Background: Failure of axon regeneration after spinal cord injury (SCI) underlies the paralysis that so profoundly affects patients’ quality of life. Many factors are involved in the regeneration failure. Chondroitin sulfate proteoglycans (CSPGs), normal constituents of the perineuronal nets in central nervous system (CNS), are secreted at the injury site and initially were thought to act as a purely physical barrier. In the past decade, the receptor-like protein tyrosine phosphatases, protein tyrosine phosphatase sigma (PTPσ), and leukocyte common antigen-related phosphatase (LAR), have been identified as transmembrane receptors for CSPGs. The two receptors for myelin-associated growth inhibitors, Nogo receptors 1 and 3 (NgR1 and NgR3) also have been found to bind with CSPGs (Sharma et al., 2012). These findings suggest that CSPGs inhibit regeneration by interacting with these receptors, initiating downstream inhibitory signaling (Figure 1).
Figure 1.
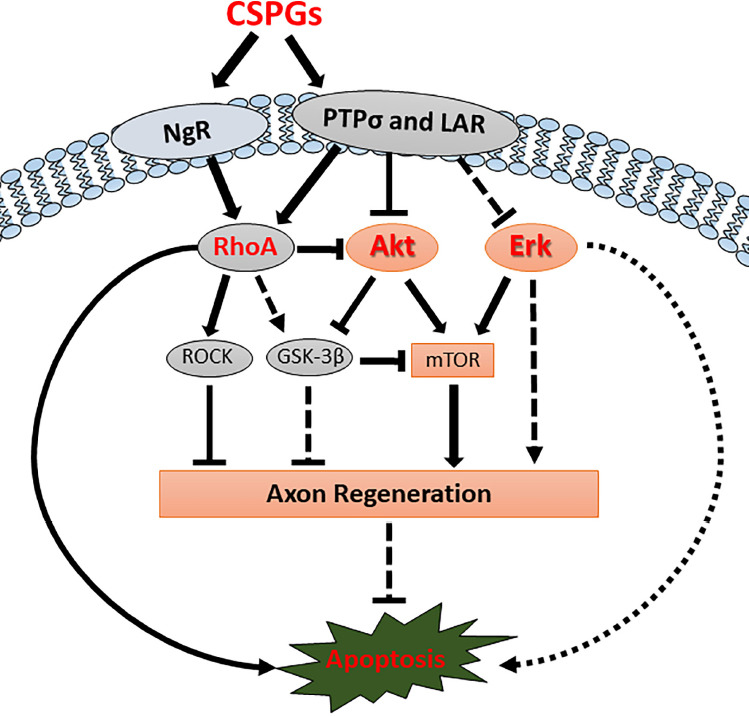
In vivo downstream signaling pathways of CSPGs and CSPG receptors.
CSPGs inhibit axon regeneration and induce retrograde apoptosis by binding CSPG receptors (PTPσ, LAR and NgRs) and affecting their downstream signaling pathways. CSPG receptors activate RhoA and inactivate Akt signaling pathways, thereby inhibiting axon regeneration and inducing retrograde neuronal apoptosis. Chondroitinase ABC treatment can remove the binding of CSPGs from CSPG receptors and re-activate the Akt signaling pathway after SCI, thus promoting axon regeneration and reducing retrograde neuronal apoptotic signaling. Erk activity has been reported to be modulated by CSPG receptors in vitro, but the specific in vivoroles after SCI are still unclear. CSPG: Chondroitin sulfate proteoglycans; Erk: extracellular regulated kinase; GSK-3β: glycogen synthase kinase-3β; LAR: leukocyte common antigen-related phosphatase; mTOR: mechanistic target of rapamycin; NgR: Nogo receptor; PTPσ: protein tyrosine phosphatase sigma; ROCK: Rho-associated kinase; SCI: spinal cord injury.
