Abstract
The pontomedullary region is responsible for both the tonic and phasic reduction of muscle activity in rapid-eye-movement sleep and contributes to the control of muscle tone in waking. This study focused on determining the time course of activity in the pontomedullary systems mediating atonia. Short-train stimulations (3 0.2-ms pulses at 330 Hz) of the pons and medulla suppressed neck and hindlimb muscle activity in decerebrate cats. We identified two distinct phases of suppression, early and late. The anatomic sites that produced each suppression were intermixed. We estimated the dividing value of the conduction velocity for reticulospinal projections responsible for early and late phases of hindlimb muscle tone suppression to be 22.8 m/s. In the medial medulla, 238 reticulospinal units, which send axons to the L1 level of the spinal cord, were identified. Pontine stimulation that suppressed hindlimb muscle tone increased the firing rate of 138 units (type I). Sixteen type I units showed a delayed response to the pontine stimulation with a latency of 10 ms or longer (type Id), whereas 122 type I units exhibited an earlier response (type Ie). Seven type Ie units had an axonal conduction velocity of <22.8 m/s, whereas the remaining 115 conducted at faster than 22.8 m/s. Early and late hindlimb muscle tone suppressions were hypothesized to be mediated through fast and slow conducting type Ie reticulospinal units. The activity of type Id neurons may contribute to the cessation of the early-phase suppression as well as to the induction, maintenance, or cessation of the late-phase suppression.
INTRODUCTION
A complete suppression of antigravity muscle tonus occurs in rapid-eye-movement (REM) sleep (Jouvet 1962). The pontine tegmentum (Hendricks et al. 1982; Henley and Morrison 1974; Jouvet and Delorme 1965; Shouse and Siegel 1992; Webster and Jones 1989) and medial medulla (Holmes and Jones 1994; Schenkel and Siegel 1989) play key roles in maintaining this state-dependent atonia. In addition to this tonic motor suppression, phasic motor activity reduction occurs in association with REMs (Gassel et al. 1964) or pontogeniculooccipital (PGO) waves (Glenn and Dement 1982; López-Rodríguez et al. 1992; Morrison and Bowker 1975; Orem 1980; Pedroarena et al. 1994; Pivik et al. 1982).
Magoun and Rhines (1946) discovered that electrical stimulation of the medial medulla produces a collapse of decerebrate rigidity. Subsequent work showed that motor activity in decerebrate cats could be suppressed by stimulating the midbrain, pontine, and medullary reticular formation both electrically and chemically (Engberg et al. 1968; Jankowska et al. 1968; Lai et al. 1987; Lai and Siegel 1988, 1990, 1991; Morales et al. 1987; Oka et al. 1993; Takakusaki et al. 1993). Polygraphically defined REM sleep with atonia (Jouvet 1962; Villablanca 1966) and REM-related phasic motor suppression (Seguin et al. 1973) can occur in the decerebrate animal as well as in human infants who have no cerebral cortex (Kohyama et al. 1995). The brain stem must contain systems mediating both tonic and phasic motor activity suppression. Some cells in the medullary inhibitory region were found to discharge at a high rate in REM sleep as well as in postural relaxation during waking (Siegel et al. 1979). In both REM sleep and waking, descending systems from the brain stem may have a role in the suppression of muscle tone.
Kanamori et al. (1980) identified two medullary reticulospinal neurons that were active during REM sleep with a conduction velocity ranging from 6 to 8 m/s. In contrast, the spike-triggered averaging technique in decerebrate cats indicated that medullary reticulospinal cells producing suppression of hindlimb motoneuronal activity conducted at 80–100 m/s (Takakusaki et al. 1994). Motor activity reduction may be mediated by more than one reticulospinal system.
In the current study, we stimulated the pons and medulla by using very short-duration stimulation trains. We found that these trains produced muscle activity reductions at both short and long latency. We identified the medullary reticulospinal neurons activated by the same short stimulation trains that induced atonia. In addition, we characterized discharge patterns and conduction velocities of these cells. The time course of discharge in these units was compared with that of the two distinct phases of muscle tone suppression.
METHODS
Surgical procedures
The experiments were performed on 23 adult cats, weighing 1.8–4.8 kg. Tracheostomy, bilateral ligation of carotid arteries, cannulation of the left carotid artery for blood pressure monitoring, insertion of wires around or into the lumbar segment of the spinal cord, and decerebration at the postmammillary-precollicular level were performed under halothane-oxygen anesthesia. All animals were allowed to recover from anesthesia for ≥3 h before the experiments began. All preparations used in this study had a mean arterial pressure of >80 mmHg. Core temperature was maintained between 37 and 38°C by a heating pad.
EMG and unit recording
The electromyogram (EMG) was recorded bilaterally from the occipitoscapularis, splenius, biventer cervicis, and gastrocnemius-soleus muscles with bipolar stainless steel electrodes. A stainless steel electrode (0.25-mm-diam shaft with 8° tapered tip, AC impedance 5 MΩ; A-M Systems, model 5710) was inserted into the medial medulla for extracellular recording of medullary reticulospinal units. Only units with signal-to-noise ratios >4:1, good isolation from other units, and stable spike amplitudes were studied. For antidromic identification of reticulospinal cells, the L1 segment of the spinal cord was stimulated with a pair of wires placed under it (Takakusaki et al. 1994) or stainless steel wires (50 μm; California Fine Wire) manually inserted to a depth of 5–6 mm toward the ventrolateral and ventromedial funiculi (Drew et al. 1986). The criteria for the antidromic identification of reticulospinal units were constant latency, high-frequency following (≥200 Hz) (Lipski 1981), and collision.
EMG signals were amplified by a Grass preamplifier (model 78D), and unit signals were amplified by an A-M Systems differential AC amplifier (model 1700). They were displayed on a polygraph and an oscilloscope and were recorded through a CED (1401) computer interface for later analysis.
Brain stem stimulation
The brain stem was stimulated with a concentric bipolar electrode (Rhodes Medical Instruments). In most experiments, stimulation consisted of three 0.2-ms cathodal rectangular pulses at 330 Hz (the total duration of each stimulus train was 6.3 ms). To determine the threshold (T) for inducing muscle tone suppression, the current intensity was increased until bilateral muscle activity reduction was visually identified in sweeps of neck and hindlimb muscles on an oscilloscope monitor. The sweep was displayed for a 200-ms period including the 50-ms period before the stimulation. The stimulus intensity used was expressed as a multiple of T. The maximum stimulus intensity used was 300 μA; stimulation was delivered once every 1–2 s.
Analysis
Rectified, digitized EMG and discriminated unit pulses were collected on a computer with a binwidth of 1 ms over a 300- to 400-ms period starting 50 ms before the stimulation and averaged for 30 trials. The 50 ms preceding the stimulus was used to calculate a baseline level of EMG activity. We measured the latency to onset, latency to trough, duration or latency to offset, and amplitude at the minimum trough of the averaged EMG waveform. The onset and offset of muscle tone suppression were defined as the points where the EMG first fell 2 SDs of the prestimulus baseline variability below the baseline level (Fig. 1A). The minimum amplitude at the trough was expressed as the percentage of the baseline value (prestimulus baseline level 100%). Thus the lower the value, the greater the magnitude of stimulation induced muscle tone suppression.
FIG. 1.
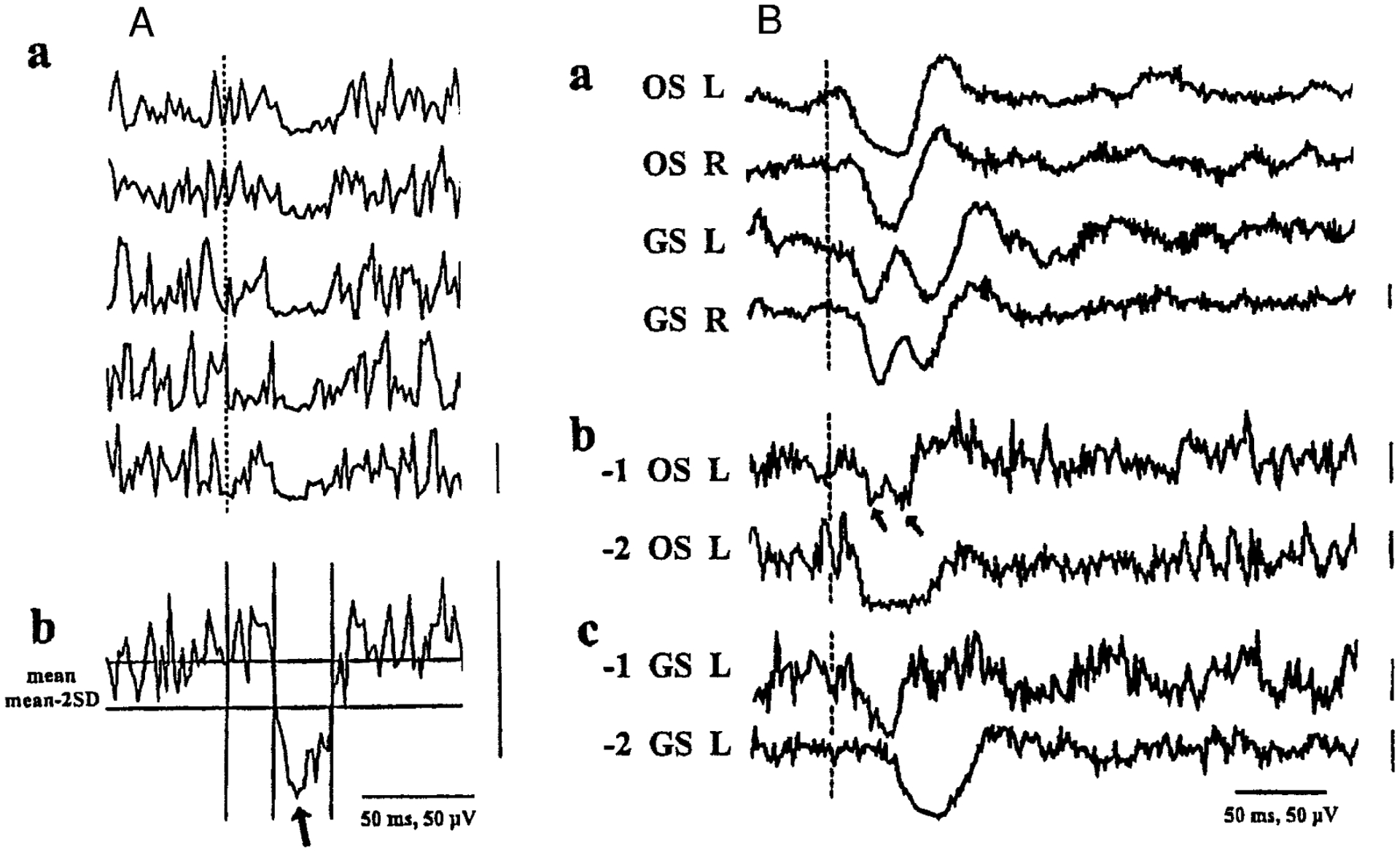
A: response of muscle activity to brain stem stimulation. Electrical stimulation [3 0.2-ms cathodal pulses at 330 Hz (6.3-ms duration), 1.5 T] is delivered to the left pons every second starting at the dotted line. a: 5 consecutive rectified electromyogram (EMG) records are taken from the right splenius muscle. b: average of 10 consecutive EMG recordings including the 5 above; top horizontal bar, mean baseline value collected from 50 ms before the stimulation; bottom horizontal bar, value of the mean minus 2 SDs. The 1st vertical line indicates the beginning of stimulation; 2nd and 3rd ones indicate the point where the waveform crosses the value of mean minus 2 SDs, each of which indicates the onset and offset of muscle tone suppression, respectively. Arrow, trough of muscle tone suppression. B: representative examples showing different types of pontine stimulation-induced EMG activities. Electrical stimulation (a, 1.7 T; b1, 2.5 T; b2, 1.7 T; c1, 2.1 T; c2, 2.5 T) is delivered at the dotted line to the left pons. Data are averaged from 30 consecutive rectified EMG sweeps. a: example of bilateral suppression of neck and hindlimb muscle tone. These 4 traces were obtained simultaneously. Hindlimb muscle tone exhibited 2 phases of suppression (type B suppression). b: 2 types of neck muscle tone suppression. Two troughs (arrows) are seen in b1, whereas no trough is determined in b2 (a saucerlike flat nadir). c: 2 types of hindlimb muscle tone suppression. c1: early-phase suppression without a subsequent late phase suppression (type E suppression). c2: late-phase suppression without a preceding early phase suppression (type L suppression). OS, occipitoscapularis muscles; GS, gastrocnemius-soleus muscles; L, left; R, right.
