Abstract
Inflammatory responses, including glial cell activation and peripheral immune cell infiltration, are involved in the pathogenesis of Parkinson’s disease (PD). These inflammatory responses appear to be closely related to the release of extracellular vesicles, such as exosomes. However, the relationships among different forms of glial cell activation, synuclein dysregulation, mitochondrial dysfunction, and exosomes are complicated. This review discusses the multiple roles played by exosomes in PD-associated inflammation and concludes that exosomes can transport toxic α-synuclein oligomers to immature neurons and into the extracellular environment, inducing the oligomerization of α-synuclein in normal neurons. Misfolded α-synuclein causes microglia and astrocytes to activate and secrete exosomes. Glial cell-derived exosomes participate in communications between glial cells and neurons, triggering anti-stress and anti-inflammatory responses, in addition to axon growth. The production and release of mitochondrial vesicles and exosomes establish a new mechanism for linking mitochondrial dysfunction to systemic inflammation associated with PD. Given the relevance of exosomes as mediators of neuron-glia communication in neuroinflammation and neuropathogenesis, new targeted treatment strategies are currently being developed that use these types of extracellular vesicles as drug carriers. Exosome-mediated inflammation may be a promising target for intervention in PD patients.
Key Words: astrocytes, exosomes, inflammation, microglia, mitochondria, neurodegeneration, neuroglia, neuron-glia communication, Parkinson's disease, synucleins
Introduction
Parkinson’s disease (PD) is the second most common degenerative disease of the central nervous system after Alzheimer’s disease (AD) (Qin et al., 2016). The neuropathological features of PD include the degeneration of dopaminergic neurons in the substantia nigra and the extensive misfolding and accumulation of α-synuclein, resulting in the formation of Lewy bodies (Dickson, 2018). Misfolded α-synuclein assembly begins in specific areas of the brain and spreads to other regions, similar to the spread of misfolded prion proteins (Jucker and Walker, 2013).
Inflammation, oxidative stress, infection, exposure to toxic substances, and other exogenous factors are considered to be likely contributors to nigral dopaminergic neurodegeneration (Arai et al., 2006). Recent evidence from PD patient autopsies and PD animal models has revealed that microglial activation, T cell infiltration, and increased levels of inflammatory factors play important roles in the degeneration of dopaminergic neurons (Whitton, 2007; Wang et al., 2015; Sanjari Moghaddam et al., 2018). However, the mechanisms underlying the initiation and spread of inflammation and the methods through which pathogenic α-synuclein protein are transported from affected cells to normal cells remain unclear.
Recently, a new mechanism of intercellular communication has emerged in the form of small extracellular vesicles (EVs). EVs can be divided into apoptotic bodies, microvesicles, and exosomes. Apoptotic bodies are 1–5 µm in diameter. Microvesicles, which range in size from 50 to 1000 nm, are produced by the direct germination and division of the plasma membrane into the extracellular space (Chan et al., 2019; Yu et al., 2020). Exosomes are bilayer, phospholipid membrane vesicles formed by the fusion of poly-vesicles and plasma membranes, with a diameter of 30–100 nm, and appear as double concave disc-shaped or cup-shaped vesicles when observed under an electron microscope (EL Andaloussi et al., 2013; Kourembanas, 2015).
Exosome-mediated, neuron-to-neuron α-synuclein transport is increasingly recognized as a potential cause of PD (Danzer et al., 2012; Chan et al., 2019). The contributions of neurogenic exosomes as active participants in the spread of neuroinflammation in neurodegenerative diseases have been increasingly recognized (Pascual et al., 2020). Neurons communicate with each other through exosomes, which can also facilitate the spread of inflammatory responses (Chan et al., 2019).
Exosome-mediated inflammatory processes are considered a promising intervention target for the treatment of PD. In this review, we discuss the role played by exosomes in mediating immune and inflammatory responses in PD, in addition to their effects on microglial activation, providing potential therapeutic strategies for delaying the progression of PD pathogenesis.
Retrieval Strategy
A computer-based online search of the PubMed database was performed to retrieve articles published up to January 31, 2021. A combination of the following text words (MeSH terms) was used to maximize search specificity and sensitivity: “Parkinson’s disease”; “inflammation”; “neuroinflammation”; “exosome”; “extracellular vesicles”; “glial cells”; “neuroglia”; “synucleins”; and “mitochondria.” The results were further screened by title and abstract, and only those studies exploring the relationship between inflammation and exosomes in PD were included to investigate the effects of exosomes on inflammatory characteristics in PD. No language or study type restrictions were applied. Articles involving only exosomes in PD without also examining inflammation were excluded.
Biological Characteristics of Exosomes
Exosomes refer to a special subtype of nanoscale EVs that were first discovered during the culturing and differentiation of sheep reticulocytes in the late 1980s (Johnstone et al., 1987) and were first detected in the blood of healthy people in 2005 (Ren et al., 2011). Exosomes are secreted from the cytoplasmic membrane into the extracellular environment due to the budding of the cytoplasmic membrane. The formation of exosomes can be roughly divided into three steps (Simons and Raposo, 2009; Bellingham et al., 2012; Rufino-Ramos et al., 2017; Hessvik and Llorente, 2018; Ortega et al., 2020), as shown in Figure 1.
Figure 1.
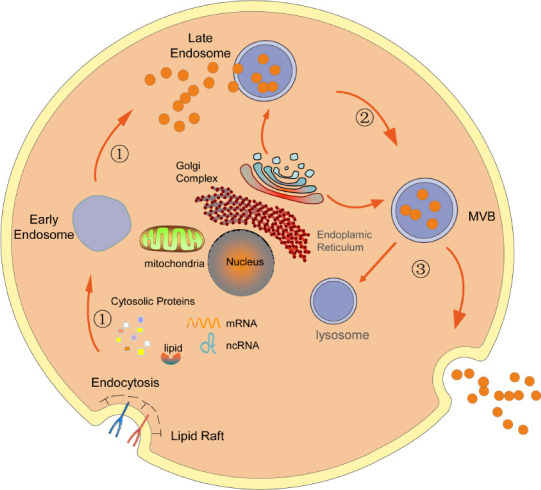
Formation of exosomes.
The formation of exosomes can be roughly divided into three steps: ① The cell membrane invaginates to form an intracellular vesicle through endocytosis, also known as an early endosome, which gradually transform into late endosomes; ② MVBs are gradually formed through the entry of some “cargo”; ③ some MVBs fuse with lysosomes and are degraded, whereas the remaining vesicles fuse with the cell membrane and are released to the extracellular space in the form of exosomes. MVB: Multivesicular bodies; ncRNA: noncoding RNA.
Exosomes are highly rich in tetraspanins (CD9, CD63, CD37, CD81, or CD82), a protein family located in microdomains of the membrane that interacts with various transmembrane and cytoplasmic signaling proteins (Caby et al., 2005; Simons and Raposo, 2009). Molecules transported within exosomes, including proteins, lipids, DNA, mRNA, and microRNAs (miRNAs), regulate the functions of recipient cells by promoting or suppressing gene expression (Chan et al., 2019). Vesicle-receptive cells can easily translate mRNA transcripts delivered through exosomes into proteins (Pascual et al., 2020). Multivesicular bodies (MVBs) are considered to serve as the aggregation site for miRNA pathway components, mature miRNAs, and target transcripts (Gibbings et al., 2009), which make MVBs candidates for the packaging of these elements into exosomes. Yu et al. (2020) reviewed the potential roles played by exosomes in the pathogenesis, diagnosis, treatment, and prognosis of PD. Exosomes appear to be involved in intercellular communications, antigen presentation, pathogen transmission, the immune response, programmed cell death, angiogenesis, the inflammatory response, and coagulation, among other processes (Simons and Raposo, 2009; Bellingham et al., 2012; Li et al., 2015; Zhang et al., 2015).
Exosomes have been proposed to serve as a new type of disease marker that can transmit information between cells, contributing to pathological mechanisms under different conditions (Danzer et al., 2012). Several studies have confirmed that exosomes play various roles in the pathogenesis of central nervous system diseases, such as PD, AD, and prion disease (Ciregia et al., 2017; Chan et al., 2019; Console et al., 2019). Exosomes in neurodegenerative diseases have been hypothesized to act as “Trojan horses,” which suggests an exosome-mediated mechanism for the transport of toxic substances from one cell to another, leading to cell death (Ghidoni et al., 2008). Several studies have proposed that exosomes may serve as potential intercellular carriers of pathogenic proteins that can lead to impaired neuronal function (Simons and Raposo, 2009; Bellingham et al., 2012).
Interestingly, several mutant genes associated with PD are also involved in the endocytic pathway, such as leucine-rich repeat kinase 2 (LRRK2) and vacuolar protein sorting-associated protein 35 (VPS35), further suggesting that exosomes play roles in the pathogenesis of PD. LRRK2 mutations lead to an abnormal increase in the number of MVBs, associated with varying morphologies, and the overexpression of LRRK2 is related to the endocytosis of synaptic vesicles and the inhibition of neuronal exocytosis (Connor-Robson et al., 2019). Previous work (Barile and Vassalli, 2017; Longoni et al., 2019; Vilaça-Faria et al., 2019; Leggio et al., 2020) summarized the roles played by exosomes in the pathogenesis of PD and supported the potential application of exosomes as biomarkers or drug carriers in PD.
