Keywords: circadian rhythms, diabetes mellitus, inflammation, ischemic stroke, long-term prognosis, neurological function, neuron, nuclear factor kappa B, stellate ganglion block, Toll like receptor 4
Abstract
Diabetes mellitus is an independent risk factor for ischemic stroke. Both diabetes mellitus and stroke are linked to systemic inflammation that aggravates patient outcomes. Stellate ganglion block can effectively regulate the inflammatory response. Therefore, it is hypothesized that stellate ganglion block could be a potential therapy for ischemic stroke in diabetic subjects. In this study, we induced diabetes mellitus in rats by feeding them a high-fat diet for 4 successive weeks. The left middle cerebral artery was occluded to establish models of ischemic stroke in diabetic rats. Subsequently, we performed left stellate ganglion block with 1% lidocaine using the percutaneous posterior approach 15 minutes before reperfusion and again 20 and 44 hours after reperfusion. Our results showed that stellate ganglion block did not decrease the blood glucose level in diabetic rats with diabetes mellitus but did reduce the cerebral infarct volume and the cerebral water content. It also improved the recovery of neurological function, increased 28-day survival rate, inhibited Toll like receptor 4/nuclear factor kappa B signaling pathway and reduced inflammatory response in the plasma of rats. However, injection of Toll like receptor 4 agonist lipopolysaccharide 5 minutes before stellate ganglion block inhibited the effect of stellate ganglion block, whereas injection of Toll like receptor 4 inhibitor TAK242 had no such effect. We also found that stellate ganglion block performed at night had no positive effect on diabetic ischemic stroke. These findings suggest that stellate ganglion block is a potential therapy for diabetic ischemic stroke and that it may be mediated through the Toll like receptor 4/nuclear factor kappa B signaling pathway. We also found that the therapeutic effect of stellate ganglion block is affected by circadian rhythm.
Introduction
Ischemic stroke and diabetes mellitus (DM) are debilitating conditions with limited therapeutic options. The pathological processes of these two conditions are closely associated; they interact with and exacerbate each other, and both diseases are related to inflammation (Moskowitz et al., 2010; Wei et al., 2016). Encouragingly, anti-inflammatories have shown multiple benefits in both diabetes and stroke (Petrovic-Djergovic et al., 2016; Wattananit et al., 2016; Rayasam et al., 2017). Many studies on inflammation in the treatment of diabetic stroke are now in progress (Petrovic-Djergovic et al., 2016; Wei et al., 2016; Fusco et al., 2019). However, the use of anti-inflammatories during routine clinical perioperative stroke procedures, especially anesthesia, may be limited. Therefore, we aim to identify an approach that is closely related to the treatment of ischemic stroke in diabetic patients.
Stellate ganglion block (SGB) is a unique anesthesia treatment that has a rapid onset, precise effects, minimal side effects and repeatability. It plays an unparalleled role in the treatment of many diseases (Haest et al., 2012; Fisher et al., 2013; Rae Olmsted et al., 2020), significantly reducing the inflammatory response (Yang et al., 2015; Chen et al., 2018). Ultrasound guidance has improved the effectiveness of SGB in ischemic stroke patients (Yoo et al., 2012; Liu et al., 2020) but there have been few studies on stroke in diabetic patients. These studies suggest that SBG may play an active role in both diabetic and non-diabetic stroke patients.
The Toll-like receptor 4/nuclear factor kappa-B (TLR4/NF-κB) pathway is a major inflammatory pathway (Fiorotto et al., 2011) and plays a crucial role in pathological features of diabetes and ischemic stroke (Liu et al., 2013; Zhang et al., 2013; Han et al., 2016; Jiang et al., 2020; Lang et al., 2021). This pathway was first discovered in the context of tumor immunity and subsequently found to play essential roles in the pathophysiology of many diseases (Muzio et al., 1998; Yang et al., 2015; Dong et al., 2018; Wang et al., 2018). It plays a vital role in the progression of inflammation (Qiao et al., 2012; Li et al., 2020a). As a cell surface protein involved in the innate immune response, TLR4 is closely related to microglia and it activates the transcription factor NF-κB that regulates cytokines and chemokines related to inflammation (Gosselin et al., 2017; Hansen et al., 2018; Choi et al., 2020). Other studies showed that microglial activation and autophagy are closely related to the TLR4/NF-κB pathway (Qin et al., 2018; Choi et al., 2020). Autophagy and activation of microglia are critical pathological processes after ischemic stroke (Qin et al., 2018; Jayaraj et al., 2019; Zhang, 2019; Deng et al., 2021). Therefore, we hypothesized that SGB could be a treatment for diabetes-associated ischemic stroke and that its neuroprotective effect might be achieved through inhibition of the TLR4/NF-κB pathway. To test this hypothesis, we inhibited and activated TLR4 using TAK242 and lipopolysaccharide (LPS), respectively, and examined whether the protective effect of SGB was related to the TLR4/NF-κB pathway. We tested for any circadian rhythms in the SGB treatment.
Materials and Methods
Animals and study design
This study was approved by the Institutional Animal Care Committee of the 2nd Affiliated Hospital of Harbin Medical University, China (approval No. SYDW2019-209) on May 8, 2019. Because there is a higher incidence of strokes in male patients than in females (Ntaios et al., 2017) and estrogen has some neuroprotective effect (Reis de Assis et al., 2021; Willsey et al., 2021), we limited our study to male rats. To reduce other extraneous variables, we used 156 3–4-week-old male clean-level Sprague-Dawley rats weighing 60–80 g that were obtained from the Department of Experimental Animals, Harbin Medical University [license No. SCXK (Hei) 2013-001]. The rats were maintained on a 12/12-hour light/dark cycle at a controlled temperature (21–24°C) and humidity (40–70%) with free access to food and water. All experiments were designed and reported according to the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Percie du Sert et al., 2020). Pentobarbital sodium (40 mg/kg; intraperitoneal injection; Siyu, Shanghai, China) was used as an anesthetic before all death surgery in this study.
The rats were divided into six groups by random number method: Sham (n = 36), middle cerebral artery occlusion (MCAO) (n = 36), SGB daytime (MCAO + SGB; n = 36), SGB night (MCAO + SGBn; n = 12), MCAO + SGB + LPS (n = 18) and MCAO + SGB + TAK242 groups (n = 18). SGB intervention in the MCAO + SGB + LPS and MCAO + SGB + TAK242 groups were the same as that in the SGB daytime group. A dietary and streptozotocin (STZ) induced diabetic rat model was generated in advance and all six-group rats were diabetic rat models. All groups apart from the sham group received the MCAO. To measure the expression of biochemical pathway components, brains were collected and analyzed by western blotting and immunofluorescence. The neurological function score, survival rate and percentage weight change were used to assess long-term neurological outcomes and survival status. 2,3,5-Triphenyl tetrazolium chloride staining of organotypic brain slices was performed to study cerebral ischemia.
The study was divided into two protocols to measure different sets of indices: (1) in one protocol, rats were studied for 28 days after surgery to measure neurological function, survival rate and percentage weight change; and (2) in the other protocol, rats were sacrificed 48 hours after injury to harvest samples for subsequent analyses, including infarct volume measurement, brain water content measurement, inflammatory cytokine detection, immunohistochemistry and western blot analysis (Additional Figure 1 (1.7MB, tif) ).
