisher Scientific. Tetraethyl orthosilicate 99.999% (TEOS) was purchased from Sigma Aldrich. Deionized (DI) water was from a Milli-Q Reference (MilliporeSigma) water purification system.
2.2. Synthesis of Microparticles
The synthesis of silver oxide particle has been previously described elsewhere.51, 52 Briefly, 600 mL of 0.01 M in DI water was stirred while 1200 mL of 0.01 M in DI water was added dropwise, and then the mixture was stirred for 10 minutes. Subsequently, 60 mL of 2 M was added dropwise. The addition of caused the solution to darken, demonstrating the synthesis of small particles. The suspension was left for 12 hours, during which time, the particles precipitated. After precipitation, the particles were washed three times with DI water and then three times with ethanol. Lastly, the supernatant liquid was decanted from the particles and particles were left to dry.
2.3. Fabrication of Transparent Silver Oxide Coatings
In order to prepare the coating, glass slides where cut into 12×12 mm pieces and cleaned serially with DI water, 70% ethanol, 6 M Nitric acid and DI water respectively. Those glass pieces acted as the -free, control samples. We prepared two different coatings that differed by the solids-loading of particles. The -coating had 5.0 mg/mL powder in suspension (1.2 mg of per mm2 of glass surface) whereas -coating had 10 mg/mL powder in suspension (2.4 mg of per mm2). To prepare the transparent silver oxide-coated surfaces, the glass pieces were plasma treated at a pressure of less than 200 mTorr and 100 W for 5 minutes. Immediately after the plasma treatment, 34 μL of a suspension of in 2.8 % (vol/vol) TEOS in ethanol solution was applied on the glass pieces. Substrates were then placed in partially sealed leveled containers to limit evaporation and the self-assembly53, 54 of the particles accordingly. After two hours, the ethanol was evaporated and samples were transferred to a leveled sealed container in contact with vapors of 8 M DI water in ethanol and 7.62 M ammonia in DI Water for 20 hours. Next, the samples were heat-treated at 50 °C for 40 min. Lastly, samples were blown with high pressure nitrogen, rinsed upside down in DI water for 10 minutes and dried with a nitrogen gas. We used cleaned glass and TEOS samples as controls in antimicrobial experiments.
2.4. Characterization of Microparticles and Coatings
The x-ray diffraction (XRD) pattern obtained from a Bruker D8 Advance diffractometer (monochromatic Cu Kα x-ray, wavelength=1.5418 Å) was used to identify the structure of the particles. X-ray photoelectron spectroscopy (XPS; PHI VersaProbe III with a monochromatic Al Kα source of 1486.6 eV) was employed to assess the chemical composition of the surface of the film. Scanning electron microscopy (JEOL JSM-IT500) and atomic force microscopy (Asylum Research 3D MFP) were utilized to examine the coating morphology and roughness respectively. ImageJ software was employed to obtain the synthesized particle size distribution through SEM images. Surface composition was assessed using electron-dispersive X-ray spectroscopy (Oxford Instruments Ultim Max 100). Optical absorbance and transmittance were measured using an Agilent model 8453 UV-VIS spectrometer. The wettability of the coatings was assessed from the contact angle (First Ten Angstroms FTA125) of 10 μL of DI water.
2.5. SARS-CoV-2 Assay
We have described the 50% tissue culture infective dose () method for SARS-CoV-2 viability tests in detail elsewhere.29, 30 Briefly, both preparation of the virus stock (Hong Kong index SARS-CoV-2 virus) and assessment of cytopathic effect utilized Vero E6 cells. These cells were cultured at 37 °C and 5% in 2% fetal bovine serum and 1% v/v penicillin−streptomycin added to Dulbecco’s modified Eagle medium. The viral transport medium consisted of 0.5% (w/v) bovine serum albumin and 0.1% (w/v) glucose in Earle’s balanced salt solution with a pH of 7.4. Control samples and coatings were disinfected with 70% ethanol in water and then dried in air at 37 °C overnight.
The antiviral properties were assessed by placing a 5 μL droplet containing 7.8 log unit TCID50/mL of SARS-CoV-2 on the test solid at 22–23 °C and 60–70% humidity, and after a predefined time, the sample was eluted in 300 μL of viral transport medium. The viable virus was then measured using assay55, 56 in quadruplicates57. Three independently produced solid surfaces were tested for each time point and the antiviral activity at each time point was obtained based on the reduction of log (virus titer).
2.6. Antibacterial Assay
Microbial Strains.
The microbial strains employed in this study were P. aeruginosa strain DSM-9644, S. aureus strain ATCC No. 6538, and a methicillin-resistant S. aureus (MRSA) strain MA43300 obtained from the Danville Community Hospital (Danville, VA).
Growth of Microbial Strains.
Bacterial strains were grown in 5 mL of Tryptic Soy Broth (TSB, BD, Sparks, MD) to mid-exponential phase at 37° C with aeration (60 rpm). Following growth, the purity and identity of the cells in the cultures was verified by streaking bacterial cultures on Tryptic Soy Agar (TSA, BD, Sparks, MD) and incubated at 37° C for 48 hr and examining colonies for species-specific traits (e.g., pigmentation and surface texture).
Preparation of Microbial Strains for Testing.
Grown cells were collected by centrifugation (5,000 g for 20 min), the supernatant medium discarded, and the cells suspended in 5 mL of sterile phosphate-buffered saline (PBS) by vortexing for 60 sec. Those suspensions were centrifuged (5,000 g for 20 min), the supernatant wash discarded, and the washed cells suspended in 5 mL of sterile PBS by vortexing for 60 sec. The number of colony-forming units (CFU)/mL of each washed suspension was measured by spreading 0.10 mL (in duplicate) of serial dilutions in PBS on TSA plates.
Measurement of Cell Number.
Cell number of PBS-suspensions of bacteria was measured as colony-forming units (CFU)/mL of suspension. This measures the number of viable cells, i.e., those cells that are able to grow into a colony. A 10-fold dilution series was prepared for each PBS suspension, 0.1 mL of each dilutions was spread on TSA in triplicate, and colonies were counted after 48 hr. incubation at 37° C. If no colonies were present for the least dilution, then we rounded this result up to one colony to enable a log transformation. We set this as the detection limit shown in figures. Any data point at the detection limit is therefore an upper bound for that measurement.
Measurement of Surface-Killing.
For each microbial strain, a 10 μL droplet of bacterial cells in PBS suspension was placed on each of 3 individual -coated or uncoated glass pieces. Immediately after drying, each glass piece was transferred to a separate sterile 50 mL centrifuge tube containing 5 mL of sterile PBS, vortexed at the highest setting for 10 sec and sonicated for 1 min. in a Branson Model 12 Ultrasonic Cleaner (Shelton, CT) and the CFU/mL of the suspension measured as described above. The process was repeated at each time point. CFU counts, corrected for dilution, are in tables in the Supporting Information.
