Abstract
Background:
To date, health-effects research on environmental stressors has rarely focused on behavioral and mental health outcomes. That lack of research is beginning to change. Science and policy experts in the environmental and behavioral health sciences are coming together to explore converging evidence on the relationship—harmful or beneficial—between environmental factors and mental health.
Objectives:
To organize evidence and catalyze new findings, the National Academy of Sciences, Engineering, and Medicine (NASEM) hosted a workshop 2–3 February 2021 on the interplay of environmental exposures and mental health outcomes.
Methods:
This commentary provides a nonsystematic, expert-guided conceptual review and interdisciplinary perspective on the convergence of environmental and mental health, drawing from hypotheses, findings, and research gaps presented and discussed at the workshop. Featured is an overview of what is known about the intersection of the environment and mental health, focusing on the effects of neurotoxic pollutants, threats related to climate change, and the importance of health promoting environments, such as urban green spaces.
Discussion:
We describe what can be gained by bridging environmental and psychological research disciplines and present a synthesis of what is needed to advance interdisciplinary investigations. We also consider the implications of the current evidence for a) foundational knowledge of the etiology of mental health and illness, b) toxicant policy and regulation, c) definitions of climate adaptation and community resilience, d) interventions targeting marginalized communities, and e) the future of research training and funding. We include a call to action for environmental and mental health researchers, focusing on the environmental contributions to mental health to unlock primary prevention strategies at the population level and open equitable paths for preventing mental disorders and achieving optimal mental health for all. https://doi.org/10.1289/EHP9889
Introduction
From psychiatric sequelae of neurotoxicants such as pesticides and heavy metals, to chronic and post-traumatic stress from climate change-driven natural disasters or legacy environmental injustice to the mental health benefits of green spaces and neighborhood amenities, the physical environment can influence mental health in important ways. To date, health-effects research on environmental stressors has rarely focused on behavioral and mental health outcomes. That is beginning to change. Science and policy experts in environmental, psychiatric, genetic, social, and behavioral epidemiology, toxicology, and neuro and developmental psychology are coming together to explore converging evidence on the relationship - harmful or beneficial - between environmental factors and mental health.
The National Academies of Sciences, Engineering, and Medicine (NASEM) Standing Committee on Emerging Science for Environmental Health Decisions is charged with scoping the field of environmental health to identify and discuss new areas of science and research methodologies with the potential to inform decision-making. In 2020, the committee identified mental health and the environment as a priority emerging science topic and appointed a multidisciplinary planning committee that met weekly for several months, culminating in a 2 day workshop on “The Interplay Between Environmental Exposures and Mental Health Outcomes,” summarized in detail elsewhere (NASEM 2021a). After the workshop, members of the standing committee and the planning committee continued to meet to further develop ideas generated at the workshop. The structure of the process followed an iterative strategy, in which the planning committee conducted an expert-guided conceptual review of the existing literature to identify potential priority areas for research and policy, refined these priorities with input from presenters and the audience at the public workshop, and individuals after the workshop organized the priority concepts into a framework and specific actions to advance the field. This commentary summarizes the framework and priorities identified through the multidisciplinary expert process. Although key papers are cited in this commentary to illustrate the concepts discussed, this paper does not constitute a systematic review of the literature on this topic.
Mental Health as an Overlooked Outcome
Mental health disorders are leading contributors to disabilities and morbidity (Vigo et al. 2016), and have considerable negative social, professional, personal, and economic consequences (Murray et al. 2020). They are also strikingly prevalent (Schaefer et al. 2017). Repeated assessments in population-representative cohorts have identified the lifetime prevalence of mental disorder diagnoses to be above 70% by age 30 y (Schaefer et al. 2017), and above 85% by age 45 y (Caspi et al. 2020). To date, relatively little research has examined the interplay of nonsocial environmental factors (e.g., toxicants, climate change, etc.) with mental health.
To assist in the goal to bridge psychological and environmental science, we briefly review here some leading approaches to identifying and measuring mental disorders. To differentiate between normal emotional experiences and psychological disorders, scientists and clinicians rely on diagnostic taxonomies, such as those in the U.S.-based Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (DSM-5) (American Psychiatric Association 2013) and the more global International Classification of Diseases (ICD)-10. Within these taxonomies, sets of disorders are grouped into clusters that share common themes, including neurodevelopmental disorders [e.g., attention deficit/hyperactivity disorder (ADHD)], anxiety disorders (e.g., specific phobias or generalized anxiety), mood disorders (e.g., major depressive disorder), schizophrenia spectrum disorders, disruptive-impulsive disorders (conduct disorder), and substance use disorders, among others (American Psychiatric Association 2013). Although the medical community frequently relies on categorical diagnoses for assessment and treatment frameworks (i.e., a person does or does not have a particular disorder according to the DSM), alternative research frameworks emphasize dimensional assessments of psychopathology, which recognize that symptoms fall along a spectrum of severity. In this way, mental health can be studied as discrete, categorical diagnoses or as quantitative levels of symptoms across a continuum of distress or impairment.
Nongenetic Drivers of Mental Health and the Potential Benefits of an Exposomic Approach
Genetic contributions to psychiatric disorders continue to be identified and characterized, and these discoveries represent important advances in our understanding of psychiatric conditions and symptoms (Geschwind and Flint 2015). However, inheritance studies clearly demonstrate that genetics alone can explain only a portion of brain or behavioral dysfunction (the heritability of depression, for example, is ; Sullivan et al. 2000), leaving the rest to nongenetic influences. If the environment includes all nongenetic components, it is necessary to consider toxicant exposures, activity, diet, physical surroundings, and social forces (McHale et al. 2018). The exposome provides an example of a holistic characterization of the environment and provides a scientific framework to uncover the wide breadth of nongenetic contributors to mental health as well as the biological consequences of exposures (Burkett and Miller 2021; Vermeulen et al. 2020). This broadly defined environmental component can range from toxicant exposures (e.g., metals, pesticides, solvents, air pollution, etc.) to psychosocial exposures (e.g., interpersonal interactions, socioeconomic factors, etc.), or combinations of and interactions between these exposures over the course of a lifetime (Vermeulen et al. 2020).
Research Challenges in Human and Animal Studies
For many environmental stressors, it is unethical to design human studies that maximize causal inference, such as experiments and randomized control trials. Most evidence thus comes from observational clinical and epidemiological studies and from animal studies. However, understanding the effects of environmental factors on mental health is difficult to approach at the bench. Where other fields of toxicology have objective biological end points that are typically easy to quantify, mental health research is limited both by the difficulty in measuring complex cognitive, psychological, and emotional states in humans and by the difficulty in replicating human-specific experiences in animals. Many mental health conditions cannot currently be evaluated in animals, such as dissociation or experiencing uncontrollable repetitive thoughts, necessitating the use of simplified indices of behavioral features as proxies for these complex conditions (Nestler and Hyman 2010).
Single toxicant exposures are translatable to animal models because equivalent exposure doses can be calculated and tested in experimental protocols (findings from such models are reviewed below). However, we know that this approach captures only part of the equation and is insufficient for addressing human-specific exposures. How does a scientist model chronic stress due to job insecurity in an animal? How does one account for known social contributors to substance use such as social networks and societal pressures in animal experiments? How can animals be used to understand the consequences of environmental and systemic racism on mental health?
As Tables 1 and 2 illustrate, the measurable neurochemical and behavioral features of mental health conditions in animal models translate with variable efficacy to human correlates. Animal models of mental health disorders are often evaluated based on their face, construct, and predictive validity (Willner 1984). Face validity refers to how well the animal model replicates the human condition based on behavioral and biological features. Construct validity refers to how authentically the etiological conditions used to create the animal model relate to the etiological conditions under which the disorder arises in humans. Finally, predictive validity refers to how well the model responds to treatments that are effective in the human condition (e.g., excessive grooming is considered a compulsive behavior in obsessive-compulsive disorder animal models because it diminishes following treatment with antidepressants). Overall, animal models can provide invaluable insights into human mental health conditions, but they will also invariably have limitations.
Table 1.
Examples of human features and animal model correlates for mental health conditions: behavioral features and correlates.
| Human behavioral features | Behavioral correlates in animal models | |
|---|---|---|
| Attention deficit/hyperactivity disorder | Hyperactivity | Increased activity in the open field test |
| Inattention | Slow reaction times and inaccuracy during operant tasks | |
| Impulsivity | Perseverance in accessing stimulus despite aversive consequence | |
| Substance use disorder | Substance seeking regardless of conflict or punishment | Animals preferentially choosing to use substances over eating or drinking |
| Pathological choice of drug over other necessities (e.g., food, water) | Animals tolerating aversive stimulus (e.g., foot shock or pharmacological agents such as histamine) to access drug | |
| Persistence of drug-associated behavior(s) during extinction paradigm | ||
| Major depressive disorder | Apathy | Impaired nest-building, disturbed grooming regimen |
| Anhedonia | Reduced preference for palatable solutions or food (e.g., sucrose water or cookies) | |
| Despair | Decreased escape attempts in forced swim test | |
| Irritability | Increased aggression when intruder animal is introduced to resident animal’s cage | |
| Obsessive compulsive disorder | Uncontrollable repetitive thoughts | NA |
| Uncontrollable repetitive behaviors | Excessive grooming | |
| Schedule induced polydipsia | ||
| Post-traumatic stress disorder | Recurring, involuntary, and intrusive memories | NA |
| Derealization or dissociation | NA | |
| Hypervigilance | Increased startle response |
Note: NA, Not Applicable.
