Abstract
Introduction
The summary of product characteristics of vaccines administered intramuscularly, including the vaccine for coronavirus SARS-CoV-2 (COVID-19) and Influenza, warned for risks of bleeding in patients treated with oral anticoagulants. We aimed to estimate the incidence of major bleeding events in this setting and to compare these risks against other vaccination routes.
Methods
This systematic review included all prospective and retrospective studies enrolling anticoagulated patients that received intramuscular vaccination, published until December 2020 in CENTRAL, MEDLINE and EMBASE. The outcomes of interest were major bleeding and haematoma related with vaccination. The incidence of the outcomes was estimated through a random-effects meta-analysis using the Freeman-Turkey transformation. The results are expressed in percentages, with 95%-confidence intervals (95%CI), limited between 0 and 100%. When studies compared intramuscular vaccination vs. other route, the data were compared and pooled using random-effects meta-analysis. Risk ratios (RR) with 95%CI were reported.
Results
Overall 16 studies with 642 patients were included. No major bleeding event was reported. The pooled incidence of haematomas following vaccination (mostly against Influenza) in patients treated with oral anticoagulants (mostly warfarin; no data with DOACs / NOACs) was 0.46% (95%CI 0-1.53%). Three studies evaluated the intramuscular vs. subcutaneous route of vaccination. Intramuscular vaccines did not increase the risk of haematoma (RR 0.53, 95%CI 0.10-2.82) compared with subcutaneous route.
Conclusions
Intramuscular vaccination in anticoagulated patients is safe with very low incidence of haematomas and the best available evidence suggests that using the intramuscular route does not increase the risk of haematomas compared with the subcutaneous route.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12959-022-00367-1.
Keywords: Bleeding; Haemorrhage; Vaccine; Flu, anticoagulation
Introduction
Oral anticoagulants are used to treat or prevent thromboembolic events. Atrial fibrillation, venous thromboembolism, and mechanical prosthesis are the main indications for the use of these drugs. Despite the invasiveness of intramuscular injections, oral anticoagulation is not discontinued in vaccination contrary to what occurs before major surgeries due to the increased risk of bleeding.[1, 2] Nevertheless, the summary of product characteristics of vaccines administered intramuscularly recommend precaution in patients with coagulation disorders, due to potential risk of bleeding after intramuscular injection.[3] This issue has raised some doubts, particularly for the recent vaccine against coronavirus SARS-CoV-2 (COVID-19).[4].
To further elucidate all stakeholders about the risks of intramuscular vaccines in anticoagulated patients, we aimed to perform a systematic review to estimate the incidence of hemorrhagic complications in this setting and to compare the hemorrhagic risks of intramuscular vaccination against other routes, namely subcutaneous.
Methods
This systematic review has been developed based on the applicable aspects of Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) guidelines and Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) Checklist.[5, 6].
Types of studies included
This systematic review aimed to enrol all interventional or observational studies, including randomized controlled trials, quasi-randomized clinical trials, cohort/nested case-control studies, case-control studies, either prospective or retrospective. Studies had to include at least one arm with anticoagulated patients receiving vaccines deemed to be administrated through intramuscular route. For eligibility we considered all types of oral anticoagulation, i.e. vitamin K antagonists (warfarin, acenocoumarol, phenprocoumon, fluindione) or direct oral anticoagulants (DOACs / NOACs: dabigatran, apixaban, edoxaban, rivaroxaban) or oral anticoagulation without specifying the used drugs. Case reports and case series of bleeding events were excluded.
Types of outcome measures
The primary outcomes were: (1) the incidence of major bleeding events; (2) the incidence of local haematoma. The secondary outcome was the increase of arm circumference as a surrogate of local complication, defined as an increase of at least 1 cm or swollen arm as defined by the investigators.
Search methods for identification of studies
We searched for studies in the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE; from inception until 18th December 2020. The full search strategy is presented in the Table 1 of the Supplementary Data Appendix.
Data extraction and risk of bias evaluation
Two reviewers (BSR and MA) screened the titles and abstracts yielded by the searches against the inclusion criteria. In a second phase, the full text reports were assessed independently by the reviewers to determine whether these met the inclusion criteria. Disagreements were solved by consensus or recurring to a third party (DC). The reasons for exclusion at this stage were recorded and are detailed in Table 2 of the Supplementary Data Appendix.