Diabetic rat model
We induced diabetes mellitus in rats by feeding them a high-fat diet consisting of 20% lard, 20% sucrose, 2.5% cholesterol, and 57.5% standard chow (Solarbio; Beijing; China) for 4 weeks (Duca et al., 2015). Next, the rats were given free access to water for one night and received an intraperitoneal injection of streptozotocin (STZ) (35 mg/kg; Sigma-Aldrich (Shanghai) Trading Co., Ltd., Shanghai, China) dissolved in 0.01 M citrate buffer (pH 4.5). After 7 days, we measured the nonfasting plasma glucose levels, and only rats with levels ≥ 11.1 mM were considered sufficiently diabetic to be allocated to experimental groups (Li et al., 2020b). The final weight of the diabetic rats involved in the experiment was in the range 200–280 g.
Middle cerebral artery occlusion model
The MCAO rat model was established as described (Longa et al., 1989). Experimental rats were administered 5% sevoflurane (VETEASY, Shenzhen, China) through a face mask and anesthesia was maintained with 2–3% sevoflurane, allowing spontaneous breathing to continue. After skin preparation and disinfection of the surgical area, 1% lidocaine (Chaohui Pharmaceutical Co., Ltd, Shanghai, China) was administered by infiltration for local anesthesia, and a midline incision of approximately 0.5 cm was made at the midpoint between the two ears along the sagittal suture between the parietal bones (Chi et al., 2018). The bregma was identified and defined as the origin of a coordinate system; a cerebral blood flow (CBF) probe was fixed 4 mm left of and 1.5 mm caudal to the bregma, and CBF was monitored by laser Doppler flowmetry (TF5000; PRIMED AB, Stockholm, Sweden). The rat was turned over slowly, and the neck was prepared and disinfected. Local anesthesia was established by infiltration of 1% lidocaine, and a parallel incision was made 3 mm left of the midline. The subcutaneous tissue was separated by blunt dissection with micro-forceps, exposing the left sternocleidomastoid muscle. The digastric, sternocleidomastoid and omohyoid muscles were gradually freed, allowing observation of the left carotid sheath between the muscles. The common carotid artery, external carotid artery and internal carotid artery were separated by blunt dissection. The communicating branch between the external carotid artery and the internal carotid artery was disconnected and electro-coagulated, as were the collateral branches of the external carotid artery. The left external carotid artery was ligated and disconnected, then the proximal end of the common carotid artery and the distal end of the internal carotid artery were clamped with vascular clips. A suture was inserted into the stump of the external carotid artery, and the CBF suddenly dropped by more than 70% of the baseline value. The suture was fixed in place and the incision was sutured. The time of suture insertion and the CBF were recorded. The suture was removed from the lumen of the vessel after 90 minutes of embolization. To achieve MCAO-reperfusion (MCAO/R) after the operation, the rats were sent to the animal post-anesthesia care unit, where they were kept warm as they recovered from anesthesia. Additionally, to ensure consistency of the MCAO models in each group, only rats with a Longa score of 2 (Belayev et al., 1996) after model establishment were selected; rats with any other score were excluded from the experiment. MCAO surgery in SGB night group was performed at night.
Stellate ganglion block technique
In this experiment, left SGB was performed using the percutaneous posterior approach after the MCAO model was successfully established (Abdi and Yang, 2005) and slightly adjusted. However, no rats died directly as a result of the SGB operation in this study. The primary surgical procedure was as follows: the MCAO model rats were anesthetized with sevoflurane (3.5% for induction and 2.0% for maintenance) through a face mask and a heating pad was used to maintain the body temperature at 37°C. After the induction of general anesthesia, the rats were connected to anesthesia masks to maintain spontaneous breathing and placed in a prone position. The cartilage of the rat C7 spinous process was palpated as a marker, and a 1-mL syringe with a short, beveled needle was advanced in the anteroposterior direction along the left side of the C7 vertebral body in the median sagittal position. When the needle tip and vertebral body made contact the needle was withdrawn approximately 0.5 mm and the plunger was retracted to confirm that no blood or cerebrospinal fluid was aspirated. Then, a local anesthetic was injected (0.3 mL of 1.0% lidocaine). The above operation was performed on all rats at the same point in treatment by the same experimenter, who was skilled in rat SGB. Once the nerve block was complete, sevoflurane administration was stopped, and the rat regained consciousness after a few minutes. At that time, we observed whether rats had ptosis, and eye fissure reduction (Figure 1A) was considered to judge that SGB was successful (Abdi and Yang, 2005). In addition, we found that the ear capillaries of some rats were dilated after SGB (Additional Figure 2 (456.5KB, tif) ). All groups were operated with SGB apart from the Sham and MCAO groups. The rats were subjected to SGB 15 minutes before reperfusion and again 20 and 44 hours after reperfusion. The time difference between the SGB night group and SGB daytime group operations was 12 hours.
Figure 1.
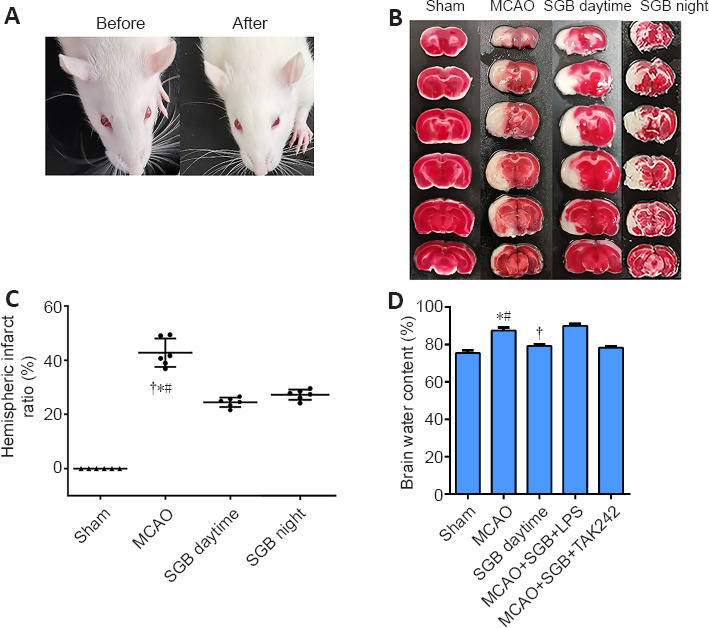
Effect of SGB on infarct volume and eye changes in ischemic stroke diabetic rats.
(A) Eye changes before and after SGB. After SGB, rats had ptosis, and eye fissure reduction. (B) Representative images of 2,3,5-triphenyl tetrazolium chloride staining. Significant hemispheric ischemia (white) was present 48 hours after MCAO, whereas it was significantly improved in the MCAO + SGB daytime group and MCAO + SGB night group. (C) Quantitative analysis of hemispheric infarct ratio. (D) Quantitative analysis of brain water content. The data are presented as the mean ± SD (n = 6). *P < 0.05, vs. Sham group; #P < 0.05, vs. MCAO + SGB daytime group; †P < 0.05, vs. MCAO + SGB + LPS group (one-way analysis of variance followed by the Student-Newman-Keuls test). LPS: Lipopolysaccharide, a Toll-like receptor 4 agonist; MCAO: middle cerebral artery occlusion; SGB: stellate ganglion block; TAK-242 (Resatorvid): a small-molecule-specific inhibitor of Toll-like receptor (TLR) 4 signaling.
