Summary:
The pontomedullary region is responsible for the reduction of muscle activity in rapid-eye-movement sleep and contributes to the control of muscle tone in waking. This study sought to clarify the nature of the interaction between the pontine and medullary reticular formation in mediating muscle tone suppression. The degree of medullary-induced neck muscle tone suppression in the decerebrate cat was assessed before and after microinjection of lidocaine into the pontine reticular formation. Medullary stimulation-induced suppression of neck muscle tone was blocked after pontine lidocaine microinjection. The maximum blockade was observed at 16.6 minutes on average after the injection, and recovery occurred within 45 minutes. We conclude that descending mechanisms from the medulla are not sufficient for the triggering of muscle tone suppression. A two-way interaction between the medulla and pons is hypothesized to play a crucial role in the control of muscle tone.
Keywords: Lidocaine, decerebrate cat, muscle tone, atonia, brainstem, reverberatory circuit, REM sleep
CHOLINERGIC STIMULATION of the rostral part of the pontine reticular formation induces rapid-eye-movement (REM) sleep with atonia in intact animals, and lesions at this pontine site cause REM sleep without atonia.1,2,3 REM sleep without atonia is also induced by lesions in the medial medulla.4,5 In the decerebrate cat, both chemical and electrical stimulation delivered to the pontine inhibitory regions, as well as to portions of the medial medulla, produce a collapse of decerebrate rigidity.6 The latency to the onset of pontine-induced muscle tone suppression is longer than that elicited by medullary stimulation in the decerebrate cat.7–9 Descending pathways that originate in the mesopontine reticular formation and project to the medullary inhibitory region10,11 appear to mediate atonia.
However, atonia is much more difficult to elicit by medullary stimulation in cats transected at the pontomedullary junction than in cats transected at the midbrain level.12 Also, chronically maintained medullary cats never have periods of atonia.13 These findings suggest that the pons may contribute to atonia elicited by medullary stimulation. In the current study, we have reversibly inactivated the pontine reticular formation by means of lidocaine microinjection. The degree of medullary-induced muscle tone suppression was assessed before and after microinjection of lidocaine.
In prior studies, we identified ventral medullary sites at which 500-msec stimulation produced a suppression of muscle tone.6 These sites correspond to the medullary inhibitory area identified by Magoun and Rhines14 with electrical stimulation. We found that activation of nonNMDA glutamate receptors at medullary inhibitory sites in the rostral medulla produced inhibition, and that blockade of these nonNMDA glutamate receptors reversed muscle atonia elicited by microinjection of agonists into the pontine reticular formation.8 We found that the caudomedial medullary atonia sites identified by electrical stimulation were activated by cholinergic agonists that were ineffective in the rostral medulla. Thus we identified a rostromedial medullary inhibitory region that was activated by glutamate and a caudomedial medullary site activated by acetylcholine. We found that lesions of these medullary regions produced REM sleep without atonia.5 We also found that, during REM sleep in the intact cat, glutamate release was increased in the rostromedial medullary region,15 while acetylcholine release was increased in the caudomedial medullary region.16 Thus, stimulation-induced muscle tone suppression in the decerebrate animal identifies sites that mediate muscle atonia in REM sleep.
Long- (500 msec) train stimulation of inhibitory sites produces an equally long-duration inhibition of muscle tone. In recent work, we have found that short- (6.3 msec) train stimulation produces a similar, though shorter lived, suppression of muscle tone.7 All sites at which long-train stimulation is effective produce inhibition with short-train stimulation. However, short-train stimulation has the advantage of revealing the waveform of the inhibitory pulse onset and offset; this is not clearly seen with long-train stimulation.7 In the current study, all motor inhibitory sites were identified with long-train stimulation, and are therefore equivalent to those shown to mediate pontine muscle tone suppression. We used both short- and long-train stimulation at these sites in the current study to get data on the effect of pontine mechanisms on inhibition induced by medullary stimulation.
