Abstract
CYP17A1 is an essential human steroidogenic enzyme, which catalyzes two sequential reactions leading to the formation of androstenedione from progesterone and dehydroepiandrosterone from pregnenolone. The second reaction is the C17-C20 bond scission, which is strongly dependent on the presence of cytochrome b5 and displays a heretofore unexplained more pronounced acceleration when 17OH-progesteone (17OH-PROG) is a substrate. The origin of the stimulating effect of cytochrome b5 on C-C bond scission catalyzed by CYP17A1 is still debated as mostly due to either the acceleration of the electron transfer to the P450 oxy complex or allosteric effects of cytochrome b5 favoring active site conformations that promote lyase activity. Using resonance Raman spectroscopy, we compared the effect of Mn-substituted cytochrome b5 (Mn-Cytb5) on the oxy complex of CYP17A1 with both proteins co-incorporated in lipid nanodiscs. For CYP17A1 with 17OH-PROG, a characteristic shift of the Fe-O mode is observed in the presence of Mn-b5, indicating reorientation of a hydrogen bond between the 17OH group of the substrate from the terminal to the proximal oxygenatom of the Fe-O-O moiety, a configuration favorable for the lyase catalysis. For 17OHpregnenolone, no such shift is observed, the favorable H-bonding orientation being present even without Mn-Cytb5. These new data provide a precise allosteric interpretation for the more pronounced acceleration seen for the 17OH-PROG substrate.
The role of cytochrome b5 (Cytb5) in cytochrome P450 catalysis has been a subject of spirited debate for more than 40 years.1 This small ~15 kDa eukaryotic heme protein is a mitochondrial and microsomal membrane protein that is well-known for its role in electron transfer in several systems, such as fatty acid elongation.2 Its role in several cytochrome P450 systems is less clear. Addition of Cytb5 to reconstituted microsomal P450 drug metabolizing assays by using cytochrome P450 reductase (CPR) can result in activation or inhibition as well as in alterations of substrate specificity.3–6 More recently, some of these varied results were successfully explained by the competition of two redox transfer partners for the same, or overlapping, binding sites on the proximal surface face of hepatic P450 CYP2B4 and CYP17A1.7,8 Other works indicated an improvement of redox coupling and an acceleration of the protonation events that are necessary for formation of the active “compound I” intermediate.9 These, as well as other investigations, have provided a large amount of information about the multiple aspects of cytochrome b5 effects, but there is still a lack of mechanistic understanding in many cases, particularly for the human CYP17A1 involved in steroid biosynthesis.
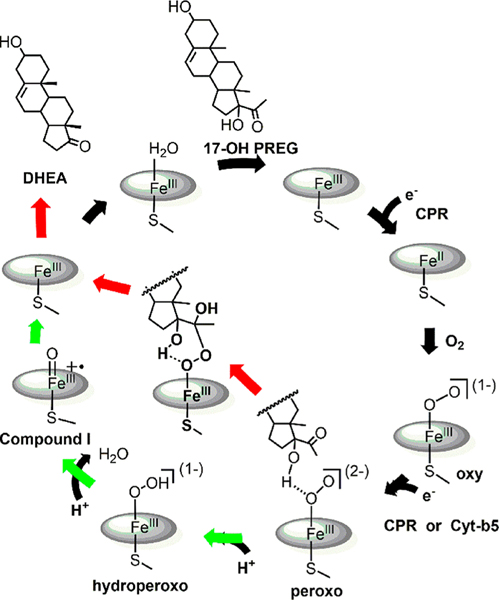
Interactions of Cytb5 with CYP17A1 attracts special attention because of a vitally important role in hormonal regulation. Human CYP17A1 is located at a branch point leading to androgen biosynthesis.10–13 This is a two-step reaction, first involving a typical P450 hydroxylation of either progesterone (PROG) or pregnenolone (PREG) at the C-17 position to produce 17α-hydroxyprogesterone (17OH-PROG) or 17α-hydroxypregnenolone (17OH-PREG). In a second round of catalysis, these hydroxylated products undergo a C17-C20 bond cleavage (lyase) reaction to form androstenedione (AD) or dehydroepiandrosterone (DHEA),14 also critical precursors for glucocorticoid biosynthesis.15 While the mechanism of this lyase reaction has been vigorously debated with arguments being made that it proceeds through a conventional compound I pathway,16 a proposed peroxo-mediated mechanism of the lyase activity, supported by recent studies on cryo-trapped intermediates17–21 and further reinforced by the present results, is shown in Scheme 1. The presence of substrate promotes the transfer of an electron from cytochrome P450 reductase (CPR), with the resulting ferrous CYP17A1 protein then binding oxygen to form the oxygenated (oxy) complex. The second electron transfer required can occur through either CPR or cytochrome b5. In the case of the carbon-carbon lyase reaction, delivery of this second electron generates a nucleophilic ferric-peroxo intermediate that attacks the susceptible carbonyl group of the lyase substrate, forming a new intermediate, whose spectroscopic properties are consistent with the peroxo-hemiketal structure depicted in the center of Scheme 1,18,20,21 which precedes an electrocyclic carbon-carbon (C-C) bond cleavage process to form the DHEA product.14,22,23
Scheme 1.
CYP17A1 Catalytic Cycle Showing a Proposed Lyase Pathway (Black and Red Arrows) for 17-OH PREG through a Peroxoanion Intermediate18,20,21 versus the Possibility of C-C Scission Mediated by Compound I (Black and Green Arrows)16 a aAn analogous scheme applies to 17OH-PROG, with the caveat that the 17-OH hydrogen bond is directed toward the terminal oxygen of the Fe-O-O fragment of the peroxo intermediate.
Significantly, it has been well documented that Cytb5 (Scheme 1) plays a crucial physiological role by enhancing CYP17A1-mediated 17,20-lyase turnover by up to ~10 fold,24–26 although the origin of this stimulation remains controversial.8,27–31 One possibility is that Cytb5 acts as a complementary electron donor, working in concert with CPR to more efficiently complete the lyase cycle24 with the first electron transfer requiring CPR and the second electron being donated by either CPR or Cytb5.7,32,33 Clearly, the presence of a reduced Cytb5 donor positioned near the newly formed oxygenated intermediate could offer a significant advantage.
In previous work we demonstrated that a redox-inactive form of Cytb5, where the heme iron was replaced with manganese (Mn-Cytb5),34 does not enhance the rate of lyase reaction catalyzed by CYP17A1 when incorporated into nanodiscs.24 In contrast, the normal iron Cytb5 increases the reaction rate by ~5-fold, thus identifying an important role of Cytb5 in electron transfer during the catalytic cycle.24 Subsequently, we performed more detailed kinetic studies to directly compare the rates of the second electron transfer to CYP17A1 from CPR and Cytb5.35 In addition, the autoxidation rates were measured for the oxy-ferrous 17OH-PREG bound-CYP17A1 in the absence or presence of Cytb5, of cytochrome P450 reductase (CPR), or both simultaneously when all are self-assembled into nanodiscs. It was observed that ferrous Cytb5 reduces oxy-ferrous CYP17A1 ~10 times faster than CPR, clearly demonstrating a direct electron transfer from Cytb5 to the oxy complex of CYP17A1. However, these results do not exclude the possibility of a significant allosteric interaction between Cytb5 and CYP17A1 when in a protein-protein complex. Evidence for such an allosteric role was recently observed in our resonance Raman (rR) data acquired for 17OH-PREG bound samples. A 3 cm−1 upshift of the Fe-S stretching mode, which carries functional implications, was noted when the redox-inactive Mn-Cytb5 was bound to ND:CYP17.24 Furthermore, several studies of Cytb5 impact on the lyase reactivity have also pointed to possible allosteric influences,7,28,31 including reports that the level of enhancement is greater for 17-OH-PROG than for 17-OH-PREG.26,36
