Abstract
Background:
Human adipose-derived stem cells (hADSCs) have gained attention lately because of their ease of harvesting and ability to be substantially multiplied in laboratory cultures. Stem cells are usually cultured under atmospheric conditions; however, preconditioning stem cells under hypoxic conditions seems beneficial.
Aim:
This systematic review aims to investigate the effect of hypoxia preconditioning and its impact on the proliferation and angiogenic capacity of the hADSCs.
Methods:
We performed a systematic review by searching PubMed, Scopus, Embase, and Google Scholar databases from all years through March 22, 2021, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Medical Subject Headings terms “adipose-derived stem cell,” “Hypoxia,” “cell proliferation,” and “angiogenesis” guided our search. Only articles written in English using experimental models comparing a preconditioned group against a control group of hADSCs with data on proliferation and angiogenic capacity were included.
Results:
Our search yielded a total of 321 articles. 11 articles met our inclusion criteria and were ultimately included in this review. Two studies induced hypoxia using hypoxia-inducible factor-1 alpha stabilizing agents, while nine reached hypoxia by changing oxygen tension conditions around the cells. Four articles conducted in-vivo studies to correlate their in-vitro findings, which proved to be consistent. Although 1 article indicated cell proliferation inhibition with hypoxia preconditioning, the remaining 10 found enhanced proliferation in preconditioned groups compared to controls. All articles showed an enhanced angiogenic capacity of hADSCs after hypoxia preconditioning.
Conclusion:
In this review, we found evidence to support hypoxia preconditioning of hADSCs before implantation. Benefits include enhanced cell proliferation with a faster population doubling rate and increased secretion of multiple angiogenic growth factors, enhancing angiogenesis capacity.
Relevance for Patients:
Although regenerative therapy is a promising field of study and treatment in medicine, much is still unknown. The potential for angiogenic therapeutics with stem cells is high, but more so, if we discover ways to enhance their natural angiogenic properties. Procedures and pathologies alike require the assistance of angiogenic treatments to improve outcome, such is the case with skin grafts, muscle flaps, skin flaps, or myocardial infarction to mention a few. Enhanced angiogenic properties of stem cells may pave the way for better outcomes and results for patients.
Keywords: adipose-derived stem cells, angiogenesis, cell hypoxia, cell proliferation, growth factors, human stem cells, regenerative medicine
1. Introduction
Multipotent stem cells are found in adult organs, with vast amounts residing in adipose tissue [1,2]. Multipotent mesenchymal stromal cells, or stem cells, are of great importance to researchers given their regenerative capacity and ability to be used in treatment for various medical conditions [3,4]. Specifically, human adipose-derived stem cells (hADSCs) have garnered significant attention given their ease of harvesting and culturing with few ethical concerns [1,2,4]. hADSCs are capable of secreting a nutrient-rich secretome that contains multiple growth factors, cytokines, and chemokines, which enhance the microenvironment around them, improving the proliferation of surrounding cells [1]. Angiogenesis is upregulated by growth factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and transforming growth factor-beta (TGF-B), all of which are known to be secreted by hADSCs [5,6].
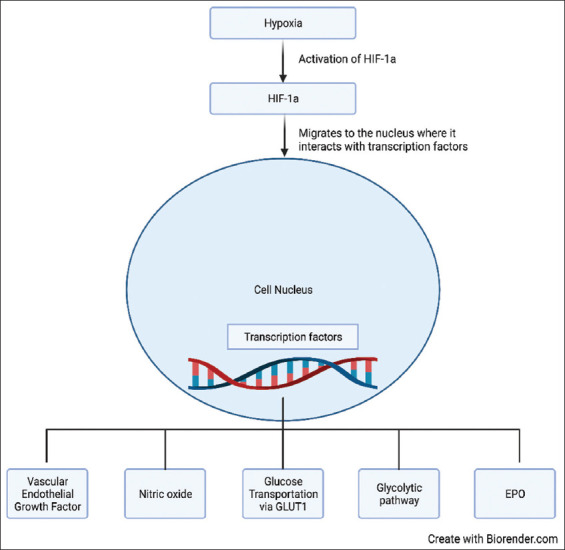
Stem cells reside in a niche, which is defined as an anatomical area that contains a specialized microenvironment that provides the stem cells with the necessary components for self-renewal and multipotency [7-9]. The niche also serves as a regulator of stem cells through specific pathways and oxygen, the latter playing an essential part in stem cell differentiation and molecular response (Figure 1) [7,9,10]. Adaptation to low oxygen tension is mediated by multiple genes, which regulate angiogenesis, proliferation and survival, glucose, and iron metabolism (Figure 2) [11-15]. Because stem cell death on transplant into target tissue is a considerable complication of regenerative medicine, several attempts have been made to enhance cell survival and viability within the transplanted niche; scientists have recently started to manipulate cell cultures by exposing them to different oxygen tensions to improve cell survival with the intent of enhancing cell survival.
Figure 1. Oxygen concentration variability. Illustrates the difference in oxygen concentration in ambient air and as it reaches body tissues.