Injection of the TLR4 inhibitor TAK242 (3 mg/kg; tail vein injection, HY-11109, MCE, Newark, NJ, USA) in the MCAO + SGB + TAK242 group and of the TLR4 agonist LPS (5 mg/kg; intraperitoneal injection, Roche, Basel, Switzerland) in the MCAO + SGB + LPS group were performed 5 minutes before SGB.
Measurement of blood sugar level
To explore the effect of SGB on the blood sugar level, we measured the blood sugar using a blood glucose meter and blood glucose test strips (Sinocare Inc, Shenzhen, China). Thirty minutes before MCAO and 45 minutes after MCAO/R, blood samples were obtained from the tail vein to measure glucose levels and recorded.
Assessment of neurological outcomes, survival rate, and percentage weight change
Neurological deficits were measured with the modified neurological severity score and the Garcia score on days 1, 3, 5, 7, 14, and 28. The modified neurological severity score (mNSS) and the Garcia score objectively and accurately assess changes in neurological function of rats through a combination of muscle strength, balance beam, orientation, limb coordination, sensory and other measures (Chi et al., 2018; Li et al., 2020b). The mNSS score is the sum of all test scores from the tail lift test, ground movement test, balance beam test, reflex and paradoxical movement test, with the lowest score 0 and the highest score 18. The higher the score, the worse the neurological function. Garcia score assesses voluntary movement in the cage for 5 minutes and includes symmetry of limb movement, symmetry of forelimb extension during tail pulling, climbing the cage wall, response to touching the trunk and response to touching the whiskers. The maximum score is 18. The lower the Garcia score, the worse the neurological function. The survival rates were also recorded. Deaths in the Sham, MCAO, SGB daytime and night groups were recorded until day 28, and in the MCAO, MCAO + SGB + LPS and MCAO + SGB + TAK242 groups until 48 hours post MCAO. All rats were exposed to the neurological function testing twice before undergoing surgery. Neurological function scores were conducted by the same investigator, who was blinded to the group assignments. Body weight was monitored daily for 28 days by animal weight scale (Mettler Toledo, Zurich, Switzerland). The weight change (%) was determined relative to the baseline weight (n = 6 rats/group).
Measurement of infarct volume
Forty-eight hours after reperfusion, rats (n = 6/group) were sacrificed. The brains were rapidly removed and sliced into 2-mm coronal sections, stained with 1% 2,3,5-triphenyl tetrazolium chloride (Biosharp, Beijing, China) for 20 minutes at 37°C, and immersed overnight in 4% paraformaldehyde. The infarct and hemisphere areas (%) were measured using ImageJ 1.8 software (Schneider et al., 2012). The hemispheric infarct ratio was calculated as (contralateral hemisphere area - ipsilateral hemisphere without infarct)/contralateral hemisphere area × 100.
Measurement of brain water content
Forty-eight hours after reperfusion, we randomly selected six rats from each group to measure their brain water content. Briefly, we separated the cerebral cortex from the rest of the brain and recorded the weight of the cerebral cortex (wet weight). Next, each specimen was dried in an electric oven at 80°C for 48 hours, and the weight was recorded (dry weight). We used the following equation to calculate the brain water content: brain water content (%) = (wet weight – dry weight)/wet weight × 100.
Measurement of immunofluorescence
To locate and quantify TLR4 and NF-κB, 48 hours after reperfusion, the rats (n = 6/group) were transcranially perfused with normal saline and 4% paraformaldehyde. The brains were postfixed in 4% paraformaldehyde at 4°C for 24 hours. The infarcted segments were cut into 10-mm transverse frozen sections. After being washed in 0.1% Triton X-100 for 20 minutes, the sections were blocked with 3% bovine albumin for 30 minutes. The sections were then incubated with primary antibodies against TLR4 (1:100; mouse; Cat# BS3489; RRID: AB_1662746; BioWorld Technology, Shanghai, China) and NF-κB (1:200; rat; Cat# BMS-33117M; Bioss, Beijing, China) at 4°C overnight and were then incubated with fluorescein isothiocyanate isomer-conjugated secondary antibodies (1:1000, goat; Cat# SA00003-2; RRID: AB_2890897; Proteintech, Wuhan, China) for 50 minutes at 25°C. Finally, the sections were incubated with 4′,6-diamidino-2-phenylindole (5192-23-4, Aladdin, Shanghai, China) for 10 minutes and visualized with an optical microscope (Nikon Eclipse Ti-SR, Tokyo, Japan) at 400× magnification. One section per animal was selected. Images were merged using ImageJ software at the same contrast level.
Western blot analysis
To quantify the protein expression levels of TLR4/NF-κB pathway, at 48 hours after reperfusion, the cerebral cortex (n = 6/group) was homogenized and centrifuged at 10,000 × g for 20 minutes at 4°C. We collected the supernatants and measured the protein concentrations with a bicinchoninic acid assay kit (Beyotime, Shanghai, China). Then, we separated the protein samples by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred them onto nitrocellulose membranes. The membranes were blocked with 5% milk powder in 0.1% Tris-buffered saline-Tween 20 and incubated with primary antibodies against TLR4 (1:2000; rat; Cat# 35463, RRID: AB_2893499; SAB, City of College Park, MD, USA), NF-κB p65 (rat; 1:2000; Cat# 48500, RRID: AB_2893499; SAB), NF-κB p100 (1:1000; rat; Cat# ab191594, RRID: AB_2893501; Abcam, London, UK), phospho-NF-κB p65 (phospho-Ser536; 1:2000; RRID: AB_2893502; rat; Cat# YP0191, RRID: AB_2893502; ImmunoWay Technology Company, Plano, TX, USA) and β-actin (1:400; rat; Cat# PR-0255, ZSGB Bio, RRID: AB_2636897; Beijing, China) overnight at 4°C. Subsequently, we washed the membranes with 0.1% Tris-buffered saline-Tween 20 and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000; goat; Cat# ZB-2301; RRID: AB_2747412; ZSGB Bio) for 1 hour at 25°C. Bands were detected with an electrochemiluminescence kit (Haigene, Shenzhen, China), and a gel imaging system (Clinx Scientific Instruments Co., Ltd, Shanghai, China) was used only for acquisition and analysis.
Measurement of inflammatory cytokine levels
Rats (n = 6/group) were anesthetized at 48 hours after reperfusion, and the femoral artery was punctured. Blood samples were collected in heparin-coated tubes and centrifuged (1000 × g for 20 minutes at 4°C). Plasma levels of interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) were detected by enzyme-linked immunosorbent assay kits (Wuhan Boster Biotechnology Ltd, Wuhan, China) according to the manufacturer's instructions.