Histology
Electrolytic lesions (50 μA, DC cathodal current for 20 s) (Lai et al. 1987) were made at the stimulating point and at the recording sites. For tracks on which several units were recorded, lesions were made at the most superficial and the deepest recording sites along the track. The brain stems were removed and stored in potassium ferrocyanide with buffered formalin solution. Serial 60-μm sections were stained with neutral red. The stimulating and recording points were verified and reconstructed according to the Berman atlas (1968).
Statistical analysis
Student’s t-test was used for comparison of waveform parameters and for assessing the significance of correlation coefficients (r). An analysis of variance (ANOVA) or a χ2 test for independence was used when necessary.
RESULTS
EMG analysis
Muscle tone suppression was induced by pontine stimulation in 21 cats and by medullary stimulation in 9 cats. We found no obvious difference in the time course of waveforms among occipitoscapularis, splenius, and biventer cervicis muscles. Therefore we treated suppression of tone in these muscles together as neck muscle tone suppression. On each trial, four muscles were monitored: ipsilateral neck, contralateral neck, ipsilateral hindlimb, and contralateral hindlimb (Fig. 1Ba). We termed each EMG recording a trace. Thus four traces were gathered on each trial.
EARLY AND LATE SUPPRESSIONS.
By stimulating the pons and medulla, we identified two phases of suppression, early and late phases.
PONTINE-INDUCED MUSCLE TONE SUPPRESSION.
We analyzed 148 trials of pontine-induced muscle tone suppression (Fig. 1B).
Neck muscles.
In 15 of 296 traces of pontine-induced neck muscle tone suppression, two troughs were distinguished in a single trace (Fig. 1Bb1). In these 15 traces, the mean latency to trough of the earlier trough was 24.0 ± 6.8 (SD) ms, one was whereas the mean latency to trough of the later 40.6 ± 7.8 ms. In another 59 traces, discrete troughs were not identified (Fig. 1Bb2). We described this type of waveform as having a saucerlike flat nadir. In the other 222 traces of pontine-induced neck muscle tone suppression, a single trough was identified.
Hindlimb muscles.
In hindlimb muscles, we identified 437 troughs. These troughs (Fig. 1, Ba, GS, and 1Bc) were divided into two types (Fig. 2), 181 early troughs and 256 late troughs with 40 ms being the dividing line. Flat nadirs were observed in 21 of 296 traces of pontine-induced hindlimb muscle tone suppression. Offsets of 4 flat nadirs were ≤40 ms of the beginning of stimulation (early nadir), whereas onsets of the other 17 flat nadirs were >40 ms after stimulation onset (late nadir). We termed a hindlimb muscle tone suppression with an early trough or an early nadir an early phase suppression and that with a late trough or a late nadir a late phase suppression. Thus among 296 traces of pontine-induced hindlimb muscle tone suppression, 23 traces had only an early phase (Fig. 1Bc1; type E suppression), 111 traces had only a late phase (Fig. 1Bc2; type L suppression), and 162 traces had both early and late phases (Fig. 1Ba; type B suppression). In type B suppression, EMG activity did not always return to a level below 2 SDs from the baseline between troughs (Fig. 1Ba, GS R). In these cases, the offset of early-phase suppression and the onset of late-phase suppression could not be determined.
FIG. 2.
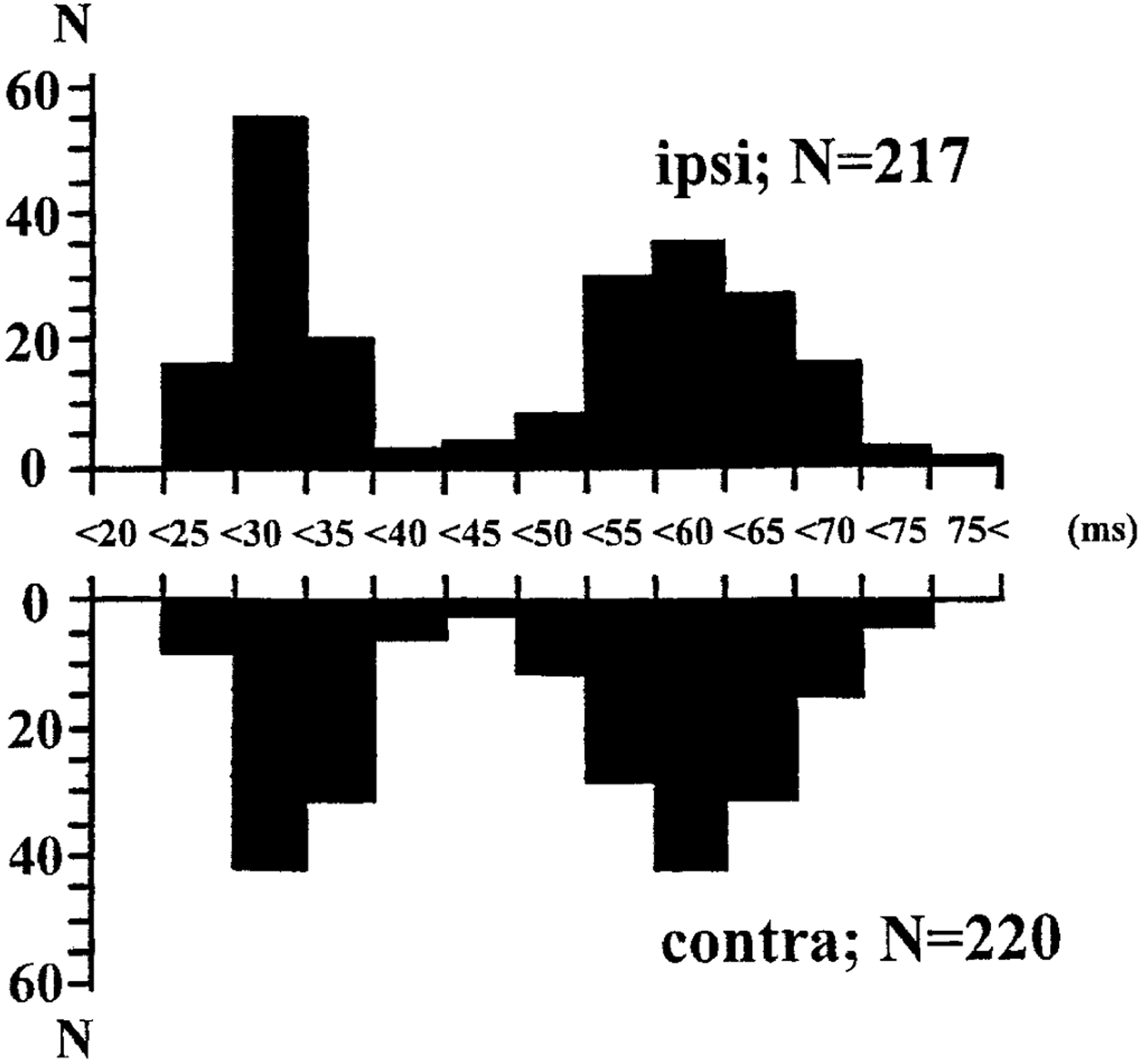
Distribution of the latency of 437 identified troughs in pontine-induced hindlimb muscle tone suppression. The distribution of 217 troughs on the side ipsilateral to the stimulation is shown in the top part, whereas that of the contralateral 220 troughs is shown in the bottom part. Each side has 2 peaks; one is <40 ms (between 25 and 30 ms) and the other is >40 ms (between 55 and 60 ms). N, number.
In pontine-induced hindlimb muscle tone suppression, the mean latencies to onset and to trough of the early-phase suppression in type E suppression to the stimulation did not differ significantly from those in type B suppression (Table 1A). Also the mean latencies to trough and to offset of the late-phase suppression in type B suppression showed no significant difference from those in type L suppression (Table 1A). Thus we concluded that the early- and late-phase hindlimb muscle tone suppressions induced by pontine stimulation were independent.
TABLE 1.
Latencies in different types of hindlimb muscle tone suppression
| Pontine-Induced Suppression | Latency to Onset | Latency to Trough | ||
|---|---|---|---|---|
| Ipsi | Contra | Ipsi | Contra | |
| Early-phase suppression | ||||
| Traces (ipsi/contra) | ||||
| Type E, 23 (14/9) | 21.0 ± 3.3 (14) | 23.6 ± 2.3 (9) | 28.6 ± 4.2 (14) | 31.0 ± 3.7 (9) |
| Type B, 162 (82/80) | 21.1 ± 3.8 (82) | 22.9 ± 3.6 (80) | 27.5 ± 3.6 (80*) | 28.9 ± 3.6 (78*) |
| P value: E vs. B | 0.86 | 0.58 | 0.45 | 0.10 |
| Latency to Trough | Latency to Offset | |||
| Ipsi | Contra | Ipsi | Contra | |
| Late-phase suppression | ||||
| Type B, 162 (82/80) | 58.1 ± 5.3 (81*) | 57.9 ± 5.4 (80) | 70.8 ± 9.2 (82) | 70.2 ± 8.5 (80) |
| Type L, 111 (52/59) | 56.0 ± 7.2 (42*) | 58.1 ± 7.2 (53*) | 69.7 ± 10.7 (52) | 68.4 ± 9.5 (59) |
| P value: B vs. L | 0.06 | 0.88 | 0.51 | 0.24 |
| Medullary induced suppression | ||||
| Type B, 27 (15/12) | 53.0 ± 14.5 (15) | 55.4 ± 18.3 (12) | 65.2 ± 6.3 (15) | 64.8 ± 7.5 (12) |
| Type L, 33 (15/18) | 48.9 ± 20.9 (15) | 53.9 ± 16.5 (18) | 66.2 ± 17.0 (15) | 70.7 ± 12.3 (18) |
| P value: B vs. L | 0.54 | 0.83 | 0.82 | 0.15 mean ± SD |
Values expressed as means ± SD; number of traces in parentheses.
Differences in numbers of latency to trough caused by traces with a flat nadir.
MEDULLARY INDUCED MUSCLE TONE SUPPRESSION.
We analyzed 30 trials of medullary induced muscle tone suppression.
Neck muscles.
In 51 of 60 traces, a single trough was identified. In another four traces, two troughs were distinguished in a single trace. The mean latency to trough of earlier troughs was 22.0 ± 8.5 ms, whereas that of later ones was 41.6 ± 10.8 ms. In the other five traces, flat nadirs were observed.
Hindlimb muscles.