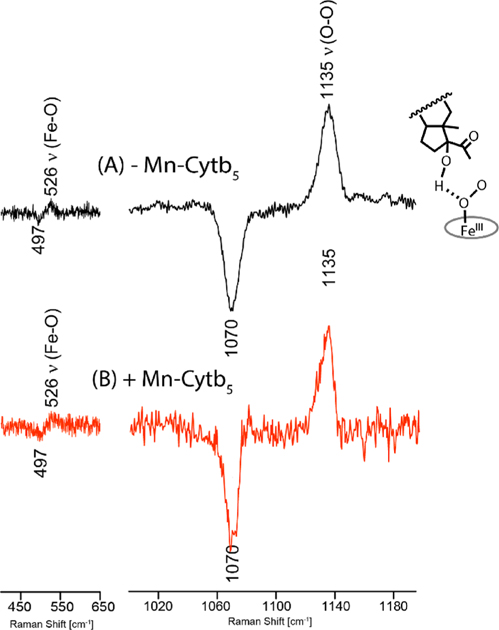
To further address these two distinct roles for Cytb5 in CYP17A1 carbon-carbon bond scission, in the present work, rR spectra were acquired for the 17-OH PREG and 17-OH PROG bound oxy complexes of CYP17A1 in nanodiscs in the absence and presence of oxidized Mn-Cytb5. It is noted that the role of nanodisc technology is especially important in these studies of enzyme/reductase interactions inasmuch as the experimental protocols (Supporting Information) employed here not only eliminate undesired CYP17 aggregation but also ensure the formation of functional dyads, ND:CYP17/Cytb5 in a membrane bilayer. In the case of 17-OH PREG (Figure 1), the υ(16O-16O) and υ(Fe-16O) modes are observed at 1135 and 526 cm−1, with the corresponding υ(18O-18O) and υ(Fe-18O) modes occurring at 1070 and 497 cm−1. These values are not changed from those observed without Mn-Cytb5. It is noted that the υ(Fe-O) mode is 9 cm−1 lower than the value (535 cm−1) observed for the sample containing the non-hydrogen-bonding PREG,17 confirming the presence of a hydrogen-bond interaction of the C17-OH group with the proximal oxygen atom (OP) of the Fe-OP-OT fragment.17,18
Figure 1.
16O-18O rR difference spectra of the 17OH-PREG bound CYP17A1 oxy complex without (A)17 and with (B) Mn-Cytb5; the spectrum in trace A was taken from raw data reported in ref 15. The [16O2-18O2] difference trace was generated by subtracting the two absolute spectral traces, where all (many) nonshifted features (i.e., those not associated with the Fe-O-O fragment) effectively cancel, with only the targeted υ(Fe-O) and υ(O-O) modes being observed.
As argued previously,17–20,22,37,38 such an arrangement, which has been shown to persist in the corresponding peroxo intermediate,18 possesses a more nucleophilic terminal oxygen (Ot) of the peroxo fragment compared to that with an H-bonding interaction with the terminal oxygen, thereby facilitating peroxo attack on the C20 carbon. Clearly, the present observation that the CYP17A1:Cytb5 complex with 17OH-PREG maintains an inherent nucleophilicity of the peroxo fragment as seen without the Cytb5 redox partner suggests that the observed enhancement of lyase efficiency by Cytb5 for this substrate is mainly ascribable to the faster second electron transfer from this redox partner, as suggested by earlier studies.24
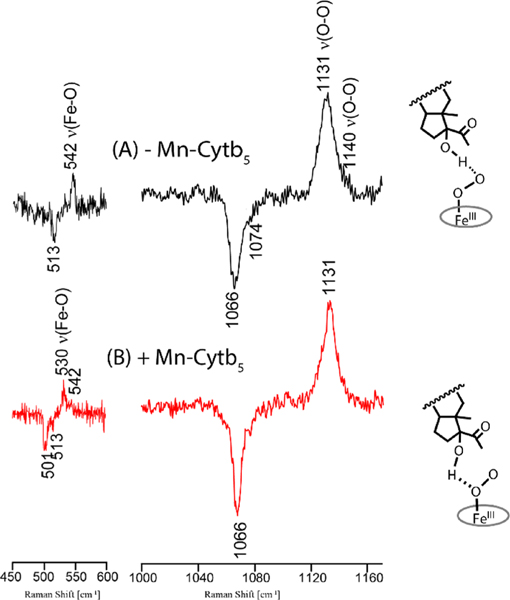
In contrast with the above data, the binding of Mn-Cytb5 to the oxygenated complex with 17OH-PROG bound CYP17A1 brings about obvious conformational changes, as evidenced in the rR difference spectra shown in Figure 2, where the υ(16O-16O) and υ(18O-18O) stretching modes occur at 1131 and 1066 cm−1, consistent with H-bonded Fe-O-O fragments.