Abstract
Polysaccharides are macromolecular complexes that have various biological activities. In vivo and in vitro studies have shown that polysaccharides play neuroprotective roles through multiple mechanisms; consequently, they have potential in the prevention and treatment of neurodegenerative diseases. This paper summarizes related research published during 2015–2020 and reviews advances in the understanding of the neuroprotective effects of bioactive polysaccharides. This review focuses on 15 bioactive polysaccharides from plants and fungi that have neuroprotective properties against oxidative stress, apoptosis, neuroinflammation, and excitatory amino acid toxicity mainly through the regulation of nuclear factor kappa-B, phosphatidylinositol-3-kinase/protein kinase B, mitogen-activated protein kinase, nuclear factor-E2-related factor 2/ hemeoxygenase-1, c-jun N-terminal kinase, protein kinase B-mammalian target of rapamycin, and reactive oxygen species-nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 3 signaling pathways. Natural bioactive polysaccharides have potential in the prevention and treatment of neurodegenerative diseases because of their advantageous characteristics, including multi-targeting, low toxicity, and synergistic effects. However, most of the recent related research has focused on cell and animal models. Future randomized clinical trials involving large sample sizes are needed to validate the therapeutic benefits of these neuroprotective polysaccharides in patients having neurodegenerative diseases.
Key Words: Alzheimer's disease, apoptosis, experimental research, neurodegeneration, neuroinflammation, neuroprotective, oxidative stress, Parkinson's disease, polysaccharides, protective mechanisms
Introduction
Neurodegenerative diseases (NDDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), and motor neuron disease, form a prominent class of progressive degenerative conditions characterized by the functional deterioration and ultimate loss of neurons. NDDs seriously affect human health and result in high rates of disability and mortality. Aging is a leading risk factor for many NDDs (Ciccocioppo et al., 2020; Popa-Wagner et al., 2020; Xu et al., 2021). The overall NDD incidence gradually increases along with age (Hou et al., 2019). Millions of people worldwide suffer from NDDs, and they have become a global public health issue as life expectancy increases. At present, pharmacological therapy is the most prevalent treatment strategy against NDDs. Unfortunately, most of the currently prescribed drugs only partially relieve the clinical symptoms and fail to prevent or alleviate the occurrence and progression of the disease. They also produce negative side effects and limitations. Therefore, it is necessary to discover and develop novel disease-modifying therapeutic candidates with better safety and efficacy profiles. NDDs are characterized by complex etiologies, diverse clinical manifestations, and large individual differences, but the progressive loss of specific neurons is a basic feature of NDDs (Fu et al., 2018). Various mechanisms, such as oxidative stress, neuroinflammation, excitatory amino acid toxicity, apoptosis, and autophagic dysfunction, are involved in the pathological processes of NDDs (Barnham et al., 2004; Li et al., 2008; Perry and Holmes, 2014). Therefore, neuronal protection through multiple mechanisms is the key to the prevention and treatment of NDDs. Numerous studies have shown that naturally sourced neuroprotective agents play important roles in the prevention and treatment of NDDs. Various bioactive compounds or components extracted from traditional Chinese medicines have shown neuroprotective activities and are effective against AD (Li et al., 2021) and amyotrophic lateral sclerosis (ALS) (Zhang et al., 2014a, b; Shang et al., 2020). Bioactive polysaccharides, as the main active ingredients of most herbal extracts, may serve as valuable natural sources for pharmaceuticals, and they have attracted attention, owing to their safety, non-toxicity, multi-targeting bioactivities, and low costs. Natural polysaccharides play important roles as autophagy modulators in the fight against AD, PD, ALS, and other NDDs (Wang et al., 2021). Modern pharmacological studies have found that polysaccharides extracted from marine organisms and their derivatives are also good therapeutic candidates against AD (Rathnayake et al., 2019). Recent studies found that Lycium barbarum polysaccharides exert neuroprotective effects and are beneficial for the treatment of AD, PD, and other NDDs through antioxidant, neuroimmunity, anti-apoptotic, autophagy, and other mechanisms (Xing et al., 2016). Acemannan, the main bioactive polysaccharide of Aloe vera, is a neuroprotective immunomodulator and antioxidant, and it improves the cognitive performances of middle-aged patients suffering from mental fatigue (Liu et al., 2019). In addition, polysaccharides extracted from plants, fungi, and animals, such as ginseng polysaccharides, Astragalus polysaccharides, lentinan, and fucoidan, have been widely used in the medical field in polysaccharide-based drugs (Yu et al., 2018).
Thus, polysaccharides, as effective substances for the treatment of NDDs, are the focus of drug research and development. Consequently, great progress has been made in the isolation and extraction of polysaccharides and in understanding their neuroprotective effects. Here, we review newly discovered neuroprotective polysaccharides. We have summarized recent research progress on neuroprotective polysaccharides and their properties, and we discuss possible underlying action mechanisms that may provide a basis for the research and development of new therapeutic strategies against NDDs.
Retrieval Strategy
This is an extensive review of the literature on the beneficial roles of polysaccharides in different models of neurodegeneration. We searched PubMed and Google Scholar to retrieve studies using the following search strategies: [polysaccharide (MeSH Terms) AND neuroprotective (MeSH Terms)] OR oxidative stress (MeSH Terms) OR apoptosis (MeSH Terms) OR neuroinflammation (MeSH Terms) OR excitatory amino acid toxicity (MeSH Terms) OR neurodegenerative diseases (MeSH Terms). Articles published from 2015 to 2020 were included. The reviewer XLX selected articles by reading titles and abstracts. Reference lists of articles were also reviewed for additional relevant studies. Related studies conducted on any species were included, and non-scientific experiments or review articles were excluded.
Bioactive Polysaccharides with Antioxidative Stress Activities
Reactive oxygen species (ROS) are by-products of metabolism that are involved in the degeneration of nerve cells by regulating the functions of different biological molecules (DNA, RNA, lipids, and proteins). The overproduction of ROS induced by oxidative stress can lead to the destruction of biomolecules and cellular structures, eventually leading to neuronal death (Gao et al., 2018). Recent studies have provided clear evidence for the neuroprotective effects of bioactive polysaccharides against oxidative stress. In vitro studies have demonstrated that bioactive polysaccharides exhibit potent reducing powers, total antioxidant capacities, and free radical-scavenging abilities. Some bioactive polysaccharides reduce the levels of ROS and associated peroxidation products in cellular and animal models under oxidative stress conditions. Moreover, bioactive polysaccharides counteract oxidative damage by enhancing the activities of multiple antioxidant enzymes, adjusting gene expression and regulation, and regulating stress-related signaling events.
