Abstract
Background:
The current state of the science regarding the care and prognosis of patients with disorders of consciousness is limited. Scientific advances are needed to improve the accuracy, relevance, and approach to prognostication, thereby providing the foundation to develop meaningful and effective interventions.
Methods:
To address this need, an interdisciplinary expert panel was created as part of the Coma Science Working Group of the Neurocritical Care Society Curing Coma Campaign.
Results:
The panel performed a gap analysis which identified seven research needs for prognostic modeling and trajectory analysis (“recovery science”) in patients with disorders of consciousness: (1) to define the variables that predict outcomes; (2) to define meaningful intermediate outcomes at specific time points for different endotypes; (3) to describe recovery trajectories in the absence of limitations to care; (4) to harness big data and develop analytic methods to prognosticate more accurately; (5) to identify key elements and processes for communicating prognostic uncertainty over time; (6) to identify health care delivery models that facilitate recovery and recovery science; and (7) to advocate for changes in the health care delivery system needed to advance recovery science and implement already-known best practices.
Conclusion:
This report summarizes the current research available to inform the proposed research needs, articulates key elements within each area, and discusses the goals and advances in recovery science and care anticipated by successfully addressing these needs.
Keywords: Brain injuries, Prognosis, Statistical models, Algorithms, Research, Outcome, Function, Recovery, Trajectorie, Recovery science, Disorders of consciousness, Coma
Introduction
Considerable resources and scientific study are invested in the emergent and intensive care of patients with severe brain injuries, yet knowledge of post-acute-care recovery trajectories remains limited, and predictions of recovery are imprecise. Major challenges include lack of large-scale data sources, early withdrawal of life-sustaining treatments (WoLST), inequities in access to care, uncertainties regarding recovery trajectories, inaccurate early prognostication, and variable perspectives on the capacity for meaningful recovery.
There is a need to align clinical practices with the current evidence and advance recovery science to improve prognostication. It is clear from the current science that late recovery and functional independence are possible for many patients with severe brain injuries and should be considered among the potential outcomes [1–5]. On the basis of these findings, some clinical guidelines have adopted a default frame of reference that a good outcome is possible [6]. Still, 70% of deaths during acute care for traumatic brain injury have been associated with WoLST [7]. Decisions to WoLST are frequently made during the early phase of intensive care unit (ICU) care [8], suggesting limitations in provider knowledge regarding the range of possible outcomes [2, 4, 5, 9]. Complicating the situation, providers tend to provide overly pessimistic prognostic information [10, 11] that may negatively influence care decisions. Although clinical guidelines [6] have incorporated the need to factor in the uncertainty of outcome in family communications during the first 28 days following traumatic and nontraumatic brain injury, this approach has not become standard practice [12, 13]. Pressure to make decisions early stems from pessimistic expectations, beliefs, communication style, health care cost considerations, organ donation needs, and hospital metrics [12–15]. Many family discussions and decisions about goals of care and aggressiveness of management may also be influenced by providers’ unconscious biases [16]. Practices related to WoLST vary widely throughout the world, further complicating analysis.
This article summarizes the results of a gap analysis focused on the science of trajectory analysis and prediction in patients with disorders of consciousness (DoC). We provide an overview of the current science on DoC prognostication and trajectory analysis (current state), the science needed to achieve the ideal state (desired state), and key scientific needs to achieve the desired state.
Procedures
Clinician-scientists (the authors) were identified on the basis of their domain expertise in DoC to join a panel that met weekly by video teleconference. The principal task was to perform a gap analysis regarding the science of trajectory analysis and prognostic modeling for patients with severe brain injury and DoC. Each author independently described the current and desired states, and the results were collated by the primary author. Themes were identified and organized. The recommendations were driven by evidence synthesis, expert opinion, and principles of feasibility and pragmatism.
Terminology
For the purposes of this article, several terms are defined below:
Cognitive motor dissociation (CMD): Refers to volitional brain activity detected by task-based functional magnetic resonance imaging (MRI) or electroencephalography in patients who, on bedside behavioral assessment, appear to be in a coma, a vegetative state (i.e., unresponsive wakefulness syndrome), or a minimally conscious state, without the ability to communicate with the examiner [17].
DoC: Medical conditions that impair consciousness (awareness and/or arousal). DoC include coma, vegetative state (i.e., unresponsive wakefulness syndrome), minimally conscious state, and CMD [17].
Endotype: A subgroup of patients with a condition who share similar biological and physiological mechanisms, clinical trajectory, outcome, and response to therapy.
Prognosis: Forecast of the likely outcome from the brain injury.
Recovery science: The science of trajectory analysis and prediction in patients with DoC.
Trajectory: The path to an outcome. There may be multiple paths to the same outcome.
WoLST: Discontinuation of medical treatments necessary to support or extend a patient’s life.
Current state of science
The current state of the science of outcome prognostication and recovery trajectories is summarized in Table 1. Identifying and addressing the current limitations is critical for advancing recovery science.
Table 1.
Overview of the current state of the science for prognostic modeling and trajectory analysis in patients with disorders of consciousness
| Incomplete understanding of the factors that determine prognosis |
| Limited knowledge about mechanisms of recovery |
| Limited knowledge regarding how to model prognosis and trajectory |
| Inconsistent scientific vocabulary (e.g., need for common data elements) |
| Paucity of integrated data sets across care continuum |
| Limited research using patient-centered outcomes |
| Paucity of knowledge regarding how to communicate prognostic uncertainties |
| Knowledge of recovery trajectories hampered by decisions to limit life-sustaining treatments |
| Care delivery systems impede the ability to translate novel research into practice |
Central to the current gaps in recovery science is that predicting outcomes and recovery trajectories for individuals with severe brain injury is often inaccurate and has a high degree of uncertainty [18]. There are insufficient data available to guide decisions adequately, and the few data available are not translated effectively to the bedside. Inaccuracies and uncertainties of prognostication are multifactorial. There is limited understanding of the biological mechanisms of recovery, the factors that determine prognosis, and how to model trajectories. The best available predictive tools and models are not accurate enough to make early individual patient care decisions, yielding, at best, a 70–80% positive predictive value [19, 20]. Some of the poor performance of models is due to an inconsistent assessment of the level of responsiveness, a key factor in predicting outcome [21]. Approximately 40% of clinical assessments of the level of consciousness are inaccurate (i.e., diagnosing individuals with demonstrable evidence of consciousness as comatose or vegetative) [22–24]. Reasons for misdiagnosis are many, including lack of practical measures for proper serial assessments, fluctuating arousal and consciousness, and failure to optimize arousal before assessment [25–28].