Statistical analysis
The sample size was based on our previously reported study (Li et al., 2020b). We used SPSS (version 21.0, IBM, Armonk, NY, USA) statistical software to analyze the data. The data are presented as the mean ± standard deviation (SD). Two-tailed t-test was used for blood glucose comparison before and after surgery. Multigroup comparisons were performed with one-way analysis of variance followed by the Student-Newman-Keuls test for comparisons brain water content, hemispheric infarct ratio, enzyme-linked immunosorbent assay, western blot and immunofluorescence results. Neurological function scores were analyzed by the Kruskal-Wallis test with Bonferroni correction. Kaplan-Meier survival curves were compared using the log-rank test. Repeated-measures analysis of variance, followed by the least significant difference test, was used for comparisons of 28-day weight change. A P-value < 0.05 was considered statistically significant.
Results
SGB does not affect the increase in blood glucose level in ischemic stroke rats
The blood glucose level increased significantly between the pre- and post-MCAO measurements. The MCAO group showed rapid blood glucose change (P < 0.05). Blood glucose after SGB (daytime) also increased (P < 0.05), indicating SGB did not affect the increase in blood glucose resulting from MCAO (Table 1).
Table 1.
Effects of MCAO and SGB on the blood glucose (mM) level in rats
| MCAO | SGB daytime | |
|---|---|---|
| 30 minutes before MCAO | 18.9±4.03* | 15.2±3.39* |
| 45 minutes after MCAO/R | 23.75±4.25 | 21.4±3.36 |
The data are presented as the mean ± SD (n = 6). *P < 0.05, vs. intraoperative (two-tailed t-test). MCAO: Middle cerebral artery occlusion; SGB: stellate ganglion block.
SGB reduces infarct volume and brain water content in ischemic stroke diabetic rats
Forty-eight hours after MCAO, the rats in the MCAO group showed prominent infarct areas and the infarct size was significantly reduced after either day or night SGB treatment (P < 0.05) (Figure 1B and C). Consistent results were obtained for brain water content that was significantly reduced after SGB treatment (Figure 1D).
SGB improves the neurological outcomes, weight and survival rate in MCAO diabetic rats
SGB administered in the daytime effectively improves long-term neurological function compared with the MCAO group. There was a significant improvement in the modified neurological severity score and Garcia score in the SGB daytime group, which showed accelerating positive neural outcomes compared with the MCAO group (Figure 2A and B). The postoperative weight changes in the SGB daytime group showed a smaller loss and a more rapid recovery (reaching the lowest value on the third day and returning to the preoperative value on the sixth day) compared with the MCAO group (Figure 2C). SGB was found to confer a significant survival benefit, the MCAO group had a lower 28-day survival rate than the SGB daytime group (P = 0.02; Figure 2D), but there was no significant difference in the survival rate between the MCAO and SGB night groups (P = 0.50; Figure 3A). However, there was some improvement in the long-term neurological functions of the SGB night group (Figure 3B and C). The mean weight in the SGB night group was lowest on day 5 and recovered to baseline on day 10 (Figure 3D), while that of the MCAO group was lowest on day 5 and recovered to baseline on day 11 (Figure 3D). There was also a significant difference in the 48 hours survival rates between the MCAO and MCAO + SGB + LPS groups. Compared with the MCAO group, the MCAO + SGB + LPS group had a higher mortality (P < 0.05;Table 2).
Figure 2.
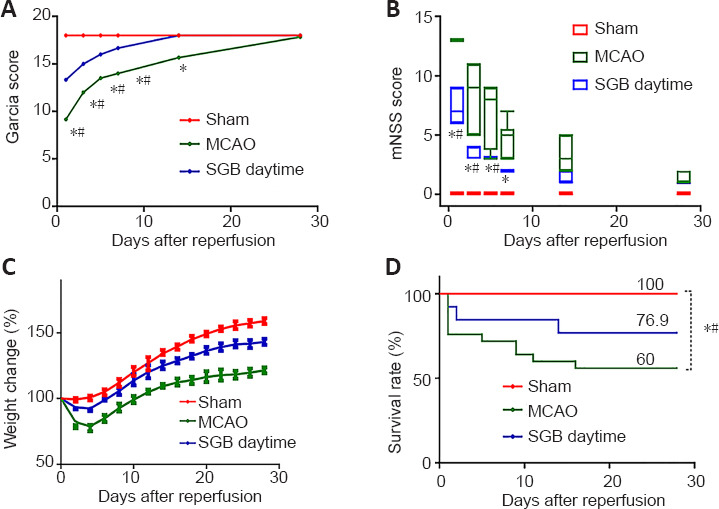
Long-term functional benefit of SGB in ischemic stroke diabetic rats.
(A) Quantitative changes of Garcia score. (B) Quantitative changes in mNSS. (C) Changes in body weight after reperfusion. The weight change (%) was determined relative to the baseline weight. The data are presented as the mean ± SD (n = 6) and were analyzed by repeated-measures analysis of variance followed by least significant difference test. (D) SGB increased the 28-day survival rate. Kaplan-Meier survival curves were compared using the log-rank test. *P < 0.05, vs. Sham group; #P < 0.05, vs. SGB daytime group. MCAO: Middle cerebral artery occlusion; mNSS: modified neurological severity scores; SGB: stellate ganglion block.
Figure 3.
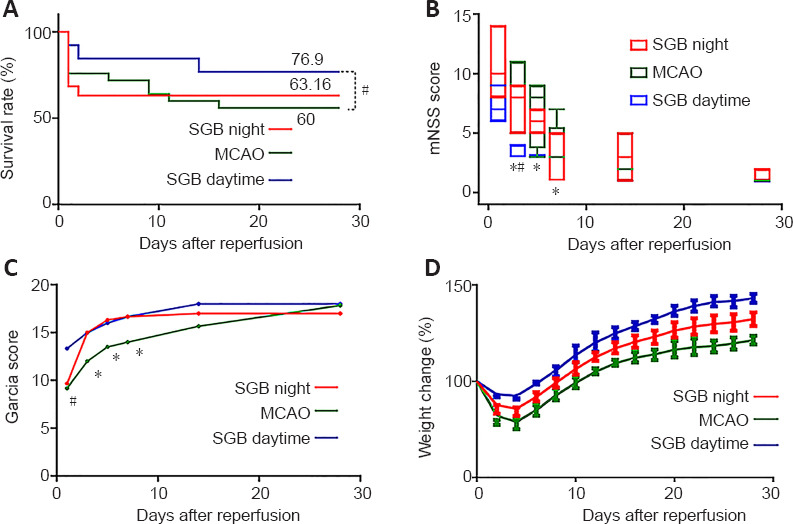
Effect of SGB performed at night or day in ischemic stroke diabetic rat.
(A) Percentage survival over 28 days after MCAO. (B) Change in mNSS over 30 days after MCAO. (C) Change in Garcia score after MCAO. (D) Percentage weight changes after MCAO. The data of weight change, mNSS and Garcia score are presented as the mean ± SD (n = 6) and were analyzed by Kruskal-Wallis test with post hoc Bonferroni correction. Kaplan-Meier survival curves were compared using the log-rank test. *P < 0.05, vs. Sham group; #P < 0.05, vs. SGB daytime group. MCAO: Middle cerebral artery occlusion; mNSS: modified neurological severity scores; SGB: stellate ganglion block.
