Abstract
The nerves of the peripheral nervous system are not able to effectively regenerate in cases of severe neural injury. This can result in debilitating consequences, including morbidity and lifelong impairments affecting the quality of the patient’s life. Recent findings in neural tissue engineering have opened promising avenues to apply fibrous tissue-engineered scaffolds to promote tissue regeneration and functional recovery. These scaffolds, known as neural scaffolds, are able to improve neural regeneration by playing two major roles, namely, by being a carrier for transplanted peripheral nervous system cells or biological cues and by providing structural support to direct growing nerve fibers towards the target area. However, successful implementation of scaffold-based therapeutic approaches calls for an appropriate design of the neural scaffold structure that is capable of up- and down-regulation of neuron-scaffold interactions in the extracellular matrix environment. This review discusses the main challenges that need to be addressed to develop and apply fibrous tissue-engineered scaffolds in clinical practice. It describes some promising solutions that, so far, have shown to promote neural cell adhesion and growth and a potential to repair peripheral nervous system injuries.
Key Words: electrospun scaffold, extracellular matrix, nerve conduit, neural tissue engineering, physical lumen filler, scaffold topography, structural support, surface interaction
Introduction
The peripheral nervous system (PNS) involves the ganglia and nerves that connect the central nervous system (CNS) with the limbs and peripheral organs. Although, the nerve cells of the PNS have some power of regeneration compared to the CNS, most PNS pathways with large injury gaps caused by severe nerve injury display inadequate regenerative capacity. As a result, the nerve defects and injuries in the PNS often lead to an irreversible loss of the damaged neurons and, consequently, a permanent impairment. Injuries to the PNS are formally recognized as serious public health issues and affect millions of individuals worldwide (Silver and Miller, 2004; Aijie et al., 2018).
Various treatments are available for repairing the PNS injuries; however, none of the known treatments are yet able to effectively restore the function of the damaged site. Nerve autografts from the patient, allografts and xenografts from cadavers and animals have been broadly investigated in research studies and trials. The effectiveness of nerve autografts is limited by an inadequate supply/source of donor cells, a mismatch between the damaged and donor nerves and a loss of function at the target area. Possible disease transmission and immunogenic responses limit the application of allografts and xenografts. The disadvantages associated with using the aforementioned grafts in nerve-regenerating procedures have encouraged researchers to explore other therapeutic approaches (Mackinnon and Hudson, 1992; Chen et al., 2015; Ashraf et al., 2018).
The extensive research developments behind the neural tissue engineering studies demonstrate that cell-based therapy treatments that focus on glial cell delivery and/or support can lead to at least moderately successful nerve regeneration in the injured PNS sites (Barton et al., 2017; Ghane et al., 2021). Glia offer a significant therapeutic potential due to their ability to repair and maintain the function of the neurons of the PNS and regenerate the nerve cells following injury or neuropathology. To illustrate, after a peripheral nerve injury, Schwann cells at the damaged site proliferate and secrete neurotrophins that elicit axonal regrowth and control the restoration of neurons (Chew et al., 2008; Cao et al., 2009; Barton et al., 2017; Ashraf et al., 2018; Tsui et al., 2019; Muangsanit et al., 2021). Transplanting exogenous Schwann cells can therefore potentially aid the endogenous repair processes. These cell-based modalities, however, require a suitable microenvironment for the delivery, survival and integration of the transplanted cells, as well as for supporting the endogenous Schwann cells and damaged neurons in order to restore and enhance cell-to-cell interactions and regeneration. As such, tissue-engineered scaffolds have been the focus of recent investigations as prominent means of re-connecting the damaged nerve tissue (Yi et al., 2019). These scaffolds can provide support to the transplanted cells and act as structural bridges to guide the neurite growth from the proximal to the distal nerve stump site and, consequently, aid the restoration of the nerve. The scaffold- and the cell-based approaches, therefore, offer a possibility of replacing the conventional nerve graft transplantation methods (Chang et al., 2008; Seidlits et al., 2008; Dutta et al., 2017; Aijie et al., 2018; Yi et al., 2019; Behtaj et al., 2020).
In order to offer suitable structural support to the PNS cells, the cell-supporting scaffolds should meet some fundamental requirements. For example, the scaffold should mimic the natural extracellular matrix (ECM) at the damaged site and display morphological, physical and mechanical properties close to the ECM, including a three-dimensional (3D) fibrillar network structure (Chen et al., 2015; Asadian et al., 2020; Behtaj et al., 2021a).
Recent advances in neural tissue engineering research have shown that the use of 3D fibrous substrates as supportive platforms is a highly promising approach that is capable of enhancing cell-to-scaffold interactions. Designing such scaffolds with structural and functional characteristics that mimic the ECM of the PNS is a key consideration that should lead towards successful neural scaffold developments. In order to draft the structural design of fibrous scaffolds to support the PNS cells, we first need to address the structural requirements that are needed for the implementation of fibrous scaffolds in clinical practice to treat the PNS injuries.
Retrieval Strategy
Bibliographic citation databases that were searched: Google Scholar, Science Direct, Scopus, ProQuest and Web of Knowledge. Search date: April–July 2021. Search terms include PNS, tissue engineered scaffolds, fibrous scaffolds, electrospinning, nerve conduit, regenerative medicine, surface topography and surface modifications.
