Abstract
Multiple sclerosis is an autoimmune treatable but not curable disease. There are a multiplicity of medications for multiple sclerosis therapy, including a class entitled disease-modifying drugs that are mainly indicated to reduce the number and severity of disease relapses. Not all patients respond well to these therapies, and minor to severe adverse effects have been reported. Vitamin D, called sunshine vitamin, is being studied as a possible light at the end of the tunnel. In this review, we recapitulated the similar immunopathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis, the immunomodulatory and neuroprotective potential of vitamin D and the state-of-art concerning its supplementation to multiple sclerosis patients. Finally, based on our and other groups’ experimental findings, we analyzed the need to consider the relevance of the route and the different time-point administration aspects for a more rational indication of this vitamin to multiple sclerosis patients.
Key Words: central nervous system, cytokines, experimental autoimmune encephalomyelitis, immunomodulation, immunopathogenesis, inflammation, multiple sclerosis, neuroprotection, regulatory T cells, vitamin D
Introduction
The simplest and widely disseminated concept of multiple sclerosis (MS) is that it is a heterogeneous, autoimmune and neurodegenerative disease characterized by inflammation, demyelination and axonal damage (Lassmann et al., 2007). It is believed that myelin-specific T cells from distinct subsets orchestrate an autoimmune attack to the central nervous system (CNS). However, the complexity of this pathology seems to go beyond the autoimmune phenomenon and includes a pivotal contribution of genetic background and environmental circumstances. Human leukocyte antigen (HLA) class II and I genes, which encode molecules crucial for antigen presentation, activating T helper and T cytotoxic lymphocytes, respectively, are relevant modifiers of disease risk. The class II HLA variants have a striking association with an increased risk of MS (odds ratio (OR)~3), particularly HLA-DRB1*15:0 in Europeans, HLA-DRB1*15:03 in African Americans and HLA-DRB1*04:05 in the Japanese population, whereas the class I HLA A*02 variant is associated with protection from the disease (OR ~0.6) (Moutsianas et al., 2015; Chi et al., 2019). There are approximately 200 non-HLA single nucleotide polymorphisms conferring modest risk of MS, predominantly inflammation-related genes (Consortium, 2017). These single nucleotide polymorphisms associated with MS are located close to these genes and regulate both adaptive and innate immune responses. These researchers found an enrichment of MS genes in microglia but not in astrocytes or neurons, suggesting that these brain-resident immune cells play a role in MS susceptibility. This contribution of genes located outside the major histocompatibility complex has been more recently sustained by the findings of an International Multiple Sclerosis Genetics Consortium, 2019 (Consortium, 2019).
Concerning the relevance of the environment, special emphasis has been placed on smoking, obesity and infectious agents such as Chlamydia pneumoniae, human herpes virus 6, Epstein-Barr virus, influenza A, measles, parainfluenza 2, varicella zoster (Olsson et al., 2017; Saberi et al., 2018) and fungi and their toxins (Benito-León et al., 2010; Saroukolaei et al., 2016). As the disease is more common in women and there are clear gender differences in terms of progression and severity, it is believed that hormonal factors contribute to regulate the evolution of this pathology (Avila et al., 2018; Ysrraelit and Correale, 2019). Another strong environmental risk factor for MS development and severity is vitamin D (VitD) levels. According to epidemiological evidence, lower serum VitD levels are associated with a higher risk of developing disease and with more severe clinical manifestations (Munger et al., 2006; Alharbi, 2015; Sintzel et al., 2018; Bäcker-Koduah et al., 2020). The name sunshine vitamin derives from the fact that most of this hormone is produced by the skin under the influence of sunlight. During exposure to ultraviolet light, 7-dehydrocholesterol is converted to pre-VitD3 by keratinocytes and dermal fibroblasts (Mostafa and Hegazy, 2015). Detailed information about its metabolism, interaction with the specific receptor (VitD receptor-VDR) and numerous biological functions are available in the literature (Bikle, 2014; Khammissa et al., 2018). This essential participation of sunlight in VitD production and its relevance in the context of MS is supported by strong and recent epidemiological evidence (Tremlett et al., 2018; Gallagher et al., 2019). Most of the protective effect of VitD in MS has been attributed to its immunomodulatory capacity and to direct neuroprotective effects (Wrzosek et al., 2013; Wei and Christakos, 2015; Sintzel et al., 2018).
Evidence from prospective studies is consistent with the hypothesis that high circulating levels of VitD are associated with a lower risk of MS. A study by Munger et al. (2006), for example, studying MS cases identified among more than 7 million US military personnel between 1992 and 2004, evidenced a decreased risk of MS among white population, but not among black and Hispanic populations, with high VitD serum levels. Another prospective study conducted in Sweden identified an association between high VitD levels during the years preceding disease onset and a decreased risk of MS (Salzer et al., 2012). Interestingly, sunlight exposure during childhood and adolescence also interferes with the risk of developing MS in adulthood. A multinational case-control study reported an association between infrequent summer outdoor activity and increased MS risk in Norway and Italy (Bjørnevik et al., 2014). Comparable findings were recognized in Tasmania (van der Mei et al., 2003), Sweden (Bäärnhielm et al., 2012) and the USA (Tremlett et al., 2018). Ethnicity also interferes with the beneficial effects of VitD or sun exposure on MS risk. In a more recent study conducted in California, higher lifetime ultraviolet radiation exposure and serum VitD levels were associated with a lower risk of MS in whites but not in Hispanics or blacks (Langer-Gould et al., 2018).
In this review, we will address the related immunopathogenesis underlying MS and EAE development and the relevance of VitD as an environmental immunomodulator, including its role in the CNS. Finally, we will cover experimental and clinical studies concerning its use as an adjunct therapy for MS control. Our experience with EAE added to that of other researchers suggests that the preclinical application of VitD in MS patients could be more effective in controlling the disease.
Search Strategy and Selection Criteria
A literature review was electronically performed using the PubMed database. The following search term combinations were used to select the articles: vitamin D and immunomodulation; multiple sclerosis and experimental autoimmune encephalomyelitis immunopathogenesis; vitamin D and neuroprotection; vitamin D and multiple sclerosis clinical trials; vitamin D and experimental autoimmune encephalomyelitis. Most elected studies (approximately 80% of all references) were published from 2011 to 2021. The remaining articles were published before 2011 and were included due to their relevance in the area.
Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis Immunopathogenesis
Much of the scientific knowledge concerning the immunopathogenesis of MS derives from investigations performed with EAE. This disease is artificially induced in rodents, mainly mice and rats, by immunization with myelin-derived proteins and peptides in the presence of complete Freund’s adjuvant and pertussis toxin (Robinson et al., 2014; Bjelobaba et al., 2018). Despite clear differences between human and animal diseases (Lassmann and Bradl, 2017), this experimental model is considered suitable for studying MS immunopathogenesis. The main stages of this pathology are briefly outlined in Figure 1.
Figure 1.

Multiple sclerosis/experimental autoimmune encephalomyelitis immunopathogenesis.
The following sequence of events has been suggested for multiple sclerosis and experimental autoimmune encephalomyelitis immunopathogenesis: activation of self-reactive T cells in secondary lymphoid organs, probably in cervical lymph nodes (1), possible licensing in the lungs but perhaps in other places (2), Th17 differentiation in the intestine (3), breakdown of central nervous system (CNS) permeability barriers (4), local reactivation and expansion of T cells and release of proinflammatory mediators (5), demyelination and neurodegeneration triggered by the inflammatory reaction (6), and regulatory mechanisms usually activated but not strong enough to control the disease (7).
Peripheral activation of myelin-specific lymphocytes
Activation of myelin-specific T cells could occur in peripheral lymphoid organs by recognition of microbial epitopes that share homology with self-antigens (molecular mimicry) (Cusick et al., 2012), release of myelin from the CNS by a local infection (Stohlman and Hinton, 2001) or by bystander activation (Waldner et al., 2004). These possibilities are probably facilitated by the higher degree of T cell receptor degeneracy that was confirmed in myelin T cells by Hemmer et al. (1998). Apart from Th1/Th17 subsets that have been more classically associated with MS (Fletcher et al., 2010), Tγδ, CD8 T cells, B lymphocytes and macrophages/microglia have also been considered essential in the immunopathogenesis of MS and EAE (Yadav et al., 2015; Zarobkiewicz et al., 2019).
Presumable T cell licensing for pathogenicity
In the last few years, a new concept has emerged from EAE studies concerning the “education” of T cells to become pathogenic and therefore to be able to reach and damage the CNS. This phenomenon has been called “licensing” or “licensing for pathogenicity” (Tan et al., 2017). Despite some controversies, the lungs and spleen seem to be the main licensing environments in the context of EAE/MS (Odoardi et al., 2012; Tan et al., 2017). This licensing process, which allows T cells to reach the target organ, is basically a change in the pattern of gene expression characterized by downregulation of genes related to proliferation/activation and upregulation of migration-promoting genes (Ransohoff, 2012). Human Th17 lymphocytes stand out among encephalitogenic effector cells because they are initially involved in the opening of the blood-brain barrier (BBB) (Kebir et al., 2007) and later in neurodegeneration. In humans, this subset is found in lesions, and transcriptomic investigation reveals that interleukin-17 is the highest-ranking gene expressed in active plaques (Lock et al., 2002).
Expansion of Th17 in the intestine
The immune system associated with the intestinal mucosa has recently been recognized as pivotal to MS and EAE development. The intestinal environment plays a crucial role in the pathogenesis of these two diseases mainly by promoting activation and acquisition of the Th17 phenotype (Ivanov et al., 2009; Cosorich et al., 2017). Th17 cells present a considerable degree of plasticity involving not only the phenotype but also their functions. It is well documented that these cells act in the defense against a variety of pathogens and in a myriad of inflammatory pathologies (Stockinger and Omenetti, 2017). It is assumed that activation of effector Th17 cells occurs mostly in the murine small intestine, independent of their ensuing function (Gaboriau-Routhiau et al., 2009; Ivanov et al., 2009). Interestingly, the development of steady-state or pathogenic Th17 cells is critically determined by microbiota composition. Segmented filamentous bacteria, for example, induce brain autoimmunity in mice by selectively promoting Th17 differentiation (Lee et al., 2011). More recently, Cosorich et al. (2017) confirmed the contribution of the intestinal milieu in promoting Th17 expansion in MS patients. They also described that a higher Th17 frequency correlated with more accentuated disease severity and dysbiosis characterized by an elevated Firmicutes/Bacteroidetes ratio, relative abundance of Streptococcus, and a decreased abundance of Prevotella strains. These findings emphasize the relevance of investigating targeted gut therapies such as dietary intervention, helminth therapy, and, more recently, fecal microbiota transplantation (Budhram et al., 2017; Brown et al., 2021). It is well established that certain dietary modifications, such as reduced intake of fats and salt and probiotic supplementation, can ameliorate EAE/MS symptoms (Valburg et al., 2021). Conversely, a high-fat/high-salt diet led to increased disability in the EAE mouse model, which was associated with augmented immune cell infiltration and oxidative stress in the CNS (Valburg et al., 2021). A probiotic mixture (VSL3) including several strains of Lactobacillus, Bifidobacterium, and Streptococcus was also tested in patients with relapsing remitting MS. This procedure reversed MS-induced alterations in the gut microbiota composition and triggered an anti-inflammatory peripheral immune response characterized by decreased expression of CD80 on monocytes and HLA-DR on dendritic cells (Tankou et al., 2018).
