Abstract
Background:
Levonadifloxacin is a novel broad-spectrum antibiotic belonging to the benzoquinolizine subclass of quinolones. It is available in intravenous as well as oral formulation for the treatment of infections caused by common Gram-positive bacterial pathogens including methicillin-resistant Staphylococcus aureus (MRSA).
Patients and Methods:
This study retrospectively assessed the real-world safety and efficacy of levonadifloxacin (oral and/or IV) in the treatment of 1229 patients across various clinical conditions. Study outcomes were clinical and microbiological success at the end of therapy.
Results:
The mean duration of levonadifloxacin therapy was 7.2 days, with a time to clinical improvement averaging at 4 days. Three hundred and three patients received oral therapy, 875 received IV, and 51 received a combination of IV followed by oral therapy. Patients were prescribed levonadifloxacin for skin and soft-tissue infections, diabetic foot infections, septicemia, catheter-related bloodstream infections, bone and joint infections, febrile neutropenia, and respiratory infections including COVID-19 pneumonia. High clinical success rates of 98.3%, 93.7%, and 96.1% with oral, IV, and IV followed by oral levonadifloxacin, respectively, were obtained. Only 11 mild adverse events were reported in 9 patients which included constipation, diarrhea, hyperglycemia, nausea, fatigue, and vomiting. Overall, 96.3% and 97.3% of investigators rated the efficacy and safety of levonadifloxacin as “good to excellent.”
Conclusions:
An excellent safety and efficacy profile of levonadifloxacin was observed in this study making it a suitable treatment option for management of various bacterial infections, including those caused by resistant Gram-positive pathogens such as MRSA and quinolone-resistant S. aureus.
Keywords: Methicillin-resistant Staphylococcus aureus, levonadifloxacin, clinical success, bacterial infections
Introduction
Gram-positive organisms are among the most common bacterial causes of clinical infections. This is primarily due to their diverse spectrum of pathology, ranging from mild skin and soft-tissue infections to life-threatening systemic sepsis, pneumonia, bloodstream infections, and meningitis.[1] Although recent global attention has focused more on multidrug-resistant (MDR) Gram-negative infections, critical analysis of therapeutic options for MDR Gram-positive infections reveals glaring gaps in current therapies. The situation is compounded by increase in population of resistant pathogens. In India, high rates of methicillin-resistant Staphylococcus aureus (MRSA) have been reported in clinical isolates with rates as high as 54.8% (ranging between 32% and 80% among the S. aureus pool).[2] Resistant bacterial infections including MRSA are not only a major health issue but also a major cause of financial burden all over the world.
In the absence of better alternative treatment options, vancomycin has been used as a first-line antibiotic for MRSA infections. This antibiotic is marred with multiple drawbacks such as slow bactericidal activity and inability to tackle higher bacterial load at the infection site, commonly observed in pneumonia and bloodstream infections. Risk of emergence and spread of MRSA clones not susceptible to vancomycin mandates maintaining high trough levels which lead to collateral effect of nephrotoxicity.[3,4] Linezolid offers an oral treatment option, however, it is essentially bacteriostatic and therefore is not recommended to treat MRSA infections in bloodstream as well as in immune-suppressed patients. Teicoplanin, which is also a glycopeptide, is associated with vancomycin-like drawbacks.[5] Daptomycin, though bactericidal, is inactivated in the lungs, therefore, it is not approved for the treatment of pneumonia.[6] Moreover, all the above mentioned MRSA antibiotics do not provide coverage of common Gram-negative pathogens necessitating the use of add-on therapies for managing polymicrobial infections which are frequently encountered in acute bacterial skin and skin structure infections (ABSSSIs) and diabetic foot infections (DFIs).[7]
Levonadifloxacin displays high activity against Gram-positive organisms such as S. aureus (methicillin-resistant, methicillin-susceptible, quinolone-resistant, and quinolone-susceptible), Streptococcus pyogenes, Enterococcus faecalis, and Streptococcus dysgalactiae spp. dysgalactiae. Levonadifloxacin has therapeutic potential against respiratory pathogens such as Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, quinolone-susceptible Gram-negative bacteria, and atypical bacteria.[8] In addition, levonadifloxacin has an excellent safety profile and does not require therapeutic drug monitoring or dose modifications in renal or hepatic impaired patients.