Table 2.
Examples of human features and animal model correlates for mental health conditions: Neuroimaging and neurochemical correlates.
| Human neuroimaging features | Neurochemical correlates in animal models | ||
|---|---|---|---|
| ADHD | fMRI | Reduced blood flow in fronto-striatal, fronto-cerebellar, and fronto-striato-parieto-cerebellar networks | Dysregulated dopamine metabolism and transmission |
| Increased blood flow in posterior parietal lobe, PCC, and regions of dlPFC | |||
| PET | Abnormal dopamine transporter binding, dopamine receptor binding, and dopamine metabolism in right caudate | Dysregulated (increased or decreased) extracellular dopamine and/or norepinephrine concentrations | |
| Decreased receptor availability in left caudate | |||
| Decreased dopamine transporter density in midbrain | Decreased spontaneously active ventral tegmental area dopaminergic neurons | ||
| DTI | Abnormal white matter structural anatomical connectivity in fronto-striatal circuitry, fronto-cerebellar circuitry, and executive functioning and attentional networks | Impaired modulation of cortico-striato-thalamo-cortical circuits | |
| SUD | fMRI | Hypoactive PFC during cognitive tasks | Increased dopamine signaling |
| rsMRI | Decreased connectivity in the default mode network | Increased activity in mesolimbic pathway | |
| PET | Reduced regional brain glucose metabolism in PFC and ACC | ||
| EEG | Altered P300 on reward processing tasks | Dysregulated hypothalamic-pituitary-adrenal activity | |
| MDD | fMRI | Increased activity in mPFC, amygdala, and hippocampus | Increased circulating glucocorticoids and decreased glucocorticoid receptors |
| Decreased activity in IPFC, and striatum | |||
| PET | Hyperactivity in the mPFC | Increased pro-inflammatory cytokines and decreased anti-inflammatory cytokines | |
| Altered metabolism and neural activity in the PCC, insula, hippocampus, and amygdala | |||
| Altered serotonin receptor binding | Decreased serotonin levels | ||
| DTI | Abnormal white matter integrity in superior longitudinal fasciculus, corpus callosum, and uncinate fasciculus | Decreased hippocampal brain derived neurotrophic factor expression | |
| MRI/VBM | Decreased volume in mPFC, IPFC, striatum, amygdala, and hippocampus | ||
| OCD | rsMRI | Abnormal functional connectivity in OFC and ACC | Altered cortico-basal ganglia-thalamo-cortical activity |
| PET/SPECT | Intrusive thought-induced hyperactivity in the OFC | Altered dopamine receptor subtype composition and dopamine receptor binding | |
| Anxiety related hyperactivity in the ACC | Disturbed redox balance | ||
| OCD stimuli-induced increased activity in OFC and ACC | Altered firing and postsynaptic currents in bed nucleus of the stria terminalis neurons | ||
| MRI/CT/VBM | Structural changes in OFC, ACC, basal ganglia, and thalamus | Altered cyclic adenosine-monophosphate phosphodiesterase signaling | |
| Altered serotonin reuptake transporter expression | |||
| PTSD | fMRI | Hyperactivity in the amygdala | Increased stress hormones |
| Hypoactivity in the mPFC and ACC | Increased epigenetic methylation of brain-derived neurotrophic factor gene in hippocampus | ||
| Reduced hippocampal activity | Upregulated corticotropin releasing factor receptors in stria terminalis | ||
| MRI/MRS | Structural changes in hippocampus, amygdala, and mPFC | Decreased hippocampal plasticity | |
| Increased cortical and hippocampal expression of glucocorticoid receptors | |||
Note: ADHD findings summarized in Weyandt et al. (2013) and Russell (2005, 2007). SUD findings summarized in Cabrera et al. (2016) and Koob and Simon (2009). MDD findings summarized in Wise et al. (2014), Krishnan and Nestler (2011), and Wang et al. (2017). OCD findings summarized in Holzschneider and Mulert (2011) and Szechtman et al. (2017). PTSD findings summarized in Holzschneider and Mulert (2011) and Borghans and Homberg (2015). Endogenous event-related potentials between 300–600 ms after cue (P300). ACC, anterior cingulate cortex; ADHD, attention deficit/hyperactivity disorder; CT, computerized tomography; d, dorsal; DTI, diffusion tensor imaging; EEG, electroencephalogram; fMRI, functional MRI; IPFC, inferior prefrontal cortex; l, lateral; m, medial; MDD, major depressive disorder; mPFC, medial prefrontal cortex; MRI, magnetic resonance imaging; MRS, magnetic resonance spectroscopy; OCD, obsessive compulsive disorder; OFC, orbitofrontal cortex; PET, positron emission tomography; PFC, prefrontal cortex; PCC, posterior cingulate cortex; PTSD, posttraumatic stress disorder; rsMRI, resting state MRI; SPECT, single photon emission computerized tomography; SUD, substance use disorder; VBM, voxel based morphometry.
Domains of Environmental Exposures and Mental Health Research
Notwithstanding unique research challenges in this field, a considerable body of evidence already suggests that the physical environment may influence mental health outcomes. Here we summarize the evidence on neurotoxicants, environmental disasters, and urban natural spaces as examples of this influence because these have the largest literature base, but there are other equally important examples that we could have selected, including psychological reactions to environmental racism and systemic injustice, species loss and climate change (solastalgia), and community initiatives to improve environmental conditions. Figure 1 ties together these diverse domains of environmental influence on mental health through an illustrative conceptual model.
Figure 1.
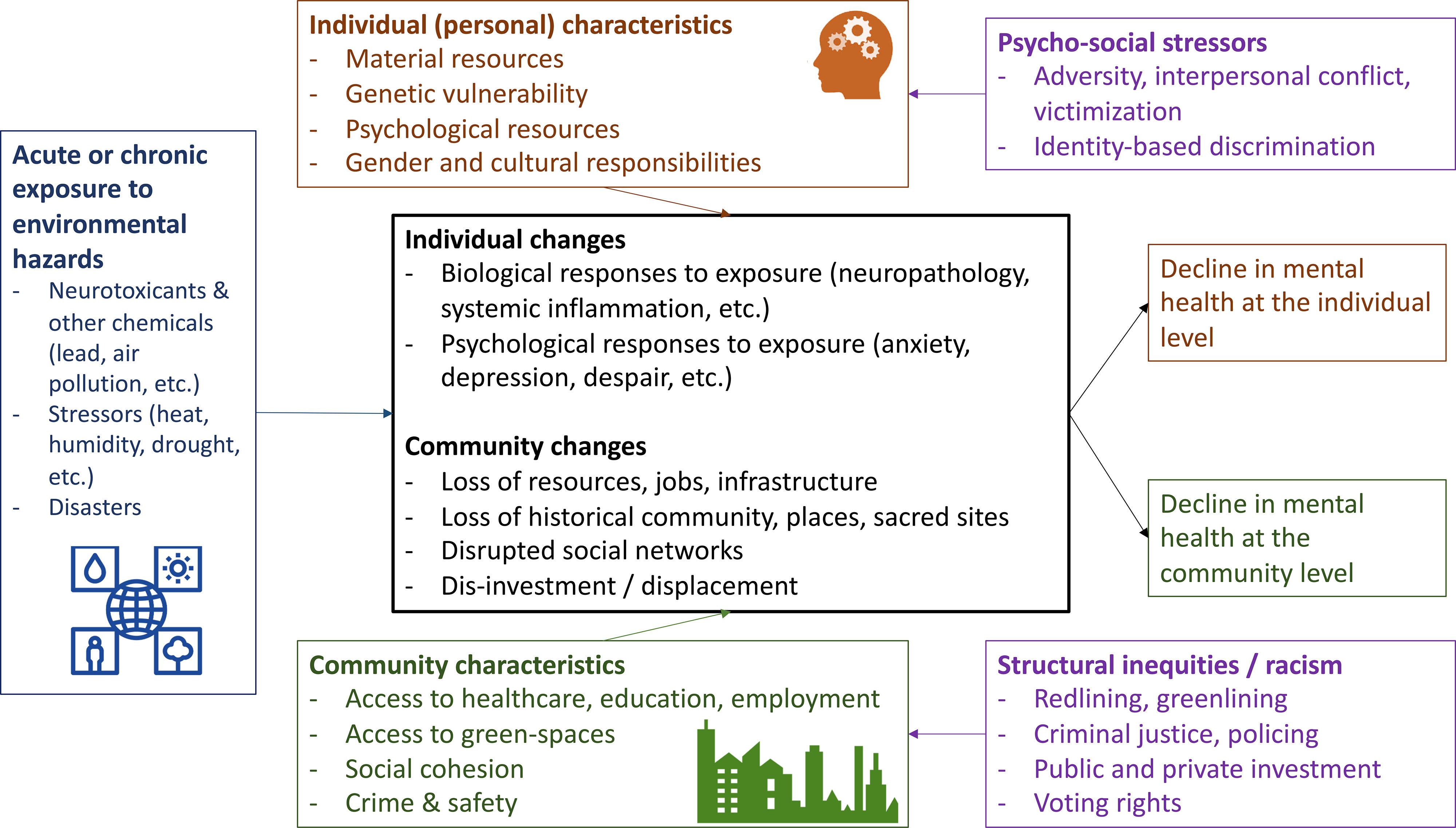
Conceptual model of the association of environmental hazards with mental health outcomes at the individual and community level.