2.7. Robustness of Coatings
The United States Environmental Protection Agency (EPA) has a required procedure for the evaluation of antibacterial coatings. We followed their procedure58 but with some minor modifications. The procedure is described more fully in in Supporting Information, but in brief, it consists of repeatedly translating a wet sponge across the coating with a mass of 0.45 kg using a Gardco, Model D10V abrasion 214 tester. The main modification to the EPA procedure was to use ethanol, because our main application was for transparent surfaces, such as electronic displays, that are usually cleaned with alcohol solutions.
We further tested the particle attachment strength by sonicating the coatings for three minutes in ethanol. The absorbance spectra of resulted suspensions were then obtained using UV-VIS to evaluate the detachment of the particles.
2.8. Statistics
All experiments were done with three independent trials. Effects were considered significant when was near or below 0.05. Linear regression was performed using Excel or MATLAB.
3. Results
3.1. Characterization of the particles
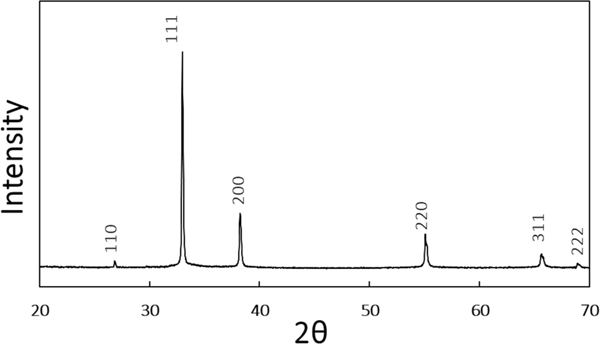
The XRD pattern (Figure 1) of the particles is consistent with the known cubic space group and the previously-observed XRD pattern of truncated octahedral silver oxide microparticles.51, 52 SEM images of the particles (Figure 2) show a morphology consistent with the literature.51, 52 The mean particle size is 1.5 μm (Supporting Information, Figure S1).
Figure 1.
XRD pattern of the particles, which is consistent with the known pattern of microparticles.51, 52 The numbers on each peak indicate the Miller Indices of the scattering planes.
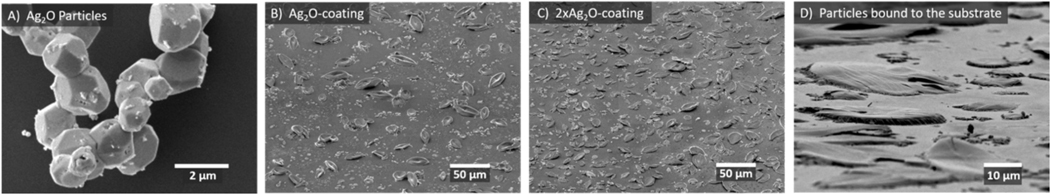
Figure 2.
SEM images of A) particles prior to fabrication of the coating. B) -coating C) -coating and D) Tilted and magnified view of C. The SEM images show that the coating of particles is uniform and is consistent with protruding from the silica matrix. Additional SEM images of controls are included in the Supporting Information, Figure S3.
3.2. The Coatings Contain at or near the surface.
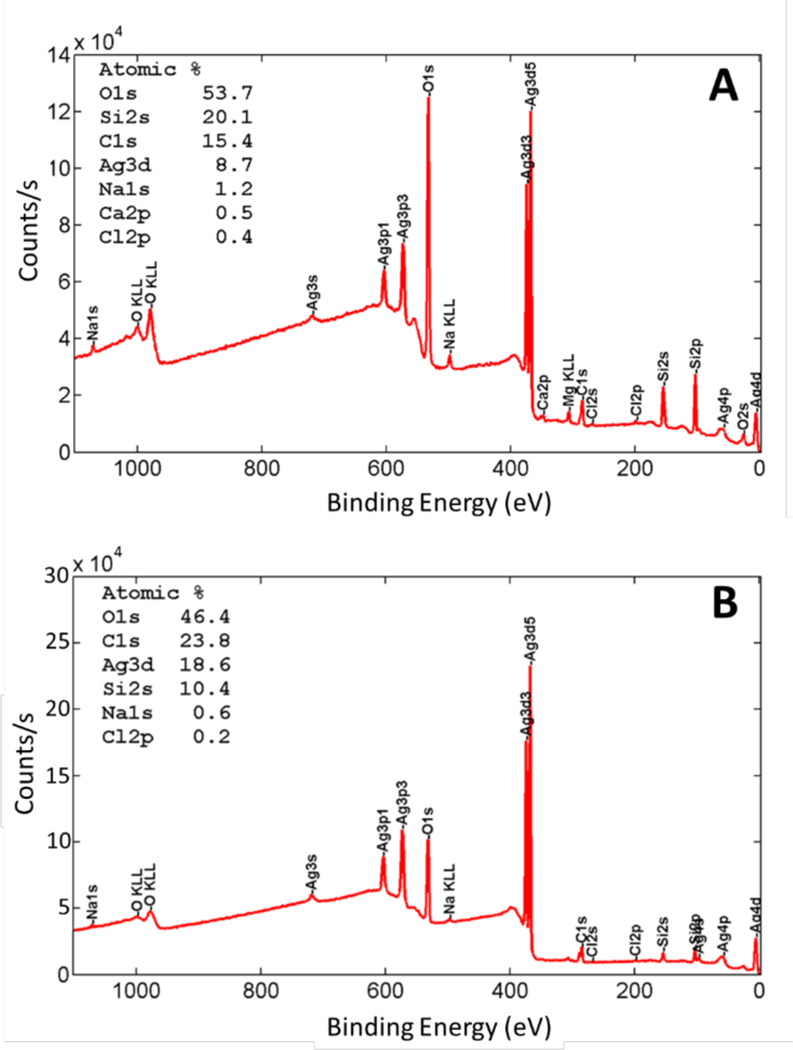
Our plan was to have the protrude from the silica matrix, such that microbes would come into contact with the surface or dissolved ions. SEM images (Figure 2B–D) are consistent with protruding from the silica matrix and show a uniform distribution of particles. EDS of individual particles shows a 2.5:1 ratio of Ag:O which is similar to a 2:1 ratio expected for (Supporting Information, Table S1). We used XPS, which assays only the top 1 nm or so of the interface, to determine whether the was exposed. The results in Figure 3, which show that the surface has about 9% silver for the -coating and 19% for the -coating, verified that the particles were at or near the surface and that the amount of silver at the surface scaled with the amount added to the coating.
Figure 3.
XPS of the silver oxide coatings showing the elemental analysis and confirming presence of silver and oxygen on the surface. A) Survey spectrum of -caoting and B) Survey spectrum -coating.
The morphology of the particles changed during film formation: the octahedral shape was transformed into lotus-leaf-type features (Figure 2B–D). These features protrude less than about 2 μm from the surrounding silica layer, which has roughness of about 10 nm (See Figure S2). Silver oxide particle are known to have partial solubility when in contact with ammonia59 or alkali,60 and the coating was exposed to ammonia vapor during formation. Therefore, the morphology change is a result of a partial dissolution/precipitation process in the presence of ammonia. Figure S4, S5 and S6 in Supporting information show optical timelapse photography demonstrating that the morphology change is mainly complete after about 12 hours, that ammonia is necessary for the reaction, and that the heat treatment does not affect the morphology.