Breakdown of the blood-brain barrier and cell migration to the CNS
In the past few years, it became evident that the crosstalk between the gut microbiota and brain has a crucial impact on neurodegenerative processes (Ma et al., 2019). CNS integrity and functionality are preserved by the BBB and the blood-cerebrospinal fluid barrier. These two barriers are located in distinct CNS compartments, and damage and/or activation of their various components will allow leukocyte infiltration and ensuing neurodegeneration in MS and EAE (Alvarez et al., 2011). The breakdown mechanisms of such barriers in these pathologies are incompletely understood but appear to involve many molecular and cellular components. The presence of proinflammatory cytokines (interferon (IFN-γ), TNF-α and interleukin (IL)-1β) disorganizes endothelial tight junctions and enhances endothelial leukocyte adhesion and the subsequent migration of cells towards the CNS parenchyma (Minagar and Alexander, 2003). Special attention has been given to the pathogenicity of Th17 cells concerning the mechanisms by which they interfere with CNS barrier permeability. Briefly, these cells can disrupt local tight junctions through the actions of IL-17 and IL-22, through release of CXCL1 and CXCL2 and consequent attraction of polymorphonuclear cells and by inducing the production of reactive oxygen species (ROS) by endothelial cells (Huppert et al., 2010; Dos Passos et al., 2016). By releasing metalloproteinases, histamine, and TNF-α, mast cells also contribute to increased CNS permeability (Elieh-Ali-Komi and Cao, 2017; Brown and Weinberg, 2018). The participation of mast cells in EAE and MS immunopathogenesis goes, however, much beyond this effect, including at least the production of cytokines and chemokines, PMN cell recruitment and interaction with microglia and T lymphocytes (Brown and Weinberg, 2018; Pinke et al., 2020a, b).
T cell reactivation, inflammation, and neurodegeneration
The inflammatory and damaging process that occurs in the CNS during EAE and MS is a complex phenomenon whose details can be found in recent and informative reviews (Freeman and Ting, 2016; Lazibat et al., 2018; McGinley et al., 2018; Karpus, 2020). A very brief description of these in situ reactions is given below. Infiltrating cells, especially γδT, Th1 and Th17 cells, are locally expanded and release cytokines that activate microglia and oligodendrocytes. Once activated, these original CNS cells and others that came from the periphery will release inflammatory mediators such as IL-8, IL-17, GM-CSF, CCL2 and enzymes that will, ultimately, trigger neurodegeneration (McGinley et al., 2018; Karpus, 2020). The role of CD8 T cells seems to be significantly different in MS and EAE models. Pathological data from patients indicate that CD8 T cells play an important role in propagating inflammation and tissue damage in established MS (Lassmann and Bradl, 2017). On the other hand, a recent investigation revealed that CD8 T cells exhibit a regulatory role in EAE by suppressing the proliferation of MOG-specific CD4 T cells (Saligrama et al., 2019). The contribution of B cells, mitochondrial dysfunction, oxidative stress and inflammasome activation have been characterized as pivotal players and potential therapeutic targets in this disease (Bhise and Dhib-Jalbut, 2016; Lang et al., 2018; Gharibi et al., 2020).
Activation of regulatory mechanisms
Regulatory T cells (Tregs) are essential to maintain peripheral tolerance to self-antigens and to suppress excessive immune responses, avoiding deleterious tissue damage (Sakaguchi et al., 2008). Tregs have emerged as central players in the control of autoreactive T cells, reducing the activity of effector CD4+T cells and the production of proinflammatory cytokines, such as IL-6, IL-17, TNF-α and IFN-γ. In addition to the well-established Foxp3+Tregs, other subtypes that lack the expression of Foxp3 are also involved in the suppression of autoimmune conditions, including IL-10-producing Tr1 cells and TGF-β-producing Th3 cells (Kitz et al., 2018). Patients with MS frequently exhibit a normal number of circulating Tregs; however, these cells present lower suppressive activity, which has been associated with reduced IL-10 production and Foxp3 expression and genetic abnormalities in CD25 and CTLA-4 gene expression or function (Danikowski et al., 2017). McGeachy et al., 2005 demonstrated for the first time the direct involvement of CD4+CD25+Treg cells in the natural resolution of EAE in C57BL/6 mice. This regulatory population in the CNS was Foxp3+and expressed other markers, such as CTLA-4 and GITR, in addition to the regulatory function ex vivo. Tregs have also been implicated in the remission phase of an acute monophasic disease in Lewis rats (Almolda et al., 2011). These animals totally recovered from leg paralysis through elimination of inflammatory cells in the CNS by apoptosis mediated by NK cells, macrophage polarization toward M2 and Treg activation (Shin et al., 2012). Similarly, in a chronic mouse model of EAE, Tregs mediated the recovery phase of the disease by controlling cytokine production, proliferation and motility of effector T cells in the CNS (Koutrolos et al., 2014). Tregs are also involved in the differential susceptibility of male and female SJL/J mice to develop relapsing remitting EAE. Male SJL mice are more resistant to autoimmune diseases by favoring the development of Th2 immune responses through augmented expression of the inhibitor markers CTLA-4 and CD62L by IL-10-secreting Tregs (Hussain et al., 2011). Based on their intrinsic regulatory mechanisms capable of controlling neuroinflammation during MS/EAE clinical manifestations, numerous immunoregulatory cell types, including Tregs, regulatory B cells, M2 macrophages and tolerogenic DCs, have been explored as novel therapeutic approaches to treat MS patients (Cheng et al., 2017).
Immunomodulatory Skills of Vitamin D
VitD is a powerful hormone derived from cholesterol that is primarily produced by the skin under the catalytic effect of ultraviolet B rays (Plum and DeLuca, 2010). Its classic role is to allow calcium and phosphate absorption in the gut to maintain adequate serum levels and to promote bone growth (Dereje et al., 2017). However, the widespread expression of VDR (Kongsbak et al., 2013) and of the enzyme 1-α-hydroxylase (CYP27B1) led to the discovery that it is also fundamental in many other physiological circuits, including immunomodulation and neuroprotection.
VitD from the diet or synthesized by the skin is biologically inactive. To perform immunomodulatory or other functions, it needs to be hydroxylated twice, one in the liver and another in the kidneys (Hewison et al., 2000). Extrarenal production of 1,25(OH)2D3has also been demonstrated in normal human tissues, including the gastrointestinal tract, skin, vasculature, placenta, brain and lymph nodes, through the expression of CYP27B1 (Hewison et al., 2007). Classical VDR is a member of the nuclear receptor family, and is found in approximately 30 different tissues, including distinct cell types such as macrophages, DCs, activated T cells, neurons and glial cells (Smolders and Damoiseaux, 2011), and can regulate the expression of more than 1000 genes. After initial interaction with 1,25(OH)2D3, VDR heterodimerizes with the retinoid-X receptor. Activated VDR binds to vitamin D-responsive elements, which are located close to gene promoters, allowing VDR complexes to regulate the expression of approximately 3% of the human genome (Clark and Mach, 2016). VitD can also act through the putative membrane receptor membrane-associated, rapid response steroid-binding to induce nongenomic actions (Landel et al., 2018).
Enhancement of phagocytic and antimicrobial ability
Broadly speaking, VitD has distinct effects on innate and specific immunity. Most of the antimicrobial monocyte/macrophage activities are upregulated by VitD. Under the effect of this vitamin, macrophages increase their phagocytic ability, produce more defensin β2 and cathelicidin antimicrobial peptide (Youssef et al., 2011) and synthesize more reactive oxygen intermediates (Ghosh et al., 2016). VitD also induces autophagy, which is a potent innate antimicrobial defense mechanism. Interestingly, autophagy is upregulated via cathelicidin, which activates the transcription of autophagy-related genes such as Beclin-1 and Atg5 (Fabri and Modlin, 2009). The VitD/VDR axis is also critical to maintain optimal expression of defensins and tight junction genes, therefore supporting intestinal integrity and eubiosis (Su et al., 2016).
Tolerization of dendritic cells
A great deal of research has been dedicated to the effect of VitD on antigen presenting cells, especially DC differentiation. This initial stage of specific immunity is critical because it will shape much of the T cell polarization into distinct subsets. It is well established that VitD has long been proposed to be able to imprint DCs with a tolerogenic profile. DCs differentiated in the presence of calcitriol produced lower amounts of IL-12 and TNF-α and higher levels of IL-10 (Griffin et al., 2001). These cells also expressed reduced amounts of class II MHC and costimulatory molecules (Széles et al., 2009). Under the influence of VitD, DCs display higher expression of inhibitory molecules, including immunoglobulin-like transcript 3 and PD-L1 (Chambers and Hawrylowicz, 2011). These tolerogenic DCs are poor inducers of T cell proliferation and activation, and they also trigger autoreactive T cell apoptosis (Colotta et al., 2017). On the other hand, VitD-modified DCs are very efficient in inducing Tregs, as will be further described.
Promotion of T lymphocyte polarization
VitD can also directly modulate T cells. These effects are complex, diverge among T cell subsets and require TCR stimulation; that is, naïve T cells do not respond to interaction with VitD (Colotta et al., 2017). Calcitriol inhibits Th1 and Th17 differentiation and the production of their related cytokines (Chang et al., 2010; Zhang et al., 2018). Despite some controversial findings, it has been shown that VitD promotes Th2 polarization (Sloka et al., 2011).
The ability of VitD to induce the differentiation of Tregs is being viewed and explored as a highly promising strategy to control autoimmune diseases (Kurniawan et al., 2020). The most well-characterized Treg lineage is CD4+CD25(high)Foxp3+T cells (Vignali et al., 2008). This vitamin can promote Treg differentiation by acting directly on CD4+T cells, downmodulating the production of proinflammatory cytokines such as IFN-γ, IL-17 and IL-21. Interestingly and very promising from a therapeutic point of view, these cells acquire phenotypical and physiological characteristics of Treg cells. They express high levels of Foxp3 and CTLA-4 and are also able to suppress the proliferation of resting cells (Jeffery et al., 2009). As stated above, Tregs can also be indirectly induced by VitD with the assistance of DCs. DCs differentiated from monocytes in the presence of VitD displayed a semimature phenotype identified by low expression of costimulatory molecules together with increased indoleamine 2,3 dioxygenase, IL-10, TRAIL and PDL-1 expression levels (Raker et al., 2015). These are very encouraging findings because these molecules are intrinsically related to the immunoregulatory ability of Treg cells. Indoleamine 2,3 dioxygenase, for example, triggers tryptophan starvation to inhibit T cell responses through the expansion of Tregs (Yan et al., 2010). Additionally, TRAIL and PDL-1 are critically involved in the induction of these classical CD4+CD25(high)Foxp3+Treg cells. VitD is also able to induce Foxp3-Tr1 cells; preferential induction of Treg Foxp3+or Tr1 cells is attributed to interaction with distinct DC subsets (van der Aar et al., 2011).
Role of Vitamin D in Neurogenesis and Neuroprotection
A growing number of studies performed in vitro, ex vivo, and employing animal models provide evidence that VitD is crucial for the healthy function of neuronal pathways. Fundamental findings in this area indicated that VitD signaling affected both the developing and adult brain (Eyles et al., 2013; Groves et al., 2014). Studies have also indicated that VitD upregulates the synthesis of several neurotrophic mediators, including nerve growth factors and neurotrophins, which are essential for neurite outgrowth, neuronal growth and survival, neurotransmission, and synaptic plasticity. By using a rat model, Pardridge et al. (1985) demonstrated that VitD enters the CNS through the blood-brain barrier. The local presence of enzymes involved in VDR metabolism and VDRs sustains local bioactivation, catabolism and biological activity (Almokhtar et al., 2016; Landel et al., 2018). This pivotal role of VitD in brain development triggered attention to diseases that were linked to VitD deficiency in early life as autistic spectrum and schizophrenia (Eyles et al., 2013) and later to pathologies more frequently linked to adult life as MS or aging as depression, Alzheimer´s disease (AD), Parkinson´s disease and amyotrophic lateral sclerosis. Epidemiological data have supported this possibility. There is, for example, a striking association between low VitD levels and dementia and AD (Chai et al., 2019). By using a mouse model of AD, (Morello et al., 2018) thoroughly investigated the role of VitD and found that its supplementation was able to improve neurogenesis and cognition. The evidence of a relationship between VitD levels and Parkinson´s disease is less strong and waits for additional investigations (Rimmelzwaan et al., 2016). Numerous studies support the notion that VitD insufficiency contributes to higher-risk MS (Pierrot-Deseilligny and Souberbielle, 2017). As will be pointed out further in this review, VitD efficiently controls EAE development, but its usage in MS patients is still a matter of contention.