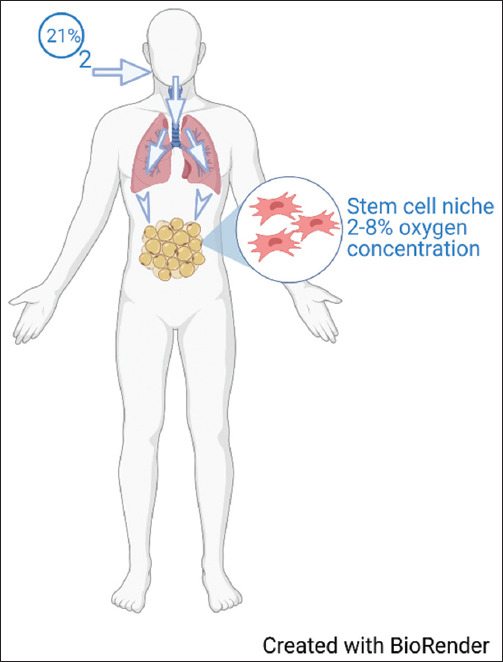
Figure 2. Hypoxia regulated genes. When a cell encounters hypoxic conditions, hypoxia inducible factor-1a is activated, this leads to its interaction with transcription factors within the nucleus which further leads to activation of downstream genes which help cells endure hypoxic conditions.
Given the clinical potential of regenerative medicine and to better understand the effect of oxygen tension on stem cells, this study aims to report the outcome of hypoxic preconditioning on the capability of hADSC’s to proliferate and produce angiogenesis. We hypothesize that adipose-derived stem cells cultured under hypoxic conditions will have increased proliferation rates and angiogenic capability as hypoxic culturing most resembles their cell niche.
2. Methods
2.1. Eligibility criteria
We searched for full-text articles of in-vivo and in-vitro experimental models comparing hypoxia versus normoxia preconditioning of hADSCs, which also included a comparison of angiogenic capacity and proliferation rate versus control groups. Articles written in a language other than English were excluded, given the risk of losing information in the translation. In addition, those that made no mention of stem cell origin or discussed cellular enhancement or mitigation by any external factor other than hypoxia were excluded, such as diabetes or other chronic conditions, given their ability to manipulate angiogenesis and proliferation.
2.2. Information sources and search strategy
A computerized search of the following databases was performed on March 22, 2021, by the first and second authors independently: PubMed (all years), Scopus (all years), Embase (all years), and Google Scholar (all years). The following Medical Subject Headings terms guided our search strategy: (“adipose-derived stem cell” OR “adipose tissue-derived mesenchymal stem cell” OR “adipose tissue-derived mesenchymal stromal cells”) AND “hypoxia” AND “cell proliferation” AND “angiogenesis.” Discordant papers were further discussed between authors before reaching a final agreement.
2.3. Study selection and data collection process
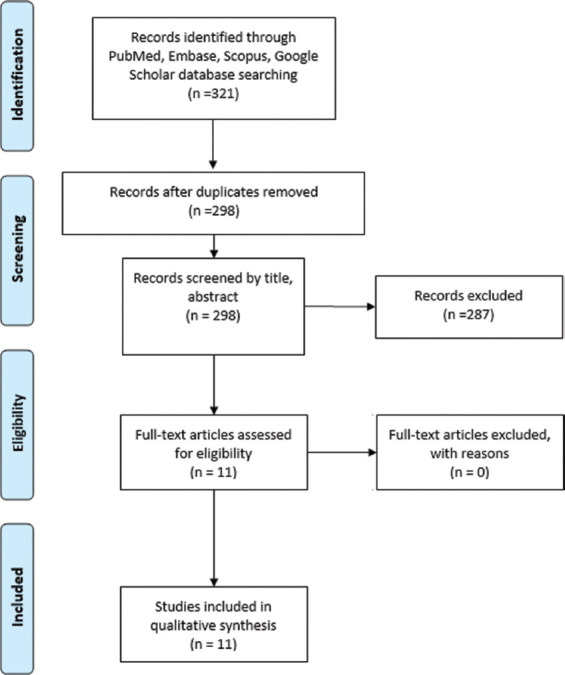
Each author independently performed the search and filtered the studies firstly by removing duplicates with the help of the computer program EndNote (Clarivate Analytics). Subsequently, the inclusion and exclusion criteria described above filtered titles and abstracts. Finally, the remaining studies were screened based on full-text readings (Figure 3).
Figure 3. Study selection flow chart. Flow chart describing the study selection process according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
2.4. Risk of bias assessment
The risk of bias in the included studies was assessed with the help of the Risk Of Bias in Non-randomized Studies of Interventions tool of the Cochrane Library. Illustrations of individualized bias and cross bias are shown in Figures 4 and 5.
Figure 4. Risk of bias graph created with RevMan 5.3 following the Risk of Bias In Non-randomized Studies of Interventions -I guidelines of the Cochrane Library. Green indicates a low risk of bias, yellow indicates an unclear risk of bias, and red indicates a high risk of bias.
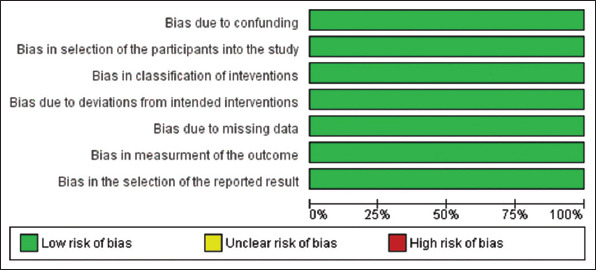
Figure 5. Risk of bias summary created with RevMan 5.3 following the Risk of Bias In Non-randomized Studies of Interventions-I guidelines of the Cochrane Library. A low risk of bias is indicated by green color, yellow indicates an unclear risk of bias, and red indicates a high risk of bias.