Role of Exosomes in Neuroinflammation in Parkinson’s Disease
The relationship between inflammation and PD
Low-grade, chronic, systemic inflammation is a sign of aging and age-related diseases, such as neurodegenerative conditions (Giunta, 2006; Hou et al., 2019). The involvement of inflammatory responses in PD was first demonstrated in the 1980s (McGeer et al., 1988). Specific fluctuations in inflammatory components occur in both sporadic and familial forms of PD (Collins et al., 2012; Deleidi and Gasser, 2013; Qin et al., 2016). The autopsy results of PD patients showed that several pro-inflammatory cytokines and chemokines, including interleukin (IL)-1β, IL-2, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, C-X-C motif ligand (CXCL)12, C-X-C motif chemokine receptor (CXCR)4, and intracellular adhesion molecule (ICAM)-1, are increased in the nigrostriatal system and other brain regions (Boka et al., 1994; Mogi et al., 1994a, b; Shimoji et al., 2009; Zhou et al., 2019). Elevated levels of nitric oxide synthase (iNOS), nuclear factor (NF)-κB, cyclooxygenase (COX)-1, COX-2, and prostaglandin E2 have also been found in the brains of PD patients (Salvioli et al., 2006). Serum levels of NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) and IL-1β were significantly increased in PD patients, and NLRP3 levels are linearly correlated with α-synuclein levels (Chatterjee et al., 2020). Elevated levels of serum inflammatory biomarkers may indicate the progression of nonmotor impairments in PD (Veselý et al., 2018). In addition, levodopa therapy in PD patients may induce oxidative stress through various mechanisms, increasing the level of inflammatory markers and leading to abnormal biological mercaptan levels, apoptosis, or possible autophagy-mediated cell death (Andican et al., 2012). Future studies should focus on oxidative stress and microglial activation during dopamine metabolism in PD patients treated with levodopa (Dorszewska et al., 2014).
Increased peripheral inflammation is also associated with PD. Pro-inflammatory cytokine levels were found to be increased in cerebrospinal fluid (CSF) and blood samples from PD patients (Lindqvist et al., 2012; Dursun et al., 2015). A regulatory role for intestinal microbiota in motor dysfunction and neuroinflammation in PD models has been proposed (Sampson et al., 2016). The pathological distribution of α-synuclein in the intestinal submucosal nerve plexus and the mucosal nerve plexus from the esophagus to the rectum has been shown in PD. Recent studies suggest that the pathological distribution of α-synuclein in the gastrointestinal tract can be activated by the intestinal microflora without the need for pathogenic or environmental triggers (Caputi and Giron, 2018). The overstimulation of the innate immune system in response to intestinal biological dysregulation or the overgrowth of intestinal bacteria may cause intestinal glial cell activation and local and systemic inflammation, ultimately triggering the development of α-synuclein pathology (Sampson et al., 2016). Relationships among PD, intestinal microbiota, and intestinal pathogenicity have suggested that PD is associated with inflammation (Villarán et al., 2010; Prigent et al., 2019; Qiao et al., 2020). During PD, systemic inflammation is related to disease severity and rate of progression, and non-neuronal changes occur even before neurodegeneration is detected (White et al., 2018).
Currently available evidence suggests that a persistent inflammatory response, glial cell activation, and T cell infiltration play important roles in dopaminergic neuronal degeneration in PD (Joshi and Singh, 2018). The production of reactive oxygen species and pro-inflammatory cytokines is considered to represent the primary cause of dopaminergic cell death in PD (Ren et al., 2016). The increased production of inflammatory cytokines and microglial activation has been reported in brain tissues from PD patients. Peripheral inflammation can also increase the inflammatory response in the brain through a variety of mechanisms, which can trigger, promote, or aggravate disease development (Liu et al., 2019). Central and peripheral inflammation occurs during the prodromal phase of the disease and persists throughout the disease.
Exosomes act as mediators of neuroinflammation in PD
Several studies have emphasized the important roles played by exosomes in neuroinflammation and neurodegenerative diseases, particularly in response to toxic or pathological insults in the brain. Exosomes have potential diagnostic value as biomarkers in PD, such as miRNA-containing exosomes (Cao et al., 2017). One study demonstrated that new serum biomarkers could be used to identify and distinguish PD patients from control individuals using only a novel, multi-marker discovery approach (sequential and orthogonalized covariance selection), which indicates that PD patients present with unique inflammatory characteristics (Calvani et al., 2020). Circulating exosomes can also migrate through the blood-brain barrier (BBB) and produce biological effects on the neurons that internalize those vesicles, resulting in the subsequent release of exosomes in the central nervous system (Alvarez-Erviti et al., 2011). Exosomes released from peripheral immune cells, such as activated monocytes and macrophages, that cross the BBB can be internalized by neurons and astrocytes, resulting in the functional transfer of neurotoxic cargo and resulting in cell damage (Gupta and Pulliam, 2014). Inflammatory cytokines, such as IL-1β and TNF-α, can directly induce the death of dopaminergic neurons.
One study (Han et al., 2019) detected a significant increase in the inflammatory cytokines TNF-α and IL-1β in serum exosomes obtained from PD patients. The intravenous or striatal injection of serum exosomes derived from PD patients can cause protein accumulation, dopaminergic neuronal degeneration, and microglial activation, which provides evidence suggesting that the exosome-mediated transport of inflammatory cytokines further damages dopaminergic neurons. Exosomes purified from the serum of mice treated with lipopolysaccharide (LPS) can induce systemic and central nervous system inflammation (Li et al., 2018). One study purified small EVs/exosomes from serum samples obtained from older PD patients and used multiple immunoassays to identify the inflammatory biomolecules that served as cargo; low-level fusion (multi-platform) and partial least squares discriminant analysis was used to identify molecular signatures that were associated with both EVs in PD circulation and systemic inflammation (Picca et al., 2020). Published studies exploring the role played by exosomes in the pathogenesis of inflammation in PD are summarized in Table 1.
Table 1.
Summaries of studies exploring the roles of exosomes in Parkinson’s disease (PD)-associated inflammation
| Authors | Country | Study subjects | Main conclusions |
|---|---|---|---|
| Han et al., 2019 | China | 20 Sporadic mild-late-stage PD patients without any α-synuclein genetic variants and 20 controls (sex-and age-matched) | (1) Significant increases in TNF-α and IL-1β levels were detected in PD patient serum exosomes. (2) PD patient serum exosomes contained a higher density of oligomeric and monomeric α-synuclein than those from controls. (3) When PD exosomes were injected into the striatum of mice, the levels of IL-1β, α-synuclein, p-synuclein, and P62 increased. (4) Mice injected with PD exosomes in the striatum showed ipsilateral rotation, and mice injected with PD exosomes by intravenous injection showed obvious motor deficits. |
| Calvani et al., 2020 | Italy | 20 PD patients and 30 age-matched controls | Higher levels of IL-8 and MIP-1β and lower levels of IL-9 and MIP-1α were detected in PD patients. |
| Picca et al., 2020 | Italy | 20 older adult PD patients and 12 age- and sex-matched controls | (1) PD patients had more circulating exosomes than the control group, and the cell flux level of MQC was severely impaired. (2) CD9, CD63, ATP5A, NDUFS3, and SDHB levels were lower in PD patients. |
| Sarkar et al., 2019 | USA | Microglial cells were primed with LPS for 3 hours and then treated with Mn for a further 24 hours and compared with unstimulated or LPS-primed microglial cells | (1) ASC loaded in the exosome can stimulate the activation of inflammatory bodies in adjacent cells. (2) Exosomes isolated from welder serum-stimulated the expression of NLRP3 and pro-IL-1β, further validating the role of exosomes in modulating inflammasome propagation. |
| Tsutsumi et al., 2019 | Japan | Rat midbrain | Exosomes were involved in the effects of microglial activation on dopaminergic neurodegeneration. |
| Harischandra et al., 2018 | USA | MN9D-SynGFP cell line and MN9D-EVGFP cell line (300 mM MnCl2 exposure for 24 h) | (1) The miRNA released by the PD cell model involves a variety of biological processes, such as mitochondrial function, inflammation, autophagy, and protein aggregation. (2) MiR-125b (known pro-inflammatory miRNA) increases significantly. |
| Picca et al., 2019 | Italy | 20 PD patients and 20 sex- and age-matched controls | Isolated sEVs were used to identify the mitochondrial components, in particular respiratory chain complex subunits and mtDNA and MQC factors. |
ASC: The NLRP3 inflammasome oligomeric complex is composed of an adapter protein ASC (apoptosis-associated speck-like protein containing a CARD); ATP5A: adenosine triphosphate 5A; IL-8: interleukin-8; IL-9: interleukin-9; LPS: lipopolysaccharide; MIP-1α: macrophage inflammatory protein 1-α; MIP-1β: macrophage inflammatory protein 1-β; MN9D-EVGFP: vector control of MN9D dopaminergic cell; MN9D-SynGFP: GFP-positive α-synuclein-expressing MN9D dopaminergic cell; MQC: mitochondrial quality control; NDUFS3: NADH ubiquinone oxidoreductase subunit S3; NLRP3: nucleotide-binding and oligomerization domain-like receptor family pyrin domain-containing 3; SDHB: succinate dehydrogenase complex iron-sulfur subunit B; TNF-α: tumor necrosis factor-α.
Evidence supports a relationship between exosomes, inflammation, and the immune response. Proteins associated with PD, such as α-synuclein, are transferred from one cell to another. α-Synuclein is continuously released into the extracellular space, triggering an inflammatory cascade (Carrière et al., 2016). Immune cells can actively secrete exosomes after stimulation or in response to exosome stimulation. The changes in exosome composition that regulate gene expression and cell function are associated with the physiological and pathological status of the cells from which they are released (Chan et al., 2019); therefore, the exosomes in the blood or CSF obtained from PD patients at different stages can serve as potential biomarkers of disease progression.
Role of Exosomes in Neuron-Glia Communications in Parkinson’s Disease
The role of glial cells in neuroinflammation in PD
Glial cells have long been considered the primary contributors of cytokine and chemokine production and serve as the major cells expressing immune receptors in the brain. Microglial cells are the innate immune cells of the central nervous system (Kreutzberg, 1996), able to cause increased brain inflammation. Recently, immunoexcitotoxicity induced by microglia has been suggested to serve as the central mechanism underlying neurodegeneration in PD pathology (Blaylock, 2017; Joshi and Singh, 2018). Filamentous α-synuclein aggregates are widely deposited in astrocytes and oligodendrocytes in PD (Wakabayashi et al., 2000; Braak et al., 2007). Postmortem and in vivo positron emission tomography scans of PD patients showed an increased inflammatory response, including microglial activation (McGeer et al., 1988; Ouchi et al., 2005) and increased levels of immune markers (Boka et al., 1994; Mogi et al., 1994b, 1996).