Annona muricata L. polysaccharide
Annona muricata, also known as Soursop, Graviola, and Guanabana, mainly exists in the tropical and subtropical regions of the world. It is a fruit tree that has long been used in traditional medicines. The fruits, seeds, and leaves of A. muricata are widely used to treat a variety of diseases (Abdul Wahab et al., 2018). Annona muricata L. polysaccharide (ALP0 is a polysaccharide component extracted from A. muricata leaves that consists of galactose (64.3%), glucose (25.37%), mannitose (9.81%), caramelized sugar (0.51%), glucosamine (0.93%), and galactosamine (0.06%). In addition, the neuroprotective effects of Annona muricata L. Polysaccharide (ALP) were first investigated using an in vitro oxidative stress model of hippocampal neurons. Kim et al. (2020) reported that ALP protects a hippocampal neuronal cell line (HT22) from H2O2-induced oxidative stress by directly inhibiting H2O2-induced ROS production, thereby inhibiting excessive MAPK and NF-κB signaling and activating PI3K/Akt-mediated activation of Nrf2 signaling. These findings suggest that ALP may be the basis for new candidate drugs in the treatment of NDDs.
Dictyophora echinovolvata polysaccharide
Dictyophora echinovolvata is an edible fungus that has been grown and consumed in China for more than a thousand years. The anti-tumor, anti-proliferation, anti-oxidation, and other biological activities of D. echinovolvata have been verified recently in different models. Yu et al. (2017) investigated the neuroprotective effects of Dictyophora echinovolvata polysaccharide (DEVP) and its possible mechanisms using a H2O2-induced PC12 cell model. They found that DEVP greatly inhibits H2O2-induced cellular neurotoxicity and reverses H2O2-induced cell morphological changes and intracellular ROS aggregation. In addition, the altered expression levels of Bax, cleaved caspase-3, cytochrome C, and Bcl-2 proteins further indicate the neuroprotective effects of DEVP through the inhibition of the mitochondrial apoptotic pathway. Thus, DEVP may be a potential candidate that prevents NDDs by combating oxidative stress and apoptosis.
Apios americana Medik flower polysaccharide
Apios americana Medik is an herb native to North America that is widely cultivated in the USA and Japan. Its nutritious tuber is a staple food of indigenous North American people (Kim et al., 2017). Previous studies mainly reported the biological activities of A. americana Medik tuber extracts (Sohn et al., 2015; Kaneta et al., 2016). Apios americana Medik flower polysaccharide (AFP-2), a purified polysaccharide, was extracted from flowers of A. americana Medik to investigate its beneficial effects on health (Chu et al., 2019). In vitro experiments showed that AFP-2 activates the intracellular antioxidant system through the Sirtl/Nrf2 signaling pathway, and it effectively reduces the accumulation of ROS and mitochondrial dysfunction caused by H2O2. At the molecular level, autophagy was found to be involved in the antioxidant process of AFP-2 in H2O2-induced PC12 cells. Further studies showed that AFP-2 activates autophagy by inhibiting the phosphorylation of the Akt-mTOR pathway. In conclusion, AFP-2 is an effective antioxidant with potential neuroprotective effects that may be applied to treat NDDs.
Perilla frutescens polysaccharide
Perilla frutescens is a traditional medicine commonly used in Southeast Asia that has therapeutic effects on many diseases. Perilla frutescens leaves, which are rich in proteins and vitamins, are highly valued for consumption and in the medical field. Polysaccharides are important bioactive components of Perilla leaves. Polysaccharide extracts isolated from P. frutescens (PEPF) exert neuroprotective effects against H2O2-induced oxidative stress in an HT22 hippocampal neuronal cell line (Byun et al., 2018). PEPF protects nerve cells against H2O2-induced oxidative damage by reducing the ROS-mediated increased intracellular Ca2+level, upregulating HO-1 protein expression, and inhibiting caspase-3. The neuroprotective effect of PEPF on HT22 hippocampal cells was achieved by activating MAPK and NF-κB and negatively regulating the PI3K/Akt pathway. Moreover, the up-regulation of Nrf2-mediated HO-1/NQO1 pathways plays important roles in the inhibitory effect of PEPF against H2O2-induced neurotoxicity. Thus, PEPF may be a promising candidate for the treatment of NDDs, and it is worthy of further investigations.
Amanita caesarea polysaccharide
Amanita caesarea is a fungi of the Amanita genus that is distributed in the Sichuan and Yunnan provinces of China. Zhu et al. (2016) isolated a water-soluble heterosaccharide (AC-1) from the fruitbody of A. caesarea, characterized its chemical structure, and evaluated its antioxidant activity. AC-1 is mainly composed of α-D-glucose and α-D-lyose in at a 2:1 ratio. The main chains are 1,4-α-D-glucose and 1,36-α-D-glucose, and the branch chain has a 1-α-D-lyxose residue. The in vitro experimental results of this study also showed that the A. caesarea polysaccharide exhibits a strong antioxidant activity. Li et al. (2019b) isolated and purified new polysaccharides, named A. caesarea polysaccharides (ACPs), that have different compositions and structures than AC-1 isolated from the fruitbody of A. caesarea, and they investigated the relationship between its neuroprotective effect and antioxidant activity. The data from both in vivo and in vitro experiments showed that ACPs have significant neuroprotective effects and anti-AD activities. The mechanisms are mainly related to the regulation of Nrf2-mediated oxidative stress. Although there are differences in the monosaccharide compositions and chemical structures between AC-1 and the ACPs, they all show promising antioxidant activities, suggesting that the polysaccharides of A. caesarea have the potential to protect the brain from neuronal damage caused by oxidative stress.
Fucoidan
Fucoidan (FPS), a sulfurated polysaccharide, is extracted from Saccharina japonica. The chemical composition of FPS is complex, and multiple fractions can be obtained during separation and purification. Various FPS fractions have broad ranges of antioxidative properties, and the differences in the sulfate/fucose content ratio determine the antioxidant activity levels (Wang et al., 2008). Several investigators have reported the neuroprotective effects of different FPS fractions both in vitro and in vivo, and they mainly function through antioxidant activities and the prevention of cell apoptosis (Wang et al., 2016). Sulfated hetero-polysaccharides (DF1s) effectively decrease lipid peroxidation and increase the levels/activities of glutathione, glutathione peroxidase, malondialdehyde, and catalase in 1-methyl-4-phenyl-1,2,36-tetrahydropyridine mice. UF, a sulfated hetero-polysaccharide prepared from DF, protects SH-SY5Y cells from H2O catalase-induced apoptosis by regulating the PI3K/Akt signaling pathway and downstream signal transduction (Wang et al., 2017). These findings indicated that FPS and its different fractions exert great neuroprotective effects and have anti-PD potential as effective drugs for the treatment of PD.