3. Results
Of the 321 articles yielded by our search, a total of 11 studies were included in this review. Studies were found to either induce hypoxia through manipulating atmospheric conditions inside a closed chamber or using a hypoxia-inducible factor-1 alpha (HIF-1a) stabilizer. In addition, studies assessed the proliferation and/or angiogenic capacity of stem cells after hypoxic exposure compared to nonexposed cells. A summary of study outcomes is found in Table 1.
Table 1. Summary of studies that measured the effects of hypoxic preconditioning on cellular proliferation and angiogenic capacity.
| Author | Cells | Type of study | Number of cells seeded | Hypoxic model | Results |
|---|---|---|---|---|---|
| Pilgaard et al. [16], 2009 Denmark |
hADSC | In-vitro | 12 rows in a 96-well culture plate to a density of 1000 to 0.5 cells per well. |
XVivo hypoxic workbench/ Ambient, 15%, 10%, 5%, 1% O2). |
• 1% and 5 oxygen may inhibit cell proliferation • Clonogenic precursor cells were preserved • Angiogenic factors were identified in hypoxic conditions |
| Weijers et al. [17], 2011 The netherlands |
hADSC | In-vitro | 3×104 cells/cm2 | Hypoxic workstation, CO2- and O2-controlled, humidifier. 20% O2, 5% CO2, or 1% O2, 5% CO2 | • hADSC have increased proliferation in 1% O2
• Reduced cell aging and preservation of stemness in low O2% |
| Valorani et al. [18], 2012 United Kingdom |
hADSC | In-vitro | 1×104 cells/cm2 | Hypoxia workstation (21% O2) or (2% O2) with 5% CO2 | • Increased hADSC expansion and viability in low O2%, • Decrease in apoptosis and necrosis in low O2% |
| Barros et al. [23], 2013 France |
hADSC | In-vitro In-vivo | 4000 cell/cm2 1×106 cell/cm2 | Hypoxic incubator at 0.5% O2 or 21% O2 for 24 h | • hADSC enhanced in-vivo neovascularization • Age of the donor decreased angiogenic benefits •Hypoxic preconditioning reversed the adverse effect of aging |
| Liu et al. [19], 2013 China |
hADSC | In-vitro | 1500 cells were seeded in a 96-well plate | Tri-gas incubator containing 5% CO2, 1% O2, for 48 h. | • Preconditioned cells have increased proliferation • Preconditioning significantly increased mRNA levels of VEGF and bFGF •Preconditioned medium stimulated the formation of capillary-like structures |
| Chen et al. [20], 2017 China |
hADSC | In-vitro In-vivo | 1×105 cells/well 1×106 cells/well | DMOG at 50, 100 and 150 μmol/L and for 2, 4 and 7 days | •Preconditioned cells had a higher survival rate and lower death rate •50% decrease in mitochondrial mass with reduction of ROS •Increased angiogenic capabilities via increased HIF-1a -> VEGF, VEGF • Significantly promoted In-vivo survival of cells |
| Oses et al. [24], 2017 Chile |
hADSC | In-vitro | 7000 cells/cm2 | Cultured for 48 h in α-MEM without FBS with either 150 μM DFX, 400 μM DFX or double-distilled water (Control) | • Increased levels of HIF-1a •Upregulation of pro-angiogenic genes (VEGF-a, Angiopoietin) •Increased concentration of pro-angiogenic factors in the secretome of hADSC |
| Xue et al. [25], 2018 China |
hADSC-derived exosomes | In-vitro In-vivo | 2×106 cells 100 mg/mL of Exosomes | 1% O2 and 21% O2 | •Preconditioned exosomes significantly improved tube forming (in-vivo and In-vitro) •Angiogenesis inhibitory gene Vash1 was significantly decreased •HIF-1a and VEGF were increased considerably 6 days after hypoxia stimulation • Angiopoietin and Flk1 were considerably increased |
| Almeria et al. [21], 2019 Austria |
hADSC-derived extracellular vesicles | In-vitro | 3000 cells/cm2 | 21% or 5% O2 for 6 days | • Higher proliferation rate in preconditioned cells •Population doubling level and cell density were significantly higher in preconditioned cells #x2022;Total HUVECs length was significantly increased in preconditioned EV group |
| Han et al. [26], 2019 China |
hADSC-derived exosomes | In-vitro In-vivo | 5×103 cells/cm2 | Tri gas incubator with O2 at 5% with 5% CO2 and balanced nitrogen. | •Preconditioned Exosomes enhanced proliferation, migration, and tube-forming capacity of HUVECs •VEGF, EGF, bFGF, angiopoietin-1 were significantly upregulated •Preconditioned group improved neovascularization in-vivo fat grafting |
| Hwang et al. [22], 2020 Republic of Korea |
hADSC | In-vitro | 2.5×103 to 1×104 per well. | Multi-gas incubator at 37°C, 5% CO2, balanced nitrogen, and 1% O2 | •Cells cultured at 1% O2 showed significantly higher proliferation at 24 and 48 h • HIF-1a was increased by hypoxia •VEGF was expressed and secreted higher in preconditioned cells |
hADSC: Human adipose-derived stem cell; mRNA: Messenger ribonucleic acid; VEGF: Vascular endothelial growth factor; bFGF: Basic fibroblast growth factor; DMOG: Dimethyloxalylglycine; ROS: Reactive oxygen species; HIF-1a: Hypoxia-inducible factor -1alpha; A-mem: Alpha-minimum essential medium; FBS: Fetal bovine serum; DFX: Deferoxamine; HUVEC: Human umbilical vein endothelial cells; EV: Extracellular vesicles; EGF: Epidermal growth factor
3.1. Proliferation
In 2009, Pilgaard et al. [16] found a higher apoptosis rate in hADSCs preconditioned with hypoxia; however, clonogenic precursor cells, also known as colony-forming unit fibroblasts, were preserved and displayed in higher quantity. In 2011, Weijers et al. [17] reported beneficial results as they found stem cell proliferation increased 1.7 fold in hypoxic cells versus. normoxic cells (P < 0.05). Population doubling during the first passage supported this result in hypoxic versus normoxic conditions (6.3 ± 0.9 vs. 4.1 ± 0.9, respectively; P < 0.01). They also reported that hypoxic preconditioning led to an increase in telomere length, with normoxic cells having telomeres that, on average, were 1.5-fold shorter (P < 0.05). Following these results, Valorani et al. [18] reported that incubation under hypoxic conditions accelerated cellular proliferation fivefold on day 14 (P < 0.01). They used annexin V and propidium iodide to confirm that hypoxia reduced cell death and raised stem cell viability (P < 0.05).