The data from the individual studies identified for inclusion was introduced into a pre-piloted form. This information included: authors, year of publication; sample size; participants’ characteristics; anticoagulant used; indication for oral anticoagulation; vaccines used, measures before and after vaccination.
The risk of bias evaluation of the included studies was performed using a scale adapted from Hoy and colleagues[7, 8]. This tool evaluates the representativeness of the sample, the sampling technique, the response rate, the data collection method, the measurement tools, the case definitions, and the statistical reporting. According to this score the risk of bias of the studies were categorised as “low risk” (7-9 points), “moderate risk” (4-6 points), or “high risk” (0-3 points).[8] For randomized controlled trial evaluating the intramuscular route against others, the Cochrane Risk of Bias Tool was applied.
Meta-analysis
STATA 12.0 and RevMan 4.3 were used to synthesize the results.
For incidence calculations we used the incidence of events in the numerator and the evaluated population in the denominator. The incidence of individual and pooled studies was estimated using the Freeman-Turkey transformation (double arcsine transformation) to adjust the limiting the CI among 0-100%.[9, 10] For the comparison of intramuscular route vs. others, we used a Mantel-Haenszel method to pool the data using risk ratios (RRs).
The random effects model was used by default. If studies reported to have zero events, we applied a correction factor of 0.5 to allow for the inclusion of those studies in the analysis.[11] Statistical heterogeneity was assessed using I2, which describes the percentage of the variabilitythat is attributable to heterogeneity rather than chance.[12] Publication bias was assessed through the Egger test [13].
Results
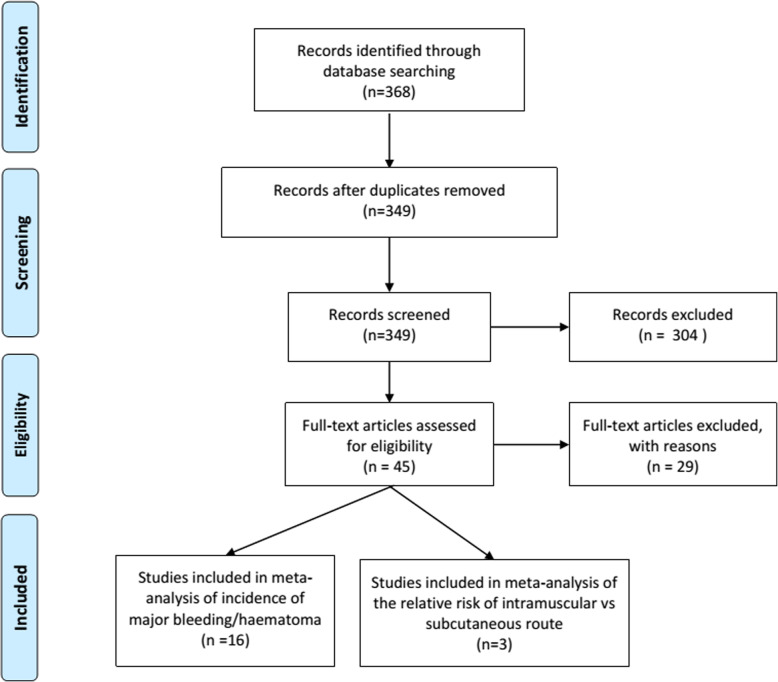
The study search yielded 368 records, from which 16 fulfilled the inclusion criteria. All the 16 studies had data (including zero events) used for the estimation of bleeding events in patients anticoagulated submitted to vaccination[14–29], and 3 studies had comparative data about the risks of intramuscular route vs. subcutaneous route [22, 24, 26] (Fig. 1). Influenza vaccination was the commonest vaccine in the included studies. Most of the studies included only patients treated with VKA. The main characteristics of the included studies are shown in Table 1.
Fig. 1.
Flowchart of studies selection
Table 1.