Bioactive Polysaccharides with Anti-neuroinflammatory Activities
Neuroinflammation is a complex reaction of the central nervous system to various factors, such as infection, trauma, toxins, and degeneration. It plays important roles in maintaining the homeostasis of the brain. However, protracted or uncontrollable neuroinflammation causes neuronal damage (Xanthos and Sandkühler, 2014). Microglia are innate immune cells in the central nervous system that are activated in several different states under physiological and pathological conditions, and they play key roles in neuroinflammatory responses (Cherry et al., 2014). Over-activated microglia release excessive pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and IL-18, as well as tumor necrosis factor α, leading to synaptic dysfunction, neuronal death, and neurogenesis inhibition (Lyman et al., 2014). According to the literature, bioactive polysaccharides have potential anti-neuroinflammatory effects, and their effective targets are involved in inhibiting the over-activation of microglia, reducing the release of pro-inflammatory factors, and regulating related signaling pathways.
Acorus tatarinowii polysaccharides
Acorus tatarinowii Schott is a common traditional Chinese medicine for the treatment of neuropsychiatric diseases, and it has been used in the clinical practice of traditional Chinese medicine for thousands of years (Zhang et al., 2019). To investigate the bioactivity of A. tatarinowii Schott, Yan et al. (2020) extracted a crude polysaccharide named ATP50, which reduces the levels of pro-inflammatory cytokines in the brain and serum. It also improves the memory and cognitive performances of scopolamine-induced amnesiatic mice. The team isolated and purified ATP50 to produce a polysaccharide with a much lower molecular weight and a well-defined structure, called ATP50-3. The neuroprotective and anti-inflammatory effects of ATP50-3 have been studied using the BV2 microglia model induced by lipopolysaccharide (LPS) (Zhong et al., 2020). ATP50-3 has a significant anti-neuroinflammatory effect that acts by inhibiting LPS-induced over-activation of pro-inflammatory BV2 microglia and by inhibiting TLR4-mediated PI3K/Akt and MyD88/NF-κB signaling pathways that reduce the levels of inflammatory mediators and cytokines. In addition, ATP50-3 protects cortical and hippocampal primary neurons from neurotoxic damage caused by LPS-activated microglia. Thus, ATP50-3 is an effective neuroprotective agent that can inhibit neuroinflammation, and it should be assessed in preclinical trials for the treatment of NDDs.
Schisandra chinensis polysaccharide
Schisandra chinensis (Turcz.) Baill is a traditional Chinese medicine commonly used for treating forgetfulness or dementia. Schisandra chinensis polysaccharides (SCPs) are the main bioactive components in the mature and dried fruit of Fructus schisandra. In an animal model of AD, SCP improves the cognition and histopathological changes in AD mice, decreases the levels of pro-inflammatory cytokines and reduces the activation of astrocytes and microglia in the hippocampus (Xu et al., 2019). The anti-AD effects of SCP were achieved mainly by activating the NF-κB/MAPK pathway to alleviate neuroinflammation. Subsequently, the homogeneous heteropolysaccharide SCP2-1 was isolated and purified from the SCPs, and its effects on microglial activation and neuroinflammation were examined in cultured cells and the LPS-induced mouse model (Xu et al., 2020a). In vitro experiments showed that SCP2-1 reverses the LPS-induced microglial polarization by upregulating lipoprotein receptor-related protein-1 and inhibiting the over-activation of NF-κB and c-Jun N-terminal kinase pathways. In addition, the in vivo experiment showed that SCP2-1 ameliorates LPS-induced cognitive impairment and neuroinflammation in mice. Moreover, Xu et al. (2020b) revealed the preliminary structure of SCP2-1 and found that the anti-neuroinflammatory activity of SCP2-1 was related to the high glucose and galactose contents and the structural characteristics of the major bonds of glucose residues (1→4)-α-D-GLC and (1→4)-β-D-GALP. These findings suggest that SCP is a potential candidate for the prevention and treatment of AD.
Antrodia camphorata polysaccharide
Antrodia camphorata is a polyporous fungus endemic to Taiwan that is rich in polysaccharides, terpenoids, steroids, and other bioactive substances. A previous study demonstrated the anti-tumor activity of A. camphorata polysaccharide (ACP) (Popović et al., 2013). The anti-neuroinflammatory effects of ACP have recently been investigated in a mouse model of PD induced by 6-hydroxydopamine (Han et al., 2019). The ACP interventions improve the motor symptoms of mice modeling PD and greatly inhibit the expression of the NLRP3 inflammatory body and its downstream inflammatory factors. In a subsequent study, Han et al. (2020) found that ACP exhibits neuroprotective effects on a 6-hydroxydopamine-induced cell model and in an animal model of PD by inhibiting the ROS-NLRP3 signaling pathway. Thus, although the composition and structural characteristics of ACP need to be further studied, these results suggest that ACP has the potential to improve the motor symptoms of PD patients and may provide new hope for PD therapy.
Bioactive Polysaccharides with Anti-apoptotic Activities
Apoptosis plays important roles in the development of the nervous system (Dekkers and Barde, 2013). Under normal physiological conditions, the relative resistance of neurons to apoptosis in the adult central nervous system prevents extensive neuron loss (Yuan et al., 2019). Apoptosis is a main pathway of neuronal death in NDDs, in which the expression levels of key apoptosis-related proteins are obviously changed (Chi et al., 2018). Bioactive polysaccharides can play protective roles by inhibiting nerve cell apoptosis. Their main mechanisms are as follows: first, they block the mitochondria-mediated apoptotic pathway by maintaining the stability of the mitochondrial internal environment and blocking the Caspase cascade reaction. In addition, they inhibit the expression of related pro-apoptotic proteins and promote the expression of anti-apoptotic proteins; second, they inhibit autophagy and apoptosis by activating the Akt-MTOR signaling pathway; and third, they inhibit the cell death receptor-mediated pathway.
Corydalis yanhusuo polysaccharide
Corydalis yanhusuo W.T. Wang is a well-known traditional Chinese medicine commonly used to relieve pain. While the biological activities of C. yanhusuo alkaloids and their derivatives have been extensively explored, there are limited studies on the bioactivities of the polysaccharide components. He et al. (2020) isolated a highly purified water-soluble neutral polysaccharide from the plant, named C. yanhusuo polysaccharide (CYP). The CYP treatments protect cells from Aβ25–35-induced cytotoxicity, reduce the release of lactate dehydrogenase and mitochondrial cytochrome c, mitigate mitochondrial dysfunction, and reverse increases in the Bax and Bcl-2 protein expression ratio, which leads to a remarkable dose-dependent elevation of cell viability. Thus, the neuroprotective effects of CYP may be achieved by inhibiting Aβ-induced apoptosis through the mitochondrial apoptotic pathway, which indicates the therapeutic potential of CYP against AD.