Levonadifloxacin received marketing authorization based on multiple phase 1, phase 2 and a single phase 3 study. Although well-designed and randomized phase 2 and phase 3 studies serve well to include patients with specific infection caused by target pathogens, controlled studies preclude the inclusion of real-world patients, who might not meet the inclusion criteria, but still present considerable diversity in terms of infection source, immunity status, comorbidities, etc., Therefore, evidence of safety and efficacy from real-world patients through postmarketing observational studies enriches the evolving clinical role of a novel antibiotic. Keeping these objectives in mind, the postmarketing, retrospective, multicentric, observational study was designed to capture the performance of levonadifloxacin in real-world patients.
Patients and Methods
Setting
Data were gathered from 117 hospitals across India as part of a large multicenter, retrospective, postmarketing, real-world, observational study (PIONEER study) that was conducted for assessment of safety and efficacy of levonadifloxacin used clinically in the therapy of varied bacterial infections.
Informed consent and ethics
As a part of the prescription-event monitoring study, data were collected from 1229 patients who received levonadifloxacin as therapy for varied bacterial infections. The study was conducted in accordance with the principles of the Declaration of Helsinki (World Medical Association)[9] and Good Clinical Practice guidelines issued by the ICMR and CDSCO, Government of India, and was registered with the Clinical Trials Registry of India (CTRI/2020/09/028152). The study documents were reviewed and approved by the Institutional Ethics Committee (IEC) of D Y Patil University School of Medicine, Navi Mumbai (DYP/IEC/06-019/2020). This being a retrospective study, patient consent was not mandatory, however, the investigators were informed to obtain informed consent wherever possible/applicable.
Study participants
Data of 1229 patients of any gender above 17 years of age who received levonadifloxacin (oral or injectable) were included in the study. A clinical diagnosis of bacterial infection was based on clinical and microbiology test results. Data collected were recorded in a study-specific data capture tool from 177 participating sites which included clinical condition on admission, comorbidities, preexisting complications, and concomitant therapy. Microbial testing data were collected where available and clinical therapy was administered at the discretion of a treating physician.
Study outcomes
The study outcomes were assessed as clinical and microbiological success at the completion of therapy. Clinical success was defined as resolution or improvement in signs and symptoms without the need of additional antimicrobial therapy, whereas persistence or worsening of signs/symptoms, the need for additional antimicrobial agents, occurrence of new infection, or death was considered clinical failure. Microbiological success was defined as the absence of organisms at follow-up microbial testing in those patients where organisms were detected at baseline or a subsequent negative culture during a follow-up microbial testing. Safety of treatment was assessed using the clinical and laboratory adverse events documentation, and investigators ranked therapy with levonadifloxacin on a global assessment for efficacy and safety based on a 5-point Likert scale of excellent, very good, good, satisfactory, and poor.
Statistical analysis
This being an observational study, there was no study hypothesis or statistical testing. Data were entered in Microsoft Office Excel Worksheet. A descriptive representation of demography and study outcome is presented along with measurement data as mean and standard deviation (SD), and categorical data as percentages.