Fibrous Scaffolds for Peripheral Nervous System Regeneration
Recent progress in neural tissue engineering has resulted in the development and fabrication of fibrous electrospun scaffolds which enable cells to interact with the scaffolds’ structure in a 3D environment and replicate the characteristics of the endogenous nerve (Yousefzadeh and Ghasemkhah, 2018). While this approach is promising, understanding the limitations of the various natural and synthetic scaffolds can help in the design of improvements needed to create an effective clinical product.
Electrospinning (ES) is a fibre fabrication method which uses electric force to draw fibre threads with diameters ranging from tens of microns down to a few hundreds of nanometers from liquid or solid polymer precursors. Fibrous scaffolds fabricated using the ES method have been shown to display a considerable variation in the scaffold morphology, and by altering the chemical composition of the spun polymer solutions and the ES processing parameters, including the pump speed, voltage, working distance, temperature, polymer viscosity, solvent types, fibre collection modes and other parameters a broad range of scaffolds with extended fibrous 3D networks and varying morphologies can be fabricated in a cost-effective and a relatively straight-forward way (Chang et al., 2008; Xue et al., 2017, Badea and Wu, 2018). In fact, almost 80 % of tissue-engineered scaffolds fabricated over the last two decades have been produced employing the ES method (Asadian et al., 2020).
Applications of Fibrous Scaffolds in Nerve Tissue Engineering
3D fibrous scaffolds as carriers for cell transplantation
Due to their fibrous and porous structure, 3D nanofibrous scaffolds can be loaded with cells to create a transplantable product and various cell-based scaffolds have been broadly investigated (Katiyar et al., 2019).
The porous 3D structure of the fibrous scaffolds can increase cell attachment and neurite growth enabling the exchange of fluid, supply of nutrients and removal of cell metabolic waste products, overall leading to an improved cell growth. The porous structure of the fibrous scaffolds is a major benefit as it can enable cell penetration throughout the scaffold and increase cell attachment; this is important as once cells are attached, they can generate stable cell-cell interactions and having a uniform distribution of cells will promote neurite extension through the construct. The porous nature of nanofibrous scaffolds also enables the exchange of fluid, supply of nutrients and removal of cell metabolic waste products all of which are important for improved cell growth. In contrast, constructs which do not have porous structures such as the solid structures of spheroid-based constructs which were trialed as cell-supporting carriers, have shown a limited capacity to provide an adequate fluid flow and sufficient nutrient exchange, which often result in the formation of necrotic cores and hypoxic microenvironment in studies were solid cell supporting structure were used (Watson et al., 2017). Consequently, the 3D characteristics of fibrous scaffolds such as porosity, volume, a relative pore size and fibre diameters and mesh densities have been recognized as playing significant roles in facilitating appropriate nutrient and gas exchange, as well as providing a 3D cellular infiltration and degradation rates (Balguid et al., 2009; Behtaj et al., 2021a). Individual fibre diameters also can have an effect on cellular processes, for example, larger diameter fibres (with diameters of a few microns) were found to simulate the morphology of blood vessels, whereas the smaller ones (sub-micron diameter fibres) replicate the topography of radial glial processes (Yang et al., 2021). In addition to the nanofibrous structure itself, vital biological cues can be incorporated within the scaffolds to further promote cell growth. In this context, the engineered substrates can be functionalized or loaded with drugs or neurotrophic factors to aid the seeded cells.
Fibrous scaffolds as a guidance conduit
Bridging and re-connecting a damaged nerve tissue is an important role of the neural scaffold. To achieve this goal, scaffolds which resemble the architecture of a nerve conduit were selected in earlier studies owing to their structural similarity to the tubular-like structures of nerves (Figure 1A and B). However, the hollow architecture can, potentially, lead to the axons extending through and along the internal surfaces of the conduit with only a few axons remaining in the centre of the tubular structure, which can limit the connectivity and nerve repair (Brushart et al., 1995; Yi et al., 2019; Ghane et al., 2021). Without an appropriate axon distribution across the whole width of the nerve conduit, there is a possibility of axon mismatching and, subsequently, disconnection in the target area (Gu et al., 2011).
Figure 1.
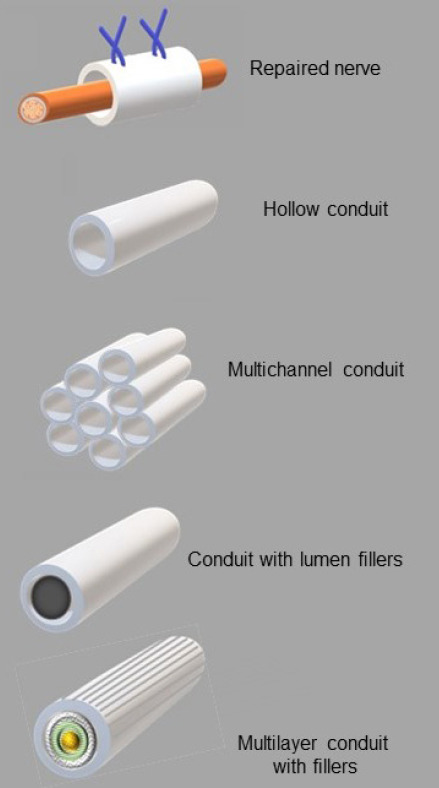
Design of various tubular constructs as nerve conduits.
