Abstract
Introduction:
Training fires may constitute a major portion of some firefighters’ occupational exposures to smoke. However, the magnitude and composition of those exposures are not well understood and may vary by the type of training scenario and fuels.
Objectives:
To understand how structure fire training contributes to firefighters’ and instructors’ select chemical exposures, we conducted biological monitoring during exercises involving combustion of pallet and straw and oriented strand board (OSB) or the use of simulated smoke.
Methods:
Urine was analyzed for metabolites of polycyclic aromatic hydrocarbons (PAHs) and breath was analyzed for volatile organic compounds (VOCs) including benzene.
Results:
Median concentrations of nearly all PAH metabolites in urine increased from pre-to 3-hr post-training for each scenario and were highest for OSB, followed by pallet and straw, and then simulated smoke. For instructors who supervised three trainings per day, median concentrations increased at each collection. A single day of OSB exercises led to a 30-fold increase in 1-hydroxypyrene for instructors, culminating in a median endof-shift concentration 3.5-fold greater than median levels measured from firefighters in a previous controlledresidential fire study. Breath concentrations of benzene increased 2 to 7-fold immediately after the training exercises (with the exception of simulated smoke training). Exposures were highest for the OSB scenario and instructors accumulated PAHs with repeated daily exercises.
Conclusions:
Dermal absorption likely contributed to the biological levels as the respiratory route was well protected. Training academies should consider exposure risks as well as instructional objectives when selecting training exercises.
Keywords: Breath, Urine, Biomarker, Occupational exposure, Fire, Combustion products
1. Introduction
Studies suggest that firefighters have increased risk for numerous types of cancer (Daniels et al., 2014, 2015; Glass et al., 2014; Pukkala et al., 2009; Tsai et al., 2015) and the International Agency for Research on Cancer (IARC) classified occupational exposure as a firefighter to be possibly carcinogenic to humans (Group 2B) (IARC, 2010). Firefighters’ exposure to chemical carcinogens during emergency fire responses may contribute to this increased risk (Daniels et al., 2015). Firefighters could also be exposed to chemical carcinogens during training fires. A recent study found a dose-response relationship between estimated exposures from training fires and cancer incidence at a fire training college in Australia (Glass et al., 2016). The high exposure group at the fire training college had increased risk of all cancers, testicular cancer, and melanoma compared to the general population.
Training fires may constitute a major portion of some firefighters’ occupational exposures to smoke. Many fire departments require routine live-fire training for their firefighters to maintain and build proficiencies and certifications. Training institutes often utilize firefighters and officers from surrounding communities, or employ dedicated personnel, to serve as instructors. Instructors often oversee 3–5 live instructional fires per day over a combined period of several weeks or even months. This could add up to as many or more live-fire exposures (albeit in a controlled setting) than what firefighters in busy fire departments experience.
Fuels used for fire training vary, but typically follow recommendations from the National Fire Protection Association (NFPA) standard NFPA 1403 Standard on Live Fire Training Evolutions in an attempt to control the risk involved with this type of training (NFPA, 2018). Such training scenarios will often include Class A materials such as pallet and straw, which tend to produce light grey smoke for obscuring visibility, as well as elevated temperatures. In recent years, many training institutes have also begun to use engineered wood products, such as oriented strand board (OSB) in addition to the pallet and straw to generate products of combustion that more closely replicate those encountered in residential structure fires (e.g., flames “rolling” across the ceiling, darker smoke and higher temperatures) (Horn et al., 2011). Some fire training institutes have begun using simulation technologies to produce training environments with no live fire. These systems typically use theatrical smoke or pepper fog for visual obscuration; they may also incorporate propane burners or an electronic display of fire glow. While simulated smoke exercises are assumed to be less hazardous than live-fire training, chemical hazards like insoluble aerosols and formaldehyde have been measured at concentrations above or just below occupational exposure limits during these exercises (NIOSH, 2013). The relative risk of these varying approaches has not been studied in an integrated manner to allow direct comparison between fire training environments.
A relatively small number of studies have investigated firefighters’ exposures during various types of live-fire training exercises, including those that used firewood, particle chipboard, plywood, OSB, diesel fuel, and heating oil as fuel sources (Feunekes et al., 1997; Kirk and Logan, 2015; Laitinen et al., 2010; Moen and Ovrebo, 1997; Stec et al., 2018). These studies generally show that firefighters can be exposed to single-ring and polycyclic aromatic hydrocarbons (PAHs) during training fires, leading to contamination of protective clothing and skin, as well as potential for biological uptake of benzene and pyrene. However, the accumulation of toxicants from repeated training exercises, especially among instructors, has not been fully characterized.
In a recent companion paper (Fent et al., In Press-a), we reported airborne contamination levels measured during firefighting exercises that used pallet and straw alone or in concert with OSB as fuel for the fires or used simulated smoke. Generally, the magnitude of contaminants measured in air were highest for the OSB exercises, followed by pallet and straw and then simulated smoke exercises. Although the participants wore self-contained breathing apparatus (SCBA) prior to entering the structure, as is typically the case for firefighters, some biological absorption could still take place via inhalation before donning respirators while outside of the structure. Dermal absorption may also be responsible for the biological absorption of toxicants. A number of firefighter exposure studies have documented absorption of toxicants despite the consistent use of SCBA, suggesting that the dermal route contributes substantially to the dose (Fent et al., 2014; Fent et al., In Press-b; Keir et al., 2017).
Building on our previous work evaluating airborne contamination (Fent et al., In Press-a), we assessed both firefighters’ and instructors’ exposures to PAHs and volatile organic compounds (VOCs) by collecting biological specimens over a five-day period of training exercises involving a) pallet and straw, b) OSB, and c) simulated smoke. This study design provides the opportunity to investigate the biological accumulation of hazardous substances in instructors over a typical workday involving routine training exercises with broad applicability in the U.S. fire service and abroad. By following the same methodology as in the previous controlled residential fires project (Fent et al., 2018; Fent et al., In Press-b), we are also able to compare findings between exercises involving training fuels and those involving furnishings typical of a residential home.
2. Methods
2.1. Participants
This study was approved by the Institutional Review Boards at the University of Illinois and the National Institute for Occupational Safety and Health (NIOSH). All participants were required to be active members of a fire department and/or fire training organization and have completed a medical evaluation consistent with National Fire Protection Association (NFPA) 1582 in the past 12 months. Firefighters with any known cardiovascular disease, who used tobacco, were younger than 18 or older than 55 years of age, had gastrointestinal complications, or pregnant were excluded from the study. All firefighters were fit tested for the SCBA mask which they used for this study within the past 12 months. Participants were also requested to avoid eating char-grilled or smoked foods 24-hr before and during each study day and were provided a standardized meal 1 h prior to reporting for pre-firefighting data collection. Twenty-four firefighters (22 male, 2 female) and ten fire instructors (9 male, 1 female) participated in the study.