Results
Patient characteristics and pretreatment data
Of the 1229 patients, 881 (71.7%) were males and 347 (28.2%) were females and gender was not specified for one patient. The mean age was 58.51 years (ranging between 17 and 89 years). Table 1 represents the demography, duration, and various indications for the use of levonadifloxacin therapy in patients. Most of the patients (n = 875, 71.2%) received IV levonadifloxacin, whereas 303 (24.7%) patients received oral therapy and 51 (4.1%) received IV therapy followed by switchover to oral levonadifloxacin. Levonadifloxacin was started empirically either as monotherapy or in combination with other antimicrobial agents. One thousand and forty-six (85.1%) patients were hospitalized, whereas 183 (14.9%) patients were treated on an outpatient basis, and the most common comorbid conditions were diabetes (18.3%) and hypertension (11.2%). Other comorbidities were malignancy (2.2%), renal disorders (2.8%), ischemic heart disease (2.3%), respiratory disorders (1.6%), thyroid disorders (2.6%), and hepatic disorders (0.9%). Lower respiratory tract infections (27.5%) and ABSSSI (18.5%) were the most common indications for the use of levonadifloxacin.
Table 1.
Demography of patients, duration, and indications for levonadifloxacin therapy
| IV, n (%) | Oral, n (%) | IV followed by oral, n (%) | Total, n (%) | |
|---|---|---|---|---|
| n | 875 | 303 | 51 | 1229 |
| Age (years) | ||||
| Mean (SD) | 59.16 (12.56) | 56.45 (13.54) | 59.61 (14.36) | 58.51 (12.93) |
| Median (range) | 60.00 (19-89) | 58.00 (17-88) | 60.00 (21-85) | 60.00 (17-89) |
| BMI (kg/m2) | ||||
| Mean (SD) | 25.50 (4.35) | 25.94 (4.36) | 25.77 (3.59) | 25.62 (4.32) |
| Median (range) | 25.10 (13.11-46.87) | 24.98 (15.78-46.87) | 25.15 (18.52-34.01) | 25.10 (13.11-46.87) |
| Duration of therapy (days) | ||||
| Mean (SD) | 6.85 (3.01) | 7.38 (2.92) | 12.14 (4.77) | 7.20 (3.22) |
| Median (range) | 6.00 (1-41) | 7.00 (2-37) | 12.00 (5-31) | 7.00 (1-41) |
| Gender | ||||
| Male | 645 (73.7) | 202 (66.7) | 34 (66.7) | 881 (71.7) |
| Female | 229 (26.2) | 101.00 (33.3) | 17.00 (33.3) | 347 (28.2) |
| Transgender | 1.00 (0.1) | 0 | 0 | 1 (0.1) |
| Indications for levonadifloxacin | ||||
| ABSSSI | 140 (16.0) | 76 (25.1) | 11 (21.6) | 227 (18.5) |
| DFI | 58 (6.6) | 42 (13.9) | 3 (5.9) | 103 (8.4) |
| Septicemia | 125 (14.3) | 25 (8.3) | 10 (19.6) | 160 (13.0) |
| LRTI (non-COVID-19) | 248 (28.3) | 79 (26.1) | 11 (21.6) | 338 (27.5) |
| COVID-19 pneumonia | 119 (13.6) | 24 (7.9) | 11 (21.6) | 154 (12.5) |
| CRBSI | 11 (1.3) | 9 (3.0) | 0 | 20 (1.6) |
| Febrile neutropenia | 11 (1.3) | 5 (1.7) | 0 | 16 (1.3) |
| Gram-positive infections | 63 (7.2) | 15 (5.0) | 0 | 78 (6.3) |
| BJI | 6 (0.7) | 4 (1.3) | 1 (2.0) | 11 (0.9) |
| Others | 94 (10.7) | 24 (7.9) | 4 (7.8) | 122 (9.9) |
SD: Standard deviation; ABSSSI: Acute bacterial skin and soft-tissue infection; BMI: Body mass index; BJI: Bone and joint infection; CRBSI: Catheter-related bloodstream infection; DFI: Diabetic foot infection; LRTI: Lower respiratory tract infection; IV: Intravenous; COVID-19: Coronavirus disease 2019
Table 2a presents the different systems involved in bacterial infection and preexisting complications in patients who were prescribed levonadifloxacin. Preexisting complications were reported in 553 (49.0%) patients, with renal impairment and septic shock being the most common complication (17.5% each), and systemic inflammatory response syndrome in 12.5% of patients. Other preexisting complications included multi-organ failure (9.9%), hepatic impairment (5.5%), and thrombocytopenia (5.5%). Culture report was positive in 71.8% of patients, with only 18.2% of cultures reported negative for bacterial growth. Gram-positive infections (54.3%) were more common than Gram-negative (26.1%) and mixed (12.7%) infections [Table 2b].
