Abstract
Ionizing radiation caused by medical treatments, nuclear events or even space flights can irreversibly damage structure and function of brain cells. That can result in serious brain damage, with memory and behavior disorders, or even fatal oncologic or neurodegenerative illnesses. Currently used treatments and drugs are mostly targeting biochemical processes of cell apoptosis, radiation toxicity, neuroinflammation, and conditions such as cognitive-behavioral disturbances or others that result from the radiation insult. With most drugs, the side effects and potential toxicity are also to be considered. Therefore, many agents have not been approved for clinical use yet. In this review, we focus on the latest and most effective agents that have been used in animal and also in the human research, and clinical treatments. They could have the potential therapeutical use in cases of radiation damage of central nervous system, and also in prevention considering their radioprotecting effect of nervous tissue.
Key Words: antioxidants, biomedical neuroprotectants, central nervous system, ionizing radiation, neuroprotection, radiation injury, radiomitigators, radioprotectants, radioprotection, therapeutics
Introduction
For a long time, human brain was thought to be highly radioresistant, nerve cells are generally considered to be less sensitive and thereby better resisting the possible damage of radiation. Radiation induces injuries to multiple cell types, also in the brain tissue. Depending on the type, more dangerous ionizing radiation can be blocked by some barriers. Nevertheless, it can enter the body externally (irradiation or radioactive contamination) and/or internally (by ingestion or inhalation), and cause significant organ damage. Major types of ionizing radiation are alpha, beta, neutron, gamma and X-rays. The effects on biological systems of any type of radiation increase with the linear energy transfer (LET). Therefore, the damage from high-LET type radiation (alpha, beta, neutrons) is bigger than that from low-LET radiation (gamma). The reason is, the tissue repair of a damage that is spread in a large area is easier than of one focused in a small area (Stabin, 2010).
The radiation danger for body and brain arises during medical radiation, cosmic space flights, and other events such as laboratory, industrial, military, or power plant nuclear accidents (Coeytaux at al., 2015). The accidents in Hiroshima, Chernobyl, Fukushima and other reactor incidents or potential use of nuclear weapons are still a harm to human health, and could be a great risk in the future as well. High doses of irradiation used in the therapy or those present in the deep space, can cause significant central nervous system (CNS) damage.
Medical radiation is used as a part of cancer treatment therapy. It is often applied together with surgery and systemic therapy. Nowadays, estimated more than 60% of all cancer patients undergo radiotherapy, with high number of female patients treated with frequent radiation for breast cancer. Perhaps also because of that, in females in comparison with male patients, could occur more severe radiation-induced cognitive decline (Bryant et al., 2017). High-LET heavy ions are used in treatment of brain tumors, and it should be also considered that the potential damage to healthy tissues surrounding the tumor can happen during the treatment.
In the deep space, astronaut is under the high-LET galactic cosmic radiation, that is of higher doses than on Earth. HZE particles in galactic cosmic radiation includes multitude of different elements, also 56Fe particles, which can cause oxidative stress in neurons, neuroinflammation, defects in neurogenesis, and cognitive impairment. When exposed to HZE particles at the relevant doses (> 30 cGy) of National Aeronautics and Space Administration, many negative effects happen in organism, that influence not only physiological but also cognitive function, and can be of a great risk to the crew in exploratory Mars missions (Patel et al., 2020).
Ionizing radiation has various negative effects on the brain tissue. Neurocognitive damage in rodents and humans relate to neural stem cell dysfunction, inflammation of brain tissue, and demyelination (Roughton et al., 2013). The dysfunction of central nervous system in patients after irradiation is complex, includes many factors, and is influenced also by individual factors such as age, sex, other health diagnosis, psychological and genetic predispositions, and possible injuries caused by other treatments such as surgery and chemotherapy (Ahles et al., 2012). Therefore, the search for effective and accessible radioprotective agents is critical, especially those without any significant toxicity or side effects. Their practical use would be much needed in all cases of radiation danger. For that reason, the aim of this review was to thoroughly research the latest literature on the use of various natural and medical options and other possibilities, that could serve in neuroprotection of the nervous tissue in all above-mentioned cases of radiation risk. A review of the most effective radioprotectants that are helpful specifically in CNS, with the focus on the brain tissue, would not only broaden the knowledge of the latest drugs used in research, but also support their application in clinical treatment.
Search Strategy and Selection Criteria
The research presented is analysis of the articles and works retrieved in PubMed database with the keywords: ionizing radiation, neuroprotectants, radioprotectants, brain radiation damage, antioxidants in CNS, cosmic radiation damage, novel neuroprotectants, radioprotective agents of CNS. The timeline criterion was applied, with the focus on the newest articles, mostly published in the past 5 years.
Pathophysiology of Radiation Toxicity in the Brain and Neural Damage
Radiation acts in human organism directly and indirectly. It can potentially directly damage critical biological molecules, and indirectly produce ions and free radicals that then interact with cellular components. In the deep space, nuclear catastrophes or some types of medical treatment, the radiation influence result in total body irradiation (TBI). In medicine, TBI has been used in hematologic stem cell transplant or bone marrow transplant preparative regimes (Wilhelm-Buchstab et al., 2020). In case of TBI, it is necessary to mention the acute radiation syndrome that describes many manifestations and symptoms that display severe damage to specific organ systems. Their clinical effects, timing and severity is highly dependent on the dose received.
In whole-body exposures, equivalent doses of radiation are transported to parts of the body. This might not have such high mortality however; it is also very damaging. The clinical effect of irradiation depends on many variables, such as radiation and exposure type, the type of tissue exposed and its sensitivity, the depth of radiation penetration in the body, and also the total absorbed dose, effective dose, and the dose rate. Radiation can cause short- and long-term negative effects in every organ system in the body (Swartz et al., 2020).
Radiation-induced brain damage includes both anatomic and functional deficits. Depending on the time of clinical expression radiation-induced brain injury could be characterized as acute, early delayed, and late delayed injury (Sah et al., 2019). Damaging effects from radiation can occur during or just after the radiation event, or with the late onset after months to years and can be irreversible. Moreover, there is also the possibility for secondary malignancies to occur. Radiation induced brain damage and all its complex pathophysiological mechanisms are yet not completely understood. Nowadays however, it is known that radiation-induced brain injury is multifactorial, depending factors such as dose, duration of irradiation, the presence of shielding. It depends of complex interactions between various brain cell types, e.g., endothelial cells, neurons, astrocytes and microglia (Miyatake et al., 2015). Furthermore, the risk of the radiation damage of the brain can be increased also by neurotoxic factors. Those change microvascular integrity or blood-brain-barrier. The neurotoxic factors include cytotoxic drugs, older age and concurrent diseases such as diabetes, neurological disorders and vascular diseases (Murray et al., 2014).
All types of ionizing radiation can be toxic to the central nervous system. Functional toxicities can have correlation to changes in the whole brain, including gray matter, white matter, ventricles, and their combinations (Prust et al., 2015). During radiation exposure, free radicals form, which causes damage in cerebellum that is responsible for locomotion. Irradiation causes increased oxidative stress leading to metabolic stress, DNA damage, and other damaging biochemical processes in brain tissue (Pariset et al., 2020).
The recent research shows, that other mechanisms of radiation-induced injury can cause additional stress and damage, such as oxidation of the lipid bilayer, changes in microvascular permeability, cell-cell junctional complex rearrangements and mitochondrial alterations (Abdel-Magied et al., 2019). Radiation can induce vascular damage, devascularization, gliosis, demyelination and white matter necrosis. The tissue toxicity incorporates coagulation necrosis of cerebral vasculature by demyelination of axons and damage to vascular endothelial cells (Greene-Schloesser et al., 2013). That further causes ischemia which progresses in N-methy-D-aspartate (NMDA) receptor stimulation and excitotoxicity.
Cranial irradiation is used for the treatment of tumors that are localized in the central nervous system. In the brain, there is a high capacity for metastasis of the tumor to seed and spread as the normal blood-brain barrier prevents systemic agents from be delivered to tumor cells. The radiotherapy still remains the main treatment option for patients with brain metastases, but it is important to mention that it can cause negative neurocognitive deficits. Patients with brain metastases often undergo whole brain irradiation (WBI) (Westover et al., 2020), that may halt the progression of metastases and prolong lifespan, but it can cause cognitive deficits. Patients after fractionated, partial or whole brain irradiation have chronic, progressive cognitive damage and show progressive deficits in information processing speed, frontal lobe executive functions, memory (also spatial memory), visual motor processing, quantitative skills, and possibly also attention (Peng et al., 2018). While the toxic radiation effects depend on total dose, fractionation schedule, and treated volume, research is showing on the difference in radiation sensitivity in various CNS subcompartments. Radiation causes inflammation and leads to activation of microglia and macrophages, which can lead to neuronal cell death. Furthermore, it increases the expression of cytotoxic molecules in brain, such as pro-inflammatory cytokines and chemokines. These processes have been involved in radiotherapy-associated brain damage (Lumniczky et al., 2017). The process of pathogenesis of radiation-induced neurocognitive damage also comprises activation of microglia in dentate gyrus, and apoptosis of neuroproliferative cells in the subgranular zone of the hippocampus. This brain region is vital for learning and memory because there the neurogenesis is still possible in the course of one’s life. After irradiation, a long-term decrease in neurogenesis in subgranular zone was observed (Ouyang et al., 2017). Also, direct irradiation of the hippocampus results in serious cognitive deficits. There are deficits of learning, memory, and spatial processing, accompanied by pronounced alteration in neurogenic microenvironment. Not only early but also late effects occur, that can manifest as chronic and irreversible cognitive impairment and dementia.
