
Keywords: Alzheimer's disease, amnestic mild cognitive impairment, blood oxygen level-dependent, default mode network, dynamic functional connectivity, frontoparietal network, resting-state functional magnetic resonance imaging, support vector machine
Abstract
Numerous studies have shown abnormal brain functional connectivity in individuals with Alzheimer's disease (AD) or amnestic mild cognitive impairment (aMCI). However, most studies examined traditional resting state functional connections, ignoring the instantaneous connection mode of the whole brain. In this case-control study, we used a new method called dynamic functional connectivity (DFC) to look for abnormalities in patients with AD and aMCI. We calculated dynamic functional connectivity strength from functional magnetic resonance imaging data for each participant, and then used a support vector machine to classify AD patients and normal controls. Finally, we highlighted brain regions and brain networks that made the largest contributions to the classification. We found differences in dynamic function connectivity strength in the left precuneus, default mode network, and dorsal attention network among normal controls, aMCI patients, and AD patients. These abnormalities are potential imaging markers for the early diagnosis of AD.
Introduction
A patient is diagnosed with dementia every 3 seconds, and the global number of people with dementia is expected to reach more than 70 million by 2030 (McDade and Bateman, 2017). Alzheimer's disease (AD) is one of the most common causes of dementia in older adults. Despite the tremendous burden for patients, their families, and societies, effective treatments for AD are still lacking (Tiwari et al., 2019). Amnestic mild cognitive impairment (aMCI) has been regarded as a transitional stage between normal aging and AD (Blennow and Hampel, 2003). Interestingly, not all aMCI patients experience further progression. Thus, studying aMCI and AD patients simultaneously might aid the discovery of biomarkers of disease progression (Drago et al., 2011). Making timely and effective diagnoses in the early stages of AD could lead to promising opportunities for interventions (Grady et al., 1988; Jack et al., 2010).
Functional magnetic resonance imaging (fMRI) is used to noninvasively obtain brain activity information by measuring blood oxygen level-dependent signals (Logothetis and Wandell, 2004; Cai et al., 2020; Huang et al., 2020; Xing et al., 2021). Previous fMRI and structural MRI studies have shown evidence of reduced gray matter volume and cortical thickness in multiple brain regions (Wenk, 2003; Suzuki et al., 2019) in the preclinical phase of AD, and these changes are strongly associated with altered cognitive function. In particular, resting-state fMRI has great potential for discovering biomarkers of AD, which could facilitate early diagnosis of AD and understanding of the underpinning mechanisms (Luo et al., 2019). Widespread alterations in connectivity among multiple brain systems have been reported in AD patients (Dennis and Thompson, 2014). For instance, AD patients exhibited decreased connectivity in the default mode network (DMN), as well altered functional connectivity between the default-mode and salience network (Schultz et al., 2017). These large-scale dynamic network abnormalities were related to reduced cognitive performance, as well as to the levels of molecular biomarkers of AD and AD-related genetic risk factors (Wang et al., 2021).
Static resting-state functional connectivity has been widely used to measure the correlation between averaged time courses in different brain regions. However, dynamic connectivity analysis is used to calculate the variability of functional connectivity over time by considering temporal fluctuations within varying windows (Hutchison et al., 2013). Dynamic functional connectivity (dFC) has certain advantages with respect to static connectivity, such as providing more useful information to distinguish patients from healthy controls (Rashid et al., 2014). One of these novel measures is dFC strength (dFCS), which represents the strength of the dynamic correlation between the time series of voxels. This measure reflects the dynamic interconnections between brain regions from the voxel perspective (Luo et al., 2019). However, few studies have investigated the variability of dFCS in AD patients, especially in the aforementioned DMN. Based on a series of previous studies, we hypothesized that, compared with cognitively normal older adults, the dFCs of the DMN would be low in patients with AD and aMCI, and that this would be associated with overall cognitive ability.
dFC features can reflect detailed changes in brain activity (Du et al., 2017). When static and dFC features were compared in terms of utility in clinical disease recognition, the dFC features were more accurate in identifying diseases, and combining static and dynamic features did not lead to a significant improvement in accuracy (Rashid et al., 2016). Numerous studies have indicated that dFC is better able to distinguish patients from controls compared with state functional connection analysis (Rashid et al., 2014; Chen et al., 2018; Yang et al., 2019), which is widely used in studies of autism (Chen et al., 2017), schizophrenia (Duan et al., 2020), and depression (Liao et al., 2018). In recent years, some investigators have used the dynamic amplitude of low frequency fluctuation signals to examine characteristics of AD (Zeng et al., 2019), but few studies have compared dFC among AD, aMCI, and normal control (NC) groups.
Rapid developments in computer science and the accumulation of brain imaging data have provided clinical researchers with new approaches for differentiating and predicting AD and aMCI (Pereira et al., 2009). One of these techniques is a machine learning algorithm called a support vector machine (SVM). SVMs map the input vectors (input data) into a high-dimensional feature space and then compute a hyperplane that divides these input vectors into two classes. SVMs have been extensively used in AD pathology research, drug development, and computer-aided diagnoses (Haller et al., 2011). For instance, Zhao et al. (2019) used a SVM to identify 257 microRNAs associated with AD. Lv and Xue (2010) improved the effectiveness of a SVM in predicting inhibitors of acetylcholinesterase, a drug for AD, using a new feature selection method. Chaves et al. (2009) obtained a diagnostic accuracy of 98.3% for early AD diagnosis using voxels in the temporal and parietal regions as features. Recently, resting-state fMRI data has become much easier to collect, making it a useful tool for discovering the biomarkers of AD. Furthermore, dynamic connectivity (e.g. dFCS) can provide more information about how neural processes change over time (Ma et al., 2020). However, no studies have employed SVMs to conduct aMCI-NC and AD-NC classifications using dFCS variability as features.
To examine our above-mentioned hypothesis, we compared differences in dFCS at the voxel level among three groups (AD, aMCI, and NC), and then explored the relationship between comprehensive cognitive performance and dFCS variability in statistically significant regions. In addition, to investigate potential dFCS variability in pre-clinical diagnoses of AD, we trained two SVM models to conduct aMCI-NC and AD-NC classifications, respectively, and visualized the weights of brain regions and brain networks that contributed to the classification.
Participants and Methods
Participants
This case-control study was approved by the Institutional Ethics Committee of the General Hospital of the Chinese PLA General Hospital (approval No. 20100317-001) on April 28, 2010 (Additional file 1 (999.4KB, pdf) ). All subjects were recruited from the Neurology Outpatient Clinic of PLA General Hospital (Beijing, China) between January 1, 2017 and March 12, 2020, and two experts (BZ and YEG) were involved in the final diagnosis of each subject. All subjects agreed to participate and signed ethical informed consent forms (Additional file 2 (70.9KB, pdf) ). They underwent standard pre-experimental physical and psychological examination batteries, neuropsychological screening, and a cranial magnetic resonance scan. A total of 107 subjects were included in this study, including 36 patients with AD, 30 patients with aMCI, and 41 individuals who were cognitively NC. This study was conducted in accordance with the STrengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (von Elm et al., 2007) (Additional file 3).
