Abstract
Retinal degeneration is a debilitating ocular complication characterized by the progressive loss of photoreceptors and other retinal neurons, which are caused by a group of retinal diseases affecting various age groups, and increasingly prevalent in the elderly. Age-related macular degeneration, diabetic retinopathy and glaucoma are among the most common complex degenerative retinal disorders, posing significant public health problems worldwide largely due to the aging society and the lack of effective therapeutics. Whilst pathoetiologies vary, if left untreated, loss of retinal neurons can result in an acquired degeneration and ultimately severe visual impairment. Irrespective of underlined etiology, loss of neurons and supporting cells including retinal pigment epithelium, microvascular endothelium, and glia, converges as the common endpoint of retinal degeneration and therefore discovery or repurposing of therapies to protect retinal neurons directly or indirectly are under intensive investigation. This review overviews recent developments of potential neuroprotectants including neuropeptides, exosomes, mitochondrial-derived peptides, complement inhibitors, senolytics, autophagy enhancers and antioxidants either still experimentally or in clinical trials. Effective treatments that possess direct or indirect neuroprotective properties would significantly lift the burden of visual handicap.
Key Words: antioxidants, autophagy enhancers, complement inhibitors, exosomes, neuropeptides, neuroprotective agents, retinal degeneration, senolytics
Introduction
Retinal degeneration (RD) is a consequence of progressive, chronic neuroretinal disorders as a result of genetic mutations and/or environmental or inflammation/vascular or acquired degenerative pathology. All conditions may lead to partial or complete visual loss throughout life, and are more prevalent in the elderly (Cremers et al., 2020). Complex multifactorial acquired RD disorders include age-related macular degeneration (AMD) (Ammar et al., 2020), diabetic retinopathy (DR) (Wang and Lo, 2018) and glaucoma (Weinreb et al., 2014). Although these retinal diseases are associated with assorted pathophysiological mechanisms, they share some common causative or contributing factors, such as genetic polymorphisms associated with increased risk or severity of disease, oxidative stress, inflammation, metabolic perturbation, and cellular senescence (Weinreb et al., 2014; Copland et al., 2018; Sahajpal et al., 2019; Lee et al., 2021).
Neuroprotective agents are pharmacological or natural substances that are intended to prevent or slow down neuronal loss and neurodegeneration by combating inflammation, oxidative stress, and apoptosis (Boia et al., 2020). Treatments using neuroprotectants via oral, intravenous, intra-arterial or intra-muscular routes are used currently to mitigate symptoms and/or relieve the pain in neurological illnesses (Upadhyay, 2014). Despite the blossoming of recent progress in gene and cell therapies, the use of neuroprotective agents remains a front-line approach for a spectrum of neurodegenerative diseases, including RD disorders (Wubben et al., 2019). The approved neuroprotectants have documented pharmacokinetics, bioactivities and demonstrate minimal adverse effects, facilitating long-term administration and compliance that are normally required for the treatment of chronic neurological diseases (Mikitsh and Chacko, 2014).
In this review, we overview therapeutic targets for the major acquired degenerative retinal diseases: AMD, DR and glaucoma. We highlight the development of novel neuroprotective agents being assessed either experimentally or in clinical trials.
Search Strategy and Selection Criteria
Studies cited in this review published from 2000 to 2021 were searched by medical and science databases, PubMed and Web of Science, using the following keywords “neuropeptides in retina”, “exosomes in retina”, “mitochondrial-derived peptides in retina”, “complement in retina”, “senescence in retina”, “oxidative stress in retina”, “autophagy in retina”, “resveratrol in retina”, “cannabis in retina” for search strategy.
Main Etiologies of Degenerative Retinal Diseases
AMD is currently the leading cause of blindness in people aged 65 and over (Wong et al., 2014). With increasing life expectancy, AMD has become a major public health challenge as the global burden is projected to reach 288 million people by 2040 (Datta et al., 2017). AMD is a progressive, polygenic and multifactorial disease of complex etiology. Characterized by drusen deposits, atrophy of the retinal pigment epithelium (RPE) and photoreceptor loss, AMD can progress to visual loss via acute wet or neovascular AMD (nAMD) or, in the majority of patients, an insidious geographic atrophy (aAMD). With age as the primary risk factor for AMD, the interplays between susceptible genes associated with complement activation (Heesterbeek et al., 2020), lipid metabolism, endocytosis and extracellular matrix organization, and environmental risk factors, particularly smoking and high-fat diet (García-Layana et al., 2017), conspire to initiate and accelerate the development of AMD. Mechanisms operating in AMD progression encompass changes in mitochondrial functions (Kaarniranta et al., 2020), elevated oxidative stress (Ruan et al., 2020), dysregulated microglia/macrophage responses (Alves et al., 2020), and decline in retinal pigment epithelial cells, choroidal endothelial cells and photoreceptors (Ma et al., 2021). For example, altered mitochondria in the RPE serve as the main source of excessive reactive oxygen species (ROS), which induces oxidative stress-related damage to the RPE: a recognized early event in AMD progression. Consequently, cellular damage induced by ROS causes a decline in housekeeping autophagy, which activates immune responses and immune-mediated inflammation. The over-activation of inflammation further provokes damage in photoreceptors, ultimately leading to vision loss (Copland et al., 2018; Wang et al., 2019). Although it has been previously thought that the photoreceptors and RPE are two mostly affected cell types in AMD, recent advances in single-cell (sc) transcriptomic atlases of the human donor eyes have defined leading AMD risk genes associated with additional cell types, such as glia, vascular cells and microglia (Menon et al., 2019; Voigt et al., 2019). For example, C-X-C motif chemokine ligand 14, WAP four-disulfide core domain 1 and calcitonin related polypeptide beta are top differential expressed genes (DEGs) of macular RPE; tissue inhibitor of metalloproteinase 3 and complement factor I (CFI) are top DEGs of Müller glia; and transforming growth factor beta receptor 1 and complement 3 (C3) are top DEGs of microglia (Menon et al., 2019; Voigt et al., 2019). The immune, metabolic and tissue responses occurring in retinal degeneration involve the integrated function of different cell types and a combination of cell-type specific risk genes.
DR is responsible for most cases of visual loss in adults aged 20–74 years (Wang and Lo, 2018). By definition, the incidence of DR is directly linked to the prevalence of diabetes, which is on a sharp rise due to population ageing, obesity and an increase in metabolic syndrome in ever urbanized societies (Lee et al., 2015). Diabetes is highly heterogeneous and recently refined into five subgroups according to clinical variables (Thomas and Philipson, 2015; Ahlqvist et al., 2018). Group I, severe autoimmune diabetes, which largely overlap with type 1 diabetes and latent autoimmune diabetes in adults, is characterized by onset at a young age, poor metabolic control, impaired insulin production and the presence of glutamic acid decarboxylase antibodies. Severe autoimmune diabetes is associated with more than 40 gene markers including AIRE gene, FoxP3, HLA-DQB1 and many others (Erlich et al., 2008; Yi et al., 2018). Group II, severe insulin-deficient diabetes, includes individuals with high hemoglobin A1C, impaired insulin secretion and moderate insulin resistance. This group has the highest incidence of retinopathy. Group III, severe insulin-resistant diabetes, is characterized by obesity and severe insulin resistance and has the highest incidence of kidney damage. Group IV, mild obesity-related diabetes, includes obese patients who fall ill at a relatively young age. Group V, mild age-related diabetes, are the largest group consisting of the most elderly patients. Mild obesity-related diabetes and mild age-related diabetes are both associated with metabolic syndrome and lifestyle factors such as obesity, stress, and lack of physical activities (Thomas and Philipson, 2015; Ahlqvist et al., 2018). Regardless the types of diabetes, long-term cellular exposure to the high sugar level in uncontrolled diabetes is the primary cause for DR, acting via the polyol pathway, advanced glycation end products (AGEs) accumulation, the protein kinase C pathway and the hexosamine pathway (Brownlee, 2005). In particular, mitochondrial dysfunction in endothelial cells is present in hyperglycemic conditions, which is an important source of superoxide production in the retina (Du et al., 2000). The massive production of free radicals in mitochondria increases oxidative stress, which induces excessive local expression of pro-inflammatory mediators. As a consequence, such chronic, low-grade inflammation can influence the immune cell activation, extracellular glutamate accumulation, imbalance of local production of neurotrophic factors and upregulation of pro-apoptotic molecules such as caspase-3, caspase-8 and Bax (Silva et al., 2009). These aberrations observed in DR patients are considered to contribute to both the onset and development of the disease. Furthermore, increasing studies have demonstrated that DR is a developing complication secondary to disruptions in not only vascular cells, but neurons and microglia in the retina (Solomon et al., 2017). Declines in retinal ganglion cells (RGCs), microglia, Müller glia, astrocytes, endothelial cells and pericytes all cause dysfunction of neurovascular unit, leading to structural defect and functional disorder (Sacks et al., 2018; Yang et al., 2020).
Glaucoma can occur in people of all ages and is the most common cause of irreversible blindness worldwide, affecting more than 64 million people (Weinreb et al., 2014). Despite various initiating pathologies or disease subtypes to be determined, increased intraocular pressure (IOP) and optic neuropathy are the key pathogenic factors for the disease severity in most patients with different subtypes of either primary or secondary glaucomas (Lusthaus and Goldberg, 2019). The increased IOP causes compression of the axonal fibres and optic nerve, which results in the death of RGCs and optic nerve degeneration. Secondary to RGC damage, changes in cone photoreceptors, bipolar cells and horizontal cells, have been defined during the development of glaucoma (Kumar et al., 2021). Therefore, the neuronal defects happen in both inner and outer retina of glaucomatous eyes. Besides, activated microglia are observed to be recruited in the compressed lamina cribrosa surrounding blood vessels in glaucomatous eyes, which express various proinflammatory cytokines and mediators such as tumor necrosis factor-α (TNF-α), transforming growth factor-β and proliferating cell nuclear antigens (Yuan and Neufeld, 2001), indicating the involvement of inflammatory responses within glaucomatous retina. There are also other risk factors associated with glaucoma, including, and not exclusively perturbation in reactive glia, neurotrophic factor deprivation, pro-apoptotic signaling activation of neurotransmitters, excitotoxicity and oxidative stress (Levkovitch-Verbin, 2015). Besides the decline of nerve growth factor, other neurotrophic factors contribute to the progression of glaucoma (Chitranshi et al., 2018). Lack of neurotrophin is one reason for the RGCs’ death in glaucoma. Brain-derived neurotrophic factor has been identified as a protector for RGCs and the optic nerve (Dekeyster et al., 2015), and potential for treatment for glaucoma. Studies have detected over 70 single nucleotide polymorphisms associated with glaucoma (Abu-Amero et al., 2015; Khawaja et al., 2018). However, genetics studies of primary open angle glaucoma (POAG) also suggest that functional alteration arisen from a single inherited gene mutation is not sufficient to explain its pathogenesis, such as gene variants in neurotrophin 4, myocilin, optineurin (Chen et al., 2012). Therefore, it is essential to acknowledge that glaucoma ensues from an additive or interactive cooperation of multiple gene-linked molecular events. Nevertheless, a certain number of POAG patients still progress despite IOP control following treatment (Garway-Heath et al., 2015). Approximately 12% patients with glaucoma with reduced/controlled IOP, will inevitably become blind due to persistent progression of glaucoma over 20 years (Khatib and Martin, 2020). There remains, therefore, urgent demand for novel neuroprotection therapies.