Studies included in the review
| Study | Sample size | Mean/ median age |
Anticoagulant | Indication for anticoagulation | Vaccine used and route(s) of administration | Follow-up |
|---|---|---|---|---|---|---|
| Patriarca 1983 | 33 | Not reported | Warfarin | Not reported |
Influenza vaccination Route not reported |
30 days |
| Lipsky 1984 | 21 | 62.5 years | Warfarin | Not reported |
Influenza vaccine Route not reported |
28-30 days |
| Kramer 1984 | 8 | Not reported | Warfarin | Not reported |
Influenza vaccine Route not reported |
21 days |
| Gomolin 1985 | 15 | Not specified (geriatric) | Warfarin | Not reported |
Influenza vaccine Route not reported |
21 days |
| Weibert 1986 | 13 | N/R | Warfarin | Not reported |
Influenza vaccine Route not reported |
14 days |
| Bussey 1988 | 24 | 60.3 years | Warfarin | Not reported |
Influenza vaccine Route not reported |
, 4 months |
| Arnold 1990 | 9 | 68 years | Warfarin | Not reported |
Influenza vaccine Route not reported |
30 days |
| Raj 1995 | 41 | 65.7 years | Warfarin | Not reported |
Influenza vaccine Route: im |
14 days |
| Delafuente 1998 | 36 | 68 years | Warfarin | Not reported |
Influenza vaccine Route: im vs. sc |
4 months |
| Paliani 2003 | 90 | 74 years | Warfarin (98%), acenocoumarol (2%) | Not reported |
Influenza vaccine Route: im |
7-10 days |
| Ballester Torrens 2005 | 59 | 72.4 years | Not specified | Atrial fibrillation (majority), valvular prosthesis (10%) |
Influenza vaccine Route: im vs. sc |
7 months |
| MacCallum 2007 |
106 (INR analysis only 78) |
73.7 years | Warfarin | Not reported |
Influenza vaccination Route not known, possibly im |
3 months |
| Casajuana 2008 | 229 | 73.6 years | Acenocoumarol (98%), warfarin (2%) | Atrial fibrillation (70%), valvular heart disease (17%), ischemic heart disease (12%) |
Influenza vaccine. Route: im vs. sc |
10 days |
| Iorio 2010 | 104 | 71.3 years | Warfarin | Atrial fibrillation (54%), venous tromboembolism (14%), aortic valve prosthesis (12%), dilated cardiomyopathy (12%), mitral valve prosthesis (6%), mitral and aortic valve prosthesis (2%) |
Influenza vaccine Route: im |
28 days |
| Van Aalsburg 2011 |
19 (im) 9 (sc) |
65 years (im) 57 years (sc) |
89% oral anticoagulants, 11% combination platelet anti-aggregate therapy | Not reported |
DTP, HepA, Hib, typhoid fever vaccine, combination of HepA and HepB Route: im HepA Route: sc |
3 days |
| Bauman 2016 | 28 | 5 years | Warfarin | Congenital heart disease (86%), Kawasaki syndrome (7%), others |
Influenza vaccine (82%), combinations of PCV, DTaP-IPV, MMR, MMRV, MenC, Hib, HepA, HepB, palivizumab Route: im, sc |
6 days (1-14 days) |
BCR British Corrected Ratio, DOACs direct oral anticoagulants, DTP diphtheria, tetanus and polio virus vaccine, DTaP-IPV diphtheria, tetanus, acellular pertussis, inactivated polio virus combination vaccine, Hib Hemophilus influenzae type B vaccine, HepA hepatitis A vaccine, HepB hepatitis B vaccine, im intramuscular, INR International Normalized Ratio, MenC conjugate meningococcal type C vaccine, MMR measles, mumps and rubella vaccine, MMRV measles, mumps rubella and varicella vaccine, PCV pneumococcal conjugate vaccine, sc subcutaneous
Risk of bias
The risk of bias of all studies was classified as moderate with score ranging between 5 and 6 (Supplementary Table 3).The major sources of bias are related to the small sample sizes, and the definition of the exposure which as deemed to be intramuscular due to the type of vaccine used in the older studies. Regarding the 3 RCTs included, the most remarkable feature of risk of bias, in particular performance bias, was the single-blinded nature of all trials (Supplementary Table 4).