−17 Significantly, the rR spectrum of the sample including Mn-Cytb5 reveals an active site rearrangement yielding a dominant fraction of the oxy complex possessing a υ(Fe-16O) mode at 530 cm−1 compared to 542 cm−1, a spectral shift consistent with transfer of the H-bonding interaction from the terminal to the proximal oxygen of the Fe-O-O fragment;17 this structure favors the carbon-carbon bond scission reaction,15–20 noting that similar conformers were described in the X-ray structures of CYP17A1 with OH-PREG and OH-PROG bound as substrates.39 The positions of these two substrates are clearly different in the crystal structures, with OH-PROG bound further from heme iron and thus not in a favorable geometry for peroxo attack on the C20 carbonyl as the first step in C17-C20 bond scission, unlike the productive conformer observed for OH-PREG. These spectral changes observed for 17OH-PROG in the presence of Mn-Cytb5 are quite consistent with a shift of the conformational equilibrium toward formation of an H-bond between the 17OH group of the substrate and the proximal oxygen atom of the Fe-O-O complex, i.e., the conformer well oriented to form the peroxo-hemiketal intermediate.22 Finally, it may initially appear surprising that binding of Cytb5 on the proximal side of CYP17 could differentially affect orientations of these two lyase substrates bound in the distal substrate binding pocket. However, recent NMR studies reported by Scott and coworkers reveal that binding of Cytb5 causes conformational changes in a region containing several isoleusine residues (I205, I206, and I238),27 all of which are in the vicinity of an asparagine (N202) that has been shown to play an important role in controlling the position of the H-bonding hydroxyl group of OH-PROG and OH-PREG substrates with respect to the Fe-O-O fragments of the oxy and peroxo intermediates.39
Figure 2.
16O-18O rR difference spectra of 17OH-PROG bound ND: CYP17A1 oxy complex without (A)17 and with (B) Mn-Cytb5; the spectrum in trace A was taken from raw data reported in ref 15. The [16O2-18O2] difference trace was generated as described in the caption to Figure 1.
In summary, earlier functional studies showed the principal role of Cytb5 on P450 catalysis by CYP17A1 is as an electron donor.24,40,41 The rR studies reported herein provide structural evidence for an additional allosteric role when 17OH progesterone (OH-PROG) is the substrate for carbon-carbon bond scission. This additional allosteric enhancement is not expected, and not seen, when 17OH-PREG is at the active site. It is satisfying to note that these new structural revelations obtained through resonance Raman spectroscopy are entirely consistent with the results derived from detailed functional studies of the enzyme with both lyase substrates. Early studies of CYP17A1 in conventional media showed that the coupling efficiency for the OH-PREG substrate is enhanced by a factor of ~8 in the presence of Cytb5, whereas the enhancement for OH-PROG was larger, ~20.26 Also, detailed studies of the nanodisc CYP17A1/Cytb5 complex reveal that the presence of Cytb5 accelerates the lyase activity by ~5-fold for 17-OH PREG but by ~7-fold for 17-OH-PROG.36 Thus, the present work confirms that, in addition to its redox partner role in CYP17A1 function, Cytb5 binding also repositions 17OH-PROG in an optimal orientation to form a peroxo-hemiketal catalytic intermediate.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge grant support from the National Institutes of Health, NIH GM118145 (S.G.S.) and R01 GM125303 (J.R.K.).