In 2013, Liu et al. [19] found that hypoxia exposure for 48 h led to a statistically significant higher proliferation rate from days 1 through 4 (P < 0.05). Chen et al. [20] took a different approach in 2017 by mimicking hypoxic conditions using the HIF-1a stabilizer dimethyloxalylglycine (DMOG). When preconditioned cells were exposed to a hypoxic environment, these cells exhibited a higher survival rate both in-vitro (P < 0.05) and in-vivo (P < 0.05). Following these results, Almeria et al. [21] in 2019 cultured hypoxic cells for 6 days, reporting a higher proliferation rate. In addition, population doubling in hypoxic conditions was increased versus normoxic cells (3.0 ± 0.4 vs. 2.3 ± 0.5), respectively; (P < 0.01), along with cell density (P < 0.01). Most recently, Hwang et al. [22] reported increased proliferation of hADSCs after hypoxic preconditioning (P < 0.05).
3.2. Angiogenesis
In 2009, Pilgaard et al. [16] concluded that cells under hypoxic preconditioning had a higher concentration of angiogenic factors, thus crucial for treating ischemic conditions. Barros et al. [23] departed from their previous conclusions that indicated an inverse relationship between the age of donors and angiogenic capacity from hADSCs. They proposed that hypoxic preconditioning could reverse this age-related difference. Hypoxia increased the secretion of VEGF in cells from both young donors (20–35 years of age) and older donors (>50 years of age) (P < 0.01). However, cells from older donors had a more significant benefit from normoxic to hypoxic conditions (P < 0.05). Furthermore, Barros and colleagues tested this theory in a mouse model with an ischemic hindlimb. They concluded that hypoxic preconditioning only seemed beneficial in cells from older donors by increasing tissue perfusion, thus demonstrating that hypoxia may reverse aging impairment.
Liu et al. [19] in 2013 reported the upregulation of HIF-1a with hypoxia preconditioning, which in turn stimulated the upregulation of VEGF and bFGF (P < 0.05). Furthermore, they said that VEGF and bFGF stimulated the formation of capillary-like structures in a Matrigel (Corning Life Sciences) assay of human umbilical vein endothelial cells (HUVECs) (P < 0.05). In 2017, Chen et al. [20] experimented with DMOG to mimic hypoxic conditions. They reported the effects of DMOG by tube formation assay in Matrigel. They concluded that the number of tubes, total tube length, and total branching points were significantly increased in the DMOG group (P < 0.05). In addition, a significant increase of HIF-1a downstream factors, such as VEGF and VEGF receptor 2, was seen in preconditioned cells (P < 0.05).
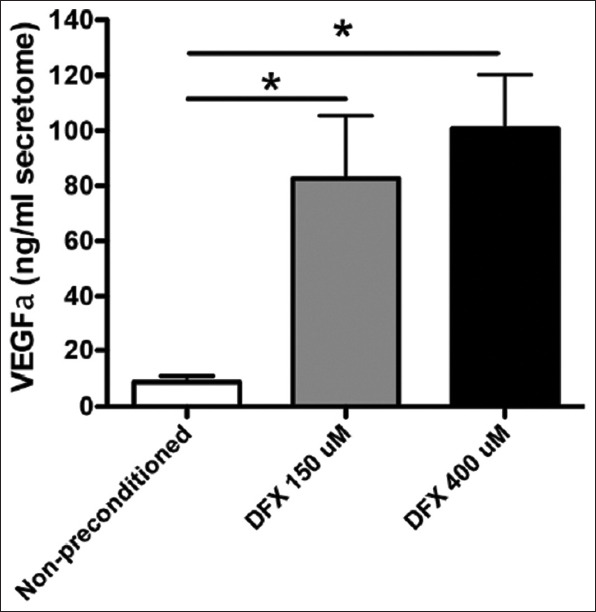
In 2017, Oses et al. [24] experimented with another type of HIF-1a stabilizer, deferoxamine (DFX). They concluded that DFX induced a dose-dependent rise of HIF-1a (P < 0.05). In addition, DFX generated a significant increase in VEGF and angiopoietin 1 mRNA levels (P < 0.05) (Figure 6). To confirm these findings, Oses and colleagues tested their hypothesis that an increase of mRNA would translate to increased secretion of these factors; they were found to be significantly elevated (P < 0.05) (Figure 7).