Psychiatric Effects of Environmental Toxicants
What is known already.
Hundreds of natural and synthetic toxicants have been implicated in human psychiatric disorders and psychological functioning; heavy metals, solvents, and pesticides represent some of the best studied classes (Table 3). Although evidence continues to emerge, the neurotoxicity of these agents has been well-characterized in epidemiological research, focusing on end points such as impairments of motor function or visuospatial ability or impairments in learning, memory, and attention (White et al. 2014). Less well-studied are the effects of these agents on psychological, noncognitive end points, such as personality, emotion regulation, impulse control, or symptoms of mental disorder. Early neuro-epidemiology studies often considered changes in mood, affect, and personality following toxicant exposures to be the result of psychological reactions to the exposure (i.e., distressing thoughts and beliefs about having been exposed), rather than the result of actual observable pathology (White et al. 2014). In the most biased designs, participants with psychiatric symptoms were excluded from observational studies to identify the “pure” effects of toxicants on cognitive and neurological outcomes.
Table 3.
Major classes of neurotoxicants and their typical exposure sources and neurotoxic action.
| Class of toxicant | Typical exposure sources | Typical neurotoxic action | Animal model examples |
|---|---|---|---|
| Metals | Metals are naturally occurring, but human exposure is usually due to current or historical uses. Current sources include mining, smelting, battery manufacturing and recycling, construction, automotive, and electronics. | Toxicity and action vary by metal and dose (e.g., lead is toxic at all levels, whereas copper is essential at low levels and harmful at high). Metals generally harm the CNS by substituting for necessary minerals (e.g., lead substitutes for calcium, which is critical to neuronal signaling), binding to and inactivating necessary enzymes (e.g., arsenic can inactivate enzymes), or generating reactive oxygen species. | Prenatal and neonatal lead exposure in mice results in learning deficits and hyperactivity that is attenuated by amphetamine or methylphenidate (Silbergeld and Goldberg 1974, 1975). |
| Organic solvents | Solvents are used as vehicle and equipment fuels; in almost all chemical and industrial processes; and as ingredients in cleaning and degreasing products, pesticides, paints, adhesives, cosmetics, coatings, and ink. | Toxicity and action vary by solvent and dose, but typically organic solvents are lipophilic and concentrate in lipid-rich brain white matter. Mechanisms of toxicity remain poorly characterized but are related to generation of toxic reactive oxygen species. Consequences can involve dysregulation of glial cells, demyelination of nerve fibers, ischemic damage, and white matter necrosis. | Adult mice acutely exposed to toluene show depressive symptoms as measured by increased time spent immobile during the tail suspension test and forced swim test, which is indicative of despair. These symptoms are not the result of an overall decrease in movement and are reversed via treatment with antidepressants (Yang 2010). |
| Pesticides | Pesticides are applied in agriculture and manufacturing processes, in parks, golf courses, rights of way, and home and garden use. | Toxicity and action vary by pesticide, but the best-studied classes, organophosphates and carbamate pesticides, inhibit acetylcholinesterase resulting in accumulation of acetylcholine and disrupted neurotransmission in the parasympathetic nervous system. | Young mice (1 month of age) exposed subchronically or chronically to glyphosate demonstrate both depressive and anxiety behaviors including decreased time spent in open arms of an elevated plus maze (Ait Bali 2017). |
Note: CNS, central nervous system.
Neurotoxicant exposures, both acute and chronic, can result in subtle and lasting mental health consequences. For example, adults occupationally exposed to metals, particularly lead, have long been known to demonstrate alterations in mood, energy, and irritability (Baker et al. 1985). Children exposed to lead demonstrate greater externalizing symptoms, such as hyperactivity and antisocial behavior (Marcus et al. 2010; Needleman et al. 1996), and, in adulthood, tend to develop more disadvantageous personality profiles (Reuben et al. 2019; Schwaba et al. 2021), schizophrenia diagnoses (Opler et al. 2004), and psychiatric symptomatology across diagnostic categories (McFarlane et al. 2013; Reuben et al. 2019).
Similar findings exist for other classes of neurotoxicants. Solvent exposure has been linked to changes in personality, motivation, and impulsivity (Condray et al. 2000; van Valen et al. 2012) and to higher rates of mood, anxiety, bipolar, and psychotic disorders (Aschengrau et al. 2012; Visser et al. 2011). Exposure to pesticides, particularly acetylcholinesterase-inhibiting organophosphates, has been linked to increases in depressive symptomatology, anxiety and depression diagnoses (Suarez-Lopez et al. 2021), suicides (Khan et al. 2019), and general neuropsychiatric symptomatology (Rauh and Margolis 2016). In addition, individual and mixtures of pollutants with neurotoxic properties such as perfluoro- and polyfluoroalkyl substances and outdoor air pollutants (e.g., nitrogen oxides and particulate matter) (de Prado-Bert et al. 2018), have been linked to risk of autism (Long et al. 2019), ADHD (Jorcano et al. 2019; Liew et al. 2015), depression, anxiety (Braithwaite et al. 2019), and schizophrenia (Newbury et al. 2019), and to elevations in risk of mental illness across diagnostic categories (Brokamp et al. 2019; Reuben et al. 2021).
What we still need to know.
How do psychological reactions to toxicant exposure (i.e., beliefs and feelings about having been exposed) interact with biological reactions (e.g., neuronal and glial cell dysfunction)?
What are the psychological mechanisms (e.g., enhanced emotional arousal, impaired cognitive control) that mediate biologically driven mood, personality, and psychopathology changes following toxicant exposures?
How do early life toxicant exposures alter life-course trajectories relevant to health, happiness, and productivity, including educational and occupational attainment, interpersonal relationship quality, and healthy aging and longevity?
How do multitoxicant exposures, both concurrent and sequential, interact to differentially alter mental health outcomes?
What genetic, sex, gender, ethnic, and cultural factors alter individual vulnerability and resilience to toxicant effects on mental health?
Environmental Disasters and Mental Health
What is known already.
Human-caused and natural environmental disasters, such as oil spills, drinking-water contamination, drought, floods, and wildfires often result in widespread mental health consequences (Beaglehole et al. 2018; Morganstein and Ursano 2020), most commonly posttraumatic stress disorder (PTSD) (Marshall et al. 2007), depression (Sastry and VanLandingham 2009), anxiety, and substance use (North et al. 2011; Heard-Garris et al. 2017). Rises in violence and suicide have been associated with the postdisaster period and increase during extreme heat events (Mares 2013). A notable proportion of individuals (e.g., ) who develop mental health sequelae after an environmental disaster go on to develop chronic psychological dysfunction (McLaughlin 2009). Meta-analyses have identified perceived health effects from the events and institutional delegitimization of community concerns as factors that increase psychological consequences, particularly from chemical disasters (Schmitt et al. 2021). Similarly, climate disasters can bring inordinate stress to communities, although studies of the specific mental health impacts are rare (Cianconi et al. 2020). Slow-moving disasters, such as drought or melting permafrost can also have clinically meaningful, rarely studied mental health effects, particularly depression and suicide, in populations directly dependent on the land, such as farmers (Guiney 2012) and indigenous populations (Obrien 2014; Middleton et al. 2020). Anxiety from the general threat of climate change can also be a stressor contributing to psychological distress (Clayton 2020).
What we still need to know.
Do the mental health outcomes from disaster vary based on the type of disaster (e.g., chronic, ongoing vs. single event, natural vs. human-generated). Do ethnic and cultural factors alter community vulnerability and resilience?
Can conceptual models of community resilience accurately predict the psychological impact of disasters? If so, can community resilience assessments help plan responses to disasters to limit mental health impacts?
When should psychological intervention be deployed in communities to mitigate the mental health impacts of disasters (e.g., pre, during, post)? Can psychological first aid–style interventions be tailored to address community resilience needs in the disaster context, at-scale?
What are the costs and benefits of community resilience investments in the short and long run? Which institutions (federal, state, local) should bear the costs?
Beneficial Outcomes of Natural Environments on Mental Health
What is known already.
Environmental factors may also help promote mental health (Palinkas et al. 2020), particularly exposure to green spaces (i.e., nature reserves, wilderness environments, and urban parks) (Barton and Rogerson 2017). A recent review of studies reported a link between exposure to natural environments and a decrease in symptoms of mental illness (Bratman et al. 2019) while increasing happiness and subjective well-being (White et al. 2013). A multidecade nationwide study in Denmark found that children raised in neighborhoods with the least green space had up to a 55% greater risk of developing a psychiatric disorder in adulthood than their peers raised in greener settings regardless of other assessed risk factors, including level of urbanization, socioeconomic factors, parental history of mental illness, and parental age (Engemann 2019). Another study found that self-reported mental health improves with every hour of contact with natural settings each week, with peak mental health reported after 3–5 h of weekly contact (White et al. 2019).