According to Berridge’s hypothesis, Vit D deficiency accelerates aging and age-related diseases (Berridge, 2017). This author proposes that this hormone controls aging by acting in a series of metabolic pathways, such as autophagy, mitochondrial dysfunction, oxidative stress, inflammation, calcium signaling, and telomere shortening. In fact, many of these processes are disturbed in these pathologies, and VitD has been able to restore their physiological status. VitD is able, for example, to reestablish cellular calcium homeostasis through the synthesis of protein binding calcium ions (Pendo and DeGiorgio, 2016) and by regulation of L-type voltage-sensitive Ca2+channel expression that together will avoid the excessive accumulation of Ca2+in nerve cells (Wrzosek et al., 2013). Excess calcium in nerve cells leads to cytoplasmic and mitochondrial membrane damage through increased release of neurotransmitters, activation of lipases and activation of nitric oxide synthase and formation of ROS (Zündorf and Reiser, 2011). Low levels of VitD have also been associated with lower antioxidant potential, as recently demonstrated by (Fan et al., 2020). According to these authors, the exacerbation observed in experimental Alzheimer-like pathologies was due to enhanced oxidative stress via the downregulation of superoxide dismutase 1, glutathione peroxidase 4 and cystine/glutamate exchanger.
Several neurodegenerative disorders have been more recently associated with mitochondrial dysfunction (Waseem et al., 2020). Interestingly, silencing VDR in different cell types initially triggered impairment of mitochondrial integrity and then cell death (Ricca et al., 2018). In addition to these more straightforward effects, VitD controls CNS homeostasis through its previously mentioned immunomodulatory ability (Fernandes de Abreu et al., 2009). An encompassing and enlightening review concerning the possible use of VitD to control the brain decay that accompanies aging and neurogenerative conditions was recently published (Farghali et al., 2020).
Vitamin D Efficacy in Multiple Sclerosis: A Controversial Issue
Available data suggest that VitD levels play a role in both the risk of developing MS and the degree of disease disability. Concerning the risk, several studies have indicated the existence of a latitudinal gradient in MS prevalence. A lower risk of developing this disease was found in geographical areas with higher exposure to sunlight (Pierrot-Deseilligny and Souberbielle, 2017). This finding is easily explained by the need for ultraviolet light skin exposure for VitD synthesis (Jeon and Shin, 2018). Many studies have revealed VitD deficiency in these patients (Suresh Kumar et al., 2013; Martinelli et al., 2014). It is important to highlight, however, that not all MS patients presented VitD deficiency. In contrast, negative associations with VitD deficiency have been described before IFN-β treatment, as observed in RRMS patients from the original OFAMS study. In these patients, the serum levels of VitD were inversely associated with radiologic disease activity (Løken-Amsrud et al., 2012) and were negatively correlated with magnetic resonance imaging (MRI) activity (Røsjø et al., 2015) only before IFN-β treatment. During IFN-β therapy, similar alterations were observed in these patients, suggesting that VitD status has no influence on IFN-β treatment effects.
Concerning the degree of disease activity, many studies also reinforced the direct relationship between lower VitD levels and more severe disease or more severe comorbidity manifestations (Thouvenot et al., 2015; Brola et al., 2016; Wawrzyniak et al., 2017). Interestingly, the possible contribution of VitD to lower MS disability rates seems to be affected by the underlying disease modifying therapy treatment; this was clear in patients receiving IFN-β-1b (Fitzgerald et al., 2015). However, until now, there has also been no consensus regarding the effect of VitD on disease severity (Nikanfar et al., 2015; Muris et al., 2016). The scientific community is still asking if this deficiency is contributing to trigger MS or if the deficiency is being caused by the disease itself. In this scenario, it has been debated whether VitD supplementation is therapeutically recommended for MS patients. A recent study based on the clinical trial called BENEFIT (Betaferon/Betaseron in Newly Emerging Multiple Sclerosis for Initial Treatment) that completed the 11-year assessment (BENEFIT-11) indicated that higher VitD serum levels predicted better cognitive performance in MS patients. Higher 25(OH)D (50 nM) levels in the first 2 years were related to 65% lower odds of poorer Paced Auditory Serial Addition Test-3 performance at year 11 (Cortese et al., 2020). Analogously, and based on the phase 3, double-blind, placebo-controlled study, called FREEDOMS (FTY720 Research Evaluating Effects of Daily Oral therapy in Multiple Sclerosis), it was verified improved MRI outcomes on percent brain volume change, proportion of patients free of new/enlarging T2 lesions, and a trend of less depression in the ‘daily users’ of VitD versus ‘non-users’ (Hongell et al., 2018).
Most of the recent systematic reviews and meta-analyses indicate that VitD supplementation had no therapeutic effects on MS patients (Jagannath et al., 2010; Mostafa and Hegazy, 2015; Zheng et al., 2018). This was partially reinforced by the study of (Kampman et al., 2012), in which weekly supplementation with 20,000 IU vitamin D3 did not result in beneficial effects on the measured MS-related outcomes, including the annualized relapse rate (ARR) and expanded disability status scale (EDSS). However, some papers have shown that VitD is safe and is at least able to promote some degree of immunomodulation. With respect to safety, a single center open-labeled randomized, controlled clinical Phase I/II pilot study conducted with 15 pregnant women with confirmed MS and low serum 25(OH)D levels reported no adverse effects after VitD supplementation (Etemadifar and Janghorbani, 2015). These pregnant women received 50,000 IU/week of VitD from 12 to 16 weeks of gestation until delivery and had fewer relapse events during pregnancy and within 6 months after delivery. In addition, the mean EDSS score did not change 6 months after delivery, whereas in the nonsupplemented group, the mean EDSS score increased from 1.3 to 1.7 (Etemadifar and Janghorbani, 2015). Concerning immunomodulation, VitD administration was able to increase TGF-β serum levels (Mahon et al., 2003) and to reduce IL-17 production by T lymphocytes, the proportion of effector memory T cells (Sotirchos et al., 2016) and TNF-α concentration in culture supernatants of anti-CD3 stimulated PBMC cultures from VitD treated RRMS patients but not in the placebo group (Rolf et al., 2019). In addition, 48 weeks of VitD supplementation preserved CD25 expression in Tregs and circulating soluble CD25 levels, contrasting with a significant reduction in the placebo group (Rolf et al., 2018).
From 2018 to the current year, at least three trials were published and showed positive results regarding VitD supplementation in MS patients. In a double-blind, placebo-controlled parallel-group study named CHOLINE (Cholecalciferol in Relapsing-Remitting MS: A Randomized Clinical Trial), conducted for 2 years, enrolled RRMS patients whose inclusion criteria were low serum 25(OH)D concentration (< 75 nM) and treatment with IFN-β-1a. All efficacy parameters favored cholecalciferol. In this study, patients who received oral 100,000 IU of cholecalciferol every other week for 96 weeks presented an ARR reduction, fewer new hypointense T1-weighted lesions, a lower volume of hypointense T1-weighted lesions, and a lower progression of EDSS (Camu et al., 2019). Based on the aforementioned findings, these authors suggested a potential therapeutic effect of cholecalciferol in RRMS patients who were being treated with IFN-β-1a and presented an original low serum level of 25(OH)D.
The SOLAR study was performed by following a group of patients similar to the CHOLINE trial, that is, RRMS subjects with low serum 25(OH)D levels (< 150 nM in this case) who were also being treated with IFN-β-1a. This phase II, randomized, double-blind, placebo-controlled, supplemented with Vigantol Oil, showed no significant difference based on evidence of disease activity (NEDA-3) status (Hupperts et al., 2019). These authors also reported that oral treatment with 14,007 IU/d cholecalciferol for 48 weeks protected the patients against the development of new MRI lesions (Hupperts et al., 2019). However, high-dose vitamin D3 as an add-on to IFN-β-1a showed no established benefit based on the primary outcome of NEDA-3, and these patients showed no difference in circulating levels of neurofilament light chain, which is a biomarker of disease activity in RRMS (Smolders et al., 2020). A similar absence of effect over the levels of neurofilament light chain had already been published by (Holmøy et al., 2019), conducted with 71 RRMS patients weekly supplemented with 20,000 IU of VitD3.
In contrast, a recent multicenter randomized/stratified actively controlled explorative phase 2 pilot trial with a double-blind intervention period of 18 months, called EVIDIMS (Efficacy of Vitamin D Supplementation in Multiple Sclerosis), enrolled RRMS patients with IFN-β-1b treatment and compared the effects of every other day high- (20,400 IU) versus low-dose (400 IU) cholecalciferol supplementation. No differences were observed in these two treatments concerning clinical (relapse rates, disability progression) and radiographical (T2-weighted lesion development, contrast-enhancing lesion development, brain atrophy) parameters. The authors concluded that the results neither support nor disprove a therapeutic benefit of high-dose VitD supplementation and suggested that a larger sample size was necessary to prove the hypothesis (Dörr et al., 2020).
To explore the association between dietary intake and physical capacity and fatigue in MS, a randomized controlled trial assessed the dietary records of patients for 4 days and correlated them with the 6-minute walk test, a VO2max test, and self-reported questionnaires assessing fatigue severity (Fatigue Severity Scale) and impact (Modified Fatigue Impact Scale) (Albrechtsen et al., 2020). In the multiple regression analyses, the absolute intake of VitD (average of 41.6 µg/d) by RRMS patients showed trends towards a positive association with VO2max but not with other parameters. The main immunological or clinical outcomes observed in patients supplemented with VitD are summarized in Table 1.
Table 1.
Clinical trials with vitamin D supplementation
| Type of study | Patients | Dose | Period | Effect |
|---|---|---|---|---|
| Double-blind design study | 17 patients | 1000 IU/d | 6 mon | Increased serum TGF-β1 levels |
| Phase I/II randomized trial (Completed) | 15 pregnant patients | 50000 IU/wk | 12 to 16 wk | Fewer relapse events and unchanged EDSS |
| Clinical trial (Completed) | 40 RRMS patients | 10400 IU or 800 IU/d | 6 mon | Reduction in the proportion of IL-17+ CD4+ cells, CD161+ CD4+ cells, and effector memory CD4+ cells in high-dose group |
| Phase 3 FREEDOMS and FREEDOMS II trials (Completed) | 1953 patients | Not registered | 674.5 ± 181.61 d | Improved MRI on brain volume and patients free of T2 lesions |
| Clinical trial (Terminated) | RRMS female patients | 4000 IU/d | 16 wk | Decreased TNF-α concentration in culture supernatants of CD3+CD4+ cells |
| Clinical trial (Completed) | 53 RRMS patients | 14000 IU/d | 48 wk | Decreased Treg CD25-expression and circulating soluble-CD25 levels period |
| Clinical trial CHOLINE (Completed) | RRMS patients with low serum VitD and therapy with IFN-β-1a | 100000 IU every other week | 96 wk | ARR reduction, less new T1-weighted lesions, lower volume of T1-weighted lesions, and a lower EDSS period |
| Clinical trial SOLAR (Completed) | RRMS patients with low serum VitD and therapy with IFN-β-1a | 14007 IU/d | 48 wk | Protective effects on development of new MRI lesions |
| Clinical trial BENEFIT (Completed) | 278 patients with clinically isolated syndrome | 50 nM | 2 yr | 65% lower odds of poorer PASAT-3 |
ARR: Annualized relapse rate; CD: cluster of differentiation; d: daily; EDSS: expanded disability status scale; IFN: interferon; IL-17: interleukin-17; IU: international unit; MRI: magnetic resonance imaging; PASAT-3: paced auditory serial addition test-3; RRMS: Relapsing Remitting Multiple Sclerosis; TGF: transforming growth factor; TNF: tumor necrosis factor; Treg: regulatory T cells; VitD: vitamin D; mths: months; wk: week; yr: year.
VitD supplementation in MS patients has been revealed to be an amazing complex subject to be addressed and elucidated. Some of the constraints that prevent this clarification are the wide and intricate activity of VitD in the body, the expected variability in each patient’s response to this hormone, the obstacles to establishing homogenous and safe clinical trials, the variation in doses, how long a patient should be treated, and what disease modifying therapy or other drugs are being used by the patient. In our opinion, the stage of the disease, as will be approached next in this review, is one of the parameters that deserves major attention.