The next generation of neural scaffolds that succeeded the earlier tubular model was the multi-channel nerve conduit comprised of multiple sub-micron-sized hollow tubes that were intended to mimic nerve fascicles within a nerve (Figure 1C). While this approach would have potentially resulted in a greater long-distance nerve regeneration compared to a single hollow conduit type, the effectiveness of the multichannel nerve conduit was found to be limited by its low permeability for the fluids to enter the interior of the multichannel structure and its high rigidity (De Ruiter et al., 2008; Yi et al., 2019).
Another approach that has been considered is to infuse nerve conduits with physical lumen fillers in which the filaments are embedded into the internal area of the nerve tubes (Figure 1D) allows the potential surfaces to grow on across the entire breadth of the conduit. The neurites growing through the filled lumen are able to retain their alignment, orientation and spatial arrangements, which reduce unwanted axon dispersions and increase the interactions with the distal nerves (Yi et al., 2019). To date, the ECM components such as collagen, laminin, fibrin, heparin and heparin sulphate, as well as micro- or nano-filaments coated and/or functionalized with the ECM components, are the most frequently used lumen fillers for this type of the conduit. These conduit fillers have been shown to promote neural growth in various models. Recent work by Entekhabi et al. (2021) employed collagen-hyaluronic acid sponge as a filler of an electrospun conduit to increase axonal growth in dorsal root ganglion cells.
The constructs with lumin fillers can also be loaded with cells prior to transplantation (Liu et al., 2021a; Onode et al., 2021). Debski et al. (2021) reported that a tubular-shaped structure made of electrospun fibres enriched with adipose-derived stem cells produce a high density of axons in a nerve gap of a rat sciatic nerve. Lee et al. (2021) suggested that transplanting olfactory ensheathing cells embedded in a nerve conduit in the form of a lumen filler can enhance the regeneration of injured PNS cells.
Despite the fact that the nanofibrous scaffolds themselves are quite promising for use as luminal fillers (Yi et al., 2019), these scaffolds with their own pseudo two dimensional geometries are still far from being able to offer a desired structure that is required for the ideal nerve conduits and as such, re-shaping fibrous sheets into a tubular design structure has been proposed as a possible solution. However, a possible damage to the sheet during the re-shaping process and a spontaneous folding of the sheet at the target site remain the main concerns if this approach to be implemented. The combination of additive fabrication technology with the application of ES methods may help to overcome the current challenges, and these technical solutions can lead to the next generation of truly 3D fibrous substrates, including carefully designed multilayer nerve scaffolds (Asadian et al., 2020; Liu et al., 2021b; Figure 1E).
Structural Design Considerations for Fibrous Scaffolds in the Peripheral Nervous System
Extracellular matrix mimicking scaffolds
The ultimate aim of scaffold fabrication is to reproduce the features of the natural ECM (Watson et al., 2017). The ECM of the PNS displays a non-homogeneous 3D structure and an intricate architecture with complex components consisting of many different proteins and polysaccharides which influence the cellular response (Hernández et al., 2007; Soman and Vijayavenkataraman, 2020). In the PNS, the Schwann cells and axons are covered by epineurium, perineurium and endoneurium, and the ECM is located in the endoneurium and the basal lamina of the Schwann cells. The peripheral nerve components across the site of an injury are illustrated in Figure 2.
Figure 2.
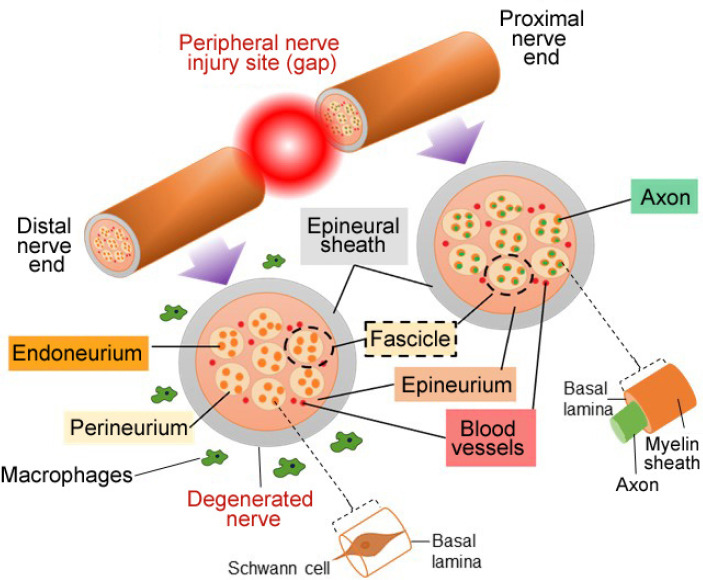
Schematic of the peripheral nerve components across an injury site.
The axons are covered by epineurium, perineurium and endoneurium; numerous axons form a nerve fascicle, with several fascicles combining to form the nerve fiber.
In the ECM, the proteins are assembled into a 3D network of nanofibres, and therefore it could be possible to mimic the ECM of the PNS by producing nanofibrous structures of chemically identical substrates. Nano-sized fibrillar features of the scaffold play a key role in increasing the cell-to-scaffold interactions and in aiding the infiltration of neuronal and glial processes, as well as by guiding the neurite outgrowth (Seidlits et al., 2008; Asadian et al., 2020). 3D nanoplatforms, with their large surface-to-volume ratios, enable cell attachment and provide in-growth sites for cell growth (Asadian et al., 2020). These 3D nano-sized architectures resemble the ECM structure quite closely by simulating the structural environment that is needed for cell growth and by modulating the neuronal behaviour, which could effectively enhance the PNS regeneration processes after injury (Cao et al., 2009; Tian et al., 2015; Aijie et al., 2018; Badea and Wu, 2018). Notably, an ideal nerve scaffold needs to display a homogeneous structure with a minimally varying tubular fibre diameter and tubular wall thickness to replicate the ECM environment and provide the rigidity, mechanical strength and biological support to bridge the lesion cavity effectively (Ghane et al., 2021).