Table 2a.
Preexisting system involvement and complications on admission (n=1229)
| n (%) | |
|---|---|
| Systems involved | |
| Abdominal | 101 (8.22) |
| Respiratory | 651 (52.97) |
| Cardiovascular | 72 (5.86) |
| Skin/soft tissue | 372 (30.27) |
| Pelvic | 32 (2.60) |
| Neurological/meningeal | 45 (3.66) |
| Retroperitoneal | 5 (0.41) |
| Other systems involved | 101 (8.22) |
| Complications | |
| Complications of infection | 553 (45.00) |
| SIRS | 153 (12.45) |
| Septic shock | 215 (17.49) |
| Multi-organ failure | 122 (9.93) |
| Renal impairment | 215 (17.49) |
| Hepatic impairment | 68 (5.53) |
| Thrombocytopenia | 68 (5.53) |
| Other complications | 33 (2.69) |
SIRS: Systemic inflammatory response syndrome
Table 2b.
Microbiological culture data (n=857)
| Organisms detected | n (%) |
|---|---|
| Gram-positive | 465 (54.26) |
| Gram-negative | 224 (26.14) |
| Atypical organisms | 64 (7.47) |
| Anaerobic organisms | 55 (6.42) |
| Polymicrobial | 109 (12.72) |
| Negative culture | 156 (18.20) |
Concomitant antimicrobial agents used in patients receiving levonadifloxacin are presented in Table 3. Meropenem/imipenem/carbapenem was the most commonly prescribed antibiotic in 32.5% of patients, followed by other beta-lactam antibiotics (9.1%). Concomitant drugs other than antimicrobial agents used were oral hypoglycemic agents (10.3%), ACE-inhibitors/Angiotensin Receptor Blockers (ARBs) (4.1%), insulin (3.3%), heparin (3.0%), and other drugs (14.5%).
Table 3.
Anti-microbial agents used as concomitant therapy
| Levonadifloxacin - IV (n=875), n (%) | Levonadifloxacin - oral (n=303), n (%) | Levonadifloxacin - IV followed by oral (n=51), n (%) | Total (n=1229), n (%) | |
|---|---|---|---|---|
| Antitubercular therapy | 1 (0.1) | 0 | 0 | 1 (0.1) |
| Antifungal | 11 (1.3) | 6 (2.0) | 0 | 17 (1.4) |
| Beta-lactams | 69 (7.9) | 35 (11.6) | 8 (15.7) | 112 (9.1) |
| Meropenem/imipenem/carbapenem | 312 (35.7) | 76 (25.1) | 12 (23.5) | 400 (32.5) |
| Glycopeptides | 2 (0.2) | 0 | 0 | 2 (0.2) |
| Remdesivir | 47 (5.4) | 8 (2.6) | 3 (5.9) | 58 (4.7) |
| Polypeptides | 33 (3.8) | 0 | 0 | 33 (2.7) |
| Macrolides | 7 (0.8) | 1 (0.3) | 0 | 8 (0.7) |
| Quinolones | 4 (0.5) | 3 (1.0) | 0 | 7 (0.6) |
| Tigecycline | 4 (0.5) | 0 (0.0) | 0 | 4 (0.3) |
| Aminoglycosides | 17 (1.9) | 5 (1.7) | 3 (5.9) | 25 (2.0) |
| Linezolid | 6 (0.7) | 9 (3.0) | 0 | 15 (1.2) |
| ART | 1 (0.1) | 0 | 0 | 1 (0.1) |
| Other drugs | 137 (15.7) | 55 (18.2) | 12 (23.5) | 204 (16.6) |
ART: Antiretroviral therapy; IV: Intravenous
Clinical and microbiological outcome
Table 4 represents clinical success at the end of therapy. More than 93.7% of patients showed clinical success at completion of therapy with IV levonadifloxacin, with a 98.3% success rate with oral levonadifloxacin. For varied indications and types of infections, the clinical success rates were from 89.3% to 100.0%. It is noteworthy that the clinical success rates for MRSA and methicillin-susceptible S. aureus (MSSA) were 96.1% and 100.0%, respectively. The microbiological success rate was 97% in patients treated with levonadifloxacin. The mean time to clinical improvement was 3.89 days, 4.03 days, and 3.86 days with IV therapy, oral therapy, and IV followed by oral therapy, respectively. The median time to improvement was 4 days (range: 1–15 days) of levonadifloxacin therapy.