The static contact angles for a 10 μL water droplet on -coating and -coating were 62 ± 7° and 67 ± 5°, respectively (See Figure S7 Supporting information). We also examined how firmly the silver particles were attached in the film by exposing the film to ultrasound while immersed in ethanol for 3 minutes. We were not able to detect any particles that were removed by ultrasound (See Figure S8 in Supporting information).
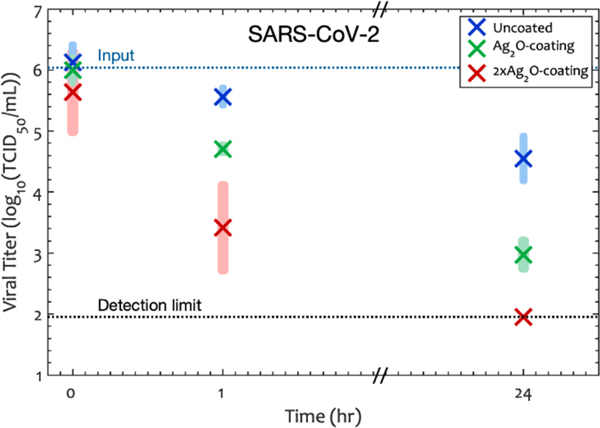
3.3. Silver Oxide Coatings inactivate SARS-CoV-2
The antiviral activity of transparent silver oxide coatings was evaluated by placing a 5 μL droplet containing SARS-CoV-2 on each coating and measuring the viable virus titer at predefined time points. The results in Figure 4 show that silver oxide particles are able to greatly accelerate the decay of SARS-CoV-2 on the coatings compared to the uncoated solid. There are two reference points for considering the effectiveness of the coatings: (1) comparison to the input microbe titer in the test droplet, which we call “inactivation” and (2) comparison to the microbe titer on the uncoated glass at the same time, which we call “Reduction”, each of which is defined as follows:
| (1) |
| (2) |
| (3) |
| (4) |
Figure 4.
SARS-CoV-2 titer as a function of time and surface density in the coating. -catong has 1.18 mg of per mm2 of glass surface whereas -coating has 3.6 mg of per mm2. Shaded rectangles represent the 95% confidence interval calculated only for the points at that condition, and × represents the average of the log of the viral titer at each time point. The results show that the coating inactivates the SARS-CoV-2 virus.
In equations (1) and (2) the titers have been made unitless by multiplying by the volume units, so the same volume units must be used for the two means. The assay does not measure numbers of virions and instead, measures the infectious dose of the virus needed to infect 50% of the tissue culture. The experimental reductions and efficiencies are listed in Table 1. ANOVA (with time and loading as factors) showed that both time (p= 7×10−19) and concentration of (p= 1×10−14) were significant factors affecting the inactivation of SARS-CoV-2.
Table 1.
log Inactivation, log survival, and log reduction of microbes after 1 hour. Inactivation and killing compare to the droplet suspension at the that was used to inoculate the solid and Reduction compares to glass at the same time point.
| SARS-CoV-2 | P. aeruginosa | S. aureus | MRSA | |||||
|---|---|---|---|---|---|---|---|---|
| Coating | Inactivation | Reduction | Survival | Reduction | Survival | Reduction | Survival | Reduction |
| 1.34 | 0.86 | −4.68 | 3.79 | −2.67 | 1.77 | −2.18 | 1.44 | |
| 2.62 | 2.15 | −4.68 | 3.79 | −3.16 | 2.26 | −3.63 | 2.89 | |
We observed a very slow inactivation of SARS-CoV-2 titer on the uncoated glass: the /mL was decreased by only 66.7% (0.48 reduction) after one hour. In contrast, on the and the coatings, the virus was inactivated by 95.4% (1.3 reduction) and 99.8% (2.6 reduction) after one hour. When we compared the performance of these two transparent coatings with uncoated glass using (eq 2) and (eq 4), the average Reduction was 86.1% for the -coating and 99.3% for the -coating after one hour. The 95% confidence interval (one tailed, heteroscedastic) indicated that the Reduction was more than 73.2% on -coating and more than 95.7% on -coating compared to uncoated glass after one hour. The absence of significant Reduction for TEOS-only samples confirmed that silver oxide is the active ingredient for inactivating SARS-CoV-2 (Figure S9 Supporting information).
The significance of the silver oxide surface density, , and time, , can be determined by a regression analysis. For this analysis we included the zero- (TEOS only) coating, the -coating and the -coating. We included a cross-term () because we hypothesized that more SARS-CoV-2 would be inactivated over time if there were a greater density of in the coating. The regression equation has the following from:
| (5) |
where , , , and are coefficients to be determined from the regression. A regression analysis using 0 and 1 h data points showed that p = 0.12 for the coefficient of concentration and so this term was deleted and the analysis was rerun with only the and terms. For the cross term, , p= 4×10−4, showing that the loss of viral titer depended on the concentration on the surface density. The half-life of the viral titer is:
| (6) |
so, the significant value of shows that the half-life of SARS-CoV-2 titer decreases with the concentration of in the coating, a major conclusion of this paper. Values of all coefficients are in Table 2.
Table 2.
Linear regression coefficients for Equation 5. C was omitted because it was not significant in any microorganism. The small values for show that the half-life of all the microorganisms decreases with concentration of silver oxide in the coating. The linear regression was run with in units of hours and is in units of mg/mm2. For SARS-CoV-2, only the 0h and 1 h data was included because all the data for the beyond 1 h was below the detection limit, so only the upper bound was known.
| SARS-CoV-2 | P. aeruginosa | S. aureus | MRSA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| variable | coefficient | value | p | value | p | value | p | value | p |
| constant | 5.904 | 3×10−18 | 5.835 | 10–15 | 5.709 | 10–19 | 6.048 | 10–20 | |
| 0.243 | 0.28 | 0.014 | 0.07 | 0.008 | 0.07 | 0.006 | 0.16 | ||
| 0.923 | 1×10–6 | 0.018 | 1.9×10−4 | 0.011 | 2.3×10−4 | 0.015 | 3.5×10−6 | ||
3.4. Silver Oxide Coatings Kill Bacteria
We tested the -coating and the -coating against three bacteria strains by placing a 10 μL droplet on the solid and measuring the CFU after a predefined period of time. Figure 5 shows the antibacterial activity of silver oxide coatings against P. aeruginosa, S. aureus and MRSA. Both coatings are extremely effective against all three bacteria as demonstrated by the death of bacteria at 1 h. We quantified the efficacy of the coatings using the following equations:
| (7) |
| (8) |
| (9) |
| (10) |
Figure 5.