ABBREVIATIONS
- 17OH-PROG
17α-hydroxyprogesterone
- 17OH-PREG
17α-hydroxypregnenolone
- AD
androstenedione
- DHEA
dehydroepiandrosterone
- CPR
cytochrome P450 reductase
- Cytb5
cytochrome b5
- Mn-Cytb5
manganese protoporphyrin IX substituted cytochrome b5
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c00581.
All experimental details including sample preparation and resonance Raman measurements (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.1c00581
Contributor Information
Yilin Liu, Department of Chemistry, Marquette University, Milwaukee, Wisconsin 53233, United States.
Ilia G. Denisov, Department of Biochemistry, University of Illinois, Urbana, Illinois 61801, United States
Stephen G. Sligar, Department of Biochemistry and Department of Chemistry, University of Illinois, Urbana, Illinois 61801, United States
James R. Kincaid, Department of Chemistry, Marquette University, Milwaukee, Wisconsin 53233, United States.
REFERENCES
- (1).Schenkman JB; Jansson I; Robiesuh KM Many Roles of Cytochrome-B5 in Hepatic Microsomes. Life Sci. 1976, 19, 611–623. [DOI] [PubMed] [Google Scholar]
- (2).Martin CE; Oh CS; Jiang YD Regulation of long chain unsaturated fatty acid synthesis in yeast. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2007, 1771, 271–285. [DOI] [PubMed] [Google Scholar]
- (3).Sergeev GV; Gilep AA; Usanov SA The role of cytochrome b (5) structural domains in interaction with cytochromes P450. Biochemistry 2014, 79, 406–416. [DOI] [PubMed] [Google Scholar]
- (4).Storbeck KH; Swart AC; Fox CL; Swart P Cytochrome b(5) modulates multiple reactions in steroidogenesis by diverse mechanisms. J. Steroid Biochem. Mol. Biol. 2015, 151, 66–73. [DOI] [PubMed] [Google Scholar]
- (5).Fluck CE, Miller WL, Eds.; Disorders of the Human Adrenal Cortex; Karger: 2008, Vol. 13, pp 1–18. [Google Scholar]
- (6).Akhtar MK; Kelly SL; Kaderbhai MA Cytochrome b(5) modulation of 17 alpha hydroxylase and 17–20 lyase (CYP17) activities in steroidogenesis. J. Endocrinol. 2005, 187, 267–274. [DOI] [PubMed] [Google Scholar]
- (7).Im S-C; Waskell L The interaction of microsomal cytochrome P450 2B4 with its redox partners, cytochrome P450 reductase and cytochrome b5. Arch. Biochem. Biophys. 2011, 507, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Estrada DF; Laurence JS; Scott EE Cytochrome P450 17A1 Interactions with the FMN Domain of Its Reductase as Characterized by NMR. J. Biol. Chem. 2016, 291, 3990–4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Pearl NM; Wilcoxen J; Im S; Kunz R; Darty J; Britt RD; Ragsdale SW; Waskell L Protonation of the Hydroperoxo Intermediate of Cytochrome P450 2B4 Is Slower in the Presence of Cytochrome P450 Reductase Than in the Presence of Cytochrome b5. Biochemistry 2016, 55, 6558–6567. [DOI] [PubMed] [Google Scholar]
- (10).Oshino N; Imai Y; Sato R A function of cytochrome b5 in fatty acid desaturation by rat liver microsomes. J. Biochem. 1971, 69, 155–167. [DOI] [PubMed] [Google Scholar]
- (11).Suzuki T; Sasano H; Sawai T; Mason JI; Nagura H Immunohistochemistry and in situ hybridization of P-45017α (17α-hydroxylase/17,20-lyase). J. Histochem. Cytochem. 1992, 40, 903–908. [DOI] [PubMed] [Google Scholar]
- (12).Vergeres G; Waskell L Cytochrome b5, its functions, structure and membrane topology. Biochimie 1995, 77, 604–620. [DOI] [PubMed] [Google Scholar]