Incidence of major bleeding or haematomas
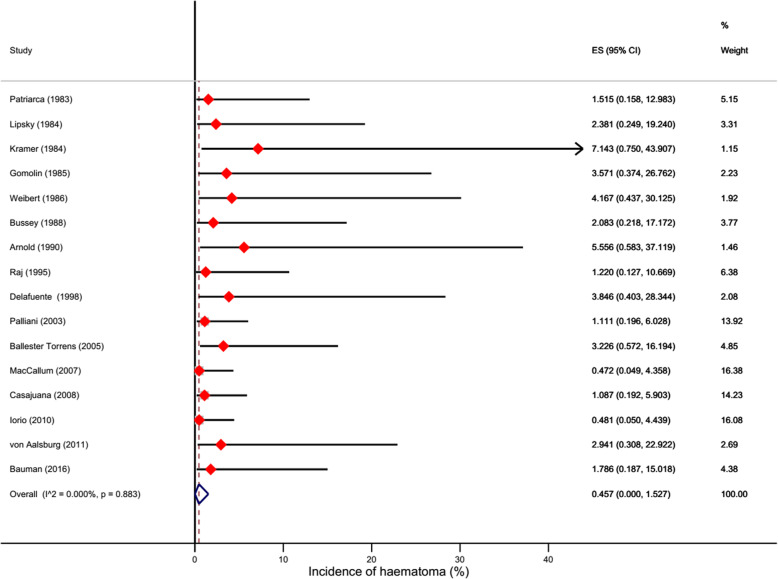
Among the included studies, no major bleeding was reported. The pooled data of 16 studies enrolling 642 anticoagulated patients showed that the estimated incidence of haematomas was 0.46% (95%CI 0-1.53%) (Fig. 2). There was no significant heterogeneity (I2=0%).
Fig. 2.
Forest plot showing the incidence of haematomas
Exploratory analyses showed that when only studies at lower risk bias were included the incidence was 0.44% (95%CI 0-1.60%). This incidence was lower but not significantly so than that estimated for higher risk of bias studies (1.80%, 95%CI 0-5.75) (Supplementary Fig. 1; Table 2). Also, different methods to handle zero events did not show substantial changes in the estimates (Table 2; Supplementary Figs. 2 and 3).
Table 2.
Results of subgroup/exploratory analyses
| Subgroup/Method | Incidence (%) | 95% Confidence interval | I2 |
|---|---|---|---|
| Moderate-Low risk of bias | 0.44 | 0.00-1.60 | 0% |
| Moderate-High risk of bias | 1.80 | 0.01-5.75 | 0% |
| Adding 0.5 to zero cells (primary approach) | 0.46 | 0.00-1.53 | 0% |
| Adding 0.1 to zero cells | 0.03 | 0.00-0.65 | 0% |
| No addition | <0.001 | 0.00-0.25 | 0% |
The Egger test was performed to raw data (i.e. without continuity correction) and it did not suggest publication bias (Supplementary Fig. 4).
Intramuscular vs. subcutaneous route for vaccination
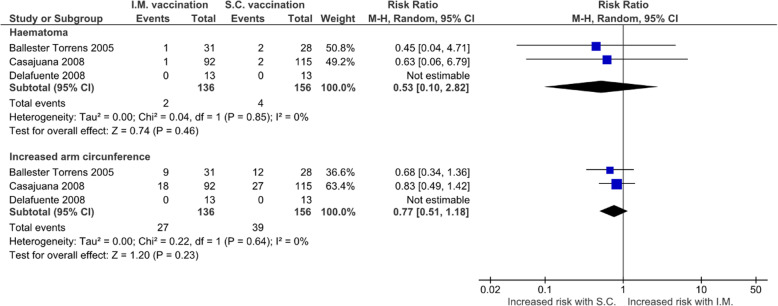
There were 3 studies reporting data about haematomas for intramuscular and subcutaneous route. Intramuscular route did not increase the risk of haematoma (RR 0.53, 95%CI 0.10-2.82; I2=0%; 2 studies, 266 patients) nor the risk of increased arm circumference (RR 0.77, 95%CI 0.51-1.18; I2=0%; 2 studies, 266 patients) (Fig. 3). Publication bias was not formally evaluated due to the small number of studies (Supplementary Fig. 5).
Fig. 3.
Forest plot showing the comparison of the risks of intramuscular vaccination and subcutaneous vaccination in anticoagulated patients
Discussion
The main results of our systematic review were: (1) There was no report of major bleeding events related with intramuscular vaccination; (2) The incidence of haematomas was very low among these patients treated with oral anticoagulants; (3) The comparative risk of haematomas through intramuscular vaccination is not higher than the subcutaneous route in patients treated with oral anticoagulants.