Coptis chinensis Franch. polysaccharide
Coptis chinensis Franch. is a long-used traditional Chinese medicine. Its ability to alleviate symptoms of NDDs have been clinically confirmed. Applications of C. chinensis Franch polysaccharide (CCP), a water-soluble polysaccharide isolated from the dried rhizome of C. chinensis Franch, can greatly prolong the lifespan of Caenorhabditis elegans, decrease the rate of paralysis, reduce Aβ deposition in the head, and upregulate the small heat-shock proteins against Aβ-induced neurotoxicity (Li et al., 2018). The neuroprotective activities of CCP and its possible mechanisms against Aβ25–35-induced cytotoxicity have been further validated in PC12 cells (Li et al., 2019a). CCP also exhibits great neuroprotective effects in Aβ25–35-treated PC12 cells, and the mechanism is related to the c-Jun N-terminal kinase-mediated apoptotic signaling pathway. Further studies in animal models are required to determine the potential of CCP as a drug candidate for AD therapy.
AAP70-1
Anemarrhena asphodeloides Bunge is known as “Zhi-mu” in traditional Chinese medicine. The pure compound and its crude extract show obvious neuroprotective activities. Anemarrhena asphodeloides polysaccharides (AAPs) are the main bioactive components isolated from A. asphodeloides. Zhang et al. (2020) isolated and purified a new polysaccharide named AAP70-1 from the AAPs. AAP70-1 is a homogeneous polysaccharide composed of glucose and fructose. Pretreatment with APP70-1 protects SH-SY5Y cells from CoCl2-induced apoptosis. The low molecular weight and small branching structure of AAP70-1, as well as the presence of fructose, may be linked to its neuroprotective effects. The data suggest that AAP70-1 has a neuroprotective potential, to some extent, but whether it can prevent or treat NDDs requires more data.
Bioactive Polysaccharides against Excitatory Amino Acid Toxicity
Glutamate, an acidic amino acid, is the main excitatory neurotransmitter in the mammalian central nervous system. Over-activation of glutamate receptors leads to excitotoxicity, mainly manifested by the continuous influx of calcium ions, ROS overproduction, mitochondrial dysfunction, and release of pro-apoptotic factors, which eventually lead to neuronal dysfunction and cell death (Armada-Moreira et al., 2020). Although the mechanisms of the anti-excitotoxicity effects of polysaccharides have not been elucidated, several recent reports suggest protective effects of polysaccharides against glutamate-induced cytotoxicity.
LBPS02
Lycium barbarum is a medicinal plant that has been used traditionally in China for thousands of years. Polysaccharides are the main bioactive ingredients of L. barbarum, which plays powerful neuroprotective roles in nervous system diseases through a variety of mechanisms, which has led to increased research (Xing et al., 2016). By purifying functional polysaccharides from L. barbarum, Kou et al. (2017) obtained a new polysaccharide component, named purified L. barbarum polysaccharide (LBPS02). The structure of LBPS02 has been characterized, and its neuroprotective effects investigated. LBPS02 treatments improve cell survival, reduce glutamate-induced mitochondrial dysfunction and ROS accumulation, and protect neurons from damage caused by glutamate stimulation. The mechanism is related to the regulation of extracellular signal-regulated kinases and protein kinase B (Akt) phosphorylation, as well as the inhibition of caspase-dependent mitochondrial signaling. These data suggest inhibitory effects of LBPS02 against glutamate-induced excitotoxicity and indicate the therapeutic potential of LBPS02 in the treatment of NDDs.
Hericium erinaceus Polysaccharides
Hericium erinaceus is an edible fungus with medicinal value that has a certain preventive effect against dementia. Hericium erinaceus polysaccharide (HEP) is a main biologically active component of H. erinaceus that exists in fruitbodies, mycelia, and culture fluids (Wang et al., 2019). HEP preconditioning induces cell differentiation, increases cell survival, inhibits ROS accumulation, blocks intracellular Ca2+ overload, and prevents mitochondrial membrane potential depolarization in glutamate-treated PC12 cells (Zhang et al., 2016). The study also found that HEP therapy ameliorates behavioral abnormalities and memory impairments in the AD mouse model. These findings indicate the great neuroprotective activities of HEP both in vivo and in vitro, which indicate it has value in the prevention and treatment of AD.
Conclusions and Future Perspectives
Summary of overview contents
At present, the rising prevalence of NDDs places a heavy burden on society and families. Owing to the complexity of the NDD pathologies and the limitations of current therapeutics, the development of innovative treatment methods has received increasing attention. After the integration and analysis of related studies published in the past 5 years, this review introduced 15 bioactive polysaccharides with neuroprotective effects, including their possible action mechanisms and application prospects in the prevention and treatment of NDDs. These studies indicate that bioactive polysaccharides have potential medicinal values in the treatment of NDDs through various mechanisms, including the inhibition of oxidant stress, neuroinflammation, cell apoptosis, and excitotoxicity (Table 1 and Figure 1). In addition, the neuroprotective effects of naturally sourced bioactive polysaccharides have many advantages, such as multi-targeting, lower toxicity levels, and potential synergistic effects (Xie et al., 2016). Therefore, bioactive polysaccharides are promising neuroprotective agents. Unfortunately, most of these studies focused on in vitro studies and only a few have been validated in animal models (Figure 2). Consequently, information on the pharmacokinetics of these biologically active polysaccharides and the differences in their mechanisms of action in vivo and in vitro are lacking. In addition, although the neuroprotective mechanisms of these agents are diverse, there may be more mechanisms waiting to be determined. Recent studies have reported a bidirectional relationship between gut microbiota and the brain, and polysaccharides regulate the composition of gut microbiota and play positive roles in the prevention and treatment of NDDs through the microbiota-gut-brain axis (Sun et al., 2020).
Table 1.