Radiation dose and age dependence have to be also taken into consideration in the evolution of neuro-cognitive impairments, that can manifest even from young age (Figure 1) (Newton et al., 2020). Early insults can cause lifelong problems, both physical and mental. The brain that is not mature enough, is highly susceptible to negative impacts on its circuit formation. Radiotherapy causes adverse neurocognitive outcome in young age survivors. They may develop motor, intellectual, visual, and psychological dysfunctions, with moderate to severe disabilities. The younger the age, higher cranial irradiation dose, larger brain volume irradiated, and prolonged time predict worse neurocognitive outcomes (Chu et al., 2020). In clinical research, it is difficult to gather much data about the group of older patients, since they are often excluded from clinical trials. Also, radiation therapy in this case requires special considerations, concerning also other comorbidities and chronic conditions they may have. Nevertheless, higher age is one of the significant predictors of cognitive decline after whole brain radiotherapy. In the recent study by Chan et al. (2020) was seen that after WBRT there is diminished cognitive function in older patients, and also that every patient over 70 years experienced cognitive decline after WBRT.
Figure 1.
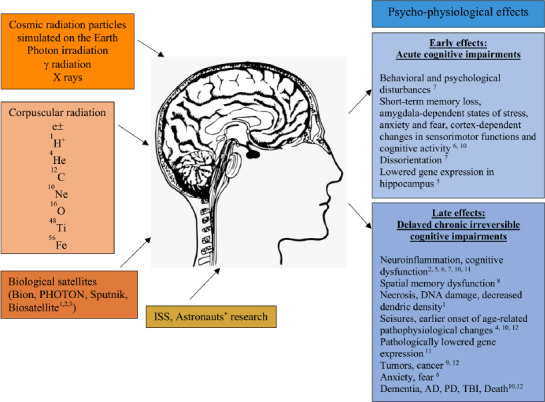
Factors of radiation impact in cosmos and psycho-physiological effects on CNS.
Graphic demonstration of various types of radiation influencing the nervous tissue in the space environment. Early and late psycho-physiological disturbances as a result. AD: Alzheimer’s disease; CNS: central nervous system; PD: Parkinson’s disease, TBI: traumatic brain injury. 1-Variset et al., 2020; 2-Parihar et al., 2015b; 3-Parihar et al., 2015a; 4-Vlkolinsky, 2010; 5-Cherry et al., 2012; 6-Nelson et al., 2016; 7-Rabin et al., 2014; 8-Britten et al., 2012; 9-Seidensaal et al., 2020; 10-Cucinotta and Cacao, 2020; 11-Cucinotta and Cacao, 2019; 12-Boice, 2019.
In standard treatment guidelines, there are many ways how to prevent and treat radiation damage. In clinical care, medicaments can be used to bind radioisotopes in the gastrointestinal tract to avoid their absorption. Among other agents, the important and often used agent is potassium iodide (Parrish and Seda, 2019). Compounds that have been used in treatment include statins, non-steroidal anti-inflammatory drugs, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, metformin, N-acetylcysteine, calcium channel blockers, beta blockers, fingolimod, and pentoxifylline (McLaughlin et al., 2017). However, not all of them are effective enough and many have serious side effects. That is why it is important to find agents that could be safely used as radioprotectants in treatment, and to avoid the toxicity in patients.
Neuroprotectants in Radiation Medicine
In radiation medicine, there are agents used in protection against ionizing radiation. Radioprotectors are used before radiation to protect cells and tissues from the damage. Other group of agents, radiomitigators are used early after the exposure to help in repairing and recovering of the tissues even before the presence of symptoms. And finally, there are therapeutics that are utilized after radiation to enhance healing of injuries and regeneration (Obrador et al., 2020). Various radioprotectants have different effect on tissues and organs. Not each one has effect in neural cells, however, there are some used in a therapy of CNS radiation damage (Figure 2).
Figure 2.
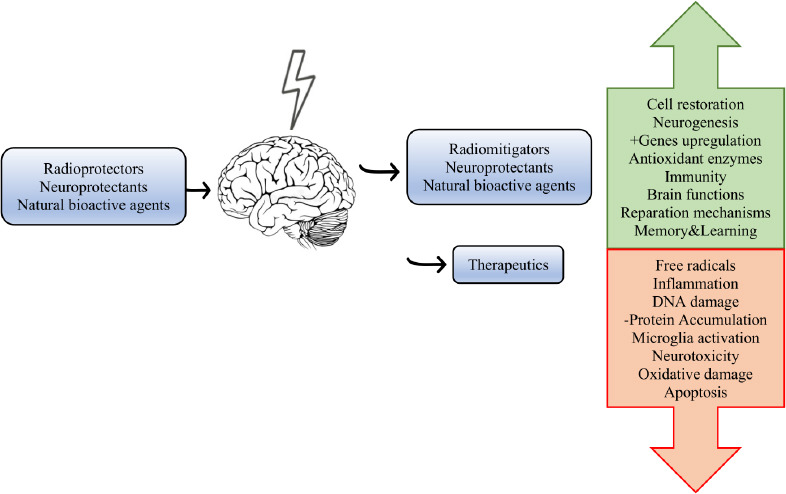
Schematic illustration of the use and effects of active agents in the radiation research and therapy.
Main types of neuroprotectants, increase of reparation processes (green) and reduction of the impairment (red) is displayed.
Neuroprotectants are a category of neuroprotective agents generally used for the protection of neuronal structure and/or function. They are used in medicine to protect against neuronal injury following acute diseases or neurodegeneration in the brain following chronic neurodegenerative diseases. The goal of neuroprotection is to prevent or slow progression of disease and also secondary injuries by ceasing or decreasing the neuronal loss (Paul and Candelario-Jalil, 2021). Mostly used neuroprotective agents are glutamate antagonists and antioxidants, which suppress excitotoxicity and oxidative stress, and also some cognitive enhancers. Recently, some nootropic drugs showed radioprotective effect in the animal research (Lyakhova et al., 2019; Severyukhin et al., 2020). Many neuroprotectants have shown positive effects on brain tissue also in cases of ionizing radiation, as though they can be also used in prevention or treatment of CNS radiation injury.
Besides them, some natural products, diet and lifestyle changes are also recommended in cases of radiation risk. In experimental researches, other strategies such as environmental enrichment, voluntary running, or chronotherapy has shown a positive effect on irradiated brain. With the use of chronotherapy, it is possible to precisely schedule time of drug/therapy administration to enhance treatment effects on the disease while reducing negative outcomes in healthy tissue, mostly by using the body’s circadian rhythms. That can be used also in treatment of patients that undergo medical radiation with very promising outcomes (Ballesta et al., 2017; Shuboni-Mulligan et al., 2019). Nowadays, the modern approaches in stem-cell transplantation could be also a promising way for mitigation of the radiation-induced cognitive decline. When oligodendrocyte progenitors derived from human embryonic stem cells were disseminated into the forebrain and cerebellum of young rats, the remyelinization and to amelioration of cognitive deficits was seen (Piao et al., 2015).
Other lifestyle changes, such as exercise can partially restore hippocampal neurogenesis and behaviour. Clinical studies show that exercise may be beneficial in children and young adults as well (Sabel et al., 2017).
Natural Radioprotectors
Antioxidants and natural agents
In search for protection that could be effective and reliable in cancer therapy and cases of space radiation, with the focus on the brain tissue, there is a need for neuroprotection against heavy-ion radiation. Estimated 70% of tissue destruction that happen during irradiation is due to free radicals, therefore it is necessary to find agents that could neutralize or eliminate free radicals. Antioxidants can stop or slow down oxidation in tissue and so reduce DNA damage that results from ionizing radiation (Brand et al., 2015). Most important antioxidant enzymes in human body are superoxide dismutase, catalase and glutathione peroxidase (SOD, CAT, GSH-Px). Radioprotective agents could generate endogene neuroprotection, influence DNA repair, lower inflammatory response, and slow down cellular division. The use of neuroprotective substances before or during radiation exposure could be a possible option to reduce radiation-induced tissue damage.