Recent development of neuroprotectants for treating retinal degeneration
To date, several drugs, biologics and small molecules have been developed and tested for treatment of RD diseases. Figure 1 is a schematic diagram representing the main groups of neuroprotective agents and known corresponding molecular targets. Table 1 lists the neuroprotective agents that are under active or recently completed trials, registered on clinicaltrials.gov. These agents were categorized based on the mechanisms of drug action and disease targets, and the status of each clinical study is also described in Table 1. Notably, each class of agents may primarily target a specific pathway or have pleiotropic effects to enhance retinal neuron survival.
Figure 1.
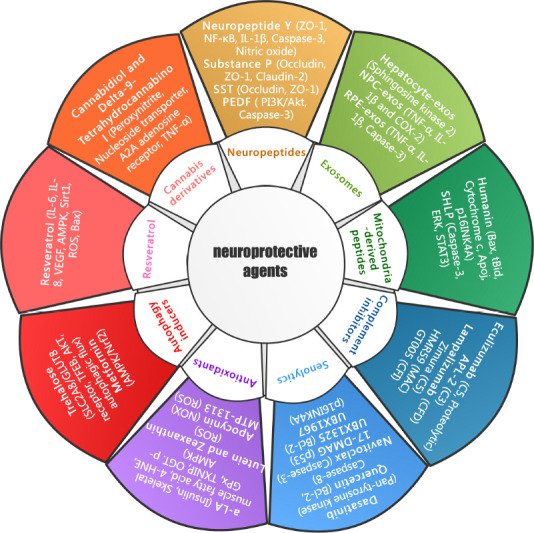
Schematic representation of neuroprotective agents and known therapeutic targets for treatment of degenerative retinal disorders.
Representative neuroprotectants classified according to salient characteristics or mechanisms of drug action, are listed around the inner circle. The major experimental or clinical drugs for each class of neuroprotectants and the corresponding molecular targets (bracketed) are indicated around the outer circle. 4-HNE: 4-Hydroxynonenal; a-LA: alpha-lipoic acid; AKT: protein kinase B; AMPK: adenosine 5‘-monophosphate (AMP)-activated protein kinase; APL-2: pegcetacoplan; BCL-2: B-cell lymphoma-2; GLUT: glutamate transporter 8; GPx: glutathione peroxidase; IL-1β: interleukin-1β; NADPH: nicotinamide adenine dinucleotide phosphate; NF-κB: nuclear factor-kappa B; NOX: NADPH oxidases; NPC: neural progenitor cells; OGT: O-linked N-acetylglucosamine transferase; PEDF: pigment epithelium-derived factor; PI3K: phosphatidylinositol 3 kinase; ROS: reactive oxygen species; RPE: retinal pigment epithelium; SHLP: small humanin-like peptides; Sirt-1: sirtuin 1; SST: somatostatin; TFEB: transcription factor EB; TNF-α: tumor necrosis factor-α; TXNIP: thioredoxin-interacting protein; VEGF: vascular endothelial growth factor; ZO-1: zonula occludens-1.
Table 1.
Current clinical trials of neuroprotectants for major degenerative retinal diseases
| Disease | NCT number | Drug name | Phase | Target classification | Mechanism | Current status |
|---|---|---|---|---|---|---|
| DR | NCT03452657 | Ranibizumab | Phase 3 | Angiogenesis | Ranibizumab combines with all VEGF-A subtypes to block cascade reactions. | Unknown |
| NCT04418427 | Aflibercept | Phase 2 | Aflibercept acts as a bait receptor to combine with VEGF-A, VEGF-B and PIGF dimer, inhibiting VEGF and PIGF at the same time. | Active, not recruiting | ||
| NCT01189461 | Pegaptanib | Phase3 | Pegaptanib mainly combines with VEGF-165 (isomer of VEGF-A), inhibits the binding of the isomer to VEGFR-2 and blocks the downstream pathway of VEGF-165, thus inhibits angiogenesis. | Completed (well tolerated with evidence of efficacy) | ||
| NCT00131144 | Octreotide | Phase 3 | Octreotide, a specific SST analogue, can effectively reduce cell death and VEGF overproduction induced by high glucose. | Completed (confirmed the safety profile of long-acting Octreotide) | ||
| NCT01702441 | Nesvacumab | Phase 1 Phase 2 |
Nesvacumab activates Tie-2 signaling and decreases vascular permeability by inhibiting Ang-2, an antagonist of Tie-2. | Completed (results pending) | ||
| NCT01702441 | SubcutaneousAKB-9778 | Phase 1 Phase 2 |
AKB-9778 activates Tie-2 signaling by inhibiting VE-PTP, a negative regulator of Tie-2. | Completed (results pending) | ||
| NCT02348918 | Luminate | Phase 2 | Luminate is an integrin inhibitor, which inhibits macular edema and improves visual acuity by blocking a variety of integrin receptors. | Completed (results pending) | ||
| NCT02511067 | Tocilizumab/Ranibizumab | Phase 2 | Inflammation | Tocilizumab blocks IL-6-emediated signaling by binding to both soluble and transmembrane IL-6 receptors. | Withdrawn | |
| NCT02314299 | MTP-131 | Phase 1 Phase 2 |
Oxidative stress | MTP-131 shows a protective effect on visual function in a diabetic mouse model by attenuating mitochondrial oxidative stress. | Completed (results pending) | |
| NCT02062034 | Ubiquinone (Q10) | Phase 2 | Coenzyme Q10 promotes RGC survival by modulating Bax and Bad protein expression and by preserving mitochondrial DNA content and mitochondrial transcription factor A/oxidative phosphorylation complex IV protein expression. | Completed (ubiquinone improves clinical outcomes and reduces oxidative stress without significant adverse events) | ||
| NCT01726075 | COLIRIOBCN070660 Placebo Brimonidine |
Phase 2 Phase 3 |
Neuropeptides | Somatostatin eye drops cause general excitation; it also increases the signal-to-noise ratio and leads to a shift in centre–surround balance towards a more dominant centre | Completed (results pending) | |
| NCT04537884 | UBX1325 | Phase 1 | Senolytics | Inhibiting the anti-apoptotic proteins BCL-2 and BCL-xL and selectively kill Senescent cells | Recruiting | |
| AMD | NCT01175395 | IBI-20089/Lucentis | Phase 1 Phase 2 |
Angiogenesis | IBI-20089/Lucentis combines with all VEGF-A subtypes to block cascade reactions. | Completed (Combination therapy IBI-20089 and ranibizumab was well-tolerated and resulted in fewer ranibizumab retreatments. Transient intraocular pressure elevation and cataract progression occurred) |
| NCT03668054 | Bevacizumab | Phase 3 | Bevacizumab combines with VEGF-A to reduce the binding of VEGF and VEGFR on endothelial cells, thus reducing the permeability of neovascularization. | Completed (well tolerated and resulted in a sustained response regarding VA improvement and CRT reduction) | ||
| NCT02727881 | Squalamine | Phase3 | Squalamine inhibits multiple angiogenic factors (VEGF, PDGF, and b-FGF). | Unknown | ||
| NCT02684578 | Metformin | Phase 2 | Autophagy | Metformin may protect the visual function of retinal degenerative mice through neuroprotective, anti-inflammatory and anti-apoptotic effects. | Recruiting | |
| NCT02247531 | Lampalizumab | Phase 3 | Complement | Antibody targeting complement factor D. | Terminated (lampalizumab did not reduce GA enlargement) | |
| NCT02686658 | Zimura | Phase 2 Phase 3 |
Aptamer targeting complement component 5. | Completed (results pending) | ||
| NCT02503332 | Pegcetacoplan (APL-2) | Phase 2 | Cyclic peptide inhibitor of complement component 3. | Completed (results pending) | ||
| NCT00935883 | Eculizumab | Phase 2 | Complement inhibition with Eculizumab to evaluate the effects of C5 inhibition on drusen and geographic atrophy | Completed (Systemic complement inhibition with eculizumab did not significantly reduce drusen volume) | ||
| NCT04756310 | Retilut | Not applicable | Resveratrol | Resveratrol inhibits NADPH oxidase-mediated production of ROS by down-regulating the expression and activity of the oxidase | Completed (results pending) | |
| Theavit | Binding to cardiolipin and protecting it from oxidation | Completed (results pending) | ||||
| NCT03891875 | Elamipretide | Phase 2 | Mitochondria | AdGVPEDF.11D is a replication deficient (E1, E3 and E4 deleted) adenovirus vector | Active, not recruiting | |
| NCT00109499 | AdGVPEDF.11D | Phase 1 | Angiogenesis | containing the gene for the PEDF protein, which has anti-angiogenic and neuroprotective efficacy. | Completed (results pending) | |
| Glaucoma | NCT00476138 | Epigallocatechin-gallate | Phase 1 Phase 2 |
Oxidative stress | By counteracting directly oxidative stress to RGC, increasing blood flow in the inner retina, counteracting glutamate toxicity, or by exerting an anti-inflammatory action on retinal tissue. | Unknown |
| NCT00626782 | Ranibizumab | Phase 2 Phase 3 |
Angiogenesis | Ranibizumab combines with all VEGF-A subtypes to block cascade reactions. | Completed (more patients in the ranibizumab group required additional glaucoma surgery during the study period) | |
| NCT00317577 | Brimonidine | Phase 2 | Autophagy | Brimonidine decreases RGC apoptosis upregulating EAAT1 and downregulating NMDA receptors. | Completed (Low-pressure glaucoma patients treated with brimonidine 0.2% who do not develop ocular allergy) | |
| NCT01254006 | Forskolin | Not applicable | Forskolin prevents RGC apoptosis induced by retinal ischemia/reperfusion by acting on the PI3K/Akt signaling pathway. | Completed (results pending) | ||
| NCT00404729 | Citicoline | Phase 4 | Metabolism | Citicoline acts as an intermediary in the synthesis of phosphatidylcholine through the activation of the biosynthesis of structural phospholipids in neuronal membranes, increases the metabolism of cerebral structures, inhibits phospholipid degradation and induces an increase in the levels of different neurotransmitters and neuromodulators, including noradrenaline in the Central Nervous System. | Completed (significantly improves retinal and cortical bioelectrical responses) | |
| NCT01408472 | CNTF | Phase 1 | Neurotrophic factor | Binding of CNTF to its receptor complex activates the JAK/STAT, MAPK/ERK, and PI3K/Akt signaling pathways. | Completed (results pending) | |
| Dry eye | NCT04213248 | Umbilical Mesenchymal Stem Cells derived Exosomes | Phase 1 Phase 2 |
Exosomes | Umbilical mesenchymal stem cells derived exosomes regulate the activity of intraocular immune cells. | Recruiting |
Ang-2: Angiopoitin 2; b-FGF: basic fibroblast growth factor; BCL-2: B-cell lymphoma-2; CNTF: ciliary neurotrophic factor; IL-6: interleukin-6; NADPH: nicotinamide adenine dinucleotide phosphate; NMDA: N-methyl-D-aspartic acid receptor; PDGF: platelet derived growth factor; PIGF: placental growth factor; RGC: retinal ganglion cell; ROS: reactive oxygen species; SST: somatostatin; VE-PTP: vascular endothelial protein tyrosine phosphatase; VEGF: vascular endothelial growth factor.