Mechanisms driving these associations are an active area of investigation (Bratman et al. 2019), and several pathways have been proposed (Markevych et al. 2017) including: a) reduction of physiological stressors impacting mental health, including heat, noise, and air pollution; b) promotion of behaviors that improve mental health, including social interaction, self-reflection, and physical activity; and c) direct restoration of cognitive resources (e.g., attention) and/or alteration of nervous system activity (e.g., activation of the parasympathetic nervous system) in ways that improve mental health. Causal inference in this domain has been hindered to date by a lack of common metrics for the assessment of green space “exposure” (Holland et al. 2021), although innovations in measurement, including wearable and geospatial technology to dynamically assess individuals’ locations and activities throughout time, hold promise for more fully characterizing the experiences of people exposed to nature settings (Barnes et al. 2018).
Exposure to nature and green spaces is now considered a mental health intervention at both the community and individual level, particularly in low-resourced areas (South et al. 2020). In a randomized control trial, such greening initiatives significantly reduced feelings of depression and self-reported poor mental health for adults living near greened lots (South et al. 2018). Randomized trials among low-income minority families show park prescriptions from pediatric health care providers can increase family park visits, reduce stress among parents, and improve resilience among youth (Razani et al. 2018, 2019).
What we still need to know.
How do sociodemographic and cultural factors affect the association of natural environment contact and mental health outcomes?
What intensity, duration, and frequency of green-environment exposure are needed to yield a mental health benefit? At what levels of green-environment deprivation do negative mental health outcomes emerge?
Which of the many proposed mechanisms of effect (e.g., stress reduction, physical activity, social engagement, attention restoration, etc.) best explain green-environment associations with specific mental health outcomes? Which exposure metrics (e.g., active park use, passive greenery exposure, etc.) best predict various mental health outcomes?
What are the systemic injustices in access to green space, where urban green spaces are typically most accessible in wealthier, higher-educated, and predominantly White neighborhoods (Nesbitt et al. 2019; Williams et al. 2020)?
What are the social, economic, and ethical implications of current interventions to restore or create green environments? Are there potential unintended consequences (e.g., can greening interventions inadvertently price residents out of their neighborhood)? Are there any adverse mental health effects of natural environment exposure?
Vulnerability and Environmental Risks in Groups and Communities
What is known already.
Previous research at the intersection of vulnerability and environmental risks has focused on understanding environmental factors that put people and communities at risk. Children (Carroquino et al. 2012; Mitro et al. 2015), the elderly, pregnant and postpartum women (Lowe et al. 2020), people with preexisting mental illness, people facing economic and social disadvantage, and first responders (Osofsky et al. 2011) are among the groups identified as being uniquely vulnerable to environmental exposures and disasters (Gee and Payne-Sturges 2004; Lowe and Rhodes 2013; La Greca 2013). Differential patterns of exposure may be exacerbated by environmental injustices that disproportionately affect Black, Indigenous, and people of color (BIPOC) (Hoover et al. 2015). BIPOC populations are more likely to live near toxic waste sites, breathe polluted air, or work in jobs that involve harmful exposures (e.g., Ard 2015; Bell and Ebisu 2012). Structural racism is now recognized as one driver of these disproportionate hazards. These same populations may have less access to a) quality information on effects of environmental exposures; b) economic and social resources to buffer stress in response to exposures and disasters; and c) timely health care for prevention or treatment of mental illness. Another vulnerability factor that has been largely overlooked until now is that environmental factors can worsen health outcomes for people with preexisting mental illness. For example, extreme heat can increase the risk of disease and death for people with mental illness, people with health comorbidities, or those moved to temporary shelters (Taioli et al. 2018) because some antipsychotic and anxiolytic drugs can impair temperature regulation (Martin-Latry 2007).
What we still need to know.
What are the direct and indirect mental health consequences of environmental harms disproportionately sited in or near BIPOC communities—e.g., refineries, Superfund sites, confined animal feeding operations—and how do they interact with other stressors, including exposure to racism and discrimination?
What do BIPOC communities most care about when it comes to the mental health effects of the environment? What priorities do they have for research and interventions in this area?
What are the features of organizations (funding, training, culture) that effectively support scientists of color conducting research into the intersections between environmental health and mental health?
What tool(s) for measuring cumulative environmental and social stressors in communities can be deployed in research and policy-making at a national level?
What are the specific vulnerabilities to environmental hazards among people experiencing mental illness?
Emerging Research Opportunities
Opportunities for environmental and mental health researchers range from including psychiatric and behavioral outcomes among the end points that toxicologists and epidemiologists study to including biomarkers of toxicant exposures in psychosocial studies of health and well-being. Advancing these opportunities will require bridging distinct disciplinary perspectives. Psychological and social phenomena should not be considered separate from biological processes; rather, the biological reality of these disorders are still too complex to fully model with existing tools. In the meantime, although much of psychiatry and psychology incorporates self- and observer-reported assessments, these methods (such as structured clinical interviews or neuropsychological tests) have well-documented reliability and validity, and biomarker, neuroimaging, and animal model correlates (Tables 1 and 2).
In addition to opportunities to merge toxicant measures with psychological end points in experimental animal studies and observational epidemiological studies, emerging research opportunities in this field include:
Omics approaches.
Genome-wide search for genetic drivers of psychiatric illness has revealed new ways to investigate disease etiology (Kitsios and Zintzaras 2009). Similar approaches have been adopted for the study of proteins, metabolites, and the microbiome. More recently, mass spectrometry methods are making it possible to measure environmental exposures in a similar manner under the full “exposome” banner (Vermeulen et al. 2020). These advances provide opportunities for determining intersections between environmental exposures, genetic predispositions, and mental health disorders. By combining multi-omic analysis of human biofluids with advanced imaging techniques (see Table 2) and psychological evaluation, it is possible to systematically study the effects of complex environmental stressors on advanced brain functions. When coupled with other omic-scale markers such as epigenetics, proteomics, and metabolomics, it becomes possible to associate biological changes with specific patterns of exposure. Another emerging area of research is the isolation and characterization of brain-derived extracellular vesicles (Brenna et al. 2021). Although primarily considered to be a source of microRNA, these vesicles can also serve as a window to toxicant deposition in brain tissue. By taking advantage of the range of omic-based approaches, we may advance the science on etiology and prevention of mental health disorders in a manner akin to recent advances using genomic approaches, such as the finding that clinically distinct disorders share common genetic risks (Geschwind and Flint 2015).
Community-based participatory research.
Community-based participatory research (CBPR) is a dual research-practice approach to deploy and study interventions in partnership with communities to improve physical and mental health and resilience. CPBR is rooted in shared decision-making power that can build trust among community members (Viswanathan et al. 2004). For example, the Harvard–UMass Boston Metropolitan Immigrant Health and Legal Status Survey (BM-IHLSS; Holmes and Marcelli 2020) tested interventions to improve neighborhood social cohesion to buffer recent immigrants against psychological distress. Academic investigators (Bateman et al. 2017) partnered with low-income neighborhood residents to plan and plant a neighborhood garden to enhance social cohesion and increase safety in the neighborhood. Similar CBPR studies have been conducted in multiple other populations (Stalker et al. 2020; Bang et al. 2014). The collaborative approach of CPBR is a strength, which ensures that the community’s voice and preferences are embedded in the structure of the research process (Hoover et al. 2015; Viswanathan et al. 2004). CBPR methods can be extended into studies of natural disaster response and resilience, industrial disasters, climate change, and mental health in disadvantaged neighborhoods so that communities are equal cocreators of the study design and questions.
New technologies.
Recent advances in assessment instruments, methodologies, and statistical approaches offer exciting opportunities to significantly advance the study of environmental exposures and mental health. For example, wearables and smart phone–based technologies are areas that offer opportunities to monitor and assess an individual’s activity, exposures, symptoms, and social interactions for early warning signs and even deliver mental health interventions (NASEM 2020). Advances in remote geospatial sensing technologies that could be relevant to mental health research were also addressed in a recent NASEM workshop (NASEM 2021b).
Ecological momentary assessment (EMA) approaches can provide temporally and/or geospatially precise links to human emotion and behavior and are among the approaches enabled by wearables and mobile technologies. Under EMA approaches, participants are prompted to provide information on their mood or psychological state through their smartphone at either random or predesignated times throughout the day. These responses can be geo-located and even structured so that participants are prompted in response to particular locations of interest (e.g., when participants arrive at their work locations).
Statistical methods that are appropriate for evaluating the combined impacts of environmental and social stressors, rather than simply controlling for covariates, are also ripe for broader deployment. Such approaches include machine learning and other data-mining techniques (Huang et al. 2018). The concept of “neighborhood-specific epigenetic markers” also offers promise by potentially allowing identification of epigenetic patterns associated with combined environmental and social stressors and mental health effects (Olden et al. 2014; Reuben et al. 2020).
A Call to Action
As summarized above, much information is already known about how environmental hazards can harm mental health (Table 3), how clean and green environments can benefit mental health, and how burdens of environmental exposures disproportionately fall on marginalized communities and communities of color. Although not exhaustive, we seek to catalyze increasing interdisciplinary action by listing some early implications of the current evidence.
Implications for foundational thinking about the etiology of mental illness.
Given the high prevalence, population burden, and lasting consequences of mental illness, it is important to move past the confines of a specific discipline to more fully identify the causes of mental health and disease. We argue that mental health researchers must expand conventional approaches to incorporate the physical environment into etiological models of mental illness as well as social factors of resilience. In turn, identifying new environmental contributors holds the potential to reveal novel modifiable targets for interventions, with implications for individual- and community-level treatment and preventive medicine. For example, mental health interventions that focus on reducing environmental exposures may be less stigmatized, more cost effective, and better tolerated than exclusively pharmacological or psychotherapeutic approaches.