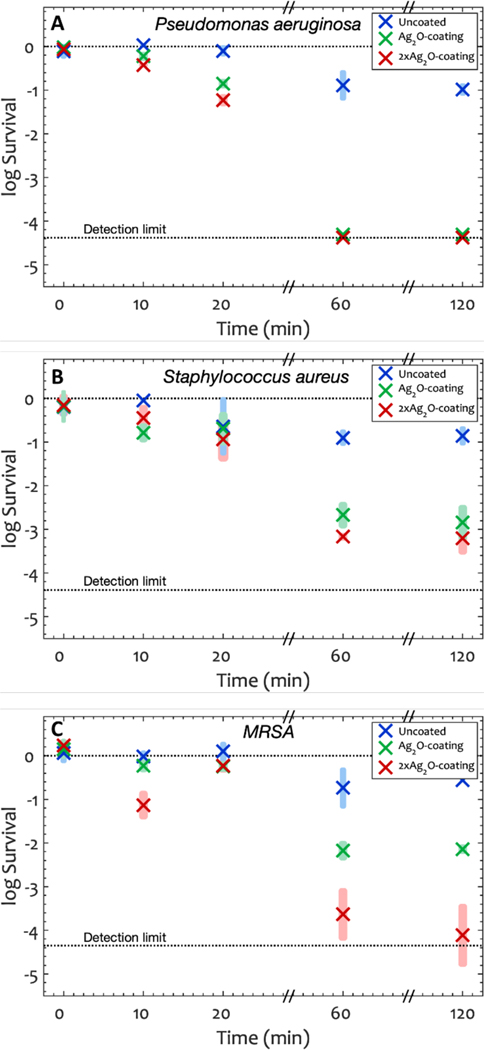
Log survival in Colony forming units (Equation 1) over time for A) P. aeruginosa, B) S. aureus and C) MRSA. Shaded rectangles represent the 95% confidence interval and × represents the average of the log of the CFU at each time point. The log input titers were 6.08, 6.09 and 6.05 for P. aeruginosa, S. aureus and MRSA respectively. After one hour, the average killing percentage of P. aeruginosa, S. aureus and MRSA were >99.9% on -coating and >99.3% on -coating. Silver oxide transparent coatings significantly reduced the CFU units of the bacteria compared to uncoated glass (ANOVA p=7×10−3).
We use the word survival for simplicity but acknowledge that the CFU assay measures those cells that can reproduce to form a colony. Table 2 lists the survival (in log units) compared to both the input of bacteria, and the Reduction compared to glass at one hour. Both coatings demonstrated excellent antibacterial activity and the results indicate that the number of viable bacteria was reduced by at least 1.8 log units (>98.7% Reduction) on the -coating and at least 2 log units (>99.3 % Reduction) on the -coating compared to glass in 1 hour. Again, the Reduction was greater with more present in the film, and there was no significant Reduction for TEOS-only coatings (See Figure S10, S11 and S12 in Supporting Information), indicating that is the active ingredient.
The significance of time and concentration was again determined by a regression analysis using Equation 5 (by replacing with CFU). Again, the effect of concentration was insignificant and was omitted in subsequent analysis. In common with SARS-CoV-2, the cross term, , shows that a greater density of in the coating leads to greater inactivation of all three bacteria over time. More precisely, a greater density of in the coating reduces the half-life of the bacteria.
The -coating demonstrated an excellent bacterial activity by reducing the viable cells of P. aeruginosa and MRSA by more than 99.9% (p=7×10−6 and p=7×10−3 respectively) after one hour, and reducing S. aureus by 99.5% (p=2×10−7) after one hour compared to uncoated glass. The -coating also showed a considerable Reduction in comparison to uncoated glass. The Reduction of viable P. aeruginosa, S. aureus and MRSA on this coating was 99.9% (p=7×10−6), 98.3% (p=6×10−6) and 96.4% (p=4×10−4) respectively compared to uncoated glass after one hour (Figure 5).
3.5. -coatings are Transparent and Retain Touch-Screen Function.
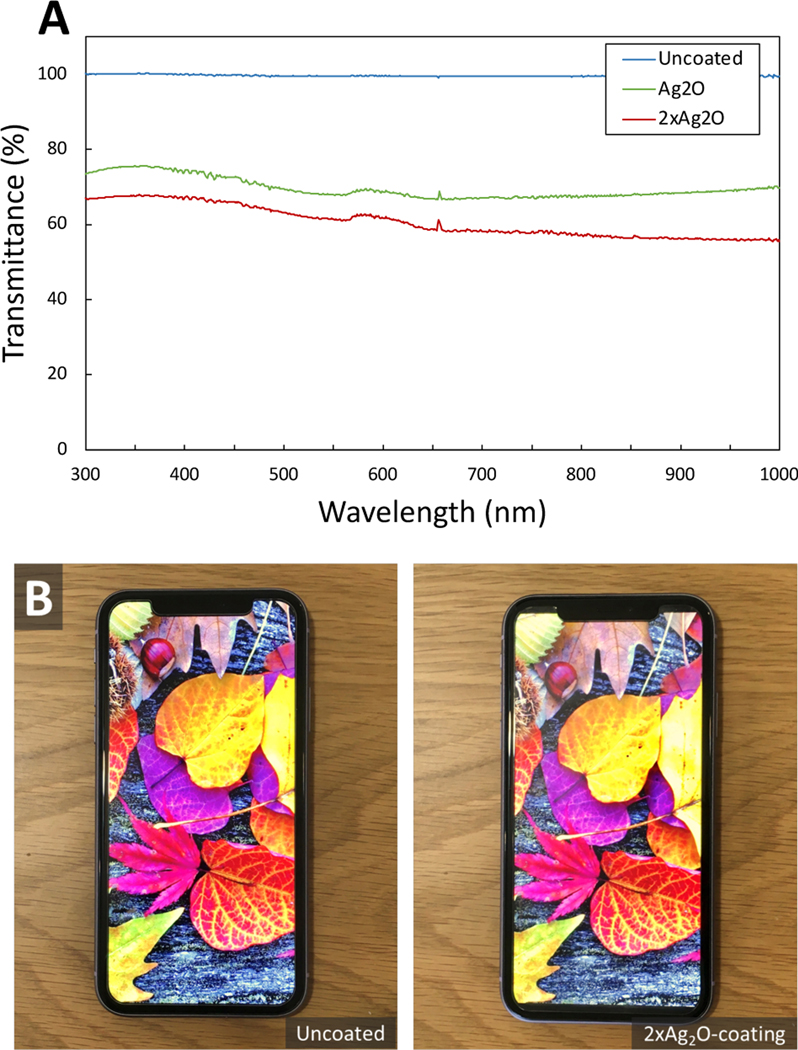
The -coatings are transparent, with about 60–75% of the transmission of glass in the visible range, and only small variation in transmission with color (Figure 6A). As a result, the colors of a smart phone screen are retained when a screen protector with the -coating is attached to a smart phone screen (Figure 6B). Importantly, the screen function is retained with the coating in place (See Video in Supporting Information).
Figure 6.
A) UV-VIS transmission spectrum of glass, -coating and -coating showing that both films demonstrate more than 60–75% transparency in the visible range (400–700 nm) B) A smart phone (iPhone 11) with uncoated and -coated screen protector (Mkeke, Amazon B07HRN9J19, tempered glass). The visual appeal and the touch screen function are retained with the antimicrobial screen protector in place.
3.6. The Antimicrobial Coatings are Resistant to Abrasion
We also conducted an EPA abrasion test (slightly modified) on the coating. After abrasion, the antimicrobial properties were unchanged, demonstrating that the coating was robust (See Figure S13 in Supporting Information).