Existing data sets have several limitations that contribute to inaccurate prognostication. Many data sets have few cases with DoC, do not assess the level of consciousness comprehensively and over time, and do not use gold standard assessments of consciousness (e.g., Coma Recovery Scale-Revised) [29]. Assessments of mortality are confounded by the inclusion of those who die following WoLST (ascertainment bias). The true potential outcome trajectories for those who experienced WoLST are not known.
Current approaches to outcome research have important limitations. Studies are often cross-sectional and fail to describe the trajectory of recovery. The outcome measurements used in population-based research are often broad and lack meaningful application to individual patients. There is a lack of widely accepted common data elements for this population, and most data sets do not include long-term follow-up years after the insult. Large data repositories are limited to unlinked data sets that are not easily translated to care and decisions at the bedside. Obstacles to linking existing data sets include technical, administrative, political, regulatory, and data ownership challenges. Current clinical practice does not support state-of-the-art data collection and data sharing methods needed to use advanced statistical approaches, such as big data multicentric machine learning methods and artificial intelligence platforms.
At a systems level, individuals with DoC have limited access to quality rehabilitation. All these factors also create major inequity of care for patients with DoC, particularly because they are fully dependent on the care culture of each ICU and are not able to voice their preference themselves.
Desired state of recovery science
Table 2 provides an overview of a vision for the future state of DoC recovery science. Achieving this vision will require a comprehensive and far-reaching program leveraging science, education, health care delivery systems, and policy.
Table 2.
Overview of the desired state of the science for prognostic modeling and trajectory analysis in patients with disorders of consciousness
| Identification of key preinjury, clinical, intervention, imaging, physiologic, molecular, and genetic factors that determine prognosis |
| Enhanced understanding of mechanisms and time course of recovery |
| More accurate modeling of prognosis and trajectory using advanced statistical methods |
| Comprehensive and widely implemented common data elements |
| Implementation of data sets for tracking patient data across the entire continuum of care |
| Patient-centered outcomes used and integrated into research and clinical practice |
| Prognostic uncertainties effectively acknowledged and communicated to families |
| Knowledge base about recovery trajectories in the absence of limitations to care, i.e., withdrawal of life-sustaining treatments |
| Integrated health care delivery systems that translate novel research into practice and facilitate access to disorders of consciousness care across the recovery continuum |
| Scientific evidence that supports the development of guidelines for withdrawal of life-sustaining treatments |
Ideally, clinical data systems should be equipped with tools to individualize a patient’s predicted trajectory in terms that provide a meaningful picture of the anticipated clinical course while accounting for limitations in diagnostic/prognostic accuracy that even advanced assessment tools might have [30]. Ideally, health infor mation will flow seamlessly across systems. Assessment of prognosis and its communication to family members should employ continuously updated population-based data that, using advanced tools, tailor predictions for individuals using meaningful long-term end points. Family discussions should specify both the predictions and the levels of confidence in the predictions.
Data elements that most accurately and precisely predict recovery are incorporated into models that are iterative, yielding incremental refinements in model prognostic performance as they incorporate new data elements and additional patient recovery trajectories. Predictors may include demographics, preinjury characteristics, injury/disease-related data, examination findings, diagnostic tools (e.g., imaging, neurophysiology, biomarkers), and social variables (e.g., insurance status, income, employment, education, primary language, family supports, location of residence, caregiver and community culture and values). Reliable, reproducible, and precise tools that can handle the significant heterogeneity are needed. This will require extensive data collection effort, including family interviews, medical record reviews, and imaging results, combined with means of reliably interpreting trajectories. Multimodal functional assessments that capture meaningful information about impairments, activities of daily living, and participation in life roles are essential to contextualize prognosis appropriately for patients and families [5, 31].