Angiogenesis inhibitors are the principal type of drugs currently being studied in trials (Table 1). Pathological angiogenesis is a recognized aspect involved in subtypes of RD disorders, including nAMD, proliferative diabetic retinopathy and neovascular glaucoma (Brownlee, 2005; Lorenz et al., 2017; Wang and Lo, 2018). Notwithstanding, limitation of anti-angiogenic strategies exists, mainly because of targeting small proportion of the patients or treating late in disease course. For example, VEGF-blocking medicines are effective for nAMD, but no licensed treatment is available for aAMD, which accounts for 80% patients with AMD (Ammar et al., 2020) and VEGF-blocking treatments have no impact on preventing aAMD progression. Proliferative diabetic retinopathy, the late stage of DR, while considered a microvascular condition, has other drivers e.g. generation of metabolic reactive intermediates, which promote neuronal and glial activation leading to secondary disruption in retinal microvasculature (Gábriel, 2013). Furthermore, anti-angiogenic preparations are an adjuvant therapy for neovascular glaucoma, but neovascular glaucoma is rare (3.9%) in all glaucoma cases (Lorenz et al., 2017).
Neuropeptides
To date, nearly 20 neuropeptides have been identified in the human retina. Neuropeptides are produced by neural elements (amacrine cells and RGC) and non-neural elements (Müller cells and the RPE), some of which are found to promote the development of RD, whereas others slow down or eliminate the progression of RD (Gábriel, 2013).
Somatostatin (SST) is a polypeptide that acts as an inhibitor of endocrine and exocrine hormone secretion in mammals. SST synthetic analogs are widely used in the clinic due to their longer half-life compared to native SST, as well as its significant antisecretory, antiproliferative and immunomodulatory activities. Human RPE contains abundant SST, while proliferative DR and DME patients have been found to have significantly low concentration of SST (Simó et al., 2002). Experimental studies have indicated that SST has an effect on DR by improving the integrity of the blood-retina barrier (Simó-Servat et al., 2018). There is a clinical trial of SST treating DR in progress (Table 1).
Neuropeptide Y (NPY), one of the most abundant peptides in the mammalian central nervous system (CNS), is present in wide-field amacrine cells and large human RGCs. In some species NPY is detected in non-neural elements too (Müller glia, endothelial cells and microglia (Alvaro et al., 2007). NPY is activated by glutamate through a purinergic paracrine mechanism, which in turn inhibits osmotic glial cell swelling, thus crucially influencing the volume homeostasis of the retina (Uckermann et al., 2006). The most important known physiological action of NPY on retinal neurons is that it inhibits the increase of intracellular Ca2+concentration in response to depolarizing stimuli through Y1, Y4 and Y5 receptors (Alvaro et al., 2009) and it inhibits adenylyl cyclase activity through Y2 receptors (Gábriel, 2013). Our recent in vivo studies have highlighted the inhibitory effects of NPY on pathological neovascularization and inflammation in the retina (Ou et al., 2020). In a Streptozotocin-induced rat DR model, hyperglycemic condition decreases the mRNA levels of NPY in the retina, as well as the protein levels of NPY and Y5 receptor (Santos-Carvalho et al., 2013). Intravitreous treatment with NPY increased retinal endothelial expression of ZO-1, accompanied by reduction of phosphorylated mitogen-activated protein kinase (MAPK) isoforms which contribute to the maintenance of vascular integrity under DR conditions (Takano et al., 2014). NPY treatment also regulates inflammatory responses in retinal microglia through inhibiting nuclear translocation of NF-κB, and production of inflammasome cytokine IL-1β and pro-inflammatory mediator nitric oxide (Ferreira et al., 2010). Moreover, NPY pretreatment can prevent N-methyl-D-aspartic acid receptor-induced retinal ganglion cell (RGC) apoptosis in the mouse glaucoma model (Alvaro et al., 2008). Through the inhibition of inflammation and neovascularization, NPY may impact AMD outcomes (Ou et al., 2020).
Substance P (SP) is a neuropeptide secreted by neurons and is involved in many biological processes, including vasodilatation, cell proliferation, anti-apoptosis and inflammatory regulation (D’Alessandro et al., 2014). There are detailed data demonstrating the circuitry of SP-positive amacrine cells in the primate retina. The SP-immunoreactive amacrine cell dendrites mostly target bipolar cell axon terminals and ganglion cell dendrites (Williams et al., 2016). SP is found to be decreased in the serum of type 1 diabetes patients, especially in those with diabetic neuropathy. Restoration of endogenous SP caused marked inhibition of the apoptosis and the activity of caspase-3 in the diabetic rats’ neurons (Troger et al., 2001; Yang et al., 2013). One of the therapeutic mechanisms of SP in treating RD has been delineated as the involvement of p38 MAPK signaling. p38 MAPK is activated by tumor necrosis factor α and interferon γ, leading to down-regulation of occludin, ZO-1 and claudin-2, which contributes to the increase in paracellular permeability in endothelial cells (Patrick et al., 2006). In our previous experiments, we found that both phosphorylated forms of MAPK (p38 and p44/42) were reduced upon SP treatment, alongside ZO-1 upregulation, supporting that SP inactivated MAPK to maintain vascular integrity (Ou et al., 2019).
Pigment epithelium-derived factor (PEDF) belongs to a serpin superfamily and naturally exists in assorted tissues, including the interphotoreceptor matrix of the eye (Michelis et al., 2021). However, the expression of PEDF was significantly reduced with degenerative retinal diseases, evidenced by decreased level in not only Bruch’s membrane and RPE of donor eyes with AMD and DR (Ogata et al., 2002; He et al., 2014), but also in the vitreous from eye of patients with AMD (Holekamp et al., 2002). Data have shown that PEDF has antiangiogenic, antioxidative, neurotrophic properties against neurodegeneration in the CNS as well as in the retina. For example, PEDF can preserve mitochondrial and barrier functions of RPE from oxidative stress, and promote retinal neuron survival against nutrient deprivation or stresses by suppressing apoptotic and inflammatory pathways through mediating PI3K/Akt signaling (Ho et al., 2006; He et al., 2014; Michelis et al., 2021). Given the therapeutic potential of PEDF in retinal neuroprotection, a Phase 1 trial using a replication-deficient adenovirus vector, Ad(GV)PEDF.11D, containing the human PEDF gene has been currently investigated for nAMD treatment (Table 1).
Exosomes
Exosomes are extracellular shuttling nanovesicles secreted from cells and packaged with diverse biomolecules cargo, such as miRNAs, mRNAs, lipids and various proteins to facilitate intercellular communication (Biasutto et al., 2013; Klingeborn et al., 2018). The role of exosomes in regulating angiogenesis, apoptosis, autophagy, and inflammation has been well established (Gurunathan et al., 2019). For example, hepatocyte-derived exosomes could transfer sphingosine kinase 2 to form sphingosine-1-phosphate in target hepatocytes, thus leading to cell proliferation and liver regeneration (Nojima et al., 2016). The fact that exosomes are released by various cell types in both normal and pathological conditions to transport nucleic acids, lipids and proteins between cells, makes exosomes an ideal drug and gene delivery carriers. In particular, exosomes possess edges over other carrier options because of their nano-scale size, low immunogenicity, long circulation time and biodegradability (Ortega et al., 2020).
Recently, the protective role of RPE-derived exosomes (RPE-Exos) in the retina has been recognized (Wang et al., 2021). Due to their minuscule dimensions and lipid membrane, RPE-Exos can readily pass across the retinal-blood barrier, making them ideal therapeutic substances to be delivered into retinal lesions (Wang et al., 2021). Subretinal delivery of RPE-Exos can benefit photoreceptor survival and enhance the retinal function in N-methyl-nitrosourea-induced RD models by suppressing inflammatory responses, prompting cell differentiation and inhibiting apoptotic cascades. Besides the protective role of RPE-Exos, studies also show that exosomes derived from mouse neural progenitor cells (NPC-exos) could be specifically internalized by retinal microglia, which protects photoreceptors from what-induced apoptosis both in vivo and in vitro. Mechanistically, RNA sequencing of microglia revealed a set of 17 miRNAs enriched in NPC-exos that inhibit inflammatory signaling pathways in microglia by decreasing the expression of TNF-α, IL-1β and COX-2 (Bian et al., 2020). Given the above, exosomes have the potential to become a therapy for RD.
Mitochondria-derived peptides
MDPs are peptides encoded by mitochondrial DNA (mtDNA) that serve as signals for organism cytoprotection and energy regulation (Yang et al., 2019b). Numerous mitochondria-derived peptides (MDPs) have been well-characterized in preclinical assessment in models of macular degeneration, including Humanin (HN), Small Humanin-Like Peptides (SHLP) and Mitochondrial Open Reading Frame of the 12S rRNA-c (Cobb et al., 2016).
HN, encoded from the 16S rRNA region of the mtDNA, is the first MDP discovered within the mammalian mitochondrial genome. In the polarized RPE, HN is predominantly expressed in the cytosol and largely localized in the mitochondria. It has been reported that exogenous HN can enter RPE cells, co-localize with BAX and block cell death. A recent study demonstrated that HN interacted with the membrane-bound Bax and tBid, prevented the recruitment of cytosolic Bax and its oligomerization on the mitochondrial outer membrane, and suppressed cytochrome c release and mitochondria-dependent apoptosis (Ma and Liu, 2018). Oxidative damage arising from intrinsic or extrinsic stress is a critical driver of cellular senescence that plays a central role in AMD progression. In an H2O2-induced human primary RPE senescence model, HN co-treatment significantly reduced the classical markers of senescence, such as senescence-associated β-Gal-positive cells, ApoJ transcripts, and p16INK4A expression (Sreekumar et al., 2016). The findings suggest that HN could be used to delay the progression of AMD (Minasyan et al., 2017).
Newly identified by silico prediction analyses, SHLPs 1–6 are peptides encoded by genes located within the same region of the 16S rRNA gene as HN. Among the biological effects of SHLPs, SHLP2 and SHLP3 were cytoprotective. As with HN, SHLP2 and SHLP3 promote cell viability and inhibit apoptosis in many cell lines cultured under serum-free conditions. Mechanistically, SHLP2 mediates neuroprotection via activation of ERK and STAT3 (Cobb et al., 2016). In a transmitochondrial cybrid cell model, in which the cybrid ARPE-19 cells contain identical nuclei but possess mitochondria from either AMD or age-matched normal subjects, SHLP2 treatment protected from cell apoptosis and oxidative damage of mtDNA in AMD cybrids (Ding et al., 2011; Nashine et al., 2018).