It is important to keep in mind that mild and severe side effects have been verified in both experimental animal studies and clinical trials. The most well-described manifestations include neuropsychiatric, gastrointestinal, cardiovascular, and renal symptoms. This subject has been addressed in detail by experts in the field (Jones, 2008; Galior et al., 2018; Taylor and Davies, 2018; Feige et al., 2019). Despite these side effects and many disappointing outcomes during clinical trials, the scientific community still believes that the usage of VitD is worthwhile to be further evaluated.
Vitamin D: Lessons from Experimental Encephalomyelitis
Despite the differences between EAE and MS, a great deal of information obtained from this model helps to understand MS immunopathogenesis and to adopt new therapeutic measures. EAE has also been widely employed to uncover the potential mechanisms by which VitD could control EAE development and, eventually, be translated to MS. Table 2 summarizes the main findings showing that the in vivo administration of this hormone protected mice against EAE development.
Table 2.
Preclinical studies with vitamin D supplementation
| Reference | Experimental model | Mechanism of VitD action |
|---|---|---|
| Lemire and Archer, 1991; Cantorna et al., 1996 | SJL/J and B10.PL mice | VitD prevented the induction and blocked the progression of relapsing EAE. |
| Cantorna et al., 1998 | B10.PL mice | Increased the levels of IL-4 and TGF-β1 transcripts in LN and CNS. |
| Mattner et al., 2000 | Biozzi AB/H mice | Reduced T cell proliferation and lower IFN-γ levels in popliteal LN. |
| Reduced the inflammatory infiltrates, demyelinated areas and axonal loss in brains and spinal cords. | ||
| Nashold et al., 2000 | B10.PL mice | Decreased macrophage accumulation in the CNS. |
| Meehan and DeLuca, 2002 | VDR null and B10.PL mice | VDR was necessary for preventing the onset of EAE development. |
| Muthian et al., 2006 | C57BL/6 and SJL/J mice | Inhibited JAK-STAT pathway in IL-12/IFN-γ axis leading to differentiation of neural antigen-specific Th1 cells. |
| Spach et al., 2006 | C57BL/6 and SJL/J mice with a disrupted IL-10 or IL-10R gene | Protective mechanism required a functional IL-10-IL-10R pathway. |
| Chang et al., 2010 | BALB/c, C57BL/6 mice, Rag2−/− DO11.10, MOG-TCR (2D2), IL-10−/− and STAT1−/− transgenic mice | Down-regulated CCR6 expression, inhibited the differentiation and migration of Th17 cells to the CNS. Reduced the total infiltration of CD4+ T cells in the CNS. |
| Sloka et al., 2011 | C57BL/6 and STAT6−/− mice | Increased the levels of GATA-3 and STAT6. |
| Grishkan et al., 2013 | C57BL/6, CD45.1+, B6(Cg)-Tyrc-2J/J and 2D2 mice | Impaired Th cell migration into the CNS and reduced the expression of the chemokine receptor CXCR3 on Th cells. |
| Li et al., 2013 | C57BL/6 mice | Reduced the inflammatory cell infiltration, inflammatory cytokine (TNF-a, INF-γ and IL-17) and TLR8 expression in the CNS. |
| Nashold et al., 2013 | B10.PL mice | Increased Helios+FoxP3+ T cells in the CNS. |
| Mohammadi-Kordkhayli et al., 2015 | C57BL/6 mice | Down-modulated the expression of IL-27 and IL-33 in the CNS. |
| Sloka et al., 2015 | C57BL/6 mice | Reduced the axonal and neuronal loss. |
| Waddell et al., 2015 | Jα18−/−, CD1d−/−, IL-4−/− and WT C57BL/6 mice | Protection from EAE mediated by VitD was partially regulated by NKT cells. |
| Zhen et al., 2015 | C57BL/6 mice | Impaired autophagic activity and neuroapoptosis, characterized by elevated expression of Beclin1, increased Bcl-2/Bax ratio, and decreased LC3-II accumulation. |
| Shirazi et al., 2017 | C57BL/6 mice | Increased the numbers of neural stem cells, oligodendrocyte precursor cells, and oligodendrocytes in disease lesions in the CNS. |
| Ahangar-Parvin et al., 2018 | C57BL/6 mice | Down-modulated IL-12 and TGF-β expression in the CNS and serum. |
| Hoepner et al., 2019 | C57BL/6 and BALB/c WT mice and danimals with altered GR signaling | Increased glucocorticoid efficacy via inhibition of mTORC1. |
| Jafarzadeh et al., 2019 | C57BL/6 mice | Down-regulated the expression of some Th17 cell-related cytokines, key inflammatory chemokines, and chemokine receptors in the CNS (IL-17, IL-23 P19, IL-23 P40, CCL20, CCL22 and CCR4). |
| de Oliveira et al., 2020 | C57BL/6 mice | Prevented neuroinflammation and reduced blood-brain barrier disruption and local macrophage/microglia activation. |
| Reduced the oxidative stress and expression of NLRP3, caspase-1, IL-1β, CX3CR1, CCL17, RORc and Tbx21 in the CNS. | ||
| Gomez-Pinedo et al., 2020 | Wistar rats | Increased remyelination by promoting oligodendrocyte lineage differentiation. |
| Spanier et al., 2020 | C57BL/6 and B6. Lyz2-Cre+Cyp27b1f/f mice | Increased CTLA-4 expression by spinal cord-infiltrating CD4+ T and Treg cells. |
| Mimura et al., 2021 | C57BL/6 mice | Reduced the expression of inflammatory parameters, and demyelination at the CNS |
| Down-regulated the proinflammatory cytokines production by lymph node-derived cells and IL-17 by gut explants and reduced intestinal inflammation. |
CCL: Chemokine ligand; CCR: chemokine receptor; CD: cluster of differentiation; CNS: central nervous system; CTLA: cytotoxic T-lymphocyte associated protein; EAE: experimental autoimmune encephalomyelitis; FoxP3: forkhead box P3; GR: glucocorticoid receptor; IFN: interferon; IL: interleukin; JAK: Janus kinase; LC3: microtubule-associated protein light chain 3; LN: lymph node; MOG: myelin oligodendrocyte glycoprotein; mTOR: mammalian target of rapamycin; NKT: natural Killer T cell; NLRP: nucleotide-biding oligomerization domain, leucine rich repeat and pyrin domain containing; STAT: signal transducer and activator of transcription; TGF: transforming growth factor; Th: T helper cell; TLR: toll-like receptor; TNF: tumor necrosis factor; Treg: regulatory T cell; VDR: vitamin D receptor; VitD: vitamin D; WT: wild-type.
One of the promising research lines in this scenario is the tolerogenic DC/Treg axis. Most of the investigations regarding the effect of VitD on DCs indicate that its in vitro addition promotes a tolerogenic profile. The adoptive transfer of these cells to EAE rats and mice was able to induce regulatory Foxp3 T cells and to reduce disease severity (Farias et al., 2013; Mansilla et al., 2015; Xie et al., 2017). Cryopreservation of DCs generated in the presence of VitD conserved their tolerogenic properties, which are relevant for human application (Mansilla et al., 2015). This strategy is very elegant and appealing and generates robust knowledge in adoptive cell transfer technology. However, it is very expensive and laborious and would be interesting to consider other alternatives based on this tolerogenic phenomenon. Leichner et al. (2017) demonstrated that topical, but not systemic, application of a VitD analog increased the proportion and absolute number of Tregs and attenuated EAE clinical scores. The literature also points to other pathways and components that can boost this VitD & Foxp3 Treg intersection. Estrogen, for example, synergizes with VitD, promoting higher CD4+Helios+Foxp3+Treg differentiation (Spanier et al., 2015). Interestingly, it was recently described that higher VitD levels were protective in females but not in C57BL/6 male mice. In contrast to this enhancing effect on Treg cell differentiation, VitD downregulates multiple signaling and metabolic pathways critical for T lymphocyte activation and further polarization towards Th1 and Th17 subsets (Nanduri et al., 2015; Zeitelhofer et al., 2017). In addition, downmodulation of proinflammatory cytokines and key chemokines and their specific receptors has been observed in VitD-treated EAE mice (Ahangar-Parvin et al., 2018; Jafarzadeh et al., 2019). Other EAE protective mechanisms, such as enhanced DC lysis by NK cells (Al-Jaderi and Maghazachi, 2015) and release of IL-4 by NKT cells (Waddell et al., 2015), have been attributed to VitD.
One of the key questions being addressed by our research team refers to the potential of VitD to work as a tolerogenic adjuvant. Initially, we demonstrated that VitD associated with MOG was able to clinically control EAE development in both prophylactic and therapeutic scenarios. Lower production of encephalitogenic cytokines and inhibition of DC maturation in peripheral lymphoid organs and less inflammation at the CNS were concomitant with this tolerogenic effect (Chiuso-Minicucci et al., 2015; Mimura et al., 2016). Interestingly, this tolerogenic vaccination with MOG plus VitD was able to control paralysis even in mice whose EAE was aggravated by previous infection with Candida albicans (Fraga-Silva et al., 2016). More recently, we reported that the association of MOG with a VitD analog (paricalcitol), applied by an epicutaneous route, was also effective in controlling EAE evolution. An enhanced proportion of Treg cells and a direct downmodulatory effect on microglial cell activation were associated with this protection (Zorzella-Pezavento et al., 2017). Among the putative benefits of VitD in the control of EAE is its ability to act directly on neural cells. In vitro and in vivo assays indicated that VitD was able to induce the proliferation of neural stem cells, enhance their differentiation into neurons and oligodendrocytes and protect neurons from killing by T cells (Gu et al., 2015; Sloka et al., 2015; Shirazi et al., 2017).
When we tested the effectiveness of VitD administered at different stages of the disease to control paralysis, a clear picture emerged, indicating that the sooner the disease was treated, better was the result. Significant control of disease disability was observed when VitD was provided 1 or 7 days after disease induction, being the earlier even more efficient (de Oliveira et al., 2020; Mimura et al., 2021). However, when therapy was postponed to 10 days postinfection, VitD lost its effectiveness (not shown). The protective outcome coincided with a stabilizing effect on BBB permeability, which is an interesting finding because a turning point in MS development is exactly BBB disruption and the subsequent transendothelial migration of activated leukocytes to the CNS. We verified that VitD stabilizing ability over the BBB is also crucially dependent upon the stage of the disease; only very precocious intervention with VitD can preserve its normal level of permeability (de Oliveira et al., 2020; Mimura et al., 2021). This competence of VitD to stabilize the BBB was already demonstrated in other scenarios, such as in vivo in a hypoxia/reoxigenation model (Won et al., 2015) and in vitro with human endothelial cells (Takahashi et al., 2017). Some of the immunomodulatory effects of VitD in experimental models of MS are depicted in Figure 2.
Figure 2.

Immunomodulatory effects of VitD in EAE.
VitD administration controls clinical EAE severity. Many immunological mechanisms are associated with protection: decreased differentiation of APCs and effector T cells, induction of Treg cells, reduction in microglial activation and CNS permeability and downregulation of intestinal Th17 differentiation. APCs: Antigen presenting cells; BBB: blood-brain barrier; CD: cluster of differentiation; CNS: central nervous system; EAE: experimental autoimmune encephalomyelitis; Foxp3: forkhead box P3; MHC: major histocompatibility complex; VitD: vitamin D.
As the results concerning VitD were observed by its intraperitoneal administration, we recently tested its per-oral efficacy. We presumed that this approach could better mimic the physiological immunoregulatory role of this hormone and considered that this is a more friendly procedure that facilitates patient adherence to therapy. As illustrated in Figure 3, per-oral delivery of VitD was also able to control paralysis development when administered 1 or 7 days after disease induction. Interestingly, unlike previous findings that indicated high calcium levels in the blood after delivery of VitD by the intraperitoneal route (de Oliveira et al., 2020), the oral route determined only a nonsignificant increase in serum calcium levels. However, efficacy was again more pronounced when therapy was delivered earlier. Taken together, our results employing the experimental model indicated that VitD administration in the early phase is clearly effective in reducing disease severity. The translation of these and of other authors’ findings to MS patients would require that their future disease development could be already predicted during the prodromal phase. Even though this still seems a challenging task, increasing evidence suggests that MS patients present various clinical manifestations, such as anxiety, depression, migraine, and lower cognitive performance, several years before diagnosis (Giovannoni, 2017; Disanto et al., 2018). According to those authors, the identification of a prodromal syndrome in MS can positively impact patient management.