A list of neuroprotective effects of bioactive polysaccharides
| Mechanism | Name | Source | Cell lines/model | Main conclusions | Application significance | Year of publication |
|---|---|---|---|---|---|---|
| Anti-oxidative stress | Sulfated hetero-polysaccharides (DF1s) | Saccharina japonica | MPTP-induced PD mice | DF1 effectively decreases lipid peroxidation and increases the level/activities of GSH, GSH-PX, MDA and CAT in MPTP mice. | DF1 may be a promising candidate for the treatment of AD. | Wang et al., 2016 |
| Dictyophora echinovolvata polysaccharide (DEVP) | Dictyophora echinovolvata | PC12 cells induced by H2 O2 | DEVP provides substantial neuroprotection against H2 O2-induced PC12 cytotoxicity by inhibiting mitochondrial apoptotic pathways. | DEVP may be a potential candidate for preclinical studies to further prevent oxidative stress and apoptosis-related neurodegenerative diseases. | Yu et al., 2017 | |
| Sulfated hetero-polysaccharides (UF) | Saccharina japonica | SH-SY5Y cells induced by H2 O2 | The UF-mediated activation of PI3K/Akt provides a new potential therapeutic strategy for neurodegenerative diseases associated with oxidative injury. | These findings contribute to a better understanding of the critical roles of UF in the treatment of PD. | Wang et al., 2017 | |
| Perilla frutescens polysaccharide (PEPF) | Perilla frutescens | HT22 cells induced by H2 O2 | PEPF suppresses H2 O2-induced neurotoxicity by activating PI3K/AKT, as well as by negatively regulating MAPKs and NF-κB pathways. PEPF, through an up-regulation of Nrf2-mediated HO-1 pathways; It plays an important role in the suppression of H2 O2-induced neurotoxicity. | PEPF-induced neuroprotective effect holds great potential for pharmacological or therapeutic strategies to treat neurodegenerative diseases, including AD and PD. | Byun et al., 2018 | |
| AFP-2 | Apios americana Medik | PC12 cells induced by H2 O2 | AFP-2 reduces ROS production and mitochondrial damage caused by hydrogen peroxide; AFP-2 significantly activates autophagy via the Akt-mTOR pathway. | AFP-2 has resistant effects on oxidation of PC12 cells, implying a potential neuroprotective effect on neurological diseases. | Chu et al., 2019 | |
| Amanita caesarea polysaccharides (ACPS) | Amanita caesarea | L-Glu induced in HT22 cells; D-gal and AlCl3 induced in mice | ACPS has protective effects on apoptotic model cells and AD mice by regulating Nrf2-mediated oxidative stress. | ACPS may be a promising candidate for the treatment of AD. | Li et al., 2019c | |
| Annona muricata L. polysaccharide (ALP) | Annona muricata | HT22 cells induced by H2 O2 | ALP inhibits oxidative stress by directly inhibiting H2 O2-induced ROS production, thereby inhibiting excessive MAPK and NF-κB signals, and indirectly activating PI3K/ AkT-mediated Nrf2 signals. | ALP represents a novel candidate for pharmacological or therapeutic strategies to treat neurodegenerative diseases. | Kim et al., 2020 | |
| Anti-neuro-inflammation | Antrodia camphorata polysaccharide (ACP) | Antrodia camphorata | 6-OHDA induced in mice | ACP reduces NLRP3 activation and the expression of related inflammatory factors to improve the neurobehavior, motility, and coordination of PD mice. | ACP has a good anti-neuroinflammatory effects and exerts a certain effect on PD. | Han et al., 2019 |
| 6-OHDA induced in MES23.5 cell and mice | At both animal and cellular levels, ACP protects dopamine neurons by inhibiting the ROS-NLRP3 signaling pathway. | ACP may be a natural drug with good application prospects in the treatment of PD. | Han et al., 2020 | |||
| ATP50-3 | Acorus tatarinowii Schott | LPS induced in BV2 cells | ATP50-3 exerts anti-neuroinflammatory and neuroprotective effects through the modulation of TLR4-mediated MyD88/NF-κB and PI3K/Akt signaling pathways. | ATP50-3 may represent a potential neuroprotectant with anti-neuroinflammatory effects for the treatment of neurodegenerative diseases. | Zhong et al., 2020 | |
| SCP2-1 | Schisandra chinensis (Turcz.) Baill | LPS induced in BV2 cells and mice | SCP2-1 suppresses M1 polarization to decrease neuroinflammation in an LRP-1-dependent manner by inhibiting the activation of JNK and NF-κB pathways. | SCP2-1 may be a new treatment of microglial dysfunction caused by the chronic inflammation associated with neurodegenerative diseases, such as AD. | Xu et al., 2020a | |
| Anti-apoptosis | Coptis chinensis Franch polysaccharide (CCP) | Coptis chinensis Franch | Aβ1–42 transgenic CL4176 Caenorhabditis elegans | CCP acts against Aβ-induced toxicity in the C. elegans AD model partly by increasing lifespan, reducing Aβ accumulation, and up-regulating HSPs. | CCP may be a potential therapeutic for AD treatment. | Li et al., 2018 |
| Aβ25–35 induced in PC12 cells | CCP acts against Aβ25–35-induced toxicity by inhibiting JNK signaling in the apoptotic pathway. | CCP might be a promising drug candidate for the prevention and/or treatment of AD. | Li et al., 2019b | |||
| Corydalis yanhusuo polysaccharide (CYP) | Aβ25–35 induced PC12 cells | CYP’s action against Aβ 25–35-induced toxicity in PC12 cells may be mediated by the inhibition of apoptosis via both mitochondrial apoptotic and death receptor pathways. | This study provides new insights into the application of CYP as a promising therapeutic agent for AD. | He et al., 2020 | ||
| AAP70-1 | Anemarrhena asphodeloides Bunge | CoCl2 induced in SH-SY5Y cells | AAP70-1 prevents and ameliorates neurological damage by reducing apoptosis. | AAP70-1 has potential as a therapeutic agent for central nervous system diseases or as an immunomodulatory agent. | Zhang et al., 2020 | |
| Anti-excitatory aminoacids | Hericium erinaceus polysaccharides (HEP) | Hericium erinaceus | Glutamate induced in PC12 cells; AlCl3- and D-gal induced in AD mice | HEP protects DPC12 cells from L-Glu-induced neurotoxicity through mitochondria-related pathways. HEP therapy ameliorates behavioral abnormalities and memory impairments in the AD mouse model. | HEP may be a neuroprotective candidate for treating or preventing AD. | Zhang et al., 2016 |
| Lycium barbarum | L-Glu induced in differentiated PC12 cells | LBPS02 suppresses L-Glu-induced neurotoxicity by regulating Akt and ERKs and by inhibiting mitochondrial apoptotic pathways. | LBPS02 may be a candidate for neurodegenerative disease treatment. | Kou et al., 2017 |
In total, 15 polysaccharides were selected from related studies published between 2015 and 2020. These polysaccharides were extracted from plants and fungi. They exert neuroprotective effects through different mechanisms and have significant neuroprotective activities. Specifically, DF1, DEVP, UF, PEPF, AFP-2, ACPs, and ALP play neuroprotective roles through antioxidative stress. ACP, ATP50-3, and SCP2-1 have potential anti-neuroinflammatory effects. CCP, CYP, and AAP70-1 play protective roles by inhibiting nerve cell apoptosis. HEP and LBPS02 exert neuroprotective effects by attenuating neuronal damage induced by glutamate excitotoxicity. AD: Alzheimer’s disease; Akt: protein kinase B; Aβ: amyloid-β protein; D-Gal: D-galactose; ERK: extracellular regulated kinase; GSH-Px: glutathione peroxidase; H2O2: hydrogen peroxide; HO-1: hemeoxygenase-1; JNK: c-jun N-terminal kinase; L-Glu: L-glutamine; LPS: lipopolysaccharide; MAPKs: mitogen-activated protein kinases; MDA: malondialdehyde; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide; MyD88: myeloid differentiation primary response protein; NF-κB: nuclear factor-Κb; NLRP3: NOD-like receptor pyrin domain containing three; Nrf2: nuclear factor-E2-related factor 2; PC12 cells: rat pheochromocytoma cell line; PD: Parkinson’s disease; PI3K: phosphatidylinositol-3kinase; ROS: reactive oxygen species; TLR4: Toll-like receptor 4; 6-OHDA: 6-hydroxydopamine.
