Abstract
Light plays an essential role in psychobiological and psychophysiological processes, such as alertness. The alerting effect is influenced by light characteristics and the timing of interventions. This meta-analysis is the first to systematically review the effect of light intervention on alertness and to discuss the optimal protocol for light intervention. In this meta-analysis, registered at PROSPERO (Registration ID: CRD42020181485), we conducted a systematic search of the Web of Science, PubMed, and PsycINFO databases for studies published in English prior to August 2021. The outcomes included both subjective and objective alertness. Subgroup analyses considered a variety of factors, such as wavelength, correlated color temperature (CCT), light illuminance, and timing of interventions (daytime, night-time, or all day). Twenty-seven crossover studies and two parallel-group studies were included in this meta-analysis, with a total of 1210 healthy participants (636 (52%) male, mean age 25.62 years). The results revealed that light intervention had a positive effect on both subjective alertness (standardized mean difference (SMD) = –0.28, 95% confidence interval (CI): –0.49 to –0.06, P = 0.01) and objective alertness in healthy subjects (SMD = –0.34, 95% CI: –0.68 to –0.01, P = 0.04). The subgroup analysis revealed that cold light was better than warm light in improving subjective alertness (SMD = –0.37, 95% CI: –0.65 to –0.10, P = 0.007, I2 = 26%) and objective alertness (SMD = –0.36, 95% CI: –0.66 to –0.07, P = 0.02, I2 = 0). Both daytime (SMD = –0.22, 95% CI: –0.37 to –0.07, P = 0.005, I2 = 74%) and night-time (SMD = –0.32, 95% CI: –0.61 to –0.02, P = 0.04, I2 = 0) light exposure improved subjective alertness. The results of this meta-analysis and systematic review indicate that light exposure is associated with significant improvement in subjective and objective alertness. In addition, light exposure with a higher CCT was more effective in improving alertness than light exposure with a lower CCT. Our results also suggest that both daytime and night-time light exposure can improve subjective alertness.
Key Words: correlated color temperature, illuminance, light, meta-analysis, objective alertness, subjective alertness, time of light intervention, wavelength
Introduction
Alertness is a psychological construct closely related to attention, and refers to a behavioral and physiological state of responsiveness to incoming stimuli (Posner, 2008). For practical reasons, alertness has been regarded as the opposite of sleepiness (Kaida et al., 2006). Alertness is essential for independent functioning in our daily lives, and is an important factor that contributes to the quality of work performance. For instance, it is essential for a student to be alert when attending a lecture, reading a textbook, or writing a report. In contrast, fatigue or daytime sleepiness can negatively affect cognitive and physiological functions, and have a series of consequences on an individual’s safety and quality of life. For instance, one study showed that fatigued train drivers performed less consistently and committed more speeding violations than well-rested drivers (Dorrian et al., 2007). Sleepiness is known to be a safety hazard in many 24/7 industries (Sallinen and Hublin, 2015). In clinical settings, several trials have reported that extended-duration shifts worked by interns were related to more medical errors and adverse events (Landrigan et al., 2004; Lockley et al., 2004; Barger et al., 2006). Epidemiological studies have indicated that the prevalence of daytime sleepiness is approximately 10% to 25% in the general community (Roehrs et al., 2011). Daytime sleepiness has become pervasive, and it is becoming a serious concern in modern society.
Accumulated evidence from animal and human studies has demonstrated an important role of light on alertness. However, the efficacy of light intervention on alertness can vary according to properties such as illumination level, light wavelength, and measures of alertness (Cajochen, 2007). With regard to the illuminance level of light, the first demonstration of a physiological effect of light was Lewy’s discovery in 1980, which indicated that at least 1000 lx of white light was required to suppress melatonin in healthy humans (Lewy et al., 1980). Cajochen and colleagues investigated the relationship between light intensity and alertness, and found increased levels of alertness with increases in illuminance level according to a logistic function (Cajochen et al., 2000). Several studies have also investigated the relationship between wavelength and alertness, and it seems that short-wavelength light is superior to long-wavelength light in improving alertness (Cajochen, 2007). This short-wavelength sensitivity corresponds closely to the spectral sensitivity of melanopsin-expressing intrinsically photosensitive retinal ganglion cells (ipRGCs λmax ~480 nm) that primarily mediate the alerting effect (Brainard et al., 2001; Thapan et al., 2001). Although mounting studies have examined the effect of light on alertness and have consistently found a beneficial effect of light on alertness, there is still no clear consensus on the optimal protocol of light intervention to improve alertness. A systematic review of evidence on light intervention is needed to explore the relationship between the efficacy of light exposure and intervention properties. Several systematic review studies have been conducted in this field. For instance, Souman et al. (2018), who reviewed 68 studies, showed that exposure to higher intensities of polychromatic white light was reported to increase subjective alertness in many studies. It also remains unclear how different properties of light contribute to the alerting effect (Souman et al., 2018). The current study improves upon previous studies in at least 2 ways. Firstly, the current study provides more reliable conclusions by employing statistical methods to quantitatively synthesize the results of multiple studies. Secondly, we focused on a healthy population with a normal sleep-wake cycle and excluded studies whose subjects were night shift workers or had disturbed sleep. Previous studies did not apply such restrictions on subjects. Stable and high levels of alertness can only be maintained when the phase relationship between the endogenous circadian timing system and the sleep/wake cycle is such that the circadian timing system opposes the wake-dependent deterioration of alertness and performance as conceptualized in the “opponent process” model (Dijk and Czeisler, 1994). Cajochen’s review concluded that light should exert its alerting action most strongly when the circadian drive for sleep is at its maximum, during sleep inertia and under high homeostatic sleep pressure (Cajochen, 2007). Therefore, it is reasonable to postulate that compared with healthy individuals, subjects with disturbed sleep cycles are more sensitive to the alerting effects of light, and the mechanism underlying alerting effects may also be different between healthy individuals and individuals with disturbed sleep rhythms.
In addition to alertness, light exerts profound effects on a wide range of physiologies and behaviors, such as entraining circadian rhythms (Aschoff et al., 1969; Czeisler et al., 1986), improving mood (Eastman et al., 1998), and affecting sleep (Chellappa et al., 2013). These non-image-forming effects are not associated with classical cones and rods, but are mainly mediated by ipRGCs (Provencio et al., 2000; Thapan et al., 2001). Accumulating evidence has suggested that ipRGCs play an essential role in the alerting effects of light. For example, one human study using 460 nm narrowband light to stimulate ipRGCs specifically improved alertness (Vandewalle et al., 2007), while the alert-improving effect of exposure to bright light was absent in mice lacking melanopsin (Milosavljevic et al., 2016), which is the photosensitive protein expressed in ipRGCs (Berson et al., 2002). The efferent projections of the ipRGCs include those to multiple hypothalamic, thalamic, striatal, brainstem, and limbic regions (Hattar et al., 2006). Among the hypothalamic regions, the most studied are the suprachiasmatic nucleus (SCN), ventral lateral preoptic area, locus coeruleus (LC), and dorsal raphe. These pathways may regulate the alerting effects of light (Lok et al., 2018).
The alerting effect of light has previously been examined in narrative reviews (Cajochen, 2007; Lok et al., 2018; Souman et al., 2018; Xu and Lang, 2018). To the best of our knowledge, the present study is the first quantitative meta-analysis to investigate the efficacy of light exposure on the alertness of healthy volunteers. We evaluated the efficacy of light exposure on subjective alertness, as well as objective performance measures of alertness. Subjective measures of alertness include standardized scales to rate the level of sleepiness or fatigue, which supposedly measure the construct of alertness. Alertness has also been assessed using several behavioral tasks that are more objective. Importantly, we evaluated the relationship between the efficacy of light and intervention properties, including wavelength, correlated color temperature (CCT), illuminance level, and timing of light intervention (daytime, night-time, or all day). The human visible light spectrum ranges from 380 nm to 700 nm, and we applied a subgroup analysis to compare the alerting effects of short wavelength (~480 nm) and long wavelength (~600 nm) light. With regard to CCT, light with a CCT of 3000 K has a yellow appearance and is described as warm light, while light with a CCT of 5000 K is described as cold light. Considering that 1000 lx is typical for indoor lighting, the subgroup analysis compared the alerting effects of low-intensity light (< 1000 lx) and high-intensity light (≥ 1000 lx). With regard to the timing of light, the studies were categorized into one of three groups-daytime light exposure (8:00–18:00), night-time light exposure (18:00–24:00), or whole-day light exposure (8:00–24:00).