Complement inhibitors
Modulation of the complement system by targeting the regulating components is now the new focus on treatment modalities for AMD, DR and glaucoma (Kassa et al., 2019a). Genetic evidence of complement association in the pathogenesis of AMD was discovered in 2005, when a common variant in complement factor H (CFH) gene was noted to be associated with a 7.4 fold increased risk of developing AMD in individuals homozygous for the risk allele (Toomey et al., 2018). Several other genetic variants in complement genes, such as CFB/C2, C3, C5and CFI, have since been associated with AMD (Heesterbeek et al., 2020). Genome-wide association studies have revealed that of the many complement components, C3 and C5 play prominent parts within the complement cascade and have risen as the leading therapeutic targets in many inflammatory diseases, including AMD (Park et al., 2019; Heesterbeek et al., 2020). Activation of C3 ultimately leads to cleavage of C5 to form key terminal fragments (C5a and C5b). The C5a fragment, found in drusen of AMD patients, is an important inflammatory activator that induces VEGF expression from RPE cells (Liu et al., 2019). C5b leads to the formation of membrane attack complex (MAC) in humans. It has been indicated that small amounts of MAC are sub-lytic and protective during inflammation but when the amount of MAC reaches a certain level, MAC becomes lytic, which leads to membrane and cell apoptosis (Kumar-Singh, 2019). Deposition of MAC in Bruch’s membrane and choriocapillaris increases significantly with aging as well as in the AMD (Jaffe et al., 2021). In light of the critical role of complement activation in the pathogenesis of AMD, the importance of cell types, in particular the microglia, that express relevant complement receptors have been acknowledged (Madeira et al., 2015). Microglia constitute the retinal resident immune cell population essential for both tissue homeostasis and pathology (Alves et al., 2020). C5a receptor (C5aR) and C3aR in microglia/macrophages are required for the cell recruitment and tissue repair following insults, but dysregulated cell response and disturbed C5aR/C3aR activation have been demonstrated to induce inflammation-associated retinal diseases, including uveitis, choroidal neovascularization and macular damage (Nozaki et al., 2006; Zhang et al., 2016). Conversely, depletion of C5aR suppresses light exposure-induced microglia migration, deleterious inflammation and preserves retinal integrity in murine models (Nozaki et al., 2006; Song et al., 2017). Understanding of complement genetic risk has driven approaches to develop therapeutics in both dry and wet AMD, including inhibitors and gene therapies targeting complement pathway (Holz et al., 2018; Lee et al., 2021). A phase II study on APL-2 treatment, a C3 inhibitor, achieved a significantly retarded progression of geographic atrophy (GA, an advanced form of dry AMD) at 12 months by 29% compared with sham treatment when administered monthly, awaiting the validation in extensive phase III clinical trials (Kassa et al., 2019b) (Table 1). Eculizumab is a monoclonal antibody that binds to C5 and blocks its proteolytic activation (Rother et al., 2007). Approved for the treatment of paroxysmal nocturnal hemoglobinuria in 2007, eculizumab has become the first complement inhibitor available to patients. Although some complement inhibitors did not show tractable effects in clinical trials for GA, there are still ongoing trials that evaluate C3 inhibition (pegcetacoplan, Apellis Pharmaceuticals; NCT03525600, NCT03525613) and C5 inhibition (avacincaptad pegol, Iveric Bio; NCT04435366) as treatments for atrophic or neovascular AMD (Kim et al., 2020) (Table 1). The use of ocular gene therapy through targeting complement components has also been under phase I trials for aAMD, including GT005 that induces CFI expression and HMR59 that expresses C59 to prevent the formation of MAC (Lee et al., 2021).
In progressive DR subjects, the vitreous proteome studies have detected several complement proteins, such as C3, CFI, CFB, C4A, C4B, C2, C4BPA, CFD, and CFH (Loukovaara et al., 2015). A recent study identified a localized elevation of C3, especially the 110 kDa activated fragment C3bα’, and a concurrent upregulation of CFH along with activated microglial infiltration in the progressive DR vitreous (Shahulhameed et al., 2020). Notably, a disease course-dependent increase in microglial-mediated activation of the alternative complement pathway from the early to late DR suggests a clinical relevance of the alternative complement pathway as a possible biomarker for the disease progression (Xu and Chen, 2016).
Although the traditional glaucoma medication focuses on IOP reduction, recent focus has been targeted to immune-mediated processes and complement regulation of the ganglion cell dendritic tree (Williams et al., 2016). For example, increased expression of several complement factors (C5, C3 CHF) in retinal protein samples of glaucoma patients was noted (Gassel et al., 2020a; Hubens et al., 2021). The increased level of protein C3 was also detected in the aqueous humor of primary open angle glaucoma patients (Liu et al., 2020). Furthermore, deposition of C3 was detected in retina and optic nerve of glaucoma model before the death of retinal ganglion cell, and such damage could develop even without IOP elevation (Noristani et al., 2016). A study has demonstrated that intravitreal injection of C5 antibody preserves optic nerve in experimental glaucoma (Gassel et al., 2020b). C1q subunit, comprised of C1qa, C1qb and C1qc, is the initiator of the classical complement cascade. Studies indicated that C1qa deficiency prevented RGC loss in glaucoma and was protective in other neurodegenerative diseases, whereas C3 deficiency showed opposite effects (Kumari et al., 2015; Harder et al., 2017).
Senolytics
Recent experimental and clinical evidence reveals multiple roles of senescence in health and disease (Lee et al., 2021). Cellular senescence is an adaptive cell process in response to stress, initiated by the activation of tumor suppressor proteins such as p53/p21CIP1 and p16INK4A/retinoblastoma protein (RB). Recent data demonstrate that senescence can occur in both mitotic and postmitotic cells, heightened with an inflammatory secretome and altered cell metabolism. The senescent state is complex with an array of characteristics. The most identified markers of cellular senescence are p21CIP1, p53, p16INK4A, chromatin and mitochondrial DNA modifications, increased senescence-associated β-galactosidase (SA-β-gal) activity, and an induction of senescence-associated secretory phenotype (SASP) (Sreekumar et al., 2020).
Aging has always been considered to be closely related to AMD. Detection of senescent RPE cells in human AMD donor eyes and old non-human primate eyes has been confirmed (Chaum et al., 2015). In addition to RPE cells, retinal neurons, choroidal endothelial cells and retinal microglia also demonstrate senescence in association with the retinal ageing and pathogenesis of AMD (Damani et al., 2011; Cabrera et al., 2016). Moreover, the protein levels of senescence markers, including p16INK4A, p21CIP1 and p53, were increased in aged human RPE from donors. Aβ has been found to be a component of drusen and also increased in aging retina, which suggests that Aβ could be an important factor in development of AMD. Recent studies demonstrated that Aβ peptide-induced RPE senescence in RD by increasing senescence markers (Liu et al., 2015). Glaucoma is more frequent with age, and there is an age-related decrease in the anterior segment outflow that induces elevated IOP and subsequent death in the RGCs. The expression of SA-β-gal, a well-studied marker for cellular senescence, has been proved to be elevated in the anterior segments from POAG donors (Liton et al., 2005). Preclinical studies also demonstrate that cellular senescence is a contributor to glaucoma development (El-Nimri et al., 2020). Furthermore, senescent (p16INK4A-expressing) cells accumulate in the retina of DR patients and during peak destructive neovascularization in a murine DR model (Crespo-Garcia et al., 2021).
Teasing apart the distinct mechanisms of cellular senescence from physiological aging has driven the development of therapeutic approaches that target chronic senescence for the treatment of age-related diseases. Senescent cells can be selectively eliminated pharmacologically using senolytic, such as a cocktail of dasatinib, a pan-tyrosine kinase inhibitor, and quercetin, a naturally occurring flavonoid (Zhu et al., 2015), or genetically inducing cell suicide via caspase 8 by gene transfection. So far, several senolytics, including dasatinib plus quercetin, navitoclax and 17-DMAG and a peptide that targets the Bcl-2- and p53-related anti-apoptotic pathways, have been demonstrated to be effective in reducing senescent cell burden in mice. UBX1325, an inhibitor of Bcl-2 protein (BCL-xL), is currently under phase I trial for diabetic macular edema, also with ongoing evaluation for AMD (Lee et al., 2021) (Table 1). Interestingly, a recent study demonstrates promising senolytic effects of another BCL-xL inhibitor, UBX1967, which can eliminate senescent endothelial cells and promotes vascular repair in a mouse model of retinopathy (Crespo-Garcia et al., 2021).
Antioxidants
The retina represents one of the highest oxygen-consuming tissues in the human body. During ageing, the retina suffers from a low-grade oxidative stress caused by environmental factors such as continuous exposure to light, intensive oxygen metabolism, and the presence of photosensitizers. The oxidative insult sustains for decades and increases in level with advancing age. Increased levels of ROS generated by chronic oxidative stress may exceed the anti-oxidation capability of the retina and lead to modification of biological macromolecules and damage of the cells (Khandhadia and Lotery, 2010).
In AMD, oxidative stress works in concert with other risk factors, such as ageing, smoking, phototoxicity, and genetic factors, leading to sub-RPE drusen deposition, RPE/photoreceptor cell death, and the resultant inflammatory and immune responses (Hanus et al., 2015). These processes may aggravate oxidative stress and inflammation, forming a vicious cycle propelling AMD pathogenesis. Antioxidant supplements and ROS scavengers have been proposed as potential therapies for assorted AMD. Nutritional supplementation (lutein, zeaxanthin, and polyunsaturated fatty acids) with antioxidants and micronutrients can effectively reduce the progression toward advanced forms of AMD (Bonds et al., 2014).
The hyperglycemic microenvironment in diabetes mellitus can promote a nonenzymatic binding of glucose to macromolecules (amino acids in proteins, lipids, and nucleic acids), forming AGEs (Sahajpal et al., 2019), a group of highly reactive compounds prevalent in diabetic vasculature. AGEs bind to their receptors, known as RAGE (Receptor for AGE), and induce the translocation of NF-κB with disorders in endothelial cell function (Stitt, 2010). NADPH oxidase is a main enzymatic source of ROS and is directly related with promoting pathological neovascularization in the retina by hyperglycaemia. The development of specific NADPH oxidase inhibitors, such as diphenyleneiodonium and apocynin, may improve DR treatment (Peng et al., 2019). Another option for DR treatment is alpha-lipoic acid (a-LA), a potent antioxidant, which improves insulin sensitivity and skeletal muscle fatty acid oxidation by activating AMP-activated protein kinase (AMPK) in diabetic patients. A recent study has shown that a-LA lowered levels of 4-HNE, which recognizes ROS modified proteins, and increased GPx levels in the retinas of diabetic mice and retinal cells treated with high glucose, showing its potential antioxidant action (Rochette et al., 2015). More importantly, a-LA inversely regulates p-AMPK and OGT or TXNIP levels, as well as protecting neuronal cells against cell death in the GCL of the diabetic retinas (Kim et al., 2018). These findings suggest that a-LA may be classified as one of the candidate molecules for prevention or suppress oxidative stress in the development of DR, though further investigations are required. MTP-131, a novel mitochondria-targeted peptide, has been identified to reduce ROS reduction and prevents oxidative damage in neuronal cell lines. Recent studies have demonstrated that MTP-131 has protective effect against oxidative damage in retinal ganglion cells(Chen et al., 2017). MTP-131 is now under phase I clinical trial for more evaluation on treating DR (Table 1).