Figure 1.
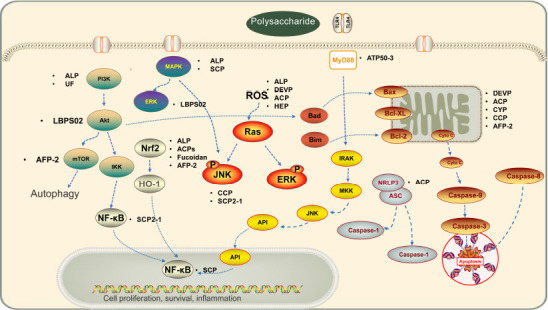
Mechanisms of action and molecular targets of naturally sourced bioactive polysaccharides.
Naturally sourced bioactive polysaccharides have great potential in the treatment of neurodegenerative diseases because of their therapeutic effects against oxidative stress, apoptosis, and neuroinflammation. These mechanisms are related to the regulation of NF-κB, PI3K/Akt, MAPK, Nrf2/HO-1, JNK, Akt-MTOR, ROS-NLRP3, and TLR4-mediated MyD88/NF-κB signaling pathways by bioactive polysaccharides. Name of polysaccharides: ACP: Antrodia camphorata polysaccharide; ACPS: Amanita caesarea polysaccharides; ALP: Annona muricata L. polysaccharide; CCP: Coptis Chinensis Franch. polysaccharide; CYP: Corydalis yanhusuo polysaccharide; DEVP: Dictyophora echinovolvata polysaccharide; FPS: Fucoidan; HEP: Hericium erinaceus polysaccharides; LBPS02: purified Lycium barbarum polysaccharide; SCP: Schisandra chinensis polysaccharide; UF: sulfated hetero-polysaccharides. Akt: protein kinase; Cyto c: cytochrome C; ERK: extracellular regulated kinase; IKK: inhibitor of nuclear factor kappa-B kinase; JNK: c-jun N-terminal kinase; L Glu: L-glutamine; LDH: lactate dehydrogenase; LPS: lipopolysaccharide; MAPK: mitogen-activated protein kinase; MyD88: myeloid differentiation primary response protein; NF-κB: nuclear factor-Κb; NLRP3: NOD-like receptor pyrin domain-containing three; Nrf2: nuclear factor-E2-related factor 2; HO-1: hemeoxygenase-1; PI3K: phosphatidylinositol-3kinase; ROS: reactive oxygen species; TLR4: Toll-like receptor 4.
Figure 2.
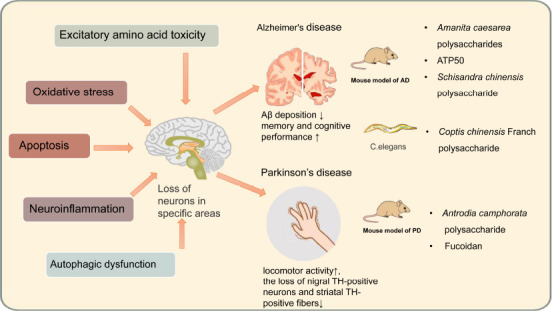
Neuroprotective effects of bioactive polysaccharides in animal models of Alzheimer’s and Parkinson’s diseases.
The progressive loss of neurons in specific regions owing to oxidative stress, neuroinflammation, excitatory amino acid toxicity, apoptosis, and autophagy dysfunction are common pathological features of neurodegenerative diseases. In vivo studies have demonstrated that bioactive polysaccharides play neuroprotective roles through these mechanisms, improving behavioral abnormalities and pathological changes in mouse and Caenorhabditis elegans models of neurodegenerative diseases.
Limitations and Prospects
Although great achievements have been made, there are still many unresolved issues in the clinical translation of polysaccharides into NDD therapies. First, the relationships between the structures and functions of bioactive polysaccharides need further clarification. Because of the complicated structures of bioactive polysaccharides and the close correlations between their biological characteristics and their chemical structures, even polysaccharides from the same source have greatly different activities. Therefore, it is necessary to further clarify the structure-activity relationships of bioactive polysaccharides. Second, although previous studies have observed the neuroprotective effects of bioactive polysaccharides in various animal models, the in vivo pharmacokinetics of these bioactive molecules, such as absorption and metabolism, still need to be further elucidated. New techniques, such as transcriptomics and metabolomics, may provide new methodological tools and approaches. Finally, much of the current research has focused on cell and animal models, but large randomized clinical trials are needed to provide more direct clinical evidence to confirm the therapeutic benefits of these neuroprotective polysaccharides for NDD patients. The purpose of this paper was to review polysaccharides with neuroprotective effects that had been discovered in the last five years, with an emphasis on their roles and molecular mechanisms in neurodegenerative diseases. Through a literature search and screening, we found that there were few existing studies on the neuroprotective effects of Ganoderma lucidum polysaccharides and that they mostly focused on their roles in ischemia/reperfusion injury models. In addition, some articles on G. lucidum polysaccharides did not explore the relevant mechanisms in depth. For these reasons, relevant literature on G. lucidum polysaccharides was not included in this paper. We will continue to monitor research on G. lucidum polysaccharides and hope to have the opportunity to discuss them in the future. Although the limitations of these studies cannot be ignored, we believe that these findings provide more possibilities for further in-depth and comprehensive studies on the neuroprotective effects of bioactive polysaccharides.
Additional file: Open peer review report 1 (85.1KB, pdf) and 2 (86.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewers: Rayudu Gopalakrishna, Keck School of Medicine of USC Cell and Neurobiology, USA; Jaroslav Hanuš, The University of Chemistry and Technology, Prague (UCT Prague), Czech Republic.
Funding: This work was supported by the Key Research and Development Support Project of Chengdu Science and Technology Bureau, No. 2019-YF05-00655-SN (to WDL) and the Key Project of the Medical Science Department, University of Electronic Science and Technology of China, No. ZYGX2020ZB035 (to WDL).