Figure 3.
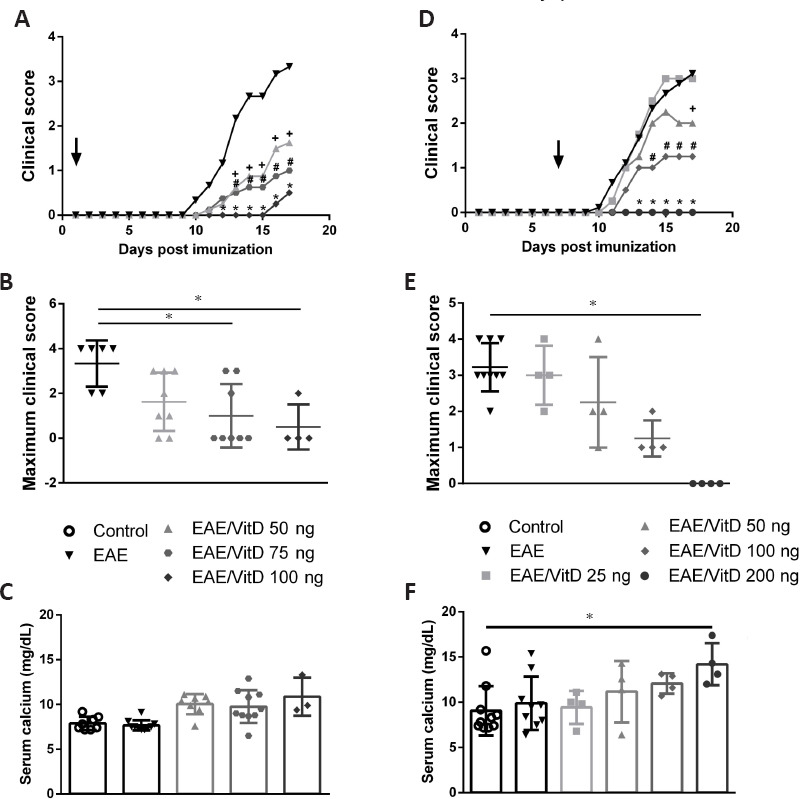
Effect of precocious and preclinical oral administration of VitD on EAE development.
Female C57BL/6 EAE mice were subjected to precocious therapy (A, B, and C) with different doses of VitD (50, 75 and 100 ng) by gavage every other day starting 1 day after disease induction with an emulsion containing MOG (150 µg) and CFA (50 µL) plus pertussis toxin (225 ng). In the preclinical therapy (D, E and F), EAE mice were treated daily with VitD (25, 50, 100 and 200 ng) by gavage starting 7 days after disease induction. Clinical disease score (A and D), maximum clinical score (B and E) and serum calcium levels (C and F) were assessed in the EAE acute phase (day 17). The results are presented as the mean ± standard error of the mean. * # + represents statistical significance between the EAE and EAE groups treated with VitD. Unpublished data. EAE: Experimental autoimmune encephalomyelitis; VitD: vitamin D.
Conclusion
Considering the aspects reviewed above, we consider that the potential use of VitD as an adjunct therapy for MS deserves to be investigated. Based on our experience with the EAE model and the plethora of data from other authors in both the experimental model and the clinical trials, we believe that better results could be obtained by a more precocious administration of this hormone. We certainly agree with other authors that emphasize the relevance of nontoxic doses, duration of the therapy and the maintenance of classical treatments during VitD supplementation.
Additional file: Open peer review report 1 (95.5KB, pdf) .
Acknowledgments:
The authors thank the Brazilian site for fCiências - Ciência e Tecnologia for providing a free image bank that was used in the assembly of Figures 1 and 2.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Anne L Astier, Université de Toulouse, France.
Funding: This work was supported by the scholarships provided by São Paulo Research Foundation (FAPESP), No. 2013/02371-6 (to LANM), No. 2013/01604-7 (to SFGZP), No. 2013/14353-2 (to TFCFS), No. 2015/06706-8 (to LANM) and No. 2019/15980-7 (to MBD) and financial support grants No. 2013/26257-8, São Paulo Research Foundation (FAPESP) and No. 307269/2017-5 and the National Council for Scientific and Technological Development (CNPq), to AS.
References
- 1.Ahangar-Parvin R, Mohammadi-Kordkhayli M, Azizi SV, Nemati M, Khorramdelazad H, Taghipour Z, Hassan Z, Moazzeni SM, Jafarzadeh A. The modulatory effects of vitamin D on the expression of IL-12 and TGF-β in the spinal cord and serum of mice with experimental autoimmune encephalomyelitis. Iran J Pathol. 2018;13:10–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Jaderi Z, Maghazachi AA. Vitamin D3 and monomethyl fumarate enhance natural killer cell lysis of dendritic cells and ameliorate the clinical score in mice suffering from experimental autoimmune encephalomyelitis. Toxins (Basel) 2015;7:4730–4744. doi: 10.3390/toxins7114730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrechtsen MT, Langeskov-Christensen M, Jørgensen MLK, Dalgas U, Hansen M. Is diet associated with physical capacity and fatigue in persons with multiple sclerosis? -results from a pilot study. Mult Scler Relat Disord. 2020;40:101921. doi: 10.1016/j.msard.2019.101921. [DOI] [PubMed] [Google Scholar]
- 4.Alharbi FM. Update in vitamin D and multiple sclerosis. Neurosciences (Riyadh) 2015;20:329–335. doi: 10.17712/nsj.2015.4.20150357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almokhtar M, Wikvall K, Ubhayasekera SJKA, Bergquist J, Norlin M. Motor neuron-like NSC-34 cells as a new model for the study of vitamin D metabolism in the brain. J Steroid Biochem Mol Biol. 2016;158:178–188. doi: 10.1016/j.jsbmb.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Almolda B, Costa M, Montoya M, González B, Castellano B. Increase in Th17 and T-reg lymphocytes and decrease of IL22 correlate with the recovery phase of acute EAE in rat. PLoS One. 2011;6:e27473. doi: 10.1371/journal.pone.0027473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Avila M, Bansal A, Culberson J, Peiris AN. The role of sex hormones in multiple sclerosis. Eur Neurol. 2018;80:93–99. doi: 10.1159/000494262. [DOI] [PubMed] [Google Scholar]
- 9.Bäärnhielm M, Hedström AK, Kockum I, Sundqvist E, Gustafsson SA, Hillert J, Olsson T, Alfredsson L. Sunlight is associated with decreased multiple sclerosis risk: no interaction with human leukocyte antigen-DRB1*15. Eur J Neurol. 2012;19:955–962. doi: 10.1111/j.1468-1331.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 10.Bäcker-Koduah P, Bellmann-Strobl J, Scheel M, Wuerfel J, Wernecke KD, Dörr J, Brandt AU, Paul F. Vitamin D and disease severity in multiple sclerosis-baseline data from the randomized controlled trial (EVIDIMS) Front Neurol. 2020;11:129. doi: 10.3389/fneur.2020.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benito-León J, Pisa D, Alonso R, Calleja P, Díaz-Sánchez M, Carrasco L. Association between multiple sclerosis and Candida species: evidence from a case-control study. Eur J Clin Microbiol Infect Dis. 2010;29:1139–1145. doi: 10.1007/s10096-010-0979-y. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ. Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J Physiol. 2017;595:6825–6836. doi: 10.1113/JP274887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhise V, Dhib-Jalbut S. Further understanding of the immunopathology of multiple sclerosis: impact on future treatments. Expert Rev Clin Immunol. 2016;12:1069–1089. doi: 10.1080/1744666X.2016.1191351. [DOI] [PubMed] [Google Scholar]
- 14.Bikle DD. Vitamin D, metabolism mechanism of action and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bjelobaba I, Begovic-Kupresanin V, Pekovic S, Lavrnja I. Animal models of multiple sclerosis: focus on experimental autoimmune encephalomyelitis. J Neurosci Res. 2018;96:1021–1042. doi: 10.1002/jnr.24224. [DOI] [PubMed] [Google Scholar]
- 16.Bjørnevik K, Riise T, Casetta I, Drulovic J, Granieri E, Holmøy T, Kampman MT, Landtblom AM, Lauer K, Lossius A, Magalhaes S, Myhr KM, Pekmezovic T, Wesnes K, Wolfson C, Pugliatti M. Sun exposure and multiple sclerosis risk in Norway and Italy: the EnvIMS study. Mult Scler. 2014;20:1042–1049. doi: 10.1177/1352458513513968. [DOI] [PubMed] [Google Scholar]
- 17.Brola W, Sobolewski P, Szczuchniak W, Góral A, Fudala M, Przybylski W, Opara J. Association of seasonal serum 25-hydroxyvitamin D levels with disability and relapses in relapsing-remitting multiple sclerosis. Eur J Clin Nutr. 2016;70:995–999. doi: 10.1038/ejcn.2016.51. [DOI] [PubMed] [Google Scholar]
- 18.Brown J, Quattrochi B, Everett C, Hong BY, Cervantes J. Gut commensals dysbiosis and immune response imbalance in the pathogenesis of multiple sclerosis. Mult Scler. 2021;27:807–811. doi: 10.1177/1352458520928301. [DOI] [PubMed] [Google Scholar]
- 19.Brown MA, Weinberg RB. Mast cells and innate lymphoid cells: underappreciated players in CNS autoimmune demyelinating disease. Front Immunol. 2018;9:514. doi: 10.3389/fimmu.2018.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budhram A, Parvathy S, Kremenchutzky M, Silverman M. Breaking down the gut microbiome composition in multiple sclerosis. Mult Scler. 2017;23:628–636. doi: 10.1177/1352458516682105. [DOI] [PubMed] [Google Scholar]
- 21.Camu W, Lehert P, Pierrot-Deseilligny C, Hautecoeur P, Besserve A, Jean Deleglise AS, Payet M, Thouvenot E, Souberbielle JC. Cholecalciferol in relapsing-remitting MS: a randomized clinical trial (CHOLINE) Neurol Neuroimmunol Neuroinflamm. 2019;6:e597. doi: 10.1212/NXI.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantorna MT, Hayes CE, DeLuca HF. 1, 25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1, 25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314–5319. [PubMed] [Google Scholar]
- 24.Chai B, Gao F, Wu R, Dong T, Gu C, Lin Q, Zhang Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: an updated meta-analysis. BMC Neurol. 2019;19:284. doi: 10.1186/s12883-019-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36. doi: 10.1007/s11882-010-0161-8. [DOI] [PubMed] [Google Scholar]
- 26.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1, 25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng Y, Sun L, Xie Z, Fan X, Cao Q, Han J, Zhu J, Jin T. Diversity of immune cell types in multiple sclerosis and its animal model: pathological and therapeutic implications. J Neurosci Res. 2017;95:1973–1983. doi: 10.1002/jnr.24023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi C, Shao X, Rhead B, Gonzales E, Smith JB, Xiang AH, Graves J, Waldman A, Lotze T, Schreiner T, Weinstock-Guttman B, Aaen G, Tillema JM, Ness J, Candee M, Krupp L, Gorman M, Benson L, Chitnis T, Mar S, et al. Admixture mapping reveals evidence of differential multiple sclerosis risk by genetic ancestry. PLoS Genet. 2019;15:e1007808. doi: 10.1371/journal.pgen.1007808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiuso-Minicucci F, Ishikawa LL, Mimura LA, Fraga-Silva TF, França TG, Zorzella-Pezavento SF, Marques C, Ikoma MR, Sartori A. Treatment with vitamin D/MOG association suppresses experimental autoimmune encephalomyelitis. PLoS One. 2015;10:e0125836. doi: 10.1371/journal.pone.0125836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark A, Mach N. Role of vitamin D in the hygiene hypothesis: the interplay between vitamin D, vitamin D receptors gut microbiota and immune response. Front Immunol. 2016;7:627. doi: 10.3389/fimmu.2016.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colotta F, Jansson B, Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J Autoimmun. 2017;85:78–97. doi: 10.1016/j.jaut.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Cortese M, Munger KL, Martínez-Lapiscina EH, Barro C, Edan G, Freedman MS, Hartung HP, Montalbán X, Foley FW, Penner IK, Hemmer B, Fox EJ, Schippling S, Wicklein EM, Kappos L, Kuhle J, Ascherio A, Group BS. Vitamin D, smoking, EBV, and long-term cognitive performance in MS: 11-year follow-up of BENEFIT. Neurology. 2020;94:e1950–1960. doi: 10.1212/WNL.0000000000009371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosorich I, Dalla-Costa G, Sorini C, Ferrarese R, Messina MJ, Dolpady J, Radice E, Mariani A, Testoni PA, Canducci F, Comi G, Martinelli V, Falcone M. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci Adv. 2017;3:e1700492. doi: 10.1126/sciadv.1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cusick MF, Libbey JE, Fujinami RS. Molecular mimicry as a mechanism of autoimmune disease. Clin Rev Allergy Immunol. 2012;42:102–111. doi: 10.1007/s12016-011-8294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danikowski KM, Jayaraman S, Prabhakar BS. Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation. 2017;14:117. doi: 10.1186/s12974-017-0892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Oliveira LRC, Mimura LAN, Fraga-Silva TFC, Ishikawa LLW, Fernandes AAH, Zorzella-Pezavento SFG, Sartori A. Calcitriol prevents neuroinflammation and reduces blood-brain barrier disruption and local macrophage/microglia activation. Front Pharmacol. 2020;11:161. doi: 10.3389/fphar.2020.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dereje S, Muradov I, Nazzal S, Nguyen T. Cholecalciferol (D3) versus ergocalciferol (D2) in older adults. Consult Pharm. 2017;32:337–339. doi: 10.4140/TCP.n.2017.337. [DOI] [PubMed] [Google Scholar]