Aligned and oriented fibers
An ideal neural network requires an appropriate direction to guide neuron growth in the repairing process (Yang et al., 2021). The alignment of fibres in the neural network is an important factor, which has been shown to have a direct influence over neurite growth and migration; neurites have been shown to grow along the fibre axes following the direction of the fibres along their lengths (Masaeli et al., 2013; Satish and Korrapati, 2017; Behtaj et al., 2021b; Ghane et al., 2021). The aligned structure of the substrates may have the ability to increase the expressions of markers for the neural progenitor cells, such as Nestin and other neurons, such as β3-tubulin and MAP2. The aligned structure and oriented 3D features can also regulate the expression of genes such as was shown with myelin-specific gene P0 expression in Schwann cells leading to the initiation of myelination (Chew et al., 2008; Aijie et al., 2018). Recently, oriented microfibres that were used as a core of a nerve conduit induced macrophages to a pro-healing phenotype which consequently promoted Schwann cell migration and myelination to increase axonal extension in an animal model of PNS nerve damage (Dong et al., 2021).
Therefore, the alignment of the scaffolds not only provides the oriented parallel guidance cue for axon growth but can also aid positive changes in various cell phenotypes to promote regeneration (Chew et al., 2008; Aijie et al., 2018; Asadian et al., 2020; Amini et al., 2021; Ghane et al., 2021).
Surface morphology
For successful neural repair and reconstruction, the interactions between the substrate surface and the neurons and the glia should be considered. Cells sense various topographic features of a substrate by receptor clustering or curvatures on the cell membrane (Santoro et al., 2017; Yang et al., 2021). Orientation, symmetry, pattern and roughness are the main features of the scaffold surface (Zhang et al., 2021). Various topographic patterns and the spatial features on a scaffold have a strong influence over the cellular behaviour, including the adhesion, migration, morphological changes, growth, differentiation and proliferation of cells (Lim and Donahue, 2007; Bramini et al., 2019; Yang et al., 2021), which all depend on the cell-to-surface receptor activation (Behtaj et al., 2020; Zhang et al., 2021). Therefore, surface topography is another key physical property that can strongly impact the biological environment of surrounding cells and tissues. Some specific topographical features such as the surface stiffness (or hardness) and the surface roughness have been shown to induce the desired signaling events by affecting the secretion of specific channel proteins from the cells (Torres et al., 2008; Blumenthal et al., 2014; Yang et al., 2021).
The topographical modification of the scaffold, including the alterations to the types, sizes and spacing of surface patterns, can be a means to guide intrinsic signaling pathways and control neural cell development (Chen et al., 2015; Zhang et al., 2021). Alteration of the scaffold surface roughness results in changes in surface energy and, consequently, enhances the adsorption and bioactivation of the ECM proteins on the surface of the scaffold (Yang et al., 2021). Common surface roughening approaches include the application of laser and plasma to the surface of synthetic or naturally inspired scaffold materials, or tools and surfaces that are used to fabricate or imprint the scaffolds. These include the use of photoablation methods and an application of conventional thermal nano- and pico-second laser light, and a non-thermal femto-second laser light irradiation, the use of ultraviolet, soft- and plasma lithography methods, electrospray and electrospinning methods and a combination of micro-modelling and lyophilization (Chen et al., 2015; Ermis et al., 2018; Ali et al., 2021). The application of these modification methods to alter the topographical features of the cell-supporting surfaces and, in addition, loading the surface with micro- or nano-sized particles result in significant changes in the surface free energy, roughness, oxidation potential and wettability characteristics and offer more favourable properties to the scaffold surface for cell adhesion and, ultimately, nerve regeneration. The subsequent neuron/axon attachment, spread and directed growth can be enhanced by increasing the interface area of the scaffold-to-cell via a higher charge transfer (Chen et al., 2015; Ghane et al., 2021). The nano-sized topography of the scaffold results in a more intimate contact between the surface and the neurons (Badea and Wu, 2018). Even though a wide range of topographical modification approaches have been used for exploring the direct interaction of cells and substrates, much uncertainty still exists about the exact effects of topographical cues on the cell fate (Santos-Ferreira et al., 2017; Bramini et al., 2019). It is, however, certain that a more detailed and clearer characterization of the cell-to-scaffold interaction mechanisms is required to modulate the cell interactions with the scaffold surface for the nerve regeneration in the PNS.
Points to Consider for Modulating the Cell Interactions with the 3D Scaffolds for the Treatment of the Injured Peripheral Nervous System Cells
A more detailed characterization of the native ECM of PNS cells and a deeper understanding of the cell-to-ECM interactions are necessary for designing novel scaffolds with suitable morphological properties. Scaffolds that closely mimic the native ECM environment would be able to incorporate different biomimetic characteristics at a molecular level to guide and align the cell growth in the desired direction.