Table 4.
Clinical success at the end of treatment
| Clinical success | ||
|---|---|---|
|
| ||
| n | n (%) | |
| Route of administration | ||
| IV | 875 | 820 (93.7) |
| Oral | 303 | 298 (98.3) |
| IV followed by oral | 51 | 49 (96.1) |
| All | 1229 | 1167 (95.0) |
| Indication for levonadifloxacin use | ||
| ABSSSI | 227 | 223 (98.2) |
| DFI | 103 | 98 (95.1) |
| Septicemia | 160 | 147 (91.9) |
| LRTI (non-COVID-19) | 338 | 322 (95.3) |
| COVID-19 pneumonia | 154 | 149 (96.8) |
| CRBSI | 20 | 18 (90.0) |
| Febrile neutropenia | 16 | 15 (93.8) |
| Gram-positive infections | 78 | 75 (96.2) |
| BJI | 11 | 11 (100.0) |
| Others | 122 | 109 (89.3) |
| All indications | 1229 | 1167 (95.0) |
ABSSSI: Acute bacterial skin and soft-tissue infection; BJI: Bone and joint infection; CRBSI: Catheter-related bloodstream infection; DFI: Diabetic foot infection; LRTI: Lower respiratory tract infection; IV: Intravenous; COVID-19: Coronavirus disease 2019
Global assessments
Figure 1 presents the global assessments for efficacy and safety at the end of therapy. Overall, investigators rated the global efficacy as “good to excellent” in 96.3% of patients, and “satisfactory” in 3.7% of patients. For global safety, investigators rated the safety as “good to excellent” in 97.3% of patients and “satisfactory” in 2.3% of patients.
Figure 1.
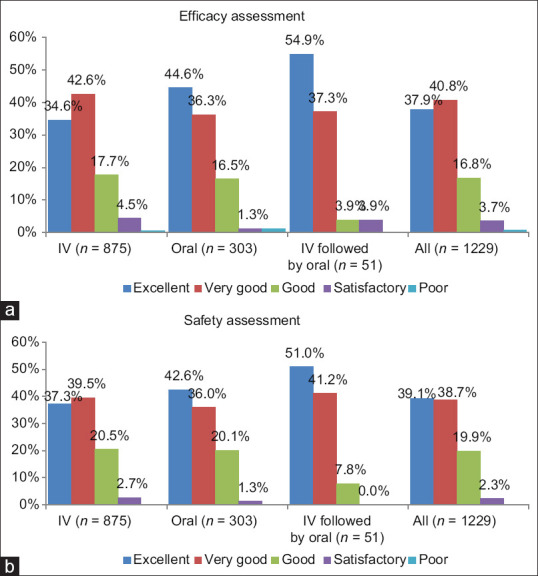
Global efficacy (a) and safety (b) assessment at the end of therapy (%, n = 1229). (a) Efficacy assessment at the end of therapy made by a treating investigator for IV levonadifloxacin, oral levonadifloxacin, and IV followed by oral levonadifloxacin and all patients based on five-point scale of excellent, very good, good, satisfactory, and poor. (b) Safety assessment at the end of therapy made by a treating investigator for IV levonadifloxacin, oral levonadifloxacin, and IV followed by oral levonadifloxacin and all patients based on five-point scale of excellent, very good, good, satisfactory, and poor
Safety
There were only 11 adverse events reported in 9 patients, 2 on IV therapy and 7 on oral therapy. The events reported were constipation (n = 2), diarrhea (n = 2), hyperglycemia (n = 1), nausea (n = 4), fatigue (n = 1), and vomiting (n = 1). All events were of mild severity. There were no serious adverse events reported in patient records.