Figure 6. Deferoxamine preconditioning increases the expression levels of pro-angiogenic factors. Total RNA was obtained from MSCs exposed to 150 μM DFX, 400 μMDFX, or the vehicle for 48 h and subjected to quantitative reverse transcriptase ± PCR analysis. White bars represent non-preconditioned MSCs, gray bars represent MSCs preconditioned with 150 μM DFX, and black bars represent MSCs preconditioned with 400μMDFX. Data are shown as mean ± SEM. n = 4 per experimental group (biological repeats). Experiments were repeated 3 times at the technical level. *P<0.05. MSCs: Mesenchymal stem cells; DFX: Deferoxamine; PCR: Polymerase chain reaction; VEGFa: Vascular endothelial growth factor-a; ANG-1: Angiopoietin-1; bFGF: Basic fibroblast growth factor; PDGF: Platelet-derived growth factor; SEM: Standard error of the mean.
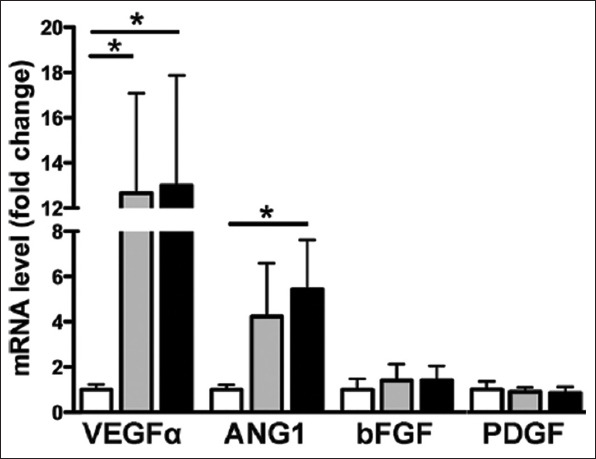
Figure 7. Deferoxamine preconditioning increases the secretion of pro-angiogenic factors. The secretomes obtained from MSCs were exposed to 150 μMDFX, 400 μMDFX, or the vehicle for 48 h. Quantification of VEGFα in MSC secretomes is shown. Data is presented as mean ± SEM. N = 4 per experimental group (biological repeats). Experiments were repeated 3 times at the technical level. *P<0.05. MSC: Mesenchymal stem cells; DFX: Deferoxamine; VEGFa: Vascular endothelial growth factor-a; SEM: standard mean error.
Xue et al. [25] used exosomes derived from hypoxia-preconditioned cells and compared them to exosomes from nonpreconditioned cells. Matrigel tube-forming assay was conducted using HUVECs. Results showed that preconditioned exosomes significantly increased tube formation at 0.5, 2, and 4 h after administration of the exosomes (P < 0.05). In addition, the Vash1 gene, an angiogenesis inhibitor, was significantly decreased in hypoxia-derived exosomes. Exosomes were implanted into mice subcutaneously and Matrigel to further explore these effects. Results showed that endothelial cells produced more tubular structures after 8 days when exposed to preconditioned-derived exosomes (P < 0.05). As seen in previous reports, VEGF expression was significantly increased (P < 0.05). In 2019, Almeria et al. [21] used a HUVEC angiogenesis assay to measure the angiogenic capacity of extracellular vehicles derived from hADSCs cultured under hypoxia compared to normoxia. Total tube length, the number of branches, branching points, and loops were significantly increased in the preconditioned group (P < 0.01).
In 2019, Han et al. [26] used exosomes secreted by hypoxia-preconditioned stem cells or normoxic cells to measure angiogenesis. Exosomes from preconditioned cells significantly increased the proliferation of HUVECs from 12 to 48 h after inoculation (P < 0.05). In addition, migration capacity from HUVECs was significantly increased from the preconditioned group (P < 0.05). Following the same pattern, the tube-like formation was increased (P < 0.05). The in-depth analysis revealed increased angiopoietin 1, VEGF, endothelial growth factor, and bFGF with their respective receptors (P < 0.05). Han and colleagues implemented a nude mice model to confirm these findings to perform fat grafting. Results demonstrated that exosomes from preconditioned stem cells dramatically increased neovascularization (P < 0.05). In addition, protein secretion of angiogenic growth factors was also significantly increased (P < 0.05).
Most recently, Hwang et al. [22] measured HIF-1a protein secreted by preconditioned versus non preconditioned cells. HIF-1a was increased in the preconditioned group (P < 0.05). In addition, upregulation of the VEGF gene and its secretion was reported with hypoxia (P < 0.05).
4. Discussion
Oxygen plays a crucial role in stem cells’ proliferation and angiogenic capacities [7,27]. The partial pressure of oxygen decreases as it enters the body through the lungs and is transported through red blood cells. Ultimately, oxygen tension drops to 2%-8% in cellular niches [27]. Cell cultures that mimic these oxygen tensions benefit stem cells, survivability, proliferation, and angiogenic capacity.