- (13).Dharia S; Slane A; Jian M; Conner M; Conley AJ; Parker CR Jr. Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol. Reprod. 2004, 71, 83–88. [DOI] [PubMed] [Google Scholar]
- (14).Akhtar M; Wright JN; Lee-Robichaud P A review of mechanistic studies on aromatase (CYP19) and 17α-hydroxylase-17,20-lyase (CYP17). J. Steroid Biochem. Mol. Biol. 2011, 125, 2–12. [DOI] [PubMed] [Google Scholar]
- (15).Sreenivasulu G; Senthilkumaran B A role for cytochrome P450 17α-hydroxylase/c17–20 lyase during shift in steroidogenesis occurring in ovarian follicles prior to oocyte maturation. J. Steroid Biochem. Mol. Biol. 2009, 115, 77–85. [DOI] [PubMed] [Google Scholar]
- (16).Guengerich FP; Yoshimoto FK Formation and Cleavage of C-C Bonds by Enzymatic Oxidation Reduction Reactions. Chem. Rev. 2018, 118, 6573–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Gregory M; Mak PJ; Sligar SG; Kincaid JR Differential Hydrogen Bonding in Human CYP17 Dictates Hydroxylation versus Lyase Chemistry. Angew. Chem., Int. Ed. 2013, 52, 5342–5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Mak PJ; Gregory MC; Denisov IG; Sligar SG; Kincaid JR Unveiling the crucial intermediates in androgen production. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 15856–15861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mak PJ; Denisov IG Spectroscopic studies of the cytochrome P450 reaction mechanisms. Biochim. Biophys. Acta, Proteins Proteomics 2018, 1866, 178–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Mak PJ; Duggal R; Denisov IG; Gregory MC; Sligar SG; Kincaid JR Human Cytochrome CYP17A1: The Structural Basis for Compromised Lyase Activity with 17-Hydroxyprogesterone. J. Am. Chem. Soc. 2018, 140, 7324–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Liu Y; Denisov I; Grinkova Y; Sligar S; Kincaid JR P450 CYP17A1 variant with a disordered proton shuttle assembly retains peroxo-mediated lyase efficiency. Chem. - Eur. J 2020, 26, 16846–16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Bonomo S; Jorgensen FS; Olsen L Mechanism of Cytochrome P450 17A1-Catalyzed Hydroxylase and Lyase Reactions. J. Chem. Inf. Model. 2017, 57, 1123–1133. [DOI] [PubMed] [Google Scholar]
- (23).Akhtar M; Corina DL; Miller SL; Shyadehi AZ; Wright JN Incorporation of Label from O-18(2) into Acetate during Side-Chain Cleavage Catalyzed by Cytochrome P-450(17-Alpha) (17-Alpha-Hydroxylase-17,20-Lyase). J. Chem. Soc., Perkin Trans 1 1994, 263–267. [Google Scholar]
- (24).Duggal R; Liu YL; Gregory MC; Denisov IG; Kincaid JR; Sligar SG Evidence that cytochrome b(5) acts as a redox donor in CYP17A1 mediated androgen synthesis. Biochem. Biophys. Res. Commun. 2016, 477, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Katagiri M; Kagawa N; Waterman MR The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch. Biochem. Biophys. 1995, 317, 343–347. [DOI] [PubMed] [Google Scholar]
- (26).Peng HM; Im SC; Pearl NM; Turcu AF; Rege J; Waskell L; Auchus RJ Cytochrome b(5) Activates the 17,20-Lyase Activity of Human Cytochrome P450 17A1 by Increasing the Coupling of NADPH Consumption to Androgen Production. Biochemistry 2016, 55, 4356–4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Estrada DF; Skinner AL; Laurence JS; Scott EE Human Cytochrome P450 17A1 Conformational Selection. J. Biol. Chem. 2014, 289, 14310–14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Auchus RJ; Lee TC; Miller WL Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J. Biol. Chem. 1998, 273, 3158–3165. [DOI] [PubMed] [Google Scholar]