Table 2.
Effects of MCAO and SGB on 48-hour survival in rats
| Group | SGB daytime | MCAO+SGB+LPS | MCAO+SGB+TAK242 |
|---|---|---|---|
| Survive | 12† | 12 | 12 |
| Total | 17 | 26 | 16 |
| 48-h survival rate (%) | 70.59 | 46.15 | 75 |
†P < 0.05, vs. MCAO + SGB + LPS group (Student’s t-test). LPS: Lipopolysaccharide, a Toll-like receptor agonist; MCAO: middle cerebral artery occlusion; SGB: stellate ganglion block; TAK-242 (Resatorvid): a smallmolecule-specific inhibitor of Toll-like receptor (TLR) 4 signaling.
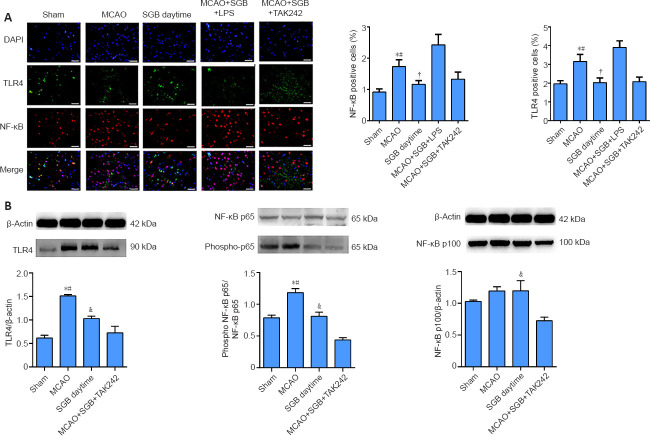
SGB inhibits the TLR4/NF-κB pathway in the ischemic penumbra of stroke diabetic rats
The TLR4 and NF-κB contents were measured by two methods. The localization of the proteins, using immunofluorescence, quantitatively showed that the TLR4 content in the ischemic penumbra of ischemic stroke diabetic rats was greatly decreased when treated with SGB (P = 0.0187; Figure 4A). SGB did not reduce the TLR4 and NF-κB positive cells when administered 5 minutes after LPS (P = 0.0047; Figure 4A). The results of western blot analysis in the ischemic penumbra showed the greatest significant difference (MCAO group vs. SGB daytime group: P < 0.0001; Figure 4B). Immunofluorescence indicated that NF-κB was present mainly in the nucleus in the MCAO group, possibly because nuclear translocation of NF-κB was enhanced in the MCAO group. The western blot results showed that NF-κB p65 phosphorylation was enhanced in the MCAO group, while SGB inhibited this phosphorylation (P = 0.0022). No significant differences in NF-κB p100 phosphorylation were observed, except between the SGB daytime and the MCAO + SGB + TAK242 groups. NF-κB p100 expression in the MCAO + SGB + TAK242 group was significantly lower than that in the SGB daytime group (Figure 4B).
Figure 4.
Immunofluorescence and western blot analysis results reflecting the effect of SGB in ischemic stroke diabetic rats.
(A) Representative images of double immunofluorescence staining of TLR4- (FITC, green) and NF-κB-positive cells (Cy3, red) and DAPI for nucleus staining (blue). The NF-κB is expressed in both the nucleus and the cytoplasm, and the red color is seen in the nucleus, overlapping with the DAPI stained nucleus. Numbers of NF-κB- and TLR4-positive cells were higher in the MCAO group than in the SGB daytime group. Scale bars: 50 µm. *P < 0.05, vs. Sham group; #P < 0.05, vs. SGB daytime group; †P < 0.05, vs. MCAO + SGB + LPS group (one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). (B) Quantitative analysis of western blots. The data are presented as the mean ± SD (n = 6). *P < 0.05, vs. Sham group; #P < 0.05, vs. SGB daytime group; †P < 0.05, vs. MCAO + SGB + TAK242 group (one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). DAPI: 4′,6-Diamidino-2-phenylindole; FITC: fluorescein isothiocyanate isomer; LPS: Lipopolysaccharide, a TLR4 agonist; MCAO: middle cerebral artery occlusion; NF-κB: nuclear factor kappa-B; SGB: stellate ganglion block; TAK-242 (Resatorvid): a small-molecule-specific inhibitor of TLR4 signaling; TLR4: Toll-like receptor 4.
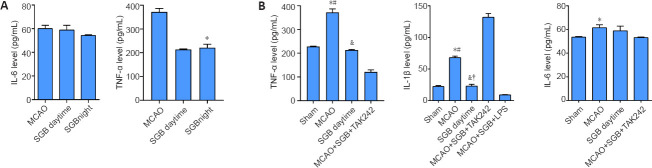
SGB inhibits the inflammation in plasma of ischemic stroke diabetic rats
Compared with SGB daytime group, the TNF-α level in SGB night group was significantly lower (P < 0.0001; Figure 5A) but there were no significant differences in the IL-6 levels between the day and night groups (P = 0.078; Figure 5A). The IL-1β and TNF-α levels in the SGB daytime group were lower than those in the MCAO group (IL-1β: P < 0.0001; TNF-α: P < 0.0001; Figure 5B). Compared with those in the SGB daytime group, the IL-1β and TNF-α levels were greatly reduced in the MCAO + SGB + TAK242 group (IL-1β: P < 0.0001; TNF-α: P < 0.0001; Figure 5B) and greatly increased in the MCAO + SGB + LPS group (IL-1β: P = 0.0006; Figure 5B).
Figure 5.
Effect of SGB on inflammation in ischemic stroke diabetic rats (enzyme-linked immunosorbent assay).
(A) Effect of nighttime SGB on inflammatory cytokines in plasma of ischemic stroke diabetic rats. (B) Quantitative analysis of IL-6, IL-1β, and TNF-α levels in plasma. The data are presented as the mean ± SD (n = 6). *P < 0.05, vs. Sham group; #P < 0.05, vs. SGB daytime group; &P < 0.05, vs. MCAO + SGB + TAK242 group; †P < 0.05, vs. MCAO + SGB + LPS group (one-way analysis of variance followed by the Student-Newman-Keuls post hoc test). IL-6: Interleukin-6, IL-1β: interleukin-1β; LPS: Lipopolysaccharide, a TLR4 agonist; MCAO: middle cerebral artery occlusion; NF-κB: nuclear factor kappa-B; SGB: stellate ganglion block; TAK-242 (Resatorvid): a small-molecule-specific inhibitor of TLR4 signaling; TLR4: Toll-like receptor 4; TNF-α: tumor necrosis factor-α.
Discussion
This study has shown that SGB can effectively improve long-term neurological function after ischemic stroke in diabetic rats and that the mechanism involves the TLR4/NF-κB pathway. We also observed diurnal differences in the effect of SGB treatment.