STROBE Statement-—checklist of items that should be included in reports of observational studies
| Item No | Recommendation | Page | |
|---|---|---|---|
| Title and abstract | 1 | (a) Indicate the study’s design with a commonly used term in the title or the abstract | 2 |
|
| |||
| (b) Provide in the abstract an informative and balanced summary of what was done and what was found | 2 | ||
|
| |||
| Introduction | |||
|
| |||
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported | 3 |
|
| |||
| Objectives | 3 | State specific objectives, including any prespecified hypotheses | 3 |
|
| |||
| Methods | |||
|
| |||
| Study design | 4 | Present key elements of study design early in the paper | 4 |
|
| |||
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection | 4 |
|
| |||
| Participants | 6 | (a) Cohort study—Give the eligibility criteria, and the sources and methods of selection of participants. Describe methods of follow-up Case-control study—Give the eligibility criteria, and the sources and methods of case ascertainment and control selection. Give the rationale for the choice of cases and controls Cross-sectional study—Give the eligibility criteria, and the sources and methods of selection of participants |
4, 5 |
|
| |||
| (b) Cohort study—For matched studies, give matching criteria and number of exposed and unexposed Case-control study—For matched studies, give matching criteria and the number of controls per case |
4, 5 | ||
|
| |||
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable | 4 |
|
| |||
| Data sources/ measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group | 4 |
|
| |||
| Bias | 9 | Describe any efforts to address potential sources of bias | 4 |
|
| |||
| Study size | 10 | Explain how the study size was arrived at | 5 |
|
| |||
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why | 6 |
|
| |||
| Statistical methods | 12 | (a) Describe all statistical methods, including those used to control for confounding | 6 |
|
| |||
| (b) Describe any methods used to examine subgroups and interactions | 6 | ||
|
| |||
| (c) Explain how missing data were addressed | 6 | ||
|
| |||
| (d) Cohort study—If applicable, explain how loss to follow-up was addressed Case-control study—If applicable, explain how matching of cases and controls was addressed Cross-sectional study—If applicable, describe analytical methods taking account of sampling strategy |
6 | ||
|
| |||
| (e) Describe any sensitivity analyses | 6 | ||
|
| |||
| Results | |||
|
| |||
| Participants | 13* | (a) Report numbers of individuals at each stage of study—eg numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up, and analysed | 7 |
|
| |||
| (b) Give reasons for non-participation at each stage | 7 | ||
|
| |||
| (c) Consider use of a flow diagram | 7 | ||
|
| |||
| Descriptive data | 14* | (a) Give characteristics of study participants (eg demographic, clinical, social) and information on exposures and potential confounders | 7 |
|
| |||
| (b) Indicate number of participants with missing data for each variable of interest | 7 | ||
|
| |||
| (c) Cohort study—Summarise follow-up time (eg, average and total amount) | 7 | ||
|
| |||
| Outcome data | 15* | Cohort study—Report numbers of outcome events or summary measures over time | |
|
| |||
| Case-control study—Report numbers in each exposure category, or summary measures of exposure | 7 | ||
|
| |||
| Cross-sectional study—Report numbers of outcome events or summary measures | |||
|
| |||
| Main results | 16 | (a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (eg, 95% confidence interval). Make clear which confounders were adjusted for and why they were included | 7, 8 |
|
| |||
| (b) Report category boundaries when continuous variables were categorized | 7, 8 | ||
|
| |||
| (c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period | |||
|
| |||
| Other analyses | 17 | Report other analyses done—eg analyses of subgroups and interactions, and sensitivity analyses | 8 |
|
| |||
| Discussion | |||
|
| |||
| Key results | 18 | Summarise key results with reference to study objectives | 7, 8 |
|
| |||
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias | 8, 9 |
|
| |||
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence | 8, 9 |
|
| |||
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results | 8, 9 |
|
| |||
| Other information | |||
|
| |||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based | 1 |
*Give information separately for cases and controls in case-control studies and, if applicable, for exposed and unexposed groups in cohort and cross-sectional studies.
Note: An Explanation and Elaboration article discusses each checklist item and gives methodological background and published examples of transparent reporting. The STROBE checklist is best used in conjunction with this article (freely available on the Web sites of PLoS Medicine at http://www.plosmedicine.org/, Annals of Internal Medicine at http://www.annals.org/, and Epidemiology at http://www.epidem.com/). Information on the STROBE Initiative is available at www.strobe-statement.org.
Selection criteria
We included AD patients who (1) met the criteria for AD diagnosis jointly developed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) (McKhann et al., 1984) and the Alzheimer's Diseases and Related Disorders Association (ADRDA) (McKhann et al., 1984); (2) had a final score of 1 or 2 on the Clinical Dementia Rating Scale (CDR) (Sperling et al., 2011); (3) had no history of use of medications that affect cognition (e.g., acetylcholinesterase inhibitors, NMDA receptor antagonists); and (4) were aged 60–80 years.
In accordance with Peterson's criteria (Petersen et al., 2014) for mild cognitive impairment, we selected aMCI patients with (1) self-reported cognitive decline or confirmed decline from a knowledgeable person, with clinically significant symptoms of cognitive decline that had persisted for at least six months, and who did not met the criteria for a dementia diagnosis; (2) a score of 0.5 on the CDR; (3) an activities of daily living score (Petersen et al., 2001) of 26 or less and no significant impairment in the ability to perform activities of daily living; and (4) a Mini-Mental State Examination (MMSE) score (Petersen, 2004) no less than 24. We selected normal controls who were over 65 years old with a CDR score of 0, activities of daily living score less than 26, and MMSE score greater than 24.
We excluded patients with a Hachinski ischemic index score (Pantoni and Inzitari, 1993) of 4 or more, hypothyroidism, vitamin B12 or folic acid deficiency, a long history (more than 5 years) of smoking and alcohol abuse, inability to complete an MRI scan (patients with contraindications to MRI scanning, i.e., severely febrile, critically ill, claustrophobic, in early pregnancy, and with metal implants in the body or metal foreign bodies) or neuropsychological testing, traumatic brain disease or a history of other brain disorders, Parkinson's syndrome, epilepsy, and those with other systemic neurological disorders that severely affect cognitive function and systemic disorders that can affect MRI scans or neuropsychological tests. We excluded patients whose fMRI images failed visual quality control or pre-processing. The trial procedure is shown in Figure 1.
Figure 1.

Patient flow chart.
AD: Alzheimer's disease; aMCI: amnestic mild cognitive impairment; fMRI: functional magnetic resonance imaging; NC: normal control.
Neuropsychological tests
All subjects underwent a series of neuropsychological tests, including the MMSE, CDR, Geriatric Depression Scale (GDS), Montreal cognitive assessment (MoCA), and the Activities of Daily Living Scale (ADLS). The MMSE is currently the most widely used scale for evaluating cognitive function. It is simple and easy to implement, and includes items about time, place, calculation, and memory. The total score ranges from 0–30 points, and lower scores reflect worse cognitive function (Petersen, 2004). The MoCA is used to quickly screen for mild cognitive dysfunction. It includes a total of eight tests measuring different cognitive domains such as memory, executive function, visual space, and attention. The maximum score is 30 points. If the patient has 12 or fewer years of education, 1 point is added to the total score. A lower score reflects worse cognitive function. The CDR is mostly used to assess the degree of cognitive impairment in patient populations, and covers memory, orientation, judgment, problem-solving, and social activities. The total score is expressed as 0, 0.5, 1, 2, or 3 points. A higher score reflects worse cognitive function (Sperling et al., 2011). We also used the GDS-15, which is currently recommended by the American Academy of Geriatrics for screening depression in older people, and has a total score of 0–15. Higher scores represent more serious depression. Patients with > 4 points on the GDS-15 were excluded from this study. Finally, we used the ADLS, which includes the basic ADL and instrumental ADL, and covers 20 activities such as eating, dressing, bathing, and handling money independently. The ADL is considered to be the most appropriate assessment of activity ability in elderly individuals. Each item is worth 1–9 points, for a total of 20–180 points. A higher score indicates worse living ability.
fMRI
Image acquisition and preprocessing
MRI images were acquired by an experienced physician using a Siemens 3.0T MRI scanner with a 20-channel cranial coil. Scanning was conducted at the outpatient clinic of the Department of Radiology, Chinese PLA General Hospital. fMRI images were acquired using a gradient echo combined with a single excitation echo planar imaging sequence (64 × 64 resolution, 2000 ms repetition time, 30 ms echo time, 30 axial layers with 1 mm layer space and 3 mm thickness, 220 mm × 220 mm field of view, 90 flip angle, and duration of 8 minutes 6 seconds). We used the following protocol to obtain better MRI quality. First, all subjects underwent safety training prior to image acquisition. The subjects wore latex earplugs and a fixed head strap throughout the scan to minimize the effects of instrument noise and involuntary head movements. They were asked to lie down and relax with their eyes closed, and to avoid systematic thinking during the scan.