METHODS
This study was approved by the animal studies committee of the University of California Los Angeles and Dept. of Veterans Affairs. The experiments were performed on three adult cats, weighing 2.5–4.2 kg. Under halothane-oxygen anesthesia, tracheotomy, bilateral ligation of carotid arteries, cannulation of the carotid artery for blood pressure monitoring, and decerebration at the precollicular level were performed.6,7 All animals were allowed to recover from anesthesia for at least 3 hours before the experiments began. These preparations maintained a mean arterial pressure of more than 80 mm Hg throughout the experiment. Core temperature was kept between 37 and 38° C by a heating pad. The decerebration in all cases produced decerebrate rigidity, characterized by limb extension and head dorsiflexion.
The electromyogram (EMG) was recorded bilaterally from neck muscles (occipitoscapularis, splenius, and biventer cervicis muscles) with bipolar stainless steel electrodes. EMG signals were amplified by a Grass preamplifier (model 78D). They were displayed on a polygraph and were recorded through a CED (1401) computer interface.
The medullary reticular formation was stimulated electrically (range of intensity 30–50 μA) with a concentric bipolar electrode (Rhodes Medical Instruments). Two types of stimulation parameters were used—long trains (200 ms trains of 0.2 ms cathodal rectangular pulses at 100 Hz) and short trains (6.3 ms trains consisting of three 0.2 ms cathodal rectangular pulses at 330 Hz). Long-train stimulation produced a brief collapse of decerebrate rigidity. Short-train stimulation was delivered 30 times, once every second. These 30 EMG signals were rectified, digitized, and collected on a computer with a bin width of 1 ms over a 250-ms period, starting 50 ms before the stimulation. They were averaged, and the amplitude at the trough of the averaged waveform was used for assessing the degree of muscle tone suppression.7 The 50 ms prestimulus period was used to calculate baseline EMG activity. The amplitude at the trough was expressed as the percentage of the baseline value (prestimulus baseline level = 100%). Thus, the lower the value, the greater the magnitude of stimulation-induced muscle tone suppression.7 Two-way analysis of variance (two-way ANOVA) and paired t tests were used for statistical analysis.
After baseline measurements were completed, lidocaine (4%, 1 μl) was microinjected into the pontine reticular formation through a 26-gauge Hamilton 1 μl microsyringe over a period of 1 minute. Bilateral injections were completed within 5 minutes. In two cats, lidocaine was injected twice, with a 2-hour interval between injections. In the other cat, the injection was performed four times, with the same 2-hour interval between injections. Injections were aimed at the pontine regions, where acetylcholine is most effective in inducing atonia.8 The decerebrate posture was maintained after lidocaine injection—ie, the limbs remained extended and the head dorsiflexed.
Prior to sacrifice, a 50-μA DC cathodal current was applied at the stimulating sites for 20 seconds.6,7 The brainstems were removed and stored in potassium ferrocyanide with buffered formalin solution. Serial 60 μm sections were stained with neutral red. The stimulating and injection points were verified and reconstructed according to the Berman atlas.17
RESULTS
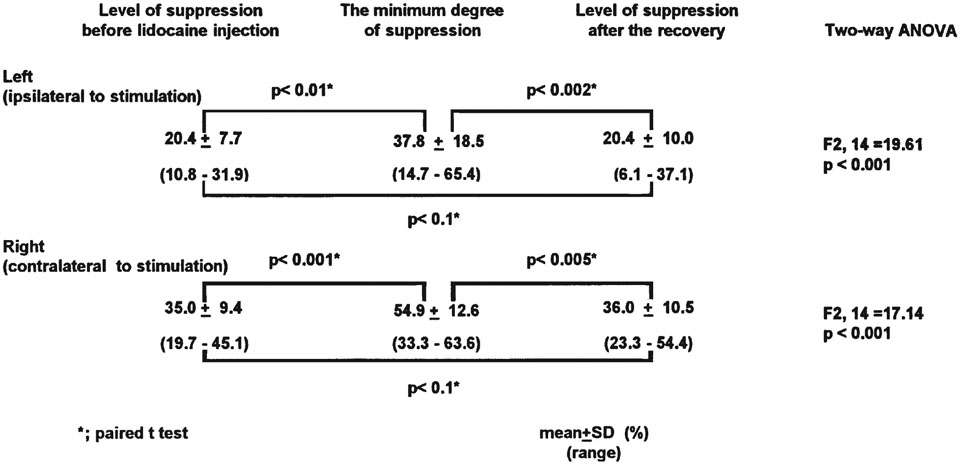
Long-train stimulation of the medullary reticular formation (Fig. 1) produced bilateral suppression of neck muscle tone (Fig. 2A-1, 2B-1, Preinjection). The averaged waveforms obtained by short-train stimulation showed a bilateral reduction of neck muscle activity (Fig. 2A-2, 2B-2, Preinjection). The mean percentage of the amplitude at the trough (prestimulus baseline level = 100%) of suppression was 20.4% (SD 7.7, n=8) for the left side (ipsilateral to the stimulation), and 35.0% (SD 9.4, n=8) for the right side (contralateral to the stimulation), respectively (Table 1).