Actions to Take
Conduct research that employs an exposome framework to systematically examine environmental, social, and biological exposures in relation to psychopathology outcomes.
Investigate the potential mechanisms linking social and cultural environments (e.g., diet, norms, responsibilities, social cohesion, family structure, etc.) to differences in mental health outcomes following environmental exposures.
Develop and test novel therapeutic interventions that target reduction of exposures to environmental contaminants, or increase exposure to beneficial environmental conditions, for the treatment or prevention of psychopathology—at both the individual and community level.
Include mental health assessments and treatment infrastructure in public responses to natural disasters and disasters created by people and policies (e.g., long-term response programming for children exposed to lead during the Flint drinking water crisis).
Implications for the regulation of toxicants.
Environmental health policy currently fails to adequately address the mental health impacts of the environment on individuals and communities. For example, communities facing health concerns around local pollution sources (Downey and Van Willigen 2005) or drinking-water contamination (Cuthbertson et al. 2016) may experience high levels of stress, anxiety, and depression. These signs of distress are either overlooked or viewed by regulators and policymakers as ancillary, or as a risk-communication challenge. The mental health impacts of environmental stressors need more focused attention for prevention, assessment, and mitigation.
Historically the challenges of studying mental health outcomes in laboratory animals, the lack of mechanistic or in vitro models, and the limitations inherent in observational epidemiology have made it difficult to regulate toxicant exposures based on mental and behavioral adverse impacts. Yet accumulated evidence, as reviewed above, demonstrates that there are both direct and indirect effects of neurotoxicants on mental functioning, making behavioral impacts critical end points for population protection. From incorporating behavioral targets in standard toxicology assessments to requiring human studies for chemical approval, research to inform future regulatory action in this sphere is a challenge that must be met.
There also remains the question of whether “secondary” symptoms arising from increased stress in a community should be considered as related to environmental contaminants (e.g., feelings of anxiety following a community-exposure event). Against a background of other chronic social stressors, and a history of environmental racism, an added stressor—such as the discovery of lead in the Flint water system—likely results in measurable increases in adverse mental health outcomes beyond the neurotoxic effect of the exposure itself (e.g., Cuthbertson et al. 2016). Measuring the cumulative impacts of toxic exposures and increased stress and the resulting impacts on mental functioning is needed to address the underlying inequities that contribute to poorer health status in many disadvantaged communities.
Actions to Take
Agencies charged with responding to environmental disasters or oversight of environmental hazards should develop and implement strategies to mitigate mental health effects in exposed communities.
Clinicians and researchers should evaluate mental health effects of neurotoxicants. Regulators should develop methods to use mental health endpoints in quantitative risk assessments.
Develop a national policy and regulatory framework to document, measure, and incorporate cumulative impacts of combined environmental and social stressors in regulatory decision making.
Implications for justice and community resilience.
The disparities in community exposure to environmental hazards and vulnerability to environmental disasters reviewed above suggest that research and governance activities concerning the environment and mental health are also matters of social equity and justice. More effort must be taken to improve vulnerable communities’ psychological resilience to environmental harms (Gray et al. 2020). Such efforts (c.f., Norris et al. 2008), could involve: a) initiating activities to improve economic and social resources in vulnerable communities before disasters/exposures; b) using social cohesion and social capital–building as part of the disaster mitigation process; c) mobilizing preexisting organizational networks and relationships to deploy resources after harmful events; and d) creating effective and trusted information and communication channels to confront uncertainty and deploy health information quickly. Other pre- and postdisaster activities should leverage known but often overlooked social components of community resilience, including cultural resilience, the social capital of local networks, and traditional heritage around local knowledge and connections to place (Clarke and Mayer 2017). It is also important for researchers to understand past controversies that have resulted in community distrust (Pinder 2002), such as the Baltimore Lead Paint study in which families were not appropriately informed about the effectiveness of different lead-abatement strategies being tested (Buchanan and Miller 2006).
Critical for targeting interventions to vulnerable communities, multiple teams from state and federal government, academia, and the nonprofit sectors have developed methods for measuring aggregate community environmental and social stressors, including the CalEnviroScreen (already being used for multiple public policy purposes; Cushing et al. 2015), Maryland EJ Screen (Driver et al. 2019), and the Healthy Places Index (Maizlish et al. 2019). Currently, no clear gold-standard method for cumulative-impacts mapping exists at a national level.
Actions to Take
Environmental health scientists should broaden their research, clinical, advocacy, and communication work to include natural disasters as priority components of environmental health.
Federal, state, and county-level policymakers should prioritize community resilience in pre-disaster planning—and be held accountable for failing to address community needs in advance of disaster events.
Impacted communities should be active partners in needs-assessments to comprehensively understand their psychosocial needs, build trust, and identify factors that will contribute to community resiliency before environmental events.
Communication about exposure, health risks, and mitigation responses should include focus on the psychological impacts of environmental events and natural disasters. These communications should be timely, transparent, accessible, consistent, and ongoing.
A national standard for cumulative environmental and social stressors assessment should be developed to facilitate research and policy approaches to mitigate mental health burdens in disadvantaged communities.
Implications for research training and funding.
Interdisciplinary collaboration is needed to answer critical research questions about the environment and mental health (questions around community vulnerabilities, gene-by-environment interactions, disruption of neurobiological pathways, or lifespan consequences of exposures). The challenge is to develop the multidisciplinary collaborative research teams of environmental health scientists, mental health professionals, and community members to disentangle these complex relationships, generate new evidence, and translate evidence into meaningful policies at local and national levels. Training programs that bridge environmental and mental health disciplines and funding programs supporting research projects examining nontraditional collaborations are essential to advance these research intersections, as are opportunities to support CBPR and other nontraditional approaches to evidence-building. Symposia cohosted by multiple National Institutes of Health (NIH) organizations and cosponsored research programs (Request for Applications, Request for Proposals, Program Announcements) could incentivize interdisciplinary collaboration, particularly from the National Institute of Mental Health (NIMH), the National Institute on Drug Abuse (NIDA), the National Institute of Neurological Disease and Stroke (NINDS), the National Institute of Child Health and Human Development (NICHD), the National Institute of Aging (NIA), and the National Institute of Environmental Health Sciences (NIEHS).
Actions to Take
Include basic training on effects of exposures to toxicants in psychology and psychiatry training programs. Include basic training on mental health effects in environmental science and toxicology training programs.
Develop and promote new funding opportunities that require interdisciplinary teams of environmental science, mental health, and social and behavioral science researchers focused not just on understanding mental health consequences of environmental exposures but also on developing tools for community engagement, intervention, and mitigation of harm.
The NIH should fund interdisciplinary research centers on mental health and the environment. These centers should include multiyear longitudinal investigation to illuminate consequences across the life span, including lifelong prospective registries in vulnerable communities.
Support training and funding in CBPR that includes community partners who take equal part in setting research questions and agendas.
Ensure investigators of color are given full access to training opportunities and supported to lead new investigations in environmental exposures and mental health. Consider targeting additional resources for BIPOC investigator recruitment and retention at early and midcareer stages.
Conclusion
Historical efforts, many funded by the NIH, have revealed a wealth of information on the molecular and cellular underpinnings of mental health disorders, but these discoveries have yet to be scaled to the population level, widespread use of pharmacotherapy notwithstanding. People who have access to more green space, better food options, cleaner air and water, and more affordable health care may experience better mental health, and these changes can be measured with advancing scientific tools as shown in Table 2. How communities are organized, cities are designed, and societies are supported all have ramifications for mental health, and these population-level factors have individual-level biological impacts. It is no longer acceptable to dismiss population-level and public mental health interventions as being unempirical or less scientific. Interventions at a population scale have the potential to improve the mental health of far more people than the individual patient approach of treating one mental disorder at a time (Albee 1982). Because environmental stressors may disproportionately affect the mental health of under-resourced communities and communities of color in the United States and globally, it is imperative to address these issues if we truly want to ameliorate chronic health disparities and provide for a healthy, just society. Environmental and mental health scientists working together to answer our call to action will shed new light on the etiology of mental illness, build new avenues for treatment and primary prevention, and begin to address the large and ever-growing unmet need for mental health services.
Acknowledgments
The authors thank the many presenters and participants at the NASEM Workshop on The Interplay Between Environmental Exposures and Mental Health Outcomes. A special thanks goes to the Workshop planning committee members and the NASEM staff members A. Andrada, K. Sawyer, J. De Mouy, and C. Rea. The NASEM Standing Committee on Emerging Science for Environmental Health Decisions is supported by funding from NIEHS.
A.R. was supported by the NIEHS, grant F31ES029358. M.A. was supported by the NIMH, grant R01MH117247 and the NIA, grant R01AG046149. G.M.S. was supported by the NIEHS, grant R21ES031501. M.B. was supported by the NIEHS, grant T32007322. G.W.M. was supported by the NIEHS, grant R01023839, and the NIDA, grant U18052498. Figure icons courtesy of M. and N. Tatah from the Noun Project.