We used DPASFA (Data Processing Assistant for Resting-State fMRI package, version 4.2; http://www.restfmri.net) for fMRI preprocessing. Briefly, the standard process is as follows. First, we excluded the first 10 volumes in each fMRI dataset to account for changes in the magnetic field stability during scanning and the subject's adaptability to the scanning environment. Second, we corrected the data for participant head movements and slice-timing (Buchanan et al., 2020). Third, we registered all images to standard Montreal Neurological Institute neuroimaging space and performed spatial correction with a full width at half maximum Gaussian kernel function to reduce individual anatomical structure differences and spatial noise (resample resolution of 3 mm × 3 mm × 3 mm and kernel length-width-height of 6 mm × 6 mm × 6 mm). Finally, we denoised the data via linear regression and a subsequent temporal band-pass filter (0.01–0.1 Hz) to regress out interfering covariates including Friston 24 motion parameters, linear drift, white matter signal, and cerebrospinal fluid signal.
dFCS
We used the DynamicBC toolkit (v1.1, www.restfmri.net/forum/DynamicBC) to calculate the global dFCS for each voxel with the sliding window approach, as shown in the flow chart in Figure 2. We set the length of the window to 50 repetitions with an overlap of 0.6. The rest of the parameters were identical to a previous study (Luo et al., 2019) (230 total repetitions were available and 7 windows were created.) For each window, we first calculated the global dFCS at each voxel as the sum of the functional connectivity between this voxel and the other voxels in the brain mask. We adopted the threshold P < 0.001 to eliminate voxels with weak correlations attributable to signal noise and removed negative correlations. Then, we obtained a series of dFCS maps corresponding to the number of windows. The variance of each dFCS map across time was calculated to measure its temporal variability. Finally, on the basis of a previous study, the variance of the dFCS map for each subject was transformed to a Z score by subtracting the mean values divided by the standard deviation of all values within the brain mask to control the global effects (Zou et al., 2008).
Figure 2.

Process of dFCS calculation.
(A) Raw fMRI. (B) Preprocessed fMRI. (C) Calculation of dFCS atlas using a sliding-window approach. (D) Final atlas of dFCS variance. dFCS: Dynamic functional connectivity strength; fMRI: functional magnetic resonance imaging.
Data analysis
Data analysis was performed using the DynamicBC toolbox in Matlab 2018b (The MathWorks, Inc., Natick, MD, USA) as described below. First, we compared demographic factors, including age, gender, and years of education using either an analysis of variance or the Chi-squared test among the AD, aMCI, and NC groups. Second, we conducted a voxel-wise one-way analysis of covariance with age, gender, and education as covariates to determine the altered variance of dFCS among the three groups. We corrected the results via threshold-free cluster enhancement correction (Smith and Nichols, 2009) at a significance level of 0.05. Third, we extracted the variance of the dFCS values with significant differences among the three groups by summing the Z scores of the clusters with statistical significance, and then conducted between-group comparisons via two-tailed two-sample t-tests. Finally, to investigate the relationship between cognitive ability and dFCS variability in statistically significant regions, we conducted a partial correlation analysis of dFCS variance with MMSE scores and MoCA, controlling for age, gender, and years of education.
To discriminate aMCI and AD from NC, we used the variance of dFCS maps as features and trained linear SVM models using LIBSVN 3.25 (https://www.npackd.org/p/libsvm/3.25). This led to a model with more interpretability than other non-linear models and less susceptibility to over-fitting. Because of the limited study population, we used 5-fold cross validation (Figure 3) to evaluate the performance of the models. In each model, four-fifths of the participants were selected as the training dataset, and the other participants were used as the test dataset. Because the classification was pair-wise, we used a two-sample t-test to select the features with P values < 0.05 in the training dataset. Although they can preserve multivariate patterns, we did not use multivariate feature selection methods such as recursive feature elimination because they are time-consuming. Before inputting the data into the model, we normalized the features using the mean values and standard deviations from the training dataset. We used the accuracy, sensitivity, specificity, area under the curve, positive predictive value, negative predictive value, and F-score to evaluate model performance. We used the permutation test to determine whether the obtained final metrics were significantly better than chance. Specifically, we ran the above prediction procedure 1000 times. For each time, we permuted the labels across the samples without replacements. The P values of the metrics were calculated by dividing the number of permutations with a higher value than the actual value for the real sample by the total number of permutations. For each sample, we also calculated the decision value, which is the distance between the samples on a hyperplane that is determined by SVM classifiers. We also used partial correlation analysis to explore the relationship between decision values and MMSE and MoCA scores.
Figure 3.
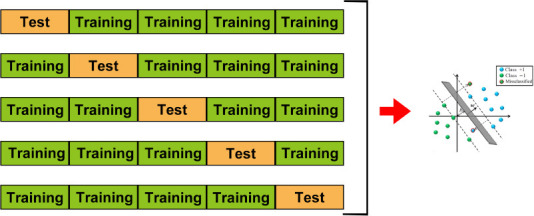
Five-fold cross validation.
The dataset was divided into five parts for 5-fold cross validation. One part was selected as the test dataset, while the remaining four parts were used as the training dataset. This was repeated five times so that each fold of the data was selected as a test set once, enabling us to obtain the predicted labels for all of the data. ‘b’ and ‘w’ in the figure are the parameters of the SVM. Different colored balls represent the samples with different labels.
Finally, we visualized the distribution of voxels contributing to the classifier by summing the weights (absolute weight)/distributions of the voxels separately on the brain region or network level using LIBSVN 3.25. Ninety brain regions were defined using the AAL template in standard space (Tzourio-Mazoyer et al., 2002). The whole brain was divided into seven networks based on a functional partition, as described by Yeo et al. (2011): visual network, somatomotor network, dorsal attention network, ventral attention network, limbic network, frontoparietal network, and DMN. The specific coordinates and detailed functions of the brain regions in these seven networks can be found in Bargmann and Marder (2013).
We also conducted a follow-up analysis. The goal of the training process for the SVM model was to determine a hyperplane and separate the different types of samples in the feature space. The coefficients defined by the hyperplane can be used to quantify the contribution of different features in the classification task, that is, the greater the absolute value of the coefficients, the greater the contribution of the corresponding features. We coded the patient sample as 1 and the control group as –1. Compared with the controls, the patient group had a higher number of features with positive coefficients in the hyperplane, that is, with positive contributions. In contrast, the eigenvalues with negative coefficients, that is, those with negative contributions, tended to be lower in the patient group.