Eighty-seven troughs were identified among 60 traces. No flat nadirs were obtained. Thirty-three traces were type L suppression, whereas 27 traces were type B suppression. Among medullary induced hindlimb muscle tone suppression, the mean latencies to trough and to offset of the late-phase suppression in type B suppression showed no significant difference from those in type L suppression (Table 1B). Therefore as in the case of pontine-induced suppressions, we concluded that early and late phases of hindlimb muscle tone suppression induced by medullary stimulation were independent.
Stimulus effects on ipsilateral and contralateral side to the stimulation
The difference of stimulus effects on waveform parameters obtained from the side ipsilateral to the stimulation and that from the side contralateral to the stimulation were analyzed. Except for the duration of the early-phase suppression of hindlimb muscle tone (type E suppression) and latency to onset of the late suppression (type L suppression), data from different types of suppression were pooled. The magnitude of muscle tone suppression at nadirs was calculated together with the amplitude at the trough.
PONTINE-INDUCED MUSCLE TONE SUPPRESSION.
In pontine-induced muscle tone suppression (Table 2), the latency to onset of neck muscle tone suppression was significantly earlier in the side contralateral to the stimulation. However, in hindlimb muscle tone suppression, the latencies to onset of both early and late phases and the latency to trough of the early phase were significantly earlier in the side ipsilateral to the stimulation. The other parameters were not statistically different for the two sides.
TABLE 2.
Waveform parameters of pontine-induced muscle tone suppression
| Latency to Onset | Duration | Latency to Trough | Amplitude* at Trough | |
|---|---|---|---|---|
| A. Neck muscles | ||||
| Ipsilateral side (range)/No. | 17.8 ± 3.1 (11–31)/148 |
25.1 ± 9.3 (4–56)/148 |
30.6 ± 5.5 (20–43)/109 |
34.3 ± 15.6 (7–77)/148 |
| Contralateral side (range)/No. | 17.0 ± 3.7 (8–32)/148 |
24.2 ± 9.4 (4–58)/148 |
29.5 ± 6.1 (17–52)/113 |
35.8 ± 14.7 (10–77)/148 |
| P value (ipsi vs. contra) | 0.04† | 0.39 | 0.14 | 0.40 |
| B. Hindlimb muscles | ||||
| Early Suppression | Latency to Onset | Duration‡ | Latency to Trough | Amplitude* at Trough |
| Ipsilateral side (range)/No. | 21.1 ± 3.7 (14–30)/96 |
15.2 ± 11.2 (6–48)/13 |
27.7 ± 3.7 (21–39)/94 |
43.3 ± 18.2 (14–79)/96 |
| Contralateral side (range)/No. | 23.0 ± 3.5 (13–32)/89 |
16.1 ± 5.6 (8–24)/9 |
29.2 ± 3.6 (22–38)/87 |
44.6 ± 15.6 (15–79)/89 |
| P value (ipsi vs. contra) | 0.001 >† | 0.82 | 0.01 >† | 0.61 |
| Late Suppression | Latency§ to Onset | Latency to Trough | Latency to Offset | Amplitude* at Trough |
| Ipsilateral side (range)/No. | 42.8 ± 8.0 (19–60)/52 |
57.4 ± 6.1 (41–72)/123 |
70.4 ± 9.8 (48–110)/134 |
44.0 ± 19.0 (2–78)/134 |
| Contralateral side (range)/No. | 46.4 ± 7.2 (24–60)/59 |
58.0 ± 6.1 (44–73)133 |
69.4 ± 9.0 (51–100)/139 |
44.5 ± 18.1 (3–80)/139 |
| P value (ipsi vs. contra) | 0.02>† | 0.46 | 0.37 | 0.89 |
Values expressed as means ± SD; number of traces in parentheses.
Prestimulus baseline level = 100%.
Significant difference.
Data obtained from type E suppression.
Data obtained from type L suppression.
MEDULLARY INDUCED MUSCLE TONE SUPPRESSION.
In medullary induced muscle tone suppression (Table 3), all parameters obtained from both sides had statistically indistinguishable values.
TABLE 3.
Waveform parameters of medullary induced muscle tone suppression
| Latency to Onset | Duration | Latency to Trough | Amplitude* at Trough | |
|---|---|---|---|---|
| A. Neck muscles | ||||
| Ipsilateral side (range)/No. | 16.4 ± 3.8 (9–24)/30 |
23.8 ± 15.9 (3–58)/30 |
25.4 ± 7.5 (14–40)/25 |
41.3 ± 16.1 (12–80)/30 |
| Contralateral side (range)/No. | 16.2 ± 4.7 (8–25)/30 |
27.0 ± 15.5 (5–70)/30 |
28.9 ± 8.6 (9–48)/26 |
33.7 ± 15.7 (10–65)/30 |
| P value (ipsi vs. contra) | 0.81 | 0.43 | 0.12 | 0.07 |
| B. Hindlimb muscles | ||||
| Early Suppression | Latency to Onset | Latency to Trough | Amplitude* at Trough | |
| Ipsilateral side (range)/No. | 20.1 ± 4.4 (14–32)/15 |
24.6 ± 5.4 (16–35)/15 |
60.3 ± 11.9 (39–79)/15 |
|
| Contralateral side (range)/No. | 22.4 ± 4.4 (16–30)/12 |
28.4 ± 3.8 (24–35)/12 |
51.7 ± 23.7 (10–76)/12 |
|
| P value (ipsi vs. contra) | 0.19 | 0.051 | 0.23 | |
| Late Suppression | Latency† to Onset | Latency to Trough | Latency to Offset | Amplitude* at Trough |
| Ipsilateral side (range)/No. | 41.3 ± 6.6 (22–49)/15 |
54.3 ± 6.8 (41–70)/30 |
66.4 ± 11.5 (52–100)/30 |
50.9 ± 17.8 (12–84)/30 |
| Contralateral side (range)/No. | 44.4 ± 3.5 (38–52)/18 |
54.6 ± 6.5 (41–67)/30 |
68.4 ± 10.9 (52–105)/30 |
54.5 ± 17.0 (15–81)/30 |
| P value (ipsi vs. contra) | 0.10 | 0.96 | 0.51 | 0.43 |
Values expressed as means ± SD; number of traces in parentheses.
Prestimulus baseline level = 100%.
Data obtained from type L suppression.
Effects of stimulus intensity
Correlation coefficients (r) between stimulus intensity and waveform parameters were assessed.
PONTINE-INDUCED MUSCLE TONE SUPPRESSION.
Neck muscles.
In pontine-induced neck muscle tone suppression, latency to onset showed significant negative correlations with stimulus intensity: r = −0.22 for the ipsilateral side (|T| = 2.68, P < 0.01, n = 148) and r = −0.20 for the contralateral side (|T| = 2.51, P < 0.02, n = 148), respectively. Other parameters (the latency to solitary troughs and duration and amplitude at trough) showed no significant correlation with stimulus intensity. In pontine-induced neck muscle tone suppression, the mean stimulus intensity required to induce two troughs (2.31 T), to produce a flat nadir (2.06 T), and to elicit a single trough (2.11 T) showed no significant difference (ANOVA).
Hindlimb muscles.
For the early phase of pontine-induced hindlimb muscle tone suppression, the latency-to-onset on the contralateral side had a significant negative correlation with stimulus intensity [r = −0.22 (|T| = 2.07, P < 0.05), n = 89], whereas that on the ipsilateral side showed no significant correlation. Latency to trough of early hindlimb muscle tone suppression was not affected by stimulus intensity. The magnitude of the suppression at trough on both sides increased with an increase of stimulus intensity [ipsi; r = −0.27 (|T| = 2.68, P < 0.01, n = 96), contra; r = −0.41 (|T| = 4.15, P < 0.001, n = 89)].
For late-phase hindlimb muscle tone suppression, the latency to trough, latency to offset, amplitude at trough, and latency to onset in type L suppression were not affected by stimulus intensity.
In pontine-induced hindlimb muscle tone suppression, the mean stimulus intensity that induced an early-phase suppression (2.28 T, 1.03 SD, n = 185) was significantly higher than that which evoked late suppression (1.83 T, 0.50 SD, n = 111) (|T| = 4.30, P < 0.001). However, an increase of stimulus intensity did not always evoke the early-phase suppression. Among 28 sets of trials that used more than 3 different stimulus intensities at the same stimulus site, 10 sets changed from type L suppression into type B with increasing stimulus intensity, whereas 15 sets did not change their types of suppression. Results in the other three sets were inconclusive.
MEDULLARY INDUCED MUSCLE TONE SUPPRESSION.
Neck muscles.
No parameter on medullary induced neck muscle tone suppression showed a significant correlation with stimulus intensity. No significant difference was obtained among the mean stimulus intensity to induce a single trough (1.60 T), to evoke two troughs (1.50 T), and to elicit a flat nadir (1.76 T) (ANOVA).
Hindlimb muscles.
In hindlimb muscles, the latency to trough of the late ipsilateral suppression had a negative correlation with stimulus intensity (r = −0.39, |T| = 2.25, P < 0.05, n = 30). The other parameters on medullary induced hindlimb muscle tone suppression showed no significant correlation with stimulus intensity. As with pontine stimulation, the mean stimulus intensity that produced type B suppression (1.63 T, 0.56 SD, n = 27) through medullary stimulation was higher than the mean stimulus intensity that induced type L suppression (1.59 T, 0.73 SD, n = 33), although no significant difference was obtained.
Stimulus sites
Of 27 pontine stimulation sites (Fig. 3A) and 10 medullary stimulation sites (Fig. 3B), 5 sites in the pons and 3 sites in the medulla (●) elicited only type L suppression. All latencies to onset and to trough of brain stem-induced muscle tone suppression (Tables 2 and 3) were shorter in the medullary induced suppressions than in the pontine-induced suppressions. However, not all of these differences were significant. Significant differences were obtained in the side ipsilateral to the stimulation of the neck muscle tone suppression [both latencies to onset (P < 0.05) and to trough (P < 0.001)] and the latency to trough of the early-phase hindlimb muscle tone suppression in the side ipsilateral to the stimulation (P < 0.01).
FIG. 3.