Growing evidence indicates that neurons can also actively initiate and maintain the innate immune response (Préhaud et al., 2005). Even in the absence of immune cells, neurons appear capable of sensing and responding to viral infections (Préhaud et al., 2005). The interaction between neurons and microglia is bidirectional. Healthy neurons support resting microglia through membrane-binding signals, such as CD200 and CX3CL1, which promote the secretion of neurotransmitters and neurotrophins (Raivich, 2005; Hanisch and Kettenmann, 2007). Activated microglia can quickly change their morphology/phenotype and migrate to injured cells, releasing a large number of neurodegenerative and neuroprotective factors (Halliday and Stevens, 2011; Yokoyama et al., 2011; Richardson and Hossain, 2013). Activated microglia produce neurotoxic molecules, including cytokines, chemokines, complement proteins, and nitric oxide (NO) (Hanisch and Kettenmann, 2007). Human leukocyte antigen DR subtype(HLA-DR) is the major histocompatibility complex II protein, which is the most widely used marker to describe the activated microglia in the diseased brain. HLA-DR positive microglia have a wide range of activation forms (Walker and Lue, 2015). One study (McGeer et al., 1988) showed the presence of HLA-DR-positive microglia and Lewy bodies in the substantia nigra of patients with PD. An increase in the numbers of microglial cells was observed in the substantia nigra, putamen, hippocampus, olfactory cortex, cingulate cortex, and temporal lobe cortex of PD patients (Imamura et al., 2003) relative to normal controls. Other studies have confirmed the proliferation of activated and reactive astrocytes in PD (Imamura et al., 2003; Miklossy et al., 2006).
Studies have identified genes associated with immunity and inflammation that are upregulated in PD, which can directly or indirectly modulate various immunobiological processes, including T cell activation, B cell response, and the innate immune response, such as the activation of macrophages, microglia, and astrocytes (Deleidi and Gasser, 2013). Studies have also shown that mutations in Parkin or LRRK2, which are associated with familial PD, can affect innate immunity (Huang and Halliday, 2012; Allen Reish and Standaert, 2015; Lee et al., 2017). LRRK2 is highly expressed in antigen-presenting cells, including microglia, monocytes, and B cells, and participates in the immune response to pathogens (Miklossy et al., 2006). Gaucher disease, which is caused by a homozygous mutation in the GBA1 gene, is characterized by a persistent systemic inflammatory response, increased levels of pro-inflammatory molecules, and the loss of neurons, accompanied by the proliferation of astrocytes and microglia (Mizukami et al., 2002). Furthermore, LRRK2 and GBA not only affect the degradation of misfolded proteins in neurons but also regulate phagocytosis and the subsequent inflammatory response of microglia (Orenstein et al., 2013). Many PD-associated genes are highly expressed in astrocytes, such as DJ-1 and α-syn, which encodes α-synuclein. DJ-1 regulates the production of NO and pro-inflammatory cytokines, inhibiting the inflammatory responses of astrocytes (Waak et al., 2009).
Microglia are considered to be the key regulatory cells of neuroinflammation. M1 microglia activated in PD regulate pro-inflammatory cytokines and induce the production of inducible nitric oxide synthase (iNOS), reactive oxygen species (ROS), and reactive nitrogen species (RNS) (Janda et al., 2018). Microglia activated by LPS trigger the activation of A1 astrocytes through TNF-α, IL-1α, and C1q (Liddelow et al., 2017). Astrocytes are regulated by immune cells, such as monocytes and T cells, which can release neurotoxic factors and lead to neuronal degeneration in PD.
Microglia and astrocytes promote neuroinflammation in the substantia nigra, resulting in the death of dopaminergic neurons in PD. The process of neuroinflammation is associated with primary immune cell infiltration and microglia, in addition to the activation of reactive glial hyperplasia and neuronal death (Lee et al., 2019). Glial cells activated under neuroinflammatory conditions release pro-inflammatory and neurotoxic factors, leading to neuronal damage and neurodegeneration. Glial cells play a dual role, providing nutritional support to neurons and can provide neuroprotective support for neurons under certain stress conditions. For example, α-synuclein treatment trigger a defensive response by microglia to protect neurons from damage; however, excessive or denatured α-synuclein treatment can result in the overactivation of microglia, and the deposition of α-synuclein in astrocytes can initiate the release of damaging microglial factors that result in neuronal cell death (Zhang et al., 2017).
Glial cell-derived exosomes in PD-associated neuroinflammation
Microglia or macrophages in the central nervous system can clear pathogens and regulate neuroinflammation (Franco and Fernandez-Suarez, 2015). Glial cells secrete exosomes in response to different stimuli, and these exosomes have both protective and toxic effects on the nervous system. Glial cell-derived exosomes (GDEs) have been proposed to mediate glial cell functions and are essential for the interaction between neurons and glial cells (Fruhbeis et al., 2012; Pascual et al., 2020). Exosome transfer to neurons is mediated by oligodendrocytes, microglia cells, and astrocytes, which may be involved in either supporting neurons or transmitting diseases (Brites and Fernandes, 2015). GDEs can promote communications between glial cells and neurons and mediate anti-stress and anti-inflammatory responses and axon growth (Li et al., 2019). GDEs mediate the functions of parental cells by transporting mutated genes, damaged proteins, and pathogens to all cell types, which can lead to the onset of PD. GDEs also regulate neuroinflammation by transporting relevant proteins and miRNAs (Li et al., 2019). Simultaneously, α-synuclein can induce microglia to produce exosomes that are rich in major histocompatibility complex class II (MHC-II) and pro-inflammatory cytokines, such as TNF inducing neuronal apoptosis in a TNF-dependent manner (Chang et al., 2013).
Compared with other types of neurons, dopaminergic neurons are particularly vulnerable to inflammatory mediators, which may be due to the fact that microglia, which are highly responsive to pro-inflammatory stimuli, are enriched in areas containing dopaminergic neurons (Lawson et al., 1990; Chistiakov and Chistiakov, 2017). Glial exosomes containing inflammatory molecules can communicate with neurons and promote the occurrence and development of PD (Pascual et al., 2020). A previous study (Han et al., 2019) found that the intravenous or striatal injection of serum exosomes derived from PD patients to mice can cause protein aggregation, dopaminergic neuronal degeneration, microglia activation, and motor deficits.
Oligodendrocytes release exosomes that contain myelin, glycolytic enzymes, and typical exosome-associated proteins, among other components, to maintain axonal integrity (Fruhbeis et al., 2013). Astrocytes are involved in maintaining BBB integrity and regulating synaptic function. Type A1 reactive astrocytes lose their neurotrophic capacity and upregulate pro-inflammatory pathways, with toxic effects on synapses and neurons (Goetzl et al., 2018). Neurons communicate with astrocytes by secreting exosomes, resulting in neuron-dependent modifications of glutamate transporter expression (Pascual et al., 2020). Astrocyte-derived exosomes can also transport misfolded pathogenic proteins or abnormally expressed miRNAs to neurons, where they act to initiate or spread neuroinflammation (Gupta and Pulliam, 2014; Pascual et al., 2020).
GDEs are generally viewed to act as active participants in the spread of PD-associated neuroinflammation. GDEs can transfer α-synuclein between neurons and glial cells, promoting the transmission of PD. GDEs can even contain immune receptors, such as Toll-like receptor 4 (TLR4) and NLRP3, which can respond to tissue damage by releasing cytokines, chemokines, and inflammatory mediators. GDEs can spread to other glial cells and neurons, resulting in gradual disease progression.
Exosome-derived α-synuclein and microglia activation in PD
The role of exosome-derived α-synuclein in PD
The aggregation of neurotoxic α-synuclein in dopaminergic neurons represents a pathological feature of PD (Rocha et al., 2018). A presynaptic neuronal protein, α-synuclein, has been observed as EV cargo (Vella et al., 2016). The vesicle-mediated exocytosis from normal cells may be the primary source of extracellular α-synuclein. The level of α-synuclein found in exosomes derived from the central nervous system of patients with PD is significantly higher than that from healthy controls and correlates with disease severity (Shi et al., 2014).
The level of α-synuclein in plasma neuronal exosomes in PD patients was also significantly higher than that in healthy controls (Niu et al., 2020). The formation of large quantities of MVBs may lead to the accumulation of exosomes rich in toxic forms of α-synuclein, which can spread the pathological protein to neighboring cells, enhancing the disease process (Gupta and Pulliam, 2014; Brundin et al., 2016; Riazifar et al., 2019). Exosomes catalyze the polymerization kinetics and promote the aggregation of α-synuclein (Grey et al., 2015). Compared with free α-synuclein monomers, α-synuclein oligomers contained in exosomes are taken up more easily by target cells and spread more efficiently. Previous work showed that exosomes could efficiently transport α-synuclein oligomers between neurons and induce the α-synuclein oligomerization in normal neurons, promoting the transmission of pathological synuclein (Danzer et al., 2012).
When soluble α-synuclein binds to microglial cell surface receptors, such as TLR2 or TLR4, it may indirectly lead to an increase in oxidative stress, activating inflammatory pathways involving NF-κB and mitogen-activated protein kinase (MAPK) (Kim et al., 2016; Zhang et al., 2017). The TLR2 subtype is considered to be highly specific for the neuroinflammatory response of PD microglia. TLR4 not only senses extracellular α-synuclein and induces the signaling cascade involved in the inflammatory response but also promotes the clearance of α-synuclein through microglial phagocytosis (Zhang et al., 2017). The induction of NF-κB in microglia and other classical inflammatory pathways is also associated with the activation of astrocytes, which rapidly upregulate inflammatory signaling molecules, such as iNOS, which produces NO (Bauernfeind et al., 2009; Rocha et al., 2018). Treatment with α-synuclein does not result in NF-κB nuclear translocation in TLR4-deficient microglia, and the release of pro-inflammatory cytokine/chemokines was blocked, suggesting a role for TLR4 in the mediation of the inflammatory response to α-synuclein (Fellner et al., 2013). Exosomes from the CSF of PD patients can induce the oligomerization of soluble α-synuclein in target cells, further aggravating the disease (Stuendl et al., 2016). Additionally, activated exosomes can increase apoptosis, further supporting the hypothesis that exosomes can mediate neurodegeneration.