The statistical methods of this study were reviewed by the biostatistician of Chinese PLA General Hospital.
Results
Demographic features of AD, aMCI, and NC groups
We found no significant differences in age, gender ratio, or years of education among the three groups (P > 0.05). However, the MMSE and MoCA scores were significantly different (P < 0.001), with the lowest scores for AD patients, highest scores for NCs, and intermediate scores for aMCI patients (Table 1).
Table 1.
Demographic and neuropsychological data for the AD, aMCI and NC groups
| Item | AD (n =36) | aMCI (n =30) | NC (n =41) | F-value | P-value |
|---|---|---|---|---|---|
| Age (yr) | 71.6±8.8 | 69.4±8.8 | 68.3±6.8 | 3.0491 | 0.2177 |
| Sex (male/female) | 16/20 | 11/19 | 21/20 | 0.0376 | 0.9814 |
| Education (yr) | 9.7±4.5 | 12.1±4.0 | 12.1±4.3 | 0.4472 | 0.7996 |
| MMSE score | 17.72±6.0 | 26.83±2.13 | 28.51±1.36 | 86.6850 | < 0.001 |
| MoCA score | 14.37±3.15 | 22.11±2.62 | 26.64±2.65 | 104.5989 | < 0.001 |
Data are expressed as mean ± SD, and were analyzed by one-way analysis of variance, except sex with number and analyzed by Chi-squared test. AD: Alzheimer’s disease; aMCI: Amnestic mild cognitive impairment; MMSE: Mini-mental state examination; MoCA: Montreal Cognitive Assessment; NC: Normal control.
Significant differences in the left precuneus among the AD, aMCI, and NC groups
Using voxel-wise analysis, we found significant differences in dFCS variance in a cluster including the left precuneus among the three groups (Figure 4A). The Montreal Neurological Institute coordinates of the peak voxel, which had an F value of 12.03, were (–3, –60, 57) and the cluster size was 6. Then, we extracted the mean variance of the dFCS in the left precuneus. A two-tailed two-sample t-test (Figure 4B) showed that the mean variances of the dFCS in the left precuneus region of patients with aMCI (P < 0.01) and AD (P < 0.001) were lower than that in the NC group.
Figure 4.
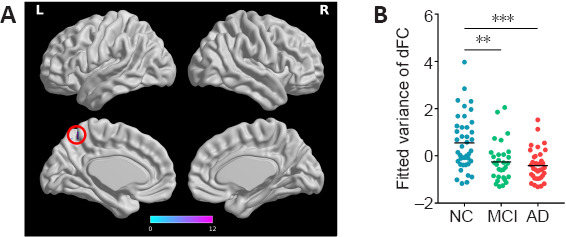
The difference in dFCS among the NC, aMCI, and AD groups.
(A) The voxel-based analysis revealed a difference in dFCS variance among the NC, aMCI, and AD groups in the left precuneus (red circle). (B) The NC group showed larger dFCS variance in the precuneus than the aMCI and AD groups. Data were analyzed using a two-tailed two-sample t-test. **P < 0.01; ***P < 0.001. AD: Alzheimer's disease; aMCI: amnestic mild cognitive impairment; dFCS: dynamic functional connectivity strength; L: left; NC: normal control R: right.
dFCS variance in the left precuneus was positively correlated with MMSE and MoCA scores
A partial correlation analysis controlling for sex, age, and education revealed that the mean variance of the dFCS in the left precuneus was significantly positively correlated with MMSE (r = 0.29, P = 0.003) and MoCA (r = 0.24, P = 0.038) scores (Figure 5).
Figure 5.
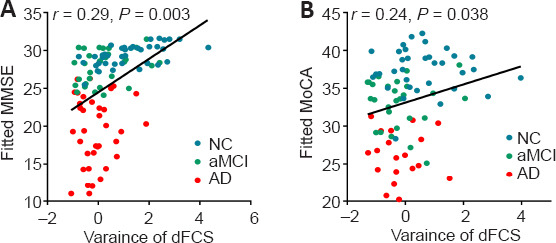
Correlations between the mean dFCS variance in the precuneus with MMSE (A) and MoCA (B) scores.
Data were analyzed via partial correlation analysis. AD: Alzheimer's disease; aMCI: amnestic mild cognitive impairment; dFCS: dynamic functional connectivity strength; MMSE: Mini-Mental Status Examination; NC: normal control.
Performance of classifiers
As shown in Table 2, except for the specificity and positive predictive value, for which the aMCI-NC classifier was better than the AD-NC classifier, the aMCI-NC classifier did not perform as well as the AD-NC classifier in all other measured domains.
Table 2.
Performance of classifying the AD and aMCI groups from the NC group
| NC vs. aMCI | NC vs. AD | |||
|---|---|---|---|---|
|
|
|
|||
| Data | P-value | Data | P-value | |
| Accuracy | 0.68 | 0.007 | 0.71 | 0.004 |
| Sensitivity | 0.40 | 0.009 | 0.72 | 0.001 |
| Specificity | 0.88 | 0.031 | 0.71 | 0.039 |
| Area under curve | 0.61 | 0.05 | 0.75 | 0.001 |
| Positive predictive value | 0.71 | 0.014 | 0.68 | 0.031 |
| Negative predictive value | 0.67 | 0.006 | 0.74 | 0.003 |
| F-score | 0.51 | 0.011 | 0.7 | 0.001 |
AD: Alzheimer’s disease; aMCI: amnestic mild cognitive impairment; NC: normal control.
We performed a partial correlation analysis to explore whether the decision values generated from the SVM classifiers were correlated with the MMSE and MoCA scores. As shown in Figure 6, the decision values were significantly correlated with the MMSE (r = 0.42, P < 0.001) and MoCA (r = 0.27, P = 0.016) scores.
Figure 6.
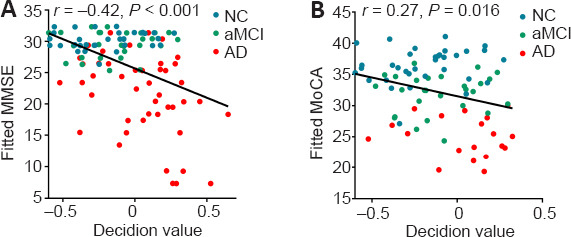
Correlations between decision value and MMSE (A) and MoCA (B) scores.
Data were analyzed via partial correlation analysis. AD: Alzheimer's disease; aMCI: amnestic mild cognitive impairment; dFCS: dynamic functional connectivity strength; MMSE: Mini-Mental Status Examination; NC: normal control.
Brain regions that most strongly contributed to classification
To identify the brain regions that most strongly contributed to classification, we calculated the mean positive and negative weights, respectively, for each region. Table 3 shows the ten regions that most negatively and positively contributed to the classification of aMCI and NC. Most of the contributive regions were in the frontal and temporal lobes, while some regions in the frontal, temporal, and occipital regions positively contributed to classification.
Table 3.