Developing multilayer scaffolds with varying properties in different layers may improve the structure (Mu et al., 2014; Amini et al., 2021). The cellular responses to these multilayer scaffolds depend on the scaffold characteristics such as flexural properties, a range and an average fiber diameter, a relative layer porosity and the number of electrospun fiber layers. Thus, controlling these parameters during the fabrication process is essential (Rahmati et al., 2021).
The nerve conduits containing the physical lumen fillers could potentially offer an appropriate path-finding for the regenerating axons and, in this regard, the cellular and molecular mechanisms of axon path-finding can be an important area of future nerve scaffold research (Gu et al., 2011; Yi et al., 2019). Also, modulating the degradation time of the filler and optimizing the regenerating process by altering matrix concentrations and densities of the lumen fillers could bring an improved regeneration rate to the PNS cells (De Luca et al., 2014).
A succinct and reliable analysis of the mechanical properties of the target nerve tissue is yet to be developed to identify and specify ideal mechanical features of the scaffolds. The increased rigidity of nerve scaffolds can exert chronic compression of the new tissue, whereas scaffolds with low rigidity may compromise the desired outcome of the transplantation procedure (Ghane et al., 2021).
Additional biochemical and physical factors such as the immobilization of the ECM proteins and growth factors onto the scaffolds may create a more conducive microenvironment for nerve cell attachment and growth. For example, a recent study of Jahromi et al. (2021) have shown that loading the combination of gold nanoparticles and brain derived neurotrophic factor encapsulated chitosan nanoparticles on a fibrous nerve conduit increased the nerve regeneration rate in a rat sciatic nerve transection model. Generating concentration gradients of such biological factors have been suggested to direct the genesis of endogenous tissues and improve the regeneration rate (Jiang et al., 2010; Nguyen and Liu, 2013; Binan et al., 2014; De Luca et al., 2014; He et al., 2016; Patel et al., 2018; Tajdaran et al., 2019).
The extensive research findings behind the cell replacement approaches have demonstrated the potential efficacy of gene and cell therapy methods, however, more work is needed to further enhance neural regeneration (Patel et al., 2018; Behtaj and Rybachuk, 2021).
Nanotechnology advances that are able to control scaffold geometry and/or manipulate the fibrous scaffold surfaces is an emerging technology. While still in early stages, it is possible that these manufacturing techniques, including a combination of 3D printing approaches with the ES technique may be able to produce a viable alternative to autografts in patients with extensive nerve defects in the near future (Nguyen and Liu, 2013; Chen et al., 2015; Ermis et al., 2018; Rahmati et al., 2021; Zhang et al., 2021).
Although this brief review does not focus on the material selection and the desired chemical properties of scaffold surfaces, it is evident that a due consideration should be given to materials selection and scaffold morphology. Both the topographical and the chemical cues are essential for the axon outgrowth and the successful implication of the fibrous scaffolds in neural tissue engineering (Katiyar et al., 2019). As such, the application of the conductive material and/or incorporation of different conductive nanoparticles to provide electrical stimulation or piezo-electrical signaling may result in an enhanced axonal outgrowth on the scaffold (Nguyen and Liu, 2013; Ashraf et al., 2018). Moreover, achieving a desire mechanical strength, degradation rates and biocompatibility of the scaffold requires both a proper morphological structure and the use of materials with appropriate chemical and physical properties (Mu et al., 2014).
Establishing safe, highly reproducible and reliable transplantation strategies to preserve scaffold architecture during the delivery and to reduce the risk of immunological rejection is another crucial consideration (Katiyar et al., 2019). Moreover, generating innovative modalities for post-surgical intervention to decrease the rehabilitation period after transplantation are also needed (Ghane et al., 2021).
Summary and Future Research Prospects
Long-distance axonal repair of the nerves of the PNS remains a challenge based on the currently used therapeutic modalities. The research has shown that creating a supportive microenvironment can bridge the nerve injury area and lead towards an improved regeneration rate (Kim et al., 2018; Katiyar et al., 2019). The current and emerging technologies are able to fabricate electrospun scaffolds with controlled 3D spatial orientation, arrangement and structure of fibers, which are highly promising in mimicking the natural ECM of the PNS (Amini et al., 2021). However, further studies focused on the functional outcomes of the neural scaffolds are required to assess their suitability before clinical applications (Kim et al., 2018; Patel et al., 2018). Advances can be made by combining the use of a defined 3D architecture and topography of the scaffolds with essential biological guidance cues for stimulating the intrinsic regenerative capacity of the neurons and the glia. Overall, an effective clinical treatment will require a collaborative input from the areas of neuroscience, genetic engineering, cell transplantation, materials science, materials engineering and nanotechnology.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Funding: This work was supported by a Garnett-Passe and Rodney Williams Memorial Foundation grant (to JE) and a National Health and Medical Research Council grant, No. APP1183799 (to JASJ and JAKE).