Exosomes are important mediators of the transmission of α-syn between cells, and α-syn can be detected both inside the exosome and on the surface of the exosomal membrane. The α-syn derived from the central nervous system is secreted from cells through exosomes in a calcium-dependent mechanism (Luo et al., 2016); therefore, the plasma exosome α-syn may reflect the state of the central nervous system. Toxic forms of aggregated α-syn and inflammatory factors can be effectively packaged into exosomes, activate the inflammatory cascade reaction, and induce the death of healthy neurons (Xia et al., 2019). The injection of exosomes containing antisense oligonucleotides 4 (Exo-ASO4) into the ventricle of α-syn A53T transgenic PD mice significantly decreased the expression of α-syn and reduced aggregation (Yang et al., 2021).
Microglia activation mediated by α-synuclein in PD
Current evidence suggests that aggregation and modification of α-synuclein are the main primary inducers of microglial activation (Lee, 2008), suggesting that α-synuclein is released during the early disease stage as an endogenous disease-related signal that activates microglia to release pro-inflammatory molecules (Chang et al., 2013; Mosley et al., 2013). Consistent with this idea, extracellular α-synuclein is absorbed by neurons and microglial cells in vitro. The delivery of α-synuclein stored in exosomes to target cells can lead to the accumulation of α-synuclein (Stuendl et al., 2016). Concentrated toxic forms of α-synuclein are generally cleared by astrocytes and microglia, but the excessive uptake of α-synuclein by glial cells can result in the formation of glial inclusions, which further aggravate the inflammatory response and lead to neurodegenerative changes (Vekrellis et al., 2011).
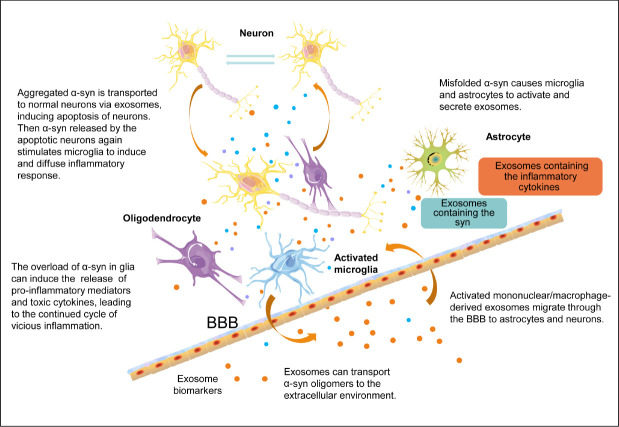
Neurons and other types of cells, including astrocytes, can internalize α-synuclein released by pathological neurons, resulting in the formation of pathological inclusion bodies and subsequent inflammation in the recipient cells (Lee et al., 2010). Under healthy conditions, astrocytes express α-synuclein at low levels or not at all (Lee et al., 2010). Misfolded α-synuclein causes microglia to activate and release toxic cytokine factors, which can metabolize and cleave natural α-synuclein, inducing misfolding and aggregation and resulting in the formation of pathological inclusion bodies (Guo et al., 2020). Misfolded synaptic proteins are released from cells and can be ingested by microglia, which causes them to be activated by the release of toxic cytokines, continuing a vicious circle. The release of various toxic cytokines by microglia can lead to the misfolding of α-synuclein in nearby cells, promoting the selective transmission and reproduction of synuclein pathology in vulnerable cell populations (Olanow et al., 2019) (Figure 2). In general, exosomes may be involved in various stages of the inflammatory process, including neuron-to-neuron communication, neuron-to-glia communication, and glia-to-glia communication. Exosomes provide an environment for α-syn aggregation and may promote the aggregation of α-syn oligomers. α-Syn induces the activation of microglia and astrocytes, and activated microglia, astrocytes, and T cells can interact to increase the release of humoral and pro-inflammatory cytokines, aggravate neuroinflammation and ROS production, and promote neuronal death (Yu et al., 2020) in a vicious cycle that exacerbates PD disease progression (Figure 2).
Figure 2.
Exosomes, α-synuclein (α-syn), and glial cells involved in the mechanisms underlying neuroinflammation and neurodegeneration in Parkinson’s disease.
The activation of microglia induced by α-syn can cause oxidative stress and a variety of pro-inflammatory changes that activate peripheral immune cells. Glial-derived exosomes containing inflammatory molecules can communicate with neurons and ultimately cause neurotoxicity. Increased neuronal death further activates the inflammatory mechanism, leading to a vicious cycle of inflammation and neuronal death. BBB: Blood-brain barrier.
Other studies have found that α-synuclein can induce microglia to secrete exosomes containing high levels of MHC and TNF-α (Chang et al., 2013), indicating that activated microglial exosomes may play important regulatory roles in α-synuclein-induced PD. Neuronal exosomes transmit misfolded α-synuclein to healthy neurons and astrocytes in PD, transport toxic α-synuclein oligomers to the extracellular environment, and induce inflammation and cell death (Lee et al., 2010). During sterile inflammation, molecules associated with aging and neuronal death may activate TLRs in neurons and glial cells to initiate a protective or harmful signaling cascade (Deleidi and Gasser, 2013). Pro-inflammatory and anti-inflammatory microglia can coexist, and anti-inflammatory molecules have been found in the CSF of PD patients, suggesting that microglial cells can play a variety of functions throughout the disease course. Microglia can both stimulate EVs to regulate synaptic transmission and enhance excitatory nerve transmission (Paolicelli et al., 2019) and produce immunomodulatory exosomes containing MHC-I, MHC-II, and inflammation-related miRNAs (Pascual et al., 2020).
These findings suggest that α-syn can positively regulate the inflammatory responses of microglia (Su et al., 2008). The overexpression of synaptophysin leads to an increase in the number of activated microglia found in the substantia nigra pars compacta, resulting in dopaminergic neuronal loss (Ren et al., 2016). Activated microglia act through different receptors to trigger different signaling pathway cascades associated with various inflammatory responses, such as NF-κB, phosphoinositide 3-phosphate (PI3K)/protein kinase B (AKT), TLR4-myeloid differentiation factor 88 (MyD88), P38, and extracellular signal-regulated kinase (ERK)1/2 MAPKs, which reduce the α-synuclein clearance ability, promoting the accumulation and transmission of α-syn and aggravating the loss of dopaminergic neurons and chronic neurodegeneration in PD (Klegeris et al., 2008; Prabhakaran et al., 2011; Cao et al., 2012; Su et al., 2014; Kim et al., 2016). Although the differential effects associated with monomeric and oligonucleotide synuclein interactions in microglia remain controversial, TLR2, TLR4, and the NLRP3 inflammasome are generally thought to be involved in the activation mechanisms of microglia (Ren et al., 2016).
Mitochondrial-Derived Vesicles and Inflammation
Mitochondrial dysfunction is related to PD, and increased oxidative stress promotes the abnormal folding and aggregation of proteins such as α-synuclein (Bouvier-Muller and Duconge, 2018). One study (Picca et al., 2019) identified specific inflammatory factors in mitochondria-derived exosomes obtained from blood samples from older PD patients. Mitochondrial DNA causes a series of inflammatory reactions through exosomes or other pathways, which participate in neuroimmunological disorders and lead to the spread of disease (Gambardella et al., 2019). Mildly damaged mitochondria can be activated by phosphatase and tensin homolog (PTEN)-induced kinase (PINK1) and Parkin to produce mitochondrial-derived vesicles (MDVs) (Mouton-Liger et al., 2017). MDVs form MVBs, which fuse with the plasma membrane and release exosomes that cause inflammation.
Current research regarding the mitochondrial indicators associated with PD exosomes has focused on mitochondrial DNA (mtDNA) and mitochondrial quality control (MQC) mechanisms (Franco-Iborra et al., 2018). In addition to the mitochondria-lysosomal axis, EV transport is considered to be an additional dimension of MQC. Proper MQC processes (i.e., mitochondrial protein stability, dynamics, and autophagy) can ensure the dynamic balance of organelles. New evidence suggests that circulating cell-free mtDNA serves as a damage-related molecular pattern (DAMP), which represents a functional link between mitochondrial damage and systemic inflammation (West et al., 2015; Picca et al., 2017). As a consequence of failed MQC, mitochondria release DAMPs that trigger inflammation by interacting with receptors involved in pathogen-related responses (Picca et al., 2019). Although the release of MDVs can trigger the clearance of dysfunctional organelles and avoids the transient release of intracellular toxic substances, aseptic and inflammatory immune responses may be induced through the activation of pattern recognition receptors on the cell membrane or in the cytoplasm (Picca et al., 2018). Extracellular vesicle transport also removes damaged organelles by producing MDVs, participating in MQC. The trafficking of EVs may also link inflammation to mitochondrial dysfunction. Extracellular mtDNA can trigger inflammatory responses by activating binding sites for the hypomethylated CpG motif, which resemble those found in bacterial DNA (Picca et al., 2019). Bacterial DNA that contains unmethylated CpG motif activates the release of pro-inflammatory cytokines by immune cells. These regions bind and activate membrane or cytoplasmic pattern recognition receptors, such as TLRs and nucleotide-binding oligomerization domain (NOD) -like receptors (Collins et al., 2004). Furthermore, the dysfunction of these mtDNA subunits may be related to insufficient protein clearance due to reduced secretion of MDVs in PD (Bouvier-Muller and Duconge, 2018). MDVs and the production and release of exosomes link PINK1 and Parkin signals to mitochondrial dysfunction and inflammation in PD (Matheoud et al., 2016; Picca et al., 2019). These observations have established a new mechanism linking mitochondrial dysfunction to systemic inflammation associated with PD.