- 38.Disanto G, Zecca C, MacLachlan S, Sacco R, Handunnetthi L, Meier UC, Simpson A, McDonald L, Rossi A, Benkert P, Kuhle J, Ramagopalan SV, Gobbi C. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol. 2018;83:1162–1173. doi: 10.1002/ana.25247. [DOI] [PubMed] [Google Scholar]
- 39.Dos Passos GR, Sato DK, Becker J, Fujihara K. Th17 cells pathways in multiple sclerosis and neuromyelitis optica spectrum disorders: pathophysiological and therapeutic implications. Mediators Inflamm. 2016;2016:5314541. doi: 10.1155/2016/5314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dörr J, Bäcker-Koduah P, Wernecke KD, Becker E, Hoffmann F, Faiss J, Brockmeier B, Hoffmann O, Anvari K, Wuerfel J, Piper SK, Bellmann-Strobl J, Brandt AU, Paul F. High-dose vitamin D supplementation in multiple sclerosis-results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Mult Scler J Exp Transl Clin. 2020;6:2055217320903474. doi: 10.1177/2055217320903474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elieh-Ali-Komi D, Cao Y. Role of mast cells in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Rev Allergy Immunol. 2017;52:436–445. doi: 10.1007/s12016-016-8595-y. [DOI] [PubMed] [Google Scholar]
- 42.Etemadifar M, Janghorbani M. Efficacy of high-dose vitamin D3 supplementation in vitamin D deficient pregnant women with multiple sclerosis: preliminary findings of a randomized-controlled trial. Iran J Neurol. 2015;14:67–73. [PMC free article] [PubMed] [Google Scholar]
- 43.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Fabri M, Modlin RL. A vitamin for autophagy. Cell Host Microbe. 2009;6:201–203. doi: 10.1016/j.chom.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Fan YG, Pang ZQ, Wu TY, Zhang YH, Xuan WQ, Wang Z, Yu X, Li YC, Guo C, Wang ZY. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic Biol Med. 2020;161:139–149. doi: 10.1016/j.freeradbiomed.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Farghali M, Ruga S, Morsanuto V, Uberti F. Can brain health be supported by vitamin D-Based supplements? A critical review. Brain Sci. 2020;10:660. doi: 10.3390/brainsci10090660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farias AS, Spagnol GS, Bordeaux-Rego P, Oliveira CO, Fontana AG, de Paula RF, Santos MP, Pradella F, Moraes AS, Oliveira EC, Longhini AL, Rezende AC, Vaisberg MW, Santos LM. Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg reducing the severity of EAE. CNS Neurosci Ther. 2013;19:269–277. doi: 10.1111/cns.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feige J, Salmhofer H, Hecker C, Kunz AB, Franzen M, Moré E, Sellner J. Life-threatening vitamin D intoxication due to intake of ultra-high doses in multiple sclerosis: a note of caution. Mult Scler. 2019;25:1326–1328. doi: 10.1177/1352458518807059. [DOI] [PubMed] [Google Scholar]
- 49.Fernandes de Abreu DA, Eyles D, Féron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34:265–277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 50.Fitzgerald KC, Munger KL, Köchert K, Arnason BG, Comi G, Cook S, Goodin DS, Filippi M, Hartung HP, Jeffery DR, O’Connor P, Suarez G, Sandbrink R, Kappos L, Pohl C, Ascherio A. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon Beta-1b. JAMA Neurol. 2015;72:1458–1465. doi: 10.1001/jamaneurol.2015.2742. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162:1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraga-Silva TF, Mimura LA, Zorzella-Pezavento SF, Ishikawa LL, França TG, Thomé R, Verinaud L, Arruda MS, Sartori A. Tolerogenic vaccination with MOG/VitD overcomes aggravating effect of C. albicans in experimental encephalomyelitis. CNS Neurosci Ther. 2016;22:807–816. doi: 10.1111/cns.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136:29–38. doi: 10.1111/jnc.13217. [DOI] [PubMed] [Google Scholar]
- 54.Gaboriau-Routhiau V, Rakotobe S, Lécuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Galior K, Grebe S, Singh R. Development of vitamin D toxicity from overcorrection of vitamin D deficiency: a review of case reports. Nutrients. 2018;10:953. doi: 10.3390/nu10080953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gallagher LG, Ilango S, Wundes A, Stobbe GA, Turk KW, Franklin GM, Linet MS, Freedman DM, Alexander BH, Checkoway H. Lifetime exposure to ultraviolet radiation and the risk of multiple sclerosis in the US radiologic technologists cohort study. Mult Scler. 2019;25:1162–1169. doi: 10.1177/1352458518783343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gharibi T, Babaloo Z, Hosseini A, Marofi F, Ebrahimi-Kalan A, Jahandideh S, Baradaran B. The role of B cells in the immunopathogenesis of multiple sclerosis. Immunology. 2020;160:325–335. doi: 10.1111/imm.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giovannoni G. The neurodegenerative prodrome in multiple sclerosis. Lancet Neurol. 2017;16:413–414. doi: 10.1016/S1474-4422(17)30127-8. [DOI] [PubMed] [Google Scholar]
- 59.Gomez-Pinedo U, Cuevas JA, Benito-Martín MS, Moreno-Jiménez L, Esteban-Garcia N, Torre-Fuentes L, Matías-Guiu JA, Pytel V, Montero P, Matías-Guiu J. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020;10:e01498. doi: 10.1002/brb3.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha, 25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grishkan IV, Fairchild AN, Calabresi PA, Gocke AR. 1, 25-Dihydroxyvitamin D3 selectively and reversibly impairs T helper-cell CNS localization. Proc Natl Acad Sci U S A. 2013;110:21101–21106. doi: 10.1073/pnas.1306072110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groves NJ, McGrath JJ, Burne TH. Vitamin D as a neurosteroid affecting the developing and adult brain. Annu Rev Nutr. 2014;34:117–141. doi: 10.1146/annurev-nutr-071813-105557. [DOI] [PubMed] [Google Scholar]
- 63.Gu SG, Wang CJ, Zhao G, Li GY. Role of vitamin D in regulating the neural stem cells of mouse model with multiple sclerosis. Eur Rev Med Pharmacol Sci. 2015;19:4004–4011. [PubMed] [Google Scholar]
- 64.Hemmer B, Vergelli M, Pinilla C, Houghten R, Martin R. Probing degeneracy in T-cell recognition using peptide combinatorial libraries. Immunol Today. 1998;19:163–168. doi: 10.1016/s0167-5699(97)01217-6. [DOI] [PubMed] [Google Scholar]
- 65.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 66.Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25:141–148. doi: 10.1677/jme.0.0250141. [DOI] [PubMed] [Google Scholar]
- 67.Hoepner R, Bagnoud M, Pistor M, Salmen A, Briner M, Synn H, Schrewe L, Guse K, Ahmadi F, Demir S, Laverick L, Gresle M, Worley P, Reichardt HM, Butzkueven H, Gold R, Metz I, Lühder F, Chan A. Vitamin D increases glucocorticoid efficacy via inhibition of mTORC1 in experimental models of multiple sclerosis. Acta Neuropathol. 2019;138:443–456. doi: 10.1007/s00401-019-02018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holmøy T, Røsjø E, Zetterberg H, Blennow K, Lindstrøm JC, Steffensen LH, Kampman MT. Vitamin D supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol Scand. 2019;139:172–176. doi: 10.1111/ane.13037. [DOI] [PubMed] [Google Scholar]
- 69.Hongell K, Silva DG, Ritter S, Meier DP, Soilu-Hänninen M. Efficacy and safety outcomes in vitamin D supplement users in the fingolimod phase 3 trials. J Neurol. 2018;265:348–355. doi: 10.1007/s00415-017-8697-3. [DOI] [PubMed] [Google Scholar]
- 70.Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- 71.Hupperts R, Smolders J, Vieth R, Holmøy T, Marhardt K, Schluep M, Killestein J, Barkhof F, Beelke M, Grimaldi LME, Group SS. Randomized trial of daily high-dose vitamin D. Neurology. 2019;93:e1906–1916. doi: 10.1212/WNL.0000000000008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hussain S, Kirwin SJ, Stohlman SA. Increased T regulatory cells lead to development of Th2 immune response in male SJL mice. Autoimmunity. 2011;44:219–228. doi: 10.3109/08916934.2010.519746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365:eaav7188. doi: 10.1126/science.aav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jafarzadeh A, Azizi SV, Arabi Z, Ahangar-Parvin R, Mohammadi-Kordkhayli M, Larussa T, Khatami F, Nemati M. Vitamin D down-regulates the expression of some Th17 cell-related cytokines key inflammatory chemokines and chemokine receptors in experimental autoimmune encephalomyelitis. Nutr Neurosci. 2019;22:725–737. doi: 10.1080/1028415X.2018.1436237. [DOI] [PubMed] [Google Scholar]
- 76.Jagannath VA, Fedorowicz Z, Asokan GV, Robak EW, Whamond L. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD008422.pub2. doi: 10.1002/14651858.CD008422.pub2. [DOI] [PubMed] [Google Scholar]
- 77.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1, 25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeon SM, Shin EA. Exploring vitamin D metabolism and function in cancer. Exp Mol Med. 2018;50:1–14. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88:582–586. doi: 10.1093/ajcn/88.2.582S. [DOI] [PubMed] [Google Scholar]
- 80.Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses disease progression and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler. 2012;18:1144–1151. doi: 10.1177/1352458511434607. [DOI] [PubMed] [Google Scholar]
- 81.Karpus WJ. Cytokines and chemokines in the pathogenesis of experimental autoimmune encephalomyelitis. J Immunol. 2020;204:316–326. doi: 10.4049/jimmunol.1900914. [DOI] [PubMed] [Google Scholar]
- 82.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khammissa RAG, Fourie J, Motswaledi MH, Ballyram R, Lemmer J, Feller L. The biological activities of vitamin D and its receptor in relation to calcium and bone homeostasis cancer immune and cardiovascular systems skin biology and oral health. Biomed Res Int. 2018;2018:9276380. doi: 10.1155/2018/9276380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitz A, Singer E, Hafler D. Regulatory T cells: from discovery to autoimmunity. Cold Spring Harb Perspect Med. 2018;8:a029041. doi: 10.1101/cshperspect.a029041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin D receptor and T cell function. Front Immunol. 2013;4:148. doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koutrolos M, Berer K, Kawakami N, Wekerle H, Krishnamoorthy G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun. 2014;2:163. doi: 10.1186/s40478-014-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kurniawan H, Soriano-Baguet L, Brenner D. Regulatory T cell metabolism at the intersection between autoimmune diseases and cancer. Eur J Immunol. 2020;50:1626–1642. doi: 10.1002/eji.201948470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Landel V, Stephan D, Cui X, Eyles D, Feron F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J Steroid Biochem Mol Biol. 2018;177:129–134. doi: 10.1016/j.jsbmb.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 89.Lang Y, Chu F, Shen D, Zhang W, Zheng C, Zhu J, Cui L. Role of inflammasomes in neuroimmune and neurodegenerative diseases: a systematic review. Mediators Inflamm. 2018;2018:1549549. doi: 10.1155/2018/1549549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Langer-Gould A, Lucas R, Xiang AH, Chen LH, Wu J, Gonzalez E, Haraszti S, Smith JB, Quach H, Barcellos LF. MS sunshine study: sun exposure but not vitamin D is associated with multiple sclerosis risk in blacks and hispanics. Nutrients. 