These results are particularly relevant for patients treated with oral anticoagulants because they are usually at high risk of cardiovascular events due to their baseline diagnosis. This high-risk group can be considered also to be at high risk of bleeding complications, and caution with previous risk/benefits ascertainments were recommended[30]. However, vaccines seem trend towards the disease prevention supporting the benefit[31–33], and our results support the absence of substantial bleeding risk. In fact, Influenza vaccination is recommended for patients with coronary disease and heart failure[34], important risk factors for atrial fibrillation, which is the most prevalent cause for needing chronic oral anticoagulation. The relevance of this topic increases with the vaccination for COVID-19 because patients with atrial fibrillation are at high-risk of mortality[35], and most of these patients are at high-risk for complications for COVID-19 and belong to priority groups.
The “COVID-19: the green book” is a British document that has guidance for vaccination anticoagulated patients.[36] It is important to mention that this book states that there are very few individuals who cannot receive the vaccines. As for care regarding patients on stable anticoagulation therapy, supratherapeutic treatment should be avoided (by confirming non-supratherapeutic International Normalised Ratio – INR in the last measure) and a fine needle (23 or 25 gauge) should be used for the vaccination, followed by firm pressure applied to the site without rubbing for at least 2 min.
These precautions are overall shared in intramuscular procedures such as the administration of botulinum toxin in neurological conditions,[37] without any safety warning. In other conditions requiring intramuscular injections, such as the administration of penicillin in patients treated with oral anticoagulants, data has shown to be safe with a low incidence of haematomas.[38].
The subcutaneous route has been considered as a possible strategy to avoid bleeding complications of vaccination in anticoagulated patients. Besides the potential problems of inadequate immunoreactivity/vaccine efficacy[39], this route did not show increased safety. In fact, in one study the subcutaneous route showed increased risk of cutaneous lesions and higher values in pain scales at 24 h.[26].
Our results are limited by the small sample sizes of the studies included. Larger population-based studies would be necessary to determine the prevalence of major bleeding events and haematomas related to intramuscular vaccination, which seems to be a rare event. The safety concerns and strict monitoring of COVID-19 vaccination could be an interesting opportunity to collect and report those data. Some studies were included deeming that the vaccination was intramuscular, however this option showed to be conservative because the studies at lower risk of bias had lower incidences of haematoma. Vitamin K antagonist are still recommended for few clinical entities, such as mechanical prosthetic heart valve or significant mitral stenosis but, nowadays, an important share of anticoagulated patients is treated with DOACs [40, 41], which were not represented in our review. Nevertheless, DOACs seem to be safer that warfarin in terms of bleeding,[42] and we cannot exclude some interaction between the vaccine and the INR in patients receiving warfarin despite many studies stating against it[25, 27, 43]. Overall, these limitations suggest that our results can be less frequent than our estimates, stressing the safety of intramuscular vaccination in this population.
Conclusions
Intramuscular vaccination in anticoagulated patients is safe, with a very low incidence of haematomas. The best available evidence suggests that using the intramuscular route does not increase the risk of haematomas compared with the subcutaneous route. Anticoagulated patients and healthcare personnel involved in vaccination should be reassured regarding intramuscular vaccinations.
Supplementary information
Acknowledgements
None.
Authors’ contributions
Conception and design: DC, FP, JJF; Acquisition of data: BSR and MA ; Analysis and interpretation: DC, BSR, MA; Drafting main manuscript: DC. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
None. This is an academic project without any governmental or non-governmental grant.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Due to methodology of systematic review and meta-analysis, ethical approval and participants consent was not needed.
Consent for publication
All authors have seen and approved the final version of the manuscript.