3.7. Efficacy after Repeated Exposure to Droplets containing Bacterial Suspension
Although coating is very robust against water and ethanol and passes the modified EPA abrasion test, the antibacterial efficacy was diminished after multiple exposures to suspensions of bacteria in droplets. Therefore, we modified the fabrication method to obtain a more robust coating. The principal change was that we increased the amount of silica in the film by increasing the TEOS from 2.8% to 20 % (vol/vol) in ethanol solution. To achieve polymerization of the greater thickness of coating, we increased the time of exposure to the vapor to 60 h and the heat treatment to 75 °C for 40 min. We refer to this modified coating as -coating. The -coating was characterized with SEM with XPS (Figure S14), which showed a lower silver content, consistent with some of the silver oxide being covered by the thicker TEOS layer. The visible spectrum showed that the transparency of -coating was retained (Figure S15).
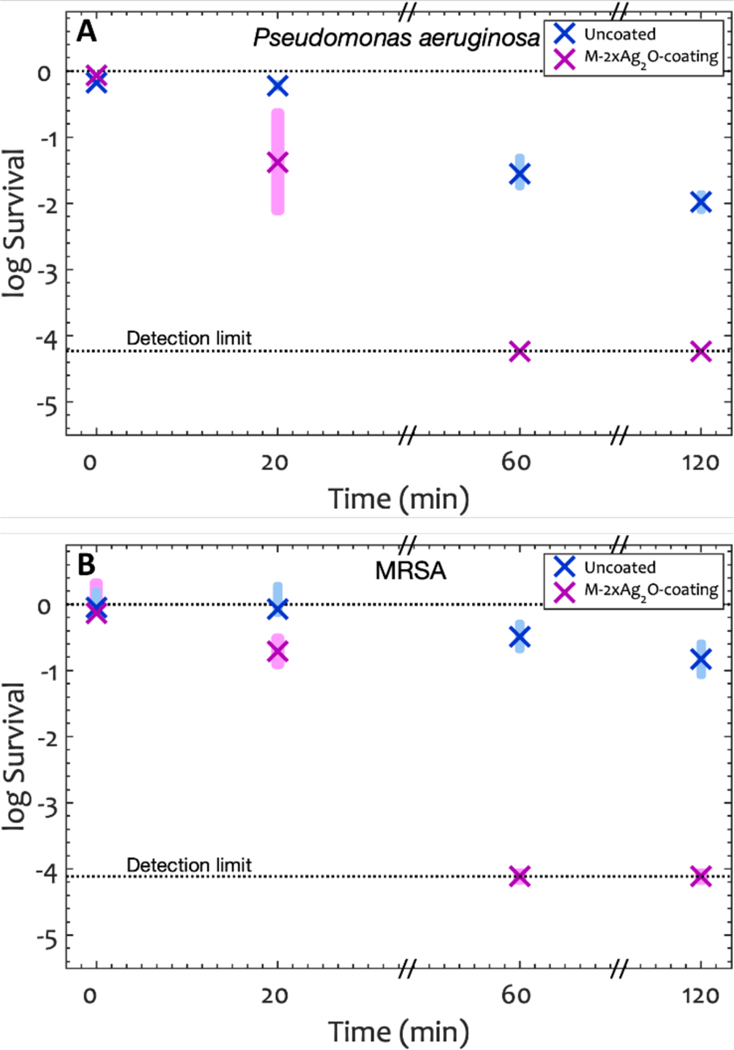
We tested the antibacterial performance of -coating against P. aeruginosa and MRSA (figure 7). After 1 h, the microbial survival was below the detection limit with > 99.99% killing, a 99.80% Reduction for P. aeruginosa and 99.97% Reduction for MRSA compared to the uncoated glass. The results were not statistically different from the -coating at any time point. We then determined the efficacy of this coating after multiple exposure to the microbe, with a series of exposure/bacterial assay/cleaning cycles (See the Supporting Information for details). More than 99.9% of P. aeruginosa were killed in 1 h after four cycles (See Figure S16 in Supporting Information). These results show the coating is still highly active after repeated exposure to the bacteria.
Figure 7.
Log Survival in Colony forming units (Equation 6) over time for A) P. aeruginosa, and B) MRSA on -coating. Shaded rectangles represent the 95% confidence interval and × represents the average of the log of the CFU at each time point. The log input titers were 5.93±0.02 for P. aeruginosa and 6.05±0.12 for MRSA. After one hour, the average killing percentage of P. aeruginosa and MRSA were 99.99% on -coating. The antibacterial results on -coating are not statistically different from the -coating results (two tailed, unpaired p>0.12).
4. Discussion
4.1. Antimicrobial Mechanism of Silver Oxide
The strong antibacterial activity61–63 of silver oxide has been attributed to silver ion species,64–66 which have a finite solubility in water (, ; , ; , ).67 The mechanism of action of silver ions is believed to be either one or a combination of 1) the generation of reactive oxygen species (ROS)64 and exertion of oxidative stress on cells, and 2) the release and penetration of ions into the microbe and damaging the cell membrane.65, 66 Park et al reported that the superoxide radical was the major form of the reactive oxygen species generated by silver ions, while was unlikely to be induced.68 These reactive oxygen species exert an oxidative stress and damage DNA accordingly which will lead to the killing of bacteria.69 Silver ions, on the other hand, lead to loss of cell viability by damaging the cell membrane.66 Minoshima et al. reported that silver oxide particles damage the viral envelope of influenza A virus and bacteriophage virus.70
There is a possibility that the efficacy of depends on light. If the bandgap of the particles were in the visible range, light could cause the excitation of electron to the conduction band, which could then act as reducing agents. We compared the antimicrobial efficacy of coating in visible light and in the dark. (See Figure S17 in Supporting Information). A student’s t-test did not resolve a significant difference between light and dark measurements (p>>0.05) and, therefore, the silver oxide coating does not require light for its antimicrobial properties.
5. Conclusion
We fabricated two silver oxide in silica coatings, and , that inactivate SARS-CoV-2 (95.4% and 99.8% in one hour) and kill P. aeruginosa, (99.99% in 1 h), S. aureus (99.78% and 99.93% in 1h), including the antibiotic-resistant strain, MRSA (99.33% and 99.98% in 1h). The coating was fabricated using a modification of the Stöber method to bind silver oxide particles to a glass substrate. The coated glass is transparent, which means that it does not degrade the aesthetic appeal of materials and it can be applied where transparency is important for function. For example, we showed that when a mobile phone screen was coated, both the screen clarity and the function of the touch-screen was retained. The combination of transparency and antimicrobial action means that the coating should find application for multi-user touchscreens, such as in check-out facilities in grocery stores.
Supplementary Material
Acknowledgements
The authors thank Stephen McCartney for assisting in capturing a part of SEM images and acknowledge use of Electron Microscopy facilities and x-ray diffraction machine within the Nanoscale Characterization and Fabrication Laboratory and Material Characterization Laboratory at Virginia Polytechnic Institute and state university. We also thank Xu Feng for capturing the XPS spectra and acknowledge the use of Surface Analysis Laboratory in Department of Chemistry at Virginia Tech, which is supported by the National Science Foundation under Grant No. CHE-1531834. This work was supported by the National Science Foundation under Grant No. CBET-1902364, the Health and Medical Research Fund (COVID190116), and the National Institute of Allergy and Infectious Diseases (contract HHSN272201400006C).