2.2. Study design
Horn et al. (2019) provides a detailed description of the study design. Briefly, two sets of five instructors (designated alpha and bravo) worked alternating days (three study days in five calendar days each). On each study day, the instructors led three training exercises with a different crew of four firefighters involved in each daily exercise (Table 1). The training exercises took ~10 min to complete with ~3 h between each daily exercise. The firefighters had about 46 h between the previous training exercise and the following pre-firefighting data collection, while the instructors had about 40 h between the last training exercise of the day and the next pre-firefighting data collection.
Table 1.
Training schedule and participant roles.
| Participant | Description | Group | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 |
|---|---|---|---|---|---|---|---|---|
| Instructors | 5 instructors, 2 assigned as stokers, and 3 assigned as officers, ~ 3-hr transpired between each daily exercise | Alpha | 3 simulated smoke exercises | Day off | 3 pallet and straw exercises | Day off | 3 OSB exercises | Day off |
| Bravo | Day off | 3 OSB exercises | Day off | 3 pallet and straw exercises | Day off | 3 simulated smoke exercises | ||
| Firefighters | 3 crews, 4 firefighters per crew, 2 firefighters assigned to attack and 2 firefighters assigned to search and rescue | Alpha | 1 simulated smoke exercise per crew | Day off | 1 pallet and straw exercise per crew | Day off | 1 OSB exercise per crew | Day off |
| Bravo | Day off | 1 OSB exercise per crew | Day off | 1 pallet and straw exercise per crew | Day off | 1 simulated smoke exercise per crew |
For all three training scenarios, the firefighters had the same objective to suppress a two-room fire and to locate and rescue two simulated occupants of the structure. The three scenarios differed primarily by fuel package and type or orientation of the structure as described below:
Pallet and straw scenario – Fires were ignited using three pine wood pallets and one bale of straw in two separate bedrooms in a single story concrete training structure. All pallets used in the study were new and had not been used for shipping or handling any materials that could potentially contaminate the wood. The structure was laid out similar to a mid-20th century single family dwelling (Supplemental Materials, Fig. S1). In all scenarios, flaming combustion was contained within the burners in the two bedrooms (did not spread to the structure or other rooms) and smoke filled the remaining rooms of the training structure, to the point of limiting visibility at crawling level. As is common in live-fire training, firefighters responded when smoke conditions reached limited visibility, which resulted in suppressing the fires when flaming combustion was still being supported by the pallets in each room. In each case, the pallets were not completely consumed prior to suppression.
OSB scenario – Fires ignited in burners using two pallets and one bale of straw along with OSB in each of two separate bedrooms in a T-shaped metal shipping container based prop (Supplemental Materials, Fig. S2). Two different types of OSB were used, identified in the paper as alpha OSB (used for the alpha groups) and bravo OSB (used for the bravo groups). Each type of OSB contained the same Engineered Wood Association APA rating for 7/16″ thickness (panel grade 24/16, exposure 1). One and half sheet of the 7/16″ alpha OSB were placed along the ceiling to provide adequate fuel supply for the training fires. Because of supply limitations, we only had access to 1/4″ sheets of the bravo OSB sheathing. One sheet of this OSB was cut in half and stacked together and then two sheets were also stacked together and placed along the ceiling. This effectively produced one and half sheets of bravo OSB with a similar thickness and orientation to the alpha OSB fuel package. According to their safety data sheets (SDS), both OSB sheathing contained phenol formaldehyde adhesive and polymeric methylene bisphenyl diisocyanate (pMDI) adhesive, but the exact volume percentage of each is unknown. The primary difference between the SDSs for the two types of OSB was that bravo OSB reported < 0.01% of free formaldehyde, while alpha OSB reported < 0.1% of free formaldehyde. Flaming combustion was isolated to the burners in each fire room and the OSB sheets along the ceiling of the rooms, while smoke migrated to the other rooms of the training structure, again banking down to the floor. For each scenario, firefighters suppressed the fires while pallet and OSB materials were still undergoing combustion as is typical in live-fire training, so these materials were not completely consumed in any trial.
Simulated smoke scenario – An electronic means of simulating a fire that also incorporated simulated smoke generation (Attack Digital Fire System, Bullex; Albany, NY) was utilized in a building constructed from metal shipping containers to have an identical layout to a mid-20th century single family dwelling (Supplemental Materials, Fig. S1). Smoke was allowed to collect throughout the structure and bank down to limit visibility, similar to the conditions common in live-fire scenarios.
The order in which the training fire environments were introduced was mirrored for the alpha and bravo groups (Table 1). Each crew was composed of two firefighters assigned to fire attack who advanced the fire hose from an engine and suppressed all active fires, and two firefighters assigned to search and rescue who performed forcible entry and then searched for and rescued two simulated victims (75 kg manikins). During each scenario, two instructors acted as stokers to light the fires and control ventilation for fire and smoke development, two instructors assigned as company officers supervised the attack teams, and the remaining instructor was the officer in charge of the search and rescue operations. The firefighters and instructors maintained these roles throughout the study.
Both the firefighters and instructors were required to wear SCBA while inside the structures during the firefighting simulation. Instructors assigned as stokers donned their SCBA masks prior to ignition, while instructors assigned as company officers and the firefighters generally donned their SCBA masks just before entry. A few donned their SCBA masks as soon as they exited the apparatus, although this was left up to the individual firefighter. Both the instructors and firefighters spent similar amounts of time inside the structures during smoky conditions (~10 min).
After each exercise, the firefighters and instructors doffed their turnout gear in a large open bay with ample ventilation and then promptly entered an adjacent climate-controlled transport container for specimen collection. Investigators performed surface sampling and wetsoap decontamination of the turnout gear as previously described (Fent et al., 2017). The firefighters’ turnout gear was decontaminated after each exercise and the instructors’ turnout gear was decontaminated at the end of each training day. Field decontamination was done because it is considered a best practice (if laundering cannot be done) and to reduce the potential for turnout gear to act as another source of exposure with subsequent use. The firefighters and instructors were also provided with cleansing wipes to use for decontaminating their skin, which all firefighters and most instructors used during rehab (within the first 10 min following each training exercise).
2.3. Urine sampling and analysis
Firefighters provided spot urine samples pre-firefighting and 3-hr post-firefighting for all training exercises (n = 24 firefighters per scenario). Previous work has indicated that 3-hr post exposure may represent peak excretion of many PAH biomarkers (Fent et al., 2014; Fent et al., In Press-b). We collected urine from instructors before the first crew’s training exercise (pre-firefighting), right after the second crew’s training exercise (~3 h after first scenario), and 3-hr after the last crew’s training exercise (~9 h after first scenario) (n = 10 instructors per scenario). The last sample collected from instructors each day represented the end-of-shift sample.
Urine samples (144 from firefighters and 90 from instructors) were shipped to the CDC National Center for Environmental Health to be analyzed for mono-hydroxylated PAH metabolites (OH-PAHs). Briefly, after enzymatic hydrolysis of conjugated OH-PAHs in urine (100 μL), the target OH-PAHs were quantified by online solid phase extraction coupled with high performance liquid chromatography-isotope dilution tandem mass spectrometry. Limits of detection (LODs) ranged from 8 to 90 ng/L, depending on the analyte (Wang et al., 2017).