Other key factors include functional imaging and electrophysiology studies that assess the brain’s response to perturbations as well as genetics and blood-based biomarkers that facilitate our understanding of personal biology, molecular response to injury, and capacity for recovery. Technology and approaches will be developed to capture meaningful, passive day-to-day data collection on the continuum of impairment, needs, and resource use, such as the deployment of wearable/portable sensors and telehealth. Applications should be developed to translate individualized prognostication algorithms into point-of-care tools readily available within electronic health records.
Unique and distinct patient groupings (endotypes) should be identified by using multisite data repositories to facilitate personalized prognostication, decision-making, and future care. A priori level of certainty could be based on these models. Figure 1 shows theoretical trajectories that individuals with brain injury may experience, based on published data using a 1-year postinjury starting point [5]. This represents only a sample of the infinite trajectories and time points possible.
Fig. 1.
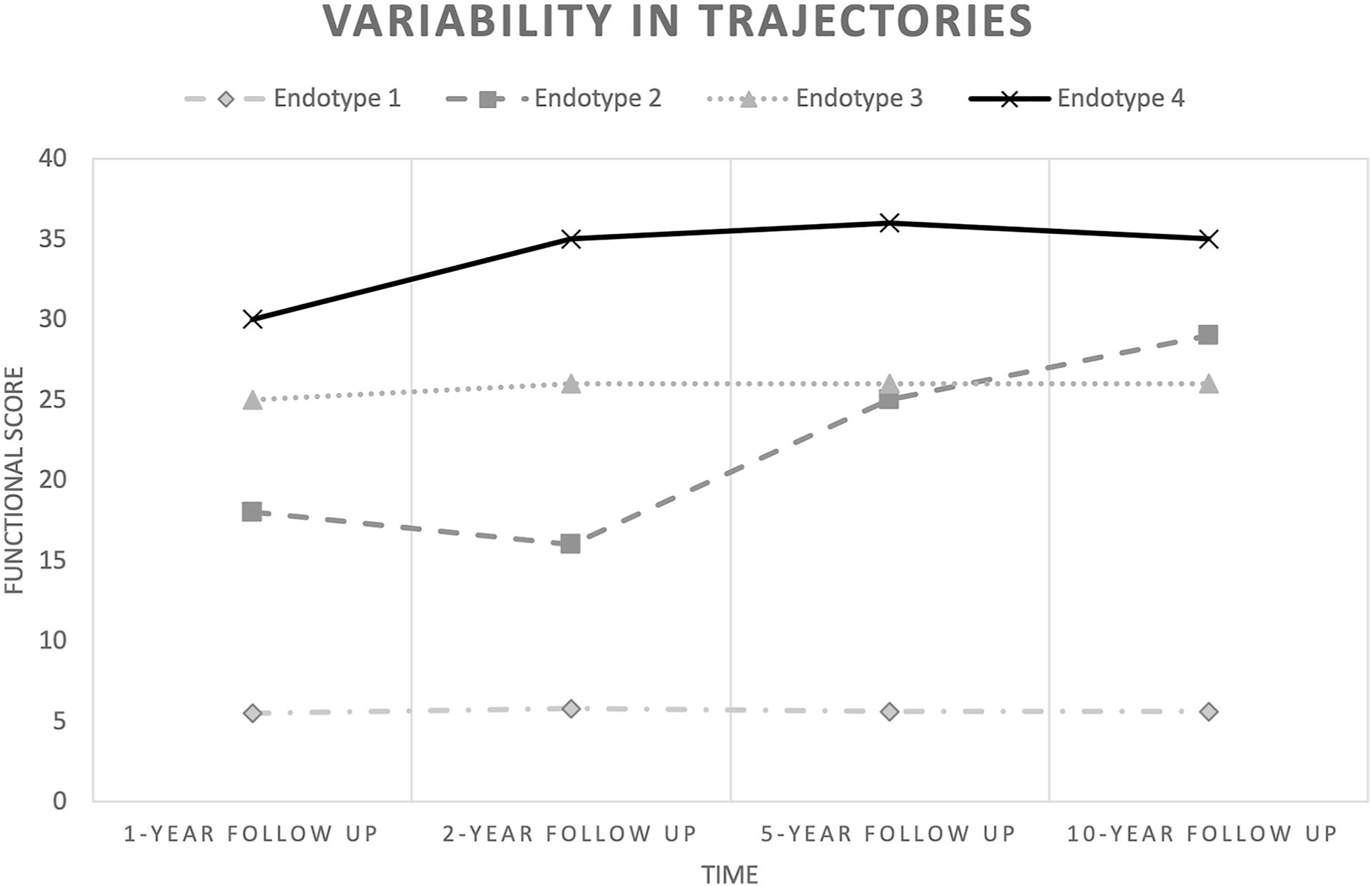
At the 2-year follow-up, endotype 2 appears markedly different from endotype 3 and appears to have declined from the 1-year follow-up. However, by the 5-year follow-up, endotype 2 progresses past endotype 3. At the present time, variables for identifying markers to assign a patient to a particular endotype are lacking. In addition, the common trajectories and meaningful markers (y axis) are not adequately identified
As a person with brain injury recovers, the trajectory may change, necessitating continuous prognostication along the recovery trajectory following an appropriate amount of observation and treatment [6]. A patient-centered model should allow for a reasonable observation period for prognostication to occur in a manner to be determined on the basis of injury pathology. Figure 1 acknowledges a wide variation in outcome paths, including those that improve rapidly and a subgroup that remains unchanged. Defining endotypes will aid in decision-making and facilitate follow-through on advanced directives regarding an acceptable outcome, wherein goals-of-care discussions should be aided by research using meaningful patient-centered outcome metrics. Many prognostic factors have predictive value at a particular moment in time or cannot be known until a particular time or recovery milestone.
Advances in prognostication will integrate research into health care delivery models designed to fit individual patient trajectories. The ideal health care delivery model addresses the following objectives (Fig. 2): (1) accommodates the diverse trajectories possible, (2) maximizes contributions from all team members, (3) enables the provision of progressively updated prognosis, (4) facilitates high-quality data collection throughout the recovery trajectory, (5) incorporates clinical trials into care delivery, and (6) respects the patient’s previously expressed values (e.g., religious beliefs) and wishes via surrogate decision-makers and advance directives.
Fig. 2.
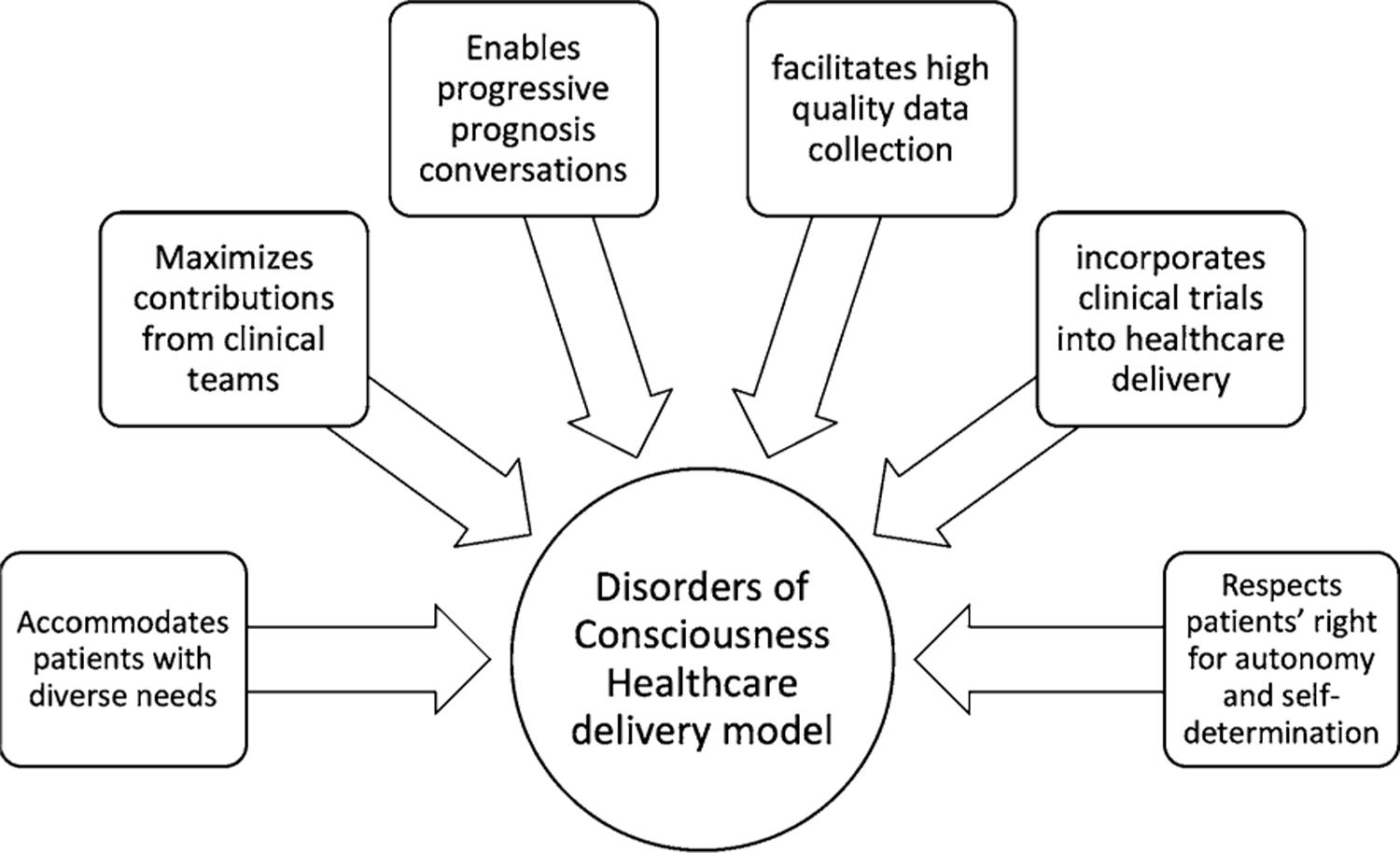
Key objectives to be addressed in an ideal health care delivery model that integrates research and prognostication
Because of the diversity of trajectories, protocols addressing the medical and rehabilitation needs of individuals with DoC require built-in flexibility that allows changes to plans as trajectory certainties become established. Acute and chronic care should ensure that patient progress is monitored and recorded and that lack of progress is adequately addressed. Implementation of interdisciplinary coma rounds is an important avenue to exchange ideas, discuss complicated cases, share perspectives, and build research and clinical capacity in real time. Available technologies, such as telemedicine, may be used to support the interdisciplinary care model as needed. Enhanced curricula may support a robust training and research agenda on DoC to bridge the gap between acute and postacute care for medical and allied health fields. Minimum competencies for DoC prognostication and treatment can guide facility-based care for individuals with prolonged DoC to reduce the incidence and time course of prolonged DoC through both better treatment and prognostication [32]. Those receiving care in an in-home setting may require ongoing monitoring for continued data collection and prognostication.