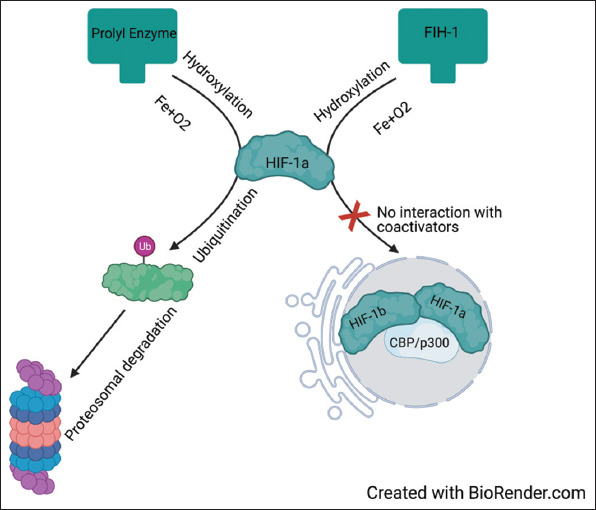
Adaptation to low oxygen tension is mediated by multiple genes, which regulate angiogenesis, proliferation and survival, glucose, and iron metabolism [11]. Of these, HIF-1a is the primary regulator of oxygen homeostasis and hypoxic adaptation [28]. Therefore, HIF-1a can be described as having an inverse relationship with oxygen tension. Under normoxic circumstances, HIF-1a is regulated by a primary and secondary pathway (Figure 8) [28].
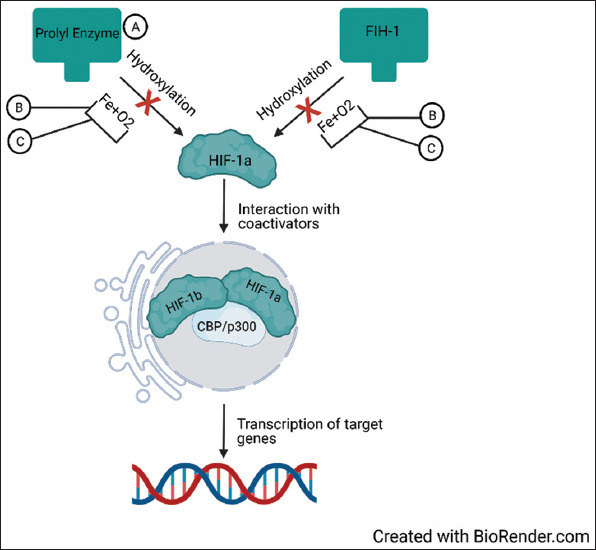
Figure 8. HIF-1a degradation in normoxia. HIF-1a is primarily degraded through two pathways. The first pathway utilizes prolyl hydroxylase enzymes to ubiquitinate HIF-1a for proteasomal degradation. The second pathway acts by hydroxylation of asparagine residues by FIH-1, preventing HIF-1a from interacting with co-activators (HIF-1b and CBP/p300) inside the nucleus. HIF-1a, hypoxia-inducible factor-1 alpha; FIH, factor inhibiting HIF-1; HIF-1b, hypoxia-inducible factor-1; CBP, CREB-binding protein; p300, E1A binding protein p300.
The primary pathway is via a family of prolyl hydroxylase enzymes, which ultimately targets HIF-1a by ubiquitination leading to proteasomal degradation [28]. The secondary regulatory way acts by hydroxylation of asparagine residues by factor inhibiting HIF-1, preventing HIF-1a from interacting within co-activators such as CREB-binding protein and E1A binding protein p300 [28]. However, under hypoxic conditions, hydroxylase substrates like oxygen and iron become scarce, limiting HIF-1a downregulation, which subsequently accumulates and translocates into the nucleus where it exerts its effects (Figure 9) [28]. Present studies have reported the contribution of hypoxia-induced HIF-1a upregulation to angiogenesis by upregulation of genes such as VEGF and angiopoietin 1 and 2, among others [29,30]. Most cells, including hADSCs, secrete these growth factors as a response to hypoxia [31].
Figure 9. HIF-1a activation by hypoxia and stabilizing agents. (A) DMOG is a prolyl hydroxylase enzyme inhibitor, thus preventing ubiquitination and proteasomal degradation of HIF-1a. (B) Oxygen and Fe are required for hydroxylation. However, hypoxia decreases oxygen and Fe availability for these reactions, reducing HIF-1a degradation. (C) Deferoxamine is a Fe chelator, thus decreasing Fe availability for hydroxylation, decreasing HIF-1a degradation. As HIF-1 is accumulating, it translocates into the nucleus, where it interacts with its co-activators (HIF-1b and CBP/p300), engaging in transcriptional activity of downstream genes. HIF-1a, hypoxia-inducible factor-1 alpha; CBP, CREB-binding protein; p300, E1A binding protein p300; Fe, iron; DMOG, dimethyloxalylglycine.
In this review, 9 of 11 studies reported hypoxia preconditioning by manipulating the atmospheric oxygen content in a closed chamber [16-19,21-23,25,26]. However, 1 study reported using DMOG as an alternative method [20]. DMOG is a prolyl hydroxylase inhibitor. It decreases HIF-1a degradation, mimicking a hypoxic response (Figure 9) [32]. An additional study mimicked hypoxia using DFX [24]. DFX increases the concentration of HIF-1a by chelating iron, which is a component necessary by hydroxylases to degrade HIF-1a (Figure 9) [28,33]. All three methods shared similar results regarding angiogenesis. All reported increased angiogenic capacity by measuring HIF-1a expression, mRNA expression of growth factors, or the quantity of secreted growth factors.
Three studies further investigated angiogenesis using HUVECs as a tube-forming assay [21,25,26]. Two of these studies used extracellular vesicles derived from hypoxia-preconditioned cells rather than actual stem cells [25,26]. Using extracellular vesicles did not affect the results as all reported increased angiogenesis.