References
- 1.Aijie C, Xuan L, Huimin L, Yanli Z, Yiyuan K, Yuqing L, Longquan S. Nanoscaffolds in promoting regeneration of the peripheral nervous system. Nanomedicine. 2018;13:1067–1085. doi: 10.2217/nnm-2017-0389. [DOI] [PubMed] [Google Scholar]
- 2.Ali B, Litvinyuk IV, Rybachuk M. Femtosecond laser micromachining of diamond: Current research status applications and challenges. Carbon. 2021;179:209–226. [Google Scholar]
- 3.Amini S, Salehi H, Setayeshmehr M, Ghorbani M. Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: Advantages and disadvantages. Polym Adv Technol. 2021 doi:10.1002/pat.5283. [Google Scholar]
- 4.Asadian M, Chan KV, Norouzi M, Grande S, Cools P, Morent R, De Geyter N. Fabrication and plasma modification of nanofibrous tissue engineering scaffolds. Nanomaterials. 2020;10:119. doi: 10.3390/nano10010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashraf R, Sofi HS, Beigh MA, Majeed S, Arjamand S, Sheikh FA. Prospects of natural polymeric scaffolds in peripheral nerve tissue-regeneration. In: Advances in experimental medicine and biology. In: Chun H, editor. Signapore: Springer; 2018. pp. 501–525. [DOI] [PubMed] [Google Scholar]
- 6.Badea S, Wu W. Nanoengineered biomaterials for bridging gaps in damaged nerve tissue. In: Nanoengineered biomaterials for regenerative medicine. In: Mozafari M, Rajadas J, Kaplan D, editors. Amsterdam, Netherlands: Elsevier; 2018. pp. 187–214. [Google Scholar]
- 7.Balguid A, Mol A, Van Marion MH, Bank RA, Bouten CVC, Baaijens FPT. Tailoring fiber diameter in electrospun poly(ε-Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng Part A. 2009;15:437–444. doi: 10.1089/ten.tea.2007.0294. [DOI] [PubMed] [Google Scholar]
- 8.Barton MJ, John JS, Clarke M, Wright A, Ekberg J. The glia response after peripheral nerve injury: a comparison between Schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. Int J Mol Sci. 2017;18:287. doi: 10.3390/ijms18020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behtaj S, Rybachuk M. Strategies on the application of stem cells based therapies for the treatment of optic neuropathies. Neural Regen Res. 2021;16:1190. doi: 10.4103/1673-5374.300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behtaj S, Öchsner A, Anissimov YG, Rybachuk M. Retinal tissue bioengineering materials and methods for the treatment of glaucoma. Tissue Eng Regen Med. 2020;17:253–269. doi: 10.1007/s13770-020-00254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behtaj S, Karamali F, Masaeli E, Anissimov YG, Rybachuk M. Electrospun PGS/PCL, PLLA/PCL, PLGA/PCL and pure PCL scaffolds for retinal progenitor cell cultivation. Bioche Eng J. 2021a;166:107846. [Google Scholar]
- 12.Behtaj S, Karamali F, Najafian S, Masaeli E, Esfahani MHN, Rybachuk M. The role of PGS/PCL scaffolds in promoting differentiation of human embryonic stem cells into retinal ganglion cells. Acta Biomater. 2021b;126:238–248. doi: 10.1016/j.actbio.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 13.Binan L, Ajji A, De Crescenzo G, Jolicoeur M. Approaches for neural tissue regeneration. Stem Cell Rev Rep. 2014;10:44–59. doi: 10.1007/s12015-013-9474-z. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal NR, Hermanson O, Heimrich B, Shastri VP. Stochastic nanoroughness modulates neuron–astrocyte interactions and function via mechanosensing cation channels. Proc Natl Acad Sci U S A. 2014;111:16124–16129. doi: 10.1073/pnas.1412740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bramini M, Rocchi A, Benfenati F, Cesca F. Neuronal cultures and nanomaterials. Adv Neurobiol. 2019;22:51–79. doi: 10.1007/978-3-030-11135-9_3. [DOI] [PubMed] [Google Scholar]
- 16.Brushart TM, Mathur V, Sood R, Koschorke GM. Dispersion of regenerating axons across enclosed neural gaps. J Hand Surg. 1995;20:557–564. doi: 10.1016/s0363-5023(05)80267-9. [DOI] [PubMed] [Google Scholar]
- 17.Cao H, Liu T, Chew SY. The application of nanofibrous scaffolds in neural tissue engineering. Adv Drug Deliv Rev. 2009;61:1055–1064. doi: 10.1016/j.addr.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Chang WC, Kliot M, Sretavan DW. Microtechnology and nanotechnology in nerve repair. Neurol Res. 2008;30:1053–1062. doi: 10.1179/174313208X362532. [DOI] [PubMed] [Google Scholar]
- 19.Chen C, Kong X, Lee IS. Modification of surface/neuron interfaces for neural cell-type specific responses: a review. Biomed Mater. 2015;11:014108. doi: 10.1088/1748-6041/11/1/014108. [DOI] [PubMed] [Google Scholar]
- 20.Chew SY, Mi R, Hoke A, Leong KW. The effect of the alignment of electrospun fibrous scaffolds on Schwann cell maturation. Biomaterials. 2008;29:653–661. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Luca AC, Lacour SP, Raffoul W, Di Summa PG. Extracellular matrix components in peripheral nerve repair: how to affect neural cellular response and nerve regeneration. Neural Regen Res. 2014;9:1943. doi: 10.4103/1673-5374.145366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Ruiter GC, Onyeneho IA, Liang ET, Moore MJ, Knight AM, Malessy MJ, Spinner RJ, Lu L, Currier BL, Yaszemski MJ. Methods for in vitro characterization of multichannel nerve tubes. J Biomed Mater Res A. 2008;84:643–651. doi: 10.1002/jbm.a.31298. [DOI] [PubMed] [Google Scholar]