The main research questions of this study were as follows: (1) Does light exposure effectively improve the alertness of healthy volunteers?; and (2) What is the relationship between the efficacy of light and intervention properties, such as wavelength, CCT, illuminance level, and timing of light exposure (daytime, night-time, or all day)?
Materials and Methods
A literature search was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses statement (PRISMA) guidelines for systematic reviews and meta-analyses (Moher et al., 2015). This systematic review protocol was registered at PROSPERO (Registration ID: CRD42020181485).
Literature search
The first author searched the Web of Science, PubMed, and PsycINFO databases for all studies investigating light intervention with healthy volunteers. The search was restricted to studies published in English, and the search period was January 1943 to August 2021. The search keywords were as follows: [bright light OR light box OR light visor OR light therapy OR light treatment OR phototherapy OR phototherapy] AND [alertness OR vigilance OR arousal OR sleepiness OR tiredness OR fatigue]. All reviews and bibliographic citations of relevant publications were examined by hand for additional references.
Inclusion and exclusion criteria
Studies were included in the meta-analysis according to the following criteria: (1) randomized controlled trials (RCTs) and nonrandomized trials that employed a crossover or parallel-group design; (2) included healthy adults aged 18 years and above; (3) at least one intervention group implemented light intervention; and (4) quantified the assessment of alertness. The exclusion criteria were as follows: (1) review, editorial material, proceedings paper, etc.; (2) participants with irregular working schedules, such as night shifts; (3) participants with major health problems indicating neurological or psychiatric disorders; (4) participants with vision or sleep problems; and (5) mean and standard deviations were not available after attempts to contact authors, and could not be calculated from descriptive data or statistical tests in the article.
Selection of studies
We imported all studies to EndNote X9 and removed duplicate records. Eligible studies were selected in two stages. Firstly, three authors (XDH, ZFH, and YMM) independently screened the title and abstract of each article identified by the systematic search. At this stage, we excluded all references that clearly did not fulfill the inclusion criteria. Secondly, the remaining full-text articles were further assessed independently by the same three authors (XDH, ZFH, and YMM). During both steps of the process, discrepancies were resolved by discussion, and decisions were made by the corresponding author (TQ). A flow chart illustrating the detailed selection process is depicted in Figure 1.
Figure 1.
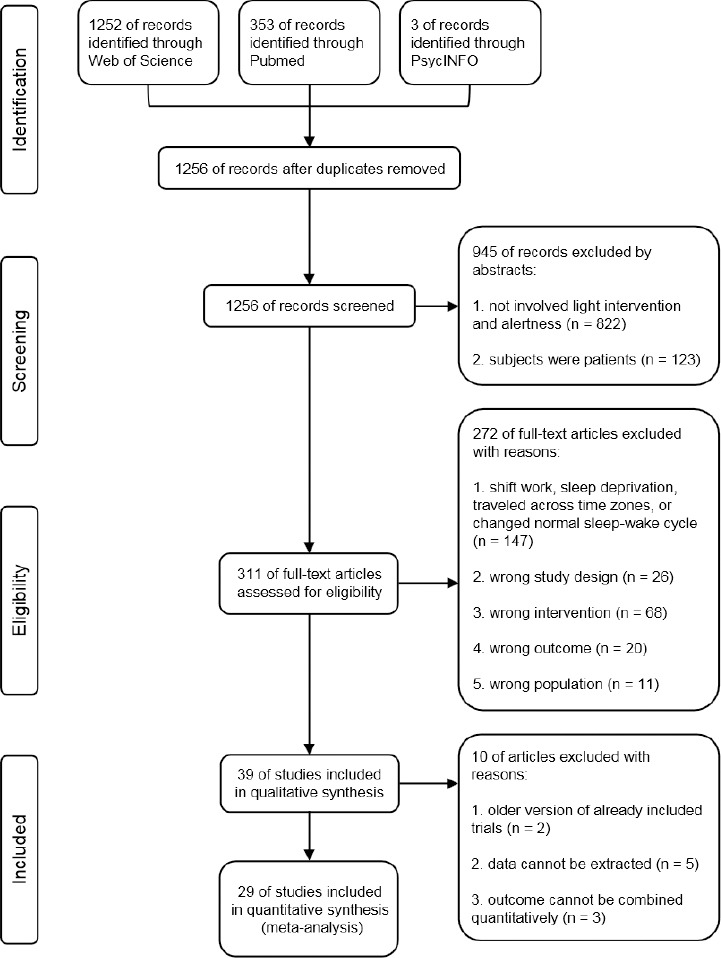
PRISMA flow diagram for the selection of studies.
Data extraction
Two authors (XDH and ZFH) independently extracted the general characteristics from the included studies, including study design, characteristics of participants, intervention characteristics, control group, and outcome measures. The extracted data were matched and discussed in a consensus meeting, and disagreements were resolved through discussions. One author (YMM) transferred data into Review Manager Software (version 5.3.0; Cochrane Collaboration, Copenhagen, Denmark) (Higgins, 2011).
Data on specific properties of light intervention were also extracted. The following light parameters were identified for the subgroup analyses: wavelength of light exposure (short wavelength ~480 nm vs. long wavelength ~600 nm), CCT of light exposure (warm light ~3000 K vs. cold light ~5000 K), and illuminance level of light exposure (low intensity < 1000 lx vs. high intensity ≥ 1000 lx). The following timing parameters of light were identified for the subgroup analyses: daytime light exposure (8:00–18:00) vs. night-time light exposure (18:00–24:00) vs. whole-day light exposure (8:00–24:00).
Risk of bias and quality assessment
The quality of the included studies was evaluated using the Quality Assessment Tool for Quantitative Studies (Effective Public Health Practice Project, 2008). This tool offers a standardized method to assess the following eight domains: selection bias; study design; adjustment for confounders; blinding; data collection method; withdrawals and dropouts; intervention integrity; and the appropriateness of the analysis to the question. Guidelines for the tool indicate that each domain be rated as strong, moderate, or weak. The final global rating for each study was quantified according to the number of “weak” ratings in each of the abovementioned domains with a final score of “strong” rating (no weak rating), “moderate” rating (one weak rating), or “weak” rating (two or more weak ratings). Assessment of the evidence was independently performed by two authors (ZFH and YMM). The results were discussed, and discrepancies were resolved by referring to the Quality Assessment Tool guidelines and dictionary (Al-Karawi and Jubair, 2016).
Data analysis
The statistical methods adopted in this study were reviewed by a statistician at Jinan University in Guangzhou, China. The statistical information extracted from each study included the mean value and standard deviation on alertness outcome measures and the number of subjects for each intervention condition. Given that the included studies quantified alertness improvement through different scales or tasks, the standardized mean difference (SMD) and its 95% confidence interval (CI) were calculated to assess the overall effect. Significance levels were determined at the 0.05 level using the z-test. Forest plots were used to demonstrate effect sizes and their 95% CI. To explore possible causes of heterogeneity, we conducted a subgroup analysis based on light characteristics (wavelength, CCT, and illuminance) and timing characteristics (daytime, night-time, or all day) using the I2statistic. If the number of studies was sufficient, we performed a homogeneity analysis using the I2statistic. If I2≤ 50%, a fixed-effects model was used. When I2> 50%, a random-effects model was used to determine the significant heterogeneity. Sensitivity analysis was conducted by eliminating “weak” rating studies. Publication bias was analyzed using funnel plots. All data were analyzed using Review Manager Software (version 5.3.0; Cochrane Collaboration, Copenhagen, Denmark) (Higgins, 2011).