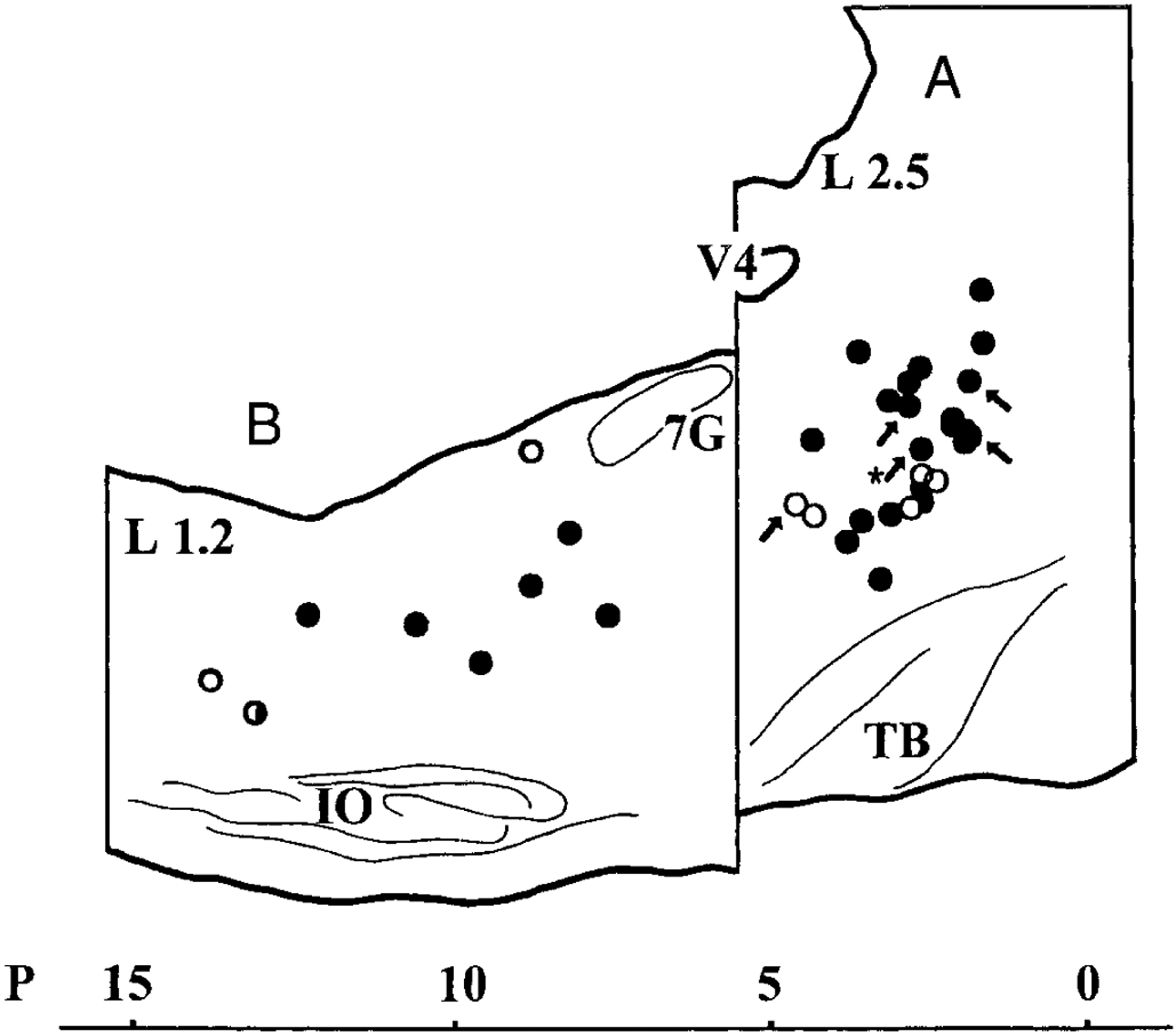
Schematic maps of pontine (A) and medullary (B) stimulus sites on parasagittal planes. Laterality of the pontine stimulation (n = 27) ranges from 1.4 to 3.8 mm (mean 2.6 ± 0.6), and that of the medullary stimulation (n = 10) ranges from 0.2 to 2.1 mm (mean 1.1 ± 0.6). All stimulation stimulation sites of the pons are transposed onto a section 2.5-mm lateral (L 2.5) from the midline, and those of the medulla are transported onto the 1.2-mm plane (L 1.2), respectively. Two pontine sites (P 2.3 mm, H −4.8 mm, L 2.7 mm and L 3.8 mm) are transposed on the same spot in A, and there are 26 circles shown in A. In B, 2 sites (P 12.8 mm, H −8.9 mm, L 1.1 mm and L 2.1 mm) are transposed on the same spot. Open circles, 5 in the pons and 2 in the medulla, indicate the sites that did not evoke the early-phase hindlimb muscle tone suppression. The site with a half-tone circle (P 12.3 mm, H −8.9 mm, L 2.1 mm) also induced only late-phase suppression. Five pontine stimulus sites with an arrow (one of them with a star indicates a site P 2.3 mm, H −4.8 mm, L 3.8 mm) were not used for unit recording studies. V4, 4th ventricle; TB, trapezoid body; 7G, the genu of the facial nerve; IO, inferior olive.
In to the stimulus sites demonstrated in Fig. 3, addition the effective stimulus sites for the occurrence of each phase of hindlimb muscle tone suppression were systematically investigated in five cats for the pons (Fig. 4) and in four cats for the medulla (Fig. 5). Identical stimulus intensity that was high enough to evoke stable waveforms (2.00–3.00 T) was used in each cat. Sites that produced type E, L, and B suppressions were intermixed.
FIG. 4.
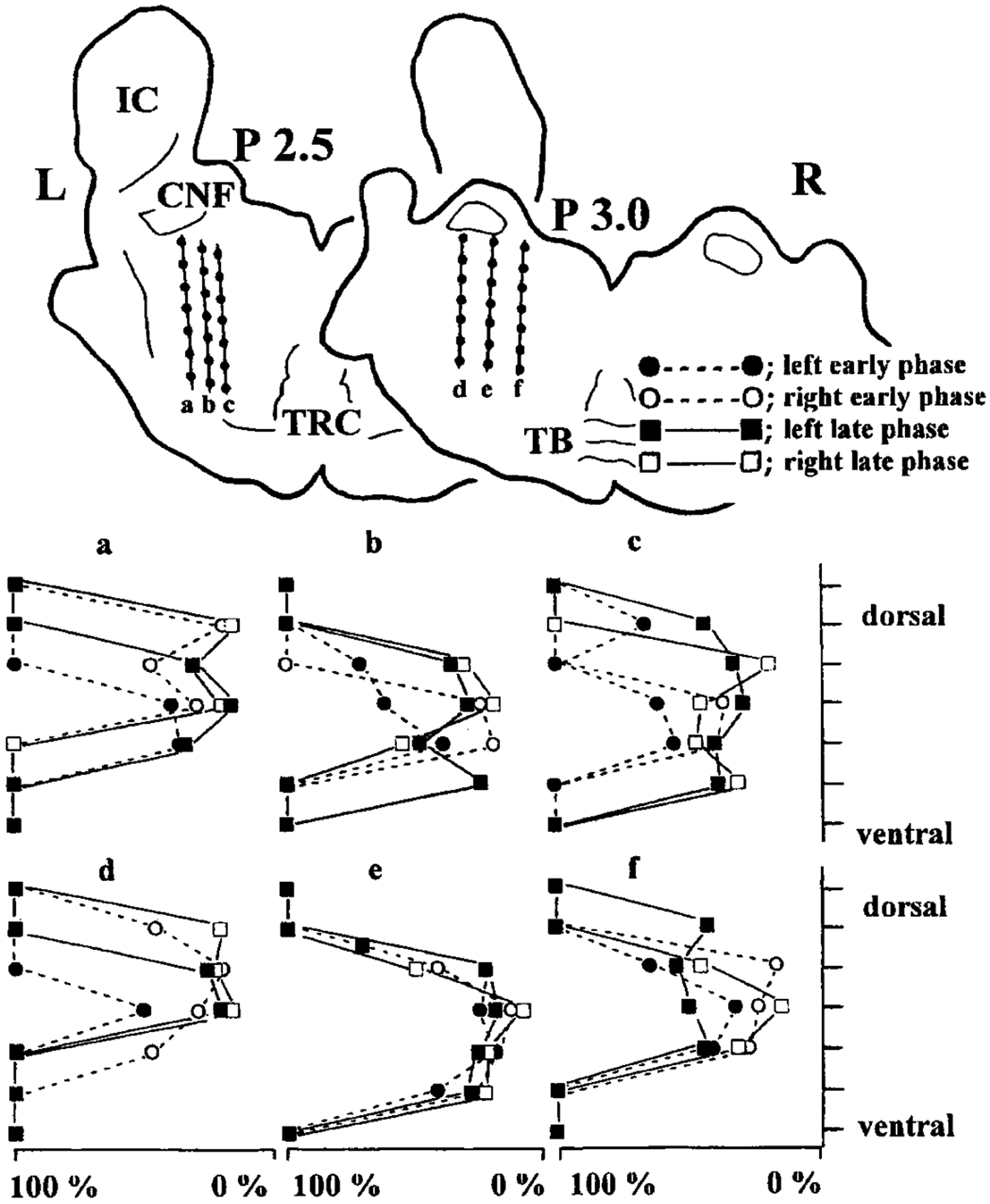
An example of effect of stimulus sites on the early and late phases of pontine-induced hindlimb muscle tone suppression. In both planes (P 2.5 and 3.0) in the top part, stimulations (2.6 T) are delivered to the left pontine reticular formation along 3 tracks (a, b, c and d, e, f). In each track, the stimulus points are indicated by closed circles. Stimulus efficacy of 7 points in each track is shown in the bottom part of the figure. Stimulus efficacy is assessed by the amplitude at trough expressed as a percentage of the baseline value before the stimulation (prestimulus baseline value 100%). Thus the smaller the percentage, the deeper the trough. filled circles, early phase of the left side; open circles, early phase of the right side; filled square, left late phase; open square, right late phase; dotted line, early phase; solid line, late phase; L, left side; R, right side, IC, inferior colliculus; CNF, cuneiform nucleus; TRC, central division of the tegmental reticular nucleus; TB, trapezoid body.
FIG. 5.
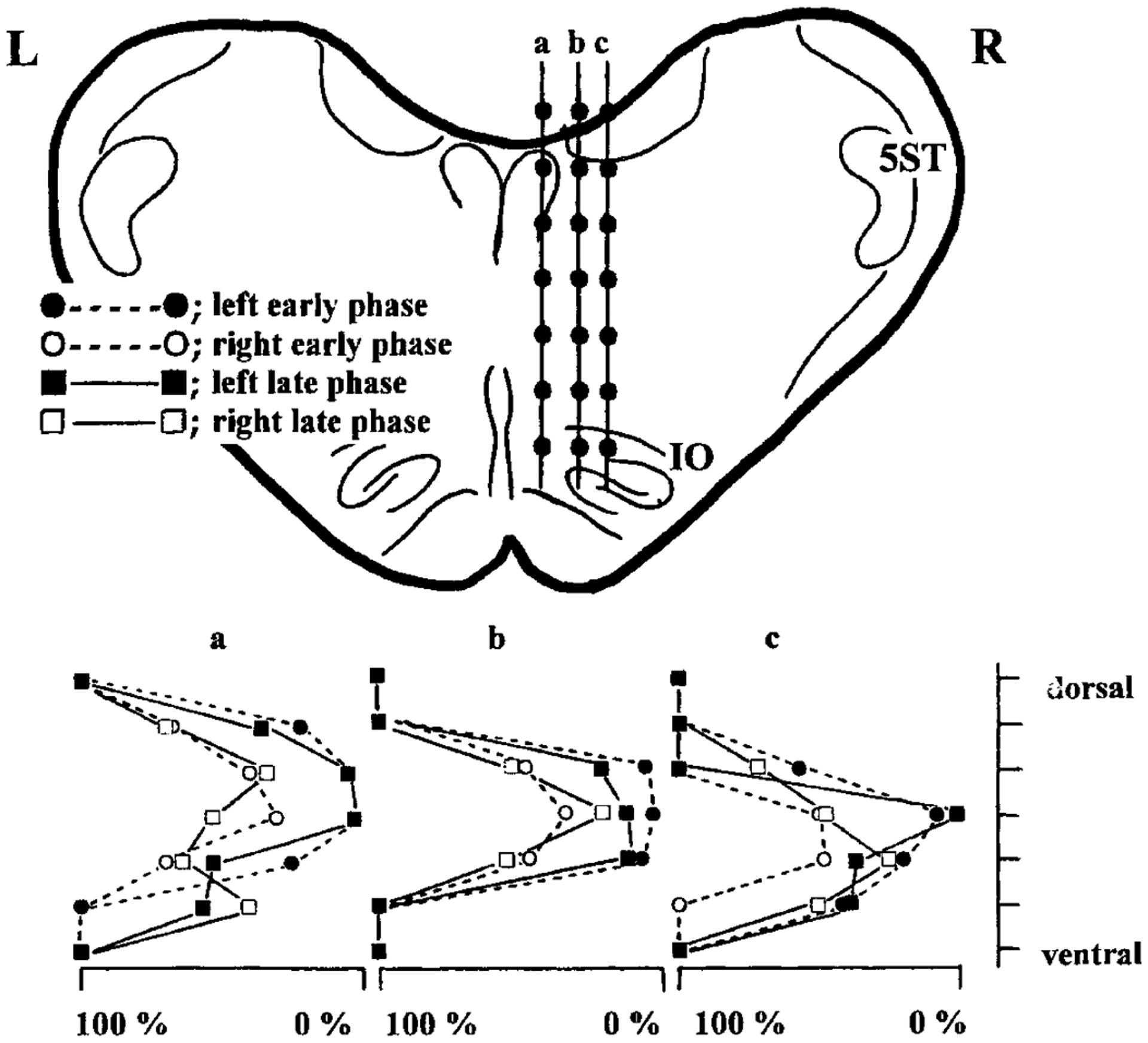
Example of effect of stimulus sites on the early and late phases of medullary induced hindlimb muscle tone suppression. Stimulations (2.3 T) are delivered to the right medullary reticular formation (P 11.0) along with 3 tracks (a, b, c). Stimulus points are expressed by closed circles in each track. Stimulus efficacy, assessed by the amplitude at trough, of every 7 points in each track are shown in the bottom part. filled circle, early phase on the left side; open circle, early phase on the right side; filled square, left late phase; open square, right late phase; dotted line, early phase; solid line, late phase, L, left side; R, right side; IO, inferior olive; 5ST, spinal trigeminal tract.