2018;10:268. doi: 10.3390/nu10030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lassmann H, Bradl M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 2017;133:223–244. doi: 10.1007/s00401-016-1631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lassmann H, Brück W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–218. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lazibat I, Rubinić Majdak M, Županić S. Multiple sclerosis: new aspects of immunopathogenesis. Acta Clin Croat. 2018;57:352–361. doi: 10.20471/acc.2018.57.02.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee YK, Menezes JS, Umesaki Y, Mazmanian SK. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2011;108:4615–4622. doi: 10.1073/pnas.1000082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leichner TM, Satake A, Harrison VS, Tanaka Y, Archambault AS, Kim BS, Siracusa MC, Leonard WJ, Naji A, Wu GF, Artis D, Kambayashi T. Skin-derived TSLP systemically expands regulatory T cells. J Autoimmun. 2017;79:39–52. doi: 10.1016/j.jaut.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lemire JM, Archer DC. 1, 25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Invest. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li B, Baylink DJ, Deb C, Zannetti C, Rajaallah F, Xing W, Walter MH, Lau KH, Qin X. 1, 25-Dihydroxyvitamin D3 suppresses TLR8 expression and TLR8-mediated inflammatory responses in monocytes in vitro and experimental autoimmune encephalomyelitis in vivo. PLoS One. 2013;8:e58808. doi: 10.1371/journal.pone.0058808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 99.Løken-Amsrud KI, Holmøy T, Bakke SJ, Beiske AG, Bjerve KS, Bjørnarå BT, Hovdal H, Lilleås F, Midgard R, Pedersen T, Benth JS, Sandvik L, Torkildsen O, Wergeland S, Myhr KM. Vitamin D and disease activity in multiple sclerosis before and during interferon-β treatment. Neurology. 2012;79:267–273. doi: 10.1212/WNL.0b013e31825fdf01. [DOI] [PubMed] [Google Scholar]
- 100.Ma Q, Xing C, Long W, Wang HY, Liu Q, Wang RF. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J Neuroinflammation. 2019;16:53. doi: 10.1186/s12974-019-1434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT. Cytokine profile in patients with multiple sclerosis following vitamin D supplementation. J Neuroimmunol. 2003;134:128–132. doi: 10.1016/s0165-5728(02)00396-x. [DOI] [PubMed] [Google Scholar]
- 102.Mansilla MJ, Sellès-Moreno C, Fàbregas-Puig S, Amoedo J, Navarro-Barriuso J, Teniente-Serra A, Grau-López L, Ramo-Tello C, Martínez-Cáceres EM. Beneficial effect of tolerogenic dendritic cells pulsed with MOG autoantigen in experimental autoimmune encephalomyelitis. CNS Neurosci Ther. 2015;21:222–230. doi: 10.1111/cns.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martinelli V, Dalla Costa G, Colombo B, Dalla Libera D, Rubinacci A, Filippi M, Furlan R, Comi G. Vitamin D levels and risk of multiple sclerosis in patients with clinically isolated syndromes. Mult Scler. 2014;20:147–155. doi: 10.1177/1352458513494959. [DOI] [PubMed] [Google Scholar]
- 104.Mattner F, Smiroldo S, Galbiati F, Muller M, Di Lucia P, Poliani PL, Martino G, Panina-Bordignon P, Adorini L. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1, 25-dihydroxyvitamin D(3) Eur J Immunol. 2000;30:498–508. doi: 10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 105.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 106.McGinley AM, Edwards SC, Raverdeau M, Mills KHG. Th17 cells, γδ T cells and their interplay in EAE and multiple sclerosis. J Autoimmun. 2018 doi: 10.1016/j.jaut.2018.01.001. doi: 10.1016/j.jaut.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 107.Meehan TF, DeLuca HF. The vitamin D receptor is necessary for 1alpha, 25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Arch Biochem Biophys. 2002;408:200–204. doi: 10.1016/s0003-9861(02)00580-5. [DOI] [PubMed] [Google Scholar]
- 108.Mimura LA, Chiuso-Minicucci F, Fraga-Silva TF, Zorzella-Pezavento SF, França TG, Ishikawa LL, Penitenti M, Ikoma MR, Sartori A. Association of myelin peptide with vitamin D prevents autoimmune encephalomyelitis development. Neuroscience. 2016;317:130–140. doi: 10.1016/j.neuroscience.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 109.Mimura LAN, Fraga-Silva TFC, Oliveira LRC, Ishikawa LLW, Borim PA, Machado CM, Júnior JACE, Fonseca DMD, Sartori A. Preclinical therapy with vitamin D3 in experimental encephalomyelitis: efficacy and comparison with paricalcitol. Int J Mol Sci. 2021;22:1914. doi: 10.3390/ijms22041914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 111.Mohammadi-Kordkhayli M, Ahangar-Parvin R, Azizi SV, Nemati M, Shamsizadeh A, Khaksari M, Moazzeni SM, Jafarzadeh A. Vitamin D modulates the expression of IL-27 and IL-33 in the central nervous system in experimental autoimmune encephalomyelitis (EAE) Iran J Immunol. 2015;12:35–49. [PubMed] [Google Scholar]
- 112.Morello M, Landel V, Lacassagne E, Baranger K, Annweiler C, Féron F, Millet P. Vitamin D improves neurogenesis and cognition in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2018;55:6463–6479. doi: 10.1007/s12035-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mostafa WZ, Hegazy RA. Vitamin D and the skin: focus on a complex relationship: a review. J Adv Res. 2015;6:793–804. doi: 10.1016/j.jare.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moutsianas L, Jostins L, Beecham AH, Dilthey AT, Xifara DK, Ban M, Shah TS, Patsopoulos NA, Alfredsson L, Anderson CA, Attfield KE, Baranzini SE, Barrett J, Binder TMC, Booth D, Buck D, Celius EG, Cotsapas C, D’Alfonso S, Dendrou CA. Class II HLA interactions modulate genetic risk for multiple sclerosis. Nat Genet. 2015;47:1107–1113. doi: 10.1038/ng.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 116.Muris AH, Smolders J, Rolf L, Thewissen M, Hupperts R, Damoiseaux J; SOLARIUM study group. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J Neuroimmunol. 2016;300:47–56. doi: 10.1016/j.jneuroim.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 117.Muthian G, Raikwar HP, Rajasingh J, Bright JJ. 1, 25 Dihydroxyvitamin-D3 modulates JAK-STAT pathway in IL-12/IFNgamma axis leading to Th1 response in experimental allergic encephalomyelitis. J Neurosci Res. 2006;83:1299–1309. doi: 10.1002/jnr.20826. [DOI] [PubMed] [Google Scholar]
- 118.Nanduri R, Mahajan S, Bhagyaraj E, Sethi K, Kalra R, Chandra V, Gupta P. The active form of vitamin D transcriptionally represses smad7 signaling and activates extracellular signal-regulated kinase (ERK) to inhibit the differentiation of a inflammatory T helper cell subset and suppress experimental autoimmune encephalomyelitis. J Biol Chem. 2015;290:12222–12236. doi: 10.1074/jbc.M114.621839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nashold FE, Miller DJ, Hayes CE. 1, 25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2000;103:171–179. doi: 10.1016/s0165-5728(99)00247-7. [DOI] [PubMed] [Google Scholar]
- 120.Nashold FE, Nelson CD, Brown LM, Hayes CE. One calcitriol dose transiently increases Helios+ FoxP3+ T cells and ameliorates autoimmune demyelinating disease. J Neuroimmunol. 2013;263:64–74. doi: 10.1016/j.jneuroim.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 121.Nikanfar M, Taheri-Aghdam AA, Yazdani M, Shaafi S, Masoudian N, Akbari H, Youhanaee P, Abbaszadeh H. Serum 25(OH) vitamin D levels is not associated with disability in multiple sclerosis patients: a case-control study. Iran J Neurol. 2015;14:17–21. [PMC free article] [PubMed] [Google Scholar]
- 122.Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C, Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flügel-Koch C, Flügel A. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 123.Olsson T, Barcellos LF, Alfredsson L. Interactions between genetic lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. 2017;13:25–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 124.Pardridge WM, Sakiyama R, Coty WA. Restricted transport of vitamin D and a derivatives through the rat blood-brain barrier. J Neurochem. 1985;44:1138–1141. doi: 10.1111/j.1471-4159.1985.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 125.Pendo K, DeGiorgio CM. Vitamin D3 for the treatment of epilepsy: basic mechanisms animal models and clinical trials. Front Neurol. 2016;7:218. doi: 10.3389/fneur.2016.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pierrot-Deseilligny C, Souberbielle JC. Vitamin D and multiple sclerosis: An update. Mult Scler Relat Disord. 2017;14:35–45. doi: 10.1016/j.msard.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 127.Pinke KH, Zorzella-Pezavento SFG, de Campos Fraga-Silva TF, Mimura LAN, de Oliveira LRC, Ishikawa LLW, Fernandes AAH, Lara VS, Sartori A. Calming down mast cells with ketotifen: a potential strategy for multiple sclerosis therapy. Neurotherapeutics. 2020a;17:218–234. doi: 10.1007/s13311-019-00775-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pinke KH, Zorzella-Pezavento SFG, Lara VS, Sartori A. Should mast cells be considered therapeutic targets in multiple sclerosis. Neural Regen Res. 2020b;15:1995–2007. doi: 10.4103/1673-5374.282238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 130.Raker VK, Domogalla MP, Steinbrink K. Tolerogenic dendritic cells for regulatory T cell induction in man. Front Immunol. 2015;6:569. doi: 10.3389/fimmu.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ransohoff RM. Immunology: Licensed in the lungs. Nature. 2012;488:595–596. doi: 10.1038/488595a. [DOI] [PubMed] [Google Scholar]
- 132.Rimmelzwaan LM, van Schoor NM, Lips P, Berendse HW, Eekhoff EM. Systematic review of the relationship between vitamin D and Parkinson’s disease. J Parkinsons Dis. 2016;6:29–37. doi: 10.3233/JPD-150615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robinson AP, Harp CT, Noronha A, Miller SD. The experimental autoimmune encephalomyelitis (EAE) model of MS: utility for understanding disease pathophysiology and treatment. Handb Clin Neurol. 2014;122:173–189. doi: 10.1016/B978-0-444-52001-2.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rolf L, Muris AH, Theunissen R, Hupperts R, Damoiseaux J, Smolders J. Vitamin D3 supplementation and the IL-2/IL-2R pathway in multiple sclerosis: Attenuation of progressive disturbances. J Neuroimmunol. 2018;314:50–57. doi: 10.1016/j.jneuroim.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 135.Rolf L, Smolders J, van den Ouweland J, Hupperts R, Damoiseaux J. Correlation of different cellular assays to analyze T cell-related cytokine profiles in vitamin D3-supplemented patients with multiple sclerosis. Mol Immunol. 2019;105:198–204. doi: 10.1016/j.molimm.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 136.Røsjø E, Myhr KM, Løken-Amsrud KI, Bakke SJ, Beiske AG, Bjerve KS, Hovdal H, Lilleås F, Midgard R, Pedersen T, Šaltytė Benth J, Torkildsen Ø, Wergeland S, Michelsen AE, Aukrust P, Ueland T, Holmøy T. Vitamin D status and effect of interferon-β1a treatment on MRI activity and serum inflammation markers in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2015;280:21–28. doi: 10.1016/j.jneuroim.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 137.Saberi A, Akhondzadeh S, Kazemi S. Infectious agents and different course of multiple sclerosis: a systematic review. Acta Neurol Belg. 2018;118:361–377. doi: 10.1007/s13760-018-0976-y. [DOI] [PubMed] [Google Scholar]