There are many antioxidants examined as a potential neuroprotectants against neuro-cognitive damage resulting after cranial irradiation. In the search for radioprotectors, successful seem to be many of them, such as vitamin C, N-acetyl cysteine, curcumin, resveratrol, cinnamic acid, even a watermelon juice. Some of them showed also neuroprotecting function against radiation (Smith et al., 2017). Most promising seems to be vitamin E and its derivates. In clinical study, patients treated with radiotherapy for nasopharyngeal carcinoma that resulted in temporal lobe radionecrosis received alpha-tocopherol or no treatment for one year. At one year, alpha-tocopherol group improved significantly in global cognitive ability (Chan et al., 2004).
Flavonoids
Flavonoids are the important group of polyphenolic substances with a big area of biological activities. They pass through the blood-brain barrier, can directly influence neuro-endocrine system and act anti-inflammatory, antithrombotic, antioxidant, antiallergic, antibacterial, analgesic and vasodilatory effects (Numakawa and Odaka, 2021).
Over a period of time, blueberry addition has showed to be helpful decreasing in suppressing the negative radiation effects and improving cognition. When diet is enriched with antioxidant fruits and vegetables, in particular blueberries, the reactive oxygen species caused neuronal tissue damage and neuroinflammation could be reduced after exposure to HZE radiation, such as in space missions. In studies with animals fed on 2% blueberry or strawberry supplemented food before irradiation, the damage after exposure to heavy particles was lowered (Shukitt-Hale et al., 2007). When young rats were exposed to 1.5 Gy or 2.5 Gy of 56Fe particles irradiation, altered gene expression was observed. This was noted in the hippocampus within 36 h after the exposure. When supplementing their diets with 2% of berries before irradiation, an up-regulation of some protective stress signal genes such as IKKe and MMK3 (apoptotic regulator genes) was observed (Shukitt-Hale et al., 2013). In another study, antioxidant-rich berry diets of animals significantly reduced neurotoxicity and dysfunction after radiation. In experiment with 100 cGy of 56Fe particle radiation, it prevented or reduced the PHF-tau proteins accumulation in hippocampus, which was enhanced after 56Fe HZE particle irradiation (Poulose et al., 2014). In later study by Poulouse et al. (2017), an antioxidant blueberry diet prevented/ameliorated HZE particle-induced cognitive and neural disturbances that are connected also with the behavioral changes. Oxidative damage was prevented in the hippocampus and frontal cortex region in the learning group on blueberry diet that was indicated before irradiation. It significantly reduced the levels of cyclooxygenase in the hippocampus and frontal cortex when 56Fe irradiation applied. Also, the reduction of radiation-induced increase in NADPH oxidase 2 in the hippocampus was seen in the learning and the memory groups (Poulose et al., 2017).
Chrysin (5,7-dihydroxyflavone) is a flavonoid extracted from propolis, honey and several plants, such as Passiflora caerulea. It can cross the blood-brain barrier and acts antiestrogenic, antitumor, antidiabetogenic, anti-hypertensive, anti-inflammatory, antioxidant, anxiolytic and is involved in cancer cell apoptosis. It shown to be helpful in reducing the increase of malondialdehyde levels and caspase-3 activity in rat brains after whole body irradiation of 5 Gy. Its administration decreased the brain-derived neurotrophic factor level, which is important in processes of synaptic plasticity, neuronal growth and survival, and it thereby decreased β-amyloid production. The study also suggests a significant increase in whole brain epinephrine and norepinephrine and serotonine levels (Mansour et al., 2017). 5,7-Dihydroxyflavone showed not only anti-oxidative properties, but a potentially neuroprotectant activity.
Other flavonoids as well have proven to be able to cross blood-brain barrier and so they can act and influence biological processes in brain tissues. Many show radioprotective effects as well.
Biomedical Agents
For prophylaxis of radiation-induced neurocognitive damage, some approved drugs seem to be promising novel agents (Table 1).
Table 1.
Neuroprotectants and biologically active substances used for prevention and treatment of radiation-induced brain damage
| Agent | Type of compound | Other usage | Effect in radiation damage | Literature |
|---|---|---|---|---|
| Vitamin C, E | Antioxidants, vitamins | Anti-inflammatory, antioxidant | DNA damage reduction, free radical scavengers, cellular protection | Brand et al., 2015; Smith et al., 2017; Fischer et al., 2018 |
| Curcumin, resveratrol, cinnamic acid, gallic acid, watermelon juice, zingerone, caffeic acid, carnosic acid, green tea extract, rutin, quercetin, α-lipoic acid, coenzyme Q10 | Phytochemicals | Anti-inflammatory, antioxidant, anti-cancerous, neuroprotection, cardiovascular benefits, antiallergic, antibacterial, antidiabetogenic, anxiolytic antitumor | Improve cognitive function, protect gene upregulation, reduce DNA damage free radical scavengers, neurotoxicity reduction, and increase neurotransmitters | Shukitt-Hale et al., 2013; Mansour et al., 2017; Poulose et al., 2017; Smith et al., 2017; Fischer et al., 2018 |
| Flavonoids | Polyphenols | |||
| VPA | Organic acid | Anti-epileptic drug, anti-seizure, antidepressant drug | Antitumor effects, DNA repair, protection against apoptosis | Thotala et al., 2015; Rahman and Nguyen, 2021 |
| Memantine | NMDA receptor antagonist | Alzheimer’s disease | Reduce WBRT cell damages, extra-cerebral endothelial cell injuries and chronic effects of radiation, improve memory | Brown et al., 2013; Wong et al., 2016 |
| Minocycline | Tetracyclin | Infections, antioxidant, anti-inflammatory | Anti-tumorigenic, neuroprotectant, protect gene upregulation, improve of cognitive abilities | Yazlovitskaya et al., 2006; Mehrotra et al., 2014 |
| Lithium | Lithium salts | Bipolar mood disorder | Cell death and neuroinflammation inhibitor, irradiation injury reduction, inhibition of apoptosis, neuroprotection | Yazlovitskaya et al., 2006; Zanni et al., 2021 |
| Fingolimod | Immunomodulator | Multiple sclerosis, anti-inflammatory, immunosupressant | Neuroprotection, cognitive abilities, and learning preservation | Stessin, et al., 2017; Metzdorf et al., 2019 |
| Ramipril | ACE inhibitor | Cardiovascular drug | Reduce WBRT cognitive impairment and alleviate the late delayed effects of WBI | Kim et al., 2008; Robbins et al., 2009 |
| Melatonine | Hormone | Sleep disorders, antioxidant | Reduction of apoptosis, neuroprotection, and improvement of cognitive abilities | Li et al., 2016; Motallebzadeh et al., 2020; Pipová Kokošová et al., 2020 |
| Amifostine | Cytoprotectant | Cancer therapy | Reduction of radiation toxicity, radioprotection, and antimutagenic effects | Kataoka et al., 1996; Little, 2000 |
| Atorvastatin | Statin | Cardiovascular | Inhibition of apoptosis | Naeimi et al., 2017 |
| Kukoamine A | Catechol | Anti-inflammatory, antioxidant, Parkinson’s disease | Neuroprotection, inhibition of oxidative stress and apoptosis after WBI, amelioration of neuroinflammation | Zhang et al., 2016, 2017 |
| NAC | Acetylated cysteine | Neurodegenerative and psychiatric disorders | Radioprotection and decreased DNA damage | Stehli et al., 2014; Velauthapillai et al., 2017 |
ACE: Angiotensin-converting enzyme; NAC: N-acetyl cysteine; NMDA: N-methyl-D-aspartate; VPA: valproic acid; WBI: whole brain irradiation; WBRT: whole brain radiotherapy.
Valproic acid
Valproic acid (VPA) is a short branched-chain carboxylic acid. It is a histone deacetylase inhibitor, clinically used in treatment of epilepsy, it is anti-seizure and antidepressant drug which shows positive effect and low toxicity. It showed antitumor effects, could reduces cognitive decline, and has radio-sensitizing effects in gliomas and radioprotection in normal brain tissue and hippocampal neurons. It acts in cases of neurological insults, such as glutamate toxicity, intracerebral hemorrhage, ischemia, and oxidative stress it modulates various cellular and tissue pathways, such as cell-cycle arrest, angiogenesis, apoptosis, differentiation, senescence and DNA repair (Rahman and Nguyen, 2021).
Intravenous application of VPA (150 mg/kg) after severe TBI in swine animal model exerts neuroprotective and prosurvival effects. The treatment showed a significant reduction in brain lesion size after isolated TBI (Biesterveld et al., 2020).
Pretreatment with VPA (300 mg/kg) in mice with cranial irradiation of 7 Gy prevented the radiation induced damage in the normal hippocampus and in the same time it sensitized malignant gliomas to radiation. VPA treatment reduced number of apoptotic cells in hippocampal neurons, lowered levels of the pro-apoptotic protein BAX and the levels of the anti-apoptotic protein Bcl-2 in HT22 cells increased, and thereby prevented radiation-induced apoptosis in mice (Thotala et al., 2015). Studies also indicate that valproic acid alone or in combination treatment can prolong the life span also in humans with brain cancer. It could improve cognition and protect against many neurological insults. Therefore, VPA could be used as a drug in patients during brain cancer treatment (Zhuo et al., 2019).