Effects of stimulus parameters on the waveform
After identifying a stimulus site that induced neck and hindlimb muscle tone suppression bilaterally with three pulses at 330 Hz, the effects of stimulus parameters (frequency and the number of pulses at 330 Hz) on the waveform were assessed. Stimulus intensity was adjusted to be high enough to induce stable waveforms with stimulation of three pulses at 330 Hz (1.50–2.50 T) and was unchanged in each cat.
FREQUENCY.
For pontine-induced muscle tone suppression, the effect of stimulation frequency was examined in five cats (8 sets of trials). Three pulses were delivered over a range of frequencies (150–1,000 Hz) with a constant intensity (1.50–2.50 T) in each set. In eight trials (4 at 1,000 Hz, 3 at 750 Hz, and 1 at 500 Hz), a neck muscle tone facilitation that was not present at 330 Hz appeared before the suppression in the side ipsilateral to the stimulation. The magnitude of neck muscle tone suppression and of both early and late phases of hindlimb muscle tone suppression showed no significant change with stimulus frequency ranging from 250 to 1,000 Hz (ANOVA).
For medullary induced muscle tone suppression, we compared waveforms with 1,000-Hz stimulation with those at 330-Hz stimulation during the course of the mapping study (Fig. 6) in two cats (4 tracks). Both early and late phases of hindlimb muscle tone suppression were obtained at 1,000-Hz stimulation, and no apparent difference was found in waveform parameters between 330- and 1,000-Hz stimulation.
FIG. 6.
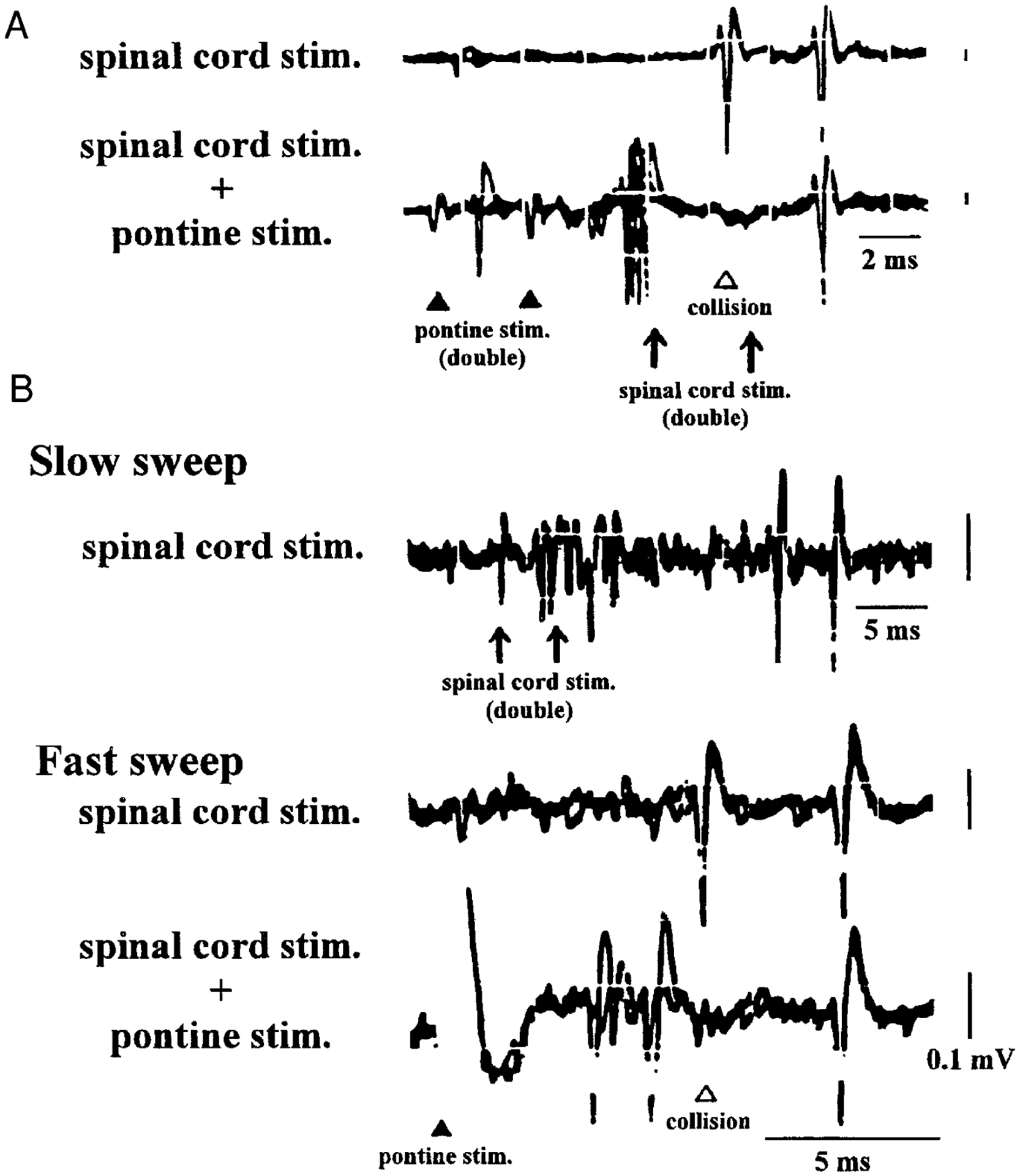
Action potentials of representative fast (A) and slow (B) conducting reticulospinal units are shown. A, top trace: action potentials activated antidromically by double spinal cord stimulation. Constant latency and the ability to follow high-frequency stimulation (330 Hz) are verified. On the bottom trace, the 1st action potentials antidromically activated by the 1st stimulation of the double spinal cord stimulation collide with action potentials orthodromically activated by double pontine stimulation. The 2nd action potential of the unit antidromically activated by the 2nd stimulation of the double spinal cord stimulation can still be identified. B: top trace shows the antidromic activation of the unit by double spinal cord stimulation (250 Hz) with a slow sweep. The 2nd and the 3rd traces present the same unit with a faster sweep with the same double spinal cord stimulation. In the 2nd trace, the constant latency and the ability to follow high-frequency stimulation can be seen. In the 3rd trace, the pontine stimulation is combined with double spinal cord stimulation. Pontine-induced spikes collide with antidromic spikes that are activated by the 1st spinal cord stimulation. Arrows, spinal cord stimulation at the L1 level; closed arrowheads, pontine stimulation; open arrowhead, pontine stimulation. Calibration: 2 (A) and 5 ms (B), 0.1 mV.
NUMBER OF PULSES.
The effect of number of stimulation pulses was investigated for pontine-induced suppression in six cats (10 sets of trials ) . After adjusting stimulus intensity to be high enough to elicit stable waveforms with three pulses at 330 Hz (1.50–2.50 T), one to four pulses were delivered at 330 Hz with a constant intensity in each cat. Stimulation with one pulse never suppressed muscle activity. Two of 10 trials with two-pulse stimulation, all 10 trials with three pulses, and 4 of 10 trials with four-pulse stimulation suppressed neck and hindlimb muscle tone bilaterally. The other six trials with four pulses elicited neck muscle tone facilitation in the side ipsilateral to the stimulation. Therefore current levels that produced muscle tone suppression with three pulses elicited a mixture of suppression and facilitation when four pulses were delivered.
Among the four trials in which neck and hindlimb muscle tone were suppressed bilaterally with 4-pulse stimulation, all 8 neck muscle tone suppressions and 9 of the 15 hindlimb muscle tone suppressions (7 early suppressions and 8 late ones) did not show discrete troughs. Rather they exhibited flat nadirs (4 early nadirs and 5 late ones), although no flat nadir was observed with three-pulse stimulation of the same sites. Therefore depending on stimulus intensity used, three-pulse stimulation but not four-pulse stimulation was suitable for inducing discrete troughs.
Estimation of conduction velocity of atonia systems
The conduction velocity of reticulospinal projections involved in the early-phase hindlimb muscle tone suppression was assumed to be X m/s and that of the late phase was presumed to be Y m/s
| (1) |
Brain stem stimulation was postulated to require Tbr ms to activate reticulospinal projections. The length of the activated reticulospinal projection was substituted for the distance from the stimulation site to the motoneuron pool innervating recording muscles (L mm), which was measured in each cat. Te represented the latency to trough of the early-phase hindlimb muscle tone suppression, and Tl stood for that of the late one. Also we assumed that it took Tperi (peripheral) ms from the arrival of responsible reticulospinal volleys at the motoneuron to the trough of EMG changes. Thus the following two formulas were obtained
| (2) |
and
| (3) |
Taking these two formulas together, the following formula was obtained
| (4) |
As shown in the previous section, the latencies to trough for both early and late phases of hindlimb muscle tone suppression evoked by pontine stimulation were not affected by stimulus intensity. Then (Tl − Te)/L was calculated in all 432 type B suppressions with 2 troughs (158 traces from the EMG study and 274 from the following unit study) induced by pontine stimulation. The formula (Eq. 4) was found to range from 0.0376 to 0.2513
| (5) |
Taken together the maximum reported conduction velocity of reticulospinal cells (160 m/s) (Eccles et al. 1976) with the formula (Eq. 1), the following formula was obtained
| (6) |
Taking Eqs. 5 and 6 together, Y was calculated to be <22.8. Thus we estimated that a reticulospinal projection involved in the late-phase suppression conducted at <22.8 m/s for hindlimb muscles. Consequently reticulospinal projections that conduct faster than this value appeared to mediate the early-phase suppression.