Modulations of CSPG levels or CSPG receptor activities have been intensively studied to determine their roles in axon regeneration after SCI. Many studies have shown that removing the polysaccharide side chains of CSPGs with chondroitinase ABC (ChABC) reduce their axon growth inhibitory effects. In rats with bilateral dorsal column lesions, ChABC treatment promoted growth of spinal axons and functional recovery (Bradbury and Carter, 2011). Digestion of CSPGs with ChABC enhanced sensory recovery after unilateral cervical rhizotomy, which was accomplished via reorganization of intact C7 primary afferent terminals – not by regeneration of severed afferents back into the spinal cord (Bradbury and Carter, 2011). Chondroitin sulphate N-acetylgalatosaminyl-transferase-1 knockout mice recover more completely from SCI than wild-type mice and even ChABC treated mice. Further investigation showed that the knockout mice have upregulated synthesis of heparan sulphate, which promotes axonal growth. ChABC treatment removes CSPGs, but does not increase heparan sulphate (Takeuchi et al., 2013), which can explain the limited efficacy of ChABC treatment in SCI. Taken together, these studies suggest that digestion of CSPGs with ChABC enhances axon sprouting and functional recovery after SCI. Besides the extracellular matrix CSPGs, there are proteoglycans integrated into the cell membrane, of which NG2/CSPG4 has been widely studied (Chelyshev et al., 2020). Unfortunately, opposite effects of NG2/CSPG4 on axon regeneration have been reported (Chelyshev et al., 2020). This needs further investigation.
On the receptor side, genetic disruption of PTPσ promoted axon growth into CSPG-rich regions of SCI, and transgenic deletion of LAR enhanced growth of descending axons into the area caudal to the lesion, and enhanced locomotor recovery after SCI (Xu et al., 2015). This also was true for systemic injection of small peptide inhibitors of LAR (Sharma et al., 2012) and PTPσ (Lang et al., 2015). The intrathecal administration of a soluble decoy receptor NgR1-Fc promoted axon growth and functional recovery in rats and non-human primates with SCI (Wang et al., 2020b). Since the ligands of NgR1 include not only CSPGs, but also Nogo-66 and MAGs, the beneficial effects might not be due solely to blocking of CSPGs. Moreover, there are substantial overlaps in the molecular signaling pathways between Nogo-66 and CSPGs.
The above concepts were derived from mammalian models requiring partial SCI, so it is unclear whether any of the reported beneficial treatment effects were due entirely to collateral sprouting by spared axons, or also involved true regeneration of injured axons. To avoid this ambiguity, experiments have been carried out in the completely transected lamprey spinal cord.
CSPGs and CSPG receptors in lamprey: In the lamprey, there are 18 pairs of individually identified reticulospinal (RS) neurons whose axons extend the entire length of spinal cord and therefore, are always axotomized by a complete spinal cord transection. Thus, if any of their axons are detected bridging a complete transection, it represents true axon regeneration, not collateral sprouting. Moreover, some spinal-projecting neurons in lamprey brain are good regenerators and some are bad. The latter often experience a very delayed form of apoptosis. These and other characteristics of the lamprey CNS make the results of SCI experiments very reliable and easy to interpret.
In the lamprey, CSPGs were widely distributed in the extracellular matrix and in cell bodies of the gray matter in lamprey CNS. After spinal cord transection, CSPG expression increased greatly at the site of injury. Digestion with ChABC reduced these high CSPG levels to those found in the un-injured spinal cord. CSPG digestion with ChABC significantly reduced retrograde apoptotic (activated caspase) signalling, and enhanced true axon regeneration at both early (in the proximal stump) and late (in the distal stump) stages post-transection (Hu et al., 2021).
PTPσ: Both PTPσ and LAR mRNAs were expressed primarily in neurons whose regeneration capacity is poor (bad regenerators) in both control brains and brains of lampreys with SCI. Surprisingly, knockdown of PTPσ in RS neurons with retrogradely-delivered morpholinos applied to the injury site did not enhance RS neuron survival or axonal regeneration (Hu et al., 2021). To explain these unexpected results, molecular off-target effects of morpholinos were unlikely. Perhaps the redundancy of CSPG receptors masks beneficial effects of in vivo PTPσ knockdown. It is also possible that PTPσ knockdown in cells at the site of injury, e.g., glia or infiltrating immune cells, might have indirect effects on RS axon regeneration. The digestion of CSPGs with ChABC at the site of SCI significantly reduced PTPσ mRNA expression in the perikarya of the axotomized identified RS neurons in brain, suggesting that the upregulation of PTPσ mRNA observed in RS neurons after SCI is partly due to the actions of elevated CSPGs (Hu et al., 2021). This finding indicates that the locally elevated CSPG levels at the site of injury affect the expression of PTPσ in brain retrogradely. A similar relationship between CSPGs and CSPG receptors has been suggested in mammals. PTPσ and LAR are concentrated in dystrophic, stabilized growth cones in vitro, and PTPσ levels are elevated in the lesion penumbra following SCI (Lang et al., 2015). Although these studies focused mainly on CSPG receptor levels locally at the site of injury, they are consistent with our findings in lampreys.