Results
General characteristics of studies
Of the 1256 studies identified during the initial stage of the literature search, 39 full-text articles were retrieved and evaluated based on the selection criteria. Twenty-nine studies satisfied the prespecified inclusion criteria, and the characteristics of these studies are shown in Table 1. Of the 29 included studies, 27 used crossover designs, and 2 used parallel-group designs. In total, 1210 participants were included in these studies; 596 participants (mean age 25.53 years, 318 (53.36%) male) received experimental light intervention and 614 participants (mean age 25.60 years, 318 (51.79%) male) received a placebo control. Experimental groups were administered light intervention with a short-wavelength, cold, or high-illuminance light, while control groups received long-wavelength, warm, or low-illuminance light.
Table 1.
Characteristics of the included trials
| Study | Study design | Participants (E/C) | Experimental | Control | Time of day | Duration | Outcomes |
|---|---|---|---|---|---|---|---|
| Kohsaka et al., 1999 | Crossover | 8/8 | Moderately bright light (1000 lx) | No light | Morning | 1 h/d, 6 d | VAS |
| O’Brien and O’Connor, 2000 | Crossover | 12/12 | 6434 lx | 1411 lx | 8:00–18:00 | 20 min | VAS |
| Cajochen et al., 2005 | Crossover | 9/9 | Monochromatic light (460 nm) | No light (0 lx) | 21:30–23:30 | 2 h | KSS |
| Crasson and Legros, 2005 | Crossover | 18/18 | Bright light (5000 lx) | Sham exposure | 13:00–14:00 | 30 min | VAS, duration-discrimination task |
| Hansen et al., 2005 | Parallel-group | 19/37 | White light (1800 lx) | Red light (100 lx) | Morning | 93 min/d, 5 d/wk, 2 wk | Harvard Cognitive Battery |
| Rüger et al., 2006 | Crossover | 12/12 | Bright light (5000 lx) | Dim light (< 10 lx) | 12:00–16:00 | 4 h | KSS, VAS |
| Takasu et al., 2006 | Crossover | 8/8 | Bright light (5000 lx) | Dim light (10 lx) | 8:00–24:00 | 16 h/d, 6 d | VAS |
| Viola et al., 2008 | Crossover | 94/94 | Blue-enriched white light (17000 K, 310.35 lx) | White light (4000 K, 421.07 lx) | 8:30–16:45 | ~8 h/d, 5 d/wk, 4 wk | KSS |
| Chellappa et al., 2012 | Crossover | 18/18 | Blue-enriched light (6500 K, 40 lx) | Control light (2500 K, 40 lx) | Evening | 2 h | KSS |
| Santhi et al., 2012 | Crossover | 22/22 | Bright blue-enhanced light (700 lx) | Near-darkness (1 lx) | 19:30–23:30 | 4 h | KSS |
| Sahin and Figueiro, 2013 | Crossover | 13/13 | Short-wavelength blue light (470 nm, 40 lx) | Dark (< 0.01 lx) | 14:30–15:30 | 1 h | KSS |
| Sahin et al., 2014 | Crossover | 13/13 | White light (3000 K, 360 lx) | Red light (630 nm, 210 lx) | Daytime | 2 h | Go/No-go task |
| Leichtfried et al., 2015 | Crossover | 33/33 | Bright light (5000 lx) | Dim light (400 lx) | 7:40–8:10 | 30 min | VAS, Sustained Attention Test |
| Okamoto and Nakagawa, 2015 | Crossover | 8/8 | Short- wavelength light (470 nm) | Darkness | 12:00–16:00 | 28 min | KSS |
| Muench et al., 2016 | Crossover | 18/18 | Mixed blue-enriched lighting (3537 K, 750 lx) | Warm -white control light (2600 K, 40 lx) | 8:00–11:00 | 3 h/d, 3 d | VAS, PVT |
| Borragan et al., 2017 | Crossover | 17/17 | Bright blue-enriched white light (460 nm, 2000 lx) | Dim orange light (600 nm, < 200 lx) | 15:00–17:00 | 20 min | KSS, VAS, PVT |
| Rahman et al., 2017 | Crossover | 16/16 | Standard fluorescent light (4100 K, 50 lx) | Blue-depleted circadian-sensitive light (50 lx) | 16:00–24:00 | 8 h | KSS, PVT |
| Rodriguez-Morilla et al., 2017 | Parallel-group | 12/12 | Blue-enriched white light (440 nm, 469 lx) | Dim light (< 1 lx) | 21:45–23:00 | 75 min | KSS, driving simulator |
| Hartstein et al., 2018 | Crossover | 40/40 | Cool experimental lighting (5000 K) | Warm experimental lighting (3500 K) | 9:00–11:00 | 20 min | Go/No-go task |
| Kazemi et al., 2018 | Crossover | 20/20 | LED (6500 K) | Compact fluorescent (3500 K) | Daytime | 2 h | KSS, Go/No-go task |
| Cajochen et al., 2019 | Crossover | 15/15 | Daylight LED (450 nm, 4000 K, 100 lx) | Conventional LED (4000 K, 100 lx) | 8:00–24:00 | 16 h | KSS |
| Choi et al., 2019 | Crossover | 15/15 | Blue-enriched white light (460 nm, 6500 K, 500 lx) | Warm white light (625 nm, 3500 K, 500 lx) | 10:00–11:00 | 1 h | KSS |
| de Zeeuw et al., 2019 | Crossover | 48/48 | High-mel light (480 nm, 3500K, 100 lx) | Dim light (< 5 lx) | 11:30–14:30 | 3 h | VAS |
| Šmotek et al., 2019 | Crossover | 12/12 | Short-wavelength light (455 nm) | Long-wavelength light (629 nm) | 12:00–15:00 | 20 min | KSS, PVT |
| Studer et al., 2019 | Crossover | 28/28 | Blue-enriched light (458 nm, 876 lx) | Red-enriched light (611 nm, 1063 lx) | Morning | 1 h | Attention network test |
| te Kulve et al., 2019 | Crossover | 12/12 | Bright light (750 lx) | Dim light (5 lx) | Night | 1 h | KSS |
| Yang et al., 2019 | Crossover | 15/15 | Bright light (6000 K, 1000 lx) | Dim light (3600 K, < 5 lx) | Night | 3 h | KSS, PVT |
| Park et al., 2020 | Crossover | 24/24 | LED (4000 K, 150 lx) | Dim light (< 10 lx) | 17:30–24:00 | 6.5 h | PVT |
| Zhou et al., 2021 | Crossover | 17/17 | Blue-enriched bright light (6500 K, 1000 lx) | Normal indoor light (4000 K, 100 lx) | 14:00–14:30 | 30 min | KSS, PVT, Go/No-go task |
E/C: number of participants received experimental intervention/number of participants received control intervention; KSS: Karolinska Sleepiness Scale; LED: Light-emitting diode; PVT: Psychomotor Vigilance Testing; VAS: Visual Analogue Scales.