Recent evidence supports that EV transport is related to inflammation and mitochondrial dysfunction. The identification of mitochondrial characteristics has indicated the presence of MDVs among EVs. The reduced secretion of MDVs was detected in PD, indicating impaired MQC flux. One consequence of a failed MQC is the release of mitochondrial-derived DAMPs, which can induce caspase-1 activation and the release of pro-inflammatory cytokines (Krysko et al., 2011). Mitochondrial-induced damage, especially the presence of cell-free mtDNA, has recently been thought to be associated with chronic inflammation (Picca et al., 2018). Cell-free mtDNA has been identified in exosomal-released molecules, and damaged mtDNA can trigger inflammation through three different signaling pathways, including TLR signaling, NLRP3 inflammasomes, and cytosolic cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING), which is a DNA sensing system-mediated pathway (Picca et al., 2018). However, changes in MQC and the release of MDV may represent signs of mitochondrial dysfunction, triggering systemic inflammation in PD. More research and exploration remain necessary.
Treatment of Parkinson’s Disease-Related Inflammation Based on Exosomes
Peripheral inflammation can be targeted, and neurodegeneration can be prevented without affecting the host’s immune system (Deleidi and Gasser, 2013). The long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) has been suggested to reduce the risk of developing sporadic PD and may delay or prevent the occurrence of PD (Bornebroek et al., 2007; Esposito et al., 2007; Rees et al., 2011). However, a meta-analysis concluded that NSAIDs did not affect the risk of PD at the population level (Poly et al., 2019). The effects and protective mechanisms of NSAIDs on the risk of PD remain controversial; therefore, new anti-inflammatory strategies should be explored.
At present, one of the most serious clinical problems is the lack of an optimized drug delivery system that will enable drugs to cross the BBB for the treatment of PD and has the ability to target specific tissues for the delivery of therapeutic doses to target regions without inducing immune responses and toxicity (van den Boorn et al., 2011). Although the viral-mediated delivery of therapeutic agents has been proposed for PD treatment, virus particles can be removed from the body by antibodies, or they may activate the immune response, making repeated administration challenging (Waehler et al., 2007).
In many pathologies, exosomes have been shown to improve diagnosis and disease surveillance, and exosomes have emerged as potential carriers for the targeted delivery of pharmacological compounds and gene therapy (Haney et al., 2015; Barile and Vassalli, 2017). After central nervous system injury, exosomes can cross the cell membrane and the BBB and transfer brain antigens to peripheral tissues, activating the innate immune response (de Rivero Vaccari et al., 2016). Exosomes are considered an ideal choice for drug delivery because of their low immunogenicity, their prion-like activity, their ability to transport molecules and interact with target cells, and the ease of manipulation for the administration of personalized drugs (Brites and Fernandes, 2015). The use of exosomes can evade immune activation, encourage uptake by target cells, and solve many other problems in the field of drug delivery, as well as prevent the degradation of cargos.
Exosome-based drug delivery systems were initially applied to AD a decade ago (Alvarez-Erviti et al., 2011). The intravenous injection of exosomes loaded with anti-BACE1 small interfering RNA targeted neurons, microglia, and oligodendrocytes into the brains of mice induced the downregulation of BACE1 expression (Alvarez-Erviti et al., 2011). One study (Sun et al., 2010) proposed the encapsulation of anti-inflammatory agents, such as curcumin, within exosomes to treat inflammation, and a subsequent study showed that curcumin loaded into exosomes and administered through the nose reduced inflammation in the brains of mice (Zhuang et al., 2011). Strategies for loading contents into exosomes continue to be optimized (Porro et al., 2019). A specific mRNA packaging device and a cytoplasmic delivery assist device have been successfully developed to significantly increase cell-to-cell communication without the need to concentrate exosomes (Kojima et al., 2018). Haney et al. (2011, 2013) also developed an exosomal-based antioxidant catalase delivery system and demonstrated that ROS levels were significantly reduced compared to non-exosome exposed macrophages. Exosome-delivered catalase has been shown to reduce neurotoxicity and neuroinflammation in both in vitro and in vivo models of PD (Haney et al., 2015; Kojima et al., 2018). One study demonstrated significant therapeutic effects by loading exosomes with dopamine in in vitro and in vivo models of PD, which significantly reduced the systemic toxicity associated with free dopamine (Qu et al., 2018).
The roles played by exosomes secreted by monocytes and macrophages in neuroinflammation provide an unprecedented opportunity for the treatment of PD, providing a mechanism that would avoid interference from mononuclear phagocytes and promoting the delivery of drugs to targeted cells. Furthermore, a method for the isolation of exosomes from bone marrow mesenchymal stem cell (MSC)-conditioned medium has been widely developed (Kourembanas, 2015). MSC-derived secretions or exosomes have been successfully used as a potential therapy to combat the progression of PD and improve symptoms, resulting in similar therapeutic effects as MSC transplantation, including reduced neuroinflammation, enhanced antioxidant capacity, and the increased expression of neurotrophic factors (Chen et al., 2020; d’Angelo et al., 2020). Exosomes carrying miRNAs or other molecules can improve the therapeutic efficacy of MSC transplantation (Porro et al., 2019). miRNA-7 targets the NLRP3 inflammasome and suppresses nigrostriatal a-syn to modulate neuroinflammation during the pathogenesis of PD (Zhou et al., 2016). Antago-miR-155 also reduced microglial activation and neuroinflammation (Thome et al., 2016).
In summary, exosomes are becoming a promising tool for PD inflammation therapy (Wani et al., 2020). The selected components of naturally secreted exosomes can be combined into nanoparticles or liposomes to enhance stability, immunogenicity, targeting, and uptake (Barile and Vassalli, 2017). Exosomes can regulate the immune system as a carrier of antigen presentation molecules but also act as an immunosuppressive factor to prevent inflammation (Fu et al., 2020; Lindenbergh et al., 2020). Future efforts should focus on improving the manufacturing process, properties, and safety issues associated with exosomes to move these therapies closer to clinical practice.
Conclusions
Exosomes have been proposed to serve as possible biomarkers and pluripotent therapeutic delivery systems for PD. Exosomes participate in the inflammatory response of PD, which involves glial cell activation and infiltration by peripheral immune cells. The relationships linking different forms of glial cell activation, α-synuclein, and exosomes are complex. Exosomes can induce both pro-inflammatory and anti-inflammatory effects and can be used as therapeutic agents. The neuroinflammatory response is regulated by immune cells (such as microglia, astrocytes, and peripheral immune cells), cytokines, and chemokines (Pascual et al., 2020). The blockade of A1 astrocyte transformation through M1 microglial activation has been proposed to serve as a protective mechanism for dopaminergic neurons in a PD disease model (Yun et al., 2018). A better understanding of how to induce different astrocytic phenotypes in a controlled manner may help control harmful neuroinflammatory responses and facilitate the development of new therapies for neuronal repair in PD.
Although changes in MQC and the release of MDVs may indicate the presence of systemic inflammation caused by mitochondrial dysfunction in PD, whether and how mitochondrial DAMPs are involved in the inflammatory response remain unclear. Although a considerable number of analytes have been detected, which may provide more insights into the relationship between EVs transport, inflammation, and mitochondrial dysfunction in PD, the causal relationship between these various mediators and the pathophysiology of PD has not yet been determined. Further exploration of exosomes containing specific genes or expressed proteins for use as potential biological markers remains necessary. How to accurately judge disease progression and how to ensure the specificity and stability of disease markers based on the detection of specific factors in exosomes remain goals of research efforts. GDEs have shown potential for triggering regeneration, in addition to pro- or anti-inflammatory and anti-stress effects for treating PD, which merits further exploration. Furthermore, how to make full use of exosomes derived from MSC, how to further enhance the therapeutic effects of exosomes, how to improve the drug loading of exosomes, and how to achieve accurate treatment remain future research directions.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Funding: This work was supported by the National Natural Science Foundation of China, No. 81960242 (to XLY); Yunnan Applied Basic Research Project of Yunnan Province of China, Nos. 2019FE001-048 (to XLY), 202001AT070001 (to XLY), “One Hundred Young and Middle-aged Academic and Technical Backbone” Training Program of Kunming Medical University, No. 60118260105 (to XLY); Miaozi Project in Science and Technology Innovation Program of Sichuan Province, No. 2020JDRC0057 (to HYH).