Research needs to reach state of science: closing the gap
The gap analysis highlighted the need for greater prognostic accuracy and improved communication over the continuum of care. Addressing this gap requires systematic research to reach the desired state. The expert panel identified seven key research goals to pursue. Below we summarize the research goals (see Table 3) as well as the resources, infrastructure, and research agenda needed to support them.
Table 3.
Research needs for prognostic modeling and trajectory analysis in patients with disorders of consciousness
| 1. Define the variables that predict outcomes |
| •Determine the key factors that predict recovery trajectories |
| 2. Define meaningful intermediate outcomes at specific time points for different endotypes |
| •Identify and define meaningful prognostication milestones along the recovery trajectory |
| 3. Describe recovery trajectories in the absence of limitations to care |
| •Identify and characterize disorders of consciousness endotypes on the basis underlying biological features, responses to treatments, and individualized outcome probability |
| •Characterize trajectories in the absence of early withdrawal of life-sustaining treatments |
| •Define the impacts of interventions, including rehabilitation, on recovery trajectories |
| 4. Harness big data access and develop analytic methods to prognosticate more accurately |
| •Develop and implement a prospective multisite data repository using common data elements |
| •Link and harmonize data across existing data sets |
| •Develop automated cloud-based bioinformatic approaches to capture meaningful passive day-to-day data collection on the continuum of impairment, recovery, needs, and resource use across participating sites |
| •Generate clinical decision algorithms based on advanced statistics that have parsed out which elements of clinical care support and optimize disorders of consciousness recovery for variable endotypes |
| •Compare traditional statistical vs. machine learning and neural network approaches to use big data to model and predict patient-based outcome trajectories |
| •Develop models that use serially collected and progressive assessments to provide a more accurate report of an individual’s prognosis and to project the likely recovery trajectory |
| •Generate clinical decision algorithms that have parsed out which elements of clinical care support and optimize disorders of consciousness recovery for variable endotypes |
| •Create freely available and interactive tools for personalized recovery trajectory predictions using distant data that follow individuals over time |
| 5. Identify key elements and processes for communicating uncertainty regarding culturally specific prognosis over time |
| •Identify key elements and uncertainties for personalized communication regarding prognosis at various milestones |
| •Determine best methods and metrics to communicate with families and evidence for how to best relate levels of certainty |
| •Test impact of training tools on unconscious bias, bioethics, and cultural sensitivity related to withdrawal of life-sustaining treatment and living with disability |
| 6. Identify health care delivery models that facilitate recovery and recovery science |
| •Identify health care delivery models that facilitate implementation of priorities 1–5 |
| •Develop a continuous care pathway supported by current best practice evidence and embed data collection in that pathway for continuous quality improvement |
| •Establish validity and effectiveness of value-based care paradigms that increase care quality and access while preventing costly complications and declines in function that require increased resource use |
| 7. Advocate for changes in the health care delivery system needed to advance recovery science and implement already-known best practices |
| •Partner with disability and advocacy groups to identify effective paths for policy development and implementation for the disorders of consciousness community |
| •Identify the core policy changes needed to support research to enhance prognostic accuracy |
| •Promote policy changes needed to integrate data collection into clinical care and link clinical and research data sets |
| •Promote research funding to establish centers of clinical excellence and research networks that facilitate clinical trials in this population |
| •Disseminate relevant, understandable, and actionable recovery science findings to the general public |
Goal 1: define the variables that predict outcomes
Existing resources:
Commonly used scales and predictive tools
Ongoing clinical trial network(s) and databases, including Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) [33], National Institute on Disability Independent Living and Rehabilitation Research (NIDILRR) TBI Model Systems [34], National Trauma Data Bank [35], Collaborative European NeuroTrauma Effectiveness Research in TBI (CENTER-TBI) [36], and EBRAINS [37]
Infrastructure needs:
A deep phenotyping Nurses’ Health or Framingham-like longitudinal study [38, 39] that focuses on patients with DoC
Research agenda
Determine the key factors that predict recovery trajectories, including the following: biological mechanisms (personal biology and genetic, proteomic, and metabolomic markers reflecting patterns of injury [e.g., inflammation] and recovery [e.g., neuronal plasticity mechanisms]), demographics, preinjury characteristics (premorbidity), injury-related data, diagnostic tools (including imaging, neurophysiology, biomarkers, and genetics), and social factors (insurance status, income, employment, education, primary language, family supports, location of residence, and caregiver and community culture and values)
Goal 2: define meaningful intermediate outcomes at specific time points for different endotypes
Existing resources:
Ongoing clinical trial network(s) and databases.
Infrastructure needs:
Define meaningful and patient-centered outcomes (including the recovery of consciousness; physical status; functional abilities; cognitive performance; psychological, emotional, and behavioral state; interpersonal relationships; caregiver burden; academic/ professional reintegration; reintegration into society; patient and caregiver quality of life; and financial impact of recovery or impairment) for the longitudinal study listed under goal 1 and future prognosis and outcome trajectory research.
Research agenda:
- Identify and define meaningful prognostication milestones along the recovery trajectory, including addressing the following issues:
- What information is necessary for the provision of care?
- What information is optimal or sufficient for WoLST?
- How early and how accurate does prognostication need to be? How should this information be used and communicated? What are the ethical implications and responsibilities associated with prognostic assessment and discussion?
- What are poor and good outcomes from the patient and family perspective and how do faith, culture, economics, and community shape these experiences?
- What outcome threshold(s) and outcome trajectories are meaningful and acceptable/unacceptable?
Goal 3: describe recovery trajectories in the absence of limitations to care
Existing resources:
In the current treatment environments, WoLST is commonly practiced hopelessly, confounding attempts to understand recovery potential and trajectories.