Creatinine was measured using a Vitros Autoanalyzer (Johnson & Johnson, New Brunswick, NJ). Cotinine, a metabolite of nicotine, was measured using the Immulite® 2000 immunoassay system (Siemens Corp., Washington, DC). Cotinine concentrations were used to confirm current non-tobacco use status of the participants and to quantify possible exposure to environmental tobacco smoke (ETS), another source of PAH exposure (Suwan-ampai et al., 2009). The vast majority of urine samples (96%) had cotinine levels consistent with non-tobacco use status and no ETS exposure (< 10 ng/mL).
2.4. Exhaled breath sampling and analysis
Exhaled breath samples were collected from firefighters before and immediately after each scenario. Previous research has suggested that peak VOC breath concentrations occur right after firefighting (Fent et al., 2014; Fent et al., In Press-b). For instructors, breath samples were collected before the first crew’s exercise and immediately after both the second and third crew’s exercise. For the simulated smoke scenario, only two instructors and two firefighter per crew (n = 4 and 12, respectively) were sampled because we expected minimal VOC exposure during this scenario. All participating firefighters (n = 24 firefighters per scenario) and instructors (n = 10 instructors per scenario) were sampled for the other scenarios.
Breath samples were collected within 3–4 min after doffing SCBA. Participants were instructed to take a deep breath in and then forcefully exhale their entire breath into the Bio-VOC™ sampler (Markes International, Inc., Cincinnati, OH), which serves to collect the final 129- mL of breath. The collected air was pushed through Markes thermal desorption tubes (Carbograph 2TD/1TD dual bed tubes). The thermal desorption tubes were capped and stored at −20 °C until shipment to the U.S. Environmental Protection Agency analytical laboratory.
The method used to analyze the breath samples for benzene, toluene, ethylbenzene, and styrene is described in detail elsewhere (Geer Wallace et al., 2017). Method detection limits ranged from 0.14 ng/ tube (styrene) to 1.1 ng/tube (ethylbenzene). The ng on tube was converted to ng/L by dividing by the total breath volume collected (129 mL) and results are reported as parts per billion by volume (ppbv).
2.5. Data analysis
We used instrumental readings for the OH-PAH metabolite results < LOD (3.7%). The OH-PAH concentrations were normalized by creatinine. To simplify the analyses, the individual OH-PAH concentrations of each parent compound were summed together to create the following variables: hydroxyphenanthrenes, hydroxynaphthalenes, and hydroxyfluorenes. In addition, some analyses were performed on the sum of all OH-PAH concentrations (∑OH-PAHs).
Benzene, toluene, ethylbenzene, and styrene were non-detectable in 21, 8.1, 69, and 44% of the breath samples. We estimated breath concentrations < LOD using ordered imputations and Q-Q plots as described in Pleil et al. (Pleil, 2016a, b). This method relies on plotting the natural log of the compound concentrations (minus non-detects) versus the Z-scores to obtain a linear best fit equation. This equation is then used to impute values for the samples with concentrations < LOD by plugging the corresponding calculated Z-scores into the obtained equation.
All statistical analyses were performed in R version 3.4.3. Quartiles were used to summarize data. Since data were skewed, values were log-transformed for all statistical analyses. The Shapiro-Wilk test for normality was performed on the log-transformed data and it was determined that data did not violate the normality assumption. The difference between pre and post measurements were calculated and, since each individual participated in multiple scenarios, mixed linear models were used to control for repeated measures in testing whether the differences were different than 0 as well as to compare differences between groups.
3. Results
3.1. Urinary excretion of PAHs after training exercises
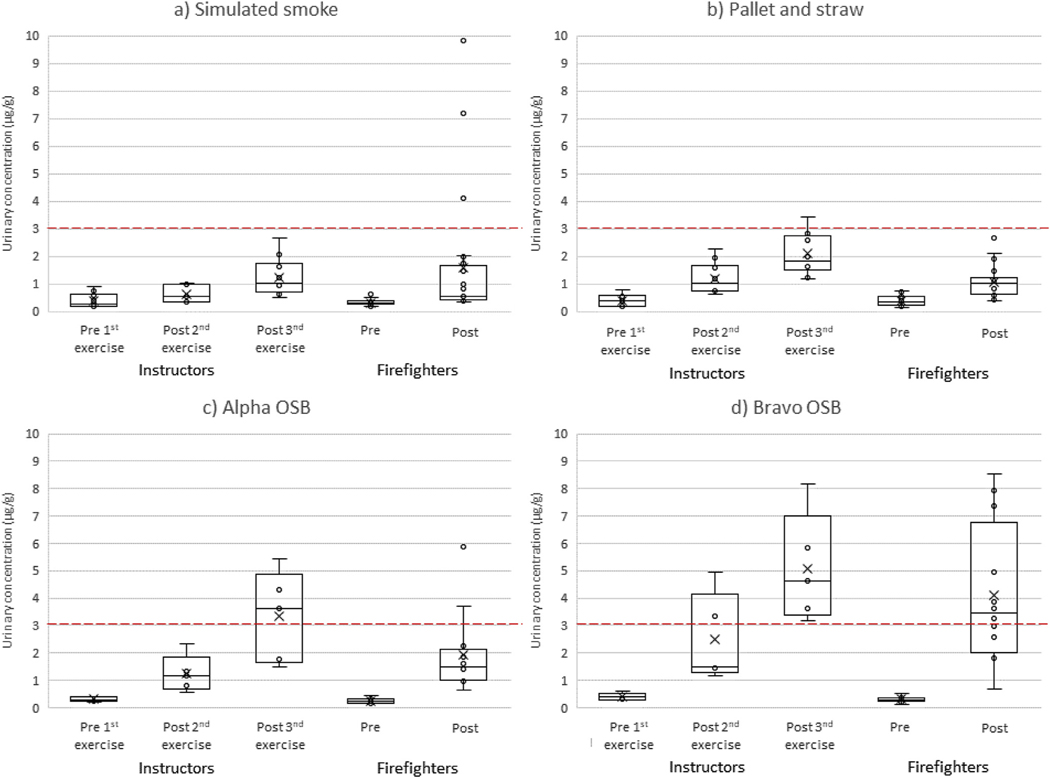
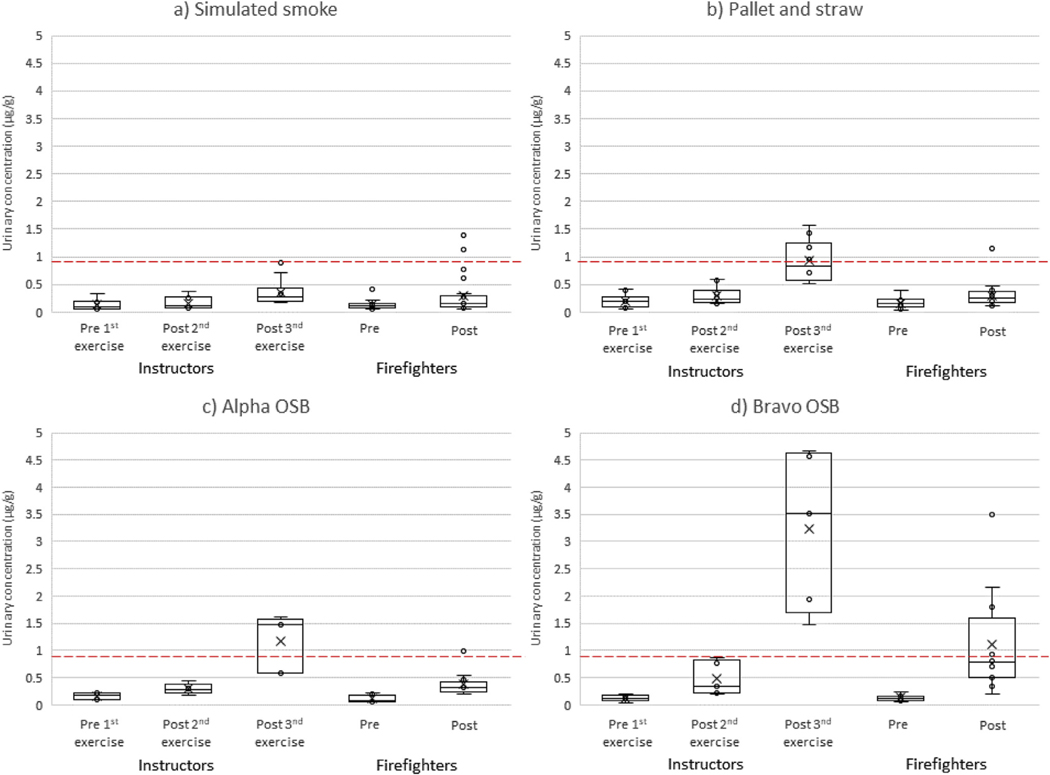
Figs. 1 and 2 provide a comparison of the hydroxyphenanthrenes and 1-hydroxypyrene results in urine over time between firefighters and instructors for the three types of scenarios, with further stratification between the two types of OSB. The Supplemental Materials provide the hydroxynaphthalenes and hydroxyfluorenes results (figs. S3–S4), as well as summary statistics for all the biomarkers that were measured (table S1). Firefighters had a significant increase in OH-PAH concentrations 3-hr after training for all scenarios (p ≤ 0.001). Furthermore, instructors’ OH-PAH concentrations increased steadily throughout each training day and by the end of the shift were significantly greater than the pre-training levels for all scenarios (p ≤ 0.001). The relative magnitude of these increases generally followed the pattern: bravo OSB > alpha OSB > pallet and straw > simulated smoke. For firefighters undergoing the bravo OSB training scenarios, hydroxyphenanthrenes had the largest pre-to 3-hr posttraining urine concentration increase on a percentage basis (median +1074%) while hydroxynaphthalenes had the largest increase on a unit basis (median +32.7 μg/g). For instructors in the bravo OSB training scenarios, 1-hydroxypyrene showed the largest pre-to end-ofshift percentage increase in concentrations (median +2860%) and hydroxynaphthalenes had the largest unit increase (median +34.3 μg/g).