Competing interests
DC has participated in educational meetings and/or attended a conferences or symposia (including travel, accommodation) with Daiichi Sankyo, Menarini, Merck Serono and Roche, in the last 3 years. MA reported participation in conferences with Boehringer-Ingelheim, AstraZeneca, Bayer, Bristol-Myers-Squibb, Grünenthal, Tecnimede, Merck Sharp & Dohme. FJP had consultant and speaker fees with Astra Zeneca, Bayer, BMS, Boehringer Ingelheim and Daiichi Sankyo. JJF is a consultant for Ipsen, GlaxoSmithKline, Novartis, Teva, Lundbeck, Solvay, Abbott, BIAL, Merck-Serono, and Merz; received grants from GlaxoSmithKline, Grunenthal, Teva, and Fundação MSD.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 2.Tafur A, Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart. 2018;104(17):1461–1467. doi: 10.1136/heartjnl-2016-310581. [DOI] [PubMed] [Google Scholar]
- 3.compendium) eem. Influvac Tetra - Summary of Product Characteristcs. (Ed.^(Eds) (2020)
- 4.Agency EM. Cominarty - Summary of Product Characteristics. (Ed.^(Eds) (2021)
- 5.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 7.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen KA, Peer N, Mills EJ, Kengne AP. A Meta-Analysis of the Metabolic Syndrome Prevalence in the Global HIV-Infected Population. PLoS One, 11(3), e0150970 (2016). [DOI] [PMC free article] [PubMed]
- 9.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 11.Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26(1):53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patriarca PA, Kendal AP, Stricof RL, Weber JA, Meissner MK, Dateno B. Influenza vaccination and warfarin or theophylline toxicity in nursing-home residents. N Engl J Med. 1983;308(26):1601–1602. doi: 10.1056/NEJM198306303082615. [DOI] [PubMed] [Google Scholar]
- 15.Lipsky BA, Pecoraro RE, Roben NJ, de Blaquiere P, Delaney CJ. Influenza vaccination and warfarin anticoagulation. Ann Intern Med. 1984;100(6):835–837. doi: 10.7326/0003-4819-100-6-835. [DOI] [PubMed] [Google Scholar]
- 16.Kramer P, Tsuru M, Cook CE, McClain CJ, Holtzman JL. Effect of influenza vaccine on warfarin anticoagulation. Clin Pharmacol Ther. 1984;35(3):416–418. doi: 10.1038/clpt.1984.52. [DOI] [PubMed] [Google Scholar]
- 17.Gomolin IH, Chapron DJ, Luhan PA. Lack of effect of influenza vaccine on theophylline levels and warfarin anticoagulation in the elderly. J Am Geriatr Soc. 1985;33(4):269–272. doi: 10.1111/j.1532-5415.1985.tb07115.x. [DOI] [PubMed] [Google Scholar]
- 18.Weibert RT, Lorentz SM, Norcross WA, Klauber MR, Jagger PI. Effect of influenza vaccine in patients receiving long-term warfarin therapy. Clin Pharm. 1986;5(6):499–503. [PubMed] [Google Scholar]
- 19.Bussey HI, Saklad JJ. Effect of influenza vaccine on chronic warfarin therapy. Drug Intell Clin Pharm. 1988;22(3):198–201. doi: 10.1177/106002808802200303. [DOI] [PubMed] [Google Scholar]
- 20.Arnold WS, Mehta MK, Roberts JS. Influenza vaccine and anticoagulation control in patients receiving warfarin. Br J Clin Pract. 1990;44(4):136–139. [PubMed] [Google Scholar]
- 21.Raj G, Kumar R, McKinney WP. Safety of intramuscular influenza immunization among patients receiving long-term warfarin anticoagulation therapy. Arch Intern Med. 1995;155(14):1529–1531. doi: 10.1001/archinte.1995.00430140104011. [DOI] [PubMed] [Google Scholar]
- 22.Delafuente JC, Davis JA, Meuleman JR, Jones RA. Influenza vaccination and warfarin anticoagulation: a comparison of subcutaneous and intramuscular routes of administration in elderly men. Pharmacotherapy. 1998;18(3):631–636. [PubMed] [Google Scholar]
- 23.Paliani U, Filippucci E, Gresele P. Significant potentiation of anticoagulation by flu-vaccine during the season 2001-2002. Haematologica. 2003;88(5):599–600. [PubMed] [Google Scholar]
- 24.Ballester Torrens Mdel M, Aballí Acosta M, Maudos Pérez MT, et al. [Intramuscular route for the administration of the anti-flu vaccine in patients receiving oral anticoagulation therapy] Med Clin (Barc) 2005;124(8):291–294. doi: 10.1157/13072321. [DOI] [PubMed] [Google Scholar]
- 25.MacCallum P, Madhani M, Mt-Isa S, Ashby D. Lack of effect of influenza immunisation on anticoagulant control in patients on long-term warfarin. Pharmacoepidemiol Drug Saf. 2007;16(7):786–789. doi: 10.1002/pds.1347. [DOI] [PubMed] [Google Scholar]