Infrastructure needs:
A deep phenotyping longitudinal study (as described above) that leverages existing brain injury treatment environments that do not perform either active or passive WoLST is needed to better understand the natural recovery course. For example, in many Middle East and Far East countries (such as Israel [ultra-Orthodox], Japan, South Korea, Singapore, and mainland China), there may be access to natural recovery data sets with clearly established inclusion/exclusion criteria. However, one concern is that even if WoLST is not offcially practiced, nonsystematic, less aggressive treatment may be provided for those deemed to have no chance for recovery.
Dedicated research staff and standardized protocols are needed to support multisite data collection and quality control.
Funding to support care for individuals with severe brain injury associated with DoC is necessary to evaluate recovery trajectories without the confound of health care cost and access barriers.
Research agenda:
Identify and characterize DoC endotypes on the basis of underlying biological features, responses to treatment, and individualized outcome probability that incorporates personal biology, premorbidity, and other individual characteristics.
- Characterize trajectories in the absence of early WoLST.
- Assess time to recovery and include long-term recovery in natural history outcome trajectory studies.
- Create data sets of trajectories of patients in which the physician team’s recommendation for WoLST was not followed.
- Characterize timing and reasons for WoLST and the impacts of standardized education training on goals of care and prognostication on WoLST rates.
Define the impacts of interventions on recovery trajectories, including providing or withholding rehabilitation (types, intensity, and level of services).
Goal 4: harness big data and develop analytic methods to prognosticate more accurately
Existing resources:
Ongoing clinical trial network(s) and databases.
Current clinical, neuromonitoring, and neuroimaging assessment tools.
Infrastructure needs:
A database that incorporates passive, automated data collection and clinical assessments across clinical pathways with multisite patient registries with injury, epidemiologic, and outcome data using common data elements for secondary analysis.
- Assessments and data collection that can be integrated into the day-to-day clinical practice and applied across the full continuum of care, from the ICU to rehabilitation, such as Simplified Evaluation of Consciousness Disorders [29] and passive movement data (e.g., heart rate variability, electroencephalography, electrophysiology, intracranial pressure, cerebral perfusion pressure, and eye-tracking data) [29].
- Multisite repository for biosamples, genetic samples, neuroimaging, and neurophysiology studies.
-
The genetics of brain injury considers both factors that influence the extent of the injury and those that impact recovery. An enhanced understanding of such factors may allow for a precision therapy approach to mitigate injury and enhance recovery on a more targeted basis [48]. This can be achieved by using genotype as a point of stratification for clinical trials [49]. Genetic information collected may target variation in inflammation, neurodegeneration, neuroplasticity, and neurotransmitters.
-
Blood-based biomarkers are linked to multiple aspects of function that are relevant to describing recovery trajectories in DoC populations [52].
Resources for prospective data management and stewardship should provide the capacity for automated data transfer and quality control. By using a system that facilitates managing patients throughout the entire continuum of care, standardized processes should be developed for data access, use, and dissemination of the results. Integration of health care cost data across the entire continuum of care should be used to establish cost-effectiveness of different treatment paradigms and resource access.
Support for harmonization of retrospective data is needed to use existing data and biorepository resources. Common data elements that can be used for probabilistic vs. deterministic matching of data records from databases collecting information about different portions of the continuum of care should be pursued [53, 54]. Data linkage with public data bases, such as the Centers for Medicare and Medicaid Services and other publicly accessible databases, may enrich cost analyses and capture information on social determinants of health [54–58].
Outcomes that are meaningful and culturally relevant must be defined [59]. Outcome measurement should be multidimensional and consider a wide range of impairment, functional activities, and participation in life roles [49]. Meaningful and culturally relevant outcomes should also include family impact and burden [60–63].
Research agenda:
Develop and implement a prospective multisite data repository using common data elements. This effort includes developing approaches to integrate the following types of data into one model to enhance precision and individualize: interview, premorbidity, epidemiologic, clinical, comorbidity, examination, imaging, diffusion tensor imaging MRI, functional MRI, electrophysiologic, biomarkers, interventions received or not received, longitudinal, and outcome. This process requires the use of the same battery of tests and outcome measures administered in a standard fashion across data sets and time and requires the ability to combine data sets and quality data.
Link and harmonize data across existing data sets. Doing so will require state-of-the-art data collection and data sharing methods needed to use advanced statistical approaches. When attempting to link siloed data sets, numerous obstacles may be encountered, including technical, administrative, political, regulatory, and data ownership challenges.
Develop automated cloud-based bioinformatics approaches to capture meaningful passive day-to-day data collection on the continuum of impairment, recovery, needs, and resource use across participating sites. This will use sensors and data platforms and tools that interface in meaningful ways.
Compare traditional statistical vs. machine learning and neural network approaches to use big data to model and predict patient-based outcome trajectories. This will bring together multidisciplinary analytic teams, including those in computational neuroscience and quantum brain science.
Develop models that use serially collected and progressive assessments to provide a more accurate report of an individual’s prognosis and project the likely recovery trajectory. This may employ item response theory and computerized assessment technology to select optimal metrics and measures of function.
- Generate clinical decision algorithms based on advanced statistics that have parsed out which elements of clinical care support and optimize DoC recovery for variable endotypes.
- Recovery curve measures, such as latent class modeling, may be used to define and support endotype definitions.
- This may employ the use and support of processes that involve treatment element extraction to define paths that positively impact specific endotypes.
Create freely available and interactive tools for personalized recovery trajectory predictions using distant data that follow persons over time. This will involve the development of research resources in commonly translatable tools that can be employed worldwide.
Goal 5: identify key elements and processes for communicating uncertainty regarding culturally specific prognosis over time.
Existing resources:
Findings from studies and systematic literature reviews may aid the development of protocols for approaching and communicating prognosis [6, 64].
Infrastructure needs:
Consensus on protocols for how and when to approach and communicate prognosis.
Training tools to educate physicians and other health care providers on effective communication about prognosis.
Training tools on unconscious bias, bioethics, and cultural sensitivity regarding goals-of-care planning and WoLST.
Training tools on unconscious bias, bioethics, and cultural sensitivity regarding multidimensional function and living with a disability.
Research agenda:
-
Identify key elements and uncertainties for personalized communication regarding prognosis at various milestones. Armed with this new knowledge, there will be a need to develop the best available evidence-based guidelines and tools to consistently implement improved bedside prognostication. For family discussions, objective data and communication tools will help deliver high-quality, evidence-based conversations informed by cultural variance.
-
Determine best methods and metrics to communicate with families and evidence to relate levels of certainty. Communication standards should include the need to update prognosis over time, communicating what is known and not known, and the limitations of certainty at each time point.