Figure 1.—
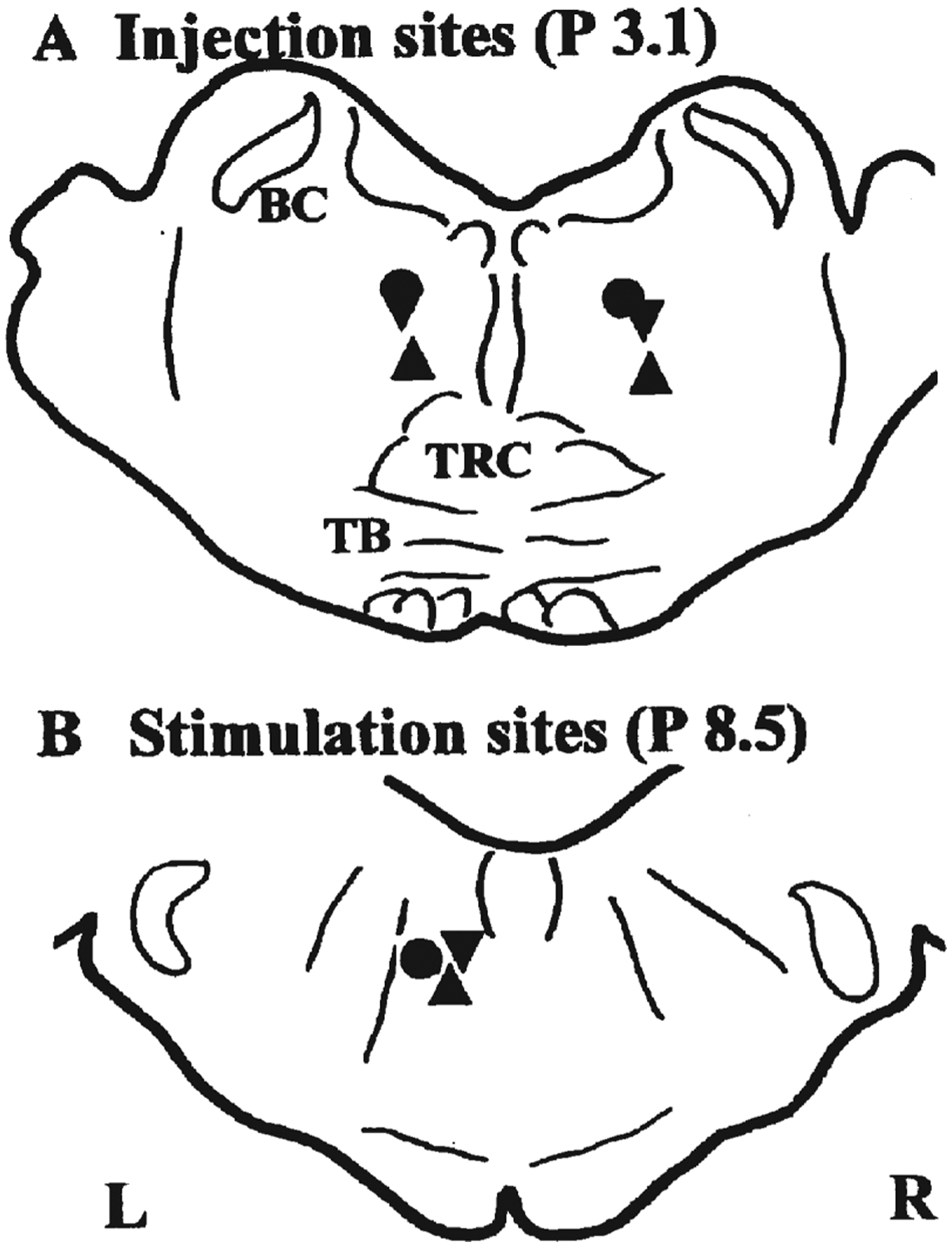
Injection sites of lidocaine (A) and stimulating sites in the medulla (B) are shown. Pontine injection sites are transposed onto a section P 3.1 mm, and medullary stimulating sites onto the P 8.5 mm plane. Circles—cat 1; triangles—cat 2; inverted triangles—cat 3. BC—brachium conjunctivum; TRC—central division of the tegmental reticular nucleus; TB—trapezoid body.
Figure 2.—
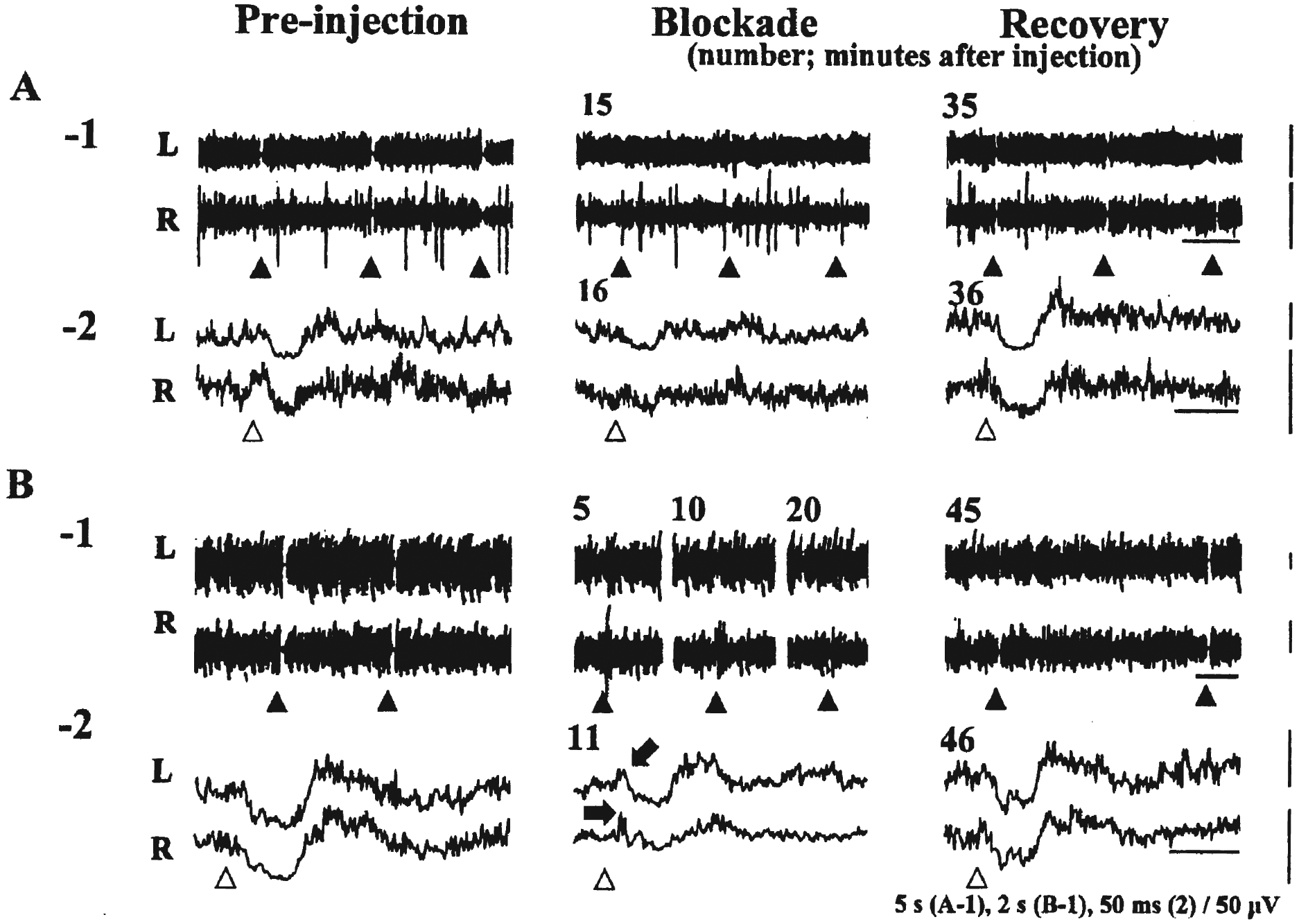
Two types of blockade of medullary-induced neck muscle tone suppression elicited by pontine lidocaine injection are shown. Long-train stimulation (200 ms trains of 0.2 ms pulses at 100 Hz) was delivered at the closed arrowheads (A-1, B-1). The averaged waveforms of rectified EMG changes induced by short-train stimulation (6.3 ms trains of three 0.2 ms pulses at 330 Hz) are shown in A-2 and B-2. Open arrowheads indicate the beginning of the short train. A constant stimulus intensity (A: 30 μA, B: 40 μA) was used during each trial. Preinjection: Both in A and B, long and short