References
- 1.Allen Reish H, Standaert D. Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J Parkinsons Dis. 2015;5:1–19. doi: 10.3233/JPD-140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood M. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 3.Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Firtiına S, Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol Belg. 2012;112:155–159. doi: 10.1007/s13760-012-0015-3. [DOI] [PubMed] [Google Scholar]
- 4.Arai H, Furuya T, Mizuno Y, Mochizuki H. Inflammation and infection in Parkinson’s disease. Histol Histopathol. 2006;21:673–678. doi: 10.14670/HH-21.673. [DOI] [PubMed] [Google Scholar]
- 5.Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellingham S, Guo B, Coleman B, Hill A. Exosomes: vehicles for the transfer of toxic proteins associated with neurodegenerative diseases. Front Physiol. 2012;3:124. doi: 10.3389/fphys.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaylock RL. Parkinson’s disease: microglial/macrophage-induced immunoexcitotoxicity as a central mechanism of neurodegeneration. Surg Neurol Int. 2017;8:65. doi: 10.4103/sni.sni_441_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch E. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 10.Bornebroek M, de Lau L, Haag M, Koudstaal P, Hofman A, Stricker B, Breteler M. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology. 2007;28:193–196. doi: 10.1159/000108110. [DOI] [PubMed] [Google Scholar]
- 11.Bouvier-Muller A, Duconge F. Nucleic acid aptamers for neurodegenerative diseases. Biochimie. 2018;145:73–83. doi: 10.1016/j.biochi.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Sastre M, Del Tredici K. Development of alpha-synuclein immunoreactive astrocytes in the forebrain parallels stages of intraneuronal pathology in sporadic Parkinson’s disease. Acta Neuropathol. 2007;114:231–241. doi: 10.1007/s00401-007-0244-3. [DOI] [PubMed] [Google Scholar]
- 13.Brites D, Fernandes A. Neuroinflammation and depression: microglia, activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. 2015;9:476. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brundin P, Ma J, Kordower JH. How strong is the evidence that Parkinson’s disease is a prion disorder. Curr Opin Neurol. 2016;29:459–466. doi: 10.1097/WCO.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caby M, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 16.Calvani R, Picca A, Landi G, Marini F, Biancolillo A, Coelho-Junior HJ, Gervasoni J, Persichilli S, Primiano A, Arcidiacono A, Urbani A, Bossola M, Bentivoglio AR, Cesari M, Bernabei R, Monaco MRL, Marzetti E. A novel multi-marker discovery approach identifies new serum biomarkers for Parkinson’s disease in older people: an EXosomes in PArkiNson Disease (EXPAND) ancillary study. Geroscience. 2020;42:1323–1334. doi: 10.1007/s11357-020-00192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao S, Standaert D, Harms A. The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J Neuroinflammation. 2012;9:259. doi: 10.1186/1742-2094-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X, Lu J, Zhao Z, Li M, Lu T, An X, Xue L. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett. 2017;644:94–99. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Caputi V, Giron Maria C. Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. Int J Mol Sci. 2018;19:1689. doi: 10.3390/ijms19061689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrière J, Bretin A, Darfeuille-Michaud A, Barnich N, Nguyen H. Exosomes released from cells infected with Crohn’s disease-associated adherent-invasive escherichia coli activate host innate immune responses and enhance bacterial intracellular replication. Inflamm Bowel Dis. 2016;22:516–528. doi: 10.1097/MIB.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 21.Chan B, Wong W, Lee M, Cho W, Yee B, Kwan Y, Tai W. Exosomes in inflammation and inflammatory disease. Proteomics. 2019;19:e1800149. doi: 10.1002/pmic.201800149. [DOI] [PubMed] [Google Scholar]
- 22.Chang C, Lang H, Geng N, Wang J, Li N, Wang X. Exosomes of BV-2 cells induced by alpha-synuclein: important mediator of neurodegeneration in PD. Neurosci Lett. 2013a;548:190–195. doi: 10.1016/j.neulet.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee K, Roy A, Banerjee R, Choudhury S, Mondal B, Halder S, Basu P, Shubham S, Dey S, Kumar H. Inflammasome and α-synuclein in Parkinson’s disease: a cross-sectional study. J Neuroimmunol. 2020;338:577089. doi: 10.1016/j.jneuroim.2019.577089. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Liang F, Gu P, Xu B, Xu H, Wang W, Hou J, Xie D, Chai X, An S. Exosomes derived from mesenchymal stem cells repair a Parkinson’s disease model by inducing autophagy. Cell Death Dis. 2020;11:288. doi: 10.1038/s41419-020-2473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chistiakov DA, Chistiakov AA. α-Synuclein-carrying extracellular vesicles in Parkinson’s disease: deadly transmitters. Acta Neurol Belg. 2017;117:43–51. doi: 10.1007/s13760-016-0679-1. [DOI] [PubMed] [Google Scholar]
- 26.Ciregia F, Urbani A, Palmisano G. Extracellular vesicles in brain tumors and neurodegenerative diseases. Front Mol Neurosci. 2017;10:276. doi: 10.3389/fnmol.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins L, Hajizadeh S, Holme E, Jonsson I, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol. 2004;75:995–1000. doi: 10.1189/jlb.0703328. [DOI] [PubMed] [Google Scholar]
- 28.Collins L, Toulouse A, Connor T, Nolan Y. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology. 2012;62:2154–2168. doi: 10.1016/j.neuropharm.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Connor-Robson N, Booth H, Martin JG, Gao B, Li K, Doig N, Vowles J, Browne C, Klinger L, Juhasz P, Klein C, Cowley SA, Bolam P, Hirst W, Wade-Martins R. An integrated transcriptomics and proteomics analysis reveals functional endocytic dysregulation caused by mutations in LRRK2. Neurobiol Dis. 2019;127:512–526. doi: 10.1016/j.nbd.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Console L, Scalise M, Indiveri C. Exosomes in inflammation and role as biomarkers. Clin Chim Acta. 2019;488:165–171. doi: 10.1016/j.cca.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 31.d’Angelo M, Cimini A, Castelli V. Insights into the effects of mesenchymal stem cell-derived secretome in Parkinson’s disease. Int J Mol Sci. 2020;21:5241. doi: 10.3390/ijms21155241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danzer K, Kranich L, Ruf W, Cagsal-Getkin O, Winslow A, Zhu L, Vanderburg C, McLean P. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Rivero Vaccari JP, Brand F, 3rd , Adamczak S, Lee SW, Perez-Barcena J, Wang MY, Bullock MR, Dietrich WD, Keane RW. Exosome-mediated inflammasome signaling after central nervous system injury. J Neurochem 136 Suppl. 2016;1:39–48. doi: 10.1111/jnc.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deleidi M, Gasser T. The role of inflammation in sporadic and familial Parkinson’s disease. Cell Mol Life Sci. 2013;70:4259–4273. doi: 10.1007/s00018-013-1352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickson D. Neuropathology of Parkinson disease. Parkinsonism Relat Disord. 2018;46(Suppl 1):S30–S33. doi: 10.1016/j.parkreldis.2017.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dorszewska J, Prendecki M, Lianeri M, Kozubski W. Molecular effects of l-dopa therapy in parkinson’s disease. Curr Genomics. 2014;15:11–17. doi: 10.2174/1389202914666131210213042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dursun E, Gezen-Ak D, Hanağası H, Bilgiç B, Lohmann E, Ertan S, Atasoy İ, Alaylıoğlu M, Araz Ö, Önal B, Gündüz A, Apaydın H, Kızıltan G, Ulutin T, Gürvit H, Yılmazer S. The interleukin 1, alpha, interleukin 1, beta, interleukin 6 and alpha-2-macroglobulin serum levels in patients with early or late onset Alzheimer’s, disease, mild cognitive impairment or Parkinson’s disease. J Neuroimmunol. 2015;283:50–57. doi: 10.1016/j.jneuroim.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 38.EL Andaloussi S, Mäger I, Breakefield X, Wood M. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 39.Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning G, Stefanova N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco-Iborra S, Vila M, Perier C. Mitochondrial quality control in neurodegenerative diseases: focus on parkinson’s disease and huntington’s disease. Front Neurosci. 2018;12:342. doi: 10.3389/fnins.2018.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franco R, Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Fruhbeis C, Frohlich D, Kramer-Albers EM. Emerging roles of exosomes in neuron-glia communication. Front Physiol. 2012;3:119. doi: 10.3389/fphys.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fruhbeis C, Frohlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Mobius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Kramer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11:e1001604. doi: 10.1371/journal.pbio.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu C, Peng P, Loschko J, Feng L, Pham P, Cui W, Lee K, Krug A, Jiang A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc Natl Acad Sci U S A. 2020;117:23730–23741. doi: 10.1073/pnas.2002345117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gambardella S, Limanaqi F, Ferese R, Biagioni F, Campopiano R, Centonze D, Fornai F. ccf-mtDNA as a potential link between the brain and immune system in neuro-immunological disorders. Front Immunol. 2019;10:1064. doi: 10.3389/fimmu.2019.01064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghidoni R, Benussi L, Binetti G. Exosomes: the Trojan horses of neurodegeneration. Med Hypotheses. 2008;70:1226–1227. doi: 10.1016/j.mehy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Gibbings D, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 49.Giunta S. Is inflammaging an auto[innate]immunity subclinical syndrome. Immun Ageing. 2006;3:12. doi: 10.1186/1742-4933-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83:544–552. doi: 10.1002/ana.25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, Linse S. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem. 2015;290:2969–2982. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo M, Wang J, Zhao Y, Feng Y, Han S, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate alpha-synuclein transmission in Parkinson’s disease. Brain. 2020;143:1476–1497. doi: 10.1093/brain/awaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halliday G, Stevens C. Glia: initiators and progressors of pathology in Parkinson’s disease. Mov Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 55.Han C, Xiong N, Guo X, Huang J, Ma K, Liu L, Xia Y, Shen Y, Li J, Jiang H, Wang L, Guo S, Xu X, Zhang G, Liu J, Cao X, Zhang Z, Lin Z, Wang T. Exosomes from patients with Parkinson’s disease are pathological in mice. J Mol Med. 2019;97:1329–1344. doi: 10.1007/s00109-019-01810-z. [DOI] [PubMed] [Google Scholar]