This experiment used a diabetic rat MCAO model. Diabetes has a high prevalence and is a risk factor for ischemic stroke. The mortality and long-term adverse outcome rates of patients with diabetes are significantly higher than those of the general population (O’Donnell et al., 2016; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Lo et al., 2020). Moreover, the primary method of clinical perioperative preparation is still controlling glycemia, but a significant multicenter data study found that merely controlling the blood sugar level after a stroke cannot effectively improve the long-term neurological function of diabetic patients (Lo et al., 2020). Therefore, it is particularly important to find an effective perioperative stroke control method for diabetic stroke patients. This experiment used a diabetic rat MCAO model to study a potential treatment and its molecular mechanism. In addition, during the MCAO modeling process, blood glucose was measured on the morning of the operation to ensure the stability of the diabetic rat model, and blood glucose monitoring was continued after the modeling was completed to eliminate the possibility that the blood glucose level was neither too high nor too low enough to affect the survival of the MCAO rats.
In experiments with rats as subjects, the commonly used methods of SGB include surgical exposure (cervical sympathetic nerve trunk transection, stellate ganglion catheterization, or direct injection of medicine) and percutaneous injection (anterior approach, posterior approach, or lateral approach) (Iwama, 1997; Abdi and Yang, 2005; Gulcu et al., 2009; Chen et al., 2012). The surgical exposure, percutaneous pre-injection approach and percutaneous injection side approach have certain limitations, including inevitably causing tissue damage, infection, and increased possibility of mortality of the subject. The posterior percutaneous approach does not have these limitations as there is less trauma, little pain and the operation is simple and has good repeatability. Thus, the posterior percutaneous approach was used to perform SGB in rats in this experiment. However, there are other potential complications resulting from SGB, including hematoma, brachial plexus injury, phrenic nerve block and pneumothorax. The use of ultrasound-guided technology for the operation can significantly reduce potential complications.
Our data indicate that SGB has an excellent therapeutic effect on the acute phase of ischemic stroke in diabetic rats. The recovery of stroke is usually assessed clinically over the following weeks and months (Shih et al., 2015). Therefore, clinical improvement after intervention should also evaluated over a relatively long observation period. Our study showed that the long-term functional advantages of SGB are very clear, and these results comprehensively demonstrated the beneficial effect of SGB on long-term (28-day) neurological outcomes and weight recovery in diabetic rats. However, we also observed a high poststroke mortality rate in diabetic rats that far exceeded that indicated in clinical data. This high mortality rate may be linked to the blood glucose level in the rat model. In our study, rats with blood glucose over 25 mM had much higher mortality rates than other diabetic rats, which may correlate with their significantly higher postoperative blood glucose levels (higher than 33.3 mM). To prevent the influence of blood glucose levels in this study, we limited the blood glucose level of diabetic rats to a range of 15–25 mM. It had been shown that nutritional support (dry granules on the cage floor, jellied food administered orally, and jellied food provided in Petri dishes), starting on the second day after MCAO, reduced the 14-day mortality rate from 59% to 15% (Chi et al., 2018). However, in our experiments, rats merely had free access to food and water after surgery and did not receive additional nutritional support.
We explored the TLR4/NF-κB pathway to better explore the molecular mechanism through which SGB improves long-term neurological function after ischemic stroke. Our study showed that SGB treatment reduced the level of NF-κB p65 (p65) phosphorylation and suppressed the inflammatory response. The activation of NF-κB generally refers to translocation of p65 from cytoplasm into nucleus after phosphorylation. We measured phosphorylated p65 (p-p65) and total p65, which showed inhibition of p-p65 after SGB. In addition, immunofluorescence showed some of the p65 overlapped with nuclei, both western blot and immunofluorescence measurements indicated that SGB was related to the transfer of NF-κB p65 to the nucleus. During this process, it was found that the level of IL-6 increased rapidly after infection and peaked at 2 hours (Fraunberger et al., 2006; Abe et al., 2010). However. our study found that the level of IL-6 had not changed significantly when we measured it 48 hours after MCAO. Therefore, we probably missed the peak level of IL-6 and that by 48 hours it had returned to base levels.
A recent study found that the difference in circadian rhythms is one reason that results from rodent studies frequently cannot translate to clinical research (Esposito et al., 2020). We found SGB in rats exhibited diurnal variation. Interestingly, daytime administration of SGB was effective in improving ischemia and long-term neurological function, promoting weight recovery, reducing mortality, and reducing inflammation. However, nighttime SGB was not effective in improving ischemia or reducing mortality, although it did improve neurological function and weight recovery. We consider that neurological function and weight recovery were related to reducing inflammation. This pattern suggests that although during both the day and the night, SGB could exert anti-inflammatory effects and improve long-term neurological function and body weight recovery, the lack of improvement in ischemia at night may be correlated with sympathetic nerve activity. In contrast to the circadian rhythm of rats, studies with patients suggest that the best time for SGB was at night. We think SGB could be useful in reducing daytime sympathetic overexcitability. This hypothesis also provides a theoretical basis for precision application of SGB and the optimization of treatment plans for clinical patients. Conversely, we found that the agonist LPS can markedly activate inflammation-related pathways, leading to a high mortality rate in the MCAO + SGB + LPS group of diabetic rats.
Compared with previous experiments, our experiment has the following innovations. First, we studied the effect of SGB in this compound model, which is highly important for the development of the field. Second, no study has addressed circadian rhythms in SGB. This study provides a new concept and theoretical basis for using SGB to optimize relevant treatment measures in clinical applications. Although the positive effects of SGB for stroke have been known since 1947 (Risteen and Volpitto, 1946), subsequent studies have focused on the effectiveness of SGB in relieving pain in patients with subacute stroke with complex localized pain syndrome. Recent studies have shown that SGB has a therapeutic effect on post-stroke pain that had failed to respond to pharmacological treatment. However, our study focused on the perioperative neuroprotection and prognosis of diabetic stroke, which was not only more comprehensive, but also innovative in studying the effect of SGB on two compound diseases. Previous studies on SGB were relatively simple, but our research explored SGB at the molecular and mechanistic levels, adding clarity and accuracy to the mechanism of SGB. Finally, we identified the optimum range for blood glucose control. When the blood glucose level exceeded 25.0 mM, the mortality rate of the MCAO model increased significantly; this observation can be a reference for related basic research. In addition, we found that the ear capillaries of some rats were dilated after SGB.
Our research also has certain limitations. The ischemia time selected in this study was 90 minutes, which is the time window from the onset of the disease to recanalization treatment for most hospitalized patients based on our observations. However, in clinical practice, some patients are treated within a different time window. In addition, the SGB technique is a minimally invasive blocking technique that can be implemented only by skilled anesthesiologists.
In conclusion, SGB is effective in improving long-term neurological outcomes and quality of life after ischemic stroke in diabetic rats and demonstrates that the mechanism is related mainly to specific inhibition of TLR4/NF-κB pathway activation. The SGB treatment is also influenced by circadian rhythms; thus, proper timing of treatment is important. SGB is widely used in various clinical diseases. However, this study provides a precise optimization strategy for the perioperative period before and after reperfusion when surgical treatment is performed on diabetic patients that suffered an ischemic stroke.
Additional files:
Additional file 1: Open peer review report 1 (82.3KB, pdf) .
Additional Figure 1 (1.7MB, tif) : Experimental timeline.
Experimental timeline.