The ten regions that contributed most to the classification of the aMCI from the NC group
| Region | Absolute weight (×10–5) |
|---|---|
| Negatively | |
| Left superior temporal gyrus | 3.94 |
| Left parahippocampal gyrus | 3.84 |
| Left rolandic sulcus | 3.59 |
| Right parahippocampal gyrus | 3.06 |
| Right Heschl’s gyrus | 2.93 |
| Right middle orbitofrontal cortex | 2.37 |
| Left superior orbitofrontal cortex | 2.36 |
| Right superior frontal gyrus | 2.32 |
| Left Heschl’s gyrus | 2.32 |
| Left amygdala | 2.31 |
| Positively | |
| Left Heschl’s gyrus | 3.46 |
| Right middle temporal pole | 3.13 |
| Left superior temporal gyrus | 3.06 |
| Right middle orbitofrontal cortex | 2.91 |
| Left fusiform gyrus | 2.83 |
| Right fusiform gyrus | 2.28 |
| Left superior occipital gyrus | 2.07 |
| Left middle orbitofrontal cortex | 2.02 |
| Right superior frontal gyrus | 1.99 |
| Left inferior temporal gyrus | 1.94 |
aMCI: Amnestic mild cognitive impairment; NC: normal control.
Pertaining to the classification of NC and AD, negatively contributive brain regions were mainly found in the bilateral temporal lobes and precuneus, while positive regions were located in the bilateral parahippocampal gyrus and orbital frontal lobes (Table 4).
Table 4.
The ten regions that contributed most to the classification of the AD from the NC group
| Region | Absolute weight (×10–5) |
|---|---|
| Negatively | |
| Left middle temporal gyrus | 4.17 |
| Right supramarginal gyrus | 3.76 |
| Left medial orbitofrontal cortex | 3.65 |
| Right rectus gyrus | 3.6 |
| Right middle temporal gyrus | 3.4 |
| Right precuneus | 3.26 |
| Right inferior temporal gyrus | 3.23 |
| Right inferior parietal gyrus | 2.14 |
| Left rectus gyrus | 2.14 |
| Left precuneus | 2.11 |
| Positively | |
| Left superior temporal gyrus | 3.94 |
| Left parahippocampal gyrus | 3.84 |
| Left rolandic operculum | 3.59 |
| Right hippocampal gyrus | 3.06 |
| Right heschl gyrus | 2.93 |
| Right middle frontal gyrus, orbital part | 2.37 |
| Left superior frontal gyrus, orbital part | 2.36 |
| Right superior frontal gyrus | 2.32 |
| Left heschl gyrus | 2.32 |
| Left amygdala | 2.31 |
AD: Alzheimer’s disease ; NC: normal control.
Brain networks that most strongly contributed to classification
We also considered the mean positive and negative weights in the brain networks (Yeo et al., 2011). In the aMCI-NC classifier, the DMN and frontoparietal network were the most contributive networks (Figure 7), whereas in the NC-AD classifier, the weights were almost all negative and mainly located in the DMN (Figure 8). For both the aMCI-NC and AD-NC classifiers, the visual network, somatomotor network, ventral attentional network, and limbic network had positive weights, with two clear phenomena. First, the weight of the positive contribution of the above networks was significantly higher in the aMCI-NC group than in the AD group. Second, the contribution weight of the somatomotor network was mainly positive, and its weight was second only to that of the DMN.
Figure 7.
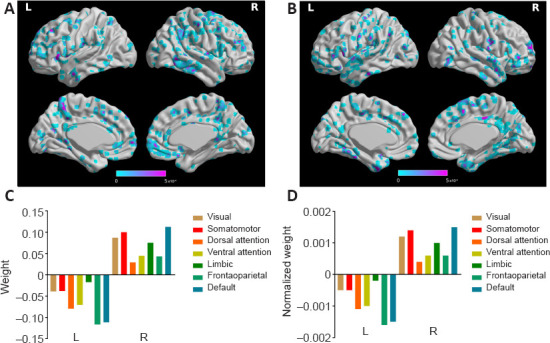
The weight of classifying the aMCI from the NC group.
(A) The distribution of negative weight. (B) The distribution of positive weight. (C) The weight of different functional networks. (D) The normalized weight (by the volume of the functional network) of different functional weights. “Default” represents the default mode areas. aMCI: Amnestic mild cognitive impairment; L: left; NC: normal control; R; right.
Figure 8.
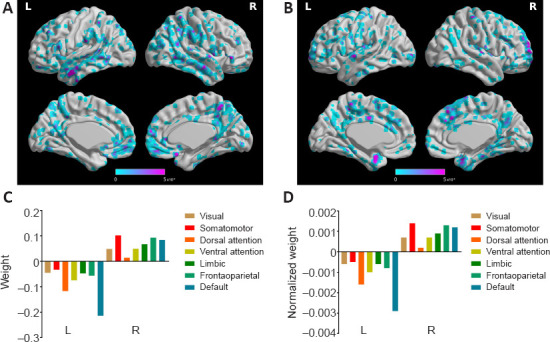
The weight of classifying the AD from the NC group.
(A) The distribution of negative weight. (B) The distribution of positive weight. (C) The weight of different functional networks. (D) The normalized weight (by the volume of the functional network) of different functional weights. AD: Alzheimer's disease; L: left; NC: normal control; R; right.
Discussion
We found that the dFCS variance in the left precuneus was significantly lower in AD and MCI patients compared with NCs, and that changes in the dFCS variance in the left precuneus were correlated with disease severity. In particular, the partial-correlation analysis showed that changes in dFCS variance in the left precuneus were linked to decreased MMSE scores after controlling for age, sex, and education as confounding variables. In addition, two SVM models were trained to classify AD from NC as well as aMCI from NC. Our results indicated that the AD-NC classifier had better performance than the aMCI-NC classifier. The mean weights across brain regions and brain networks imply that the temporal lobe plays a significant role in the development of AD and MCI, and that the DMN and frontoparietal network play prominent roles in the progression of AD. Together, our results suggest that these neural measures of dynamic connectivity strength may serve as biomarkers that could be used in the diagnosis, treatment, and ongoing assessment of AD.
Altered dFCS variability in the left precuneus in AD patients
Our results indicate that dFCS variance in the left precuneus was significantly reduced in AD and MCI patients compared with healthy older people. The precuneus is the superior parietal part of the medial surface of each cerebral hemisphere, and is located in front of the cuneus (the superior part of the occipital lobe). The precuneus is a main node of the DMN, and part of the precuneus was included in the frontal parietal control network in our study. The precuneus has been associated with various cognitive functions including episodic memory, self-reflection, and other aspects of consciousness (Cavanna and Trimble, 2006). Interestingly, the precuneus shows both functional and structural lateralization. For instance, compared with the right precuneus, the left precuneus was found to exhibit a greater degree of atrophy during normal aging and AD progression (Love and Miners, 2016). Similarly, imaging studies have indicated that the left precuneus is more vulnerable and more susceptible to various neurological disorders, including AD (Fusar-Poli et al., 2011). Furthermore, the functional connectivity of the left precuneus was found to be more vulnerable in AD patients than NCs during memory tasks (Berthoz, 1997). Consistent with the aforementioned findings, we found the most significant dFCS variability in the left precuneus in patients with AD. This dysfunction in the left precuneus may be underpinned by the accumulation of Aβ and tau proteins in this region in early AD patients (Miners et al., 2016; Baghel et al., 2019). Together, our findings suggest that abnormalities in dFCS variability in the left precuneus have potential as a noninvasive marker for early AD, and that early clinical interventions targeting this region may slow AD or aMCI progression.
In the present study, the mean MMSE score was lowest among AD patients, and we found a positive correlation between mean MMSE score and dFCS variability in the left precuneus after correcting for confounding variables. The MMSE is a rapid and comprehensive measure of overall cognitive function, and is widely used in clinical settings to screen for dementia (Tombaugh and McIntyre, 1992). A series of multicenter validation studies has shown that MMSE scores can accurately describe the trajectory of cognitive change in patients during the course of dementia development (Bergeron et al., 2017). For instance, a previous study found that increased MMSE scores predicted proneness to clinical symptoms in patients with AD (Li et al., 2016). By extending the results of that study, our findings suggest that changes in dFCS variability in the left precuneus might predict declines in cognitive ability during AD progression.