Discussion
This postmarketing study gathered real-world evidence for safety and efficacy of levonadifloxacin in bacterial infections across multiple indications. The patients were not controlled using predefined inclusion/exclusion criteria and were treated with levonadifloxacin based on a clinician's judgment pertaining to severity of infection, prior experience with other antimicrobials as well as local etiology and resistance rates. Levonadifloxacin showed high efficacy and received remarkable safety ratings in this study reflecting the safety and efficacy noted previously in a wide range of preclinical and clinical studies.
Although levonadifloxacin is active against Gram-positive bacteria, it demonstrates potent activity against S. aureus, including the resistant strains such as MRSA, quinolone-resistant S. aureus (QRSA), vancomycin-intermediate S. aureus, and vancomycin-resistant S. aureus.[10] This is attributable to its unique mechanism of action as compared to other fluoroquinolones, mainly preferential targeting of DNA gyrase (gyrA).[11] Furthermore, levonadifloxacin has a potent cidal activity against MRSA/MSSA intracellularly which was tested in THP-1 macrophages, and hence, both oral and IV levonadifloxacin can offer enhanced therapeutic benefit in treating persistent MRSA infections.[12] In a relatively recent in vitro study, levonadifloxacin showed potent inhibition (MIC50/90:0.25/0.5 mg/L) of contemporary S. aureus isolates collected from a large Indian tertiary care hospital.[13] After a successful Phase 3 clinical trial in India, IV and oral formulations of levonadifloxacin were approved for the indication of ABSSSI including DFI with concurrent bacteremia. This was due to comparable clinical cure rates seen in Phase 3 trial with linezolid when administered to patients suffering from ABSSSI. The clinical cure rates observed in the modified-intention-to-treat (mITT) populations were 91.0% (87.8% with linezolid) with IV and 95.2% (93.6% with linezolid) with oral treatment.[14] Interestingly, the PIONEER study showed similar clinical success rates for treatment of ABSSSI with levonadifloxacin therapy (oral/IV). Impressive clinical success rates were observed in DFI and bone and joint infection (BJI) patients as well. These infections are generally characterized by biofilm formation and presence of high density of bacteria at the site of infection and/or on implanted medical devices.[15] Early intervention with appropriate targeted therapy is a prerequisite to tackle such infections. Previous studies have shown that levonadifloxacin is bactericidal to biofilm-embedded MRSA reducing the viable count and effectively eradicating biofilms.[16] Furthermore, differential mechanism of action with preferential DNA gyrase inhibition, not being a substrate for the NorA efflux pump, having a rapid bactericidal action against high-inoculum cultures and slow-growing staphylococci, contribute toward its resistance suppression features. In addition, antimicrobial coverage against atypical and anaerobic pathogens is therapeutically beneficial in treatment of DFI.