- (29).Guryev OL; Gilep AA; Usanov SA; Estabrook RW Interaction of Apo-cytochrome b5 with Cytochromes P4503A4 and P45017A: Relevance of Heme Transfer Reactions. Biochemistry 2001, 40, 5018–5031. [DOI] [PubMed] [Google Scholar]
- (30).Zhang H; Im S-C; Waskell L Cytochrome b5 Increases the Rate of Product Formation by Cytochrome P450 2B4 and Competes with Cytochrome P450 Reductase for a Binding Site on Cytochrome P450 2B4. J. Biol. Chem. 2007, 282, 29766–29776. [DOI] [PubMed] [Google Scholar]
- (31).Bhatt MR; Khatri Y; Rodgers RJ; Martin LL Role of cytochrome b5 in the modulation of the enzymatic activities of cytochrome P450 17 alpha-hydroxylase/17,20-lyase (P450 17A1). J. Steroid Biochem. Mol. Biol. 2017, 170, 2–18. [DOI] [PubMed] [Google Scholar]
- (32).Zhang H; Hamdane D; Im S-C; Waskell L Cytochrome b5 Inhibits Electron Transfer from NADPH-Cytochrome P450 Reductase to Ferric Cytochrome P450 2B4. J. Biol. Chem. 2008, 283, 5217–5225. [DOI] [PubMed] [Google Scholar]
- (33).Gilep AA; Sushko TA; Usanov SA At the crossroads of steroid hormone biosynthesis: The role, substrate specificity and evolutionary development of CYP17. Biochim. Biophys. Acta, Proteins Proteomics 2011, 1814, 200–209. [DOI] [PubMed] [Google Scholar]
- (34).Morgan ET; Coon MJ Effects of cytochrome b5 on cytochrome P-450-catalyzed reactions. Studies with manganese-substituted cytochrome b5. Drug Metab. Dispos. 1984, 12, 358–364. [PubMed] [Google Scholar]
- (35).Duggal R; Denisov IG; Sligar SG Cytochrome b(5) enhances androgen synthesis by rapidly reducing the CYP17A1 oxy-complex in the lyase step. FEBS Lett. 2018, 592, 2282–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Khatri Y; Gregory MC; Grinkova YV; Denisov IG; Sligar SG Active site proton delivery and the lyase activity of human CYP17A1. Biochem. Biophys. Res. Commun. 2014, 443, 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ogliaro F; de Visser SP; Cohen S; Sharma PK; Shaik S Searching for the second oxidant in the catalytic cycle of cytochrome P450: A theoretical investigation of the iron(III)-hydroperoxo species and its epoxidation pathways. J. Am. Chem. Soc. 2002, 124, 2806–2817. [DOI] [PubMed] [Google Scholar]
- (38).Harris DL; Loew GH Theoretical investigation of the proton assisted pathway to formation of cytochrome P450 compound I. J. Am. Chem. Soc. 1998, 120, 8941–8948. [Google Scholar]
- (39).Petrunak EM; DeVore NM; Porubsky PR; Scott EE Structures of Human Steroidogenic Cytochrome P450 17A1 with Substrates. J. Biol. Chem. 2014, 289, 32952–32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Bonfils C; Balny C; Maurel P Direct evidence for electron transfer from ferrous cytochrome b5 to the oxyferrous intermediate of liver microsomal cytochrome P-450 LM2. J. Biol. Chem. 1981, 256, 9457–9465. [PubMed] [Google Scholar]
- (41).Imai Y; Sato R The roles of cytochrome b5 in a reconstituted N-demethylase system containing cytochrome P-450. Biochem. Biophys. Res. Commun. 1977, 75, 420–426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.