Classification of AD according to changes in brain regions and networks
Previous studies have found that AD patients tend to have altered medial temporal lobe structures, especially hippocampal atrophy, enlarged ventricles, widened capsules, and other signs of brain atrophy. These changes can extend to the frontal lobe, parietal lobe, and cerebellum with disease progression (Villemagne et al., 2018). In the present study, the brain regions that contributed to aMCI and NC classification were mainly distributed in the frontal lobe and temporal lobe, and these brain regions were included in the default network. At the same time, activity in the frontal lobes (including the orbitofrontal cortex), temporal lobes, and occipital lobes in aMCI and AD patients was enhanced compared with that in NCs. Previous studies have also found that MCI patients had excessive activation in the medial temporal lobe region, with reduced functional connectivity between the medial temporal lobe and other brain regions. This indicates that neuronal degeneration may increase functional connectivity in the medial temporal lobe, which may support performance in some subsystems (Alsop et al., 2010; Pasquini et al., 2015). This region of the orbitofrontal cortex has functional connections with the thalamus, temporal lobe, amygdala, and olfactory system (Aggleton, 2012). In this study, we found different degrees of functional enhancement according to the stage of aMCI and AD. At the onset of AD, multiple brain regions are thought to compensate for neurological changes, thus upholding olfaction, feeding, auditory perception, and the reward system. This suggests that compensatory mechanisms are an indispensable part of the pathophysiological mechanisms of AD. Our brain network-level analysis revealed differences in brain networks between NCs and patients. For the aMCI-NC classifiers, the DMN and frontoparietal network made large cumulative contributions, whereas only the DMN showed a large contribution among the AD-NC classifiers. In addition, compared with the other brain networks, the frontoparietal network made a large contribution in terms of both classifiers, although this was less than the contribution of the DMN.
A series of functional connectivity studies identified that AD and MCI patients are susceptible to changes in the DMN. This is consistent with our findings regarding the prominent contribution of the DMN in both classification models. The DMN is essential for memory, self-related cognitive processes, and cognitive control, which are involved in many cognitive functions in healthy individuals (Greicius et al., 2003). Disrupted DMN activity in AD and MCI patients has been found to contribute to the prominent decline of cognitive abilities. Our study provides new evidence regarding the role of DMN dynamics in distinguishing between AD, MCI, and healthy controls. The DMN plays an important role in self-related processing such as mind-wandering, which is essential for multiple cognitive functions (Vidaurre et al., 2017). Recently, dynamic changes in DMN functional connectivity were significantly implicated in flexible neural computation during both resting-state and cognitive tasks (Jones et al., 2011). Our results extend these findings by suggesting that altered DMN dynamics may contribute to the development of AD by disrupting flexible neural computation.
Our findings regarding abnormalities in dFCS variability in the frontoparietal network in AD and MCI patients are consistent with previous studies. The frontoparietal network is the key component of the triple network structure (Cocchi et al., 2013). It flexibly interacts with the DMN and the salience network to allocate cognitive resources according to task demands in various environments (Cocchi et al., 2013). Altered frontoparietal network connectivity has been associated with disrupted working memory and executive control in many neurodegenerative disease including AD and MCI. For instance, a previous task-based fMRI-based study found that MCI patients exhibited stronger neural activity in the frontal and parietal regions than healthy controls during memory encoding and retrieval, working memory, executive function, and perception-related tasks (Chand et al., 2017). Similarly, a review summarizing 75 fMRI studies reported that MCI patients showed hypoactivation in the frontoparietal cortex relative to healthy controls (Li et al., 2015). Our findings support and extend the conclusions of some studies proposing that AD and MCI share the same neural compensation mechanisms, and therefore, that activity patterns in brain networks should be similar between MCI and AD patients. Furthermore, our results suggest that altered dynamics in the frontoparietal network may be an essential element of AD pathology, which may be related to disrupted flexibility of cognitive control.
In the aMCI-NC classifier, the visual network, somatomotor network, ventral attention network, and edge-centric functional network made large positive contributions to classification. In contrast, in the AD-NC classifier, the positive contribution was relatively low among all networks. This indicates that the brain may enhance the variability of various networks to compensate for cognitive decline in the early stage of aMCI. However, by the late stage of AD, the variability of functional connectivity had decreased, which may signal occurrence of functional compensation. This is consistent with literature indicating that compensation mostly occurs in the MMCI stage, but not in the AD stage (Delli Pizzi et al., 2019; Skouras et al., 2019).
Limitations
This study had several limitations. First, when we calculated dFCS, we summed the values of all voxels rather than the calculated mean value. This may have created a bias towards larger networks because they have more voxels. Second, the results of our analysis suggested that the performance of the classifier in terms of AD diagnosis was not particularly high, which may have been related to the sample size of the study. However, dFCS is still a good model for studying the pathophysiological mechanisms of AD, and multivariate statistical analysis can serve as a supplement. Our effect size may have been higher if we had used a structural modality. However the goal of our study was to examine this application of functional modalities. Our results may have been limited by the small sample size. Future studies with larger sample sizes are needed.
Considering our relatively small sample size, and our plan to consider the contribution of all of the voxels in terms of dFCS variability in the classification system, we used SVM models because they have computational simplicity and efficiency for small samples (Zendehboudi et al., 2018). However, SVMs have several limitations. For instance, the performance of an SVM decreases when the dimensionality of the features exceeds the number of training data points. The SVM can neither divide the data points on the hyperplane nor describe the points outside the hyperplane in terms of attributes reflecting the distance from the point to the hyperplane. As a result, we could not fully consider the variability of dFCS among the AD patients in this study. Finally, using cross-validation combined with permutation tests, as we did in this study, is the most common solution for small sample problems in almost all machine learning studies. However, the uncertainty of the point estimates obtained using this validation method is unknown, and this uncertainty seems to be greater in biomedical settings (Rodríguez-Pérez et al., 2018). Therefore, future small sample studies should use this method in conjunction with advanced forms such as Bayesian confidence intervals.
Previous studies have shown that AD causes the cerebral cortices, including the temporal, frontal, and parietal lobes, to shrink over time (Chard et al., 2002; Li et al., 2016; Pini et al., 2016). This differs from what we found in the group comparisons. Considering that AD patients in traditional autopsy studies tend to have a more severe disease course than the patients in this study, changes in dFCS in the left precuneus might precede massive gray matter atrophy in other regions such as the temporal lobe. This conjecture should be examined in future longitudinal studies with larger samples. Our classification analysis indicated that the DMN including the temporal lobe contributed to the discrimination of AD patients from healthy controls. This indicates that the temporal lobe in the AD patients in this study may have undergone slight alterations that were not yet sufficient to distinguish between AD patients and healthy controls independently. Together, our findings suggest that clinicians should be concerned about abnormalities in the functional connectivity of the left precuneus. Because of the relatively small sample size in the present study, systematic, large-sample, longitudinal studies focusing on the left precuneus are needed to further validate our results and uncover more details regarding AD pathology.