In the current study, levonadifloxacin was successfully used for the treatment of several other indications such as pneumonia, BJIs, and febrile neutropenia. Potent in vitro activity of levonadifloxacin has been demonstrated against pathogens commonly associated with community-acquired pneumonia. Moreover, pulmonary pharmacokinetic study showed a remarkable penetration of levonadifloxacin into epithelial lining fluid and alveolar macrophages (7.7 and 2 times the unbound plasma concentration, respectively), indicating the clinical utility of levonadifloxacin in the treatment of respiratory infections caused by extracellular and intracellular pathogens.[17] Owing to dysregulated immune response, secondary pulmonary infections involving Gram-positive and Gram-negative pathogens are commonly seen in COVID-19 patients causing higher mortality.[18] In this study, levonadifloxacin showed promising results when used as IV and/or oral therapy for treatment of secondary bacterial pneumonia in COVID-19 patients displaying clinical success rates of up to 96.8%.
Safety of levonadifloxacin is well documented and no serious adverse events were reported in this study. Being devoid of potential adverse effects such as phototoxicity, prolongation of QT interval, hepatotoxicity, and nephrotoxicity in combination with broad-spectrum activity, levonadifloxacin offers a suitable therapeutic option for management of complex and serious bacterial infections. This study provides evidence on utility of levonadifloxacin in diverse clinical conditions across centers in India, however, it has a few limitations. As this was an observational study, there was lack of control on different confounding factors and study monitoring. Secondly, this retrospective study might have limited applicability, and a prospective study should be carried out.
Conclusions
The excellent safety and efficacy profile of levonadifloxacin makes it a desirable treatment modality for management of various bacterial infections, including those caused by resistant pathogens such as MRSA and QRSA. Features of levonadifloxacin such as availability of both IV and oral form, minimal drug–drug interactions, and lack of need to adjust dosages in renal and hepatic impaired patients along with a broad spectrum of coverage, make it a suitable agent that attends to several unmet clinical needs of physicians.
Ethical clearance
The study documents were reviewed and approved by the Institutional Ethics Committee (IEC) of D Y Patil University School of Medicine, Navi Mumbai (DYP/IEC/06-019/2020).
Trial registration no
CTRI/2020/09/028 152 (Registered on: September 30, 2020).
Financial support and sponsorship
This study was sponsored by Wockhardt Ltd.
Conflicts of interest
Nil.
Acknowledgements
The authors would like to thank all the investigators for their contribution in the conduct of the study. We would also like to acknowledge efforts of Mr. Amber Misra, Mr. Nitin Bhadana, Mr. Suraj Tewari & Mr. Lyndon D'souza in data collection and manuscript writing.
References
- 1.Menichetti F. Current and emerging serious Gram-positive infections. Clin Microbiol Infect. 2005;11(Suppl 3):22–8. doi: 10.1111/j.1469-0691.2005.01138.x. [DOI] [PubMed] [Google Scholar]
- 2.Laxminarayan R, Chaudhury RR. Antibiotic resistance in India: Drivers and opportunities for action. PLoS Med. 2016;13:e1001974. doi: 10.1371/journal.pmed.1001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro JF, Hahn SR, Gonçalves J, Fresco P. Vancomycin therapeutic drug monitoring and population pharmacokinetic models in special patient subpopulations. Pharmacol Res Perspect. 2018;6:e00420. doi: 10.1002/prp2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamoner W, Prado IR, Balbi AL, Ponce D. Vancomycin dosing, monitoring and toxicity: Critical review of the clinical practice. Clin Exp Pharmacol Physiol. 2019;46:292–301. doi: 10.1111/1440-1681.13066. [DOI] [PubMed] [Google Scholar]