Reticulospinal units
Discharge characteristics of medullary reticulospinal units that send their axons to the lumbar segment of the spinal cord (L1) were determined during pontine stimulation. Hindlimb muscle tone was recorded simultaneously. In the medullary reticular formation of 19 decerebrate cats, 238 reticulospinal units were identified. Pontine stimulation was delivered to 22 sites (Fig. 3A). Discharge patterns of units were assessed at a stimulus intensity strong enough to elicit a consistent EMG response. Stimulus intensities used ranged from 1.50 to 3.75 T with a mean value of 2.26 T (0.56 SD). Action potentials of representative units are shown (Fig. 6). Among 238 trials (476 traces), 274 traces belonged to type B suppression, 150 traces to type L, and the other 52 traces to type E suppression. According to the χ2 test, the proportion of each type of suppression in the unit recording study showed no significant difference from that in the EMG study with pontine stimulation.
DISCHARGE PATTERNS.
The discharge patterns of 238 units were classified into two types, N and I. Type N units did not change their discharge pattern after the pontine stimulation (n = 100, 42.0%), whereas type I units increased their firing after the stimulation (Fig. 7A). Among type I units, 122 units showed an earlier response than the other 16 units. These 122 units that responded to the pontine stimulation within 10 ms were termed type Ie units. Type Ie-A units ceased their discharge within 10 ms after the beginning of the stimulation (n = 59, 24.8%), whereas type Ie-B units often fired beyond 10 ms after the beginning of the stimulation (n = 63, 26.5%). The peaks of discharge of type Ie-B units occurred between 5 and 22 ms after the beginning of the stimulation with an average value of 11.7 ms. Most type Ie units (90/122 units) responded to pontine stimulation with a latency of <2.5 ms (Fig. 7B). Sixteen units that showed delayed activation were termed type Id units (n = 16, 6.7%). They began to fire >10 ms after the beginning of the stimulation. The peaks of their firing occurred between 13 and 32 ms after the beginning of the stimulation with a mean value of 24.3 ms. All type I units were diffusely distributed in the medial medulla from the rostral to caudal areas (Fig. 8).
FIG. 7.
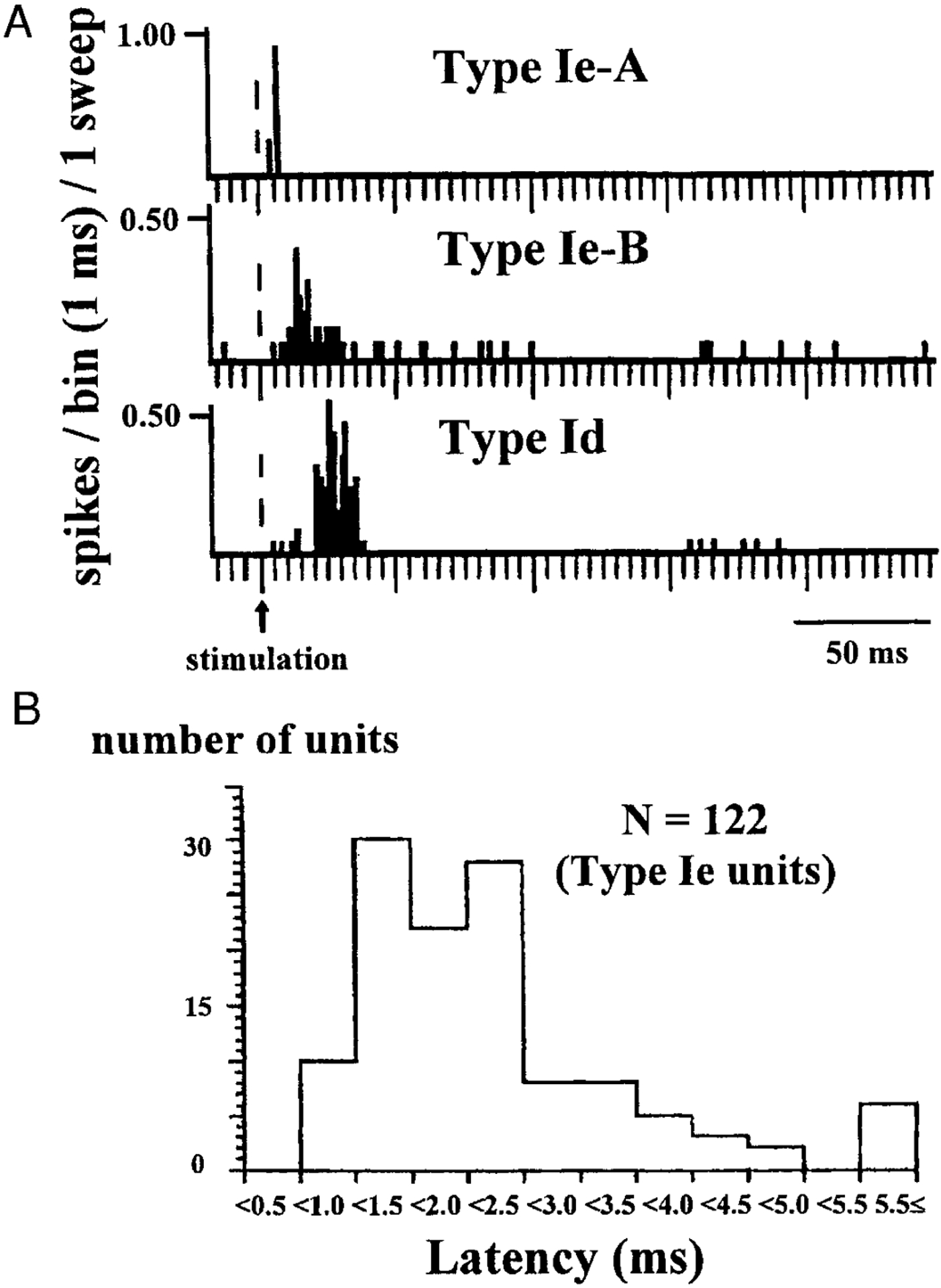
A: examples of discharge patterns of reticulospinal neurons that are activated by pontine atonia inducing stimulation (type I units). They are classified into 3 types. Type Ie-A units cease firing within 10 ms after the beginning of the stimulation, type Ie-B units sustain firing even 10 ms after the beginning of the stimulation, and type Id units begin to increase firing 10 ms after the beginning of the stimulation. Peak of the firing of this type Ie-B unit shown is obtained at 13 ms after the beginning of the stimulation and that of the type Id unit in this figure is obtained at 23 ms after the beginning of the stimulation, respectively. B: histogram of response latency of 122 type Ie medullary reticulospinal units to pontine stimulation. Ninety of 122 units respond with latency of <2.5 ms to the pontine stimulation.
FIG. 8.
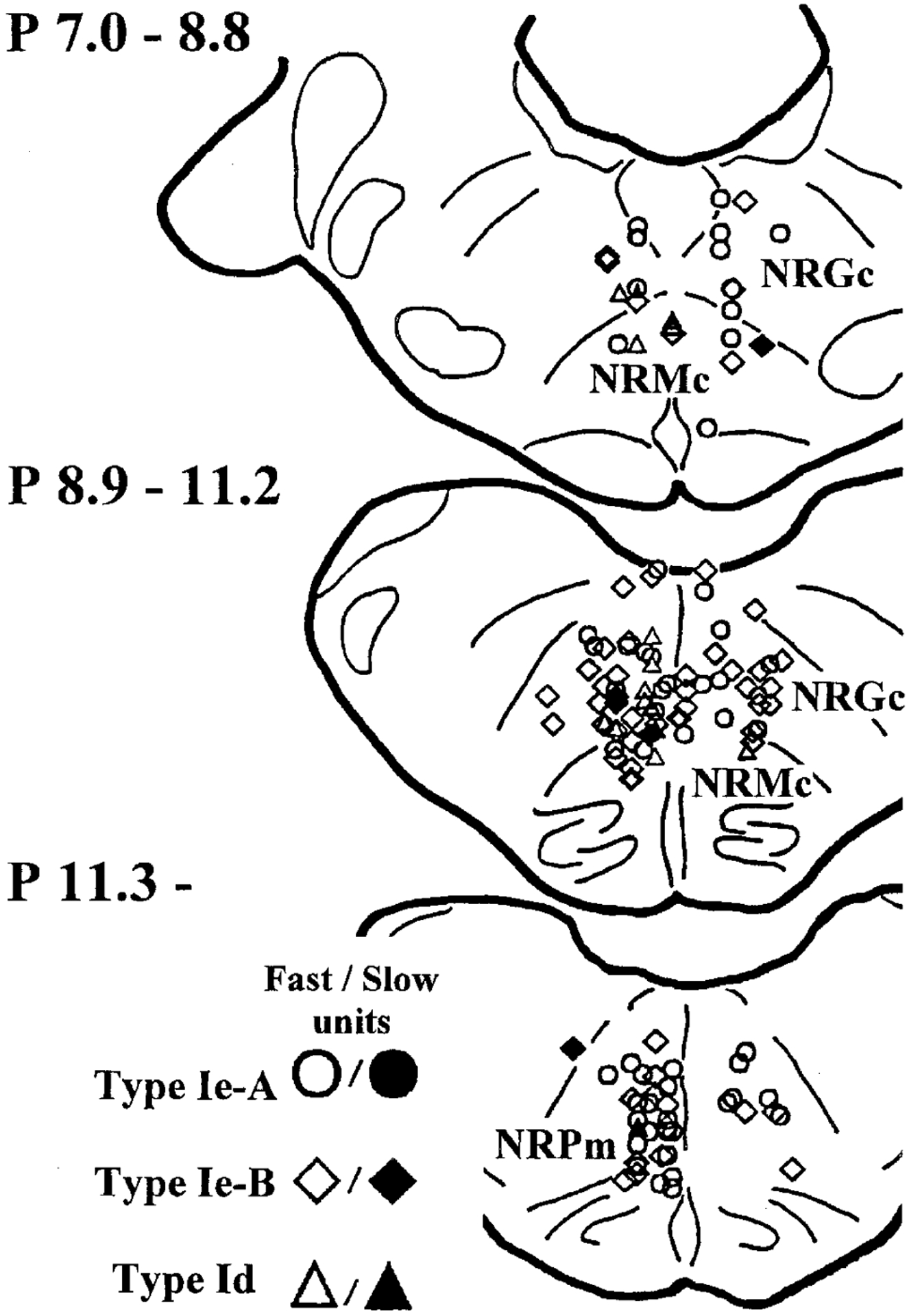
Location of type I units (n = 138). They are diffusely distributed in the medial medulla. Open symbols, fast units (conduction velocity ≥22.8 m/s); filled symbols, slow units (conduction velocity <22.8 m/s); circles, type Ie-A units; diamonds, type Ie-B units; triangles, type Id. NRGc, nucleus reticularis gigantocellularis; NRMc, nucleus reticularis magnocellularis; NRPm, nucleus reticularis paramedianus.
SLOW AND FAST CONDUCTING RETICULOSPINAL UNITS.
The conduction velocity of 238 medullary reticulospinal units identified ranged from 10.1 to 141.2 m/s with an average value of 71.8 ± 28.6 m/s. The average conduction velocity of type I units (73.5 ± 29.0 m/s) showed no significant difference from that of type N units (69.4 ± 28.0 m/s). Among 138 type I units, 9 units had a conduction velocity of <22.8 m/s (Table 4). All the trials during which these nine slow conducting type I units were recorded exhibited late-phase suppressions in bilateral hindlimb muscle tone (type L or B suppressions). The proportion of slow conducting units among 122 type Ie units was higher in the nucleus reticularis magnocellularis (NRMc; 18.2%, 4/22) than in both the gigantocellularis (NRGc; 3.4%, 2/59) and the paramedianus (NRPm, 2.4%, 1/41) [χ2 test, P (χ2 ≥ 8.32) < 0.02]. The proportion of fast conducting units was highest in the NRPm (97.6%, 40/41). The conduction velocity of 16 type Id units ranged from 12.6 to 103.2 m/s (mean 55.7 ± 30.6 m/s).