Retrograde apoptosis: We previously found that axotomized neurons known to be bad regenerators in lamprey brain eventually die by a very delayed form of TUNEL-positive apoptosis. To dissect the mechanisms involved in this delayed apoptosis, we adapted the use of fluorescently-labeled inhibitors of caspase activity (FLICA) to image apoptotic signaling as early as 1 week after SCI. The number of identified RS neurons containing activated caspases increased significantly post-TX, compared to controls. Digestion of CSPGs with ChABC significantly reduced the number of caspase-positive RS neurons post-TX, and this was accompanied by a reduction in PTPσ mRNA expression in the perikarya of the axotomized RS neurons (Hu et al., 2021). This had been suggested previously by the selective expression of PTPσ in “bad regenerator, bad survivor” RS neurons, which became FLICA-positive after SCI. Thus, PTPσ might play a role in retrograde neuronal apoptosis.
Akt: Knockout of phosphatase and tensin homolog (PTEN) promotes potent CNS axon regeneration after injury, and the signaling molecules downstream of PTEN that mediate this effect have been studied extensively. Akt is a critical pro-survival molecule and its activation is sufficient to promote optic nerve regeneration, but the regeneration is not as robust as that with PTEN deletion. Akt plays similar roles in the regeneration of lamprey CNS axons after SCI. ChABC treatment greatly promoted axonal regeneration after SCI, and this was accompanied by widespread enhancement of Akt activation (pAkt-308) in individual identified RS neurons (Hu et al., 2021) (Figure 1). ChABC treatment protected identified RS neurons from retrograde apoptosis. The Akt activation pattern in RS neurons post-TX correlated with the observed effect of ChABC treatment on neuronal survival. Thus, Akt activation probably contributes to the beneficial effect of ChABC treatment in protecting RS neurons from retrograde apoptosis after SCI.
Erk: In in vitro neuronal cultures, extracellular regulated kinase (Erk) is one of the signalling molecules downstream of CSPGs and their receptors PTPσ and LAR. CSPG application reduced Erk activity in neurons, but deletion of either PTPσ or LAR alone did not eliminate the Erk inactivation by CSPGs, indicating that Erk signalling mediates actions of both of these receptors in neurons (Ohtake et al., 2016) (and perhaps other unidentified receptors that may mediate CSPG-induced inhibition of axon growth). Deletion of both PTPσ and LAR resulted in greater enhancement of axon growth than either alone, indicating that although they both inactivate Erk, other elements of their downstream signalling diverge Figure 1. Systemic inhibition of PTPσ with small peptides showed that Erk is involved in peripheral axon regeneration. Studies on Drosophila larvae showed that specifically activating the Erk and Akt signalling pathways enhanced axon regeneration of the sensory neurons in both PNS and CNS (Wang et al., 2020a). These reports strongly suggest that Erk might be involved in the downstream signalling pathway after ChABC treatment (Hu et al., 2021). We also examined the expression pattern of Erk after SCI in the lamprey (Jin et al., 2020). Erk activation increased rapidly within axons and local cell bodies, most heavily within the 1–2 mm closest to the TX site at between 3 and 6 hours post-TX. Activated Erk colocalized with the retrograde transport motor protein dynein in membranous granules distributed along the axons, indicating that activated Erk may function as a retrograde signal in the lamprey CNS. But unlike descriptions in mammalian neurons in vitro and in PNS, these granules did not stain for vimentin in axotomized lamprey RS neurons. The detailed roles played by Erk and other signalling molecules in SCI, and how they are affected by ChABC treatment remain to be worked out.