trains suppressed neck muscle tone bilaterally. Blockade: Both in A and B, long trains failed to reduce muscle activity. Short trains produced a reduction of muscle tone, though with a lesser degree of magnitude of suppression as compared with waveforms obtained before lidocaine injection. In B but not in A, long trains elicited excitation, and short trains also contained muscle facilitation (arrows) prior to the attenuated suppression. Recovery: Both in A and B, long and short trains again produced neck muscle tone suppression bilaterally. L: left (ipsilateral), R: right (contralateral). Calibration: the bars below long-train stimulations (1); 5 seconds, below short-train stimulation (2); 50 ms.
Table 1.—
Changes of amplitude at trough of medullary-induced neck muscle tone suppression before and after pontine lidocaine injection.
|
After identifying the medullary sites that suppressed neck muscle activity bilaterally, lidocaine was microinjected into the pontine reticular formation (Fig. 1). We stimulated the medulla every 5 to 10 minutes with a constant stimulus intensity with both long and short trains. After lidocaine injection, long-train stimulation failed to reduce neck muscle tone in all eight trials (Fig. 2A-1, 2B-1, Blockade). Short-train stimulation allowed us to better describe the waveform of the attenuation of muscle tone suppression after lidocaine injection. The magnitude of muscle tone suppression induced by short-train stimulation was decreased after the lidocaine injection (Fig. 2A-2, 2B-2, Blockade). In three trials in two different animals, a muscle tone facilitation appeared prior to the attenuated suppression (Fig. 2B-1 and 2, Blockade). On average, the minimum degree of muscle tone suppression with short-train stimulation was obtained at 16.6 minutes after the beginning of the lidocaine injection (range 6–26 minutes). At this point, the mean percentage of the amplitude at the trough was 37.8% (SD 18.5) for the left side (ipsilateral to the stimulation), and 54.9% (SD 12.6) for the right side (contralateral to the stimulation), respectively (Table 1).