Fig. 1.
Urinary concentrations of hydroxyphenanthrenes by participant type and collection period for training scenarios using a) simulated smoke, b) pallet and straw, c) alpha OSB, and d) bravo OSB. The lower quartile, median, and upper quartile are shown with the box and whiskers (excluding outliers 1.5 times greater or less than the upper and lower quartiles). The mean of the distribution is shown by X. A red dashed line representing the median 3-hr post-firefighting concentration (3.1 μg/g) measured from attack and search firefighters during our residential fire study (Fent et al., In Press-b) is provided for comparison. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 2.
Urinary concentrations of 1-hydroxypyrene by participant type and collection period for training scenarios using a) simulated smoke, b) pallet and straw, c) alpha OSB, and d) bravo OSB. The lower quartile, median, and upper quartile are shown with the box and whiskers (excluding outliers 1.5 times greater or less than the upper and lower quartiles). The mean of the distribution is shown by X. A red dashed line representing the median 6-hr post-firefighting concentration (0.81 μg/g) measured from attack and search firefighters during our residential fire study (Fent et al., In Press-b) is provided for comparison. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We compared the pre-to 3-hr post training change in OH-PAHs for firefighters to the pre 1st exercise to post 2nd exercise change in OHPAHs for instructors, as the timing of these biological samples were similar. Although instructors completed two training exercises between their urine collection sessions, exposures from their second training exercise was unlikely to contribute to their post 2nd exercise urine concentrations because of the timing of the collections (Fent et al., 2014). The change in urine concentrations did not differ significantly between firefighters and instructors (for all scenarios combined) except for 1-hydroxypyrene (firefighters +103%, instructors +46%, p = 0.015) and hydroxyphenanthrenes (firefighters +234%, instructors +188%, p = 0.047). Stratifying by type of scenario, only hydroxyphenanthrenes during the bravo OSB scenario differed significantly (p = 0.026) between firefighters (+1074%) and instructors (+316%). Importantly, we found no differences between firefighters and instructors for the change in ∑OH-PAHs. In a related paper, we found that instructors had lower air concentrations of total PAHs than firefighters, but instructors had longer duration exposures, which could explain the similar magnitude of absorption in comparison to firefighters (Fent et al., In Press-a).
To further explore the impact of repeated exposures on OH-PAH concentrations, we compared instructors’ pre-to end-of-shift change in concentrations (for all scenarios combined) to firefighters’ pre-to 3-hr post-training change in concentrations (for all scenarios combined). Differences were statistically significant for 1-hydroxypyrene (firefighters +103%, instructors +397%, p < 0.001) and hydroxyphenanthrenes (firefighters +234%, instructors +480%, p = 0.046).These findings suggest cumulative exposures to PAHs in the instructors from overseeing multiple training exercises in a day.
The simulated smoke exercises are not expected to have produced PAHs because no combustion took place, which is supported by our previously published air sampling results (Fent et al., In Press-a). To further investigate why the participants experienced biological uptake of PAHs during these exercises, we compared the urinary concentrations of the alpha (started with simulated smoke) and bravo (ended with simulated smoke) participants to each other (Supplemental Materials, table S2) to determine whether the order of the scenarios had any effect. We found significantly greater (p < 0.001) pre-to 3-hr posttraining increases in the ∑OH-PAHs during the simulated smoke scenario for the bravo firefighters (+538%) than the alpha firefighters (+48%). Similarly, the bravo instructors had significantly greater (p = 0.023) pre-to end-of-shift increases (+248%) in the ∑OH-PAHs than the alpha instructors (+89%). As is commonly the case, the hydroxynaphthalenes were the dominant species in the ∑OH-PAHs. These results suggest another source of PAHs was present during the simulated smoke training that was more abundant during the bravo exercises. However, it is unclear what the source of this contamination was and why this effect was more pronounced in firefighters than instructors.
We also explored the effect of job assignment by comparing the change in urine concentrations of OH-PAHs between the fire attack and search positions for the firefighters and between stoker and company officer positions for the instructors (data not shown). The changes in ∑OH-PAHs were similar between these comparison groups, with p-values > 0.4 for stoker vs. Officer instructors and p-values > 0.13 for attack vs. search firefighters.