VPA, either used alone or with other agents, can influence brain tissue tumor growth, and show a strong neuroprotective effect also in cases with ionizing radiation.
Memantine
Memantine is a NMDA receptor competitive antagonist, and is currently used in patients with Alzheimer’s disease. Radiation can cause neuronal NMDA receptor stimulation and excitotoxicity. In this context, inhibition of the NMDA receptor by memantine could reduce whole brain radiation induced cognitive damage.
In some recent studies, memantine showed neuroprotection from radiation-induced excitotoxicity. In patients it prevented radiation induced normal-appearing white matter vasculature permeabilization. The study with adult patients who recieved WBRT of 30 Gy and 37.5 Gy shows reduction in WBRT neuronal and endotelian damages. When memantine was applied (5mg up to 10 mg) with radiotherapy it reduced extra-cerebral endothelial cell damage and the rate of chronic radiation side effects in patients, and so it could help with long-term secondary toxicities from irradiation (Wong et al., 2016). Another clinical study of patients undergoing whole brain radiotherapy that received memantine (20 mg per day) or placebo while in treatment or after that for 6 months showed, that it improved delayed memory recall at 6months, and overall cognition (Brown et al., 2013). Memantine applied before and during whole brain radiotherapy significantly delayed time to cognitive failure and lowered the rate of decline in memory, cognitive function, and processing speed. In addition, brain edema, size of brain infarct, and vascular changes of brain tissue of patients decreased (Lynch, 2019).
Memantine has a safety profile with high tolerability, which makes it a promising drug for future use in cases of brain radiation damage.
Minocycline
Minocycline is a semi-synthetic tetracycline derivative. It has been used in a many bacterial infections but has many biological actions not related to its anti-microbial properties. As other agents showing neuroprotective actions, it can efficiently cross the blood-brain barrier and protected brain tissue in researches on cerebral ischemia, traumatic brain injury and other nervous system related pathologies (Kim and Suh, 2009). It has been observed to be neuroprotective in acute CNS injuries and neurodegenerative diseases. This is probably because of its antioxidant activity. Minocycline also shows anti-apoptotic, anti-inflammatory and anti-tumorigenic effects, free radical scavenging properties and acts in microglial activation and thereby rescues neurogenesis (Bassett et al., 2021).
Intraperitoneal injection of minocycline (45 mg/kg) influenced brain cytokine levels, it increased the levels of IL-10, IL-15 and vascular endothelial growth factor (VEGF) in mice receiving 1-, 2- and 3-Gy of radiation which shows immuno- and anti-inflammatory effect of the drug, and its anti-apoptosis and neuroprotective properties. In mammalian models, it stimulates the production of anti-inflammatory and neuroprotective cytokines. Also, it “normalized” expression of genes that were up-regulated by radiation and could preserve viability of neural cells such as astrocytes (Mehrotra et al., 2014). Minocycline intervention (90 mg/kg) ameliorated radiation-induced cognition impairment after single dose of 20 Gy of whole-brain irradiation in rats, improved learning and memory, decreased radiation-induced neuronal apoptosis as a result of radiation. The minocycline treatment act also in the newborn and immature neurons in the dentate subgranular zone and can protect from radiation-induced apoptosis and depletion of newborn neurons (Zhang et al., 2014).
The oral administration of minocycline and its relatively long half-life supports its practical use in cases of a nuclear event or during deep space missions.
Lithium
Lithium has many antiapoptotic activities, and it is commonly used in the treatment of bipolar mood disorder. It shows neuroprotective and radioprotective effects as well, such as inhibition of stem or progenitor cell death and enhancing their neurogenesis, preservation of synaptic plasticity, and reduction of neuroinflammation and irradiation injury. Therefore, it could be used for treatment of radiation damage (Zanni et al., 2021). It protects against a variety of cytotoxic processes, such as oxygen and glucose deprivation, glutamate-mediated excitotoxicity (Taler et al., 2020).
Lithium was applied two weeks before, during and one month after radiotherapy, without any toxical effects (Carret et al., 2016). Since the protection is activated after treatment longer that a couple of days with maximal effect after 6 to 7 days, the lithium application requires pretreatment. This neuroprotection has been observed at therapeutic concentrations of LiCl, at 50 mg/kg LiCl with an almost maximal effect at 100 mg/kg. On the molecular basis, the mechanisms involve among others activation of antiapoptotic cell signaling pathway phosphatidylinositol 3-kinase/Akt, that leads to the inhibition of glycogen synthase kinase-3h. Lithium showed the effect in decreasing levels of the proapoptotic proteins p53 and Bax, together with increasing levels of the prosurvival protein Bcl-2 (Jope, 2003). In experiment with 7 Gy of irradiation in 2-week-old pups of mice treatment with 3 mM of LiCl before cranial irradiation was observed better performance in the Morris water maze. Lithium could decrease cognitive deficits after cranial irradiation. LiCl treatment decreased radiation-induced apoptosis of hippocampal neurons, and by inhibiting of the apoptosis it increased survival of irradiated hippocampal neurons. Pretreatment with lithium changed radiation-induced response of proteins, upstream and downstream of GSK-3 beta, including activation of Akt and protein accumulation of h-catenin and cyclin D1 in HT-22 cells which may lead to protection from radiation-induced apoptosis (Yazlovitskaya et al., 2006).
Other research with lithium pretreatment (LiCl in saline, 1 and 2 mmol/kg intraperitoneally) in male Wistar rat pups, where single absorbed dose of 6 Gy was applied, showed prevention from the radiation-induced cognitive decline. It also reduced signs of hypothalamus-pituitary dysfunction, with the doses used approximated to those in humans, without any signs of toxicity. Lithium treatment prevented irradiation-induced acute inflammatory reactions 6 hours after irradiation, and hippocampal cell death. When administered before and after irradiation, it prevented or delayed cell death at 6 and 24 hours after irradiation. It also prevented inflammatory response which was seen by cytokine and chemokine expression, which was reduced at 6 hours after irradiation. Increase in brain-derived neurotrophic factor levels was arrested by as well (Zhou et al., 2017).
Neuroprotection with lithium could enhance protection of normal neuronal tissue during in cases of medical radiation, and suggests a treatment complementary to cranial radiotherapy that help to decrease the irradiation-induced brain injury and cognitive damage.
Fingolimod
Fingolimod, sphingosine-1-phosphate receptor modulator (FTY720), can also be a novel therapeutic used in prevention of irradiation-induced cognitive dysfuncion. Sphingosine-1-phosphate receptors are expressed on many cells of the nervous system and are important in neuronal development and angiogenesis. Fingolimod can cross the blood-brain-barrier and acts neuroprotective, showed to reduce excitotoxicity and microglial activation in CNS (Wang et al., 2021).
In mice after a single dose of 6 Gy X-ray irradiation, pretreatment fingolimod (1 mg/kg per day orally) reduced radiation-induced cell death, microglia activation and partly rescued radiation-induced inhibition of neurogenesis. It decreased the activated microglia in the dentate gyrus 24 hours after irradiation, and almost reached the values of non-irradiated control group. This was observed also four weeks after irradiation. FTY720 treatment of neural progenitor cells (NPCs) before X-ray exposure led to an ameliorated cell death both in vitro and in vivo. Observed also was a suppression of microglia activation in the dentate gyrus 24 hours after the exposure. The results in vivo showed the number of neuronal progenitor cells increased 4 weeks after the exposure, which indicates a neuroprotection (Metzdorf et al., 2019). FTY 720 used at nanomolar concentrations, which correspond to drug levels in the brain, increased the viability of irradiated neural stem cells (NSCs) in a dose-dependent manner. Its administration also promoted neuronal differentiation, counteracting the radiation-induced suppression of NSC neurogenic potential. In contrast, FTY 720 did not radioprotect human glioma cells and led to decreased viability in the breast cancer cell line (Stessin et al., 2012). In another study, it partially restored neurogenesis in the dentate gyrus, 7 weeks post-irradiation and improved the Morris water maze test results after 7 Gy cranial irradiation in mice, and completely restored the learning ability to control levels. This is showing the effect of fingolimod on improving cognition and learning after irradiation (Stessin et al., 2017).
The shown safety profile of Fingolimod, and neuroprotective results from recent researches imply its potential use in the clinic, mostly for patients receiving whole brain radiation therapy.
Ramipril
Ramipril belongs to the angiotensin-converting enzyme inhibitors, that can influence memory and learning. The renin-angiotensin system is involved in many pathophysiological processes in the central nervous system, also in TBI. Angiotensin-converting enzyme inhibitors inhibit the activity of enzymes involved in the renin-angiotensin system and thereby diminish the negative outcome of the damage. Their benefit is that they are well-tolerated drugs and could selectively modulate radiation-induced normal tissue injury. Ramipril was proven to improve various types of learning and memory, also showed positive effect in lowering the intensity of radiation injury in rats (Erpolat et al., 2020).