References
- Ait Bali Y, Ba-Mhamed S, Bennis M. 2017. Behavioral and immunohistochemical study of the effects of subchronic and chronic exposure to glyphosate in mice. Front Behav Neurosci 11:146, PMID: , 10.3389/fnbeh.2017.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albee GW. 1982. Preventing psychopathology and promoting human potential. Am Psychologist 37(9):1043–1050, PMID: , 10.1037/0003-066X.37.9.1043. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publications. 10.1176/appi.books.9780890425596. [DOI] [Google Scholar]
- Ard K. 2015. Trends in exposure to industrial air toxins for different racial and socioeconomic groups: a spatial and temporal examination of environmental inequality in the U.S. from 1995 to 2004. Soc Sci Res 53:375–390, PMID: , 10.1016/j.ssresearch.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Aschengrau A, Weinberg JM, Janulewicz PA, Romano ME, Gallagher LG, Winter MR, et al. . 2012. Occurrence of mental illness following prenatal and early childhood exposure to tetrachloroethylene (PCE)-contaminated drinking water: a retrospective cohort study. Environ Health Glob Access Sci Source 11:2, PMID: , 10.1186/1476-069X-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EL, White RF, Pothier LJ, Berkey CS, Dinse GE, Travers PH, et al. . 1985. Occupational lead neurotoxicity: improvement in behavioural effects after reduction of exposure. Br J Ind Med 42(8):507–516, PMID: , 10.1136/oem.42.8.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang M, Curley L, Kessel A, Marin A, Suzukovich ES III, Strack G. 2014. Muskrat theories, tobacco in the streets, and living Chicago as Indigenous land. Environ Educ Res 20(1):37–55, 10.1080/13504622.2013.865113. [DOI] [Google Scholar]
- Barnes MR, Donahue ML, Keeler BL, Shorb CM, Mohtadi TZ, Shelby LJ. 2018. Characterizing nature and participant experience in studies of nature exposure for positive mental health: an integrative review. Front Psychol 9:2617, PMID: , 10.3389/fpsyg.2018.02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton J, Rogerson M. 2017. The importance of greenspace for mental health. BJPsych Int 14, 4:79–81, PMID: , 10.1192/s2056474000002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman LB, Fouad MN, Hawk B, Osborne T, Bae S, Eady S, et al. . 2017. Examining neighborhood social cohesion in the context of community-based participatory research: descriptive findings from an academic-community partnership. Ethn Dis 27(suppl 1):329–336, PMID: , 10.18865/ed.27.S1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaglehole B, Mulder RT, Frampton CM, Boden JM, Newton-Howes G, Bell CJ. 2018. Psychological distress and psychiatric disorder after natural disasters: systematic review and meta-analysis. Br J Psychiatry 213(6):716–722, PMID: , 10.1192/bjp.2018.210. [DOI] [PubMed] [Google Scholar]
- Bell ML, Ebisu K. 2012. Environmental inequality in exposures to airborne particulate matter components in the United States. Environ Health Perspect 120(12):1699–1704, PMID: , 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghans B, Homberg JR. 2015. Animal models for posttraumatic stress disorder: an overview of what is used in research. World J Psychiatry 5(4):387–396, PMID: , 10.5498/wjp.v5.i4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, Hayes JF. 2019. Air pollution (particulate matter) exposure and associations with depression, anxiety, bipolar, psychosis and suicide risk: a systematic review and Meta-analysis. Environ Health Perspect 127(12):126002, PMID: , 10.1289/EHP4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratman GN, Anderson CB, Berman MG, Cochran B, de Vries S, Flanders J, et al. . 2019. Nature and mental health: an ecosystem service perspective. Sci Adv 5(7):7, PMID: , 10.1126/sciadv.aax0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenna S, Krisp C, Altmeppen HC, Magnus T, Puig B. 2021. Brain-derived extracellular vesicles in health and disease: a methodological perspective. Int J Mol Sci 22(3):1365, PMID: , 10.3390/ijms22031365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp C, Strawn JR, Beck AF, Ryan P. 2019. Pediatric psychiatric emergency department utilization and fine particulate matter: a case-crossover study. Environ. Health Perspect 127:97006, PMID: , 10.1289/EHP4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan DR, Miller GF. 2006. Justice and fairness in the Kennedy Krieger Institute lead paint study: the ethics of public health research on less expensive, less effective interventions. Am J Public Health 96(5):781–787, PMID: , 10.2105/AJPH.2005.063719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Miller GW. 2021. Using the exposome to understand environmental contributors to psychiatric disorders. Neuropsychopharmacology 46(1):263–264, PMID: , 10.1038/s41386-020-00851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera EA, Wiers CE, Lindgren E, Miller G, Volkow ND, Wang GJ. 2016. Neuroimaging the effectiveness of substance use disorder treatments. J Neuroimmune Pharmacol 11(3):408–433, PMID: , 10.1007/s11481-016-9680-y. [DOI] [PubMed] [Google Scholar]
- Carroquino MJ, Posada M, Landrigan PJ. 2012. Environmental toxicology: children at risk. In: Environmental Toxicology. Laws E, ed. New York, NY: Springer, 239–291, 10.1007/978-1-4614-5764-0_11. [DOI] [Google Scholar]
- Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, et al. . 2020. Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw Open 3(4):e203221, PMID: , 10.1001/jamanetworkopen.2020.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianconi P, Betrò S, Janiri L. 2020. The impact of climate change on mental health: a systematic descriptive review. Front Psych 11:1–15, PMID: , 10.3389/fpsyt.2020.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HE, Mayer B. 2017. Community recovery following the deepwater horizon oil spill: toward a theory of cultural resilience. Soc Nat Resour 30(2):129–144, PMID: , 10.1080/08941920.2016.1185556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton S. 2020. Climate anxiety: psychological responses to climate change. J Anxiety Disord 74:102263, PMID: , 10.1016/j.janxdis.2020.102263. [DOI] [PubMed] [Google Scholar]
- Condray R, Morrow LA, Steinhauer SR, Hodgson M, Kelley M. 2000. Mood and behavioral symptoms in individuals with chronic solvent exposure. Psychiatry Res 97(2–3):191–206, PMID: , 10.1016/S0165-1781(00)00217-1. [DOI] [PubMed] [Google Scholar]
- Cushing L, Faust J, August LM, Cendak R, Wieland W, Alexeeff G. 2015. Racial/ethnic disparities in cumulative environmental health impacts in California: evidence from a statewide environmental justice screening tool (CalEnviroScreen 1.1). Am J Public Health 105(11):2341–2348, PMID: , 10.2105/AJPH.2015.302643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson CA, Newkirk C, Ilardo J, Loveridge S, Skidmore M. 2016. Angry, scared, and unsure: mental health consequences of contaminated water in Flint, Michigan. J Urban Health 93(6):899–908, PMID: , 10.1007/s11524-016-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Prado-Bert P, Mercader EMH, Pujol J, Sunyer J, Mortamais M. 2018. The effects of air pollution on the brain: a review of studies interfacing environmental epidemiology and neuroimaging. Curr Environ Health Rep 5(3):351–364, PMID: , 10.1007/s40572-018-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey L, Van Willigen M. 2005. Environmental stressors: the mental health impacts of living near industrial activity. J Health Soc Behav 46(3):289–305, PMID: , 10.1177/002214650504600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver A, Mehdizadeh C, Bara-Garcia S, Bodenreider C, Lewis J, Wilson S. 2019. Utilization of the Maryland environmental justice screening tool: a Bladensburg, Maryland case study. Int J Env Res Public Health 16(3):348, PMID: , 10.3390/ijerph16030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engemann K, Pedersen CB, Arge L, Tsirogiannis C, Mortensen PB, Svenning JC. 2019. Residential green space in childhood is associated with lower risk of psychiatric disorders from adolescence into adulthood. Proc Natl Acad Sci USA 116(11):5188–5193, PMID: , 10.1073/pnas.1807504116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee GC, Payne-Sturges DC. 2004. Environmental health disparities: a framework integrating psychosocial and environmental concepts. Environ Health Perspect 112(17):1645–1653, PMID: , 10.1289/ehp.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Flint J. 2015. Genetics and genomics of psychiatric disease. Science 439(6255):1489–1494, PMID: , 10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B, Hanna F, Reifels L. 2020. The integration of mental health and psychosocial support and disaster risk reduction: a mapping and review. Int J Env Res Public Health 17(6):1900, PMID: , 10.3390/ijerph17061900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney R. 2012. Farming suicides during the Victorian drought: 2001–2007. Aust J Rural Health 20(1):11–15, PMID: , 10.1111/j.1440-1584.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- Heard-Garris NJ, Roche J, Carter P, Abir M, Walton M, Zimmerman M, et al. . 2017. Voices from Flint: community perceptions of the Flint water crisis. J Urban Health 94(6):776–779, PMID: , 10.1007/s11524-017-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland I, DeVille NV, Browning M, Buehler RM, Hart JE, Hipp JA, et al. . 2021. Measuring nature contact: a narrative review. Int J Env Res Public Health 18(8):4092, PMID: , 10.3390/ijerph18084092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LM, Marcelli EA. 2020. Neighborhood social cohesion and serious psychological distress among Brazilian immigrants in Boston. Community Ment Health J 56(1):149–156, PMID: , 10.1007/s10597-019-00468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzschneider K, Mulert C. 2011. Neuroimaging in anxiety disorders. Dialogues Clin Neurosci 13(4):453–461, PMID: , 10.31887/DCNS.2011.13.4/kholzschneider. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover E, Renauld M, Edelstein MR, Brown P. 2015. Social science collaboration with environmental health. Environ Health Perspect 123(11):1100–1106, PMID: , 10.1289/ehp.1409283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Wang A, Morello-Frosch R, Lam J, Sirota M, Padula A, Woodruff , et al. . 2018. Cumulative risk and impact modeling on environmental chemical and social stressors. Curr Environ Health Rep 5(1):88–99, PMID: , 10.1007/s40572-018-0180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcano A, Lubczyńska MJ, Pierotti L, Altug H, Ballester F, Cesaroni G, et al. . 2019. Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ Int 131:104927, PMID: , 10.1016/j.envint.2019.104927. [DOI] [PubMed] [Google Scholar]