Future perspectives: The ChABC itself has the characteristics of thermo-in-stability, which limits its long-lasting efficacy inside the human bodies (temperature is about 37°C). In the past few years, different methods of delivery, such as nanoparticles or synthetic scaffolds, have been explored and shown promising results in animal SCI models, but these methods still need further optimization to achieve better efficacy (Muir et al., 2019). ChABC treatment for SCI is not yet in clinical trials, while the enzyme has been assessed in a phase III trial in Japan as an alternative to surgical intervention for lumbar disc herniation, in which the ChABC is injected into the lumbar disc to digest the nucleus pulposus (Muir et al., 2019). This shed light for the potential use of ChABC for SCI treatment in humans.
In summary, a successful approach to promoting CNS axon regeneration should combine manipulations of both intracellular and extracellular mechanisms, for example, to remove extracellular inhibitors, enhance expression of intracellular pro-regeneration molecules, and promote the formation of functional synapses by newly-regenerating axons (Figure 1). A better understanding of the mechanisms that bridge the environmental and intraneuronal influences on axon regeneration will allow us to design better treatments that enhance recovery from SCI.
References
- 1.Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull. 2011;84:306–316. doi: 10.1016/j.brainresbull.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Chelyshev YA, Kabdesh IM, Mukhamedshina YO. Extracellular matrix in neural plasticity and regeneration. Cell Mol Neurobiol. 2020 doi: 10.1007/s10571-020-00986-0. doi: 10.1007/s10571-020-00986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu J, Rodemer W, Zhang G, Jin LQ, Li S, Selzer ME. Chondroitinase ABC promotes axon regeneration and reduces retrograde apoptosis signaling in lamprey. Front Cell Dev Biol. 2021;9:653638. doi: 10.3389/fcell.2021.653638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin LQ, John BH, Hu J, Selzer ME. Activated Erk is an early retrograde signal after spinal cord injury in the lamprey. Front Neurosci. 2020;14:580692. doi: 10.3389/fnins.2020.580692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang BT, Cregg JM, DePaul MA, Tran AP, Xu K, Dyck SM, Madalena KM, Brown BP, Weng YL, Li S, Karimi-Abdolrezaee S, Busch SA, Shen Y, Silver J. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muir E, De Winter F, Verhaagen J, Fawcett J. Recent advances in the therapeutic uses of chondroitinase ABC. Exp Neurol. 2019;321:113032. doi: 10.1016/j.expneurol.2019.113032. [DOI] [PubMed] [Google Scholar]
- 7.Ohtake Y, Wong D, Abdul-Muneer PM, Selzer ME, Li S. Two PTP receptors mediate CSPG inhibition by convergent and divergent signaling pathways in neurons. Sci Rep. 2016;6:37152. doi: 10.1038/srep37152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma K, Selzer ME, Li S. Scar-mediated inhibition and CSPG receptors in the CNS. Exp Neurol. 2012;237:370–378. doi: 10.1016/j.expneurol.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeuchi K, Yoshioka N, Higa Onaga S, Watanabe Y, Miyata S, Wada Y, Kudo C, Okada M, Ohko K, Oda K, Sato T, Yokoyama M, Matsushita N, Nakamura M, Okano H, Sakimura K, Kawano H, Kitagawa H, Igarashi M. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun. 2013;4:2740. doi: 10.1038/ncomms3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Fan H, Li F, Skeeters SS, Krishnamurthy VV, Song Y, Zhang K. Optical control of ERK and AKT signaling promotes axon regeneration and functional recovery of PNS and CNS in Drosophila. eLife. 2020a;9:e57395. doi: 10.7554/eLife.57395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Zhou T, Maynard GD, Terse PS, Cafferty WB, Kocsis JD, Strittmatter SM. Nogo receptor decoy promotes recovery and corticospinal growth in non-human primate spinal cord injury. Brain. 2020b;143:1697–1713. doi: 10.1093/brain/awaa116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu B, Park D, Ohtake Y, Li H, Hayat U, Liu J, Selzer ME, Longo FM, Li S. Role of CSPG receptor LAR phosphatase in restricting axon regeneration after CNS injury. Neurobiol Dis. 2015;73:36–48. doi: 10.1016/j.nbd.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