Table 2 provides a comparison between the U.S. general non-smoking adult population urine concentrations of individual OH-PAHs to the firefighters’ median 3-hr post-training concentrations and instructors’ end-of-shift concentrations. These time points represent the peak excretion identified within the constraints of this study. It is important to note that the firefighters and instructors started each study day with many of the OH-PAH concentrations above (≤2-fold) general population medians. Regardless of the scenario, the firefighters’ and instructors “peak” concentrations of individual OH-PAHs were significantly higher (p < 0.05) than their pre-training concentrations (with the exception of 1-hydroxynaphthalene measured during the simulated smoke scenario). In addition, many of the firefighters’ and instructors’ peak urine concentrations after live-fire scenarios (OSB and pallet and straw) were greater than the respective 95th percentiles of the general population.
Table 2.
Comparison of urinary OH-PAH metabolite concentrations measured from firefighters 3 h after each training exercise and measured from instructors at the end of each shift to the non-smoking adult general population (μg/g creatinine).
| OH-PAH biomarker | NHANES 2011–2012 data for 20–49 year old non- smokersa | Firefighters' median 3-hr post-firefighting concentrations by scenario | Instructors' median end-of-shift concentrations by scenario | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Median | 95th percentile | Simulated smoke (n = 24) | Pallet and straw (n = 24) | Alpha OSB (n = 12) | Bravo OSB (n = 12) | Simulated smoke (n = 10) | Pallet and straw (n = 10) | Alpha OSB (n = 5) | Bravo OSB (n = 5) | |
| 1-Hydroxynaphthalene | 1.0 | 6.2 | 2.2 | 3.6 | 8.6 | 21 | 3.3 | 6.8 | 17 | 22 |
| 2-Hydroxynaphthalene | 3.7 | 16.4 | 8.4 | 7.5 | 12 | 20 | 13 | 14 | 18 | 17 |
| 1-Hydroxyphenanthrene | 0.12 | 0.47 | 0.23 | 0.38 | 0.49 | 1.3 | 0.32 | 0.72 | 1.4 | 1.5 |
| 2-Hydroxyphenanthrene and 3-hydroxyphenanthreneb | 0.13 | 0.48 | 0.33 | 0.55 | 0.93 | 2.3 | 0.67 | 1.2 | 2.2 | 3.0 |
| 1-Hydroxypyrene | 0.10 | 0.33 | 0.15 | 0.26 | 0.32 | 0.78 | 0.27 | 0.84 | 1.5 | 3.5 |
| 2-Hydroxyfluor ene | 0.18 | 0.64 | 0.45 | 0.55 | 0.96 | 1.5 | 0.88 | 1.3 | 1.5 | 2.2 |
| 3-Hydroxyfluor ene | 0.07 | 0.25 | 0.18 | 0.19 | 0.29 | 0.45 | 0.21 | 0.58 | 0.68 | 0.92 |
Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, March 2018, Volume Two (NCEH, 2018).
2-Hydroxyphenanthrene and 3-hydroxyphenanthrene were reported separately for 2011–2012 NHANES data. Thus, 2013–2014 NHANES summary statistics are provided for 2-hydroxyphenanthrene and 3-hydroxyphenanthrene (combined) for general population 20 years and older (which may include smokers). Values that are bolded were higher than the median concentrations measured from attack and search firefighters in our residential fire study (Fent et al., In Press-b).
3.2. Exhaled breath concentrations of VOCs after training
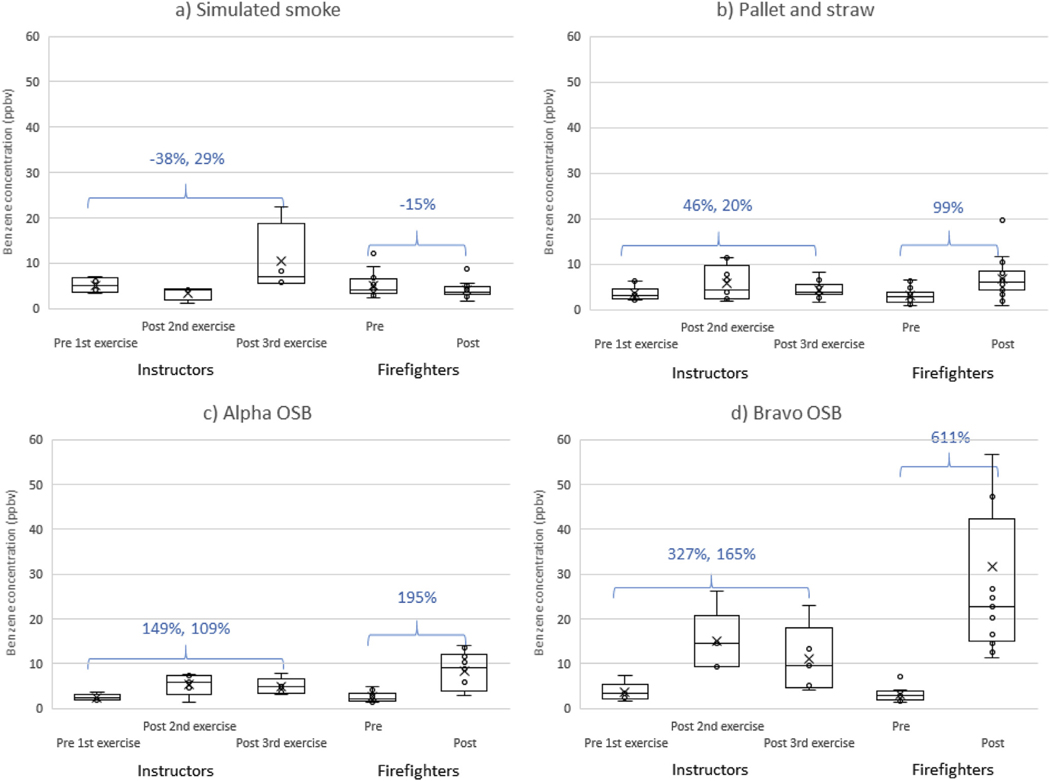
Table 3 provides the percent change in exhaled breath concentrations of VOCs for firefighters and instructors by training scenario, and Fig. 3 provides the specific results for benzene. In general, exhaled breath concentrations increased from the pre-training levels for all scenarios except the simulated smoke exercises, which had mixed results (although most VOC concentrations declined). For firefighters, the change in breath concentrations during bravo OSB training scenario was significantly greater than the alpha OSB training scenario for all analytes (p < 0.05). A similar pattern was found for instructors when comparing the change in breath concentrations of benzene (pre 1st exercise to post 2nd exercise) by the two types of OSB; however, the difference was not statistically significant (p = 0.161). The change in breath concentrations for instructors and firefighters generally did not differ (for all scenarios combined), with one exception for styrene (instructor pre 1st exercise to post 3rd exercise change +599% vs. Firefighter pre to post change +79%, p < 0.001). As with the OH-PAHs, job assignment for firefighters and instructors did not affect the change in exhaled breath concentrations (p > 0.05 for all VOCs and scenario combinations).