Ramipril, is able to modulate radiation-induced optic neuropathy (Kim et al., 2004), and prevent or reduce fractionated whole-brain radiation-induced cognitive damage (Robbins et al., 2009). Ramipril influences the brain renin-angiotensin system directly, because of its ability to cross the blood-brain barrier. By inhibition of the renin-angiotensin system, it is possible to alleviate the development of late delayed effects after whole brain radiation in doses above the necrotic threshold (Kim et al., 2008).
In male rats, the application of ramipril (15 mg/L of ramipril/water) before, during and after fractionated whole brain radiation injury prevented the decline in perirhinal cortex-dependent cognitive function. It also arrested the increase in activated microglia in the dentate gyrus after irradiation, and increased systemic levels of Ang I. This ability to influence Ang I, also in plasma, suggests angiotensin-converting enzyme inhibition (Lee et al., 2012).
In another study, ramipril (1.5 mg/kg in water) was applied 24 hours after 10 Gy WBI for 12 weeks, and it reduced the negative effect on neurogenesis in the dentate gyrus in rats. As an addition, it lowered the basal rate of granule cell neurogenesis in control rats, indicating that Ang II participates in maintaining granule cell neurogenesis (Jenrow et al. 2010). Some of the most radio-sensitive cells of the CNS are the neural progenitor cells. Atorvastatin combined with ramipril administered 24 hours after exposing rats to 10 Gy of WBI synergistically mitigated destruction of doublecortin positive neural progenitor cells (Jenrow et al., 2011).
Administration of ramipril seems to prevent the fractionated whole-brain irradiation disturbance of cognitive function, and influences other key processes in the neural cells. It might be therefore used as a radioprotector with very positive outcomes.
Melatonin
Melatonin (MEL), N-acetyl-5-methoxytryptamine, is a hormone synthesized in pineal gland. Melatonin is a small, lipophilic molecule, can penetrate the blood-brain-barrier, it can effortlessly pass through biomembranes, which means its high bioavailability. It is a strong free radical scavenger that impede inflammation and apoptosis in brain tissue injuries. It is produced during the dark phase of the day, and therefore has an important function in the regulation of the circadian rhythms. Melatonin and its metabolites are effective scavengers of almost all reactive oxygen and nitrogen species and powerful antioxidants, with neuroprotective effects in the CNS (Rancan et al., 2018; Salman et al., 2021).
From many researches it is obvious, that it could act neuroprotective in irradiation-induced apoptosis and oxidative stress in the CNS. Pretreatment with melatonin at different concentrations before ionizing radiation may reduce oxidative damage through the regulation of SOD by lowering O2– as well as of GPx and CAT by decreasing H2O2. Melatonin reduces radiation-induced apoptosis in other parts of the body as well. In CNS, it increases the antioxidant capacity of the tissue (Li et al., 2016). It was found, that melatonin relieved the signs of radiation injury in the normal cells (Najafi et al., 2017), and also the brain injuries caused by X- and gamma-irradiation (Manda and Reiter, 2010). Its application (100 mg/kg) prior to radiation reduced the processes of apoptosis in various organs such as the subventricular zone (SVZ), hippocampus, peripheral blood lymphocytes in rats (Naseri et al., 2017). Melatonin also preserved adult hippocampal neurogenesis and cognition after irradiation (Manda and Reiter, 2010).
It was noted, that melatonin treatment protects from the DNA damage after gamma-radiation, showed anti-apoptotic effect, prevented oxidative stress in the brain of the irradiated rats after whole-body exposed to 4-Gy γ-radiation. Also, it increased the release of neurotransmitters, antioxidants, and anti-inflammatory factors (El-Missiry et al., 2021).
After single radiation dose of 25 G WBI in rats, melatonin pretreatment lowered the NO level significantly in the brainstem tissues. Also, it increased antioxidant enzymes in the brainstem (Karbownik and Reiter, 2000). Caspase-3 is an irreversible apoptotic marker, this protein acts in the late stage of apoptosis (Kiang et al., 2017). The pretreatment reduced the level of expression and activation of caspase-3 after radiation, and thereby stopped X-radiation-mediated apoptosis in the brainstem. In addition, after 4-Gy whole-brain X-irradiation it acted neuroprotective against apoptosis in hippocampus (Motallebzadeh et al., 2020).
In recent studies, in irradiated animals with melatonin treatment there was measured a higher number of surviving mature neurons than without it. In a study with two-month-old rats, melatonin-treated, the number of NeuN-positive cells was increased in the CA1 region after irradiation. It also seemed to stimulate immune reactions, with strongly elevated level of reactive oxygen species in irradiated group treated with melatonin, reaching the value of healthy animals. There was also the recovery in hippocampal-dependent memory recognition in irradiated animals with treatment. Melatonin treatment (4 mg/kg in drinking water) could prevent the decline of the proliferative activity in the hilus of juvenile rats, the loss of hippocampal neurons and increase cognition after ionizing irradiation (Pipová Kokošová et al., 2020).
Melatonin applied before 25 Gy single dose irradiation decreased the oxidative stress in SVZ of irritated rats, as well as the levels of malondialdehyde, and stimulated the CAT. It increased the anti-oxidant level and prevented the production of free radicals (Naseri et al., 2017). The injection of 100 mg/kg was administered before radiation of 1000 cGy (cobalt-60 source), which led to the significant reduction of the DNA fragmentation and lipid peroxidation in the brain (Ündeğer et al. 2004). Observed also was an inhibition the cerebellum cell apoptosis in mice brains with 2 Gy 56Fe particle irradiation (Manda and Reiter, 2010).
In other study, when mice were irradiated with 4 Gy carbon ion radiation, an extensive decline in the apoptotic brain cells was measured after the use of melatonin. This indicates, that melatonin could stop apoptosis in brain tissue after carbon ion irradiation, it could suppress the cytochrome c release, and thereby stop the processes of cell death. The study showed also prevention of the reduction in antioxidant enzyme activities (SOD and CAT) as well as enhancing Nrf2 activation (Liu et al., 2012).
Since melatonin is a natural product, it shows low toxicity and therefore, safety and availability of use. It could be possibly used also for astronauts during long-term flights in deep space.
Conclusion
Exposure to ionizing radiation results in serious CNS malfunctions and cognitive deficits, with many morphological and also clinical manifestations. The prevention strategies or treatment options of early and late effects of radiation would strongly improve quality of life. In the past years, many new drugs have been in development for the possible use as prophylaxis or therapeutics of radiation induced brain damage. Not each one has yet been clinically approved for the treatment, however there are many very promising agents. In this review, we provided the view on the novel agents that have proved efficacy for treatment of radiation induced brain damage. They all show a great potential, however it is important to test all of the side effects and other possible interactions of those and also of the other medical drugs that are now in the developmental stages, to prove their safety and efficacy in the clinical medicine. It is also important to continue the research of CNS to fully understand the radiation-induced cognitive impairment and damage of the nervous tissue to broaden the knowledge of its mechanisms, detection and thereby also to improve the development and the selection of the new agents, that can help to prevent or effectively treat radiation damage.
Acknowledgments:
I want to express my deep gratitude to Prof. Aleksandr Ivanov for scientific expertise and kind guidance, and to Dr. Aleksandr Bugay Laboratory of Radiation Biology, Joint Institute for Nuclear Research, Dubna, Russia for advice and support.