- 23.Dębski T, Kijeńska-Gawrońska E, Zołocińska A, Siennicka K, Słysz A, Paskal W, Włodarski PK, Święszkowski W, Pojda Z. Bioactive nanofiber-based conduits in a peripheral nerve gap management—an animal model study. Int J Mol Sci. 2021;22:5588. doi: 10.3390/ijms22115588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong X, Liu S, Yang Y, Gao S, Li W, Cao J, Wan Y, Huang Z, Fan G, Chen Q, Wang H, Zhu M, Kong D. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials. 2021;272:120767. doi: 10.1016/j.biomaterials.2021.120767. [DOI] [PubMed] [Google Scholar]
- 25.Dutta RC, Dey M, Dutta AK, Basu B. Competent processing techniques for scaffolds in tissue engineering. Biotechnol Adv. 2017;35:240–250. doi: 10.1016/j.biotechadv.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Entekhabi E, Haghbin Nazarpak M, Shafieian M, Mohammadi H, Firouzi M, Hassannejad Z. Fabrication and in vitro evaluation of 3D composite scaffold based on collagen/hyaluronic acid sponge and electrospun polycaprolactone nanofibers for peripheral nerve regeneration. J Biomed Mater Res A. 2021;109:300–312. doi: 10.1002/jbm.a.37023. [DOI] [PubMed] [Google Scholar]
- 27.Ermis M, Antmen E, Hasirci V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact Mater. 2018;3:355–369. doi: 10.1016/j.bioactmat.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghane N, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Das O, Ramakrishna S. Regeneration of the peripheral nerve via multifunctional electrospun scaffolds. J Biomed Mater Res A. 2021;109:437–452. doi: 10.1002/jbm.a.37092. [DOI] [PubMed] [Google Scholar]
- 29.Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 30.He L, Tian L, Sun Y, Zhang Y, Xue W, So KF, Ramakrishna S, Wu W. Nano-engineered environment for nerve regeneration: Scaffolds functional molecules and stem cells. Curr Stem Cell Res Ther. 2016;11:605–617. doi: 10.2174/1574888x10666151001114735. [DOI] [PubMed] [Google Scholar]
- 31.Hernández JCR, Sánchez MS, Soria JM, Ribelles JLG, Pradas MM. Substrate chemistry-dependent conformations of single laminin molecules on polymer surfaces are revealed by the phase signal of atomic force microscopy. Biophys J. 2007;93:202–207. doi: 10.1529/biophysj.106.102491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jahromi M, Razavi S, Seyedebrahimi R, Reisi P, Kazemi M. Regeneration of rat sciatic nerve using PLGA conduit containing rat ADSCs with controlled release of BDNF and gold nanoparticles. J Mol Neurosci. 2021;71:746–760. doi: 10.1007/s12031-020-01694-6. [DOI] [PubMed] [Google Scholar]
- 33.Jiang X, Lim SH, Mao Hai-Quan HQ, Chew SY. Current applications and future perspectives of artificial nerve conduits. Exp Neurol. 2010;223:86–101. doi: 10.1016/j.expneurol.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Katiyar KS, Das S, Burrell JC, Kacy Cullen D. Scaffolds for bridging sciatic nerve gaps. In: Handbook of tissue engineering scaffolds. In: Mozafari M, Sefat F, Atala A, editors. San Diego: Elsevier Science & Technology; 2019. pp. 67–93. [Google Scholar]
- 35.Kim JI, Kim CS, Park CH. Harnessing nanotopography of electrospun nanofibrous nerve guide conduits (NGCs) for neural tissue engineering. Adv Exp Med Biol. 2018;1078:395–408. doi: 10.1007/978-981-13-0950-2_20. [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Kim YH, Kim BY, Jang DH, Choi SW, Joen SH, Kim H, Lee SU. Peripheral nerve regeneration using a nerve conduit with olfactory ensheathing cells in a rat model. Tissue Eng Regen Med. 2021;18:453–465. doi: 10.1007/s13770-020-00326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim JY, Donahue HJ. Cell sensing and response to micro-and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13:1879–1891. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Xie YY, Wang LD, Tai CX, Chen D, Mu D, Cui YY, Wang B. A multi-channel collagen scaffold loaded with neural stem cells for the repair of spinal cord injury. Neural Regen Res. 2021a;16:2284–2292. doi: 10.4103/1673-5374.310698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Yan J, Liu J, Wang Y, Yin J, Fu J. Fabrication of a dual-layer cell-laden tubular scaffold for nerve regeneration and bile duct reconstruction. Biofabrication. 2021b:13. doi: 10.1088/1758-5090/abf995. [DOI] [PubMed] [Google Scholar]
- 40.Mackinnon SE, Hudson AR. Clinical application of peripheral nerve transplantation. Plast Reconstr Surg. 1992;90:695–699. doi: 10.1097/00006534-199210000-00024. [DOI] [PubMed] [Google Scholar]
- 41.Masaeli E, Morshed M, Nasr-Esfahani MH, Sadri S, Hilderink J, van Apeldoorn A, van Blitterswijk CA, Moroni L. Fabrication characterization and cellular compatibility of poly (hydroxy alkanoate) composite nanofibrous scaffolds for nerve tissue engineering. PLoS One. 2013;8:e57157. doi: 10.1371/journal.pone.0057157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mu Y, Wu F, Lu Y, Wei L, Yuan W. Progress of electrospun fibers as nerve conduits for neural tissue repair. Nanomedicine. 2014;9:1869–1883. doi: 10.2217/nnm.14.70. [DOI] [PubMed] [Google Scholar]