References
- 1.Abdul Wahab S, Jantan I, Haque M, Arshad L. Annona muricata exploring the Leaves of L. as a source of potential anti-inflammatory and anticancer agents. Front Pharmacol. 2018;9:661. doi: 10.3389/fphar.2018.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armada-Moreira A, Gomes J, Pina C, Savchak O, Gonçalves-Ribeiro J, Rei N, Pinto S, Morais T, Martins R, Ribeiro F, Sebastião A, Crunelli V, Vaz S. Going the Extra (Synaptic) mile: excitotoxicity as the road toward neurodegenerative diseases. Front Cell Neurosci. 2020;14:90. doi: 10.3389/fncel.2020.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnham K, Masters C, Bush A. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 4.Byun EB, Cho EJ, Kim YE, Kim WS, Byun EH. Neuroprotective effect of polysaccharide separated from Perilla frutescens Britton var. acuta Kudo against H(2)O(2)-induced oxidative stress in HT22 hippocampus cells. Biosci Biotechnol Biochem. 2018;82:1344–1358. doi: 10.1080/09168451.2018.1460572. [DOI] [PubMed] [Google Scholar]
- 5.Ciccocioppo F, Bologna G, Ercolino E, Pierdomenico L, Simeone P, Lanuti P, Pieragostino D, Del Boccio P, Marchisio M, Miscia S. Neurodegenerative diseases as proteinopathies-driven immune disorders. Neural Regen Res. 2020;15:850–856. doi: 10.4103/1673-5374.268971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherry J, Olschowka J, O’Banion M. Neuroinflammation and M2 microglia: the good the bad and the inflamed. J Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi H, Chang H, Sang T. Neuronal cell death mechanisms in major neurodegenerative diseases. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Q, Chen M, Song D, Li X, Yang Y, Zheng Z, Li Y, Liu Y, Yu L, Hua Z, Zheng X. Apios americana Medik flowers polysaccharide (AFP-2) attenuates H(2)O(2) induced neurotoxicity in PC12 cells. Int J Biol Macromol. 2019;123:1115–1124. doi: 10.1016/j.ijbiomac.2018.11.078. [DOI] [PubMed] [Google Scholar]
- 9.Dekkers M, Barde Y. Developmental biology. Programmed cell death in neuronal development. Science. 2013;340:39–41. doi: 10.1126/science.1236152. [DOI] [PubMed] [Google Scholar]
- 10.Fu H, Hardy J, Duff K. Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018;21:1350–1358. doi: 10.1038/s41593-018-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao QH, Fu X, Zhang R, Wang Z, Guo M. Neuroprotective effects of plant polysaccharides: a review of the mechanisms. Int J Biol Macromol. 2018;106:749–754. doi: 10.1016/j.ijbiomac.2017.08.075. [DOI] [PubMed] [Google Scholar]
- 12.Han C, Guo L, Yang Y, Li W, Sheng Y, Wang J, Guan Q, Zhang X. Study on antrodia camphorata polysaccharide in alleviating the neuroethology of PD mice by decreasing the expression of NLRP3 inflammasome. Phytother Res. 2019;33:2288–2297. doi: 10.1002/ptr.6388. [DOI] [PubMed] [Google Scholar]
- 13.Han C, Shen H, Yang Y, Sheng Y, Wang J, Li W, Zhou X, Guo L, Zhai L, Guan Q. Antrodia camphorata polysaccharide resists 6-OHDA-induced dopaminergic neuronal damage by inhibiting ROS-NLRP3 activation. Brain Behav. 2020;10:e01824. doi: 10.1002/brb3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Xu W, Qin Y. Structural characterization and neuroprotective effect of a polysaccharide from Corydalis yanhusuo. Int J Biol Macromol. 2020;157:759–768. doi: 10.1016/j.ijbiomac.2020.01.180. [DOI] [PubMed] [Google Scholar]
- 15.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch S, Croteau D, Bohr V. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 16.Kaneta H, Koda M, Saito S, Imoto M, Kawada M, Yamazaki Y, Momose I, Shindo K. Biological activities of unique isoflavones prepared from Apios americana Medik. Biosci Biotechnol Biochem. 2016;80:774–778. doi: 10.1080/09168451.2015.1127132. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Kim H, Kang S, Kim Y, Jin C. Soluble epoxide hydrolase inhibitory activity of components isolated from apios americana medik. Molecules. 2017;22:1432. doi: 10.3390/molecules22091432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WS, Kim YE, Cho EJ, Byun EB, Park WY, Song HY, Kim K, Park SH, Byun EH. Neuroprotective effect of Annona muricata-derived polysaccharides in neuronal HT22 cell damage induced by hydrogen peroxide. Biosci Biotechnol Biochem. 2020;84:1001–1012. doi: 10.1080/09168451.2020.1715201. [DOI] [PubMed] [Google Scholar]
- 19.Kou L, Du M, Zhang C, Dai Z, Li X, Zhang B, Hu X. Polysaccharide purified from Lycium barbarum protects differentiated PC12 cells against L Glu induced toxicity via the mitochondria associated pathway. Mol Med Rep. 2017;16:5533–5540. doi: 10.3892/mmr.2017.7289. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Zhang X, Le W. Altered macroautophagy in the spinal cord of SOD1 mutant mice. Autophagy. 2008;4:290–293. doi: 10.4161/auto.5524. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Wu Z, Le W. Traditional Chinese medicine for dementia. Alzheimers Dement. 2021;17:1066–1071. doi: 10.1002/alz.12258. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wang B, Liu C, Zhu X, Zhang P, Yu H, Li Y, Li Z, Li M. Inhibiting c-Jun N-terminal kinase (JNK)-mediated apoptotic signaling pathway in PC12 cells by a polysaccharide (CCP) from Coptis chinensis against amyloid-β (Aβ)-induced neurotoxicity. Int J Biol Macromol. 2019a;134:565–574. doi: 10.1016/j.ijbiomac.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Guan S, Liu C, Chen X, Zhu Y, Xie Y, Wang J, Ji X, Li L, Li Z, Zhang Y, Zeng X, Li M. Neuroprotective effects of Coptis chinensis Franch polysaccharide on amyloid-beta (Aβ)-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease (AD) I Int J Biol Macromol. 2018;113:991–995. doi: 10.1016/j.ijbiomac.2018.03.035. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Chen X, Zhang Y, Liu X, Wang C, Teng L, Wang D. Protective roles of Amanita caesarea polysaccharides against Alzheimer’s disease via Nrf2 pathway. Int J Biol Macromol. 2019b;121:29–37. doi: 10.1016/j.ijbiomac.2018.09.216. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Cui Y, Pi F, Cheng Y, Guo Y, Qian H. Aloe vera extraction purification structural characteristics biological activities and pharmacological applications of acemannan a polysaccharide from: a review. Molecules. 2019;24:1554. doi: 10.3390/molecules24081554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyman M, Lloyd D, Ji X, Vizcaychipi M, Ma D. Neuroinflammation: the role and consequences. Neurosci Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Perry V, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 28.Popa-Wagner A, Dumitrascu DI, Capitanescu B, Petcu EB, Surugiu R, Fang WH, Dumbrava DA. Dietary habits lifestyle factors and neurodegenerative diseases. Neural Regen Res. 2020;15:394–400. doi: 10.4103/1673-5374.266045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popović V, Živković J, Davidović S, Stevanović M, Stojković D. Mycotherapy of cancer: an update on cytotoxic and antitumor activities of mushrooms bioactive principles and molecular mechanisms of their action. Curr Top Med Chem. 2013;13:2791–2806. doi: 10.2174/15680266113136660198. [DOI] [PubMed] [Google Scholar]