Twenty-one studies (Kohsaka et al., 1999; Cajochen et al., 2005; Crasson and Legros, 2005; Hansen et al., 2005; Rüger et al., 2006; Takasu et al., 2006; Viola et al., 2008; Chellappa et al., 2012; Sahin and Figueiro, 2013; Leichtfried et al., 2015; Okamoto and Nakagawa, 2015; Borragan et al., 2017; Rahman et al., 2017; Hartstein et al., 2018; Kazemi et al., 2018; Choi et al., 2019; de Zeeuw et al., 2019; Šmotek et al., 2019; Studer et al., 2019; Yang et al., 2019; Zhou et al., 2021) were included in the subgroup analyses on light characteristics; 6 studies were classified into the wavelength group (Cajochen et al., 2005; Sahin and Figueiro, 2013; Okamoto and Nakagawa, 2015; de Zeeuw et al., 2019; Šmotek et al., 2019; Studer et al., 2019), 7 studies were classified into the CCT group (Viola et al., 2008; Chellappa et al., 2012; Rahman et al., 2017; Hartstein et al., 2018; Kazemi et al., 2018; Choi et al., 2019; Yang et al., 2019), and 8 studies were classified into the illuminance group (Kohsaka et al., 1999; Crasson and Legros, 2005; Hansen et al., 2005; Rüger et al., 2006; Takasu et al., 2006; Leichtfried et al., 2015; Borragan et al., 2017; Zhou et al., 2021). Of the 8 studies that were not included in the subgroup analyses on light characteristics, 2 did not use monochromatic light, and 6 used experimental and control light that were either higher or lower than the grouping definition of CCT or illuminance. Twenty-seven studies were included in subgroup analyses on timing characteristics (Kohsaka et al., 1999; O’Brien and O’Connor, 2000; Cajochen et al., 2005; Crasson and Legros, 2005; Hansen et al., 2005; Rüger et al., 2006; Takasu et al., 2006; Viola et al., 2008; Chellappa et al., 2012; Santhi et al., 2012; Sahin and Figueiro, 2013; Sahin et al., 2014; Leichtfried et al., 2015; Okamoto and Nakagawa, 2015; Muench et al., 2016; Borragan et al., 2017; Rodriguez-Morilla et al., 2017; Hartstein et al., 2018; Kazemi et al., 2018; Cajochen et al., 2019; Choi et al., 2019; de Zeeuw et al., 2019; Šmotek et al., 2019; Studer et al., 2019; te Kulve et al., 2019; Yang et al., 2019; Zhou et al., 2021); 19 studies comprised the daytime group (Kohsaka et al., 1999; O’Brien and O’Connor, 2000; Crasson and Legros, 2005; Hansen et al., 2005; Rüger et al., 2006; Viola et al., 2008; Sahin and Figueiro, 2013; Sahin et al., 2014; Leichtfried et al., 2015; Okamoto and Nakagawa, 2015; Muench et al., 2016; Borragan et al., 2017; Hartstein et al., 2018; Kazemi et al., 2018; Choi et al., 2019; de Zeeuw et al., 2019; Šmotek et al., 2019; Studer et al., 2019; Zhou et al., 2021), 6 comprised the night-time group (Cajochen et al., 2005; Chellappa et al., 2012; Santhi et al., 2012; Rodriguez-Morilla et al., 2017; te Kulve et al., 2019; Yang et al., 2019), and 2 comprised the whole-day group (Takasu et al., 2006; Cajochen et al., 2019). Two studies were not included in the subgroup analyses on timing characteristics because the intervention lasted from the afternoon to the evening.
Improvement in alertness was quantified by employing objective tests in 15 studies (Crasson and Legros, 2005; Hansen et al., 2005; Sahin et al., 2014; Leichtfried et al., 2015; Muench et al., 2016; Borragan et al., 2017; Rahman et al., 2017; Rodriguez-Morilla et al., 2017; Hartstein et al., 2018; Kazemi et al., 2018; Šmotek et al., 2019; Studer et al., 2019; Yang et al., 2019; Park et al., 2020; Zhou et al., 2021), standardized scales in 24 studies (Kohsaka et al., 1999; O’Brien and O’Connor, 2000; Cajochen et al., 2005; Crasson and Legros, 2005; Rüger et al., 2006; Takasu et al., 2006; Viola et al., 2008; Chellappa et al., 2012; Santhi et al., 2012; Sahin and Figueiro, 2013; Leichtfried et al., 2015; Okamoto and Nakagawa, 2015; Muench et al., 2016; Borragan et al., 2017; Rahman et al., 2017; Rodriguez-Morilla et al., 2017; Kazemi et al., 2018; Cajochen et al., 2019; Choi et al., 2019; de Zeeuw et al., 2019; Šmotek et al., 2019; te Kulve et al., 2019; Yang et al., 2019; Zhou et al., 2021), and both objective tests and standardized scales in 10 studies (Crasson and Legros, 2005; Leichtfried et al., 2015; Muench et al., 2016; Borragan et al., 2017; Rahman et al., 2017; Rodriguez-Morilla et al., 2017; Kazemi et al., 2018; Šmotek et al., 2019; Yang et al., 2019; Zhou et al., 2021). The objective tests included Psychomotor Vigilance Testing (PVT, 7 studies) (Muench et al., 2016; Borragan et al., 2017; Rahman et al., 2017; Šmotek et al., 2019; Yang et al., 2019; Park et al., 2020; Zhou et al., 2021), Go/No-Go task (3 studies) (Sahin et al., 2014; Hartstein et al., 2018; Kazemi et al., 2018), and some other attention tasks (driving simulator, Harvard Cognitive Battery, sustained attention test, attention network test, and duration-discrimination task, 5 studies in total) (Crasson and Legros, 2005; Hansen et al., 2005; Leichtfried et al., 2015; Rodriguez-Morilla et al., 2017; Studer et al., 2019), while the subjective scales included the Karolinska Sleepiness Scale (KSS, 17 studies) (Cajochen et al., 2005; Rüger et al., 2006; Viola et al., 2008; Chellappa et al., 2012; Santhi et al., 2012; Sahin and Figueiro, 2013; Okamoto and Nakagawa, 2015; Borragan et al., 2017; Rahman et al., 2017; Rodriguez-Morilla et al., 2017; Kazemi et al., 2018; Cajochen et al., 2019; Choi et al., 2019; Šmotek et al., 2019; te Kulve et al., 2019; Yang et al., 2019; Zhou et al., 2021) and the Visual Analogue Scales (VAS, 9 studies) (Kohsaka et al., 1999; O’Brien and O’Connor, 2000; Crasson and Legros, 2005; Rüger et al., 2006; Takasu et al., 2006; Leichtfried et al., 2015; Muench et al., 2016; Borragan et al., 2017; de Zeeuw et al., 2019).
Risk of bias and quality assessment
Among the 29 studies, 6 scored a “strong” global final rating (O’Brien and O’Connor, 2000; Crasson and Legros, 2005; Viola et al., 2008; Rahman et al., 2017; Hartstein et al., 2018; Studer et al., 2019), 21 scored a “moderate” global final rating (Kohsaka et al., 1999; Cajochen et al., 2005; Rüger et al., 2006; Chellappa et al., 2012; Santhi et al., 2012; Sahin and Figueiro, 2013; Sahin et al., 2014; Leichtfried et al., 2015; Okamoto and Nakagawa, 2015; Muench et al., 2016; Borragan et al., 2017; Rodriguez-Morilla et al., 2017; Kazemi et al., 2018; Cajochen et al., 2019; Choi et al., 2019; de Zeeuw et al., 2019; Šmotek et al., 2019; te Kulve et al., 2019; Yang et al., 2019; Park et al., 2020; Zhou et al., 2021), and 2 scored a “weak” global final rating (Hansen et al., 2005; Takasu et al., 2006). The 21 “moderate” studies had “weak” scorings in the blinding criteria, as they did not mention whether a blinding method was used. One study had “weak” scoring in the selection bias criteria as the method of grouping was not illustrated, and “weak” scoring in the withdrawal and dropout criteria as the sample size decreased without any reason provided (Hansen et al., 2005). Another study had “weak” scoring in the selection bias criteria, as the method of grouping was not illustrated, and “weak” scoring in the blinding criteria, as they did not mention whether the blinding method was used (Takasu et al., 2006). The results are summarized in Additional Table 1.
Additional Table 1.
The quality assessment of the included trials
| Study | Selection bias | Study design | Confounders | Blinding | Data collection | Withdrawals and dropouts | Intervention integrity | Analysis appropriate to question | Global rating |
|---|---|---|---|---|---|---|---|---|---|
| Kohsakaetal., 1999 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| O’Brien and O’Connor, 2000 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Cajochen et al., 2005 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 2 |
| Crasson and Legros, 2005 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Hansen et al., 2005 3 | 3 | 1 | 1 | 2 | 1 | 3 | 1 | 1 | 3 |
| Ruger et al., 2006 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Takasu et al., 2006 | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 3 |
| Viola et al., 2008 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| Chellappa et al., 2012 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Santhietal., 2012 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Sahin and Figueiro, 2013 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Sahinetal., 2014 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Leichtfried et al., 2015 1 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 2 |
| Okamoto and Nakagawa, 2015 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Muenchetal., 2016 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Borragan et al., 2017 1 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 1 | 2 |
| Rahman etal., 2017 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Rodriguez-Morilla et al., 2017 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Hartstein etal., 2018 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| Kazemi etal., 2018 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Cajochen etal., 2019 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Choi etal., 2019 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| deZeeuwetal., 2019 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Smotek etal., 2019 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Studer etal., 2019 1 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 1 | 1 |
| teKulve etal., 2019 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Yang etal., 2019 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Park et al., 2020 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
| Zhou etal., 2021 1 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 |
1: Strong; 2: moderate; 3: weak.