TABLE 4.
Discharge pattern and location of medullary reticulospinal units identified
| Group | NRGc | NRMc | NRPm | Total |
|---|---|---|---|---|
| Type N | 66 (4/62) | 22 (3/19) | 12 (0/12) | 100 (7/93) |
| Type I | 67 (3/64) | 29 (4/25) | 42 (2/40) | 138 (9/129) |
| Ie | 59 (2/57) | 22 (4/18) | 41 (1/40) | 122 (7/115) |
| Ie-A | 25 (0/25) | 9 (0/9) | 25 (0/25) | 59 (0/59) |
| Ie-B | 34 (2/32) | 13 (4/9) | 16 (1/15) | 63 (7/56) |
| Id | 8 (1/7) | 7 (0/7) | 1 (1/0) | 16 (2/14) |
| Total | 133 (7/126) | 51 (7/44) | 54 (2/52) | 238 (16/222) |
Values within parentheses represent number of units (slow units*/fast units). NRGc, nucleus reticularis gigantocellularis; NRMc, nucleus reticularis magnocellularis; NRPm, nucleus reticularis paramedianus.
Units with a conduction velocity of <22.8 m/s.
DISCUSSION
In the current study, short-train stimulations of the brain stem were found to suppress neck and hindlimb muscle activity of decerebrate cats with two distinct phases, early and late. The dividing value of the conduction velocity for reticulospinal projections responsible for early and late phases of hindlimb muscle tone suppression was estimated to be 22.8 m/s. Pontine stimulation that suppressed hindlimb muscle tone increased the firing rate of 138 of 238 reticulospinal units identified in the medial medulla.
Early and late phases of brain stem–induced motor suppression
The medullary inhibitory region was discovered by using long-train stimulation (Magoun and Rhines 1946). Later, long trains also contributed to identifying the inhibitory regions in the midbrain and pons (Lai and Siegel 1990; Oka et al. 1993). However, long-train stimulations cannot differentiate the two atonia systems that we demonstrated in this study.
The latency to trough and latency to offset of the late-phase hindlimb muscle tone suppression induced by both pontine and medullary stimulation showed no statistical difference between type B and type L suppressions. The time course of the late-phase suppression was not affected by the presence of the early suppression. Therefore despite occurring at latencies of >40 ms, the late-phase suppression is concluded to be the result of an inactivation of motoneurons by descending systems rather than the result of a local reflex because of changes in muscle tone induced by the early-phase suppression. Also the latency to trough of pontine-induced early-phase suppression of hindlimb muscles in type E suppression was not significantly different from that of the early phase in type B suppression. We concluded that the appearances of the early and late phases of hindlimb muscle tone suppression were independent. Two troughs could also be identified in the neck muscle tone suppression induced by stimulating the pons and medulla. Brain stem stimulation of decerebrate cats suppresses neck and hindlimb muscle activity with both short and long latencies.
Through intracellular recordings, Peterson et al. (1978) obtained two types of inhibitory postsynaptic potentials (IPSPs) in neck motoneurons by stimulating the pontomedullary reticular formation: early IPSPs with short latencies of ≤1.3 ms and late ones with longer latencies including IPSPs with a latency of >2.0 ms. They attributed these differences in the latency of IPSPs to the number of synapses in pathways from the brain stem to the motoneurons. Corresponding to the long latency response we see in hindlimb muscles, IPSPs were recorded in lumbar motoneurons after stimulating the pedunculopontine tegmental nucleus of the decerebrate cat (Takakusaki et al. 1997) and by stimulating the pontine (Fung et al. 1982) and medullary (Chase et al. 1986) reticular formation during REM sleep. Corresponding to our early-phase suppression, Drew and Rossignol (1990) reported inhibitory responses of hindlimb muscles in waking cats (the mean latency to onset 20 ms), and Engberg et al. (1968) reported a medullary induced inhibitory action on lumbar motoneurons. Engberg et al. (1968) estimated this inhibitory action to conduct at faster than 20 m/s. Consistent with this estimation, we determined the dividing value of the conduction velocity for reticulospinal projections responsible for early and late phases of hindlimb muscle tone suppression to be 22.8 m/s.
Consistent with our current observation, both early and late phases of membrane hyperpolarization were identified simultaneously through intracellular recordings of lumbar motoneurons by using short trains delivered to the brain stem. Fung et al. (1982) induced both early (latency 2–15 ms) and late (38–53 ms) phases of synaptic response during REM sleep by stimulating the pontine reticular formation. Sakamoto et al. (1985) elicited both early (latency 4–6 ms) and late (30–40 ms) membrane responses in decerebrate cats by stimulating the dorsal part of the mid-pontine tegmental field and reported that earlier responses were more consistently observed than later ones. In intact cats, an early hyperpolarizing response without a subsequent late response was also obtained during waking and non-REM sleep states by similar pontine stimulation (Fung et al. 1982). An earlier response was more easily elicited than a later one in these intracellular recording studies, although they did not describe details of the stimulus intensity. In contrast to our observation, these studies did not observe peripheral muscle activity or the time course of brain stem neuronal activation during atonia eliciting stimulation.
Reticulospinal systems that mediate atonia
More than one-half of medullary reticulospinal units identified (type I units, 138/238) were activated by pontine stimulation that induced atonia. This unit population appears to serve as the final common path for motor inhibition. According to our estimate from the EMG study, we hypothesize that the fast-conducting type Ie units that conduct at ≥22.8 m/s mediate the early-phase suppression of hindlimb muscles, whereas the slow-conducting type Ie units that conduct at <22.8 m/s are involved in the late suppression. Depending on the conduction velocity, the presumed role of type Id units could extend from the cessation of the early-phase suppression to the induction, maintenance, and even the cessation of the late-phase suppression. We hypothesize that brain stem stimulation evokes muscle tone reduction and activates these several subpopulations of type I units simultaneously, resulting in IPSPs in motoneurons (Chase et al. 1986; Fung et al. 1982) and subsequent EMG changes corresponding to these IPSPs.
The delayed activation seen in types Ie-B and Id units could be caused by sensory inputs elicited by induced EMG changes or by an interaction between the pons and medulla (Siegel et al. 1986). After transection at the pontomedullary level, stimulation of the medullary inhibitory region produces little motor suppression (Siegel et al. 1983). Pontine neurons may recruit medullary neurons mediating atonia, or reticulospinal cells in the pons (Matsuyama et al. 1997) may directly participate in muscle tone suppression.
Iwakiri et al. (1995) found medullary reticulospinal cells whose activity was suppressed by pontine stimulation that reduced postural muscle tone. Siegel et al. (1992) found that muscle tone suppression seen in cataplexy was correlated with cessation of discharge in the majority of pontine and medullary neurons. The cessation of activity of noradrenergic cells in the locus coeruleus (Wu et al. 1996) may also play a role in muscle tone suppression.
Fast conducting neurons (80–100 m/s) that inhibit motoneuronal excitability were identified in the dorsal medulla (NRGc) (Takakusaki et al. 1994). We found that the proportion of fast type Ie units was highest in the caudal medulla (NRPm). Our previous studies implicated this region in muscle tone suppression (Lai and Siegel 1988; Kodama et al. 1992; Shiromani et al. 1990). Two slow conducting units (6–8 m/s) that fire specifically during REM sleep were found in the ventral medulla and were hypothesized to be involved in muscle atonia (Kanamori et al. 1980). Consistent with this report, we found that the proportion of type Ie slow conducting medullary reticulospinal neurons was highest in the ventral medulla (NRMc). This is also consistent with the localization of cataplexy-on units in narcoleptic dogs (Siegel et al. 1991).
The number of slow conducting reticulospinal units we identified was low in comparison with the amplitude and consistency of the late-phase hindlimb muscle tone suppressions we obtained. Also we did not see reticulospinal neurons with conduction velocity of <10 m/s, although such slow conducting cells do exist (West and Wolstencroft 1983). We could identify more slowly conducting reticulospinal neurons by adding stimulation of more rostral portions of the spinal cord for antidromic identification. Under the stimulation conditions used in the current study, very long latency responses elicited from caudal spinal stimulation could not easily be discriminated. However, other possibilities must be kept in mind to explain the low number of slow conducting reticulospinal neurons identified in the current study. The late-phase suppression of muscle tone might be mediated by the delayed activation of fast conducting reticulospinal projections. Interaction between the pons and medulla (Siegel et al. 1986) could cause this kind of delay. In fact, we found reticulospinal units that showed delayed activation (types Ie-B and Id) in the medullary reticular formation. Similar processes causing a delay in muscle responses might also occur within the spinal cord.
Neuronal mechanisms mediating early and late atonia
Yeomans (1990) proposed that slow conducting fibers reduced response to high-frequency stimulation compared with fast fibers because of longer absolute refractory periods in the slow fibers. However, we could not differentiate the two phases of suppression with 330- and 1,000-Hz stimulation. In cases of pontine-induced hindlimb muscle tone suppression, the average stimulus intensity needed to induce early suppression was higher than that required for evoking late suppression, although an increase of stimulus intensity at the same stimulus sites where the late suppression was induced did not always evoke the early-phase suppression. This indicates that neural elements involved in the early suppression have a larger current-distance constant than those responsible in the late one (Tehovnik 1996). According to Hentall et al. (1984), the current-distance constant is negatively correlated with the conduction velocity. Consequently, although paradoxically, this would indicate that the neural elements involved in the early-phase suppression conduct slower than the elements implicated in the late phase suppression. Although a spatially denser presence of slow fibers at the stimulus site might explain our results, the appearance of later responses was found to depend on behavioral states (Fung et al. 1982). Neuronal mechanisms that cause the state dependency of neuronal activity remain obscure, and these mechanisms could be altered in the decerebrate animals we used. The precise mechanisms that produce the early and late suppressions remain to be determined.
It also remains unclear if atonia systems act directly on motoneurons or through spinal interneurons. An inhibitory effect on lumbar motoneurons induced by pontine carbachol injection was proposed to be mediated via spinal segmental inhibitory interneurons (Takakusaki et al. 1994), whereas a monosynaptic inhibition on spinal motoneurons through glycinergic reticulospinal cells was found in the lamprey (Wannier et al. 1995). In cats, medullary glycinergic neurons are likely to mediate motor suppression in REM sleep (Rampon et al. 1997), although a role for reticulospinal projections containing γ-aminobutyric acid was also suggested (Jones et al. 1991).
Functional implications
The distinct early- and late-phase suppressions we demonstrated may have separate functional roles. By comparing two papers that described PGO wave-related IPSPs (López-Rodríguez et al. 1992; Pedroarena et al. 1994), it can be calculated that it took 20–30 ms for volleys mediating these phasic motor suppressions to conduct from the brain stem to the lumbar segment of the spinal cord. Taking into account the distance between these two sites (an average value among our 23 cats was 278 mm), PGO wave-related phasic IPSPs are likely to be mediated primarily by the slow conducting system. The fast conducting inhibitory reticulospinal system is proposed to set the activity of axial and proximal muscles related to postural fixation (Mori et al. 1995).
We demonstrated two phases of motor activity reduction in neck and hindlimb muscles elicited bilaterally by short-train stimulation. Our method may be useful in further studies that analyze and discriminate between the anatomic and neurochemical substrates of these two atonia systems. Characterization of the morphology and neurochemistry of the neurons implicated in each reticulospinal system in the decerebrate model may clarify the distinct roles of each system in motor control.