- Khan N, Kennedy A, Cotton J, Brumby S. 2019. A pest to mental health? Exploring the link between exposure to agrichemicals in farmers and mental health. Int J Env Res Public Health 16(8):1327, PMID: , 10.3390/ijerph16081327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitsios G, Zintzaras E. 2009. Genome-Wide association studies: hypothesis-“free” or “engaged”? Transl Res 154(4):161–164, PMID: , 10.1016/j.trsl.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Simon EJ. 2009. The neurobiology of addiction: where we have been and where we are going. J Drug Issues 39(1):115–132, PMID: , 10.1177/002204260903900110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. 2011. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7:121–147, PMID: , 10.1007/7854_2010_108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Greca AM, Lai BS, Llabre MM, Silverman WK, Vernberg EM, Prinstein MJ. 2013. Children’s post-disaster trajectories of PTS symptoms: predicting chronic distress. Child Youth Care Forum 42(4):351–369, PMID: , 10.1007/s10566-013-9206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. . 2015. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case–control study in the Danish National Birth Cohort. Environ Health Perspect 123(4):367–373, PMID: , 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Ghisari M, Kjeldsen L, Wielsøe M, Nørgaard-Pedersen B, Mortensen EL, et al. . 2019. Autism spectrum disorders, endocrine disrupting compounds, and heavy metals in amniotic fluid: a case–control study. Mol Autism 10:1, PMID: , 10.1186/s13229-018-0253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Raker EJ, Zacher ML. 2020. Extremes in context: a life-course approach to disaster mental health. One Earth 2(6):497–499, PMID: , 10.1016/j.oneear.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SR, Rhodes JE. 2013. Trajectories of psychological distress among low-income, female survivors of Hurricane Katrina. Am J Orthopsychiatry 83(2 pt 3):398–412, PMID: , 10.1111/ajop.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizlish N, Delaney T, Dowling H, Chapman DA, Sabo R, Woolf S, et al. . 2019. California Healthy Places Index: frames matter. Public Health Rep 134(4):354–362, PMID: , 10.1177/0033354919849882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DK, Fulton JJ, Clarke EJ. 2010. Lead and conduct problems: a meta-analysis. J Clin Child Adolesc Psychol 39(2):234–241, PMID: , 10.1080/15374411003591455. [DOI] [PubMed] [Google Scholar]
- Mares D. 2013. Climate change and levels of violence in socially disadvantaged neighborhood groups. J Urban Health 90(4):768–783, PMID: , 10.1007/s11524-013-9791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markevych I, Schoierer J, Hartig T, Chudnovsky A, Hystad P, Dzhambov AM, et al. . 2017. Exploring pathways linking greenspace to health: theoretical and methodological guidance. Environ Res 158:301–317, PMID: , 10.1016/j.envres.2017.06.028. [DOI] [PubMed] [Google Scholar]
- Marshall GN, Schell TL, Elliott MN, Rayburn NR, Jaycox LH. 2007. Psychiatric disorders among adults seeking emergency disaster assistance after a wildland-urban interface fire. Psychiatr Serv 58(4):509–514, PMID: , 10.1176/ps.2007.58.4.509. [DOI] [PubMed] [Google Scholar]
- Martin-Latry K, Goumy MP, Latry P, Gabinski C, Bégaud B, Faure I, et al. . 2007. Psychotropic drugs use and risk of heat-related hospitalisation. Eur Psychiatry 22(6):335–338, PMID: , 10.1016/j.eurpsy.2007.03.007. [DOI] [PubMed] [Google Scholar]
- McFarlane AC, Searle AK, Van Hooff M, Baghurst PA, Sawyer MG, Galletly C, et al. . 2013. Prospective associations between childhood low-level lead exposure and adult mental health problems: the Port Pirie cohort study. Neurotoxicology 39:11–17, PMID: , 10.1016/j.neuro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- McHale C, Osborne G, Morello-Frosch R, Salmon A, Sandy M, Solomon G, et al. . 2018. Assessing health risks from multiple environmental stressors: moving from G × E to I × E. Mutat Res Rev Mutat Res 775:11–20, PMID: , 10.1016/j.mrrev.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Fairbank JA, Gruber MJ, Jones RT, Lakoma MD, Pfefferbaum B, et al. . 2009. Serious emotional disturbance among youths exposed to Hurricane Katrina 2 years post-disaster. J Am Acad Child Adolesc Psychiatry 48(11):1069–1078, PMID: , 10.1097/CHI.0b013e3181b76697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J, Cunsolo A, Jones-Bitton A, Wright CJ, Harper SL. 2020. Indigenous mental health in a changing climate: a systematic scoping review of the global literature. Environ Res Lett 15(5):053001, 10.1088/1748-9326/ab68a9. [DOI] [Google Scholar]
- Mitro SD, Johnson T, Zota AR. 2015. Cumulative chemical exposures during pregnancy and early development. Curr Environ Health Rep 2(4):367–378, PMID: , 10.1007/s40572-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstein JC, Ursano RJ. 2020. Ecological disasters and mental health: causes, consequences, and interventions. Front Psychiatry 11:1–15, PMID: , 10.3389/fpsyt.2020.00001/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M, et al. . 2020. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1223–1249, PMID: , 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASEM (National Academies of Sciences, Engineering, and Medicine). 2021a. The Interplay Between Environmental Exposures and Mental Health Outcomes: Proceedings of a Workshop—In Brief. Washington, DC: The National Academies Press. 10.17226/26201. [DOI] [PubMed] [Google Scholar]
- NASEM. 2021b. Leveraging Advances in Remote Geospatial Technologies to Inform Precision Environmental Health Decisions: Proceedings of a Workshop—In Brief. Washington, DC: The National Academies Press. 10.17226/26265. [DOI] [PubMed] [Google Scholar]
- NASEM. 2020. Mobile Technology for Adaptive Aging: Proceedings of a Workshop—In Brief. Washington, DC: The National Academies Press. 10.17226/25908. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. 1996. Bone lead levels and delinquent behavior. JAMA 275(5):363–369, PMID: . [PubMed] [Google Scholar]
- Nesbitt L, Meitner JM, Girling C, Sheppard SRJ, Lu Y. 2019. Who has access to urban vegetation? A spatial analysis of distributional green equity in 10 US cities. Landscape Urban Plan 181:51–79, 10.1016/j.landurbplan.2018.08.007. [DOI] [Google Scholar]
- Nestler EJ, Hyman SE. 2010. Animal models of neuropsychiatric disorders. Nat Neurosci 13(10):1161–1169, PMID: , 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbury JB, Arseneault L, Beevers S, Kitwiroon N, Roberts S, Pariante CM, et al. . 2019. Association of air pollution exposure with psychotic experiences during adolescence. JAMA Psychiatry 76(6):614–623, PMID: , 10.1001/jamapsychiatry.2019.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris FH, Stevens SP, Pfefferbaum B, Wyche KF, Pfefferbaum RL. 2008. Community resilience as a metaphor, theory, set of capacities, and strategy for disaster readiness. Am J Community Psychol 41(1–2):127–150, PMID: , 10.1007/s10464-007-9156-6. [DOI] [PubMed] [Google Scholar]
- North CS, Ringwalt CL, Downs D, Derzon J, Galvin D. 2011. Postdisaster course of alcohol use disorders in systematically studied survivors of 10 disasters. Arch Gen Psychiatry 68(2):173–180, PMID: , 10.1001/archgenpsychiatry.2010.131. [DOI] [PubMed] [Google Scholar]
- Obrien LV, Berry HL, Coleman C, Hanigan IC. 2014. Drought as a mental health exposure. Environ Res 131:181–187, PMID: , 10.1016/j.envres.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Olden K, Yu-Sheng L, Gruber D, Sonawane B. 2014. Epigenome: biosensor of cumulative exposure to chemical and nonchemical stressors related to environmental justice. Am J Public Health 104(10):1816–1821, PMID: , 10.2105/AJPH.2014.302130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opler MGA, Brown AS, Graziano J, Desai M, Zheng W, Schaefer C, et al. . 2004. Prenatal lead exposure, delta-aminolevulinic acid, and schizophrenia. Environ Health Perspect 112(5):548–552, PMID: , 10.1289/ehp.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osofsky HJ, Osofsky JD, Arey J, Kronenberg ME, Hansel T, Many M. 2011. Hurricane Katrina’s first responders: the struggle to protect and serve in the aftermath of the disaster. Disaster Med Public Health Prep 5(S2):S214–S219, PMID: , 10.1001/dmp.2011.53. [DOI] [PubMed] [Google Scholar]
