Fusidic acid (FA) is an active antimicrobial agent against Gram-positive bacteria, which has been used for susceptible staphylococcal infections. It is generally well-tolerated and the adverse effects are usually limited to gastrointestinal discomfort, diarrhea, and skin rashes.1 Here, we describe the case of FA-associated thrombocytopenia and neutropenia.
A 15-year-old girl was transferred from a regional medical center with diffuse, non-fading petechial rash on the body which had been observed during the preceding 24 hours. It was learnt that she had been receiving oral FA therapy once a day for about 10 days to treat acneiform lesions on her face.
Her past medical history and family history were unremarkable for coagulopathies, malignancies, or any other chronic diseases. A complete blood count (CBC) test that was performed prior to the start of FA treatment was learnt to be completely normal. Her general condition was good, and the vital signs were within the normal limits. Upon physical examination, there were acneiform rashes tending to merge on the face, and non-itchy diffuse petechiae on the anterior and posterior surfaces of the trunk and on the dorsum of the feet, that do not fade with pressing There was no other pathological physical examination finding.
The laboratory evaluation of CBC parameters revealed bicytopenia, with white blood cell (WBC) count of 1400/µL (absolute neutrophil count: 400/µL, lymphocyte count: 600/µL), and a platelet count of 3000/µL. The hemoglobin level was 12.8 g/dL, and the mean platelet volume was 13.7 fL. The biochemical parameters were within normal limits. The C-reactive protein and procalcitonin levels were 19.2 ng/mL and 0.15 ng/mL, and the erythrocyte sedimentation rate was 31 mm/hour. The prothrombin time and activated partial thromboplastin time were 13.5 and 37.8 seconds, with an international normalized ratio of 1 : 1; the fibrinogen and D-dimer levels were 5.0 mg/dL and 1.9 µg/mL, respectively. The results of the direct Coombs test, viral serology (Hepatitis A, B, C, HIV, EBV, CMV, Toxoplasma, and Rubella), blood and urine cultures, and a nasopharyngeal swab-SARS-CoV-2 polymerase chain reaction were negative. Peripheral blood smear revealed normochromic-normocytic erythrocytes with lymphomonocytosis, and very few, large, single platelets.
FA was stopped immediately, and 1 unit of ABO blood group-matched apheresis platelet concentrate was given, but there was no improvement in the platelet count. Given that she had no constitutional symptoms, and a completely normal CBC that had been obtained 1 month earlier, immune bicytopenia was considered as the possible diagnosis and a 1 g/kg of intravenous immunoglobulin (IVIG) was administered. A CBC that was repeated 24 hours after IVIG revealed a platelet count of 8000/µL. Considering the presence of neutropenia and unresolving platelet count after IVIG therapy, an iliac bone marrow aspirate was obtained to exclude other bone marrow diseases. The examination of bone marrow aspirate revealed normal cellularity and morphology in all 3 lineages, and an increased number of megakaryocytes, with no atypical cells. No additional medication was given, as she had no active bleeding. After 72 hours, her CBC revealed resolution in bicytopenia, with WBC count of 4100/µL (absolute neutrophil count: 1700/µL, lymphocyte count: 1600/µL), and a platelet count of 73 000/µL. She was discharged without any complications. The post-discharge CBC tests at first and third week were normal (Figures 1 and 2).
Figure 1.
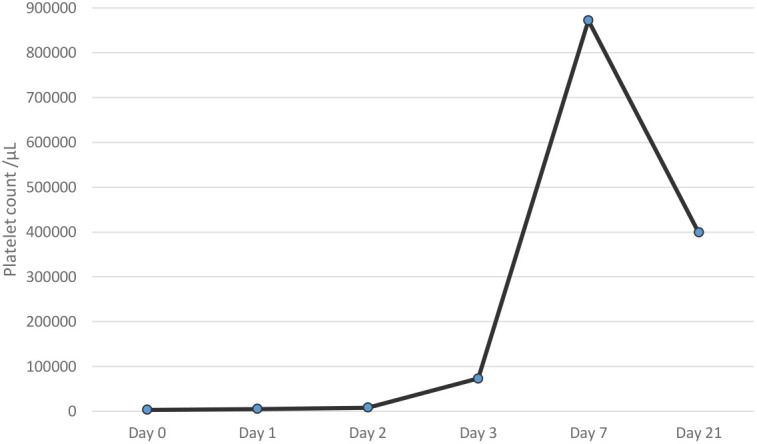
Alteration of platelet counts of the patient. Day 0: the day of admission to the pediatric emergency department.
Figure 2.

Alteration of neutrophil counts of the patient. Day 0: the day of admission to the pediatric emergency department.
There are limited data about the hematologic side effects due to FA. So far, there have been 14 cases reported in the literature.2-9 For these cases, cytopenia developed within 4-49 days after the start of FA therapy, and resolved within 2-9 days after cessation of the drug.2-9 Likewise, our patient presented with cytopenia at the 10th day of FA therapy, and cytopenia resolved within 3 days, and normal blood counts were achieved 7 days after the discontinuation of FA. The use of IVIG in our patient might have a role in the resolution of cytopenia. On the other hand, most of the patients described in the literature were reported to have spontaneous recovery within 2-9 days of cessation of FA. Most of the cases reported in the literature were adults who had chronic illnesses and received intravenous FA, with other medications.2-9 In contrast, our patient received no other medication except for FA, and had no chronic disease which could cause confusion in predicting the etiology of cytopenia. The time between the start of FA therapy and the detection of bicytopenia, as well as the normalization of CBC parameters with cessation of the drug, strongly suggested a cause and effect relationship between FA and cytopenia.