Table 3.
Median change in exhaled breath concentrations during training scenarios.
| Scenario | Participant | Comparison | n | Benzene | Toluene | Ethyl benzene | Styrene | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| ppbv | % | ppbv | % | ppbv | % | ppbv | % | ||||
| Simulated smoke | Firefighters | Pre to post | 12 | −0.6 | −15 | −0.0 | +0 | +0.0 | +172 | −0.1 | −28 |
| Instructors | Pre 1st to post 2nd exercise | 4 | −2.1 | −38 | −1.0 | −45 | −0.2 | −44 | −1.0 | −65 | |
| Pre 1st to post 3rd exercise | 4 | +1.4 | +29 | +1.3 | +51 | −0.1 | −17 | +2.9 | +176 | ||
| Pallet and straw | Firefighters | Pre to post | 24 | +2.8 | +99 | +0.4 | +55 | +0.1 | +553 | +0.1 | +99 |
| Instructors | Pre 1st to post 2nd exercise | 10 | +0.9 | +46 | +0.2 | +34 | +0.1 | +773 | +0.2 | +259 | |
| Pre 1st to post 3rd exercise | 10 | +0.6 | +20 | +0.3 | +40 | +0.2 | +1134 | +1.0 | +1831 | ||
| Alpha OSB | Firefighters | Pre to post | 12 | +6.6 | +195 | +1.2 | +105 | +0.1 | +63 | −0.0 | −1 |
| Instructors | Pre 1st to post 2nd exercise | 5 | +3.6 | +149 | +0.7 | +73 | +0.1 | +25 | +0.2 | +25 | |
| Pre 1st to post 3rd exercise | 5 | +3.0 | +109 | +1.0 | +63 | +0.0 | +4 | +3.8 | +572 | ||
| Bravo OSB | Firefighters | Pre to post | 12 | +18.0 | +611 | +3.1 | +292 | +0.4 | +669 | +0.8 | +460 |
| Instructors | Pre 1st to post 2nd exercise | 5 | +11.0 | +327 | +1.4 | +162 | +0.2 | +244 | +0.2 | +51 | |
| Pre 1st to post 3rd exercise | 5 | +5.9 | +165 | +1.2 | +125 | +0.2 | +281 | +1.9 | +550 | ||
Bolded values represent statistical significance at p < 0.05.
Fig. 3.
Exhaled breath concentrations of benzene by participant type and collection period for training scenarios using a) simulated smoke, b) pallet and straw, c) alpha OSB, and d) bravo OSB. The percent change from pre-training levels are provided for instructors and firefighters.
4. Discussion
This study improves our understanding of firefighters’ and instructors’ exposures during training exercises commonly performed at training institutes in the United States and many other countries around the world. The most important results of this study are: 1) firefighters and instructors are exposed to combustion byproducts even when wearing SCBA throughout the training exercise, and 2) firefighters and instructors undergoing training exercises involving OSB experienced higher exposures than pallet and straw (alone) as the fuel source. Furthermore, there is strong evidence of instructors’ increasing cumulative exposure to PAHs with repeated training exercises. This is an important finding because instructors commonly oversee numerous live-fire training exercises during a single day and such activity may be repeated many days over the course of a year.
For the OSB training exercise, two types of OSB were used. It is not possible to be certain of the relative proportion of different adhesives in the two OSB products as this is proprietary information. Median area air concentrations of methyl isocyanate, phenyl isocyanate, and MDI during the bravo OSB exercises were higher than the alpha OSB exercises, although the differences were not statistically significant (Fent et al., 2019a). This could suggest that the bravo OSB (with < 0.01% free formaldehyde) contained higher amounts of pMDI adhesives than the alpha OSB (with < 0.1% free formaldehyde). Differences in the types and amounts of adhesives used in the alpha and bravo OSB could have influenced the production of PAHs during combustion. PAH emissions could be further affected by the slightly different amounts of OSB used (i.e., 1.5 sheets of 7/16″ OSB for alpha vs. effectively 1.5 sheets of 1/2” OSB for bravo). However, the OSB sheathing was not fully consumed in the fires, so the exposure was not limited by the mass of fuel in any of the OSB scenarios. Other factors such as ventilation and temperature could also affect the production of combustion byproducts; although these factors were standardized to the extent possible among the different scenarios.
PAH and benzene exposures from the bravo OSB exercises (based on urine OH-PAH and breath VOC concentrations) were consistently higher than the alpha OSB exercises, which may be expected if the bravo OSB contained higher quantities of adhesives. Previously, we showed that the bravo OSB produced significantly (p < 0.01) higher personal air concentrations of total PAHs and benzene than the alpha OSB. If OSB is to be used for live fire training burns, OSB with the least amount of synthetic adhesives should be selected when possible. However, this information is not always readily available from the suppliers.
Prior to this study, we investigated firefighters’ exposures during controlled residential fires involving household furnishings using the same methodology. We hypothesized that the firefighters and instructors would have lower PAH and benzene exposures from training fires than residential fires involving a variety of typical synthetic materials including foams, plastics, and textiles. On a per training fire basis, exposures were generally below what was measured from attack and search firefighters during the residential fire study (who conducted similar firefighting tasks and timeframes as in the current study) for all training scenarios other than the bravo OSB exercises. For example, firefighters and instructors had higher median urinary concentrations of hydroxynaphthalenes and hydroxyfluorenes ~3-hr after the bravo OSB exercises than reported during the same time period in the residential fire study (see Figs. S3 and S4 in Supplemental Materials).
It is important to consider the pre-to post-firefighting unit changes for breath results when comparing the training and residential fire studies because background levels on the breath tubes varied. For example, the 611% increase in exhaled breath concentrations of benzene for firefighters after the bravo OSB exercises is due to a unit increase of 18 ppbv, which is less than the median increase found for attack and search firefighters in the residential fire study (+24 ppbv). Interestingly, we found very little, if any, increase in breath concentrations of toluene, ethylbenzene, and styrene in the residential fire study (e.g., < 0.45 ppb increase for attack and search firefighters). However, for the present training fire study, we generally found marked increases in these VOCs in breath for all scenarios except for the simulated smoke exercises (Table 3). While median personal air concentrations of these VOCs were higher for the training fires (OSB and pallet and straw) than the residential fires (Fent et al., 2019a), the levels were at least an order of magnitude below applicable occupational exposure limits (ACGIH, 2018; NIOSH, 2010). Personal air concentrations of benzene for both the training fires (other than simulated smoke) and residential fires, on the other hand, were well above the NIOSH short-term exposure limit (1 ppm) (National Institute for Occupational Safety and Health, 2010), and may present a greater concern for toxicity, especially with repeated exposures.