Overall intervention effects on subjective alertness
The meta-analysis of the pooled data from the 24 studies with subjective measures showed that light intervention can significantly improve subjective alertness (SMD = –0.28, 95% CI: –0.49 to –0.06, P = 0.01, I2= 58%, REM; Figure 2). The effect size was robust, as assessed by the sensitivity analyses in which the “weak” studies with low quality were excluded (SMD = –0.28, 95% CI: –0.50 to –0.06, P = 0.01, I2= 60%, REM). Using the funnel plot analysis of the outcome, the results showed that the funnel plot distribution was basically symmetrical, which indicated that the result was stable and reliable (Figure 3).
Figure 2.
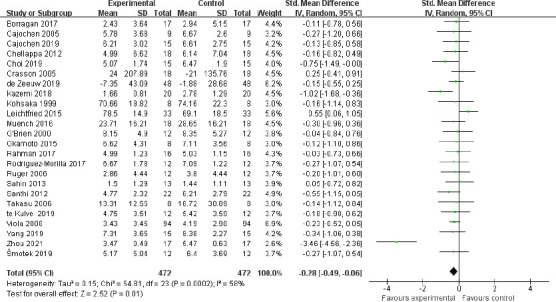
Meta-analysis of subjective alertness.
The forest plot was drawn from the effect sizes and 95% confidence intervals (CIs) of 24 independent studies using subjective scales. Each dash represents the 95% CI, and the green rectangle represents the standardized mean difference (SMD). The prismatic symbol at the bottom represents the comprehensive result of the included studies, which does not intersect with SMD = 0 and is on the left, indicating that the experimental intervention was significantly effective.
Figure 3.

Funnel plot assessing publication bias of studies with subjective measures.
The vertical line represents the meta-analysis summary estimate, and the scatter plot represents single studies. In the absence of publication bias, studies will be distributed symmetrically right and left of the vertical line. SE(SMD), standard error of the SMD; SMD: Standardized mean difference.
Subgroup analyses on subjective alertness
Data were subgrouped based on the light characteristics (wavelength, CCT, and illuminance) and timing characteristics (daytime, night-time, or all day). The results are summarized in Table 2. Data were stratified by the wavelength; compared with dim light, short-wavelength light did not significantly improve subjective alertness (SMD = –0.13, 95% CI: –0.44 to 0.19, P = 0.43, I2 = 0%). Data were stratified by the CCT; the cold light yielded a greater improvement in subjective alertness than did warm light (SMD = –0.37, 95% CI: –0.65 to –0.10, P = 0.007, I2 = 26%). Data were stratified by the illuminance; the effect of exposure to high-intensity light on alertness was not significantly different to that of exposure to low-intensity light (SMD = –0.40, 95% CI: –0.17 to 0.38, P = 0.31, I2 = 86%). Data were stratified by the timing of light exposure; the results demonstrated a significant effect size in subjective alertness for light intervention during daytime (SMD = –0.22, 95% CI: –0.37 to –0.07, P = 0.005, I2 = 74%) and night-time (SMD = –0.32, 95% CI: –0.61 to –0.02, P = 0.04, I2 = 0), but not for whole-day light intervention (SMD = –0.14, 95% CI: –0.71 to 0.44, P = 0.65, I2 = 0).
Table 2.
Subgroup analysis of the efficacy of light intervention on subjective alertness
| Subgroups | N | Sample size | I2 (%) | SMD (95% CI) | P-value |
|---|---|---|---|---|---|
| Parameters of light | 17 | 738 | 70 | –0.30 (–0.59, –0.01) | 0.04 |
| Wavelength | 4 | 156 | 0 | –0.13 (–0.44, 0.19) | NS |
| Correlated Color Temperature | 6 | 356 | 26 | –0.37 (–0.65, –0.10) | 0.007 |
| Illuminance | 7 | 226 | 86 | –0.40 (–1.17, 0.38) | NS |
| Timing of light intervention | 23 | 912 | 60 | –0.23 (–0.37, –0.10) | 0.0005 |
| Daytime | 15 | 690 | 74 | –0.22 (–0.37, –0.07) | 0.005 |
| Night-time | 6 | 176 | 0 | –0.32 (–0.61, –0.02) | 0.04 |
| Whole-day | 2 | 46 | 0 | –0.14 (–0.71, 0.44) | NS |
CI: Confidence interval; N: number of studies; NS: not significant; SMD: standardized mean difference.
Overall intervention effects on objective alertness
Although 15 studies used objective tests to assess alertness, data from 14 studies could be quantitatively combined. The aggregated data from these 14 studies showed that light intervention effectively improved objective alertness (SMD = –0.34, 95% CI = –0.68 to –0.01, P = 0.04, I2 = 74%, REM; Figure 4). The effect size was robust, as assessed by the sensitivity analyses in which the “weak” studies with low quality were excluded (SMD = –0.36, 95% CI = –0.73 to 0.00, P = 0.05, I2 = 75%, REM). The funnel plot results showed that the two sides of the graph were not completely symmetrical, which indicated a possible publication bias (Figure 5).
Figure 4.
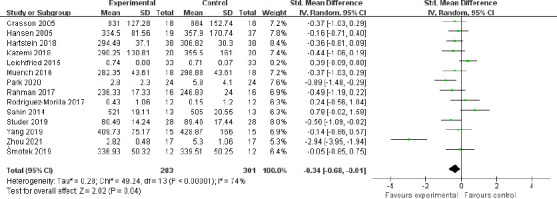
Meta-analysis of objective alertness.
The forest plot was drawn from the effect sizes and 95% confidence intervals (CIs) of 14 independent studies using objective tests. The overall effect size revealed that light intervention effectively improved objective alertness.
Figure 5.
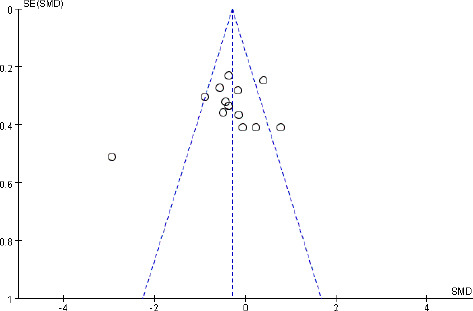
Funnel plot assessing publication bias of studies with objective tests.
There was some evidence of asymmetry on the funnel plot, indicating a possible publication bias.
Subgroup analyses on objective alertness
Studies were subgrouped according to characteristics (wavelength, CCT, and illuminance) and timing characteristics (daytime, night-time, or whole-day). The results are summarized in Table 3. Data were stratified by the wavelength; compared with long-wavelength light, short-wavelength light did not significantly improve objective alertness (SMD = –0.39, 95% CI: –0.86 to 0.07, P = 0.10, I2 = 7%). Data were stratified by the CCT; the cold light yielded a greater improvement in objective alertness than warm light (SMD = –0.36, 95% CI: –0.66 to –0.07, P = 0.02, I2 = 0). Data were stratified by the illuminance; the results showed that the effect of exposure to high-intensity light was not significantly different to that of exposure to low-intensity light (SMD = –0.69, 95% CI: –1.77 to 0.39, P = 0.21, I2 = 91%). Data were stratified by the timing of light exposure; the results revealed no significant effect of daytime light exposure (SMD = -0.35, 95% CI: –0.78 to 0.08, P = 0.11, I2 = 79%) or night-time light exposure (SMD = 0.03, 95% CI: –0.51 to 0.56, P = 0.93, I2 = 0) on objective alertness.
Table 3.