- 30.Rathnayake A, Abuine R, Kim Y, Byun H. Anti-alzheimer’s materials isolated from marine bio-resources: a review. Curr Alzheimer Res. 2019;16:895–906. doi: 10.2174/1567205016666191024144044. [DOI] [PubMed] [Google Scholar]
- 31.Shang H, Zhang J, Fu Z, Liu Y, Li S, Chen S, Le W. Therapeutic effects of hirsutella sinensis on the disease onset and progression of amyotrophic lateral sclerosis in SOD1 transgenic mouse model. CNS Neurosci Ther. 2020;26:90–100. doi: 10.1111/cns.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sohn S, Lee S, Cui J, Jang H, Kang T, Kim J, Kim I, Lee D, Choi S, Yoon I, Chung J, Nam J. Apios americana medik extract alleviates lung inflammation in influenza virus H1N1- and endotoxin-induced acute lung injury. J Microbiol Biotechnol. 2015;25:2146–2152. doi: 10.4014/jmb.1508.08017. [DOI] [PubMed] [Google Scholar]
- 33.Sun Q, Cheng L, Zeng X, Zhang X, Wu Z, Weng P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int J Biol Macromol. 2020;164:1484–1492. doi: 10.1016/j.ijbiomac.2020.07.208. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Zhang Q, Zhang Z, Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Liu H, Jin W, Zhang H, Zhang Q. Structure-activity relationship of sulfated hetero/galactofucan polysaccharides on dopaminergic neuron. Int J Biol Macromol. 2016;82:878–883. doi: 10.1016/j.ijbiomac.2015.10.042. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Liu H, Zhang X, Li X, Geng L, Zhang H, Zhang Q. Sulfated hetero-polysaccharides protect SH-SY5Y cells from H2O2-induced apoptosis by affecting the PI3K/Akt signaling pathway. Mar Drugs. 2017;15:110. doi: 10.3390/md15040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang XY, Zhang DD, Yin JY, Nie SP, Xie MY. Recent developments in Hericium erinaceus polysaccharides: extraction purification structural characteristics and biological activities. Crit Rev Food Sci Nutr. 2019;59(sup1):S96–115. doi: 10.1080/10408398.2018.1521370. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Liu J, Zhu Z, Su C, Sreenivasmurthy S, Iyaswamy A, Lu J, Chen G, Song J, Li M. Traditional Chinese medicine compounds regulate autophagy for treating neurodegenerative disease: a mechanism review. Biomed Pharmacother. 2021;133:110968. doi: 10.1016/j.biopha.2020.110968. [DOI] [PubMed] [Google Scholar]
- 39.Xanthos D, Sandkühler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 40.Xie J, Jin M, Morris G, Zha X, Chen H, Yi Y, Li J, Wang Z, Gao J, Nie S, Shang P, Xie M. Advances on bioactive polysaccharides from medicinal plants. Crit Rev Food Sci Nutr. 2016:60–84. doi: 10.1080/10408398.2015.1069255. [DOI] [PubMed] [Google Scholar]
- 41.Xing X, Liu F, Xiao J, So KF. Neuro-protective mechanisms of Lycium barbarum. Neuromolecular Med. 2016;18:253–263. doi: 10.1007/s12017-016-8393-y. [DOI] [PubMed] [Google Scholar]
- 42.Xu M, Wang J, Zhang X, Yan T, Wu B, Bi K, Jia Y. Polysaccharide from Schisandra chinensis acts via LRP-1 to reverse microglia activation through suppression of the NF-κB and MAPK signaling. J Ethnopharmacol. 2020a;256:112798. doi: 10.1016/j.jep.2020.112798. [DOI] [PubMed] [Google Scholar]
- 43.Xu M, Yan T, Fan K, Wang M, Qi Y, Xiao F, Bi K, Jia Y. Polysaccharide of Schisandra Chinensis Fructus ameliorates cognitive decline in a mouse model of Alzheimer’s disease. J Ethnopharmacol. 2019;237:354–365. doi: 10.1016/j.jep.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 44.Xu M, Yan T, Gong G, Wu B, He B, Du Y, Xiao F, Jia Y. Purification structural characterization and cognitive improvement activity of a polysaccharides from Schisandra chinensis. Int J Biol Macromol. 2020b;163:497–507. doi: 10.1016/j.ijbiomac.2020.06.275. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y, Jin MZ, Yang ZY, Jin WL. Microglia in neurodegenerative diseases. Neural Regen Res. 2021;16:270–280. doi: 10.4103/1673-5374.290881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Y, Shen M, Song Q, Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20:19–33. doi: 10.1038/s41583-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Wang Z, Liu Y, Huang J, Liang S, Wu H, Xu Y. Bioactivities of serotonin transporter mediate antidepressant effects of Acorus tatarinowii Schott. J Ethnopharmacol. 2019;241:111967. doi: 10.1016/j.jep.2019.111967. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, An S, Hu W, Teng M, Wang X, Qu Y, Liu Y, Yuan Y, Wang D. The neuroprotective properties of hericium erinaceus in glutamate-damaged differentiated PC12 cells and an Alzheimer’s disease mouse model. Int J Mol Sci. 2016;17:1810. doi: 10.3390/ijms17111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Zhang Q, An L, Zhang J, Li Z, Zhang J, Li Y, Tuerhong M, Ohizumi Y, Jin J, Xu J, Guo Y. A fructan from Anemarrhena asphodeloides Bunge showing neuroprotective and immunoregulatory effects. Carbohydr Polym. 2020;229:115477. doi: 10.1016/j.carbpol.2019.115477. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X, Chen S, Song L, Tang Y, Shen Y, Jia L, Le W. MTOR-independent autophagic enhancer trehalose prolongs motor neuron survival and ameliorates the autophagic flux defect in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2014a;10:588–602. doi: 10.4161/auto.27710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Hong Y, Xu D, Feng Y, Zhao L, Ruan K, Yang X. A review of experimental research on herbal compounds in amyotrophic lateral sclerosis. Phytother Res. 2014b;28:9–21. doi: 10.1002/ptr.4960. [DOI] [PubMed] [Google Scholar]
- 53.Zhong J, Qiu X, Yu Q, Chen H, Yan C. A novel polysaccharide from Acorus tatarinowii protects against LPS-induced neuroinflammation and neurotoxicity by inhibiting TLR4-mediated MyD88/NF-κB and PI3K/Akt signaling pathways. Int J Biol Macromol. 2020;163:464–475. doi: 10.1016/j.ijbiomac.2020.06.266. [DOI] [PubMed] [Google Scholar]
- 54.Zhu Y, Ding X, Wang M, Hou Y, Hou W, Yue C. Structure and antioxidant activity of a novel polysaccharide derived from Amanita caesarea. Mol Med Rep. 2016;14:3947–3954. doi: 10.3892/mmr.2016.5693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