At a mean time of 30.6 minutes (range; 25–45 minutes) after the beginning of the lidocaine injection, long trains again suppressed neck muscle tone bilaterally (Fig. 2A-1, 2B-1, Recovery). As assessed by short trains, the average percentage of the amplitude at the trough was found to return to 20.4% (SD 10.0) in the left (ipsilateral) side and 36.0% (SD 10.5) in the right (contralateral) side, respectively (Table 1).
The changes in the percentage of the amplitude at the trough were significant on both sides (two-way ANOVA, ipsi: F2, 14=19.61 [p<0.001], contra: F2, 14=17.14 [p<0.001]) (Table 1).
In two cats, the GABA agonist muscimol (0.1%, 1 μl) was injected after two bilateral lidocaine injections were performed. The muscimol produced the same blockade of medullary inhibition seen after lidocaine. However, presumably owing to the persistence of muscimol effects, no recovery of muscle tone suppression was seen in the 2-hour postinjection observation period. No consistent changes were observed in muscle tone level or in blood pressure as seen on the polygraph recording after lidocaine or muscimol injection.
DISCUSSION
Martin has studied the extent of reversible inactivation produced by microinjection of lidocaine (4%, 1 μl) in the cerebral cortex of the rat.18 Lidocaine was found to spread with a maximum average radius of 1.7 mm within the first 20 minutes. A reduction in glucose utilization was identified within an average radius of 1.4 mm. The level of labeled lidocaine decreased to nearly background levels by 60 minutes. On the basis of these results, it appears that the lidocaine we microinjected spread over the core of the pontine reticular formation, and reduced the excitability of neurons in this area as well as in the descending and ascending fibers of passage in the region. The maximum blocking effect (at 16.6 minutes on average) and the recovery (within 45 minutes) after lidocaine injection we obtained were consistent with Martin’s observation.18
In the current study, reversible inactivation of the pontine reticular formation was found to produce a temporary blockade of muscle tone suppression evoked by stimulating the medullary reticular formation. Short-train stimulation demonstrated that the pontine lidocaine injection did not eliminate the reduction of rectified EMG level, but reduced the degree of muscle tone suppression (disinhibition or an interruption of disfacilitation). There are several possible explanations for this effect. The first is that neuronal elements in the pons tonically raise the excitability of the reticulospinal systems that mediate atonia. Indeed, injections of carbachol and atropine sulfate into the oral pontine reticular formation were reported respectively to increase and decrease the firing rate of medullary reticulospinal neurons that suppress the excitability of lumbar motoneurons innervating hindlimb muscles.19 The second possibility is that medullary stimulation activates pontine elements that directly or multisynaptically descend to the spinal cord, producing inhibition of motoneurons. Finally, medullary stimulation may activate pontine neurons that further excite cells in the medullary inhibitory regions, producing a positive feedback loop. Cholinergic projections from the medulla to the cholinoceptive pontine site have been identified.20,21 Also, both electrical and chemical stimulation of the medial medulla produces an increase of acetylcholine release in the pontine reticular formation, along with shortened REM-sleep latency.22,23 The medullary reticular formation can modulate activity of the pontine reticular formation region implicated in atonia.
Short-train stimulation suggested that increased facilitation contributed to the lidocaine-induced attenuation of medullary-induced neck muscle tone suppression (Fig. 2B). This facilitation was not present in the EMG before lidocaine injection. Neuronal elements in the pons may tonically reduce the excitability of reticulospinal systems involved in motor facilitation.