Autophagy inducers
Autophagy is a highly conserved cellular process that delivers damaged organelles and aggregate-prone molecules to autophagosomes for subsequent lysosomal catabolism, which plays a central part in cellular and energy homoeostasis and disease (Barbosa et al., 2018). In the eye, autophagy is highly active in the RPE, and increasing evidence suggests that impaired autophagy is associated with altered proteostasis, lipofuscin accumulation, metabolic disruption and increased oxidative stress (Liu et al., 2016; Golestaneh et al., 2017). Strategies counteracting oxidative stress and enhancing autophagy have attempted in AMD treatment (Yang et al., 2019a). Autophagy is regulated by mTOR or AMPK-dependent pathways that are amenable to chemical perturbations (Menzies et al., 2017). Among the molecular compounds that regulate autophagic activities, trehalose and metformin are the most well-studied (Hernández-Zimbrón et al., 2018).
Trehalose is a disaccharide of glucose and a food constituent produced by different organisms, executing its natural role as an energy source to withstand environmental stress by maintaining cellular integrity, especially cell membranes (Lee et al., 2018). Trehalose enhances autophagy by activation of AMPK, largely through inhibition of SLC2A8/GLUT8 receptor (DeBosch et al., 2016; Mayer et al., 2016). Additionally, trehalose activates TFEB (transcription factor EB) through inhibition of AKT, increases autophagic flux and slows down DR progression (Palmieri et al., 2017; Lotfi et al., 2018).
The first-line medication for type-2 diabetes, metformin has shown a protective effect on RD, including DR (Han et al., 2018), glaucoma (Li et al., 2020) and AMD (Qu et al., 2020). Current research has shown that eye drops of metformin prevent fibrosis after glaucoma filtration surgery in rats via activating AMPK/Nrf2 signaling pathway (Li et al., 2020). A clinical trial is currently underway to evaluate the safety and efficacy of metformin in decreasing GA progression in a small group of non-diabetic patients with dry AMD (ClinicalTrials.gov Identifier: NCT02684578). Future studies are required to investigate the effects of these repurposing drugs, including trehalose and metformin on RD in large-scale multicenter clinical trials (Table 1).
Resveratrol
Resveratrol is a biologically active stilbenoid and a plant polyphenolic compound commonly found in grape (Vitis vinifera) skin and seeds. Beyond its initial use in cancer therapies due to its regulation in the inflammatory response and multiple ageing-related biological processes (de la Lastra and Villegas, 2005), resveratrol displayed benefits to attenuate the progression of DR (Huang et al., 2020). Resveratrol not only directly prevents hyperglycemia-induced inflammation and gap junction degradation in the retina by inhibiting the expression of IL-6, IL-8 and VEGF in the RPE (Josifovska et al., 2020), but also suppresses the NF-κB- controlled inflammation by increasing AMPK activity via Sirt-1 activation in the mouse model of DR (Kubota et al., 2011). In addition, ex-vivo experiments have shown that treatment with resveratrol improves cell viability and decreases ROS generation in RPE-derived cells from the nAMD patients (Chan et al., 2015). In the ischemia-reperfusion injury of glaucoma model in mice, resveratrol prevents RGC death by blocking the Bax-caspase-3-dependent apoptotic pathway and suppressing gliosis-related inflammation in the retina (Luo et al., 2018). So far, the findings suggest that resveratrol has the therapeutic potential for RD, though more studies are required (Table 1).
Cannabis derivatives
Recently, a chemical extracted from the Cannabis sativa plant, cannabidiol (CBD), has emerged as a potent protective agent for the retina, where cannabinoid receptors CB1 and CB2 are present (Zantut et al., 2020). Their endogenous counterparts, including endocannabinoid, the binding receptors and their metabolic enzymes, together constitute the endocannabinoid system in the retina. The balanced composition of this system plays important neuroprotective and neuro-regenerative roles upon intrinsic or extrinsic insults. In addition to the expression of both CB1 and CB2 receptors in human RPE cells, CB1 receptors are also expressed in the outer segments of photoreceptors, the inner plexiform layer, outer plexiform layer, inner nuclear layer and ganglion cell layer. The two primary endocannabinoid ligands, 2-arachidonoylglycerol (2-AG) and anandamide, are also detected in the human retina (Matias et al., 2006).
Studies across different species including human have demonstrated the therapeutic effect of CBDs in lowing the intraocular pressure in glaucoma, as well as in the cell protection of both glaucoma and DR (Rapino et al., 2018). CBD has also been used in clinic, it has been shown that acute cannabis intoxication improves nighttime vision and scotopic sensitivity in glaucoma patients (Chen et al., 2005). In an NMDA excitotoxic rat model, Delta-9-Tetrahydrocannabinol and CBD are found to protect the retina by decreasing peroxynitrite levels and oxidative stress-related substances in neurons (El-Remessy et al., 2003). CBD blocks retinal inflammation by equilibrating nucleoside transporter and A2A adenosine receptor and suppresses lipopolysaccharide-induced TNF-α release (Liou et al., 2008). CBD treatment also reduces neurotoxicity, inflammation, and blood-retinal barrier breakdown in diabetic animals by inhibiting p38-MAPK (El-Remessy et al., 2006). However, the potential benefit of ocular hypotensive and neuroprotective effects of CBD are curtailed because of possible systemic and ophthalmic side effects (Wang and Danesh-Meyer, 2021).
Conclusion
RD progresses through perturbation in multiple pathways including cellular metabolism, oxidative stress, cellular senescence, autophagy housekeeping and associated inflammatory responses both locally and systemically. As such these pathways are prime targets for developing ultimately neuroprotective therapies, despite challenges exist en route to prevention and delaying of the diseases leading to RD. Firstly, while we cautiously hail the enrichment of our arsenal of strategies combating RD, considering the cell diversity, genetic complexity and convergence of affected pathways, it may be that an identification of a cross-regulatory drug or a portfolio consisting of poly-drugs in low doses of each, together with gene or cell therapies, could create improved outcomes for RD and other age-associated diseases. Secondly, under certain stresses, the different types of neurons, endothelial cells and resident macrophages in the retina may not necessarily change in a synchronized way. The heterogeneity can be reflected by the diverse contribution of cell subgroups to the course of degeneration. In light of that, regimes accurately matching the tempo-spatial aspects of the degenerative program in the eye should be carefully designed. Thirdly, similar to other neurological disorders, RD diseases are typical chronic conditions, drug treatment usually requires long term and frequent administration. Drug accessibility crossing the blood-retinal barrier is an issue requiring special addressing. Therefore, coupling therapy with an efficient drug delivery is essential to ensure bioavailability and selective targeting, as well as controlled drug release with minimized dosing frequency. For instance, a single or cargo of small molecules targeting particular cell types, such as photoreceptors and vascular cells, could be encapsulated into or conjugated with nanoparticle or nanoscale PEGylated lysosomes to enable intraocular and even intracellular targeting (Gahlaut et al., 2015; Himawan et al., 2019).
Acknowledgments:
The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Abu-Amero K, Kondkar AA, Chalam KV. An updated review on the genetics of primary open angle glaucoma. Int J Mol Sci. 2015;16:28886–28911. doi: 10.3390/ijms161226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P, Wessman Y, Shaat N, Spégel P, Mulder H, Lindholm E, Melander O, Hansson O, Malmqvist U, Lernmark Å, Lahti K, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 3.Alvaro AR, Rosmaninho-Salgado J, Ambrósio AF, Cavadas C. Neuropeptide Y inhibits [Ca2+]i changes in rat retinal neurons through NPY, Y1, Y4 and Y5 receptors. J Neurochem. 2009;109:1508–1515. doi: 10.1111/j.1471-4159.2009.06079.x. [DOI] [PubMed] [Google Scholar]
- 4.Alvaro AR, Martins J, Costa AC, Fernandes E, Carvalho F, Ambrósio AF, Cavadas C. Neuropeptide Y protects retinal neural cells against cell death induced by ecstasy. Neuroscience. 2008;152:97–105. doi: 10.1016/j.neuroscience.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Alvaro AR, Rosmaninho-Salgado J, Santiago AR, Martins J, Aveleira C, Santos PF, Pereira T, Gouveia D, Carvalho AL, Grouzmann E, Ambrósio AF, Cavadas C. NPY in rat retina is present in neurons in endothelial cells and also in microglial and Müller cells. Neurochem Int. 2007;50:757–763. doi: 10.1016/j.neuint.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Alves CH, Fernandes R, Santiago AR, Ambrósio AF. Microglia contribution to the regulation of the retinal and choroidal vasculature in age-related macular degeneration. Cells. 2020;9:1217. doi: 10.3390/cells9051217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammar MJ, Hsu J, Chiang A, Ho AC, Regillo CD. Age-related macular degeneration therapy: a review. Curr Opin Ophthalmol. 2020;31:215–221. doi: 10.1097/ICU.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 8.Barbosa MC, Grosso RA, Fader CM. Hallmarks of aging: an autophagic perspective. Front Endocrinol (Lausanne) 2018;9:790. doi: 10.3389/fendo.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bian B, Zhao C, He X, Gong Y, Ren C, Ge L, Zeng Y, Li Q, Chen M, Weng C, He J, Fang Y, Xu H, Yin ZQ. Exosomes derived from neural progenitor cells preserve photoreceptors during retinal degeneration by inactivating microglia. J Extracell Vesicles. 2020;9:1748931. doi: 10.1080/20013078.2020.1748931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biasutto L, Chiechi A, Couch R, Liotta LA, Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp Cell Res. 2013;319:2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boia R, Ruzafa N, Aires ID, Pereiro X, Ambrósio AF, Vecino E, Santiago AR. Neuroprotective strategies for retinal ganglion cell degeneration: current status and challenges Ahead. Int J Mol Sci. 2020;21:2262. doi: 10.3390/ijms21072262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, Clemons TE, Fine LJ, Chew EY. Effect of long-chain ω-3 fatty acids and lutein + zeaxanthin supplements on cardiovascular outcomes: results of the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. 2014;174:763–771. doi: 10.1001/jamainternmed.2014.328. [DOI] [PubMed] [Google Scholar]