Conclusion
In this study, we found significant abnormalities in dFCS variability in the left precuneus among AD and MCI patients, which were associated with reduced overall cognitive performance. Furthermore, SVM classification showed that dFCS variability in the DMN and frontoparietal network could be used as significant features to classify AD-HC and MCI-HC. Our findings build upon previous static functional connectivity studies of AD and highlight the important role of dynamics of the precuneus, DMN, and frontoparietal network in flexible computation during the resting-state. Alterations in these neural processes may contribute to the development of AD. Furthermore, our results suggest the potential of dFCS variability, especially in the precuneus, DMN, and frontoparietal network, as an AD imaging marker for the early diagnosis of AD. Considering the relatively small sample size and methodological limitations, the results of this study need to be validated by future research.
Additional files:
Additional file 1 (999.4KB, pdf) : Hospital ethics approval (Chinese).
Additional file 2 (70.9KB, pdf) : Informed consent form (Chinese).
Additional file 3: STROBE checklist.
Additional file 4 (105.5KB, pdf) : Open peer review report 1.
Footnotes
P-Reviewer: McDonough IM; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y
Conflicts of interest: There are no conflicts of interest.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open peer reviewer: Ian M McDonough, The University of Alabama, USA.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81471120; and Fund Projects in Technology of the Foundation Strengthening Program of China, No. 2019-JCJQ-JJ-151 (both to XZ).
References
- 1.Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–1596. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Alsop DC, Dai W, Grossman M, Detre JA. Arterial spin labeling blood flow MRI: its role in the early characterization of Alzheimer's disease. J Alzheimers Dis. 2010;20:871–880. doi: 10.3233/JAD-2010-091699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baghel V, Tripathi M, Parida G, Gupta R, Yadav S, Kumar P, Dey AB, Damle NA, Kumar R, Bal C. In vivo assessment of tau deposition in Alzheimer disease and assessing its relationship to regional brain glucose metabolism and cognition. Clin Nucl Med. 2019;44:e597–e601. doi: 10.1097/RLU.0000000000002791. [DOI] [PubMed] [Google Scholar]
- 4.Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron D, Flynn K, Verret L, Poulin S, Bouchard RW, Bocti C, Fülöp T, Lacombe G, Gauthier S, Nasreddine Z, Laforce RJ. Multicenter validation of an MMSE-MoCA conversion table. J Am Geriatr Soc. 2017;65:1067–1072. doi: 10.1111/jgs.14779. [DOI] [PubMed] [Google Scholar]
- 6.Berthoz A. Parietal and hippocampal contribution to topokinetic and topographic memory. Philos Trans R Soc Lond B Biol Sci. 1997;352:1437–1448. doi: 10.1098/rstb.1997.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan CR, Bastin ME, Ritchie SJ, Liewald DC, Madole JW, Tucker-Drob EM, Deary IJ, Cox SR. The effect of network thresholding and weighting on structural brain networks in the UK Biobank. Neuroimage. 2020;211:116443. doi: 10.1016/j.neuroimage.2019.116443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Y, Xie M, Su Y, Tong Z, Wu X, Xu W, Li J, Zhao F, Dang C, Chen G, Lan L, Shen J, Zheng Y. Aberrant functional and causal connectivity in acute tinnitus with sensorineural hearing loss. Front Neurosci. 2020;14:592. doi: 10.3389/fnins.2020.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 11.Chand GB, Wu J, Hajjar I, Qiu D. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017;7:401–412. doi: 10.1089/brain.2017.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 13.Chaves R, Ramírez J, Górriz JM, López M, Salas-Gonzalez D, Alvarez I, Segovia F. SVM-based computer-aided diagnosis of the Alzheimer's disease using t-test NMSE feature selection with feature correlation weighting. Neurosci Lett. 2009;461:293–297. doi: 10.1016/j.neulet.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Nomi JS, Uddin LQ, Duan X, Chen H. Intrinsic functional connectivity variance and state-specific under-connectivity in autism. Hum Brain Mapp. 2017;38:5740–5755. doi: 10.1002/hbm.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Sun D, Shi Y, Jin W, Wang Y, Xi Q, Ren C. Alterations of static functional connectivity and dynamic functional connectivity in motor execution regions after stroke. Neurosci Lett. 2018;686:112–121. doi: 10.1016/j.neulet.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi L, Zalesky A, Fornito A, Mattingley JB. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn Sci. 2013;17:493–501. doi: 10.1016/j.tics.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Delli Pizzi S, Punzi M, Sensi SL. Functional signature of conversion of patients with mild cognitive impairment. Neurobiol Aging. 2019;74:21–37. doi: 10.1016/j.neurobiolaging.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Dennis EL, Thompson PM. Functional brain connectivity using fMRI in aging and Alzheimer's disease. Neuropsychol Rev. 2014;24:49–62. doi: 10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drago V, Babiloni C, Bartrés-Faz D, Caroli A, Bosch B, Hensch T, Didic M, Klafki HW, Pievani M, Jovicich J, Venturi L, Spitzer P, Vecchio F, Schoenknecht P, Wiltfang J, Redolfi A, Forloni G, Blin O, Irving E, Davis C, et al. Disease tracking markers for Alzheimer's disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26(Suppl 3):159–199. doi: 10.3233/JAD-2011-0043. [DOI] [PubMed] [Google Scholar]
- 20.Du Y, Pearlson GD, Lin D, Sui J, Chen J, Salman M, Tamminga CA, Ivleva EI, Sweeney JA, Keshavan MS, Clementz BA, Bustillo J, Calhoun VD. Identifying dynamic functional connectivity biomarkers using GIG-ICA: Application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Hum Brain Mapp. 2017;38:2683–2708. doi: 10.1002/hbm.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan X, Hu M, Huang X, Su C, Zong X, Dong X, He C, Xiao J, Li H, Tang J, Chen X, Chen H. Effect of risperidone monotherapy on dynamic functional connectivity of insular subdivisions in treatment-naive, first-episode schizophrenia. Schizophr Bull. 2020;46:650–660. doi: 10.1093/schbul/sbz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, Mc Guire P, Sacchetti E. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–1185. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Grady CL, Haxby JV, Horwitz B, Sundaram M, Berg G, Schapiro M, Friedland RP, Rapoport SI. Longitudinal study of the early neuropsychological and cerebral metabolic changes in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1988;10:576–596. doi: 10.1080/01688638808402796. [DOI] [PubMed] [Google Scholar]
- 24.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haller S, Lovblad KO, Giannakopoulos P. Principles of classification analyses in mild cognitive impairment (MCI) and Alzheimer disease. J Alzheimers Dis 26 Suppl. 2011;3:389–394. doi: 10.3233/JAD-2011-0014. [DOI] [PubMed] [Google Scholar]
- 26.Huang Y, Xu L, Kuang L, Wang W, Cao J, Xiao MN. Abnormal brain activity in adolescents with Internet addiction who attempt suicide: an assessment using functional magnetic resonance imaging. Neural Regen Res. 2020;15:1554–1559. doi: 10.4103/1673-5374.274346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, Bernstein MA, Gunter JL, Pankratz VS, Aisen PS, Weiner MW, Petersen RC, Shaw LM, Trojanowski JQ, Knopman DS. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain. 2010;133:3336–3348. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones DT, Machulda MM, Vemuri P, McDade EM, Zeng G, Senjem ML, Gunter JL, Przybelski SA, Avula RT, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr Age-related changes in the default mode network are more advanced in Alzheimer disease. Neurology. 2011;77:1524–1531. doi: 10.1212/WNL.0b013e318233b33d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Jia J, Yang Z. Mini-Mental State Examination in elderly Chinese: a population-based normative study. J Alzheimers Dis. 2016;53:487–496. doi: 10.3233/JAD-160119. [DOI] [PubMed] [Google Scholar]