References
- 1.Abdel-Magied N, Abdel-Aziz N, Shedid SM, Ahmed AG. Modulating effect of tiron on the capability of mitochondrial oxidative phosphorylation in the brain of rats exposed to radiation or manganese toxicity. Environ Sci Pollut Res Int. 2019;26:12550–12562. doi: 10.1007/s11356-019-04594-4. [DOI] [PubMed] [Google Scholar]
- 2.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA. Systems Chronotherapeutics. Pharmacol Rev. 2017;69:161–199. doi: 10.1124/pr.116.013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassett B, Subramaniyam S, Fan Y, Varney S, Pan H, Carneiro AMD, Chung CY. Minocycline alleviates depression-like symptoms by rescuing decrease in neurogenesis in dorsal hippocampus via blocking microglia activation/phagocytosis. Brain Behav Immun. 2021;91:519–530. doi: 10.1016/j.bbi.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Biesterveld BE, Pumiglia L, Iancu A, Shamshad AA, Remmer HA, Siddiqui AZ, O’Connell RL, Wakam GK, Kemp MT, Williams AM, Pai MP, Alam HB. Valproic acid treatment rescues injured tissues after traumatic brain injury. J Trauma Acute Care Surg. 2020;89:1156–1165. doi: 10.1097/TA.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boice JD. The million person study relevance to space exploration and mars. Int J Radiat Biol. 2019:1–9. doi: 10.1080/09553002.2019.1589020. [DOI] [PubMed] [Google Scholar]
- 7.Brand M, Sommer M, Ellmann S, Wuest W, May MS, Eller A, Vogt S, Lell MM, Kuefner MA, Uder M. Influence of different antioxidants on X-Ray induced DNA double-strand breaks (DSBs) using γ-H2AX immunofluorescence microscopy in a preliminary study. PLoS One. 2015;10:e0127142. doi: 10.1371/journal.pone.0127142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britten RA, Davis LK, Johnson AM, Keeney S, Siegel A, Sanford LD, Singletary SJ, Lonart G. Low (20 cGy) doses of 1 GeV/u (56)Fe--particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat Res. 2012;177:146–151. doi: 10.1667/rr2637.1. [DOI] [PubMed] [Google Scholar]
- 9.Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, Choucair A, Fox S, Suh JH, Roberge D, Kavadi V, Bentzen SM, Mehta MP, Watkins-Bruner D, Radiation Therapy Oncology Group (RTOG) Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized double-blind placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant AK, Banegas MP, Martinez ME, Mell LK, Murphy JD. Trends in radiation therapy among cancer survivors in the United States 2000-2030. Cancer Epidemiol Biomarkers Prev. 2017;26:963–970. doi: 10.1158/1055-9965.EPI-16-1023. [DOI] [PubMed] [Google Scholar]
- 11.Carret AS, Crevier L, Samson Y, Ellezam B, Décarie JC, Charpentier AM. MB-23 Recurrent SHH/TP53-mutant medulloblastoma treated with a combination of lithium and radiation therapy. Neuro Oncol. 2016;18:101. [Google Scholar]
- 12.Chan AS, Cheung MC, Law SC, Chan JH. Phase II study of alpha-tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer. 2004;100:398–404. doi: 10.1002/cncr.11885. [DOI] [PubMed] [Google Scholar]
- 13.Chan M, Ferguson D, Ni Mhurchu E, Yuan R, Gondara L, McKenzie M, Olson R, Thiessen B, Lalani N, Ma R, Nichol A. Patients with pretreatment leukoencephalopathy and older patients have more cognitive decline after whole brain radiotherapy. Radiat Oncol. 2020;15:271. doi: 10.1186/s13014-020-01717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherry JD, Liu B, Frost JL, Lemere CA, Williams JP, Olschowka JA, O’Banion MK. Galactic cosmic radiation leads to cognitive impairment and increased aβ plaque accumulation in a mouse model of Alzheimer’s disease. PLoS One. 2012;7:e53275. doi: 10.1371/journal.pone.0053275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu C, Gao Y, Lan X, Lin J, Thomas AM, Li S. Stem-cell therapy as a potential strategy for radiation-induced brain injury. Stem Cell Rev Rep. 2020;16:639–649. doi: 10.1007/s12015-020-09984-7. [DOI] [PubMed] [Google Scholar]
- 16.Coeytaux K, Bey E, Christensen D, Glassman ES, Murdock B, Doucet C. Reported radiation overexposure accidents worldwide 1980-2013: a systematic review. PLoS One. 2015;10:e0118709. doi: 10.1371/journal.pone.0118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cucinotta FA, Cacao E. Risks of cognitive detriments after low dose heavy ion and proton exposures. Int J Radiat Biol. 2019;95:985–998. doi: 10.1080/09553002.2019.1623427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cucinotta FA, Cacao E. Predictions of cognitive detriments from galactic cosmic ray exposures to astronauts on exploration missions. Life Sci Space Res (Amst) 2020;25:129–135. doi: 10.1016/j.lssr.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 19.El-Missiry MA, Shabana S, Ghazala SJ, Othman AI, Amer ME. Melatonin exerts a neuroprotective effect against γ-radiation-induced brain injury in the rat through the modulation of neurotransmitters inflammatory cytokines oxidative stress and apoptosis. Environ Sci Pollut Res Int. 2021;28:31108–31121. doi: 10.1007/s11356-021-12951-5. [DOI] [PubMed] [Google Scholar]
- 20.Fischer N, Seo EJ, Efferth T. Prevention from radiation damage by natural products. Phytomedicine. 2018;47:192–200. doi: 10.1016/j.phymed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K, Kim JH. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenrow KA, Liu J, Brown SL, Kolozsvary A, Lapanowski K, Kim JH. Combined atorvastatin and ramipril mitigate radiation-induced impairment of dentate gyrus neurogenesis. J Neurooncol. 2011;101:449–456. doi: 10.1007/s11060-010-0282-x. [DOI] [PubMed] [Google Scholar]
- 23.Jope RS. Lithium and GSK-3: one inhibitor two inhibitory actions multiple outcomes. Trends Pharmacol Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 24.Karbownik M, Reiter RJ. Antioxidative effects of melatonin in protection against cellular damage caused by ionizing radiation. Proc Soc Exp Biol Med. 2000;225:9–22. doi: 10.1177/153537020022500102. [DOI] [PubMed] [Google Scholar]
- 25.Kataoka Y, Perrin J, Hunter N, Milas L, Grdina DJ. Antimutagenic effects of amifostine: clinical implications. Semin Oncol. 1996;23:53–57. [PubMed] [Google Scholar]
- 26.Kiang JG, Smith JT, Anderson MN, Elliott TB, Gupta P, Balakathiresan NS, Maheshwari RK, Knollmann-Ritschel B. Hemorrhage enhances cytokine complement component 3 and caspase-3 and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury. PLoS One. 2017;12:e0184393. doi: 10.1371/journal.pone.0184393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 28.Kim JH, Brown SL, Jenrow KA, Ryu S. Mechanisms of radiation-induced brain toxicity and implications for future clinical trials. J Neurooncol. 2008;87:279–286. doi: 10.1007/s11060-008-9520-x. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Brown SL, Kolozsvary A, Jenrow KA, Ryu S, Rosenblum ML, Carretero OA. Modification of radiation injury by ramipril inhibitor of angiotensin-converting enzyme on optic neuropathy in the rat. Radiat Res. 2004;161:137–142. doi: 10.1667/rr3124. [DOI] [PubMed] [Google Scholar]
- 30.Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, Mustafa R, Riddle DR, Zhao W, Chan MD, Robbins ME. Chronic administration of the angiotensin-converting enzyme inhibitor ramipril prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Zhang G, Meng Z, Wang L, Liu H, Liu Q, Buren B. Neuroprotective effect of acute melatonin treatment on hippocampal neurons against irradiation by inhibition of caspase-3. Exp Ther Med. 2016;11:2385–2390. doi: 10.3892/etm.2016.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Zhang L, Zhang H, Liu B, Wu Z, Zhao W, Wang Z. Exogenous melatonin modulates apoptosis in the mouse brain induced by high-LET carbon ion irradiation. J Pineal Res. 2012;52:47–56. doi: 10.1111/j.1600-079X.2011.00917.x. [DOI] [PubMed] [Google Scholar]
- 34.Lumniczky K, Szatmári T, Sáfrány G. Ionizing radiation-induced immune and inflammatory reactions in the brain. Front Immunol. 2017;8:517. doi: 10.3389/fimmu.2017.00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyakhova KN, Kolesnikova IA, Budennaya NN, Severiukhin YS, Bychkova TM, Nikitenko OV et al. The effect of the medical drug “Semaks” on the vital status and brain damage of mice after irradiation by protons. Radiats Biol Radioecol. 2019;2:191–199. [Google Scholar]
- 36.Lynch M. Preservation of cognitive function following whole brain radiotherapy in patients with brain metastases: complications treatments and the emerging role of memantine. J Oncol Pharm Pract. 2019;25:657–662. doi: 10.1177/1078155218798176. [DOI] [PubMed] [Google Scholar]