Acknowledgments
This study was supported by Uehara Memorial Foundation, National Institutes of Health Grants HL-41370 and NS-14610, and the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- Berman AL The Brain Stem of the Cat. Madison, WI: Univ. of Wisconsin Press, 1968. [Google Scholar]
- Chase MH, Morales FR, Boxer PA, Fung SJ, and Soja PJ Effect of stimulation of the nucleus reticularis gigantocellularis on the membrane potential of cat lumbar motoneurons during sleep and wakefulness. Brain Res. 386: 237–244, 1986. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, and Rossignol S Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J. Neurophysiol 55: 375–401, 1986. [DOI] [PubMed] [Google Scholar]
- Drew T and Rossignol S Functional organization within the medullary reticular formation of intact unanesthetized cat. II. Electromyographic activity evoked by microstimulation. J. Neurophysiol 64: 782–795, 1990. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Nicoll RA, Rantucci T, Táboríkoá H, and Willey TJ Topographic studies on medial reticular nucleus. J. Neurophysiol 39: 109–118, 1976. [DOI] [PubMed] [Google Scholar]
- Engberg I, Lundberg A, and Ryall RW Reticulospinal inhibition of transmission in reflex pathways. J. Physiol. (Lond.) 194: 201–223, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Boxer PA, Morales FR, and Chase MH Hyperpolarizing membrane responses induced in lumbar motoneurons by stimulation of the nucleus reticularis pontis oralis during active sleep. Brain Res. 248: 267–273, 1982. [DOI] [PubMed] [Google Scholar]
- Gassel MM, Marchiafava PL, and Pompeiano O Tonic and phasic inhibition of spinal reflexes during deep desynchronized sleep in unrestrained cats. Arch. Ital. Biol 102: 471–499, 1964. [PubMed] [Google Scholar]
- Glenn LL and Dement WC Motoneurons properties during electromyogram pauses in sleep. Brain Res. 243: 11–23, 1982. [DOI] [PubMed] [Google Scholar]
- Hendricks JC, Morrison AR, and Mann GL Different behaviors during paradoxical sleep without atonia depend on pontine lesions site. Brain Res. 239: 81–105, 1982. [DOI] [PubMed] [Google Scholar]
- Henley K and Morrison AR A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat. Acta Neurobiol. Exp 34: 215–232, 1974. [PubMed] [Google Scholar]
- Hentall ID, Zorman G, Kansky S, and Fields HL Relations among threshold, spike height, electrode distance, and conduction velocity in electrical stimulation of certain medullospinal neurons. J. Neurophysiol 51: 968–977, 1984. [DOI] [PubMed] [Google Scholar]
- Holmes CJ and Jones BE Importance of cholinergic, GABAergic, serotonergic and other neurons in the medial medullary reticular formation for sleep-wake states studied by cytotoxic lesions in the cat. Neuroscience 62: 1179–1200, 1994. [DOI] [PubMed] [Google Scholar]
- Iwakiri H, Oka T, Takakusaki K, and Mori S Stimulus effects of the medial pontine reticular formation and the mesencephalic locomotor region upon medullary reticulospinal neurons in acute decerebrate cats. Neurosci. Res 23: 47–53, 1995. [PubMed] [Google Scholar]
- Jankowska E, Lund S, Lundberg A, and Pompeiano O Inhibitory effects evoked through ventral reticulospinal pathways. Arch. Ital. Biol 106: 124–140, 1968. [PubMed] [Google Scholar]
- Jones BE, Holmes CJ, Rodriguez-Veiga E, and Mainville L GABA-synthesizing neurons in the medulla; their relationship to serotonin-containing and spinally projecting neurons in the cat. J. Comp. Neurol 312: 1–19, 1991. [DOI] [PubMed] [Google Scholar]
- Jouvet M Recherches sur les structures nerveuses et les mechanismes responsables des differentes phases du sommeil physiologique. Arch. Ital. Biol 100: 125–206, 1962. [PubMed] [Google Scholar]
- Jouvet M and Delorme JF Locus coeruleus et sommeil paradoxal. C. R. Seances Soc. Biol. Fil 159: 895–899, 1965. [Google Scholar]
- Kanamori N, Sakai K, and Jouvet M Neuronal activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res. 189: 251–255, 1980. [DOI] [PubMed] [Google Scholar]
- Kodama T, Lai YY, and Siegel JM Enhancement of acetylcholine release during REM sleep in the caudo-medial medulla measured by in vivo microdialysis. Brain Res. 580: 348–350, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama J, Shimohira M, Hasegawa T, Kouji T, and Iwakawa Y Phasic motor activity reduction occurring with horizontal rapid eye movements during active sleep in human. Exp. Brain Res 107: 137–144, 1995. [DOI] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Medullary regions mediating atonia. J. Neurosci 8: 4790–4796, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Muscle tone suppression and stepping produced by stimulation of midbrain and rostral pontine reticular formation. J. Neurosci 10: 2727–2734, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY and Siegel JM Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J. Neurosci 11: 2931–2937, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai YY, Siegel JM, and Wilson WJ Effect of blood pressure on medial medulla-induced muscle atonia. Am. J. Physiol 252 (Heart Circ. Physiol. 21): H1249–1257, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J Antidromic activation of neurons as an analytic tool in the study of the central nervous system. J. Neurosci. Methods 4: 1–32, 1981. [DOI] [PubMed] [Google Scholar]
- López-Rodríguez F, Chase MH, and morale FR PGO-Related potentials in lumbar motoneurons during active sleep. J. Neurophysiol 68: 109–116, 1992. [DOI] [PubMed] [Google Scholar]
- Magoun HW and Rhines R An inhibitory mechanisms in the bulbar reticular formation. J. Neurophysiol 9: 165–171, 1946. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Nakajima K, and Mori S Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: anterograde PHA-L tracing study. J. Comp. Neurol 377: 234–250, 1997. [PubMed] [Google Scholar]
- Morales FR, Engelhardt JK, Soja PJ, Pereda AE, and Chase MH Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J. Neurophysiol 57: 1118–1129, 1987. [DOI] [PubMed] [Google Scholar]
- Mori S, Iwakiri H, Homma Y, Yokoyama T, and Matsuyama K Neuroanatomical and neurophysiological bases of postural control. Adv. Neurol 67: 289–303, 1995. [PubMed] [Google Scholar]
- Morrison AR and Bowker RM The biological significance of PGO spikes in the sleeping cat. Acta Neurobiol. Exp 35: 821–840, 1975. [PubMed] [Google Scholar]
- Oka T, Iwakiri H, and Mori S Pontine-induced generalized suppression of postural muscle tone in a reflexively standing acute decerebrate cat. Neurosci. Res 17: 127–140, 1993. [DOI] [PubMed] [Google Scholar]
- Orem L Neuronal mechanisms of respiration in REM sleep. Sleep 3: 251–267, 1980. [DOI] [PubMed] [Google Scholar]
- Pedroarena C, Castillo P, Chase MH, and Morales FR The control of jaw-opener motoneurons during active sleep. Brain Res. 653: 31–38, 1994. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Pitts NG, and Fukushima K Reticulospinal excitation and inhibition of neck motoneurons. Exp. Brain Res 32: 471–489, 1978. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Metz J, and Rechtschaffen A Spinal reflex and lateral geniculate nucleus activity during sleep: quantitative relationships. Exp. Neurol 77: 142–162, 1982. [DOI] [PubMed] [Google Scholar]
- Rampon C, Morales FR, Fort P, Luppi PH, Sampogna S, and Chase MH Glycine immunohistochemistry and c-fos expression in the cervical spinal cord of cats during carbachol-induced active sleep. Soc. Neurosci. Abstr 23: 310.17, 1997. [Google Scholar]
- Sakamoto T, Matsuyama K, and Takakusaki K Postsynaptic potential in soleus alpha-motoneurons elicited by stimulating the dorsal part of the mid-pontine tegmental field. Neurosci. Res 1, Suppl.: 66, 1985. [Google Scholar]
- Schenkel E and Siegel JM Rem sleep without atonia after lesions of the medial medulla. Neurosci. Lett 98: 159–165, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin JJ, Magherini PVC, and Pompeiano O Cholinergic mechanisms related to REM sleep. III. Tonic and phasic inhibition of monosynaptic reflexes induced by an anticholinesterase in the decerebrate cat. Arch. Ital. Biol 111: 1–23, 1973. [PubMed] [Google Scholar]
- Shiromani PJ, Lai YY, and Siegel JM Descending projections from the dorsolateral pontine tegmentum to the paramedian reticular nucleus of the caudal medulla in the cat. Brain Res. 517: 224–228, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse MN and Siegel JM Pontine regulation of REM sleep components in cats: integrity of the pedunculopontine tegmentum (PPT) is important for phasic events but unnecessary for atonia during REM sleep. Brain Res. 571: 50–63, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Chiu C, Dement WC, Mignot E, and Lufkin R Activity of medial mesopontine units during cataplexy and sleep-waking states in the narcoleptic dog. J. Neurosci 12: 1640–1646, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, Mignot E, and Chiu C Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla. Science 252: 1315–1318, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, and Tomaszewski KS Rostral brainstem contributes to medullary inhibition of muscle tone. Brain Res. 268: 344–348, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Tomaszewski KS, and Nienhuis R Behavioral states in the chronic medullary and midpontine cat. Electroencephalogr. Clin. Neurophysiol 63: 274–288, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM, Wheeler RL, and Mcginty DJ Activity of medullary reticular formation neurons in the unrestrained cat during waking and sleep. Brain Res. 179: 49–60, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki K, Habaguchi T, Nagaoka T, and Sakamoto T Stimulus effects of the pedunculopontine tegmental nucleus (PPN) on hindlimb motoneurons in cats. Soc. Neurosci. Abstr 23: 299.1, 1997. [Google Scholar]
- Takakusaki K, Matsuyama K, Kobayashi Y, Kohyama J, and Mori S Pontine microinjection of carbachol and critical zone for inducing postural atonia in reflexively standing decerebrate cats. Neurosci. Lett 153: 185–188, 1993. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shimoda N, Matsuyama K, and Mori S Discharge properties of medullary reticulospinal neurons during postural changes induced by intra-pontine injections of carbachol, atropine and serotonin, and their functional linkages to hindlimb motoneurons in cats. Exp. Brain Res 99: 361–374, 1994. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Methods 65: 1–17, 1996. [DOI] [PubMed] [Google Scholar]
- Villablanca J Behavioral and polygraphic study of “sleep” and ‘wakefulness’ in chronic decerebrate cats. Electroencephalogr. Clin. Neurophysiol 21: 562–577, 1966. [DOI] [PubMed] [Google Scholar]
- Wannier T, Orlovsky G, and Grillner S Reticulospinal neurons provide mono-synaptic glycinergic inhibition of spinal neurones in lamprey. Neuroreport 6: 1597–1600, 1995. [DOI] [PubMed] [Google Scholar]
- Webster HH and Jones BE Neurotoxic lesions of the dorsolateral pontine tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res. 458: 285–302, 1989. [DOI] [PubMed] [Google Scholar]
- West DC and Wolstencroft JH Strength-duration characteristics of myelinated and non-myelinated bulbospinal axons in the cat spinal cord. J. Physiol. (Lond.) 337: 37–50, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M-F, Gulyani S, Mignot E, and Siegel JM Activity of REM-off cells during cataplexy in the narcoleptic dog (Abstract). Sleep Res. 25: 40, 1996. [Google Scholar]
- Yeomans JS Principles of Brain Stimulation. New York: Oxford, 1990, p. 95–115. [Google Scholar]