- 31.Li HJ, Hou XH, Liu HH, Yue CL, He Y, Zuo XN. Toward systems neuroscience in mild cognitive impairment and Alzheimer's disease: a meta-analysis of 75 fMRI studies. Hum Brain Mapp. 2015;36:1217–1232. doi: 10.1002/hbm.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao W, Li J, Duan X, Cui Q, Chen H, Chen H. Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Hum Brain Mapp. 2018;39:4105–4118. doi: 10.1002/hbm.24235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- 34.Love S, Miners JS. Cerebrovascular disease in ageing and Alzheimer's disease. Acta Neuropathol. 2016;131:645–658. doi: 10.1007/s00401-015-1522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, He H, Duan M, Huang H, Hu Z, Wang H, Yao G, Yao D, Li J, Luo C. Dynamic functional connectivity strength within different frequency-band in schizophrenia. Front Psychiatry. 2019;10:995. doi: 10.3389/fpsyt.2019.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lv W, Xue Y. Prediction of acetylcholinesterase inhibitors and characterization of correlative molecular descriptors by machine learning methods. Eur J Med Chem. 2010;45:1167–1172. doi: 10.1016/j.ejmech.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Wu D, Mai Y, Xu G, Tian J, Jiang G. Functional connectome fingerprint of sleep quality in insomnia patients: Individualized out-of-sample prediction using machine learning. Neuroimage Clin. 2020;28:102439. doi: 10.1016/j.nicl.2020.102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDade E, Bateman RJ. Stop Alzheimer's before it starts. Nature. 2017;547:153–155. doi: 10.1038/547153a. [DOI] [PubMed] [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 40.Miners JS, Palmer JC, Love S. Pathophysiology of hypoperfusion of the precuneus in early Alzheimer's disease. Brain Pathol. 2016;26:533–541. doi: 10.1111/bpa.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pantoni L, Inzitari D. Hachinski's ischemic score and the diagnosis of vascular dementia: a review. Ital J Neurol Sci. 1993;14:539–546. doi: 10.1007/BF02339212. [DOI] [PubMed] [Google Scholar]
- 42.Pasquini L, Scherr M, Tahmasian M, Meng C, Myers NE, Ortner M, Mühlau M, Kurz A, Förstl H, Zimmer C, Grimmer T, Wohlschläger AM, Riedl V, Sorg C. Link between hippocampus’ raised local and eased global intrinsic connectivity in AD. Alzheimers Dement. 2015;11:475–484. doi: 10.1016/j.jalz.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Pereira F, Mitchell T, Botvinick M. Machine learning classifiers and fMRI: a tutorial overview. Neuroimage. 2009;45:S199–209. doi: 10.1016/j.neuroimage.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 47.Pini L, Pievani M, Bocchetta M, Altomare D, Bosco P, Cavedo E, Galluzzi S, Marizzoni M, Frisoni GB. Brain atrophy in Alzheimer's disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Rashid B, Damaraju E, Pearlson GD, Calhoun VD. Dynamic connectivity states estimated from resting fMRI Identify differences among Schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci. 2014;8:897. doi: 10.3389/fnhum.2014.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rashid B, Arbabshirani MR, Damaraju E, Cetin MS, Miller R, Pearlson GD, Calhoun VD. Classification of schizophrenia and bipolar patients using static and dynamic resting-state fMRI brain connectivity. Neuroimage. 2016;134:645–657. doi: 10.1016/j.neuroimage.2016.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Pérez R, Fernández L, Marco S. Overoptimism in cross-validation when using partial least squares-discriminant analysis for omics data: a systematic study. Anal Bioanal Chem. 2018;410:5981–5992. doi: 10.1007/s00216-018-1217-1. [DOI] [PubMed] [Google Scholar]
- 51.Schultz AP, Chhatwal JP, Hedden T, Mormino EC, Hanseeuw BJ, Sepulcre J, Huijbers W, LaPoint M, Buckley RF, Johnson KA, Sperling RA. Phases of hyperconnectivity and hypoconnectivity in the default mode and salience networks track with amyloid and tau in clinically normal individuals. J Neurosci. 2017;37:4323–4331. doi: 10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skouras S, Falcon C, Tucholka A, Rami L, Sanchez-Valle R, Lladó A, Gispert JD, Molinuevo JL. Mechanisms of functional compensation, delineated by eigenvector centrality mapping, across the pathophysiological continuum of Alzheimer's disease. Neuroimage Clin. 2019;22:101777. doi: 10.1016/j.nicl.2019.101777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 54.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki H, Venkataraman AV, Bai W, Guitton F, Guo Y, Dehghan A, Matthews PM. Associations of regional brain structural differences with aging, modifiable risk factors for dementia, and cognitive performance. JAMA Netw Open. 2019;2:e1917257. doi: 10.1001/jamanetworkopen.2019.17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiwari S, Atluri V, Kaushik A, Yndart A, Nair M. Alzheimer's disease: pathogenesis, diagnostics, and therapeutics. Int J Nanomedicine. 2019;14:5541–5554. doi: 10.2147/IJN.S200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 58.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 59.Vidaurre D, Smith SM, Woolrich MW. Brain network dynamics are hierarchically organized in time. Proc Natl Acad Sci U S A. 2017;114:12827–12832. doi: 10.1073/pnas.1705120114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Villemagne VL, Doré V, Burnham SC, Masters CL, Rowe CC. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat Rev Neurol. 2018;14:225–236. doi: 10.1038/nrneurol.2018.9. [DOI] [PubMed] [Google Scholar]
- 61.von Elm E, Altman DG, Egger M, Pocock SJ, Gtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies? PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, He C, Wang Z, Zhang Z, Xie C. Dynamic connectivity alteration facilitates cognitive decline in Alzheimer's disease spectrum. Brain Connect. 2021;11:213–224. doi: 10.1089/brain.2020.0823. [DOI] [PubMed] [Google Scholar]
- 63.Wenk GL. Neuropathologic changes in Alzheimer's disease. J Clin Psychiatry 64 Suppl. 2003;9:7–10. [PubMed] [Google Scholar]
- 64.Xing XX, Zheng MX, Hua XY, Ma SJ, Ma ZZ, Xu JG. Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging. Neural Regen Res. 2021;16:388–393. doi: 10.4103/1673-5374.290912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang S, Meng Y, Li J, Fan YS, Du L, Chen H, Liao W. Temporal dynamic changes of intrinsic brain activity in schizophrenia with cigarette smoking. Schizophr Res. 2019;210:66–72. doi: 10.1016/j.schres.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 66.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zendehboudi A, Baseer MA, Saidur R. Application of support vector machine models for forecasting solar and wind energy resources: A review. J Clean Prod. 2018;199:272–285. [Google Scholar]
- 68.Zeng Q, Luo X, Li K, Wang S, Zhang R, Hong H, Huang P, Jiaerken Y, Xu X, Xu J, Wang C, Zhou J, Zhang M. Distinct spontaneous brain activity patterns in different biologically-defined Alzheimer's disease cognitive stage: a preliminary study. Front Aging Neurosci. 2019;11:350. doi: 10.3389/fnagi.2019.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao T, Wang D, Hu Y, Zhang N, Zang T, Wang Y. Identifying Alzheimer's disease-related miRNA based on semi-clustering. Curr Gene Ther. 2019;19:216–223. doi: 10.2174/1566523219666190924113737. [DOI] [PubMed] [Google Scholar]
- 70.Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