-
Test impact of training tools on unconscious bias, bioethics, and cultural sensitivity related to WoLST and living with a disability. Communication protocols may facilitate eliciting acceptable thresholds of care and treatment goals from families/caregivers. Communication training tools may include simulation technology to aid clinicians’ implementation.
Goal 6: identify health care delivery models that facilitate recovery and recovery science.
Existing resources:
Multiple health care delivery models with dyssynchronous data collection and variability in evidence-based metrics of care
Infrastructure needs:
Incorporation of comprehensive data-based assessments with common measures, prognostic assessment tools, patient-specific treatment protocols, and pragmatic and translatable research protocols into care settings
Incorporation of data collection and prognostication in the home and subacute settings to better describe the trajectories and identify the families’ needs at various stages of recovery
Further implementation of telehealth-based services that allow ongoing prognostic data capture and assessment over time
Enhanced capacity and consistency for DoC care and prognostic communication with an emphasis on implementation science are necessary
Research agenda:
Identify health care delivery models that facilitate the implementation of goals 1–5
Develop a continuous care pathway supported by current best practice evidence and embed data collection in that pathway for continuous quality improvement
Establish validity and effectiveness of value-based care paradigms that increase care quality and access while preventing costly complications and declines in function that require increased resource use
Goal 7: advocate for changes in the health care delivery system needed to advance recovery science and implement already-known best practices.
Existing resources:
Global differences in policy and health care institutions
Infrastructure needs:
Research to establish stakeholder needs and barriers to health care access and compliance as well as to community and caregiver resources that influence recovery trajectories
A paradigm shift from overly pessimistic clinician and public expectations of outcome to acknowledgment of the evidence of the range of outcomes possible and degree of uncertainty
Policy agenda:
- Partner with disability and advocacy groups to identify effective paths for policy development and implementation for the DoC community:
- Employ consumers and participatory action research concepts to identify key partnerships to facilitate the necessary structural and funding changes for research progress.
- Identify the core policy changes needed to support research to enhance prognostic accuracy:
- Define the key elements necessary to facilitate research support by evaluating the needs of national, international, public, and private interests.
- Promote policy changes needed to integrate data collection into clinical care and link clinical and research data sets:
- Aggregate leaders in data management and systems define the key policy, and regulatory elements need to facilitate data capture and meaningfulness for DoC research.
- Promote research funding to establish centers of clinical excellence and research networks that facilitate clinical trials in this population:
- Define the key national and international changes needed to establish centers of excellence and linked research networks
Disseminate relevant, understandable, and actionable recovery science findings to the general public for active translation of research into practice
Immediate actionable steps:
Begin to address and resolve the infrastructure needs.
Work with key stakeholders to create awareness of the desired state vision and corresponding research and policy needs.
Conclusions
A gap analysis driven by evidence synthesis, expert opinion, and principles of feasibility and pragmatism arrived at key research needs to advance prognostication in DoC. The interdisciplinary Coma Science Work Group on Trajectory Analysis and Prediction, as part of the Neurocritical Care Society Curing Coma Campaign, has detailed a path for advancing the state of science, including infrastructure, clinical care model development, provider education, advocacy, and policy development, to support our collective research capacity to optimize recovery trajectories for individuals with DoC. Research goals needed to achieve this desired state of science are to (1) define variables that predict outcomes, (2) define meaningful intermediate outcomes at specific time points for different endotypes, (3) describe recovery trajectories in the absence of limitations to care, (4) harness big data and develop analytic methods to prognosticate more accurately, (5) identify key elements and processes for communicating uncertainty regarding prognosis over time, (6) identify health care delivery models that facilitate recovery and recovery science, and (7) advocate for changes in the health care delivery system needed to advance recovery science and implement already-known best practices.
As the needs of recovery science are addressed, the study of its implementation will be essential. If successful, we believe this road map will advance the state of the science and meaningfully impact survivors of DoC and their families.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the Curing Coma Campaign collaborators participating in the overall program, as listed in Supplementary Table 1.
Conflict of interest
The authors do not possess any relevant conflicts of interest to disclose. Dr. Hammond reports grants from NIDILRR, personal fees from Avanir, personal fees from Lash Publishing, Inc, personal fees from Demos Publishing, outside the submitted work. Drs. Katta-Charles, Wagner, Egawa, Laureys, and Diringer have nothing to disclose. Dr. Russell reports personal fees from Merz, personal fees from Allergan, outside the submitted work. Dr. Zafonte reports past royalties from (1) Oakstone for an educational CD- Physical Medicine and Rehabilitation a Comprehensive Review; (2) Springer/Demos publishing for serving as co-editor of the text Brain Injury Medicine. Dr Zafonte serves on or previously served on the Scientific Advisory Board of Myomo, Oxeia Biopharma, ElMINDA and Biodirection. Dr. Claassen reports grants from NINDS R01 NS106014, grants from McDonnell Foundation, grants from NINDS R03 NS112760, other from iCE Neurosystems, outside the submitted work. Dr. Puybasset reports grants from EU- CenterTBI, grants from French ANR, during the conduct of the study; personal fees from BRAINTALE, outside the submitted work; In addition, Dr. Puybasset has a patent WO2012160316 issued. Dr. Stevens reports other from Edwards Lifesciences outside the submitted work.
Source of support
The Curing Coma Campaign is supported by the Neurocritical Care Society.
Footnotes
Supplementary Information
The online version contains supplementary material available at https://doi.org/10.1007/s12028-021-01289-y.