Some cells in the medullary inhibitory regions were found to discharge at a high rate in REM sleep as well as in postural relaxation during waking.24 An interaction between the pontine and medullary reticular formations is hypothesized to be crucial to the control of muscle activity not only in the decerebrate animal and in REM sleep, but also in waking in the intact animal. This interaction may also have a role in the triggering of cataplexy in the narcoleptic animal.25
ACKNOWLEDGMENTS
This study was supported by Uehara Memorial Foundation, HL41370, NS14610 and the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Siegel JM. Brainstem mechanisms generating REM sleep. In Kryger MH, Roth T, Dement WC, eds. Principles and practice of sleep medicine. Philadelphia: Saunders, 1994:125–44. [Google Scholar]
- 2.Mori S Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog in neurobiol 28 (1987) 161–195. [DOI] [PubMed] [Google Scholar]
- 3.Morrison AR. Paradoxical sleep without atonia. Arch Ital Biol 126 (1988) 275–289. [PubMed] [Google Scholar]
- 4.Holmes CJ, Jones BE. Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 1994;62:1179–1200. [DOI] [PubMed] [Google Scholar]
- 5.Schenkel E, Siegel JM. REM sleep without atonia after lesions of the medial medulla. Neurosci Lett 1989; 98:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai YY, Siegel JM, Wilson WJ. Effect of blood pressure on medial medulla-induced muscle atonia. Am J Physiol 1987;252:H1249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohyama J, Lai YY, Siegel JM. Conduction velocity of the reticulospinal system mediating muscle tone suppression. J Neurophysiol (in press). [Google Scholar]
- 8.Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci 1988;8:4790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai YY, Siegel JM. Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J Neurosci 1990; 10:2727–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai K, Sastre JP, Salvert D, Touret M, Jouvet M. Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: An HRP study. Brain Res 1979;176:233–54. [DOI] [PubMed] [Google Scholar]
- 11.Shiromani PJ, Lai YY, Siegel JM. Descending projections from the dorsolateral pontine tegmentum to the paramedian reticular nucleus of the caudal medulla in the cat. Brain Res 1990;517:224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel JM, Nienhuis R, Tomaszewski KS. Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res 1983; 268:344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegel JM, Tomaszewski KS, Nienhuis R. Behavioral states in the chronic medullary and midpontine cat. Electroencephalogr Clin Neurophysiol 1986;63:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J Neurophysiol 1946;9:165–171. [DOI] [PubMed] [Google Scholar]
- 15.Kodama T, Lai YY, Siegel JM. Enhanced glutamate release during REM sleep in the rostromedial medulla as measured by in vivo microdialysis Brain Res 780:178–181, 1998. [PMC free article] [PubMed] [Google Scholar]
- 16.Kodama T, Lai YY, Siegel JM. Enhancement of acetylcholine release during REM sleep in the caudo-medial medulla measured by in vivo microdialysis. Brain Res 1992;580:348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berman AL. The Brain Stem of the Cat. Madison: University of Wisconsin Press, 1968. [Google Scholar]
- 18.Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 1991;127:160–4. [DOI] [PubMed] [Google Scholar]
- 19.Takakusaki K, Shimoda N, Matsuyama K, Mori S. Discharge properties of medullary reticulospinal neurons during postural changes induced by intrapontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp Brain Res 1994;99:361–74. [DOI] [PubMed] [Google Scholar]
- 20.Holmes CJ, Jones BE. Distribution of cholinergic, GABAergic, serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat. Neuroscience 1994;62:1155–78. [DOI] [PubMed] [Google Scholar]
- 21.Sakai K Physiological properties and afferent connections of the locus coeruleus and adjacent tegmental neurons involved in the generation of paradoxical sleep in the cat. Prog Brain Res 1991;88:31–45. [DOI] [PubMed] [Google Scholar]
- 22.Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsal tegmental field of the cat brain stem. Neurosci Lett 1990;114:277–82. [DOI] [PubMed] [Google Scholar]
- 23.Kodama T, Siegel JM. Brainstem acetylcholine release and REM sleep. In Kumar M, Mallick HN, Nayar U (Eds.) Sleep Wakefulness, Wiley Eastern, New Delhi, 1993, 51–56. [Google Scholar]
- 24.Siegel JM, Wheeler RL, McGinty DJ. Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res 1979;179:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, Chiu C. Neuronal activity in narcolepsy: identification of cataplexy related cells in the medial medulla. Science 1991;252:1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]