- 13.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 14.Cabrera AP, Bhaskaran A, Xu J, Yang X, Scott HA, Mohideen U, Ghosh K. Senescence increases choroidal endothelial stiffness and susceptibility to complement injury: implications for choriocapillaris loss in AMD. Invest Ophthalmol Vis Sci. 2016;57:5910–5918. doi: 10.1167/iovs.16-19727. [DOI] [PubMed] [Google Scholar]
- 15.Chan CM, Huang CH, Li HJ, Hsiao CY, Su CC, Lee PL, Hung CF. Protective effects of resveratrol against UVA-induced damage in ARPE19 cells. Int J Mol Sci. 2015;16:5789–5802. doi: 10.3390/ijms16035789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaum E, Winborn CS, Bhattacharya S. Genomic regulation of senescence and innate immunity signaling in the retinal pigment epithelium. Mamm Genome. 2015;26:210–221. doi: 10.1007/s00335-015-9568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Matias I, Dinh T, Lu T, Venezia S, Nieves A, Woodward DF, Di Marzo V. Finding of endocannabinoids in human eye tissues: implications for glaucoma. Biochem Biophys Res Commun. 2005;330:1062–1067. doi: 10.1016/j.bbrc.2005.03.095. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Ng T, Fan A, Leung D, Zhang M, Wang N, Zheng Y, Liang X, Chiang S, Tam P, Pang C. Evaluation of NTF4 as a causative gene for primary open-angle glaucoma. Mol Vis. 2012;18:1763–1772. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Liu B, Ma J, Ge J, Wang K. Protective effect of mitochondria targeted peptide MTP 131 against oxidative stress induced apoptosis in RGC 5 cells. Mol Med Rep. 2017;15:2179–2185. doi: 10.3892/mmr.2017.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chitranshi N, Dheer Y, Abbasi M, You Y, Graham SL, Gupta V. Glaucoma pathogenesis and neurotrophins: focus on the molecular and genetic basis for therapeutic prospects. Curr Neuropharmacol. 2018;16:1018–1035. doi: 10.2174/1570159X16666180419121247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cobb LJ, Lee C, Xiao J, Yen K, Wong RG, Nakamura HK, Mehta HH, Gao Q, Ashur C, Huffman DM, Wan J, Muzumdar R, Barzilai N, Cohen P. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis insulin sensitivity and inflammatory markers. Aging. 2016a;8:796–809. doi: 10.18632/aging.100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Copland DA, Theodoropoulou S, Liu J, Dick AD. A perspective of AMD through the eyes of immunology. Invest Ophthalmol Vis Sci. 2018;59:Amd83–amd92. doi: 10.1167/iovs.18-23893. [DOI] [PubMed] [Google Scholar]
- 23.Cremers FPM, Lee W, Collin RWJ, Allikmets R. Clinical spectrum genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog Retin Eye Res. 2020;79:100861. doi: 10.1016/j.preteyeres.2020.100861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crespo-Garcia S, et al. Pathological angiogenesis in retinopathy engages cellular senescence and is amenable to therapeutic elimination via BCL-xL inhibition. Cell Metab. 2021;33:818–832. doi: 10.1016/j.cmet.2021.01.011. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro A, Cervia D, Catalani E, Gevi F, Zolla L, Casini G. Protective effects of the neuropeptides PACAP substance P and the somatostatin analogue octreotide in retinal ischemia: a metabolomic analysis. Mol Biosyst. 2014;10:1290–1304. doi: 10.1039/c3mb70362b. [DOI] [PubMed] [Google Scholar]
- 26.Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin Eye Res. 2017;60:201–218. doi: 10.1016/j.preteyeres.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Proc Nutr Soc. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 29.DeBosch BJ, Heitmeier MR, Mayer AL, Higgins CB, Crowley JR, Kraft TE, Chi M, Newberry EP, Chen Z, Finck BN, Davidson NO, Yarasheski KE, Hruz PW, Moley KH. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci Signal. 2016;9:ra21. doi: 10.1126/scisignal.aac5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dekeyster E, Geeraerts E, Buyens T, Van den Haute C, Baekelandt V, De Groef L, Salinas-Navarro M, Moons L. Tackling glaucoma from within the brain: an unfortunate interplay of BDNF and TrkB. PLoS One. 2015;10:e0142067. doi: 10.1371/journal.pone.0142067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M, Mace BE, Sullivan P, Jamison JA, Kelly U, Harrabi O, Bollini SS, Dilley J, Kobayashi D, Kuang B, Li W, Pons J, Lin JC, Bowes Rickman C. Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2011;108:E279–287. doi: 10.1073/pnas.1100901108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–12226. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Nimri NW, Moore SM, Zangwill LM, Proudfoot JA, Weinreb RN, Skowronska-Krawczyk D, Baxter SL. Evaluating the neuroprotective impact of senolytic drugs on human vision. Sci Rep. 2020;10:21752. doi: 10.1038/s41598-020-78802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, Caldwell RB, Caldwell RW, Green K, Liou GI. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Remessy AB, Al-Shabrawey M, Khalifa Y, Tsai NT, Caldwell RB, Liou GI. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am J Pathol. 2006;168:235–244. doi: 10.2353/ajpath.2006.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the type 1 diabetes genetics consortium families. Diabetes. 2008;57:1084–1092. doi: 10.2337/db07-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferreira R, Xapelli S, Santos T, Silva AP, Cristóvão A, Cortes L, Malva JO. Neuropeptide Y modulation of interleukin-1{beta} (IL-1{beta})-induced nitric oxide production in microglia. J Biol Chem. 2010;285:41921–41934. doi: 10.1074/jbc.M110.164020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gábriel R. Neuropeptides and diabetic retinopathy. Br J Clin Pharmacol. 2013;75:1189–1201. doi: 10.1111/bcp.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gahlaut N, Suarez S, Uddin MI, Gordon AY, Evans SM, Jayagopal A. Nanoengineering of therapeutics for retinal vascular disease. Eur J Pharm Biopharm. 2015;95:323–330. doi: 10.1016/j.ejpb.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Layana A, Cabrera-López F, García-Arumí J, Arias-Barquet L, Ruiz-Moreno JM. Early and intermediate age-related macular degeneration: update and clinical review. Clin Interv Aging. 2017;12:1579–1587. doi: 10.2147/CIA.S142685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garway-Heath DF, Crabb DP, Bunce C, Lascaratos G, Amalfitano F, Anand N, Azuara-Blanco A, Bourne RR, Broadway DC, Cunliffe IA, Diamond JP, Fraser SG, Ho TA, Martin KR, McNaught AI, Negi A, Patel K, Russell RA, Shah A, Spry PG, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295–1304. doi: 10.1016/S0140-6736(14)62111-5. [DOI] [PubMed] [Google Scholar]
- 42.Gassel C, Reinehr S, Gomes S, Dick H, Joachim S. Preservation of optic nerve structure by complement inhibition in experimental glaucoma. Cell Tissue Res. 2020a;382:293–306. doi: 10.1007/s00441-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gassel CJ, Reinehr S, Gomes SC, Dick HB, Joachim SC. Preservation of optic nerve structure by complement inhibition in experimental glaucoma. Cell Tissue Res. 2020b;382:293–306. doi: 10.1007/s00441-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golestaneh N, Chu Y, Xiao YY, Stoleru GL, Theos AC. Dysfunctional autophagy in RPE a contributing factor in age-related macular degeneration. Cell Death Dis. 2017;8:e2537. doi: 10.1038/cddis.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation characterization biological function and multifarious therapeutic approaches of Exosomes. Cells. 2019;8:307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han J, Li Y, Liu X, Zhou T, Sun H, Edwards P, Gao H, Yu F, Qiao X. Metformin suppresses retinal angiogenesis and inflammation in vitro and in vivo. PLoS One. 2018;13:e0193031. doi: 10.1371/journal.pone.0193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanus J, Anderson C, Wang S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev. 2015;24:286–298. doi: 10.1016/j.arr.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harder JM, Braine CE, Williams PA, Zhu X, MacNicoll KH, Sousa GL, Buchanan RA, Smith RS, Libby RT, Howell GR, John SWM. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U S A. 2017;114:E3839–e3848. doi: 10.1073/pnas.1608769114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He Y, Leung KW, Ren Y, Pei J, Ge J, Tombran-Tink J. PEDF improves mitochondrial function in RPE cells during oxidative stress. Invest Ophthalmol Vis Sci. 2014;55:6742–6755. doi: 10.1167/iovs.14-14696. [DOI] [PubMed] [Google Scholar]
- 50.Heesterbeek T, Lechanteur Y, Lorés-Motta L, Schick T, Daha M, Altay L, Liakopoulos S, Smailhodzic D, den Hollander A, Hoyng C, de Jong E, Klevering B. Complement activation levels are related to disease stage in AMD. Invest Ophthalmol Vis Sci. 2020a;61:18. doi: 10.1167/iovs.61.3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hernández-Zimbrón LF, Zamora-Alvarado R, Ochoa-De la Paz L, Velez-Montoya R, Zenteno E, Gulias-Cañizo R, Quiroz-Mercado H, Gonzalez-Salinas R. Age-related macular degeneration: new paradigms for treatment and management of AMD. Oxid Med Cell Longev. 2018;2018:8374647. doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Himawan E, Ekström P, Buzgo M, Gaillard P, Stefánsson E, Marigo V, Loftsson T, Paquet-Durand F. Drug delivery to retinal photoreceptors. Drug Discov Today. 2019;24:1637–1643. doi: 10.1016/j.drudis.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho TC, Yang YC, Cheng HC, Wu AC, Chen SL, Tsao YP. Pigment epithelium-derived factor protects retinal pigment epithelium from oxidant-mediated barrier dysfunction. Biochem Biophys Res Commun. 2006;342:372–378. doi: 10.1016/j.bbrc.2006.01.164. [DOI] [PubMed] [Google Scholar]
- 54.Holekamp NM, Bouck N, Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol. 2002;134:220–227. doi: 10.1016/s0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- 55.Holz FG, Sadda SR, Busbee B, Chew EY, Mitchell P, Tufail A, Brittain C, Ferrara D, Gray S, Honigberg L, Martin J, Tong B, Ehrlich JS, Bressler NM, Investigators CaSS. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and spectri phase 3 randomized clinical trials. JAMA Ophthalmol. 2018;136:666–677. doi: 10.1001/jamaophthalmol.2018.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. doi: 10.1016/j.biopha.2019.109767. [DOI] [PubMed] [Google Scholar]
- 57.Hubens W, Beckers H, Gorgels T, Webers C. Increased ratios of complement factors C3a to C3 in aqueous humor and serum mark glaucoma progression. Exp Eye Res. 2021;204:108460. doi: 10.1016/j.exer.2021.108460. [DOI] [PubMed] [Google Scholar]
- 58.Jaffe G, Westby K, Csaky K, Monés J, Pearlman J, Patel S, Joondeph B, Randolph J, Masonson H, Rezaei K. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal phase 2/3 trial. Ophthalmology. 2021;128:576–586. doi: 10.1016/j.ophtha.2020.08.027. [DOI] [PubMed] [Google Scholar]
- 59.Josifovska N, Albert R, Nagymihály R, Lytvynchuk L, Moe MC, Kaarniranta K, Veréb ZJ, Petrovski G. Resveratrol as inducer of autophagy pro-survival and anti-inflammatory stimuli in cultured human RPE cells. Int J Mol Sci. 2020;21:813. doi: 10.3390/ijms21030813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaarniranta K, Uusitalo H, Blasiak J, Felszeghy S, Kannan R, Kauppinen A, Salminen A, Sinha D, Ferrington D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog Retin Eye Res. 2020;79:100858. doi: 10.1016/j.preteyeres.2020.100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kassa E, Ciulla TA, Hussain RM, Dugel PU. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. 2019a;19:335–342. doi: 10.1080/14712598.2019.1575358. [DOI] [PubMed] [Google Scholar]