References
- 1.Estraneo A, Moretta P, Loreto V, Lanzillo B, Santoro L, Trojano L. Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state. Neurology. 2010;75:239–45. [DOI] [PubMed] [Google Scholar]
- 2.Nakase-Richardson R, Whyte J, Giacino JT, Pavawalla S, Barnett SD, Yablon SA, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. 2012;29(1):59–65. [DOI] [PubMed] [Google Scholar]
- 3.Katz DI, Polyak M, Coughlan D, Nichols M, Roche A. Natural history of recovery from brain injury after prolonged disorders of consciousness: outcome of patients admitted to inpatient rehabilitation with 1–4 year follow-up. Prog Brain Res. 2009;177:73–88. [DOI] [PubMed] [Google Scholar]
- 4.Whyte J, Nakase-Richardson R, Hammond FM, McNamee S, Giacino JT, Kalmar K, et al. Functional outcomes in traumatic disorders of consciousness: 5-year outcomes from the National Institute on Disability and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch Phys Med Rehabil. 2013;94(10):1855–60. [DOI] [PubMed] [Google Scholar]
- 5.Hammond FM, Giacino JT, Nakase Richardson R,Sherer M, Zafonte RD, Whyte J, et al. Disorders of consciousness due to traumatic brain injury: functional status ten years post-injury. J Neurotrauma. 2019;36(7):1136–46. [DOI] [PubMed] [Google Scholar]
- 6.Giacino JT, Katz DI, Schiff ND, Whyte J, Ashman EJ, Ashwal S,et al. Practice guideline update recommendations summary: disorders of consciousness: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology. 2018;91(10):450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turgeon AF, Lauzier F, Simard JF, Scales DC, Burns KE, Moore L,et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183(14):1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turgeon AF, Lauzier F, Burns KE, Meade MO, Scales DC, Zarychanski R, et al. Determination of neurologic prognosis and clinical decision making in adult patients with severe traumatic brain injury: a survey of Canadian intensivists, neurosurgeons, and neurologists. Crit Care Med 2013;41(4):1086–93. [DOI] [PubMed] [Google Scholar]
- 9.Kowalski RG, Hammond FM, Weintraub AH, Nakase-Richardson R, Zafonte RD, Whyte J, et al. Recovery of consciousness and functional outcome in moderate and severe traumatic brain injury. JAMA Neurol. 2021;78(5):548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammond F, Natarajan S, Toomer A, Huynh T, Norton J. Accuracy of predicting 6-month outcome at 48 hours following severe traumatic brain injury: a pilot study [abstract]. J Neurotrauma. 2009;26(8):A1–101. [Google Scholar]
- 11.Izzy S, Compton R, Carandang R, Hall W, Muehlschlegel S. Self-fulfilling prophecies. NeuroCrit Care. 2013;19(3):347–63. [DOI] [PubMed] [Google Scholar]
- 12.Souter MJ, Blissitt PA, Blosser S, Bonomo J, Greer D, Jichici D, et al. Recommendations for the critical care management of devastating brain injury: prognostication, psychosocial, and ethical management : a position statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care 2015;23(1):4–13. [DOI] [PubMed] [Google Scholar]
- 13.Truog R New guidelines on severe brain injury complicate already difficult decisions. Boston Globe. 2019. [Google Scholar]
- 14.Fins JJ. Disorders of consciousness and disordered care: families, caregivers, and narratives of necessity. Arch Phys Med Rehabil. 2013;94(10):1934–9. [DOI] [PubMed] [Google Scholar]
- 15.Lilley EJ, Scott JW, Weissman JS, Krasnova A, Salim A, Haider AH, Cooper Z. End-of-life care in older patients after serious or severe traumatic brain injury in low-mortality hospitals compared with all other hospitals. JAMA Surg. 2018;153(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rohaut B, Claassen J. Decision making in perceived devastating brain injury: a call to explore the impact of cognitive biases. Br J Anaesth. 2018;120(1):5–9. [DOI] [PubMed] [Google Scholar]
- 17.Edlow BL, Claassen J, Schiff ND, Greer DM. Recovery from disorders of consciousness: mechanisms, prognosis and emerging therapies. Nat Rev Neurol. 2021;17(3):135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Donnell JC, Browne KD, Kilbaugh TJ, Chen HI, Whyte J, Cullen DK. Challenges and demand for modeling disorders of consciousness following traumatic brain injury. Neurosci Biobehav Rev. 2019;98:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Povlishock JT, editor; Brain Trauma Foundation; American Association of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care. Early indicators of prognosis in severe traumatic brain injury [Special section]. J Neurotrauma. 2000; 17(6–7):555–627. [Google Scholar]
- 20.Geurts M, Macleod MR, van Thiel GJ, van Gijn J, Kappelle LJ, van der Worp HB. End-of-life decisions in patients with severe acute brain injury. Lancet Neurol. 2014;13(5):515–24. [DOI] [PubMed] [Google Scholar]
- 21.Giacino JT, Kalmar K. The vegetative and minimally conscious states: a comparison of clinical features and functional outcome. J Head Trauma Rehabil. 1997;12(4):36–51. [Google Scholar]
- 22.Schnakers C, Vanhaudenhuyse A, Giacino J, Ventura M, Boly M, Majerus S, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Childs NL, Mercer WN, Childs HW. Accuracy of diagnosis of persistent vegetative state. Neurology. 1993;43:1465–7. [DOI] [PubMed] [Google Scholar]
- 24.Andrews K, Murphy L, Munday R, Littlewood C. Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit. BMJ. 1996;313:13–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wannez S, Heine L, Thonnard M, Gosseries O, Laureys S; Coma Science Group collaborators. The repetition of behavioral assessments in diagnosis of disorders of consciousness. Ann Neurol. 2017;81(6):883–9. [DOI] [PubMed] [Google Scholar]
- 26.Giacino JT, Ashwal S, Childs N, Cranford R, Jennett B, Katz DI, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58(3):349–53. [DOI] [PubMed] [Google Scholar]
- 27.Seel RT, Sherer M, Whyte J, Katz DI, Giacino JT, Rosenbaum AM, et al. ; American Congress of Rehabilitation Medicine; Brain Injury-Interdisciplinary Special Interest Group; Disorders of Consciousness Task Force. Assessment scales for disorders of consciousness: evidence-based recommendations for clinical practice and research. Arch Phys Med Rehabil. 2010;91(12):1795–813. [DOI] [PubMed] [Google Scholar]
- 28.Giacino JT, Schnakers C, Rodriguez-Moreno D, Kalmar K, Schiff N, Hirsch J. Behavioral assessment in patients with disorders of consciousness: gold standard or fool’s gold? Prog Brain Res. 2009;177:33–48. [DOI] [PubMed] [Google Scholar]
- 29.Aubinet C, Cassol H, Bodart O, Sanz LRD, Wannez S, Martial C, et al. Simplified Evaluation of CONsciousness Disorders (SECONDs) in individuals with severe brain injury: a validation study. Ann Phys Rehabil Med. 2020. 10.1016/j.rehab.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Edlow BL, Fins JJ. Assessment of covert consciousness in the intensive care unit: clinical and ethical considerations. J Head Trauma Rehabil. 2018;33(6):424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur J Phys Rehabil Med. 2010;46(4):549–56. [PubMed] [Google Scholar]
- 32.Giacino JT, Whyte J, Nakase-Richardson R, Katz DI, Arciniegas DB, Blum S, et al. Minimum competency recommendations for programs that provide rehabilitation services for persons with disorders of consciousness: a position statement of the American Congress of Rehabilitation Medicine and the National Institute on Disability, Independent Living and Rehabilitation Research Traumatic Brain Injury Model Systems. Arch Phys Med Rehabil. 2020;101(6):1072–89. [DOI] [PubMed] [Google Scholar]
- 33.Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB,et al. Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J Neurotrauma. 2013;30(22):1831–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dijkers MP, Marwitz JH, Harrison-Felix C. thirty years of national institute on disability, independent living, and rehabilitation research traumatic brain injury model systems center research-an update. J Head Trauma Rehabil. 2018;33(6):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American College of Surgeons. About NTDB. https://www.facs.org/Quality-Programs/Trauma/TQP/center-programs/NTDB/about. Accessed 4 Jan 2021.