- 56.Haney M, Klyachko N, Zhao Y, Gupta R, Plotnikova E, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov A, Batrakova E. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haney M, Zhao Y, Li S, Higginbotham S, Booth S, Han H, Vetro J, Mosley R, Kabanov A, Gendelman H, Batrakova E. Cell-mediated transfer of catalase nanoparticles from macrophages to brain, endothelial, glial and neuronal cells. Nanomedicine (Lond) 2011;6:1215–1230. doi: 10.2217/nnm.11.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haney M, Zhao Y, Harrison E, Mahajan V, Ahmed S, He Z, Suresh P, Hingtgen S, Klyachko N, Mosley R, Gendelman H, Kabanov A, Batrakova E. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One. 2013;8:e61852. doi: 10.1371/journal.pone.0061852. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Hanisch U, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 60.Harischandra DS, Ghaisas S, Rokad D, Zamanian M, Jin H, Anantharam V, Kimber M, Kanthasamy A, Kanthasamy AG. Environmental neurotoxicant manganese regulates exosome-mediated extracellular miRNAs in cell culture model of Parkinson’s disease: relevance to alpha-synuclein misfolding in metal neurotoxicity. Neurotoxicology. 2018;64:267–277. doi: 10.1016/j.neuro.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hessvik N, Llorente A. Current knowledge on exosome biogenesis and release. Cellular Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch S, Croteau D, Bohr V. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 63.Huang Y, Halliday G. Aspects of innate immunity and Parkinson’s disease. Front Pharmacol. 2012;3:33. doi: 10.3389/fphar.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- 65.Janda E, Boi L, Carta A. Microglial phagocytosis and its regulation: a therapeutic target in parkinson’s disease. Front Mol Neurosci. 2018;11:144. doi: 10.3389/fnmol.2018.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnstone R, Adam M, Hammond J, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 67.Joshi N, Singh S. Updates on immunity and inflammation in Parkinson disease pathology. J Neurosci Res. 2018;96:379–390. doi: 10.1002/jnr.24185. [DOI] [PubMed] [Google Scholar]
- 68.Jucker M, Walker L. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim C, Lee H, Masliah E, Lee S. Non-cell-autonomous neurotoxicity of α-synuclein through microglial toll-like receptor 2. Exp Neurobiol. 2016;25:113–119. doi: 10.5607/en.2016.25.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klegeris A, Pelech S, Giasson B, Maguire J, Zhang H, McGeer E, McGeer P. Alpha-synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol Aging. 2008;29:739–752. doi: 10.1016/j.neurobiolaging.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Kojima R, Bojar D, Rizzi G, Hamri G, El-Baba M, Saxena P, Ausländer S, Tan K, Fussenegger M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9:1305. doi: 10.1038/s41467-018-03733-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kourembanas S. Exosomes: vehicles of intercellular signaling biomarkers and vectors of cell therapy. Ann Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 73.Kreutzberg G. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 74.Krysko D, Agostinis P, Krysko O, Garg A, Bachert C, Lambrecht B, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Lawson L, Perry V, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 76.Lee H, James W, Cowley S. LRRK2 in peripheral and central nervous system innate immunity: its link to Parkinson’s disease. Biochem Soc Trans. 2017;45:131–139. doi: 10.1042/BST20160262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;285:9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee S. Origins and effects of extracellular alpha-synuclein: implications in Parkinson's disease. J Mol Neurosci. 2008;34:17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 79.Lee Y, Lee S, Chang S, Lee J. Significant roles of neuroinflammation in Parkinson’s disease: therapeutic targets for PD prevention. Arch Pharm Res. 2019;42:416–425. doi: 10.1007/s12272-019-01133-0. [DOI] [PubMed] [Google Scholar]
- 80.Leggio L, Paternò G, Vivarelli S, L’Episcopo F, Tirolo C, Raciti G, Pappalardo F, Giachino C, Caniglia S, Serapide M, Marchetti B, Iraci N. Extracellular vesicles as nanotherapeutics for Parkinson’s disease. Biomolecules. 2020;10:1327. doi: 10.3390/biom10091327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li H, Luo Y, Zhu L, Hua W, Zhang Y, Zhang H, Zhang L, Li Z, Xing P, Zhang Y, Hong B, Yang P, Liu J. Glia-derived exosomes: promising therapeutic targets. Life Sci. 2019;239:116951. doi: 10.1016/j.lfs.2019.116951. [DOI] [PubMed] [Google Scholar]
- 82.Li J, Wang B, Kodali M, Chen C, Kim E, Patters B, Lan L, Kumar S, Wang X, Yue J, Liao F. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflamm. 2018;15:8. doi: 10.1186/s12974-017-1038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li M, Rai A, DeCastro G, Zeringer E, Barta T, Magdaleno S, Setterquist R, Vlassov A. An optimized procedure for exosome isolation and analysis using serum samples: application to cancer biomarker discovery. Methods. 2015;87:26–30. doi: 10.1016/j.ymeth.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 84.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindenbergh M, Wubbolts R, Borg E, van ’t Veld E, Boes M, Stoorvogel W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J Extracell Vesicles. 2020;9:1798606. doi: 10.1080/20013078.2020.1798606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with Parkinson’s disease -correlations with inflammatory cytokines in serum. PLoS One. 2012;7:e47387. doi: 10.1371/journal.pone.0047387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu Y, Sun M, Cai C, Ren C, Niu H. Research progress on neural mechanism of peripheral inflammation in Parkinson’s disease. Sheng Li Xue Bao. 2019;71:732–740. [PubMed] [Google Scholar]
- 88.Longoni B, Fasciani I, Kolachalam S, Pietrantoni I, Marampon F, Petragnano F, Aloisi G, Coppolino MF, Rossi M, Scarselli M, Maggio R. Neurotoxic and neuroprotective role of exosomes in Parkinson’s disease. Curr Pharm Des. 2019;25:4510–4522. doi: 10.2174/1381612825666191113103537. [DOI] [PubMed] [Google Scholar]
- 89.Luo H, Zhang J, Miao F. Effects of pramipexole treatment on the α-synuclein content in serum exosomes of Parkinson’s disease patients. Exp Ther Med. 2016;12:1373–1376. doi: 10.3892/etm.2016.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matheoud D, Sugiura A, Bellemare-Pelletier A, Laplante A, Rondeau C, Chemali M, Fazel A, Bergeron JJ, Trudeau LE, Burelle Y, Gagnon E, McBride HM, Desjardins M. Parkinson’s disease-related proteins PINK1 and Parkin repress mitochondrial antigen presentation. Cell. 2016;166:314–327. doi: 10.1016/j.cell.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 91.McGeer P, Itagaki S, Boyes B, McGeer E. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 92.Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 93.Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, Liu Y, Werth N, Sandhoff R, Sandhoff K, Proia R. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109:1215–1221. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1, beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994b;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- 95.Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1,beta,IL-2,IL-4,IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- 96.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994a;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 97.Mosley RL, Codolo G, Plotegher N, Pozzobon T, Brucale M, Tessari I, Bubacco L, de Bernard M. Triggering of inflammasome by aggregated α-synuclein an inflammatory response in synucleinopathies. PLoS One. 2013;8:e55375. doi: 10.1371/journal.pone.0055375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mouton-Liger F, Jacoupy M, Corvol J, Corti O. PINK1/Parkin-dependent mitochondrial surveillance: from pleiotropy to Parkinson’s disease. Front Mol Neurosci. 2017;10:120. doi: 10.3389/fnmol.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Niu M, Li Y, Li G, Zhou L, Luo N, Yao M, Kang W, Liu J. A longitudinal study on alpha-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur J Neurol. 2020;27:967–974. doi: 10.1111/ene.14208. [DOI] [PubMed] [Google Scholar]
- 100.Olanow CW, Savolainen M, Chu Y, Halliday GM, Kordower JH. Temporal evolution of microglia and alpha-synuclein accumulation following foetal grafting in Parkinson’s disease. Brain. 2019;142:1690–1700. doi: 10.1093/brain/awz104. [DOI] [PubMed] [Google Scholar]
- 101.Orenstein S, Kuo S, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig L, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo A. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ortega A, Martinez-Arroyo O, Forner M, Cortes R. Exosomes as drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics. 2020;13:3. doi: 10.3390/pharmaceutics13010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 104.Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience. 2019;405:148–157. doi: 10.1016/j.neuroscience.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 105.Pascual M, Ibáñez F, Guerri C. Exosomes as mediators of neuron-glia communication in neuroinflammation. Neural Regen Res. 2020;15:796–801. doi: 10.4103/1673-5374.268893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Picca A, Guerra F, Calvani R, Marini F, Biancolillo A, Landi G, Beli R, Landi F, Bernabei R, Bentivoglio AR, Monaco MRL, Bucci C, Marzetti E. Mitochondrial signatures in circulating extracellular vesicles of older adults with parkinson’s disease: results from the EXosomes in PArkiNson’s Disease (EXPAND) Study. J Clin Med. 2020;9:504. doi: 10.3390/jcm9020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Picca A, Guerra F, Calvani R, Bucci C, Lo Monaco M, Bentivoglio A, Landi F, Bernabei R, Marzetti E. Mitochondrial-derived vesicles as candidate biomarkers in parkinson’s disease: rationale design and methods of the EXosomes in PArkiNson disease (EXPAND) study. Int J Mo Sci. 2019;20:2373. doi: 10.3390/ijms20102373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Picca A, Lezza AMS, Leeuwenburgh C, Pesce V, Calvani R, Landi F, Bernabei R, Marzetti E. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci. 2017;18:933. doi: 10.3390/ijms18050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Picca A, Lezza AMS, Leeuwenburgh C, Pesce V, Calvani R, Bossola M, Manes-Gravina E, Landi F, Bernabei R, Marzetti E. Circulating mitochondrial DNA at the crossroads of mitochondrial dysfunction and inflammation during aging and muscle wasting disorders. Rejuvenation Res. 2018;21:350–359. doi: 10.1089/rej.2017.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Poly TN, Islam MMR, Yang HC, Li YJ. Non-steroidal anti-inflammatory drugs and risk of Parkinson’s disease in the elderly population: a meta-analysis. Eur J Clin Pharmacol. 2019;75:99–108. doi: 10.1007/s00228-018-2561-y. [DOI] [PubMed] [Google Scholar]
- 111.Porro C, Panaro MA, Lofrumento DD, Hasalla E, Trotta T. The multiple roles of exosomes in Parkinson’s disease: an overview. Immunopharmacol Immunotoxicol. 2019;41:469–476. doi: 10.1080/08923973.2019.1650371. [DOI] [PubMed] [Google Scholar]