- 37.Manda K, Reiter RJ. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog Neurobiol. 2010;90:60–68. doi: 10.1016/j.pneurobio.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Mansour SZ, Moawed F, Elmarkaby SM. Protective effect of 5,7-dihydroxyflavone on brain of rats exposed to acrylamide or γ-radiation. J Photochem Photobiol B. 2017;175:149–155. doi: 10.1016/j.jphotobiol.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 39.McLaughlin MF, Donoviel DB, Jones JA. Novel indications for commonly used medications as radiation protectants in spaceflight. Aerosp Med Hum Perform. 2017;88:665–676. doi: 10.3357/AMHP.4735.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehrotra S, Pecaut MJ, Gridley DS. Minocycline modulates cytokine and gene expression profiles in the brain after whole-body exposure to radiation. In Vivo. 2014;28:21–32. [PubMed] [Google Scholar]
- 41.Metzdorf J, Hobloss Z, Schlevogt S, Ayzenberg I, Stahlke S, Pedreiturria X, Haupeltshofer S, Gold R, Tönges L, Kleiter I. Fingolimod for irradiation-induced neurodegeneration. Front Neurosci. 2019;13:699. doi: 10.3389/fnins.2019.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, Kuroiwa T. Pathophysiology diagnosis and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo) 2015;55:50–59. doi: 10.2176/nmc.ra.2014-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motallebzadeh E, Tameh AA, Zavareh S, Farhood B, Aliasgharzedeh A, Mohseni M. Neuroprotective effect of melatonin on radiation-induced oxidative stress and apoptosis in the brainstem of rats. J Cell Physiol. 2020;235:8791–8798. doi: 10.1002/jcp.29722. [DOI] [PubMed] [Google Scholar]
- 44.Murray D, McBride WH, Schwartz JL. Radiation biology in the context of changing patterns of radiotherapy. Radiat Res. 2014;182:259–272. doi: 10.1667/RR13740.1. [DOI] [PubMed] [Google Scholar]
- 45.Naeimi RA, Talebpour Amiri F, Khalatbary AR, Ghasemi A, Zargari M, Ghesemi M, Hosseinimehr SJ. Atorvastatin mitigates testicular injuries induced by ionizing radiation in mice. Reprod Toxicol. 2017;72:115–121. doi: 10.1016/j.reprotox.2017.06.052. [DOI] [PubMed] [Google Scholar]
- 46.Najafi M, Shirazi A, Motevaseli E, Geraily G, Norouzi F, Heidari M, Rezapoor S. The melatonin immunomodulatory actions in radiotherapy. Biophys Rev. 2017;9:139–148. doi: 10.1007/s12551-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naseri S, Moghahi S, Mokhtari T, Roghani M, Shirazi AR, Malek F, Rastegar T. Radio-protective effects of melatonin on subventricular zone in irradiated rat: decrease in apoptosis and upregulation of nestin. J Mol Neurosci. 2017;63:198–205. doi: 10.1007/s12031-017-0970-5. [DOI] [PubMed] [Google Scholar]
- 48.Nelson GA, Simonsen L, Huff JL. Risk of acute and late central nervous system effects from radiation exposure. Evid Rep Houston: NASA. 2016:68. [Google Scholar]
- 49.Newton J, Brown T, Corley C, Alexander T, Trujillo M, McElroy T, Ntagwabira F, Wang J, Byrum SD, Allen AR. Cranial irradiation impairs juvenile social memory and modulates hippocampal physiology. Brain Res. 2020;1748:147095. doi: 10.1016/j.brainres.2020.147095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Numakawa T, Odaka H. Brain-derived neurotrophic factor signaling in the pathophysiology of Alzheimer’s disease: beneficial effects of flavonoids for neuroprotection. Int J Mol Sci. 2021;22:5719. doi: 10.3390/ijms22115719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obrador E, Salvador R, Villaescusa JI, Soriano JM, Estrela JM, Montoro A. Radioprotection and radiomitigation: from the bench to clinical practice. Biomedicines. 2020;8:461. doi: 10.3390/biomedicines8110461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ouyang YB, Ning S, Adler JR, Maciver B, Knox SJ, Giffard R. Alteration of interneuron immunoreactivity and autophagic activity in rat hippocampus after single high-dose whole-brain irradiation. Cureus. 2017;9:e1414. doi: 10.7759/cureus.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parihar VK, Allen B, Tran KK, Macaraeg TG, Chu EM, Kwok SF, Chmielewski NN, Craver BM, Baulch JE, Acharya MM, Cucinotta FA, Limoli CL. What happens to your brain on the way to Mars. Sci Adv. 2015a;1:e1400256. doi: 10.1126/sciadv.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parihar VK, Allen BD, Tran KK, Chmielewski NN, Craver BM, Martirosian V, Morganti JM, Rosi S, Vlkolinsky R, Acharya MM, Nelson GA, Allen AR, Limoli CL. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid Redox Signal. 2015b;22:78–91. doi: 10.1089/ars.2014.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pariset E, Malkani S, Cekanaviciute E, Costes SV. Ionizing radiation-induced risks to the central nervous system and countermeasures in cellular and rodent models. Int J Rad Biol. 2020 doi: 10.1080/09553002.2020.1820598. doi: 10.1080/09553002.2020.1820598. [DOI] [PubMed] [Google Scholar]
- 56.Parrish JS, Seda G. Disasters resulting from radiologic and nuclear events. Crit Care Clin. 2019;35:619–631. doi: 10.1016/j.ccc.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Patel ZS, Brunstetter TJ, Tarver WJ, Whitmire AM, Zwart SR, Smith SM, Huff JL. Red risks for a journey to the red planet: the highest priority human health risks for a mission to Mars. NPJ Microgravity. 2020;6:33. doi: 10.1038/s41526-020-00124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paul S, Candelario-Jalil E. Emerging neuroprotective strategies for the treatment of ischemic stroke: an overview of clinical and preclinical studies. Exp Neurol. 2021;335:113518. doi: 10.1016/j.expneurol.2020.113518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng XC, Huang JR, Wang SW, Liu L, Liu ZZ, Sethi G, Ren BX, Tang FR. Traditional Chinese medicine in neuroprotection after brain insults with special reference to radioprotection. Evid Based Complement Alternat Med. 2018;2018:2767208. doi: 10.1155/2018/2767208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, Gutin P, Uryu K, Tchieu J, Soulet D, Tabar V. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pipová Kokošová N, Kisková T, Vilhanová K, Štafuriková A, Jendželovský R, Račeková E, Šmajda B. Melatonin mitigates hippocampal and cognitive impairments caused by prenatal irradiation. Eur J Neurosci. 2020;52:3575–3594. doi: 10.1111/ejn.14687. [DOI] [PubMed] [Google Scholar]
- 62.Poulose SM, Bielinski DF, Carrihill-Knoll KL, Rabin BM, Shukitt-Hale B. Protective effects of blueberry- and strawberry diets on neuronal stress following exposure to (56)Fe particles. Brain Res. 2014;1593:9–18. doi: 10.1016/j.brainres.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 63.Poulose SM, Rabin BM, Bielinski DF, Kelly ME, Miller MG, Thanthaeng N, Shukitt-Hale B. Neurochemical differences in learning and memory paradigms among rats supplemented with anthocyanin-rich blueberry diets and exposed to acute doses of 56Fe particles. Life Sci Space Res (Amst) 2017;12:16–23. doi: 10.1016/j.lssr.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Prust MJ, Jafari-Khouzani K, Kalpathy-Cramer J, Polaskova P, Batchelor TT, Gerstner ER, Dietrich J. Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology. 2015;85:683–691. doi: 10.1212/WNL.0000000000001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabin BM, Shukitt-Hale B, Carrihill-Knoll KL, Gomes SM. Comparison of the effects of partial- or whole-body exposures to 16O particles on cognitive performance in rats. Radiat Res. 2014;181:251–257. doi: 10.1667/RR13469.1. [DOI] [PubMed] [Google Scholar]
- 66.Rahman M, Nguyen H. Valproic Acid. Treasure Island (FL): StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 67.Rancan L, Paredes SD, García C, González P, Rodríguez-Bobada C, Calvo-Soto M, Hyacinthe B, Vara E, Tresguerres J. Comparison of the effect of melatonin treatment before and after brain ischemic injury in the inflammatory and apoptotic response in aged rats. Int J Mol Sci. 2018;19:2097. doi: 10.3390/ijms19072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu FC, Brown WR, Wheeler KT, Olson J, Zhao W. The AT1 receptor antagonist L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73:499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roughton K, Boström M, Kalm M, Blomgren K. Irradiation to the young mouse brain impaired white matter growth more in females than in males. Cell Death Dis. 2013;4:e897. doi: 10.1038/cddis.2013.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabel M, Sjölund A, Broeren J, Arvidsson D, Saury JM, Gillenstrand J, Emanuelson I, Blomgren K, Lannering B. Effects of physically active video gaming on cognition and activities of daily living in childhood brain tumor survivors: a randomized pilot study. Neurooncol Pract. 2017;4:98–110. doi: 10.1093/nop/npw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sah SK, Samuel VP, Dahiya S, Singh Y, Gilhotra RM, Gupta G, Mishra A, Sharma RK, Kumar GS, SreeHarsha N, Chellappan DK, Dua K. A contemporary biological pathway of islet amyloid polypeptide for the management of diabetic dementia. Chem Biol Interact. 2019;306:117–122. doi: 10.1016/j.cbi.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 72.Salman M, Kaushik P, Tabassum H, Parvez S. Melatonin provides neuroprotection following traumatic brain injury-promoted mitochondrial perturbation in wistar rat. Cell Mol Neurobiol. 2021;41:765–781. doi: 10.1007/s10571-020-00884-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seidensaal K, Harrabi SB, Uhl M, Debus J. Re-irradiation with protons or heavy ions with focus on head and neck skull base and brain malignancies. Br J Radiol. 2020;93:20190516. doi: 10.1259/bjr.20190516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Severyukhin YS, Lalkovičová M, Kolesnikova IA, Utina DM, Lyakhova KN, Gaevsky VN. The effect of piracetam on behavioral reactions of adult rats and morphological changes in the brain after whole body fractionated gamma irradiation: an exploratory study. Radiat Environ Biophys. 2021;60:73–86. doi: 10.1007/s00411-020-00886-3. [DOI] [PubMed] [Google Scholar]