- 5.Svetitsky S, Leibovici L, Paul M. Comparative efficacy and safety of vancomycin versus teicoplanin: Systematic review and meta-analysis. Antimicrob Agents Chemother. 2009;53:4069–79. doi: 10.1128/AAC.00341-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman JA, Mortin LI, Vanpraagh AD, Li T, Alder J. Inhibition of daptomycin by pulmonary surfactant: In vitro modeling and clinical impact. J Infect Dis. 2005;191:2149–52. doi: 10.1086/430352. [DOI] [PubMed] [Google Scholar]
- 7.Esposito S, Ascione T, Pagliano P. Management of bacterial skin and skin structure infections with polymicrobial etiology. Expert Rev Anti Infect Ther. 2019;17:17–25. doi: 10.1080/14787210.2019.1552518. [DOI] [PubMed] [Google Scholar]
- 8.Bhagwat SS, Nandanwar M, Kansagara A, Patel A, Takalkar S, Chavan R, et al. Levonadifloxacin, a novel broad-spectrum anti-MRSA benzoquinolizine quinolone agent: Review of current evidence. Drug Des Devel Ther. 2019;13:4351–65. doi: 10.2147/DDDT.S229882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association. France: The World Medical Association; 2018. [Last accessed on 2021 Sep 12]. WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. Available from: https://www.wma.net/policies-post/wma-declaration-of-helsinkiethical-principles-for-medical-research-involving-human-subjects . [Google Scholar]
- 10.Bakthavatchalam YD, Rao SV, Isaac B, Manesh A, Nambi S, Swaminathan S, et al. A comparative assessment of clinical pharmacological and antimicrobial profile of novel anti-methicillin-resistant Staphylococcus aureus agent levonadifloxacin. Indian J Med Microbiol. 2019;37:478–87. doi: 10.4103/ijmm.IJMM_20_34. [DOI] [PubMed] [Google Scholar]
- 11.Bhagwat SS, Mundkur LA, Gupte SV, Patel MV, Khorakiwala HF. The anti-methicillin-resistant Staphylococcus aureus quinolone WCK 771 has potent activity against sequentially selected mutants, has a narrow mutant selection window against quinolone-resistant Staphylococcus aureus, and preferentially targets DNA gyrase. Antimicrob Agents Chemother. 2006;50:3568–79. doi: 10.1128/AAC.00641-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois J, Dubois M. Levonadifloxacin (WCK 771) exerts potent intracellular activity against Staphylococcus aureus in THP-1 monocytes at clinically relevant concentrations. J Med Microbiol. 2019;68:1716–22. doi: 10.1099/jmm.0.001102. [DOI] [PubMed] [Google Scholar]
- 13.Bakthavatchalam YD, Shankar A, Muniyasamy R, Peter JV, Marcus Z, Triplicane Dwarakanathan H, et al. Levonadifloxacin, a recently approved benzoquinolizine fluoroquinolone, exhibits potent in vitro activity against contemporary Staphylococcus aureus isolates and Bengal Bay clone isolates collected from a large Indian tertiary care hospital. J Antimicrob Chemother. 2020;75:2156–9. doi: 10.1093/jac/dkaa142. [DOI] [PubMed] [Google Scholar]
- 14.Bhatia A, Mastim M, Shah M, Gutte R, Joshi P, Kumbhar D, et al. Efficacy and safety of a novel broad-spectrum Anti-MRSA agent levonadifloxacin compared with linezolid for acute bacterial skin and skin structure infections: A phase 3, openlabel, randomized study. J Assoc Physicians India. 2020;68:30–6. [PubMed] [Google Scholar]
- 15.Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4:e01067. doi: 10.1016/j.heliyon.2018.e01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tellis M, Joseph J, Khande H, Bhagwat S, Patel M. In vitro bactericidal activity of levonadifloxacin (WCK 771) against methicillin-and quinolone-resistant Staphylococcus aureus biofilms. J Med Microbiol. 2019;68:1129–36. doi: 10.1099/jmm.0.000999. [DOI] [PubMed] [Google Scholar]
- 17.Rodvold KA, Gotfried MH, Chugh R, Gupta M, Yeole R, Patel A, et al. Intrapulmonary pharmacokinetics of levonadifloxacin following oral administration of alalevonadifloxacin to healthy adult subjects. Antimicrob Agents Chemother. 2018;62:e02297–17. doi: 10.1128/AAC.02297-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Zhang Y, Wu J, Li Y, Zhou X, Li X, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9:1958–64. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]