Subgroup analysis of the efficacy of light intervention on objective alertness
| Subgroups | N | Sample size | I2 (%) | SMD (95% CI) | P-value |
|---|---|---|---|---|---|
| Parameters of light | 10 | 450 | 75 | –0.44 (–0.83, –0.07) | 0.03 |
| Wavelength | 2 | 80 | 7 | –0.39 (–0.86, 0.07) | NS |
| Correlated color temperature | 4 | 178 | 0 | –0.36 (–0.66, –0.07) | 0.02 |
| Illuminance | 4 | 192 | 91 | –0.69 (–1.77, 0.39) | NS |
| Timing of light intervention | 12 | 504 | 75 | –0.29 (–0.66, 0.09) | NS |
| Daytime | 10 | 450 | 79 | –0.35 (–0.78, 0.08) | NS |
| Night-time | 2 | 54 | 0 | 0.03 (–0.51, 0.56) | NS |
CI: Confidence interval; N: number of studies; NS: not significant; SMD: standardized mean difference.
Discussion
In this meta-analysis, we included 29 studies with a total of 1210 healthy participants enrolled in light intervention and control arms. Since these studies used the same or similar methods to quantify changes in alertness, the results were combined based on subjective alertness (the KSS or VAS) and objective alertness (the PVT, Go/No-Go task or other attention tasks). The main results were as follows: (1) light intervention significantly improved subjective and objective alertness; (2) light exposure with a higher CCT was more effective than light exposure with a lower CCT; and (3) both daytime and night-time light exposure improved subjective alertness.
Our meta-analysis results revealed that light exposure with a higher CCT improved both subjective and objective alertness when compared with a lower CCT light. This is consistent with another meta-analysis, which reported that that workplace lighting with a high CCT improved alertness in daytime workers (Pachito et al., 2018). The amount of blue light in the light source spectrum increases with color temperature. It is thought that exposure to light with a higher CCT has stronger non-imaging function effects (Brainard et al., 2001; Rahman et al., 2014). Light exposure with a high CCT can induce greater melatonin suppression (Chellappa et al., 2011). Considering that melatonin is only present at night, this cannot explain why light has alerting effects during the daytime. One explanation for the effects of light on alertness is a mediating effect of thermophysiology, which is thought to be mediated by core body temperature (CBT) (te Kulve et al., 2018). Thermophysiology may mediate the effect of light on alertness in a variety of ways. Indeed, the neural pathways that are activated by light and temperature are connected to each other in the hypothalamus. On the one hand, the pathway transmitting thermal signals is connected to the medial preoptic area, which plays an important role in the regulation of body temperature and alertness. On the other hand, the pathway transmitting light signals is connected with the SCN, which plays an important role in controlling the circadian rhythm of CBT (Scheer et al., 2005) and energy expenditure (heat production) (Coomans et al., 2013). According to Saper et al. (2005), light signals transmitted by the SCN can be sent to the medial preoptic area via the dorsal subparaventricular zone. Nevertheless, the circadian rhythm of thermoregulation requires integration of the input from the SCN concerning circadian timing. Therefore, light- and temperature-transmitting systems interact with each other, which may underlie the effect of thermophysiology in the regulation of light’s effect on alertness (te Kulve et al., 2016). Nevertheless, a higher CBT has been associated with a stronger state of alertness (Wright et al., 2002; Kraeuchi, 2007). To date, many studies have investigated the effects of CCT on mental activity, the central nervous system, and alertness. These studies have shown that a high CCT can increase both parasympathetic and sympathetic nervous system activity (Mukae and Sato, 1992).
Different mechanisms have been proposed to explain the alerting effect of light, which depends on the timing of light exposure. One mechanism is related to melatonin suppression, which occurs only during biological night-time. Moreover, the baseline alertness state has been shown to follow a circadian rhythm and thus depends on the time of day (Schmidt et al., 2007; Wright et al., 2012). In the light literature, most studies have been conducted during the biological night, and these studies have consistently found increased alertness by light exposure. Our results revealed that both daytime and night-time light exposure can improve subjective alertness. No clear pattern for the timing of light intervention can be discerned from the results for objective alertness. Attenuating SCN-dependent mechanisms responsible for promoting and maintaining cortical and behavioral arousal have been implicated for night-time light exposure (Dijk and Czeisler, et al., 1995; Lavie, 1997). Nevertheless, due to the small sample size of daytime studies, the alerting effects of daytime light are less conclusive than the alerting effects of night-time light (Lok et al., 2018).
Importantly, the current results revealed that light intervention improved both subjective and objective alertness. In light studies, subjective alertness may introduce bias by simply asking individuals to rate their levels of sleepiness or fatigue, particularly when the participants may expect to be more alert in a lit environment than in darkness. These placebo effects should be taken into consideration when using subjective measures of alertness (Cajochen, 2007). In addition, self-report measures tend to be unreliable. In contrast to subjective alertness, performance measures of alertness are objective in light intervention studies. The most widely used performance measure of alertness is the PVT, which is based on reaction time to a visual or auditory stimulus. The PVT is valid, reliable, and sensitive, but it measures sustained attention rather than alertness, per se. Nevertheless, a recent review called for a multimeasure approach to evaluate the effect of light intervention on alertness (Lok et al., 2018). Although subjective and objective alertness measured by performance may differ, even within the same intervention (Van Dongen et al., 2003; Van Dongen et al., 2004; Zhou et al., 2012), the current results confirmed that light intervention improved both subjective and objective alertness.
It is well known that ipRGCs play an important role in the light-regulated effects on non-visual responses. IpRGCs signals project to the SCN (the biological clock) and then to a variety of brain areas involved in alertness, mood, and cognition. For instance, irradiance information can be transferred to the forebrain through a multisynaptic pathway involving the LC, which receives indirect SCN inputs from the dorsomedial hypothalamus (DMH) (Aston-Jones et al., 2001). Furthermore, a light-induced change in activity detected in the thalamus has been found to be related to the improvement in subjective alertness induced by light exposure (Vandewalle et al., 2006). The effect of light on the thalamus likely results in widespread cortical effects. The rodent intergeniculate leaflet of the thalamus, which corresponds to the human ventral lateral geniculate nucleus, receives strong retinal innervations (Muscatand Morin, 2006). It has strong connections with the SCN and projects to and receives afferents from numerous brainstem and hypothalamic nuclei involved in arousal regulation. The modulation of cortical activity by light results from the recruitment of multiple (multisynaptic) pathways. These modifications of cortical function can in turn lead to behavioral changes such as alertness (Vandewalle et al., 2009).
Limitations
This meta-analysis has several limitations. Firstly, the overall quality of the included studies was not particularly high, and no studies met the RCT standard. Future well-designed RCTs are needed to advance our knowledge of light intervention. Secondly, we assumed that different types of performance tasks were comparable across studies and could be combined. However, distinct tasks may reflect a construct somewhat different from alertness. For instance, three studies used the Go/No-Go task, which is generally considered to measure inhibitory control, which can only partially reflect alertness. Finally, the outcome measure of our review only included subjective scales and objective performance tests. Many studies have used physiological measures, such as electroencephalogram, pupil fluctuations, and CBT, to assess the alerting effects of light. These studies were excluded during the literature search and selection process, because their dynamic real-time indicators were difficult to quantitatively combine.
Conclusions
Our findings suggest that light exposure is associated with significant improvement in subjective alertness, as measured by both standardized scales and objective alertness assessed using performance tasks. The results of the subgroup analyses suggest that light exposure with a higher CCT more effectively improves alertness than does light exposure with a lower CCT. With regard to the timing of light intervention, both daytime and night-time light exposure improved subjective alertness. The quality of evidence was moderate or high for most outcomes.
Additional file:
Additional Table 1: The quality assessment of the included trials.
Footnotes
Conflicts of interest: The authors do not have any conflicts of interest to disclose.
Editor note: KFS is Editor-in Chief of Neural regeneration research. He is not involved in decisions about the paper which he has written himself or colleagues or whoever relate to products or services in which the editor has an interest. The submission is subject to the journal’s standard procedures, with peer-review handled independently of the relevant editor and their research groups.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Funding: This work was supported by the National Natural Science Foundation of China, No. 82172530 (to QT); Science and Technology Program of Guangdong, No. 2018B030334001 (to CRR); Guangzhou Science and Technology Project, No. 202007030012 (to QT).