Footnotes
Supporting Information
Synthesized particle size distribution (Figure S1). AFM images of coating (Figure S2). Surface elemental analysis of coating by EDS (Table S1). SEM images of uncoated glass and TEOS coated glass (Figure S3). Time lapse of sol-gel reaction (Figure S4). The effect of ammonia on the morphology of silver oxide particles (Figure S5). The effect of heat treatment on the morphology of silver oxide particles (Figure S6). Contact angle images of silver oxide coatings (Figure S7). Test of attachment of particles using ultra sonication (Figure S8). The Comparison of SARS-CoV-2 titer Log(TCID50/mL) data on TEOS and glass over time (Figure S9). The Comparison of log decrease in P. aeruginosa colony forming units on TEOS and glass over time (Figure S10). The Comparison of log decrease in S. aureus colony forming units on TEOS and glass over time (Figure S11). The Comparison of log decrease in MRSA colony forming units on TEOS and glass over time (Figure S12). The effect of abrasion in the antibacterial properties of silver oxide coating (Figure S13). Characterization of -coating (Figure S14). UV-VIS transmission spectrum of uncoated glass and M-2xAg2O-coating (Figure S15). The effect of multiple bacteria exposure/sonicating/vortexing/ titration/cleaning on M-2xAg2O coating (Figure S16). The effect of absence of light in the antibacterial properties of silver oxide coating (Figure S17). SARS-CoV-2 /mL assay results for Figure 4 (Table S2). Pseudomonas aeruginosa CFU assay results for Figure 5 (Table S3). Staphylococcus aureus CFU assay results for Figure 5 (Table S4). MRSA CFU assay results for Figure 5 (Table S5). P. aeruginosa CFU assay results for Figure 7 (Table S6). MRSA CFU assay results for Figure 7 (Table S7). P. aeruginosa CFU assay results for Figure S13 (Table S8). P. aeruginosa CFU assays results for Figure S17 (Table S9). MRSA CFU assays results for Figure S17 (Table S10).
Conflict of Interest
WD declares part ownership in a startup company that intends to produce surface coatings. Other authors declare no conflict of interest.
References
- (1).Andersen BM Microbes, Transmission Routes and Survival Outside the Body. In Prevention and Control of Infections in Hospitals, Springer, 2019; pp 23–28. [Google Scholar]
- (2).Antonovics J; Wilson AJ; Forbes MR; Hauffe HC; Kallio ER; Leggett HC; Longdon B; Okamura B; Sait SM; Webster JP The Evolution of Transmission Mode. Philosophical Transactions of the Royal Society B: Biological Sciences 2017, 372 (1719), 20160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Weiner LM; Webb AK; Limbago B; Dudeck MA; Patel J; Kallen AJ; Edwards JR; Sievert DM Antimicrobial-resistant Pathogens Associated with Healthcare-associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. infection control & hospital epidemiology 2016, 37 (11), 1288–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Solberg CO Spread of Staphylococcus aureus in Hospitals: Causes and Prevention. Scandinavian journal of infectious diseases 2000, 32 (6), 587–595. [DOI] [PubMed] [Google Scholar]
- (5).Xiao S; Jones RM; Zhao P; Li Y. The Dynamic Fomite Transmission of Methicillin-resistant Staphylococcus aureus in Hospitals and the Possible Improved Intervention Methods. Building and Environment 2019, 161, 106246. [Google Scholar]
- (6).Davis KA; Stewart JJ; Crouch HK; Florez CE; Hospenthal DR Methicillin-resistant Staphylococcus aureus (MRSA) Nares Colonization at Hospital Admission and Its Effect on Subsequent MRSA Infection. Clinical Infectious Diseases 2004, 39 (6), 776–782. [DOI] [PubMed] [Google Scholar]
- (7).Harris SR; Feil EJ; Holden MT; Quail MA; Nickerson EK; Chantratita N; Gardete S; Tavares A; Day N; Lindsay JA Evolution of MRSA During Hospital Transmission and Intercontinental Spread. Science 2010, 327 (5964), 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Page K; Wilson M; Parkin IP Antimicrobial Surfaces and Their Potential in Reducing the Role of the Inanimate Environment in the Incidence of Hospital-acquired Infections. Journal of materials chemistry 2009, 19 (23), 3819–3831. [Google Scholar]
- (9).Desai R; Pannaraj PS; Agopian J; Sugar CA; Liu GY; Miller LG Survival and Transmission of Community-Associated Methicillin-resistant Staphylococcus aureus from Fomites. American journal of infection control 2011, 39 (3), 219–225. [DOI] [PubMed] [Google Scholar]
- (10).COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Johns Hopkins University, 2020. https://coronavirus.jhu.edu/map.html (accessed December 27, 2021). [Google Scholar]
- (11).Coutard B; Valle C; de Lamballerie X; Canard B; Seidah N; Decroly E. The Spike Glycoprotein of The New Coronavirus 2019-nCoV Contains A Furin-Like Cleavage Site Absent in Cov of The Same Clade. Antiviral research 2020, 176, 104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Prather KA; Wang CC; Schooley RT Reducing Transmission of SARS-CoV-2. Science 2020, 368 (6498), 1422–1424. [DOI] [PubMed] [Google Scholar]
- (13).Sia SF; Yan L-M; Chin AW; Fung K; Choy K-T; Wong AY; Kaewpreedee P; Perera RA; Poon LL; Nicholls JM Pathogenesis and Transmission of SARS-CoV-2 in Golden Hamsters. Nature 2020, 583 (7818), 834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Meiksin A. Dynamics of COVID-19 Transmission Including Indirect Transmission Mechanisms: A Mathematical Analysis. Epidemiology & Infection 2020, 148, e257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Harbourt DE; Haddow AD; Piper AE; Bloomfield H; Kearney BJ; Fetterer D; Gibson K; Minogue T. Modeling the Stability of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) on Skin, Currency, and Clothing. PLoS neglected tropical diseases 2020, 14 (11), e0008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Cromer AL; Latham SC; Bryant KG; Hutsell S; Gansauer L; Bendyk HA; Steed R; Carney MC Monitoring and Feedback of Hand Hygiene Compliance and the Impact on Facility-acquired Methicillin-resistant Staphylococcus aureus. American journal of infection control 2008, 36 (9), 672–677. [DOI] [PubMed] [Google Scholar]