- 75.Shuboni-Mulligan DD, Breton G, Smart D, Gilbert M, Armstrong TS. Radiation chronotherapy-clinical impact of treatment time-of-day: a systematic review. J Neurooncol. 2019;145:415–427. doi: 10.1007/s11060-019-03332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shukitt-Hale B, Carey AN, Jenkins D, Rabin BM, Joseph JA. Beneficial effects of fruit extracts on neuronal function and behavior in a rodent model of accelerated aging. Neurobiol Aging. 2007;28:1187–1194. doi: 10.1016/j.neurobiolaging.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 77.Shukitt-Hale B, Lau FC, Cheng V, Luskin K, Carey AN, Carrihill-Knoll K, Rabin BM, Joseph JA. Changes in gene expression in the rat hippocampus following exposure to 56Fe particles and protection by berry diets. Cent Nerv Syst Agents Med Chem. 2013;13:36–42. doi: 10.2174/1871524911313010006. [DOI] [PubMed] [Google Scholar]
- 78.Smith TA, Kirkpatrick DR, Smith S, Smith TK, Pearson T, Kailasam A, Herrmann KZ, Schubert J, Agrawal DK. Radioprotective agents to prevent cellular damage due to ionizing radiation. J Transl Med. 2017;15:232. doi: 10.1186/s12967-017-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stabin MG. Springer. ISBN-13: 978-1441923912. Springer-Verlag New York, USA: 2010. Radiation protection and dosimetry: an introduction to health physics. [Google Scholar]
- 80.Stehli J, Fuchs TA, Ghadri JR, Gaemperli O, Fiechter M, Kaufmann PA. Antioxidants prevent DNA double-strand breaks from X-ray-based cardiac examinations: a randomized double-blinded placebo-controlled trial. J Am Coll Cardiol. 2014;64:117–118. doi: 10.1016/j.jacc.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 81.Stessin AM, Banu MA, Clausi MG, Berry N, Boockvar JA, Ryu S. FTY720/fingolimod an oral S1PR modulator mitigates radiation induced cognitive deficits. Neurosci Lett. 2017;658:1–5. doi: 10.1016/j.neulet.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 82.Stessin AM, Gursel DB, Schwartz A, Parashar B, Kulidzhanov FG, Sabbas AM, Boockvar J, Nori D, Wernicke AG. FTY720 sphingosine 1-phosphate receptor modulator selectively radioprotects hippocampal neural stem cells. Neurosci Lett. 2012;516:253–258. doi: 10.1016/j.neulet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Swartz HM, Flood AB, Singh VK, Swarts SG. Scientific and logistical considerations when screening for radiation risks by using biodosimetry based on biological effects of radiation rather than dose: the need for prior measurements of homogeneity and distribution of dose. Health Phys. 2020;119:72–82. doi: 10.1097/HP.0000000000001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taler M, Aronovich R, Henry Hornfeld S, Dar S, Sasson E, Weizman A, Hochman E. Regulatory effect of lithium on hippocampal blood-brain barrier integrity in a rat model of depressive-like behavior. Bipolar Disord. 2020;23:55–65. doi: 10.1111/bdi.12962. [DOI] [PubMed] [Google Scholar]
- 85.Thotala D, Karvas RM, Engelbach JA, Garbow JR, Hallahan AN, DeWees TA, Laszlo A, Hallahan DE. Valproic acid enhances the efficacy of radiation therapy by protecting normal hippocampal neurons and sensitizing malignant glioblastoma cells. Oncotarget. 2015;6:35004–35022. doi: 10.18632/oncotarget.5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Undeger U, Giray B, Zorlu AF, Oge K, Baçaran N. Protective effects of melatonin on the ionizing radiation induced DNA damage in the rat brain. Exp Toxicol Pathol. 2004;55:379–384. doi: 10.1078/0940-2993-00332. [DOI] [PubMed] [Google Scholar]
- 87.Velauthapillai N, Barfett J, Jaffer H, Mikulis D, Murphy K. Antioxidants taken orally prior to diagnostic radiation exposure can prevent DNA injury. J Vasc Interv Radiol. 2017;28:406–411. doi: 10.1016/j.jvir.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 88.Vlkolinsky R, Titova E, Krucker T, Chi BB, Staufenbiel M, Nelson GA, Obenaus A. Exposure to 56Fe-particle radiation accelerates electrophysiological alterations in the hippocampus of APP23 transgenic mice. Radiat Res. 2010;173:342–352. doi: 10.1667/RR1825.1. [DOI] [PubMed] [Google Scholar]
- 89.Wang B, Tanaka K, Ji B, Ono M, Fang Y, Ninomiya Y, Maruyama K, Izumi-Nakajima N, Begum N, Higuchi M, Fujimori A, Uehara Y, Nakajima T, Suhara T, Ono T, Nenoi M. Total body 100-mGy X-irradiation does not induce Alzheimer’s disease-like pathogenesis or memory impairment in mice. J Radiat Res. 2014;55:84–96. doi: 10.1093/jrr/rrt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang CC, Kuo JR, Wang SJ. Fingolimod inhibits glutamate release through activation of S1P1 receptors and the G protein βγ subunit-dependent pathway in rat cerebrocortical nerve terminals. Neuropharmacology. 2021;185:108451. doi: 10.1016/j.neuropharm.2021.108451. [DOI] [PubMed] [Google Scholar]
- 91.Westover KD, Mendel JT, Dan T, Kumar K, Gao A, Pulipparacharuv S, Iyengar P, Nedzi L, Hannan R, Anderson J, Choe KS, Jiang W, Abdulrahman R, Rahimi A, Folkert M, Laine A, Presley C, Cullum CM, Choy H, Ahn C, Timmerman R. Phase II trial of hippocampal-sparing whole brain irradiation with simultaneous integrated boost for metastatic cancer. Neuro Oncol. 2020;22:1831–1839. doi: 10.1093/neuonc/noaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilhelm-Buchstab T, Leitzen C, Schmeel LC, Simon B, Koch D, Schmeel FC, Schoroth F, Garbe S, Röhner F, Wolf D, Schüller H, Schild HH, Müdder T. Total body irradiation: significant dose sparing of lung tissue achievable by helical tomotherapy. Z Med Phys. 2020;30:17–23. doi: 10.1016/j.zemedi.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 93.Wong P, Leppert IR, Roberge D, Boudam K, Brown PD, Muanza T, Pike GB, Chankowsky J, Mihalcioiu C. A pilot study using dynamic contrast enhanced-MRI as a response biomarker of the radioprotective effect of memantine in patients receiving whole brain radiotherapy. Oncotarget. 2016;7:50986–50996. doi: 10.18632/oncotarget.9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yazlovitskaya EM, Edwards E, Thotala D, Fu A, Osusky KL, Whetsell WO Jr, Boone , B , Shinohara ET, Hallahan DE. Lithium treatment prevents neurocognitive deficit resulting from cranial irradiation. Cancer Res. 2006;66:11179–11186. doi: 10.1158/0008-5472.CAN-06-2740. [DOI] [PubMed] [Google Scholar]
- 95.Zanni G, Goto S, Fragopoulou AF, Gaudenzi G, Naidoo V, Di Martino E, Levy G, Dominguez CA, Dethlefsen O, Cedazo-Minguez A, Merino-Serrais P, Stamatakis A, Hermanson O, Blomgren K. Lithium treatment reverses irradiation-induced changes in rodent neural progenitors and rescues cognition. Mol Psychiatry. 2021;26:322–340. doi: 10.1038/s41380-019-0584-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L, Li K, Sun R, Zhang Y, Ji J, Huang P, Yang H, Tian Y. Minocycline ameliorates cognitive impairment induced by whole-brain irradiation: an animal study. Radiat Oncol. 2014;9:281. doi: 10.1186/s13014-014-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Y, Cheng Z, Wang C, Ma H, Meng W, Zhao Q. Neuroprotective effects of kukoamine a against radiation-induced rat brain injury through inhibition of oxidative stress and neuronal apoptosis. Neurochem Res. 2016;41:2549–2558. doi: 10.1007/s11064-016-1967-0. [DOI] [PubMed] [Google Scholar]
- 98.Zhang Y, Gao L, Cheng Z, Cai J, Niu Y, Meng W, Zhao Q. Kukoamine a prevents radiation-induced neuroinflammation and preserves hippocampal neurogenesis in rats by inhibiting activation of NF-κB and AP-1. Neurotox Res. 2017;31:259–268. doi: 10.1007/s12640-016-9679-4. [DOI] [PubMed] [Google Scholar]
- 99.Zhou K, Xie C, Wickström M, Dolga AM, Zhang Y, Li T, Xu Y, Culmsee C, Kogner P, Zhu C, Blomgren K. Lithium protects hippocampal progenitors cognitive performance and hypothalamus-pituitary function after irradiation to the juvenile rat brain. Oncotarget. 2017;8:34111–34127. doi: 10.18632/oncotarget.16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhuo C, Xun Z, Hou W, Ji F, Lin X, Tian H, Zheng W, Chen M, Liu C, Wang W, Chen C. Surprising anticancer activities of psychiatric medications: old drugs offer new hope for patients with brain cancer. Front Pharmacol. 2019;10:1262. doi: 10.3389/fphar.2019.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]