References
- 1.Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J Affect Disord. 2016;198:64–71. doi: 10.1016/j.jad.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Aschoff J, Pöppel E, Wever R. Circadian rhythms in men under the influence of light-dark cycles of various periods. Pflugers Arch. 1969;306:58–70. doi: 10.1007/BF00586611. [DOI] [PubMed] [Google Scholar]
- 3.Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 4.Barger LK, Ayas NT, Cade BE, Cronin JW, Rosner B, Speizer FE, Czeisler CA. Impact of extended-duration shifts on medical errors adverse events and attentional failures. PLoS Med. 2006;3:2440–2448. doi: 10.1371/journal.pmed.0030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 6.Borragan G, Deliens G, Peigneux P, Leproult R. Bright light exposure does not prevent the deterioration of alertness induced by sustained high cognitive load demands. J Environ Psychol. 2017;51:95–103. [Google Scholar]
- 7.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–464. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Cajochen C, Freyburger M, Basishvili T, Garbazza C, Rudzik F, Renz C, Kobayashi K, Shirakawa Y, Stefani O, Weibel J. Effect of daylight LED on visual comfort melatonin mood waking performance and sleep. Light Res Technol. 2019;51:1044–1062. [Google Scholar]
- 10.Cajochen C, Münch M, Kobialka S, Kräuchi K, Steiner R, Oelhafen P, Orgül S, Wirz-Justice A. High sensitivity of human melatonin alertness thermoregulation and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 11.Cajochen C, Zeitzer JM, Czeisler CA, Dijk DJ. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 12.Chellappa SL, Steiner R, Blattner P, Oelhafen P, Goetz T, Cajochen C. Non-visual effects of light on melatonin alertness and cognitive performance: Can blue-enriched light keep us alert. PLoS One. 2011;6:e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chellappa SL, Steiner R, Oelhafen P, Lang D, Gotz T, Krebs J, Cajochen C. Acute exposure to evening blue-enriched light impacts on human sleep. J Sleep Res. 2013;22:573–580. doi: 10.1111/jsr.12050. [DOI] [PubMed] [Google Scholar]
- 14.Chellappa SL, Viola AU, Schmidt C, Bachmann V, Gabel V, Maire M, Reichert CF, Valomon A, Götz T, Landolt HP, Cajochen C. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 2012;97:E433–437. doi: 10.1210/jc.2011-2391. [DOI] [PubMed] [Google Scholar]
- 15.Choi K, Shin C, Kim T, Chung HJ, Suk HA-O. Awakening effects of blue-enriched morning light exposure on university students’ physiological and subjective responses. Sci Rep. 2019;9:345. doi: 10.1038/s41598-018-36791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coomans CP, van den Berg SA, Lucassen EA, Houben T, Pronk AC, van der Spek RD, Kalsbeek A, Biermasz NR, Willems van Dijk K, Romijn JA, Meijer JH. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62:1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crasson M, Legros JJ. Absence of daytime 50Hz 100 microT(rms) magnetic field or bright light exposure effect on human performance and psychophysiological parameters. Bioelectromagnetics. 2005;26:225–233. doi: 10.1002/bem.20070. [DOI] [PubMed] [Google Scholar]
- 18.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sánchez R, Ríos CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 19.de Zeeuw J, Papakonstantinou A, Nowozin C, Stotz S, Zaleska M, Hadel S, Bes F, Muench M, Kunz D. Living in biological darkness: objective sleepiness and the pupillary light responses are affected by different metameric lighting conditions during daytime. J Biol Rhythms. 2019;34:410–431. doi: 10.1177/0748730419847845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 21.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity sleep structure electroencephalographic slow waves and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorrian J, Hussey F, Dawson D. Train driving efficiency and safety: examining the cost of fatigue. J Sleep Res. 2007;16:1–11. doi: 10.1111/j.1365-2869.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 23.Eastman CI, Young MA, Fogg LF, Liu LW, Meaden PM. Bright light treatment of winter depression - A placebo-controlled trial. Arch Gen Psychiatry. 1998;55:883–889. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- 24.Effective Public Health Practice Project (2008) Quality assessment tool for quantitative studies. national collaborating centre for methods and tools. Hamilton, ON: McMaster University; [Google Scholar]
- 25.Hansen M, Janssen I, Schiff A, Zee PC, Dubocovich ML. The impact of school daily schedule on adolescent sleep. Pediatrics. 2005;115:1555–1561. doi: 10.1542/peds.2004-1649. [DOI] [PubMed] [Google Scholar]
- 26.Hartstein LE, Durniak MT, Karlicek RF, Jr, Berthier NE. A comparison of the effects of correlated colour temperature and gender on cognitive task performance. Light Res Technol. 2018;50:1057–1069. [Google Scholar]
- 27.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP. Cochrane handbook for systematic reviews of interventions. London: Cochrane Collaboration; 2011. [Google Scholar]
- 29.Kaida K, Takahashi M, Akerstedt T, Nakata A, Otsuka Y, Haratani T, Fukasawa K. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clin Neurophysiol. 2006;117:1574–1581. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 30.Kazemi R, Choobineh A, Taheri S, Rastipishe P. Comparing task performance visual comfort and alertness under different lighting sources: an experimental study. EXCLI J. 2018;17:1018–1029. doi: 10.17179/excli2018-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohsaka M, Fukuda N, Honma H, Kobayashi R, Sakakibara S, Koyama E, Nakano T, Matsubara H. Effects of moderately bright light on subjective evaluations in healthy elderly women. Psychiatry Clin Neurosci. 1999;53:239–241. doi: 10.1046/j.1440-1819.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- 32.Kraeuchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol Behav. 2007;90:236–245. doi: 10.1016/j.physbeh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, Katz JT, Lilly CM, Stone PH, Lockley SW, Bates DW, Czeisler CA. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351:1838–1848. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 34.Lavie P. Melatonin: role in gating nocturnal rise in sleep propensity. J Biol Rhythms. 1997;12:657–665. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- 35.Leichtfried V, Mair-Raggautz M, Schaeffer V, Hammerer-Lercher A, Mair G, Bartenbach C, Canazei M, Schobersberger W. Intense illumination in the morning hours improved mood and alertness but not mental performance. Appl Ergon. 2015;46(Pt A):54–59. doi: 10.1016/j.apergo.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 37.Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, Landrigan CP, Rothschild JM, Katz JT, Lilly CM, Stone PH, Aeschbach D, Czeisler CA, Harvard Work Hours HSG. Effect of reducing interns’ weekly work hours on sleep and attentional failures. New Engl J Med. 2004;351:1829–1837. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 38.Lok R, Smolders KCHJ, Beersma DGM, de Kort YAW. Light Alertness and alerting effects of white light: a literature overview. J Biol Rhythms. 2018;33:589–601. doi: 10.1177/0748730418796443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milosavljevic N, Cehajic-Kapetanovic J, Procyk CA, Lucas RJ. Chemogenetic activation of melanopsin retinal ganglion cells induces signatures of arousal and/or anxiety in mice. Curr Biol. 2016;26:2358–2363. doi: 10.1016/j.cub.2016.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muench M, Nowozin C, Regente J, Bes F, De Zeeuw J, Haedel S, Wahnschaffe A, Kunz D. Blue-enriched morning light as a countermeasure to light at the wrong time: effects on cognition sleepiness sleep and circadian phase. Neuropsychobiology. 2016;74:207–218. doi: 10.1159/000477093. [DOI] [PubMed] [Google Scholar]
- 42.Mukae H, Sato M. The effect of color temperature of lighting sources on the autonomic nervous functions. Ann Physiol Anthropol. 1992;11:533–538. doi: 10.2114/ahs1983.11.533. [DOI] [PubMed] [Google Scholar]