Drug-related immune thrombocytopenia is caused by accelerated platelet, and rarely, megakaryocyte destruction from drug-dependent, platelet-reactive antibodies.10,11 El Kassar et al. showed that an Ig-G antibody specifically recognizing the platelet glycoprotein IIb/IIIa only in the presence of FA was identified in the serum of their patient.9 The laboratory investigation showed an antibody–hapten reaction rather than a toxic effect of FA on stem cells and megakaryocytes.1,9,10 These findings support an immune mechanism for thrombocytopenia that is caused by FA. We could not perform antibody testing, but consistent with the literature; our patient’s bone marrow examination showed megakaryocyte hyperplasia, and there was no improvement after the transfusion of platelet concentrates, which suggests peripheral destruction. For this reason, the normalization of blood count parameters after cessation of FA, together with bone marrow aspirate findings, and in the light of previously reported cases, we conclude that the thrombocytopenia detected in our case is FA-induced immune thrombocytopenia.
Our case was also found to have neutropenia that resolved with cessation of FA. The exact mechanism by which FA causes neutropenia is not clearly understood in the literature.2 Most of the cases which were reported to develop neutropenia were treated with another antibiotic in combination with FA, especially flucloxacillin or vancomycin, potential drugs which may also induce neutropenia.2,4,6,7,9 However, the normal bone marrow findings with no myelosuppression, and the prompt recovery of neutropenia with cessation of FA in our patient also suggested a possible immune mechanism for neutropenia.
Reversible thrombocytopenia and/or neutropenia can be caused by FA. Clinicians should be vigilant of the possibility of the serious hematological side effects of the drug. It seems sensible to monitor CBCs regularly for patients who are treated with FA, to avoid such adverse reactions.
Funding Statement
The authors declared that this study has received no financial support.
Footnotes
Informed Consent: Written informed consent was obtained from the parents of the patient who participated in this case.
Peer Review: Externally peer-reviewed.
Author Contribution: Concept- N.Ş.; K.A.Ç.B.; Design- N.Ş., Ö.T., M.D.; Supervision- Ö.T., D.Y., M.D.; Materials- K.A.Ç.B., Ş.A.; Data Collection and/or Processing: N.Ş., Ş.A., K.A.Ç.B.; Analysis and/or Interpretation: Ş.A., Ö.T.; Literature review: N.Ş., Ö.T.; Writing: N.Ş.; Critical review: D.Y., Ö.T., M.D.
Conflict of Interest: The authors have no conflict of interest to declare.
References
- 1. Christiansen K. Fusidic acid adverse drug reactions. Int J Antimicrob Agents. 1999;12(suppl 2):S3–S9.. 10.1016/s0924-8579(98)00068-5) [DOI] [PubMed] [Google Scholar]
- 2. Damali Amiri N, Wijenaike N. Flucloxacillin and fusidic acid-associated neutropenia in a patient with periaortic abscess: rare side effects of commonly used antibiotics. BMJ Case Rep. 2015;2015:bcr2014208324. 10.1136/bcr-2014-208324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liao YM, Chiu CF, Ho MW, Hsueh CT. Fusidic-acid induced leukopenia and thrombocytopenia. J Chin Med Assoc. 2003;66(7):429–432.. [PubMed] [Google Scholar]
- 4. Vial T, Gontier D, Pinède L. et al. Neutropenia during treatment with fusidic acid: analysis of 5 cases. Therapie. 1995;50(5):447–450.. [PubMed] [Google Scholar]
- 5. He ZF, Chen L, Zhang JP, Wang QQ. Hepatotoxicity and hematologic complications induced by fusidic acid in a patient with hepatitis B cirrhosis: a case report. Medicine. 2019;98(45):e17852. 10.1097/MD.0000000000017852) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Revell P, Nicholson F, Pearson TC. Granulocytopenia due to fusidic acid. Lancet. 1988;2(8608):454–455.. 10.1016/s0140-6736(88)90444-8) [DOI] [PubMed] [Google Scholar]
- 7. Evans DI. Granulocytopenia due to fusidic acid. Lancet. 1988;2(8615):851. 10.1016/s0140-6736(88)92814-0) [DOI] [PubMed] [Google Scholar]
- 8. Leibowitz G, Golan D, Yeshurun D, Brezis M. Leucopenia and thrombocytopenia due to fusidic acid. Postgrad Med J. 1991;67(788):591–592.. 10.1136/pgmj.67.788.591-a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. El-Kassar N, Kalfon P, Fromont P. et al. Fusidic acid induced acute immunologic thrombocytopenia. Br J Haematol. 1996;93(2):427–431.. 10.1046/j.1365-2141.1996.5211064.x) [DOI] [PubMed] [Google Scholar]
- 10. Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357(6):580–587.. 10.1056/NEJMra066469) [DOI] [PubMed] [Google Scholar]
- 11. Arnold DM, Nazi I, Warkentin TE. et al. Approach to the diagnosis and management of drug-induced immune thrombocytopenia. Transfus Med Rev. 2013;27(3):137–145.. 10.1016/j.tmrv.2013.05.005) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a