- Palinkas LA, O’Donnell ML, Lau W, Wong M. 2020. Strategies for delivering mental health services in response to global climate change: a narrative review. Int J Env Res Public Health 17(22):8562, PMID: , 10.3390/ijerph17228562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder L. 2002. Commentary on the Kennedy Krieger institute lead paint repair and maintenance study. Neurotoxicol Teratol 24(4):477–479, PMID: , 10.1016/S0892-0362(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE. 2016. Research review: environmental exposures, neurodevelopment, and child mental health–new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry 57(7):775–793, PMID: , 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani N, Morshed S, Kohn MA, Wells NM, Thompson D, Alqassari M, et al. . 2018. Effect of park prescriptions with and without group visits to parks on stress reduction in low-income parents: SHINE randomized trial. PLoS One 13(2):e0192921, PMID: , 10.1371/journal.pone.0192921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani N, Niknam K, Wells NM, Thompson D, Hills NK, Kennedy G, et al. . 2019. Clinic and park partnerships for childhood resilience: a prospective study of park prescriptions. Health Place 57:179–185, PMID: , 10.1016/j.healthplace.2019.04.008. [DOI] [PubMed] [Google Scholar]
- Reuben A, Arseneault L, Beddows A, Beevers SD, Latham RM, Moffitt TE, et al. . 2021. Association of air pollution exposure in childhood and adolescence with psychopathology at the transition to adulthood. JAMA Netw Open 4(4):e217508. 10.1001/jamanetworkopen.2021.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Schaefer JD, Moffitt TE, Broadbent J, Harrington H, Houts RM, et al. . 2019. Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiatry 76(4):418–425, PMID: , 10.1001/jamapsychiatry.2018.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Sugden K, Arseneault L, Danese A, Fisher HL, Moffitt TE, et al. . 2020. Association of neighborhood disadvantage in childhood with DNA methylation in young adulthood. JAMA Netw Open 3(6):e206095, PMID: , 10.1001/jamanetworkopen.2020.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell VA. 2007. Neurobiology of animal models of attention-deficit hyperactivity disorder. J Neurosci Methods 161(2):185–198, PMID: , 10.1016/j.jneumeth.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Russell VA, Sagvolden T, Johansen EB. 2005. Animal models of attention-deficit hyperactivity disorder. Behav Brain Funct 1:9, PMID: , 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry N, VanLandingham M. 2009. One year later: mental illness prevalence and disparities among New Orleans residents displaced by Hurricane Katrina. Am J Public Health 99(S3):S725–S731, PMID: , 10.2105/AJPH.2009.174854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer JD, Caspi A, Belsky DW, Harrington H, Houts R, Horwood LJ, et al. . 2017. Enduring mental health: prevalence and prediction. J Abnorm Psychol 126(2):212–224, PMID: , 10.1037/abn0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt HJ, Calloway EE, Sullivan D, Clausen W, Tucker PG, Rayman J, et al. . 2021. Chronic environmental contamination: a systematic review of psychological health consequences. Sci Total Environ 772:145025, PMID: , 10.1016/j.scitotenv.2021.145025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaba T, Bleidorn W, Hopwood CJ, Gebauer JE, Rentfrow PJ, Potter J, et al. . 2021. The impact of childhood lead exposure on adult personality: evidence from the United States, Europe, and a large-scale natural experiment. Proc Natl Acad Sci USA 118(29):e2020104118, PMID: , 10.1073/pnas.2020104118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Goldberg AM. 1974. Lead-induced behavioral dysfunction: an animal model of hyperactivity. Exp Neurol 42(1):146–157, PMID: , 10.1016/0014-4886(74)90013-2. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Goldberg AM. 1975. Pharmacological and neurochemical investigations of lead-induced hyperactivity. Neuropharmacology 14(5–6):431–444, PMID: , 10.1016/0028-3908(75)90026-X. [DOI] [PubMed] [Google Scholar]
- South EC, Hohl BC, Kondo MC, MacDonald JM, Branas CC. 2018. Effect of greening vacant land on mental health of community-dwelling adults: a cluster randomized trial. JAMA Netw Open 1(3):e180298, PMID: , 10.1001/jamanetworkopen.2018.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South EC, Kondo MC, Razani N.. 2020. Nature as a community health tool: the case for healthcare providers and systems. Am. J. Preventive Medicine 59(4):606–610, PMID: , 10.1016/j.amepre.2020.03.025. [DOI] [PubMed] [Google Scholar]
- Stalker KC, Brown ME, Evans C, Hibdon J, Telep C. 2020. Addressing crime, violence, and other determinants of health through community-based participatory research and implementation science. Am J Community Psychol 66(3–4):392–403, PMID: , 10.1002/ajcp.12438. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Nguyen A, Klas J, Gahagan S, Checkoway H, Lopez-Paredes D, et al. . 2021. Associations of acetylcholinesterase inhibition between pesticide spray seasons with depression and anxiety symptoms in adolescents, and the role of sex and adrenal hormones on gender moderation. Expo Health 13(1):51–64, PMID: , 10.1007/s12403-020-00361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. 2000. Genetic epidemiology of major depression: review and meta-analysis. AJP 157(10):1552–1562, PMID: , 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Ahmari SE, Beninger RJ, Eilam D, Harvey BH, Edemann-Callesen H, et al. . 2017. Obsessive-compulsive disorder: insights from animal models. Neurosci Biobehav Rev 76(pt B):254–279, PMID: , 10.1016/j.neubiorev.2016.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taioli E, Tuminello S, Lieberman-Cribbin W, Bevilacqua K, Schneider S, Guzman M, et al. . 2018. Mental health challenges and experiences in displaced populations following Hurricane Sandy and Hurricane Harvey: the need for more comprehensive interventions in temporary shelters. J Epidemiol Community Health 72(10):867–870, PMID: , 10.1136/jech-2018-210626. [DOI] [PubMed] [Google Scholar]
- van Valen E, van Thriel C, Akila R, Nilson LN, Bast-Pettersen R, Sainio M, et al. . 2012. Chronic solvent-induced encephalopathy: European consensus of neuropsychological characteristics, assessment, and guidelines for diagnostics. Neurotoxicology 33(4):710–726, PMID: , 10.1016/j.neuro.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Vermeulen R, Schymanski E, Barabási AL, Miller GW. 2020. The exposome and health: where chemistry meets biology. Science 367(6476):392–396, PMID: , 10.1126/science.aay3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigo D, Thornicroft G, Atun R. 2016. Estimating the true global burden of mental illness. Lancet Psychiatry 3(2):171–178, PMID: , 10.1016/S2215-0366(15)00505-2. [DOI] [PubMed] [Google Scholar]
- Visser I, Wekking EM, de Boer AGEM, de Joode EA, van Hout MSE, van Dorsselaer S, et al. . 2011. Prevalence of psychiatric disorders in patients with chronic solvent induced encephalopathy (CSE). Neurotoxicology 32(6):916–922, PMID: , 10.1016/j.neuro.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Ammerman A, Eng E, Garlehner G, Lohr KN, Griffith D, et al. . 2004. Community‐based participatory research: assessing the evidence. Evid Rep Technol Assess (Summ) 99:1–8, PMID: . [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Timberlake MA II, Prall K, Dwivedi Y. 2017. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 77:99–109, PMID: , 10.1016/j.pnpbp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyandt L, Swentosky A, Gudmundsdottir BG. 2013. Neuroimaging and ADHD: fMRI, PET, DTI findings, and methodological limitations. Dev Neuropsychol 38(4):211–225, PMID: , 10.1080/87565641.2013.783833. [DOI] [PubMed] [Google Scholar]
- White MP, Alcock I, Grellier J, Wheeler BW, Hartig T, Warber SL, et al. . 2019. Spending at least 120 minutes a week in nature is associated with good health and wellbeing. Sci Rep 9(1):7730, PMID: , 10.1038/s41598-019-44097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MP, Alcock I, Wheeler BW, Depledge MH. 2013. Would you be happier living in a greener urban area? A fixed-effects analysis of panel data. Psychol Sci 24(6):920–928, PMID: , 10.1177/0956797612464659. [DOI] [PubMed] [Google Scholar]
- White RF, Krengel M, Grashow R. 2014. Neurotoxicology. In: Clinical Neuropsychology: A Pocket Handbook for Assessment. Parsons MW, Hammeke TA, eds. 3rd ed. Washington, DC: American Psychological Association, 338–362. [Google Scholar]
- Williams TG, Logan TM, Zuo CT, Liberman KD, Guikema SD. 2020. Parks and safety: a comparative study of green space access and inequity in five US cities. Landscape Urban Plan 201:103841, 10.1016/j.landurbplan.2020.103841. [DOI] [Google Scholar]
- Willner P. 1984. The validity of animal models of depression. Psychopharm 83(1):1–16, PMID: , 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Wise T, Cleare AJ, Herane A, Young AH, Arnone D. 2014. Diagnostic and therapeutic utility of neuroimaging in depression: an overview. Neuropsychiatr Dis Treat 10:1509–1522, PMID: , 10.2147/NDT.S50156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kim SH, Kim JC, Shin T, Moon C. 2010. Toluene induces depression-like behaviors in adult mice. Toxicol Res 26(4):315–320, PMID: , 10.5487/TR.2010.26.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]