- 43.Muscat L, Morin LP. Intergeniculate leaflet: contributions to photic and non-photic responsiveness of the hamster circadian system. Neuroscience. 2006;140:305–320. doi: 10.1016/j.neuroscience.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien PM, O’Connor PJ. Effect of bright light on cycling performance. Med Sci Sports Exerc. 2000;32:439–447. doi: 10.1097/00005768-200002000-00027. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto Y, Nakagawa S. Effects of daytime light exposure on cognitive brain activity as measured by the ERP P300. Physiol Behav. 2015;138:313–318. doi: 10.1016/j.physbeh.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 46.Pachito DV, Eckeli AL, Desouky AS, Corbett MA, Partonen T, Rajaratnam SM, Riera R. Workplace lighting for improving alertness and mood in daytime workers. Cochrane Database Syst Rev. 2018;3:CD012243. doi: 10.1002/14651858.CD012243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HR, Choi SJ, Jo H, Cho JW, Joo EY. Effects of evening exposure to light from organic light-emitting diodes on melatonin and sleep. J Clin Neurol. 2020;16:401–407. doi: 10.3988/jcn.2020.16.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Posner MI. Measuring alertness. Ann N Y Acad Sci. 2008;1129:193–199. doi: 10.1196/annals.1417.011. [DOI] [PubMed] [Google Scholar]
- 49.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman SA, Flynn-Evans EE, Aeschbach D, Brainard GC, Czeisler CA, Lockley SW. Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37:271–281. doi: 10.5665/sleep.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rahman SA, St Hilaire MA, Lockley SW. The effects of spectral tuning of evening ambient light on melatonin suppression alertness and sleep. Physiol Behav. 2017;177:221–229. doi: 10.1016/j.physbeh.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodriguez-Morilla B, Madrid JA, Molina E, Correa A. Blue-enriched white light enhances physiological arousal but not behavioral performance during simulated driving at early night. Front Psychol. 2017;8:1–13. doi: 10.3389/fpsyg.2017.00997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roehrs T, Carskadon MA, Dement WC, Roth T. Daytime sleepiness and alertness. In: Principles and practice of sleep medicine. In: Kryger MH, Roth T, Dement WC, editors. 5th ed. San Diego: Elsevier Inc; 2011. pp. 42–53. [Google Scholar]
- 54.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Time-of-day-dependent effects of bright light exposure on human psychophysiology: comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–1420. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- 55.Sahin L, Figueiro MG. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol Behav. 2013;116:1–7. doi: 10.1016/j.physbeh.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 56.Sahin L, Wood BM, Plitnick B, Figueiro MG. Daytime light exposure: Effects on biomarkers measures of alertness and performance. Behav Brain Res. 2014;274:176–185. doi: 10.1016/j.bbr.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Sallinen M, Hublin C. Fatigue-inducing factors in transportation operators. Rev Hum Factors Ergon. 2015;10:138–173. [Google Scholar]
- 58.Santhi N, Thorne HC, van der Veen DR, Johnsen S, Mills SL, Hommes V, Schlangen LJM, Archer SN, Dijk D-J. The spectral composition of evening light and individual differences in the suppression of melatonin and delay of sleep in humans. J Pineal Res. 2012;53:47–59. doi: 10.1111/j.1600-079X.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 59.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 60.Scheer FA, Pirovano C, Van Someren EJ, Buijs RM. Environmental light and suprachiasmatic nucleus interact in the regulation of body temperature. Neuroscience. 2005;132:465–477. doi: 10.1016/j.neuroscience.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]
- 62.Šmotek M, Vlček P, Saifutdinova E, Kopřivová J. Objective and subjective characteristics of vigilance under different narrow-bandwidth light conditions: do shorter wavelengths have an alertness-enhancing effect. Neuropsychobiology. 2019;78:238–248. doi: 10.1159/000502962. [DOI] [PubMed] [Google Scholar]
- 63.Souman JL, Tinga AM, te Pas SF, van Ee R, Vlaskamp BNS. Acute alerting effects of light: A systematic literature review. Behav Brain Res. 2018;337:228–239. doi: 10.1016/j.bbr.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 64.Studer P, Brucker JM, Haag C, Van Doren J, Moll GH, Heinrich H, Kratz O. Effects of blue- and red-enriched light on attention and sleep in typically developing adolescents. Physiol Behav. 2019;199:11–19. doi: 10.1016/j.physbeh.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Takasu NN, Hashimoto S, Yamanaka Y, Tanahashi Y, Yamazaki A, Honma S, Honma K. Repeated exposures to daytime bright light increase nocturnal melatonin rise and maintain circadian phase in young subjects under fixed sleep schedule. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1799–1807. doi: 10.1152/ajpregu.00211.2006. [DOI] [PubMed] [Google Scholar]
- 66.te Kulve M, Schellen L, Schlangen LJ, van Marken Lichtenbelt WD. The influence of light on thermal responses. Acta Physiol (Oxf) 2016;216:163–185. doi: 10.1111/apha.12552. [DOI] [PubMed] [Google Scholar]
- 67.te Kulve M, Schlangen L, Schellen L, Souman JL, Lichtenbelt WvM. Correlated colour temperature of morning light influences alertness and body temperature. Physiol Behav. 2018;185:1–13. doi: 10.1016/j.physbeh.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 68.te Kulve M, Schlangen LJM, van Marken Lichtenbelt WD. Early evening light mitigates sleep compromising physiological and alerting responses to subsequent late evening light. Sci Rep. 2019;9:16064. doi: 10.1038/s41598-019-52352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod non-cone photoreceptor system in humans. J Physiol. 2001;535(Pt 1):261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- 71.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 72.Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, Albouy G, Darsaud A, Desseilles M, Dang-Vu TT, Peigneux P, Luxen A, Dijk DJ, Maquet P. Daytime light exposure dynamically enhances brain responses. Curr Biol. 2006;16:1616–1621. doi: 10.1016/j.cub.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 73.Vandewalle G, Schmidt C, Albouy G, Sterpenich V, Darsaud A, Rauchs G, Berken PY, Balteau E, Degueldre C, Luxen A, Maquet P, Dijk DJ. Brain responses to violet, blue, and green monochromatic light exposures in humans: prominent role of blue light and the brainstem. PLoS One. 2007;2:e1247. doi: 10.1371/journal.pone.0001247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vandewalle G, Maquet P, Dijk DJ. Light as a modulator of cognitive brain function. Trends Cogn Sci. 2009;13:429–438. doi: 10.1016/j.tics.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Viola AU, James LM, Schlangen LJ, Dijk DJ. Blue-enriched white light in the workplace improves self-reported alertness performance and sleep quality. Scand J Work Environ Health. 2008;34:297–306. doi: 10.5271/sjweh.1268. [DOI] [PubMed] [Google Scholar]
- 76.Wright KP Jr, Hull JT, Czeisler CA. Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1370–1377. doi: 10.1152/ajpregu.00205.2002. [DOI] [PubMed] [Google Scholar]
- 77.Wright KP, Lowry CA, Lebourgeois MK. Circadian and wakefulness-sleep modulation of cognition in humans. Front Mol Neurosci. 2012;5:50. doi: 10.3389/fnmol.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu Q, Lang CP. Revisiting the alerting effect of light: A systematic review. Sleep Med Rev. 2018;41:39–49. doi: 10.1016/j.smrv.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 79.Yang MQ, Chen QW, Zhu YY, Zhou Q, Geng YG, Lu CC, Wang GF, Yang CM. The effects of intermittent light during the evening on sleepiness sleep electroencephalographic spectral power and performance the next morning. Light Res Technol. 2019;51:1159–1177. [Google Scholar]
- 80.Zhou X, Ferguson SA, Matthews RW, Sargent C, Darwent D, Kennaway DJ, Roach GD. Mismatch between subjective alertness and objective performance under sleep restriction is greatest during the biological night. J Sleep Res. 2012;21:40–49. doi: 10.1111/j.1365-2869.2011.00924.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhou Y, Chen Q, Luo X, Li L, Ru T, Zhou G. Does bright light counteract the post-lunch Dip in subjective states and cognitive performance among undergraduate students. Front Public Health. 2021;9:652849. doi: 10.3389/fpubh.2021.652849. [DOI] [PMC free article] [PubMed] [Google Scholar]