- 36.CENTER-TBI Web site. https://www.center-tbi.eu/. Accessed 4 Jan 2021.
- 37.EBRAINS Web site. https://ebrains.eu/. Accessed 4 Jan 2021. [Google Scholar]
- 38.Bao Y, Bertoia ML, Lenart EB, Stampfer MJ, Willett WC, Speizer FE,et al. Origin, methods, and evolution of the three nurses’ health studies. Am J Public Health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet. 2014;383(9921):999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil 2004;85(12):2020–9. [DOI] [PubMed] [Google Scholar]
- 41.Harris K, Linsenmeyer M, Weppner J, Breisinger T, Yukevich AM, Galang G, et al. Motor first: a proposed approach to the JFK Coma Recovery Scale-Revised for the consulting physician [abstract]. Am J Phys Med Rehabil. 2020;99 Suppl 3:a80. [Google Scholar]
- 42.Kondziella D, Bender A, Diserens K, van Erp W, Estraneo A, Formisano R, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27(5):741–56. [DOI] [PubMed] [Google Scholar]
- 43.Velly L, Perlbarg V, Boulier T, Adam N, Delphine S, Luyt CE, et al. Use of brain diffusion tensor imaging for the prediction of long-term neurological outcomes in patients after cardiac arrest: a multicentre, international, prospective, observational, cohort study. Lancet Neurol 2018;17(4):317–26. [DOI] [PubMed] [Google Scholar]
- 44.Comanducci A, Boly M, Claassen J, De Lucia M, Gibson RM, Juan E, et al. Clinical and advanced neurophysiology in the prognostic and diagnostic evaluation of disorders of consciousness: review of an IFCN-endorsed expert group. Clin Neurophysiol 2020;131(11):2736–65. [DOI] [PubMed] [Google Scholar]
- 45.Olsen A, Babikian T, Bigler ED, Caeyenberghs K, Conde V, Dams-O’Connor K, et al. Toward a global and reproducible science for brain imaging in neurotrauma: the ENIGMA adult moderate/severe traumatic brain injury working group. Brain Imaging Behav. 2021;15(2):526–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Claassen J, Doyle K, Matory A, Couch C, Burger KM, Velazquez A, et al. Detection of brain activation in unresponsive patients with acute brain injury. N Engl J Med. 2019;380(26):2497–505. [DOI] [PubMed] [Google Scholar]
- 47.Engemann DA, Raimondo F, King JR, Rohaut B, Louppe G, Faugeras F, et al. Robust EEG-based cross-site and cross-protocol classification of states of consciousness. Brain. 2018;141(11):3179–92. [DOI] [PubMed] [Google Scholar]
- 48.Laskowitz D, Grant G, editors. Translational research in traumatic brain injury. Boca Raton (FL): CRC Press/Taylor and Francis Group; 2016. [PubMed] [Google Scholar]
- 49.Wagner AK. TBI Rehabilomics research: an exemplar of a biomarker-based approach to precision care for populations with disability. Curr Neurol Neurosci Rep. 2017;17(11):84. [DOI] [PubMed] [Google Scholar]
- 50.Wagner AK, Kumar RG. TBI Rehabilomics research: conceptualizing a humoral triad for designing effective rehabilitation interventions. Neuropharmacology. 2019;145(Pt B):133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner AK. Rehabilomics: a conceptual framework to drive biologics research. PMR. 2011;3(6 Suppl 1):S28–30. [DOI] [PubMed] [Google Scholar]
- 52.Wagner AK, Zitelli KT. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology. 2013;20(1):39–48. [DOI] [PubMed] [Google Scholar]
- 53.Kumar RG, Wang Z, Kesinger MR, Newman M, Huynh TT, Niemeier JP, et al. Probabilistic matching of deidentified data from a trauma registry and a traumatic brain injury model system center: a follow-up validation study. Am J Phys Med Rehabil. 2018;97(4):236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kesinger MR, Kumar RG, Ritter AC, Sperry JL, Wagner AK. Probabilistic matching approach to link deidentified data from a trauma registry and a traumatic brain injury model system center. Am J Phys Med Rehabil. 2017;96(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evans E, Gutman R, Shan M, Devone F, Kumar R, Resnik L, et al. Factors associated with successful discharge among older adults with traumatic brain injury in skilled nursing facilities. Arch Phys Med Rehabil. 2020;101(11):E12–13. [Google Scholar]
- 56.Evans E, Gutman R, Davone F, Thomas K. Patient and injury characteristics of older adults with TBI receiving post-acute care in skilled nursing and inpatient rehabilitation facilities. Arch Phys Med Rehabil. 2020;101(11):E105. [Google Scholar]
- 57.Corrigan JD, Bogner JA. Neighborhood characteristics and outcomes after traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):912–21. [DOI] [PubMed] [Google Scholar]
- 58.Corrigan JD, Bogner J, Pretz C, Mellick D, Kreider S, Whiteneck GG, et al. Use of neighborhood characteristics to improve prediction of psychosocial outcomes: a traumatic brain injury model systems investigation. Arch Phys Med Rehabil. 2012;93(8):1350–8.e2. [DOI] [PubMed] [Google Scholar]
- 59.Agency for Healthcare Research and Quality. 2010 national healthcare disparities report. 2011. https://archive.ahrq.gov/research/findings/nhqrdr/nhdr10/nhdr10.pdf. Accessed 4 Jan 2021. [Google Scholar]
- 60.Leonardi M, Giovannetti AM, Pagani M, Raggi A, Sattin D; National Consortium Functioning and Disability in Vegetative and in Minimal Conscious State Patients. Burden and needs of 487 caregivers of patients in vegetative state and in minimally conscious state: results from a national study. Brain Inj 2012;26(10):1201–10. [DOI] [PubMed] [Google Scholar]
- 61.Soeterik SM, Connolly S, Playford ED, Duport S, Riazi A. The psychological impact of prolonged disorders of consciousness on caregivers: a systematic review of quantitative studies. Clin Rehabil. 2017;31(10):1374–85. [DOI] [PubMed] [Google Scholar]
- 62.Steppacher I, Kissler J. A problem shared is a problem halved? Comparing burdens arising for family caregivers of patients with disorders of consciousness in institutionalized versus at home care. BMC Psychol. 2018;6(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gonzalez-Lara LE, Munce S, Christian J, Owen AM, Weijer C, Webster F. The multiplicity of caregiving burden: a qualitative analysis of families with prolonged disorders of consciousness. Brain Inj. 2021;35(2):200–8. [DOI] [PubMed] [Google Scholar]
- 64.Wijdicks EF, Hijdra A, Young GB, Bassetti CL, Wiebe S; Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