- 112.Préhaud C, Mégret F, Lafage M, Lafon M. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol. 2005;79:12893–12904. doi: 10.1128/JVI.79.20.12893-12904.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prabhakaran K, Chapman G, Gunasekar P. α-Synuclein overexpression enhances manganese-induced neurotoxicity through the NF-κB-mediated pathway. Toxicol Mech Methods. 2011;21:435–443. doi: 10.3109/15376516.2011.560210. [DOI] [PubMed] [Google Scholar]
- 114.Prigent A, Gonzales J, Durand T, Le Berre-Scoul C, Rolli-Derkinderen M, Neunlist M, Derkinderen P. Acute inflammation down-regulates alpha-synuclein expression in enteric neurons. J Neurochem. 2019;148:746–760. doi: 10.1111/jnc.14656. [DOI] [PubMed] [Google Scholar]
- 115.Qiao C, Sun M, Jia X, Li Y, Zhang B, Zhao L, Shi Y, Zhou Z, Zhu Y, Cui C, Shen Y. Sodium butyrate exacerbates Parkinson’s disease by aggravating neuroinflammation and colonic inflammation in MPTP-induced mice model. Neurochem Res. 2020;45:2128–2142. doi: 10.1007/s11064-020-03074-3. [DOI] [PubMed] [Google Scholar]
- 116.Qin X, Zhang S, Cao C, Loh Y, Cheng Y. Aberrations in peripheral inflammatory cytokine levels in parkinson disease: a systematic review and meta-analysis. JAMA Neurol. 2016;73:1316–1324. doi: 10.1001/jamaneurol.2016.2742. [DOI] [PubMed] [Google Scholar]
- 117.Qu M, Lin Q, Huang L, Fu Y, Wang L, He S, Fu Y, Yang S, Zhang Z, Zhang L, Sun X. Dopamine-loaded blood exosomes targeted to brain for better treatment of Parkinson’s disease. J Control Release. 2018;287:156–166. doi: 10.1016/j.jconrel.2018.08.035. [DOI] [PubMed] [Google Scholar]
- 118.Raivich G. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 2005;28:571–573. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 119.Rees K, Stowe R, Patel S, Ives N, Breen K, Clarke C, Ben-Shlomo Y. Non-steroidal antiinflammatory drugs as disease-modifying agents for Parkinson's disease: evidence from observational studies. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008454.pub2. doi:10.1002/14651858.CD008454.pub2. [DOI] [PubMed] [Google Scholar]
- 120.Ren W, Tian Z, Yin F, Sun J, Zhang J. Extracellular α-synuclein--a possible initiator of inflammation in Parkinson’s disease. Pharmazie. 2016;71:51–55. [PubMed] [Google Scholar]
- 121.Ren Y, Yang J, Xie R, Gao L, Yang Y, Fan H, Qian K. Exosomal-like vesicles with immune-modulatory features are present in human plasma and can induce CD4+ T-cell apoptosis in vitro. Transfusion. 2011;51:1002–1011. doi: 10.1111/j.1537-2995.2010.02909.x. [DOI] [PubMed] [Google Scholar]
- 122.Riazifar M, Mohammadi MR, Pone EJ, Yeri A, Lässer C, Segaliny AI, McIntyre LL, Shelke GV, Hutchins E, Hamamoto A, Calle EN, Crescitelli R, Liao W, Pham V, Yin Y, Jayaraman J, Lakey JRT, Walsh CM, Van Keuren-Jensen K, Lotvall J, et al. Stem cell-derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13:6670–6688. doi: 10.1021/acsnano.9b01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Richardson JR, Hossain MM. Microglial ion channels as potential targets for neuroprotection in Parkinson’s disease. Neural Plast. 2013;2013:587418. doi: 10.1155/2013/587418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rocha EM, De Miranda B, Sanders LH. Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson's disease. Neurobiol Dis. 2018;109:249–257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 125.Rufino-Ramos D, Albuquerque P, Carmona V, Perfeito R, Nobre R, Pereira de Almeida L. Extracellular vesicles: novel promising delivery systems for therapy of brain diseases. J Controlled Release. 2017;262:247–258. doi: 10.1016/j.jconrel.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 126.Salvioli S, Capri M, Valensin S, Tieri P, Monti D, Ottaviani E, Franceschi C. Inflamm-aging cytokines and aging: state of the art new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr Pharm Design. 2006;12:3161–3171. doi: 10.2174/138161206777947470. [DOI] [PubMed] [Google Scholar]
- 127.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanjari Moghaddam H, Ghazi Sherbaf F, Mojtahed Zadeh M, Ashraf-Ganjouei A, Aarabi MH. Association between peripheral inflammation and DATSCAN data of the striatal nuclei in different motor subtypes of parkinson Disease. Front Neurol. 2018;9:234. doi: 10.3389/fneur.2018.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sarkar S, Rokad D, Malovic E, Luo J, Harischandra D, Jin H, Anantharam V, Huang X, Lewis M, Kanthasamy A, Kanthasamy A. Manganese activates NLRP3 inflammasome signaling and propagates exosomal release of ASC in microglial cells. Sci Signal. 2019;12:eaat9900. doi: 10.1126/scisignal.aat9900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128:639–650. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shimoji M, Pagan F, Healton E, Mocchetti I. CXCR4 and CXCL12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotoxic Res. 2009;16:318–328. doi: 10.1007/s12640-009-9076-3. [DOI] [PubMed] [Google Scholar]
- 132.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 133.Stuendl A, Kunadt M, Kruse N, Bartels C, Moebius W, Danzer KM, Mollenhauer B, Schneider A. Induction of α-synuclein aggregate formation by CSF exosomes from patients with Parkinson’s disease and dementia with Lewy bodies. Brain. 2016;139:481–494. doi: 10.1093/brain/awv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Su X, Chen Q, Chen W, Chen T, Li W, Li Y, Dou X, Zhang Y, Shen Y, Wu H, Yu C. Mycoepoxydiene inhibits activation of BV2 microglia stimulated by lipopolysaccharide through suppressing NF-κB, ERK 1/2 and toll-like receptor pathways. Int Immunopharmacol. 2014;19:88–93. doi: 10.1016/j.intimp.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 135.Su X, Maguire-Zeiss K, Giuliano R, Prifti L, Venkatesh K, Federoff H. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thome A, Harms A, Volpicelli-Daley L, Standaert D. microRNA-155 regulates alpha-synuclein-induced inflammatory responses in models of Parkinson disease. J Neurosci. 2016;36:2383–2390. doi: 10.1523/JNEUROSCI.3900-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsutsumi R, Hori Y, Seki T, Kurauchi Y, Sato M, Oshima M, Hisatsune A, Katsuki H. Involvement of exosomes in dopaminergic neurodegeneration by microglial activation in midbrain slice cultures. Biochem Biophys Res Commun. 2019;511:427–433. doi: 10.1016/j.bbrc.2019.02.076. [DOI] [PubMed] [Google Scholar]
- 139.van den Boorn J, Schlee M, Coch C, Hartmann G. SiRNA delivery with exosome nanoparticles. Nat Biotechnol. 2011;29:325–326. doi: 10.1038/nbt.1830. [DOI] [PubMed] [Google Scholar]
- 140.Vekrellis K, Xilouri M, Emmanouilidou E, Rideout HJ, Stefanis L. Pathological roles of α-synuclein in neurological disorders. Lancet Neurol. 2011;10:1015–1025. doi: 10.1016/S1474-4422(11)70213-7. [DOI] [PubMed] [Google Scholar]
- 141.Vella L, Hill A, Cheng L. Focus on extracellular vesicles: exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int J Mol Sci. 2016;17:173. doi: 10.3390/ijms17020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Veselý B, Dufek M, Thon V, Brozman M, Királová S, Halászová T, Koriťáková E, Rektor I. Interleukin 6 and complement serum level study in Parkinson’s disease. J Neural Transm. 2018;125:875–881. doi: 10.1007/s00702-018-1857-5. [DOI] [PubMed] [Google Scholar]
- 143.Vilaça-Faria H, Salgado A, Teixeira F. Mesenchymal stem cells-derived exosomes: a new possible therapeutic strategy for Parkinson’s disease. Cells. 2019;8:118. doi: 10.3390/cells8020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Villarán R, Espinosa-Oliva A, Sarmiento M, De Pablos R, Argüelles S, Delgado-Cortés M, Sobrino V, Van Rooijen N, Venero J, Herrera A, Cano J, Machado A. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson's disease. J Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 145.Waak J, Weber S, Waldenmaier A, Görner K, Alunni-Fabbroni M, Schell H, Vogt-Weisenhorn D, Pham T, Reumers V, Baekelandt V, Wurst W, Kahle P. Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J. 2009;23:2478–2489. doi: 10.1096/fj.08-125153. [DOI] [PubMed] [Google Scholar]
- 146.Waehler R, Russell S, Curiel D. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- 148.Walker DG, Lue LF. Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res Ther. 2015;7:56. doi: 10.1186/s13195-015-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wani T, Mohi-Ud-Din R, Mir R, Itoo A, Mir K, Fazli A, Pottoo F. Exosomes harnessed as nanocarriers for cancer therapy - current status and potential for future clinical applications. Curr Mol Med. 2020 doi: 10.2174/1566524020666200915111618. doi: 10.2174/1566524020666200915111618. [DOI] [PubMed] [Google Scholar]
- 151.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–557. doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.White AJ, Wijeyekoon RS, Scott KM, Gunawardana NP, Hayat S, Solim IH, McMahon HT, Barker RA, Williams-Gray CH. The peripheral inflammatory response to alpha-synuclein and endotoxin in Parkinson’s disease. Front Neurol. 2018;9:946. doi: 10.3389/fneur.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharm. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, Kou L, Yin S, Liu L, Huang J, Xiong N, Wang T. Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis. 2019;10:174. doi: 10.1038/s41419-019-1404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang J, Luo S, Zhang J, Yu T, Fu Z, Zheng Y, Xu X, Liu C, Fan M, Zhang Z. Exosome-mediated delivery of antisense oligonucleotides targeting α-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol Dis. 2021;148:105218. doi: 10.1016/j.nbd.2020.105218. [DOI] [PubMed] [Google Scholar]
- 156.Yokoyama H, Uchida H, Kuroiwa H, Kasahara J, Araki T. Role of glial cells in neurotoxin-induced animal models of Parkinson’s disease. Neurol Sci. 2011;32:1–7. doi: 10.1007/s10072-010-0424-0. [DOI] [PubMed] [Google Scholar]
- 157.Yu H, Sun T, An J, Wen L, Liu F, Bu Z, Cui Y, Feng J. Potential roles of exosomes in parkinson’s disease: from pathogenesis diagnosis and treatment to prognosis. Front Cell Dev Biol. 2020;8:86. doi: 10.3389/fcell.2020.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018;24:931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking sorting and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang Q, Heng Y, Yuan Y, Chen N. Pathological α-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol Lett. 2017;265:30–37. doi: 10.1016/j.toxlet.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 161.Zhou T, Lin D, Chen Y, Peng S, Jing X, Lei M, Tao E, Liang Y. α-Synuclein accumulation in SH-SY5Y cell impairs autophagy in microglia by exosomes overloading miR-19a-3p. Epigenomics. 2019;11:1661–1677. doi: 10.2217/epi-2019-0222. [DOI] [PubMed] [Google Scholar]
- 162.Zhou Y, Lu M, Du R, Qiao C, Jiang C, Zhang K, Ding J, Hu G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol Neurodegener. 2016;11:28. doi: 10.1186/s13024-016-0094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell R, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang H. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]