- 62.Kassa E, Ciulla T, Hussain R, Dugel P. Complement inhibition as a therapeutic strategy in retinal disorders. Expert Opin Biol Ther. 2019b;19:335–342. doi: 10.1080/14712598.2019.1575358. [DOI] [PubMed] [Google Scholar]
- 63.Khandhadia S, Lotery A. Oxidation and age-related macular degeneration: insights from molecular biology. Expert Rev Mol Med. 2010;12:e34. doi: 10.1017/S146239941000164X. [DOI] [PubMed] [Google Scholar]
- 64.Khatib TZ, Martin KR. Neuroprotection in glaucoma: towards clinical trials and precision medicine. Curr Eye Res. 2020;45:327–338. doi: 10.1080/02713683.2019.1663385. [DOI] [PubMed] [Google Scholar]
- 65.Khawaja AP, Cooke Bailey JN, Wareham NJ, Scott RA, Simcoe M, Igo RP, Song YE, Wojciechowski R, Cheng CY, Khaw PT, Pasquale LR, Haines JL, Foster PJ, Wiggs JL, Hammond CJ, Hysi PG. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018;50:778–782. doi: 10.1038/s41588-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim BJ, Mastellos DC, Li Y, Dunaief JL, Lambris JD. Targeting complement components C3 and C5 for the retina: Key concepts and lingering questions. Prog Retin Eye Res. 2020:100936. doi: 10.1016/j.preteyeres.2020.100936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim Y, Kim M, Choi M, Lee D, Roh G, Kim H, Kang S, Cho G, Hong E, Choi W. Alpha-lipoic acid reduces retinal cell death in diabetic mice. Biochem Biophys Res Commun. 2018;503:1307–1314. doi: 10.1016/j.bbrc.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 68.Klingeborn M, Stamer WD, Bowes Rickman C. Polarized exosome release from the retinal pigmented epithelium. Adv Exp Med Biol. 2018;1074:539–544. doi: 10.1007/978-3-319-75402-4_65. [DOI] [PubMed] [Google Scholar]
- 69.Kubota S, Ozawa Y, Kurihara T, Sasaki M, Yuki K, Miyake S, Noda K, Ishida S, Tsubota K. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci. 2011;52:9142–9148. doi: 10.1167/iovs.11-8041. [DOI] [PubMed] [Google Scholar]
- 70.Kumar-Singh R. The role of complement membrane attack complex in dry and wet AMD - From hypothesis to clinical trials. Ex Eye Res. 2019;184:266–277. doi: 10.1016/j.exer.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Kumar S, Ramakrishnan H, Viswanathan S, Akopian A, Bloomfield SA. Neuroprotection of the inner retina also prevents secondary outer retinal pathology in a mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2021;62:35. doi: 10.1167/iovs.62.9.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumari R, Astafurov K, Genis A, Danias J. Differential effects of c1qa ablation on glaucomatous damage in two sexes in DBA/2NNia mice. PLoS One. 2015;10:e0142199. doi: 10.1371/journal.pone.0142199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee H, Yoon Y, Lee S. Mechanism of neuroprotection by trehalose: controversy surrounding autophagy induction. Cell Death Dis. 2018;9:712. doi: 10.1038/s41419-018-0749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee KS, Lin S, Copland DA, Dick AD, Liu J. Cellular senescence in the aging retina and developments of senotherapies for age-related macular degeneration. J Neuroinflammation. 2021;18:32. doi: 10.1186/s12974-021-02088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy diabetic macular edema and related vision loss. Eye (London) 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levkovitch-Verbin H. Retinal ganglion cell apoptotic pathway in glaucoma: Initiating and downstream mechanisms. Prog Brain Res. 2015;220:37–57. doi: 10.1016/bs.pbr.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Leng Y, Jiang Q, Wang Z, Luo P, Zhang C, Chen L, Wang Y, Wang H, Yue X, Shen C, Zhou Y, Shi C, Xie L. Eye Drops of metformin prevents fibrosis after glaucoma filtration surgery in rats activating AMPK/Nrf2 signaling pathway. Front Pharmacol. 2020;11:1038. doi: 10.3389/fphar.2020.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S, Khan S, Khalifa Y. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49:5526–5531. doi: 10.1167/iovs.08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu C, Cao L, Yang S, Xu L, Liu P, Wang F, Xu D. Subretinal injection of amyloid-β peptide accelerates RPE cell senescence and retinal degeneration. Int J Mol Med. 2015;35:169–176. doi: 10.3892/ijmm.2014.1993. [DOI] [PubMed] [Google Scholar]
- 81.Liu H, Anders F, Funke S, Mercieca K, Grus F, Prokosch V. Proteome alterations in aqueous humour of primary open angle glaucoma patients. Int J Ophthalmol. 2020;13:176–179. doi: 10.18240/ijo.2020.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, Copland DA, Theodoropoulou S, Chiu HA, Barba MD, Mak KW, Mack M, Nicholson LB, Dick AD. Impairing autophagy in retinal pigment epithelium leads to inflammasome activation and enhanced macrophage-mediated angiogenesis. Sci Rep. 2016;6:20639. doi: 10.1038/srep20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu K, Ma L, Lai T, Brelen M, Tam P, Tham C, Pang C, Chen L. C5Evaluation of the association of with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Eye (London) 2019;6:34. doi: 10.1186/s40662-019-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorenz K, Scheller Y, Bell K, Grus F, Ponto KA, Bock F, Cursiefen C, Flach J, Gehring M, Peto T, Silva R, Tal Y, Pfeiffer N. A prospective randomised placebo-controlled double-masked three-armed multicentre phase II/III trial for the Study of a Topical Treatment of Ischaemic Central Retinal Vein Occlusion to Prevent Neovascular Glaucoma - the STRONG study: study protocol for a randomised controlled trial. Trials. 2017;18:128. doi: 10.1186/s13063-017-1861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lotfi P, Tse DY, Di Ronza A, Seymour ML, Martano G, Cooper JD, Pereira FA, Passafaro M, Wu SM, Sardiello M. Trehalose reduces retinal degeneration neuroinflammation and storage burden caused by a lysosomal hydrolase deficiency. Autophagy. 2018;14:1419–1434. doi: 10.1080/15548627.2018.1474313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loukovaara S, Nurkkala H, Tamene F, Gucciardo E, Liu X, Repo P, Lehti K, Varjosalo M. Quantitative proteomics analysis of vitreous humor from diabetic retinopathy patients. J Proteome Res. 2015;14:5131–5143. doi: 10.1021/acs.jproteome.5b00900. [DOI] [PubMed] [Google Scholar]
- 87.Luo H, Zhuang J, Hu P, Ye W, Chen S, Pang Y, Li N, Deng C, Zhang X. Resveratrol delays retinal ganglion cell loss and attenuates gliosis-related inflammation from ischemia-reperfusion injury. Invest Ophthalmol Vis Sci. 2018;59:3879–3888. doi: 10.1167/iovs.18-23806. [DOI] [PubMed] [Google Scholar]
- 88.Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust. 2019;210:180–187. doi: 10.5694/mja2.50020. [DOI] [PubMed] [Google Scholar]
- 89.Ma N, Yang X, Qi C, Yu Q, Zhu C, Ren H. Farrerol enhances Nrf2-mediated defense mechanisms against hydrogen peroxide-induced oxidative damage in human retinal pigment epithelial cells by activating akt and MAPK. Oxid Med Cell Longev. 2021;2021:8847844. doi: 10.1155/2021/8847844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ma ZW, Liu DX. Humanin decreases mitochondrial membrane permeability by inhibiting the membrane association and oligomerization of Bax and Bid proteins. Acta Pharmacol Sin. 2018;39:1012–1021. doi: 10.1038/aps.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Madeira MH, Boia R, Santos PF, Ambrósio AF, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015;2015:673090. doi: 10.1155/2015/673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matias I, Wang JW, Moriello AS, Nieves A, Woodward DF, Di Marzo V. Changes in endocannabinoid and palmitoylethanolamide levels in eye tissues of patients with diabetic retinopathy and age-related macular degeneration. Prostaglandins Leukot Essent Fatty Acids. 2006;75:413–418. doi: 10.1016/j.plefa.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Mayer AL, Higgins CB, Heitmeier MR, Kraft TE, Qian X, Crowley JR, Hyrc KL, Beatty WL, Yarasheski KE, Hruz PW, DeBosch BJ. SLC2A8 (GLUT8) is a mammalian trehalose transporter required for trehalose-induced autophagy. Sci Rep. 2016;6:38586. doi: 10.1038/srep38586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Menon M, Mohammadi S, Davila-Velderrain J, Goods BA, Cadwell TD, Xing Y, Stemmer-Rachamimov A, Shalek AK, Love JC, Kellis M, Hafler BP. Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat Commun. 2019;10:4902. doi: 10.1038/s41467-019-12780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Menzies FM, Fleming A, Caricasole A, Bento CF, Andrews SP, Ashkenazi A, Füllgrabe J, Jackson A, Jimenez Sanchez M, Karabiyik C, Licitra F, Lopez Ramirez A, Pavel M, Puri C, Renna M, Ricketts T, Schlotawa L, Vicinanza M, Won H, Zhu Y, et al. Autophagy and neurodegeneration: pathogenic mechanisms and therapeutic opportunities. Neuron. 2017;93:1015–1034. doi: 10.1016/j.neuron.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 96.Michelis G, German OL, Villasmil R, Soto T, Rotstein NP, Politi L, Becerra SP. Pigment epithelium-derived factor (PEDF) and derived peptides promote survival and differentiation of photoreceptors and induce neurite-outgrowth in amacrine neurons. J Neurochem. 2021 doi: 10.1111/jnc.15454. doi: 10.1111/jnc.15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mikitsh JL, Chacko AM. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect Medicin Chem. 2014;6:11–24. doi: 10.4137/PMC.S13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minasyan L, Sreekumar PG, Hinton DR, Kannan R. Protective mechanisms of the mitochondrial-derived peptide humanin in oxidative and endoplasmic reticulum stress in RPE cells. Oxid Med Cell Longev. 2017;2017:1675230. doi: 10.1155/2017/1675230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nashine S, Cohen P, Nesburn AB, Kuppermann BD, Kenney MC. Characterizing the protective effects of SHLP2 a mitochondrial-derived peptide in macular degeneration. Sci Rep. 2018;8:15175. doi: 10.1038/s41598-018-33290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, Lentsch AB. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noristani R, Kuehn S, Stute G, Reinehr S, Stellbogen M, Dick HB, Joachim SC. Retinal and optic nerve damage is associated with early glial responses in an experimental autoimmune glaucoma model. J Mol Neurosci. 2016;58:470–482. doi: 10.1007/s12031-015-0707-2. [DOI] [PubMed] [Google Scholar]
- 102.Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ogata N, Nishikawa M, Nishimura T, Mitsuma Y, Matsumura M. Unbalanced vitreous levels of pigment epithelium-derived factor and vascular endothelial growth factor in diabetic retinopathy. Am J Ophthalmol. 2002;134:348–353. doi: 10.1016/s0002-9394(02)01568-4. [DOI] [PubMed] [Google Scholar]