- (17).Bhalla A; Pultz NJ; Gries DM; Ray AJ; Eckstein EC; Aron DC; Donskey CJ Acquisition of Nosocomial Pathogens on Hands After Contact with Environmental Surfaces Near Hospitalized Patients. Infection Control & Hospital Epidemiology 2004, 25 (2), 164–167. [DOI] [PubMed] [Google Scholar]
- (18).Lei H; Xiao S; Cowling BJ; Li Y. Hand Hygiene and Surface Cleaning Should Be Paired for Prevention of Fomite Transmission. Indoor Air 2020, 30 (1), 49–59. [DOI] [PubMed] [Google Scholar]
- (19).Lei H; Jones RM; Li Y. Exploring Surface Cleaning Strategies in Hospital to Prevent Contact Transmission of Methicillin-resistant Staphylococcus aureus. BMC infectious diseases 2017, 17 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Monge FA; Jagadesan P; Bondu V; Donabedian PL; Ista L; Chi EY; Schanze KS; Whitten DG; Kell AM Highly Effective Inactivation of SARS-CoV-2 by Conjugated Polymers and Oligomers. ACS Appl. Mater. Interfaces 2020, 12 (50), 55688–55695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hosseini M; Behzadinasab S; Benmamoun Z; Ducker WA The Viability of SARS-COV-2 on Solid Surfaces. Curr. Opin. Colloid Interface Sci 2021, 101481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Van Doremalen N; Bushmaker T; Morris DH; Holbrook MG; Gamble A; Williamson BN; Tamin A; Harcourt JL; Thornburg NJ; Gerber SI Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. New England Journal of Medicine 2020, 382 (16), 1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chin AW; Chu JT; Perera MR; Hui KP; Yen H-L; Chan MC; Peiris M; Poon LL Stability of SARS-CoV-2 in Different Environmental Conditions. The Lancet Microbe 2020, 1 (4), e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Kramer A; Schwebke I; Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC infectious diseases 2006, 6 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Williams C; Davis DL Methicillin-resistant Staphylococcus aureus fomite survival. American Society for Clinical Laboratory Science 2009, 22 (1), 34–38. [PubMed] [Google Scholar]
- (26).Yuan W; Ji J; Fu J; Shen J. A Facile Method to Construct Hybrid Multilayered Films as A Strong and Multifunctional Antibacterial Coating. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2008, 85 (2), 556–563. [DOI] [PubMed] [Google Scholar]
- (27).Heidenau F; Mittelmeier W; Detsch R; Haenle M; Stenzel F; Ziegler G; Gollwitzer H. A Novel Antibacterial Titania Coating: Metal Ion Toxicity and in Vitro Surface Colonization. Journal of Materials Science: Materials in Medicine 2005, 16 (10), 883–888. [DOI] [PubMed] [Google Scholar]
- (28).Rakowska PD; Tiddia M; Faruqui N; Bankier C; Pei Y; Pollard AJ; Zhang J; Gilmore IS Antiviral Surfaces and Coatings and Their Mechanisms of Action. Commun. Mat 2021, 2 (1), 1–19. [Google Scholar]
- (29).Behzadinasab S; Chin A; Hosseini M; Poon LL; Ducker WA A Surface Coating that Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12 (31), 34723–34727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hosseini M; Chin AW; Behzadinasab S; Poon LL; Ducker WA Cupric Oxide Coating That Rapidly Reduces Infection by SARS-CoV-2 via Solids. ACS Appl. Mater. Interfaces 2021, 13 (5), 5919–5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hutasoit N; Kennedy B; Hamilton S; Luttick A; Rashid RAR; Palanisamy S. SARS-CoV-2 (COVID-19) Inactivation Capability of Copper-Coated Touch Surface Fabricated by Cold-Spray Technology. Manufacturing Letters 2020, 25, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Hosseini M; Behzadinasab S; Chin AW; Poon LL; Ducker WA Reduction of Infectivity of SARS-CoV-2 by Zinc Oxide Coatings. ACS Biomaterials Science & Engineering 2021, 7 (11), 5022–5027. [DOI] [PubMed] [Google Scholar]
- (33).Olsen M; Campos M; Lohning A; Jones P; Legget J; Bannach-Brown A; McKirdy S; Alghafri R; Tajouri L. Mobile Phones Represent A Pathway for Microbial Transmission: A Scoping Review. Travel medicine and infectious disease 2020, 101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Karabay O; Koçoglu E; Tahtaci M. The Role of Mobile Phones in the Spread of Bacteria Associated with Nosocomial Infections. J Infect Dev Ctries 2007, 1 (1), 72–73. [Google Scholar]
- (35).Deusenbery C; Wang Y; Shukla A. Recent Innovations in Bacterial Infection Detection and Treatment. ACS Infectious Diseases 2021, 7 (4), 695–720. [DOI] [PubMed] [Google Scholar]
- (36).Alkekhia D; Hammond PT; Shukla A. Layer-by-layer Biomaterials for Drug Delivery. Annual review of biomedical engineering 2020, 22, 1–24. [DOI] [PubMed] [Google Scholar]
- (37).Salwiczek M; Qu Y; Gardiner J; Strugnell RA; Lithgow T; McLean KM; Thissen H. Emerging Rules for Effective Antimicrobial Coatings. Trends in Biotechnology 2014, 32 (2), 82–90. [DOI] [PubMed] [Google Scholar]
- (38).Cloutier M; Mantovani D; Rosei F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends in biotechnology 2015, 33 (11), 637–652. [DOI] [PubMed] [Google Scholar]
- (39).Akiyama T; Miyamoto H; Yonekura Y; Tsukamoto M; Ando Y; Noda I; Sonohata M; Mawatari M. Silver Oxide-containing Hydroxyapatite Coating Has in vivo Antibacterial Activity in the Rat Tibia. Journal of Orthopaedic Research 2013, 31 (8), 1195–1200. [DOI] [PubMed] [Google Scholar]
- (40).Johnson JR; Roberts PL; Olsen RJ; Moyer KA; Stamm WE Prevention of Catheter-associated Urinary Tract Infection with A Silver Oxide-coated Urinary Catheter: Clinical and Microbiologic Correlates. Journal of Infectious Diseases 1990, 162 (5), 1145–1150. [DOI] [PubMed] [Google Scholar]
- (41).Babu PJ; Doble M; Raichur AM Silver Oxide Nanoparticles Embedded Silk Fibroin Spuns: Microwave Mediated Preparation, Characterization and Their Synergistic Wound Healing and Antibacterial Activity. Journal of colloid and interface science 2018, 513, 62–71. [DOI] [PubMed] [Google Scholar]
- (42).Shahrbabak MSN; Sharifianjazi F; Rahban D; Salimi A. A Comparative Investigation on Bioactivity and Antibacterial Properties of Sol-gel Derived 58S Bioactive Glass Substituted by Ag and Zn. Silicon 2019, 11 (6), 2741–2751. [Google Scholar]
- (43).Manikandan V; Velmurugan P; Park J-H; Chang W-S; Park Y-J; Jayanthi P; Cho M; Oh B-T Green Synthesis of Silver Oxide Nanoparticles and Its Antibacterial Activity Against Dental Pathogens. 3 Biotech 2017, 7 (1), 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Kim MH; Park H; Nam HC; Park SR; Jung J-Y; Park WH Injectable Methylcellulose Hydrogel Containing Silver Oxide Nanoparticles for Burn Wound Healing. Carbohydrate polymers 2018, 181, 579–586. [DOI] [PubMed] [Google Scholar]