ELISA: enzyme linked immunosorbent assay; MCAO: middle cerebral artery occlusion; mNSS: modified neurological severity scores; TTC: 2,3,5-triphenyl tetrazolium chloride.
Additional Figure 2 (456.5KB, tif) : The ear capillaries after stellate ganglion block.
The ear capillaries after stellate ganglion block.
Footnotes
P-Reviewer: Lipov E; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
Funding: The study was approved by Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province, No. LBH-Q18074 (to WCY).
Conflicts of interest: The authors declare that they have no conflicts of interest.
Author statement: This paper has been posted as a preprint on Research Square with doi: https://doi.org/10.21203/rs.3.rs-126758/v1, which is available from: https://assets.researchsquare.com/files/rs-126758/v1/d122bdaf-daa7-4cfc-b10e-985bf742eca1.pdf?c=1631866737.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Eugene Lipov, University of Illinois at Chicago, USA.
Funding: The study was approved by Postdoctoral Scientific Research Developmental Fund of Heilongjiang Province, No. LBH-Q18074 (to WCY).
References
- 1.Abdi S, Yang Z. A novel technique for experimental stellate ganglion block in rats. Anesth Analg. 2005;101:561–565. doi: 10.1213/01.ANE.0000159169.12425.50. [DOI] [PubMed] [Google Scholar]
- 2.Abe R, Oda S, Sadahiro T, Nakamura M, Hirayama Y, Tateishi Y, Shinozaki K, Hirasawa H. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit Care. 2010;14:R27. doi: 10.1186/cc8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Stroke. 1996;27:1616–1623. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Guo L, Lang H, Hu X, Jing S, Luo M, Xu G, Zhou Z. Effect of a stellate ganglion block on acute lung injury in septic rats. Inflammation. 2018;41:1601–1609. doi: 10.1007/s10753-018-0803-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen YQ, Hu GX, Fu Q, Jin XJ. Effects of stellate ganglion block on blood pressure in spontaneously hypertensive rats. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012;41:65–68. doi: 10.3785/j.issn.1008-9292.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Chi L, Du K, Liu D, Bo Y, Li W. Electroacupuncture brain protection during ischemic stroke: A role for the parasympathetic nervous system. J Cereb Blood Flow Metab. 2018;38:479–491. doi: 10.1177/0271678X17697988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, Zhang B, Yue Z. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11:1386. doi: 10.1038/s41467-020-15119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng YH, Dong LL, Zhang YJ, Zhao XM, He HY. Enriched environment boosts the post-stroke recovery of neurological function by promoting autophagy. Neural Regen Res. 2021;16:813–819. doi: 10.4103/1673-5374.297084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong LY, Chen F, Xu M, Yao LP, Zhang YJ, Zhuang Y. Quercetin attenuates myocardial ischemia-reperfusion injury via downregulation of the HMGB1-TLR4-NF-κB signaling pathway. Am J Transl Res. 2018;10:1273–1283. [PMC free article] [PubMed] [Google Scholar]
- 10.Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TKT. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esposito E, Li W, E TM, Park JH, Şencan I, Guo S, Shi J, Lan J, Lee J, Hayakawa K, Sakadžić S, Ji X, Lo EH. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582:395–398. doi: 10.1038/s41586-020-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, Strazzabosco M. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-κB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508.e5. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher WI, Johnson AK, Elkins GR, Otte JL, Burns DS, Yu M, Carpenter JS. Risk factors, pathophysiology, and treatment of hot flashes in cancer. CA Cancer J Clin. 2013;63:167–192. doi: 10.3322/caac.21171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraunberger P, Wang Y, Holler E, Parhofer KG, Nagel D, Walli AK, Seidel D. Prognostic value of interleukin 6, procalcitonin, and C-reactive protein levels in intensive care unit patients during first increase of fever. Shock. 2006;26:10–12. doi: 10.1097/01.shk.0000215319.06866.bd. [DOI] [PubMed] [Google Scholar]
- 15.Fusco R, Scuto M, Cordaro M, D’Amico R, Gugliandolo E, Siracusa R, Peritore AF, Crupi R, Impellizzeri D, Cuzzocrea S, Di Paola R. N-palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway. Int J Mol Sci. 2019;20:4845. doi: 10.3390/ijms20194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, Jaeger BN, O’Connor C, Fitzpatrick C, Pasillas MP, Pena M, Adair A, Gonda DD, Levy ML, Ransohoff RM, Gage FH, Glass CK. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356:eaal3222. doi: 10.1126/science.aal3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gulcu N, Gonca E, Kocoglu H. A lateral percutaneous technique for stellate ganglion blockade in rats. Anesth Analg. 2009;108:1701–1704. doi: 10.1213/ane.0b013e31819c6018. [DOI] [PubMed] [Google Scholar]
- 19.Haest K, Kumar A, Van Calster B, Leunen K, Smeets A, Amant F, Berteloot P, Wildiers H, Paridaens R, Van Limbergen E, Weltens C, Janssen H, Peeters S, Menten J, Vergote I, Morlion B, Verhaeghe J, Christiaens MR, Neven P. Stellate ganglion block for the management of hot flashes and sleep disturbances in breast cancer survivors: an uncontrolled experimental study with 24 weeks of follow-up. Ann Oncol. 2012;23:1449–1454. doi: 10.1093/annonc/mdr478. [DOI] [PubMed] [Google Scholar]
- 20.Han LP, Li CJ, Sun B, Xie Y, Guan Y, Ma ZJ, Chen LM. Protective effects of celastrol on diabetic liver injury via TLR4/MyD88/NF-κB signaling pathway in type 2 diabetic rats. J Diabetes Res 2016. 2016 doi: 10.1155/2016/2641248. 2641248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hansen DV, Hanson JE, Sheng M. Microglia in Alzheimer's disease. J Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwama H. Can superior cervical ganglionectomy be an experimental model of stellate ganglion block? Masui. 1997;46:565–567. [PubMed] [Google Scholar]
- 23.Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang LJ, Xu ZX, Wu MF, Dong GQ, Zhang LL, Gao JY, Feng CX, Feng X. Resatorvid protects against hypoxic-ischemic brain damage in neonatal rats. Neural Regen Res. 2020;15:1316–1325. doi: 10.4103/1673-5374.272615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang GP, Li C, Han YY. Rutin pretreatment promotes microglial M1 to M2 phenotype polarization. Neural Regen Res. 2021;16:2499–2504. doi: 10.4103/1673-5374.313050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Xu X, Wang Z, Wang Y, Luo L, Cheng J, Chen SF, Liu H, Wan Q, Wang Q. Exercise ameliorates post-stroke depression by inhibiting PTEN elevation-mediated upregulation of TLR4/NF-κB/NLRP3 signaling in mice. Brain Res. 2020a;1736:146777. doi: 10.1016/j.brainres.2020.146777. [DOI] [PubMed] [Google Scholar]