- 138.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 139.Saligrama N, Zhao F, Sikora MJ, Serratelli WS, Fernandes RA, Louis DM, Yao W, Ji X, Idoyaga J, Mahajan VB, Steinmetz LM, Chien YH, Hauser SL, Oksenberg JR, Garcia KC, Davis MM. Opposing T cell responses in experimental autoimmune encephalomyelitis. Nature. 2019;572:481–487. doi: 10.1038/s41586-019-1467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012;79:2140–2145. doi: 10.1212/WNL.0b013e3182752ea8. [DOI] [PubMed] [Google Scholar]
- 141.Saroukolaei SA, Ghabaee M, Shokri H, Badiei A, Ghourchian S. The role of Candida albicans in the severity of multiple sclerosis. Mycoses. 2016;59:697–704. doi: 10.1111/myc.12489. [DOI] [PubMed] [Google Scholar]
- 142.Shin T, Ahn M, Matsumoto Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anat Cell Biol. 2012;45:141–148. doi: 10.5115/acb.2012.45.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shirazi HA, Rasouli J, Ciric B, Wei D, Rostami A, Zhang GX. 1, 25-Dihydroxyvitamin D. Exp Mol Pathol. 2017;102:515–521. doi: 10.1016/j.yexmp.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sintzel MB, Rametta M, Reder AT. Vitamin D and multiple sclerosis: a comprehensive review. Neurol Ther. 2018;7:59–85. doi: 10.1007/s40120-017-0086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sloka S, Silva C, Wang J, Yong VW. Predominance of Th2 polarization by vitamin D through a STAT6-dependent mechanism. J Neuroinflammation. 2011;8:56. doi: 10.1186/1742-2094-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sloka S, Zhornitsky S, Silva C, Metz LM, Yong VW. 1, 25-Dihydroxyvitamin D3 protects against immune-mediated killing of neurons in culture and in experimental autoimmune encephalomyelitis. PLoS One. 2015;10:e0144084. doi: 10.1371/journal.pone.0144084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Smolders J, Damoiseaux J. Vitamin D as a T-cell modulator in multiple sclerosis. Vitam Horm. 2011;86:401–428. doi: 10.1016/B978-0-12-386960-9.00018-6. [DOI] [PubMed] [Google Scholar]
- 148.Smolders J, Mimpen M, Oechtering J, Damoiseaux J, van den Ouweland J, Hupperts R, Kuhle J. Vitamin D3 supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol Scand. 2020;141:77–80. doi: 10.1111/ane.13185. [DOI] [PubMed] [Google Scholar]
- 149.Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, Gocke A, Steinman L, Mowry EM, Calabresi PA. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86:382–390. doi: 10.1212/WNL.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spach KM, Nashold FE, Dittel BN, Hayes CE. IL-10 signaling is essential for 1, 25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030–6037. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 151.Spanier JA, Nashold FE, Mayne CG, Nelson CD, Hayes CE. Vitamin D and estrogen synergy in Vdr-expressing CD4(+) T cells is essential to induce Helios(+)FoxP3(+) T cells and prevent autoimmune demyelinating disease. J Neuroimmunol. 2015;286:48–58. doi: 10.1016/j.jneuroim.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 152.Spanier JA, Nashold FE, Nelson CD, Praska CE, Hayes CE. Vitamin D3-mediated resistance to a multiple sclerosis model disease depends on myeloid cell 1, 25-dihydroxyvitamin D3 synthesis and correlates with increased CD4+ T cell CTLA-4 expression. J Neuroimmunol. 2020;338:577105. doi: 10.1016/j.jneuroim.2019.577105. [DOI] [PubMed] [Google Scholar]
- 153.Stockinger B, Omenetti S. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 2017;17:535–544. doi: 10.1038/nri.2017.50. [DOI] [PubMed] [Google Scholar]
- 154.Stohlman SA, Hinton DR. Viral induced demyelination. Brain Pathol. 2001;11:92–106. doi: 10.1111/j.1750-3639.2001.tb00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Su D, Nie Y, Zhu A, Chen Z, Wu P, Zhang L, Luo M, Sun Q, Cai L, Lai Y, Xiao Z, Duan Z, Zheng S, Wu G, Hu R, Tsukamoto H, Lugea A, Liu Z, Pandol SJ, Han YP. Vitamin D signaling through induction of paneth cell defensins maintains gut microbiota and improves metabolic disorders and hepatic steatosis in animal models. Front Physiol. 2016;7:498. doi: 10.3389/fphys.2016.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suresh Kumar R, Syed S, Anand Kumar A, Subha Kumari KN, Sajitha K. Serum vitamin d levels in Indian patients with multiple sclerosis. Indian J Clin Biochem. 2013;28:255–258. doi: 10.1007/s12291-012-0264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Széles L, Keresztes G, Töröcsik D, Balajthy Z, Krenács L, Póliska S, Steinmeyer A, Zuegel U, Pruenster M, Rot A, Nagy L. 1, 25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 158.Tan C, Wandu WS, Lee RS, Hinshaw SH, Klinman DM, Wawrousek E, Gery I. Shedding new light on the process of “licensing” for pathogenicity by Th lymphocytes. J Immunol. 2017;198:681–690. doi: 10.4049/jimmunol.1502108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tankou SK, Regev K, Healy BC, Tjon E, Laghi L, Cox LM, Kivisäkk P, Pierre IV, Hrishikesh L, Gandhi R, Cook S, Glanz B, Stankiewicz J, Weiner HL. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018;83:1147–1161. doi: 10.1002/ana.25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Taylor PN, Davies JS. A review of the growing risk of vitamin D toxicity from inappropriate practice. Br J Clin Pharmacol. 2018;84:1121–1127. doi: 10.1111/bcp.13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thouvenot E, Orsini M, Daures JP, Camu W. Vitamin D is associated with degree of disability in patients with fully ambulatory relapsing-remitting multiple sclerosis. Eur J Neurol. 2015;22:564–569. doi: 10.1111/ene.12617. [DOI] [PubMed] [Google Scholar]
- 162.Tremlett H, Zhu F, Ascherio A, Munger KL. Sun exposure over the life course and associations with multiple sclerosis. Neurology. 2018;90:e1191–1199. doi: 10.1212/WNL.0000000000005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Valburg C, Sonti A, Stern JN, Najjar S, Harel A. Dietary factors in experimental autoimmune encephalomyelitis and multiple sclerosis: a comprehensive review. Mult Scler. 2021;27:494–502. doi: 10.1177/1352458520923955. [DOI] [PubMed] [Google Scholar]
- 164.van der Aar AM, Sibiryak DS, Bakdash G, van Capel TM, van der Kleij HP, Opstelten DJ, Teunissen MB, Kapsenberg ML, de Jong EC. Vitamin D3 targets epidermal and dermal dendritic cells for induction of distinct regulatory T cells. J Allergy Clin Immunol. 2011;127:1532–1540. doi: 10.1016/j.jaci.2011.01.068. [DOI] [PubMed] [Google Scholar]
- 165.van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, Butzkueven H, Kilpatrick T. Past exposure to sun skin phenotype and risk of multiple sclerosis: case-control study. BMJ. 2003;327:316. doi: 10.1136/bmj.327.7410.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Waddell A, Zhao J, Cantorna MT. NKT cells can help mediate the protective effects of 1, 25-dihydroxyvitamin D3 in experimental autoimmune encephalomyelitis in mice. Int Immunol. 2015;27:237–244. doi: 10.1093/intimm/dxu147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J Clin Invest. 2004;113:990–997. doi: 10.1172/JCI19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Waseem R, Shamsi A, Kazim S, Islam A. An insight into mitochondrial dysfunction and its implications in neurological diseases. Curr Drug Targets. 2020 doi: 10.2174/1389450121999201230204050. doi: 10.2174/1389450121999201230204050. [DOI] [PubMed] [Google Scholar]
- 170.Wawrzyniak S, Mikołajewska E, Kuczko-Piekarska E, Niezgodzińska-Maciejek A, Goch A. Association of vitamin D status and clinical and radiological outcomes in a treated MS population in Poland. Brain Behav. 2017;7:e00609. doi: 10.1002/brb3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wei R, Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients. 2015;7:8251–8260. doi: 10.3390/nu7105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Won S, Sayeed I, Peterson BL, Wali B, Kahn JS, Stein DG. Vitamin D prevents hypoxia/reoxygenation-induced blood-brain barrier disruption via vitamin D receptor-mediated NF-kB signaling pathways. PLoS One. 2015;10:e0122821. doi: 10.1371/journal.pone.0122821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wrzosek M, Łukaszkiewicz J, Jakubczyk A, Matsumoto H, Piątkiewicz P, Radziwoń-Zaleska M, Wojnar M, Nowicka G. Vitamin D and the central nervous system. Pharmacol Rep. 2013;65:271–278. doi: 10.1016/s1734-1140(13)71003-x. [DOI] [PubMed] [Google Scholar]
- 174.Xie Z, Chen J, Zheng C, Wu J, Cheng Y, Zhu S, Lin C, Cao Q, Zhu J, Jin T. 1, 25-dihydroxyvitamin D. Immunology. 2017;152:414–424. doi: 10.1111/imm.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S. Advances in the immunopathogenesis of multiple sclerosis. Curr Opin Neurol. 2015;28:206–219. doi: 10.1097/WCO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 176.Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, Cullimore ML, Rostami A, Xu H. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol. 2010;185:5953–5961. doi: 10.4049/jimmunol.1001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Youssef DA, Miller CW, El-Abbassi AM, Cutchins DC, Cutchins C, Grant WB, Peiris AN. Antimicrobial implications of vitamin D. Dermatoendocrinol. 2011;3:220–229. doi: 10.4161/derm.3.4.15027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ysrraelit MC, Correale J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology. 2019;156:9–22. doi: 10.1111/imm.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zarobkiewicz MK, Kowalska W, Roliński J, Bojarska-Junak AA. γδ T lymphocytes in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 2019;330:67–73. doi: 10.1016/j.jneuroim.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 180.Zeitelhofer M, Adzemovic MZ, Gomez-Cabrero D, Bergman P, Hochmeister S, N’diaye M, Paulson A, Ruhrmann S, Almgren M, Tegnér JN, Ekström TJ, Guerreiro-Cacais AO, Jagodic M. Functional genomics analysis of vitamin D effects on CD4+ T cells in vivo in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2017;114:E1678–1687. doi: 10.1073/pnas.1615783114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang Z, Chen F, Li J, Luo F, Hou T, Xu J, Sun D. 1, 25(OH)2D3 suppresses proinflammatory responses by inhibiting Th1 cell differentiation and cytokine production through the JAK/STAT pathway. Am J Transl Res. 2018;10:2737–2746. [PMC free article] [PubMed] [Google Scholar]
- 182.Zhen C, Feng X, Li Z, Wang Y, Li B, Li L, Quan M, Wang G, Guo L. Suppression of murine experimental autoimmune encephalomyelitis development by 1, 25-dihydroxyvitamin D3 with autophagy modulation. J Neuroimmunol. 2015;280:1–7. doi: 10.1016/j.jneuroim.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 183.Zheng C, He L, Liu L, Zhu J, Jin T. The efficacy of vitamin D in multiple sclerosis: a meta-analysis. Mult Scler Relat Disord. 2018;23:56–61. doi: 10.1016/j.msard.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 184.Zorzella-Pezavento SFG, Mimura LAN, Fraga-Silva TFC, Ishikawa LLW, França TGD, Sartori A. Experimental autoimmune encephalomyelitis is successfully controlled by epicutaneous administration of MOG plus vitamin D analog. Front Immunol. 2017;8:1198. doi: 10.3389/fimmu.2017.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zündorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