- 104.Ortega A, Martinez-Arroyo O, Forner MJ, Cortes R. Exosomes as Drug delivery systems: endogenous nanovehicles for treatment of systemic lupus erythematosus. Pharmaceutics. 2020;13:3. doi: 10.3390/pharmaceutics13010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ou K, Mertsch S, Theodoropoulou S, Wu J, Liu J, Copland DA, Schrader S, Liu L, Dick AD. Restoring retinal neurovascular health via substance P. Exp Cell Res. 2019;380:115–123. doi: 10.1016/j.yexcr.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ou K, Copland DA, Theodoropoulou S, Mertsch S, Li Y, Liu J, Schrader S, Liu L, Dick AD. Treatment of diabetic retinopathy through neuropeptide Y-mediated enhancement of neurovascular microenvironment. J Cell Mol Med. 2020;24:3958–3970. doi: 10.1111/jcmm.15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palmieri M, Pal R, Nelvagal HR, Lotfi P, Stinnett GR, Seymour ML, Chaudhury A, Bajaj L, Bondar VV, Bremner L, Saleem U, Tse DY, Sanagasetti D, Wu SM, Neilson JR, Pereira FA, Pautler RG, Rodney GG, Cooper JD, Sardiello M. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases. Nat Commun. 2017;8:14338. doi: 10.1038/ncomms14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park DH, Connor KM, Lambris JD. The challenges and promise of complement therapeutics for ocular diseases. Front Immunol. 2019;10:1007. doi: 10.3389/fimmu.2019.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patrick DM, Leone AK, Shellenberger JJ, Dudowicz KA, King JM. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol. 2006;6:2. doi: 10.1186/1472-6793-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peng J, Xiong S, Ding L, Peng J, Xia X. Diabetic retinopathy: Focus on NADPH oxidase and its potential as therapeutic target. Eur J Pharmacol. 2019;853:381–387. doi: 10.1016/j.ejphar.2019.04.038. [DOI] [PubMed] [Google Scholar]
- 111.Qu S, Zhang C, Liu D, Wu J, Tian H, Lu L, Xu G, Liu F, Zhang J. Metformin protects ARPE-19 cells from glyoxal-induced oxidative stress. Oxid Med Cell Longev. 2020;2020:1740943. doi: 10.1155/2020/1740943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rapino C, Tortolani D, Scipioni L, Maccarrone M. Neuroprotection by (endo) cannabinoids in glaucoma and retinal neurodegenerative diseases. Curr Neuropharmacol. 2018;16:959–970. doi: 10.2174/1570159X15666170724104305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rochette L, Ghibu S, Muresan A, Vergely C. Alpha-lipoic acid: molecular mechanisms and therapeutic potential in diabetes. Can J Physiol Pharmacol. 2015;93:1021–1027. doi: 10.1139/cjpp-2014-0353. [DOI] [PubMed] [Google Scholar]
- 114.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 115.Ruan Y, Jiang S, Musayeva A, Gericke A. Oxidative stress and vascular dysfunction in the retina: therapeutic strategies. Antioxidants (Basel) 2020;9:761. doi: 10.3390/antiox9080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, Shazam Hussain M, Jansen O, Jayaraman MV, Khalessi AA, Kluck BW, Lavine S, Meyers PM, Ramee S, Rüfenacht DA, Schirmer CM, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–632. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 117.Sahajpal N, Goel R, Chaubey A, Aurora R, Jain S. Pathological perturbations in diabetic retinopathy: hyperglycemia AGEs oxidative stress and inflammatory pathways. Curr Protein Pept Sci. 2019;20:92–110. doi: 10.2174/1389203719666180928123449. [DOI] [PubMed] [Google Scholar]
- 118.Santos-Carvalho A, Elvas F, Alvaro AR, Ambrósio AF, Cavadas C. Neuropeptide Y receptors activation protects rat retinal neural cells against necrotic and apoptotic cell death induced by glutamate. Cell Death Dis. 2013;4:e636. doi: 10.1038/cddis.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shahulhameed S, Vishwakarma S, Chhablani J, Tyagi M, Pappuru R, Jakati S, Chakrabarti S, Kaur I. A systematic investigation on complement pathway activation in diabetic retinopathy. Front Immunol. 2020;11:154. doi: 10.3389/fimmu.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Silva KC, Rosales MA, Biswas SK, Lopes de Faria JB, Lopes de Faria JM. Diabetic retinal neurodegeneration is associated with mitochondrial oxidative stress and is improved by an angiotensin receptor blocker in a model combining hypertension and diabetes. Diabetes. 2009;58:1382–1390. doi: 10.2337/db09-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simó-Servat O, Hernández C, Simó R. Somatostatin and diabetic retinopathy: an evolving story. Endocrine. 2018;60:1–3. doi: 10.1007/s12020-018-1561-0. [DOI] [PubMed] [Google Scholar]
- 122.Simó R, Lecube A, Sararols L, García-Arumí J, Segura RM, Casamitjana R, Hernández C. Deficit of somatostatin-like immunoreactivity in the vitreous fluid of diabetic patients: possible role in the development of proliferative diabetic retinopathy. Diabetes Care. 2002;25:2282–2286. doi: 10.2337/diacare.25.12.2282. [DOI] [PubMed] [Google Scholar]
- 123.Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL, Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the american diabetes association. Diabetes Care. 2017;40:412–418. doi: 10.2337/dc16-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Song D, Sulewski ME, Wang C, Song J, Bhuyan R, Sterling J, Clark E, Song WC, Dunaief JL. Complement C5a receptor knockout has diminished light-induced microglia/macrophage retinal migration. Mol Vis. 2017;23:210–218. [PMC free article] [PubMed] [Google Scholar]
- 125.Sreekumar PG, Hinton DR, Kannan R. The emerging role of senescence in ocular disease. Oxid Med Cell Longev. 2020;2020:2583601. doi: 10.1155/2020/2583601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR. The Mitochondrial-derived peptide humanin protects rpe cells from oxidative stress senescence and mitochondrial dysfunction. Invest Ophthalmol Vis Sci. 2016;57:1238–1253. doi: 10.1167/iovs.15-17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stitt A. AGEs and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2010;51:4867–4874. doi: 10.1167/iovs.10-5881. [DOI] [PubMed] [Google Scholar]
- 128.Takano K, Kojima T, Sawada N, Himi T. Role of tight junctions in signal transduction: an update. EXCLI J. 2014;13:1145–1162. [PMC free article] [PubMed] [Google Scholar]
- 129.Thomas CC, Philipson LH. Update on diabetes classification. Med Clin North Am. 2015;99:1–16. doi: 10.1016/j.mcna.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 130.Toomey C, Johnson L, Bowes Rickman C. Complement factor H in AMD: Bridging genetic associations and pathobiology. Prog Retin Eye Res. 2018;62:38–57. doi: 10.1016/j.preteyeres.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Troger J, Neyer S, Heufler C, Huemer H, Schmid E, Griesser U, Kralinger M, Kremser B, Baldissera I, Kieselbach G. Substance P and vasoactive intestinal polypeptide in the streptozotocin-induced diabetic rat retina. Invest Ophthalmol Vis Sci. 2001;42:1045–1050. [PubMed] [Google Scholar]
- 132.Uckermann O, Wolf A, Kutzera F, Kalisch F, Beck-Sickinger AG, Wiedemann P, Reichenbach A, Bringmann A. Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: activation by neuropeptide Y. J Neurosci Res. 2006;83:538–550. doi: 10.1002/jnr.20760. [DOI] [PubMed] [Google Scholar]
- 133.Upadhyay RK. Drug delivery systems CNS protection and the blood brain barrier. Biomed Res Int. 2014;2014:869269. doi: 10.1155/2014/869269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Voigt AP, Mulfaul K, Mullin NK, Flamme-Wiese MJ, Giacalone JC, Stone EM, Tucker BA, Scheetz TE, Mullins RF. Single-cell transcriptomics of the human retinal pigment epithelium and choroid in health and macular degeneration. Proc Natl Acad Sci U S A. 2019;116:24100–24107. doi: 10.1073/pnas.1914143116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang MTM, Danesh-Meyer HV. Cannabinoids and the eye. Surv Ophthalmol. 2021;66:327–345. doi: 10.1016/j.survophthal.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 136.Wang S, Wang X, Cheng Y, Ouyang W, Sang X, Liu J, Su Y, Liu Y, Li C, Yang L, Jin L, Wang Z. Autophagy dysfunction cellular senescence and abnormal immune-inflammatory responses in AMD: from mechanisms to therapeutic potential. Oxid Med Cell Longev. 2019;2019:3632169. doi: 10.1155/2019/3632169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang W, Lo ACY. Diabetic retinopathy: pathophysiology and treatments. Int J Mol Sci. 2018;19:1816. doi: 10.3390/ijms19061816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Y, Zhang Q, Yang G, Wei Y, Li M, Du E, Li H, Song Z, Tao Y. RPE-derived exosomes rescue the photoreceptors during retina degeneration: an intraocular approach to deliver exosomes into the subretinal space. Drug Deliv. 2021;28:218–228. doi: 10.1080/10717544.2020.1870584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, John SW, Howell GR. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. 2016;11:26. doi: 10.1186/s13024-016-0091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2:106–116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 142.Wubben TJ, Zacks DN, Besirli CG. Retinal neuroprotection: current strategies and future directions. Curr Opin Ophthalmol. 2019;30:199–205. doi: 10.1097/ICU.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 143.Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016;787:94–104. doi: 10.1016/j.ejphar.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang JH, Guo Z, Zhang T, Meng XX, Xie LS. Restoration of endogenous substance P is associated with inhibition of apoptosis of retinal cells in diabetic rats. Regul Pept. 2013;187:12–16. doi: 10.1016/j.regpep.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 145.Yang S, Zhang J, Chen L. The cells involved in the pathological process of diabetic retinopathy. Biomed Pharmacother. 2020;132:110818. doi: 10.1016/j.biopha.2020.110818. [DOI] [PubMed] [Google Scholar]
- 146.Yang X, Pan X, Zhao X, Luo J, Xu M, Bai D, Hu Y, Liu X, Yu Q, Gao D. Autophagy and Age-Related Eye Diseases. Biomed Res Int. 2019a;2019:5763658. doi: 10.1155/2019/5763658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yang Y, Gao H, Zhou H, Liu Q, Qi Z, Zhang Y, Zhang J. The role of mitochondria-derived peptides in cardiovascular disease: Recent updates. Biomed Pharmacother. 2019b;117:109075. doi: 10.1016/j.biopha.2019.109075. [DOI] [PubMed] [Google Scholar]
- 148.Yi L, Swensen AC, Qian WJ. Serum biomarkers for diagnosis and prediction of type 1 diabetes. Transl Res. 2018;201:13–25. doi: 10.1016/j.trsl.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yuan L, Neufeld AH. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64:523–532. doi: 10.1002/jnr.1104. [DOI] [PubMed] [Google Scholar]
- 150.Zantut PRA, Veras MM, Yariwake VY, Takahashi WY, Saldiva PH, Young LH, Damico FM, Fajersztajn L. Effects of cannabis and its components on the retina: a systematic review. Cutan Ocul Toxicol. 2020;39:1–9. doi: 10.1080/15569527.2019.1685534. [DOI] [PubMed] [Google Scholar]
- 151.Zhang L, Bell BA, Yu M, Chan CC, Peachey NS, Fung J, Zhang X, Caspi RR, Lin F. Complement anaphylatoxin receptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis. J Leukoc Biol. 2016;99:447–454. doi: 10.1189/jlb.3A0415-157R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]