- 27.Li TT, Yang WC, Wang YZ, Sun T, Cao HL, Chen JF, Li WZ. Effects of a high concentration of hydrogen on neurological function after traumatic brain injury in diabetic rats. Brain Res. 2020b;1730:146651. doi: 10.1016/j.brainres.2020.146651. [DOI] [PubMed] [Google Scholar]
- 28.Liu MH, Tian J, Su YP, Wang T, Xiang Q, Wen L. Cervical sympathetic block regulates early systemic inflammatory response in severe trauma patients. Med Sci Monit. 2013;19:194–201. doi: 10.12659/MSM.883833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q, Zhong Q, Tang G, Ye L. Ultrasound-guided stellate ganglion block for central post-stroke pain: a case report and review. J Pain Res. 2020;13:461–464. doi: 10.2147/JPR.S236812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo JW, Crawford JD, Samaras K, Desmond DW, Köhler S, Staals J, Verhey FRJ, Bae HJ, Lee KJ, Kim BJ, Bordet R, Cordonnier C, Dondaine T, Mendyk AM, Lee BC, Yu KH, Lim JS, Kandiah N, Chander RJ, Yatawara C, et al. Association of prediabetes and type 2 diabetes with cognitive function after stroke. Stroke. 2020;51:1640–1646. doi: 10.1161/STROKEAHA.119.028428. [DOI] [PubMed] [Google Scholar]
- 31.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muzio M, Natoli G, Saccani S, Levrero M, Mantovani A. The human toll signaling pathway: divergence of nuclear factor kappaB and JNK/SAPK activation upstream of tumor necrosis factor receptor-associated factor 6 (TRAF6) J Exp Med. 1998;187:2097–2101. doi: 10.1084/jem.187.12.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntaios G, Lip GYH, Vemmos K, Koroboki E, Manios E, Vemmou A, Rodríguez-Campello A, Cuadrado-Godia E, Roquer J, Arnao V, Caso V, Paciaroni M, Diez-Tejedor E, Fuentes B, Pérez Lucas J, Arauz A, Ameriso SF, Pertierra L, Gómez-Schneider M, Hawkes MA, et al. Age- and sex-specific analysis of patients with embolic stroke of undetermined source. Neurology. 2017;89:532–539. doi: 10.1212/WNL.0000000000004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 36.Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Hurst V, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:e3000411. doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circul Res. 2016;119:142–158. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao H, Zhang X, Zhu C, Dong L, Wang L, Zhang X, Xing Y, Wang C, Ji Y, Cao X. Luteolin downregulates TLR4, TLR5, NF-κB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res. 2012;1448:71–81. doi: 10.1016/j.brainres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Qin C, Liu Q, Hu ZW, Zhou LQ, Shang K, Bosco DB, Wu LJ, Tian DS, Wang W. Microglial TLR4-dependent autophagy induces ischemic white matter damage via STAT1/6 pathway. Theranostics. 2018;8:5434–5451. doi: 10.7150/thno.27882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rae Olmsted KL, Bartoszek M, Mulvaney S, McLean B, Turabi A, Young R, Kim E, Vandermaas-Peeler R, Morgan JK, Constantinescu O, Kane S, Nguyen C, Hirsch S, Munoz B, Wallace D, Croxford J, Lynch JH, White R, Walters BB. Effect of stellate ganglion block treatment on posttraumatic stress disorder symptoms: a randomized clinical trial. JAMA Psychiatry. 2020;77:130–138. doi: 10.1001/jamapsychiatry.2019.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rayasam A, Hsu M, Hernández G, Kijak J, Lindstedt A, Gerhart C, Sandor M, Fabry Z. Contrasting roles of immune cells in tissue injury and repair in stroke: The dark and bright side of immunity in the brain. Neurochem Int. 2017;107:104–116. doi: 10.1016/j.neuint.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Reis de Assis D, Szabo A, Requena Osete J, Puppo F, O’Connell KS, A Akkouh I, Hughes T, Frei E, A Andreassen O, Djurovic S. Using iPSC models to understand the role of estrogen in neuron-glia interactions in schizophrenia and bipolar disorder. Cells. 2021;10:209. doi: 10.3390/cells10020209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Risteen WA, Volpitto PP. Role of stellate ganglion block in certain neurologic disorders. South Med J. 1946;39:431–435. doi: 10.1097/00007611-194605000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shih CC, Liao CC, Sun MF, Su YC, Wen CP, Morisky DE, Sung FC, Hsu CY, Lin JG. A retrospective cohort study comparing stroke recurrence rate in ischemic stroke patients with and without acupuncture treatment. Medicine (Baltimore) 2015;94:e1572. doi: 10.1097/MD.0000000000001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Zheng F, Gao G, Yan S, Zhang L, Wang L, Cai X, Wang X, Xu D, Wang J. MiR-548a-3p regulates inflammatory response via TLR4/NF-κB signaling pathway in rheumatoid arthritis. J Cell Biochem. 2018 doi: 10.1002/jcb.26659. doi: 10.1002/jcb.26659. [DOI] [PubMed] [Google Scholar]
- 47.Wattananit S, Tornero D, Graubardt N, Memanishvili T, Monni E, Tatarishvili J, Miskinyte G, Ge R, Ahlenius H, Lindvall O, Schwartz M, Kokaia Z. Monocyte-derived macrophages contribute to spontaneous long-term functional recovery after stroke in mice. J Neurosci. 2016;36:4182–4195. doi: 10.1523/JNEUROSCI.4317-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei X, Song H, Yin L, Rizzo MG, Sidhu R, Covey DF, Ory DS, Semenkovich CF. Fatty acid synthesis configures the plasma membrane for inflammation in diabetes. Nature. 2016;539:294–298. doi: 10.1038/nature20117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willsey HR, Exner CRT, Xu Y, Everitt A, Sun N, Wang B, Dea J, Schmunk G, Zaltsman Y, Teerikorpi N, Kim A, Anderson AS, Shin D, Seyler M, Nowakowski TJ, Harland RM, Willsey AJ, State MW. Parallel in vivo analysis of large-effect autism genes implicates cortical neurogenesis and estrogen in risk and resilience. Neuron. 2021;109:788–804. doi: 10.1016/j.neuron.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X, Shi Z, Li X, Li J. Impacts of stellate ganglion block on plasma NF-κB and inflammatory factors of TBI patients. Int J Clin Exp Med. 2015;8:15630–15638. [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo SD, Jung SS, Kim HS, Yun DH, Kim DH, Chon J, Hong DW. Efficacy of ultrasonography guided stellate ganglion blockade in the stroke patients with complex regional pain syndrome. Ann Rehabil Med. 2012;36:633–639. doi: 10.5535/arm.2012.36.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Fu B, Zhang X, Chen L, Zhang L, Zhao X, Bai X, Zhu C, Cui L, Wang L. Neuroprotective effect of bicyclol in rat ischemic stroke: Down-regulates TLR4, TLR9, TRAF6, NF-κB, MMP-9 and up-regulates claudin-5 expression. Brain Res. 2013;1528:80–88. doi: 10.1016/j.brainres.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S. Microglial activation after ischaemic stroke. Stroke Vasc Neurol. 2019;4:71–74. doi: 10.1136/svn-2018-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental timeline.
ELISA: enzyme linked immunosorbent assay; MCAO: middle cerebral artery occlusion; mNSS: modified neurological severity scores; TTC: 2,3,5-triphenyl tetrazolium chloride.
The ear capillaries after stellate ganglion block.