Whereas the firefighters only participated in one training exercise per day, the instructors supervised three exercises per day. For both of the OSB scenarios, instructors’ end-of-shift median urine concentrations of all OH-PAHs were above the concentrations measured 3-hr after firefighting from attack and search firefighters in the residential fire study. In particular, 1-hydroxypyrene was 3.5-fold greater (Fig. 2), which is 35-times higher than the general population median. Of the PAH urine metabolites examined in this study, 1-hydroxypryene correlates most closely with the higher molecular weight PAHs (≥4 rings), many of which tend to be excreted in feces. Of these higher molecular weight PAHs, benzo[a]pyrene is a known human carcinogen widely considered the most toxic PAH (IARC, 2010). Our previous work shows that the composition of airborne PAHs were consistent across the OSB and pallet and straw scenarios, with benzo[a]pyrene constituting ~1% of the mixture. In contrast, naphthalene was the most abundant PAH measured in air, constituting 66–68% of the mixture (Fent et al., 2019a).
For all scenarios, firefighters’ and instructors’ OH-PAH urine concentrations (reported in Table 2) were greater than the medians for non-smoking adults in the general population. Nearly all of the OHPAHs measured after the OSB scenarios (and a few metabolites during the pallet and straw scenario) exceeded the 95th percentiles of the general population. The median end-of-shift concentration of 1-hydroxypryene measured from instructors after the bravo OSB scenario (3.5 μg/g) is within the range of average concentrations in gas workers (0.3–7.7 μg/g) and road pavers (1.2–3.5 μg/g), who are among the more exposed worker populations (Huang et al., 2004). Maximal urinary excretion of 1-hydroxypyrene following ingestion of PAHs has been estimated at 5.5 h (Li et al., 2012), and we previously found maximal excretion 6 h after firefighting (Fent et al., 2019b); thus, our study design may not have captured the peak urinary concentration of this metabolite. It is also important to note that PAHs and VOCs only represent a portion of the combustion products that are produced during fires, so caution should be exercised when interpreting the concentrations of only these compounds in firefighters relative to other populations. In addition, the firefighters and most of the instructors in this study used cleansing wipes post-training and all showered within an hour of completing their exercises each day. We have shown previously that commercial cleansing wipes can remove a median of 54% of PAH contamination from skin (Fent et al., 2017). Without these measures, we expect that exposures would have been even greater.
One unexpected finding from this study was the increase in ∑OHPAHs during the simulated smoke exercises. Upon closer examination, we found statistically significant differences between the alpha and bravo groups (p ≤ 0.002), where the bravo firefighters and instructors experienced higher post-training increases in urine OH-PAHs. Efforts were taken to minimize the firefighters’ and instructors’ exposures from peripheral sources at the training institute. For example, no live-fire training was permitted on the IFSI campus during the week other than the training required for the study. All turnout gear had been laundered before the start of the study, and field decontamination (using water, dish soap, and scrubbing) was used to clean the gear during the study. Although this type of decontamination has been shown to be effective (removing a median of 85% of PAHs on the outer shell) (Fent et al., 2017), some residual contamination will remain on the turnout gear. Moreover, field decontamination does little for contaminants on the inner liner of the gear that can directly contact firefighters’ skin.
Because the alpha participants performed simulated smoke training first, any PAHs on their turnout gear (post-laundering) should have been low, although, laundering may not remove 100% of PAH contaminants (Mayer et al., 2018). The bravo participants, however, performed simulated smoke training last. As such, their gear would have received contamination from the OSB and pallet and straw scenarios performed 4 and 2 days prior, respectively. Thus, PAHs not removed via field decontamination could have been available for biological uptake upon subsequent use of the turnout gear as suggested by Stec et al. (2018). Any residual naphthalene could off-gas and be inhaled by the participants when not wearing SCBA. In addition to contaminated turnout gear, other sources of PAH exposure at the training institute (e.g., contaminants deposited in turnout gear storage area) cannot be ruled out. It is also important to note that the sample sizes (statistical power) were small, especially for the instructors (where n = 5 for each comparison group). Further research on how contaminated gear contributes to firefighters’ exposure to PAHs is warranted.
5. Conclusions
Biological monitoring can be affected by a number of factors, such as physiological makeup and metabolism of workers, work-rate intensity and duration, and PPE use and maintenance. Thus, it is prudent to be cautious when comparing results between studies. Overall, this study suggests that live-fire training may expose firefighters and instructors to hazardous chemicals. Their dose will depend on the number of training fires and type of fuel package. Instructors’ PAH exposures may be higher from repeated training fires than responding to a single emergency residential fire. Likewise, training fires will result in the uptake of benzene and other VOCs. Contamination on turnout gear may also contribute to the biological uptake of PAHs upon subsequent use. Exposures from training fires over time could increase firefighters’ and instructors’ risk of developing certain types of cancer. Efforts should be taken to reduce these exposures, including donning SCBA before approaching the structure, cleaning skin as quickly as possible (preferably immediately after exiting the structure), laundering turnout gear after live-fire training (or field decontamination if laundering cannot be done), showering as soon as possible following training, and selecting training fuels to provide realistic training while limiting unnecessary exposures for firefighters and instructors.
Supplementary Material
Acknowledgements
The authors would like to thank all the individuals who assisted in collecting data for this study, including Kenneth Sparks and Kelsey Babik at NIOSH; Yuesong Wang, Nikki Pittman, and Debra Trinidad at NCEH; Jacob DeBlois and Margaret Morrisey at Skidmore College; and Richard Kesler, Sean Burke, Sue Blevins, as well as the field staff and support team at the Illinois Fire Service Institute. We also thank Jen Roberts at NIOSH for her help in conditioning the breath tubes for collection. We are especially grateful to the firefighters and instructors who participated in this study. This study was funded through a U.S. Department of Homeland Security, Assistance to Firefighters Grant (EMW-2014-FP-00590). The U.S. Environmental Protection Agency (EPA) analyzed the breath samples for the study but were not involved in funding the main project. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of NIOSH, Centers for Disease Control and Prevention, or EPA. Mention of trade names and commercial products does not constitute endorsement or recommendation for use.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijheh.2019.06.006.
Conflicts of interest
The authors declare that they have no competing financial interest in relation to the work described.