- 26.Casajuana J, Iglesias B, Fàbregas M, et al. Safety of intramuscular influenza vaccine in patients receiving oral anticoagulation therapy: a single blinded multi-centre randomized controlled clinical trial. BMC Blood Disord. 2008;8:1. doi: 10.1186/1471-2326-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iorio A, Basileo M, Marcucci M, et al. Influenza vaccination and vitamin K antagonist treatment: a placebo-controlled, randomized, double-blind crossover study. Arch Intern Med. 2010;170(7):609–616. doi: 10.1001/archinternmed.2010.49. [DOI] [PubMed] [Google Scholar]
- 28.van Aalsburg R, van Genderen PJ. Vaccination in patients on anticoagulants. Travel Med Infect Dis. 2011;9(6):310–311. doi: 10.1016/j.tmaid.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Bauman ME, Hawkes M, Bruce A, Siddons S, Massicotte P. Immunizations in Children Requiring Warfarin Therapy. J Pediatr Hematol Oncol. 2016;38(8):e329-e332. doi: 10.1097/MPH.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 30.Ringwald J, Strobel J, Eckstein R. Travel and oral anticoagulation. J Travel Med. 2009;16(4):276–283. doi: 10.1111/j.1708-8305.2009.00304.x. [DOI] [PubMed] [Google Scholar]
- 31.Caldeira D, Ferreira JJ, Costa J. Influenza vaccination and prevention of cardiovascular disease mortality. Lancet. 2018;391(10119):426–427. doi: 10.1016/S0140-6736(18)30143-0. [DOI] [PubMed] [Google Scholar]
- 32.Marques Antunes M, Duarte GS, Brito D, et al. Pneumococcal vaccination in adults at very high risk or with established cardiovascular disease: systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2021;7(1):97–106. doi: 10.1093/ehjqcco/qcaa030. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues BS, David C, Costa J, Ferreira JJ, Pinto FJ, Caldeira D. Influenza vaccination in patients with heart failure: a systematic review and meta-analysis of observational studies. Heart. 2020;106(5):350–357. doi: 10.1136/heartjnl-2019-315193. [DOI] [PubMed] [Google Scholar]
- 34.Rodrigues BS, Alves M, Duarte GS, Costa J, Pinto FJ, Caldeira D. The impact of influenza vaccination in patients with cardiovascular disease: An overview of systematic reviews. Trends Cardiovasc Med, (2020). [DOI] [PubMed]
- 35.Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Atrial fibrillation and the risk of 30-day incident thromboembolic events, and mortality in adults ≥ 50 years with COVID-19. Journal of Arrhythmia, n/a(n/a)). [DOI] [PMC free article] [PubMed]
- 36.England PH. COVID-19: The green book, chapter 14a. (Ed.^(Eds) (2020)
- 37.Boulias C, Ismail F, Phadke CP, et al. A Delphi-Based Consensus Statement on the Management of Anticoagulated Patients With Botulinum Toxin for Limb Spasticity. Arch Phys Med Rehabil. 2018;99(11):2183–2189. doi: 10.1016/j.apmr.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 38.Fox E, Misko J, Rawlins M, Manning L. The risk of intramuscular haematoma is low following injection of benzathine penicillin G in patients receiving concomitant anticoagulant therapy. J Thromb Thrombolysis. 2020;50(1):237–238. doi: 10.1007/s11239-019-02013-6. [DOI] [PubMed] [Google Scholar]
- 39.Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis. 1972;125(6):656–664. doi: 10.1093/infdis/125.6.656. [DOI] [PubMed] [Google Scholar]
- 40.Caldeira D, Ferreira JJ, Pinto FJ. The era of the novel oral anticoagulants in Portugal. Rev Port Cardiol. 2017;36(7-8):577–578. doi: 10.1016/j.repc.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and Variation in Oral Anticoagulant Choice in Patients with Atrial Fibrillation, 2010-2017. Pharmacotherapy. 2018;38(9):907–920. doi: 10.1002/phar.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldeira D, Rodrigues FB, Barra M, et al. Non-vitamin K antagonist oral anticoagulants and major bleeding-related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta-analysis. Heart. 2015;101(15):1204–1211. doi: 10.1136/heartjnl-2015-307489. [DOI] [PubMed] [Google Scholar]
- 43.Jackson ML, Nelson JC, Chen RT, Davis RL, Jackson LA. Vaccines and changes in coagulation parameters in adults on chronic warfarin therapy: a cohort study. Pharmacoepidemiol Drug Saf. 2007;16(7):790–796. doi: 10.1002/pds.1386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.