Proliferation was also studied as an effect of hypoxic preconditioning. Although no study using DFX as a hypoxic agent reported proliferation, the other two models shared similar reports with increased proliferation. However, Pilgaard et al. [16] reported contrasting results, with proliferation being inhibited by hypoxia, as the 1% oxygen tension group displayed numerous detached cells along with cells with condensed nuclei indicating apoptosis. Moreover, they also found that low oxygen tension groups had a higher capacity to support the growth of cell colonies with higher colony-forming units. Although Pilgaard et al.’s study preserved clonogenic precursor cells and their proportions of colony-forming progenitors are within the range of previously published work, the inhibition of culture proliferation draws questions regarding this finding. In addition, Pilgaard et al. did not adhere to the standard medium used in the culture of adipose-derived stem cells, which the authors mention as a possible confounding area for the proliferation of cells, as the medium has been shown to affect the proliferation rate. Hence, caution should be excised when analyzing these results. Further studies exploring the effects of different mediums on different cell lineages should be performed to standardize mediums and their consequences.
Four studies performed in-vivo testing to translate their in-vitro findings, with all four reporting increased angiogenesis [20,23,25,26]. Only Chen et al. [20] determined the proliferation of stem cells in an in-vivo model, increasing proliferation rate and survival.
Although regenerative medicine has great potential, it still takes its first steps into therapeutic means. Understanding the basic principles and effects of stem cells and regenerative medicine may catapult stem cells and their regenerative capabilities into everyday therapeutic use. This study successfully identified oxygen as an essential factor in the upregulation of angiogenic and proliferative capabilities in hADSCs. Further studies require testing hypoxic culturing on different stem cell lineages to determine the effect and compare it against the impact on adipose-derived stem cells. Gaining this knowledge may bridge the gap between low cell survival in the target tissue and increase the future therapeutic effect of stem cells.
5. Conclusion
Based on the included studies, growing cells in a hypoxic condition is a more natural process, thus culturing hADSCs under hypoxic conditions may have a beneficial role and positively impact regenerative medicine. Benefits include enhanced cell proliferation with a faster population doubling rate and increased secretion of multiple angiogenic growth factors, which improves the capacity for angiogenesis. Similar findings achieved between oxygen-dependent models and alternative models indicate that HIF-1a may have a critical role in enhancing angiogenesis and proliferation of HADSC.
6. Limitations
This study has several limitations. Since only studies published in English were included in this review, some studies may have been missed. Other limitations include the potential bias of misinterpreting data and results, and the study selection process, the latter being a potential source of bias common to systematic reviews.
7. Disclaimers
Figures 1-5 and 8,9 were created using the BioRender.com license
Figures 6 and 7 and their text were created by © 2017 Oses et al. [24] and are re-used under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium. Figures and text have been re-structured to fulfill the scope of this article.
Acknowledgments
This study was supported in part by the Mayo Clinic Center for Individualized Medicine, The Plastic Surgery Foundation, and the Mayo Clinic Center for Regenerative Medicine.
Conflict of Interest
The authors declare no conflict of interest
References
- [1].Mazini L, Rochette L, Admou B, Amal S, Malka G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int J Mol Sci. 2020;21:1306. doi: 10.3390/ijms21041306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ntege EH, Sunami H, Shimizu Y. Advances in Regenerative Therapy:A Review of the Literature and Future Directions. Regen Ther. 2020;14:136–53. doi: 10.1016/j.reth.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Caplan AI. Mesenchymal stem cells in regenerative medicine. In: Atala A, Lanza R, Mikos AG, Nerem R, editors. Principles of Regenerative Medicine. 3rd ed. Boston: Academic Press; 2019. pp. 219–27. Ch. 15. [Google Scholar]
- [5].Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res Ther. 2017;8:125. doi: 10.1186/s13287-017-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation. 2004;109:1292–8. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- [7].Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in Stem Cell Biology:A Critical Component of the Stem Cell Niche. Cell Stem Cell. 2010;7:150–61. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- [8].Jones DL, Wagers AJ. No Place Like Home:Anatomy and Function of the Stem Cell Niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- [9].Li L, Xie T. Stem Cell Niche:Structure and Function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- [10].Simon MC, Keith B. The Role of Oxygen Availability in Embryonic Development and Stem Cell Function. Nat Rev Mol Cell Biol. 2008;9:285–96. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bárdos JI, Ashcroft M. Negative and Positive Regulation of HIF-1:A Complex Network. Biochim Biophys Acta. 2005;1755:107–20. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- [12].Denko N, Schindler C, Koong A, Laderoute K, Green C, Giaccia A. Epigenetic Regulation of Gene Expression in Cervical Cancer Cells by the Tumor Microenvironment. Clin Cancer Res. 2000;6:480. [PubMed] [Google Scholar]