References
- ACGIH, 2018. Threshold limit values for chemical substances and physical agents and biological exposure indices. In: American Conference of Governmental Industrial Hygienists, Cincinnati, OH. [Google Scholar]
- Daniels RD, Bertke S, Dahm MM, Yiin JH, Kubale TL, Hales TR, Baris D, Zahm SH, Beaumont JJ, Waters KM, Pinkerton LE, 2015. Exposure-response relationships for select cancer and non-cancer health outcomes in a cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup.Environ. Med 72, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RD, Kubale TL, Yiin JH, Dahm MM, Hales TR, Baris D, Zahm SH, Beaumont JJ, Waters KM, Pinkerton LE, 2014. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup. Environ. Med 71, 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Alexander B, Roberts J, Robertson S, Toennis C, Sammons D, Bertke S, Kerber S, Smith D, Horn G, 2017. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg 14, 801–814. [DOI] [PubMed] [Google Scholar]
- Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, Mueller C, Horn GP, Dalton J, 2014. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann. Occup. Hyg 58, 830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Evans DE, Babik K, Striley C, Bertke S, Kerber S, Smith D, Horn GP, 2018. Airborne contaminants during controlled residential fires. J. Occup. Environ. Hyg 15, 399–412. [DOI] [PubMed] [Google Scholar]
- Fent KW, Mayer A, Bertke S, Kerber S, Smith D, Horn GP, 2019a. Understanding airborne contaminants produced by different fuel packages during training fires. J. Occup. Environ. Hyg In Press-a 10.1080/15459624.2019.1617870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Toennis C, Sammons D, Robertson S, Bertke S, Calafat AM, Pleil JD, Geer Wallace MA, Kerber S, Smith DL, Horn GP, 2019b. Firefighters absorption of PAHs and VOCs during controlled residential fires by job assignment and fire attack tactic. J. Expo. Sci. Environ. Epidemiol In Press-b https://www.nature.com/articles/s41370-019-0145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feunekes FD, Jongeneelen FJ, vd Laan H, Schoonhof FH, 1997. Uptake of polycyclic aromatic hydrocarbons among trainers in a fire-fighting training facility. Am.Ind. Hyg. Assoc. J 58, 23–28. [DOI] [PubMed] [Google Scholar]
- Geer Wallace MA, Pleil JD, Mentese S, Oliver KD, Whitaker DA, Fent KW, 2017. Calibration and performance of synchronous SIM/scan mode for simultaneous targeted and discovery (non-targeted) analysis of exhaled breath samples from firefighters. J. Chromatogr. A 1516, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D, Sim M, Pircher S, Del Monaco A, Dimitriadis C, Miosge J, 2014. Final Report Australian Firefighters’ Health Study. Monash Centre for Occupational and Environmental Health. [Google Scholar]
- Glass DC, Del Monaco A, Pircher S, Vander Hoorn S, Sim MR, 2016. Mortality and cancer incidence at a fire training college. Occup. Med. (Lond.) 66, 536–542. [DOI] [PubMed] [Google Scholar]
- Horn GP, Gutzmer S, Fahs CA, Petruzzello SJ, Goldstein E, Fahey GC, Fernhall B, Smith DL, 2011. Physiological recovery from firefighting activities in rehabilitation and beyond. Prehosp. Emerg. Care 15, 214–225. [DOI] [PubMed] [Google Scholar]
- Horn GP, Stewart JW, Kesler RM, DeBlois JP, Kerber S, Fent KW, Scott WS, Fernhall B, Smith DL, 2019. Firefighter and fire instructor’s physiological responses and safety in various training fire environments. Saf. Sci 116, 287–294. [Google Scholar]
- Huang W, Grainger J, Patterson DG Jr., Turner WE, Caudill SP, Needham LL, Pirkle JL, Sampson EJ, 2004. Comparison of 1-hydroxypyrene exposure in the US population with that in occupational exposure studies. Int. Arch. Occup. Environ. Health 77, 491–498. [DOI] [PubMed] [Google Scholar]
- IARC, 2010. Monographs on the Evaluation of the Carcinogenic Risks to Humans: Some Non-heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures, vol. 92 World Health Organization, International Agency for Research on Cancer, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- IARC, 2010. Painting, Firefighting, and Shiftwork, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 98 World Health Organization, Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Keir JLA, Akhtar US, Matschke DMJ, Kirkham TL, Chan HM, Ayotte P, White PA, Blais JM, 2017. Elevated exposures to polycyclic aromatic hydrocarbons and other organic mutagens in ottawa firefighters participating in emergency, on-shift fire suppression. Environ. Sci. Technol 51, 12745–12755. [DOI] [PubMed] [Google Scholar]
- Kirk KM, Logan MB, 2015. Firefighting instructors’ exposures to polycyclic aromatic hydrocarbons during live fire training scenarios. J. Occup. Environ. Hyg 12, 227–234. [DOI] [PubMed] [Google Scholar]
- Laitinen J, Makela M, Mikkola J, Huttu I, 2010. Fire fighting trainers’ exposure to carcinogenic agents in smoke diving simulators. Toxicol. Lett 192, 61–65. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjodin A, 2012. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem. Res. Toxicol 25, 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AC, Fent KW, Bertke S, Horn GP, Smith DL, Kerber S, La Guardia MJ, 2018. Firefighter hood contamination: efficiency of laundering to remove PAHs and FRs. J. Occup. Environ. Hyg 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen BE, Ovrebo S, 1997. Assessment of exposure to polycyclic aromatic hydrocarbons during firefighting by measurement of urinary 1-hydroxypyrene. J. Occup. Environ. Med 39, 515–519. [DOI] [PubMed] [Google Scholar]
- NIOSH, 2010. In: Barsen M. (Ed.), Pocket Guide to Chemical Hazards, (Cincinnati, OH: ). [Google Scholar]
- NCEH, 2018. Fourth National Report on Human Exposure to Environmental Chemicals:Updated Tables, March 2018, Volume Two. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. National Center for Environmental Health, Atlanta, GA. [Google Scholar]
- NFPA, 2018. NFPA 1403, Standard on Live Fire Training Evolutions. 2018. National Fire Protection Association (NFPA), Quincy, MA. [Google Scholar]
- NIOSH, 2013. In: Fent KW, Musolin K, Methner M. (Eds.), Health Hazard Evaluation Report: Evaluation of Chemical Exposures during Fire Fighter Training Exercises Involving Smoke Simulant. U.S. Department of Health and Human Services, Centers for Disease Control, National Institute for Occupational Safety and Health, Cincinnati, OH NIOSH HETA No. 2012–0028-3190. [Google Scholar]
- Pleil JD, 2016a. Imputing defensible values for left-censored ‘below level of quantitation’ (LoQ) biomarker measurements. J. Breath Res 10, 045001. [DOI] [PubMed] [Google Scholar]
- Pleil JD, 2016b. QQ-plots for assessing distributions of biomarker measurements and generating defensible summary statistics. J. Breath Res 10, 035001. [DOI] [PubMed] [Google Scholar]
- Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparen P, Tryggvadottir L, Weiderpass E, Kjaerheim K, 2009. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol. (Madr.) 48, 646–790. [DOI] [PubMed] [Google Scholar]
- Stec AA, Dickens KE, Salden M, Hewitt FE, Watts DP, Houldsworth PE, Martin FL, 2018. Occupational exposure to polycyclic aromatic hydrocarbons and elevated cancer incidence in firefighters. Sci. Rep 8, 2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwan-ampai P, Navas-Acien A, Strickland PT, Agnew J, 2009. Involuntary tobacco smoke exposure and urinary levels of polycyclic aromatic hydrocarbons in the United States, 1999 to 2002. Cancer Epidemiol. Biomark. Prev 18, 884–893. [DOI] [PubMed] [Google Scholar]
- Tsai RJ, Luckhaupt SE, Schumacher P, Cress RD, Deapen DM, Calvert GM, 2015. Risk of cancer among firefighters in California, 1988–2007. Am. J. Ind. Med 58, 715–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Meng L, Pittman EN, Etheredge A, Hubbard K, Trinidad DA, Kato K, Ye X, Calafat AM, 2017. Quantification of urinary mono-hydroxylated metabolites of polycyclic aromatic hydrocarbons by on-line solid phase extraction-high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem 409, 931–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.