- (45).Fakhri A; Tahami S; Nejad PA Preparation and Characterization of Fe3O4- Quantum Dots Decorated Cellulose Nanofibers as A Carrier of Anticancer Drugs for Skin Cancer. Journal of Photochemistry and Photobiology B: Biology 2017, 175, 83–88. [DOI] [PubMed] [Google Scholar]
- (46).Vu AA; Robertson SF; Ke D; Bandyopadhyay A; Bose S. Mechanical and Biological Properties of ZnO, SiO2, and Ag2O Doped Plasma Sprayed Hydroxyapatite Coating for Orthopaedic and Dental Applications. Acta biomaterialia 2019, 92, 325–335. [DOI] [PubMed] [Google Scholar]
- (47).Hench LL; West JK The Sol-gel Process. Chemical reviews 1990, 90 (1), 33–72. [Google Scholar]
- (48).Ghimire PP; Jaroniec M. Renaissance of Stöber Method for Synthesis of Colloidal Particles: New Developments and Opportunities. Journal of Colloid and Interface Science 2020, 584, 838–865. [DOI] [PubMed] [Google Scholar]
- (49).Stöber W; Fink A; Bohn E. Controlled Growth of Monodisperse Silica Spheres in The Micron Size Range. Journal of colloid and interface science 1968, 26 (1), 62–69. [Google Scholar]
- (50).Chang Y-R; Taylor S; Duncan S; Mazilu DA; Ritter A; Ducker WA Fabrication of Stabilized Colloidal Crystal Monolayers. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2017, 514, 185–191. [Google Scholar]
- (51).Chen Y-J; Chiang Y-W; Huang MH Synthesis of Diverse Crystals and Their Facet-Dependent Photocatalytic Activity Examination. ACS Appl. Mater. Interfaces 2016, 8 (30), 19672–19679. [DOI] [PubMed] [Google Scholar]
- (52).Wang X; Wu H-F; Kuang Q; Huang R-B; Xie Z-X; Zheng L-S Shape-dependent Antibacterial Activities of Ag2O Polyhedral Particles. Langmuir 2010, 26 (4), 2774–2778. [DOI] [PubMed] [Google Scholar]
- (53).Rabani E; Reichman DR; Geissler PL; Brus LE Drying-mediated Self-assembly of Nanoparticles. Nature 2003, 426 (6964), 271–274. [DOI] [PubMed] [Google Scholar]
- (54).Grosso D; Cagnol F; Soler-Illia G. d. A.; Crepaldi EL; Amenitsch H; Brunet-Bruneau A; Bourgeois A; Sanchez C. Fundamentals of Mesostructuring Through Evaporation-induced Self-assembly. Advanced Functional Materials 2004, 14 (4), 309–322. [Google Scholar]
- (55).Malenovska H. Virus Quantitation by Transmission Electron Microscopy, TCID50, and the Role of Timing Virus Harvesting: A Case Study of Three Animal Viruses. J. Virol. Methods 2013, 191 (2), 136–140. [DOI] [PubMed] [Google Scholar]
- (56).Chan K; Lai S; Poon L; Guan Y; Yuen K; Peiris J. Analytical Sensitivity of Rapid Influenza Antigen Detection Tests for Swine-origin Influenza Virus (H1N1). J. Clin. Virol 2009, 45 (3), 205–207. [DOI] [PubMed] [Google Scholar]
- (57).Reed LJ; Muench H. A Simple Method of Estimating Fifty Percent Endpoints. American Journal of Epidemiology 1938, 27 (3), 493–497. [Google Scholar]
- (58).Interim Method for Evaluating the Efficacy of Antimicrobial Surface Coatings. EPA. 10/2/2020. https://www.epa.gov/pesticide-analytical-methods/antimicrobial-testing-methods-procedures-interim-method-evaluating (accessed August 25, 2021). [Google Scholar]
- (59).Britton H; Williams WG Electrometric Studies of The Precipitation of Hydroxides. Part XIII. The Constitution of Aqueous Solutions of Silver Oxide in Ammonia, mono-, di-, and tri-methylamine and-ethylamine, Pyridine And Ethylenediamine; with A Note on the Dissociation Constants of The Amines. Journal of the Chemical Society (Resumed) 1935, 796–801. [Google Scholar]
- (60).Johnston HL; Cuta F; Garrett AB The Solubility of Silver Oxide in Water, in Alkali And in Alkaline Salt Solutions. The Amphoteric Character of Silver Hydroxide. Journal of the American Chemical Society 1933, 55 (6), 2311–2325. [Google Scholar]
- (61).Allahverdiyev AM; Abamor ES; Bagirova M; Rafailovich M. Antimicrobial Effects of TiO2 And Ag2O Nanoparticles Against Drug-resistant Bacteria and Leishmania Parasites. Future microbiology 2011, 6 (8), 933–940. [DOI] [PubMed] [Google Scholar]
- (62).Negi H; Saravanan PR; Agarwal T; Zaidi MGH; Goel R. In Vitro Assessment of Nanoparticles Toxicity Against Gram-positive and Gram-negative Bacteria. The Journal of general and applied microbiology 2013, 59 (1), 83–88. [DOI] [PubMed] [Google Scholar]
- (63).Rokade AA; Patil MP; Yoo SI; Lee WK; Park SS Pure Green Chemical Approach for Synthesis of Nanoparticles. Green Chemistry Letters and Reviews 2016, 9 (4), 216–222. [Google Scholar]
- (64).Rajabi A; Ghazali MJ; Mahmoudi E; Baghdadi AH; Mohammad AW; Mustafah NM; Ohnmar H; Naicker AS Synthesis, Characterization, and Antibacterial Activity of Ag2O-loaded Polyethylene Terephthalate Fabric via Ultrasonic Method. Nanomaterials 2019, 9 (3), 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Bellantone M; Williams HD; Hench LL Broad-spectrum Bactericidal Activity of -doped Bioactive Glass. Antimicrobial agents and chemotherapy 2002, 46 (6), 1940–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Li D; Chen S; Zhang K; Gao N; Zhang M; Albasher G; Shi J; Wang C. The Interaction of Ag2O Nanoparticles with Escherichia coli: Inhibition–sterilization Process. Scientific reports 2021, 11 (1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Feitknecht W; Schindler P. Solubility Constants of Metal Oxides, Metal Hydroxides and Metal Hydroxide Salts in Aqueous Solution. Pure and Applied Chemistry 1963, 6 (2), 125–206. [Google Scholar]
- (68).Park H-J; Kim JY; Kim J; Lee J-H; Hahn J-S; Gu MB; Yoon J. Silver-ion-mediated Reactive Oxygen Species Generation Affecting Bactericidal Activity. Water research 2009, 43 (4), 1027–1032. [DOI] [PubMed] [Google Scholar]
- (69).Makvandi P; Wang C. y.; Zare EN; Borzacchiello A; Niu L. n.; Tay R. Metal-based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Advanced Functional Materials 2020, 30 (22), 1910021. [Google Scholar]
- (70).Minoshima M; Lu Y; Kimura T; Nakano R; Ishiguro H; Kubota Y; Hashimoto K; Sunada K. Comparison of The Antiviral Effect of Solid-State Copper and Silver Compounds. Journal of hazardous materials 2016, 312, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.