- 43.Muangsanit P, Roberton V, Costa E, Phillips JB. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021;126:224–237. doi: 10.1016/j.actbio.2021.03.039. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen TY, Liu H. A review of current advances in biomaterials for neural tissue regeneration. Recent Pat Biomed Eng. 2013;6:29–39. [Google Scholar]
- 45.Onode E, Uemura T, Takamatsu K, Yokoi T, Shintani K, Hama S, Miyashima Y, Okada M, Nakamura H. Bioabsorbable nerve conduits three-dimensionally coated with human induced pluripotent stem cell-derived neural stem/progenitor cells promote peripheral nerve regeneration in rats. Sci Rep. 2021;11:1–13. doi: 10.1038/s41598-021-83385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel NP, Lyon KA, Huang JH. An update-tissue engineered nerve grafts for the repair of peripheral nerve injuries. Neural Regen Res. 2018;13:764–774. doi: 10.4103/1673-5374.232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rahmati M, Mills DK, Urbanska AM, Saeb MR, Venugopal JR, Ramakrishna S, Mozafari M. Electrospinning for tissue engineering applications. Prog Mater Sci. 2021;117:100721. [Google Scholar]
- 48.Santoro F, Zhao W, Joubert LM, Duan L, Schnitker J, van de Burgt Y, Lou HY, Liu B, Salleo A, Cui L. Revealing the cell–material interface with nanometer resolution by focused ion beam/scanning electron microscopy. ACS Nano. 2017;11:8320–8328. doi: 10.1021/acsnano.7b03494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos-Ferreira TF, Borsch O, Ade M. Rebuilding the missing Part—A review on photoreceptor transplantation. Front Syst Neurosci. 2017;10:105. doi: 10.3389/fnsys.2016.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satish A, Korrapati PS. Tailored release of triiodothyronine and retinoic acid from a spatio-temporally fabricated nanofiber composite instigating neuronal differentiation. Nanoscale. 2017;9:14565–14580. doi: 10.1039/c7nr05918c. [DOI] [PubMed] [Google Scholar]
- 51.Seidlits SK, Lee JY, Schmidt CE. Nanostructured scaffolds for neural applications. Nanomedicine. 2008;3:183–199. doi: 10.2217/17435889.3.2.183. [DOI] [PubMed] [Google Scholar]
- 52.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 53.Soman SS, Vijayavenkataraman S. Perspectives on 3d bioprinting of peripheral nerve conduits. Int J Mol Sci. 2020;21:1–16. doi: 10.3390/ijms21165792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tajdaran K, Chan K, Gordon T, Borschel GH. Matrices scaffolds and carriers for protein and molecule delivery in peripheral nerve regeneration. Exp Neurol. 2019;319:112817. doi: 10.1016/j.expneurol.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Tian L, Prabhakaran MP, Ramakrishna S. Strategies for regeneration of components of nervous system: scaffolds cells and biomolecules. Regen Biomater. 2015;2:31–45. doi: 10.1093/rb/rbu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Torres AJ, Wu M, Holowka D, Baird B. Nanobiotechnology and cell biology: micro-and nanofabricated surfaces to investigate receptor-mediated signaling. Annu Rev Biophys. 2008;37:265–288. doi: 10.1146/annurev.biophys.36.040306.132651. [DOI] [PubMed] [Google Scholar]
- 57.Tsui C, Koss K, Churchward MA, Todd KG. Biomaterials and glia: Progress on designs to modulate neuroinflammation. Acta Biomaterialia. 2019;83:13–28. doi: 10.1016/j.actbio.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Watson PMD, Kavanagh E, Allenby G, Vassey M. Bioengineered 3D glial cell culture systems and applications for neurodegeneration and neuroinflammation. SLAS Discov. 2017;22:583–601. doi: 10.1177/2472555217691450. [DOI] [PubMed] [Google Scholar]
- 59.Xue J, Xie J, Liu W, Xia Y. Electrospun nanofibers: new concepts materials and applications. Acc Chem Res. 2017;50:1976–1987. doi: 10.1021/acs.accounts.7b00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang CY, Huang WY, Chen LH, Liang NW, Wang HC, Lu J, Wang X, Wang TW. Neural tissue engineering: the influence of scaffold surface topography and extracellular matrix microenvironment. J Mater Chem B. 2021;9:567–584. doi: 10.1039/d0tb01605e. [DOI] [PubMed] [Google Scholar]
- 61.Yi S, Xu L, Gu X. Scaffolds for peripheral nerve repair and reconstruction. Exp Neurol. 2019;319:112761. doi: 10.1016/j.expneurol.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Yousefzadeh M, Ghasemkhah F. Design of porous, core-shell, and hollow nanofibers. In: Handbook of nanofibers. In: Barhocem A, editor. Berlin, Germany: Springer International Publishing; 2018. pp. 1–58. [Google Scholar]
- 63.Zhang W, Yang Y, Cui B. New perspectives on the roles of nanoscale surface topography in modulating intracellular signaling. Curr Opin Solid State Mater Sci. 2021;25:100873. doi: 10.1016/j.cossms.2020.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]