- [13].Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch C, et al. Candidate genes for the hypoxic tumor phenotype. Cancer Res. 2000;60:883–7. [PubMed] [Google Scholar]
- [14].Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of Novel Hypoxia Dependent and Independent Target Genes of the von Hippel-Lindau (VHL) Tumour Suppressor by mRNA Differential Expression Profiling. Oncogene. 2000;19:6297–305. doi: 10.1038/sj.onc.1204012. [DOI] [PubMed] [Google Scholar]
- [15].Lal A, Peters H, St Croix B, et al. Transcriptional Response to Hypoxia in Human Tumors. J Natl Cancer Inst. 2001;93:1337–43. doi: 10.1093/jnci/93.17.1337. [DOI] [PubMed] [Google Scholar]
- [16].Pilgaard L, Lund P, Duroux M, Lockstone H, Taylor J, Emmersen J, et al. Transcriptional Signature of Human Adipose Tissue-derived Stem Cells (hASCs) Preconditioned for Chondrogenesis in Hypoxic Conditions. Exp Cell Res. 2009;315:1937–52. doi: 10.1016/j.yexcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- [17].Weijers EM, Van Den Broek LJ, Waaijman T, Van Hinsbergh VW, Gibbs S, Koolwijk P. The Influence of Hypoxia and Fibrinogen Variants on the Expansion and Differentiation of Adipose Tissue-Derived Mesenchymal Stem Cells. Tissue Eng Part A. 2011;17:2675–85. doi: 10.1089/ten.tea.2010.0661. [DOI] [PubMed] [Google Scholar]
- [18].Valorani MG, Montelatici E, Germani A, Biddle A, D'Alessandro D, Strollo R, et al. Pre-Culturing Human Adipose Tissue Mesenchymal Stem Cells under Hypoxia Increases their Adipogenic and Osteogenic Differentiation Potentials. Cell Prolif. 2012;45:225–38. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu L, Gao J, Yuan Y, Chang Q, Liao Y, Lu F. Hypoxia Preconditioned Human Adipose Derived Mesenchymal Stem Cells Enhance Angiogenic Potential Via Secretion Of Increased VEGF and bFGF. Cell Biol Int. 2013;37:551–60. doi: 10.1002/cbin.10097. [DOI] [PubMed] [Google Scholar]
- [20].Chen C, Tang Q, Zhang Y, Dai M, Jiang Y, Wang H, et al. Metabolic Reprogramming by HIF-1 Activation Enhances Survivability of Human Adipose-derived Stem Cells in Ischaemic Microenvironments. Cell Prolif. 2017;50:e12363. doi: 10.1111/cpr.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Almeria C, Weiss R, Roy M, Tripisciano C, Kasper C, Weber V, et al. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation In Vitro. Front Bioeng Biotechnol. 2019;7:292. doi: 10.3389/fbioe.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hwang OK, Noh YW, Hong JT, Lee JW. Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-derived Stem Cells Via Vascular Endothelial Growth Factor. Tissue Eng Regen Med. 2020;17:335–50. doi: 10.1007/s13770-020-00265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barros SD, Dehez S, Arnaud E, Barreau C, Cazavet A, Perez G, et al. Aging-Related Decrease of Human ASC Angiogenic Potential is Reversed by Hypoxia Preconditioning through ROS Production. Mol Ther. 2013;21:399–408. doi: 10.1038/mt.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Oses C, Olivares B, Ezquer M, Acosta C, Bosch P, Donoso M, et al. Preconditioning of Adipose Tissue-Derived Mesenchymal Stem Cells with Deferoxamine Increases the Production of Pro-Angiogenic, Neuroprotective and Anti-Inflammatory Factors:Potential Application in the Treatment of Diabetic Neuropathy. PLoS One. 2017;12:e0178011. doi: 10.1371/journal.pone.0178011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Xue C, Shen Y, Li X, Li B, Zhao S, Gu J, et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018;27:456–65. doi: 10.1089/scd.2017.0296. [DOI] [PubMed] [Google Scholar]
- [26].Han Y, Ren J, Bai Y, Pei X, Han Y. Exosomes from Hypoxia-Treated Human Adipose-Derived Mesenchymal Stem Cells Enhance Angiogenesis through VEGF/VEGF-R. Int J Biochem Cell Biol. 2019;109:59–68. doi: 10.1016/j.biocel.2019.01.017. [DOI] [PubMed] [Google Scholar]
- [27].Mas-Bargues C, Sanz-Ros J, Román-Domínguez A, Inglés M, Gimeno-Mallench L, El Alami M, et al. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int J Mol Sci. 2019;20:1195. doi: 10.3390/ijms20051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis:Applications and Therapies. Biomed Res Int. 2015;2015:549412. doi: 10.1155/2015/549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Semenza GL. Angiogenesis in Ischemic and Neoplastic Disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- [30].Greijer AE, van der Groep P, Kemming D, Shvarts A, Semenza GL, Meijer GA, et al. Up-regulation of Gene Expression by Hypoxia is Mediated Predominantly by Hypoxia-inducible Factor 1 (HIF-1) J Pathol. 2005;206:291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- [31].Yuan Y, Gao J, Liu L, Lu F. Role of Adipose-derived Stem Cells in Enhancing Angiogenesis Early after Aspirated fat Transplantation:Induction or Differentiation? Cell Biol Int. 2013;37:547–50. doi: 10.1002/cbin.10068. [DOI] [PubMed] [Google Scholar]
- [32].Milkiewicz M, Pugh CW, Egginton S. Inhibition of Endogenous HIF Inactivation Induces Angiogenesis in Ischaemic Skeletal Muscles of Mice. J Physiol. 2004;560(Pt 1):21–6. doi: 10.1113/jphysiol.2004.069757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang GL, Semenza GL. Desferrioxamine Induces Erythropoietin Gene Expression and Hypoxia-inducible Factor 1 DNA-binding Activity:Implications for Models of Hypoxia Signal Transduction. Blood. 1993;82:3610–5. [PubMed] [Google Scholar]


