Abstract
Ribosome stalls can result in ribosome collisions that elicit quality control responses, one function of which is to prevent ribosome frameshifting, an activity that entails the interaction of the conserved yeast protein Mbf1 with uS3 on colliding ribosomes. However, the full spectrum of factors that mediate frameshifting during ribosome collisions is unknown. To delineate such factors in the yeast Saccharomyces cerevisiae, we used genetic selections for mutants that affect frameshifting from a known ribosome stall site, CGA codon repeats. We show that the general translation elongation factor eEF3 and the integrated stress response (ISR) pathway components Gcn1 and Gcn20 modulate frameshifting in opposing manners. We found a mutant form of eEF3 that specifically suppressed frameshifting, but not translation inhibition by CGA codons. Thus, we infer that frameshifting at collided ribosomes requires eEF3, which facilitates tRNA–mRNA translocation and E-site tRNA release in yeast and other single cell organisms. In contrast, we found that removal of either Gcn1 or Gcn20, which bind collided ribosomes with Mbf1, increased frameshifting. Thus, we conclude that frameshifting is suppressed by Gcn1 and Gcn20, although these effects are not mediated primarily through activation of the ISR. Furthermore, we examined the relationship between eEF3-mediated frameshifting and other quality control mechanisms, finding that Mbf1 requires either Hel2 or Gcn1 to suppress frameshifting with wild-type eEF3. Thus, these results provide evidence of a direct link between translation elongation and frameshifting at collided ribosomes, as well as evidence that frameshifting is constrained by quality control mechanisms that act on collided ribosomes.
Keywords: translation, frameshifting, ribosome quality control, eEF3, integrated stress response, general amino acid control
INTRODUCTION
Ribosomes not only accurately translate an open reading frame into the specified amino acid sequence, but also display the plasticity to accommodate regulatory events during elongation. To this end, ribosomes elongate the nascent chain at variable speeds due to the mRNA and nascent proteins, with high rates maximizing protein production, and lower rates and stalls assisting with regulatory events, such as protein folding, localization, protein–protein interactions, and programmed frameshifting (Collart and Weiss 2020). Ribosomes also stall during translation for a variety of reasons, including sequences and structures encoded in the mRNA, the composition of the nascent peptide, damage to the mRNA, and stochastic events (Doma and Parker 2006; Dimitrova et al. 2009; Letzring et al. 2013; Simms et al. 2014; Brandman and Hegde 2016; Gamble et al. 2016; Joazeiro 2017).
Some ribosomes fail to efficiently resume translation after these stalls and must be resolved by means other than continued translation. Organisms in all kingdoms have developed quality control mechanisms that act on mRNAs with stalled ribosomes (Brandman et al. 2012; Samatova et al. 2020; D'Orazio and Green 2021). In some cases, ribosome stalls result in ribosome collisions that have been implicated as the trigger for quality control responses, which act to remove stalled ribosomes from the mRNA, degrade defective mRNAs and incomplete proteins, prevent loss of reading frame by the stalled ribosome, and activate global stress response pathways (see Meydan and Guydosh 2021).
Two nonribosomal proteins conserved in eukaryotes regulate key local responses to ribosome stalls. Yeast Hel2 (ZNF598 in humans) (Garzia et al. 2017; Juszkiewicz et al. 2018; Ikeuchi et al. 2019) ubiquitinates 40S ribosomal proteins uS10 and uS3 (eS10, uS10, uS3 in humans) (Garzia et al. 2017; Juszkiewicz and Hegde 2017; Matsuo et al. 2017; Sundaramoorthy et al. 2017), promoting disassembly of ribosomal subunits of the lead ribosome by Slh1/Rqt2 (ASCC3 in humans) (Matsuo et al. 2017, 2020; Juszkiewicz et al. 2020b), recruitment of an endonuclease to the mRNA (D'Orazio et al. 2019; Glover et al. 2020), degradation of the mRNA by No-Go decay (NGD) (Doma and Parker 2006; Brandman et al. 2012; Saito et al. 2015; Brandman and Hegde 2016; Matsuo et al. 2017) and recognition of the released 60S subunit by the ribosome quality control (RQC) complex (Shao et al. 2013; Lyumkis et al. 2014; Shen et al. 2015). The RQC complex then targets the nascent peptide for degradation by ubiquitination (Brandman et al. 2012; Shao et al. 2013; Shao and Hegde 2014). The second factor, yeast Mbf1 (EDF1 in humans) prevents the leading ribosome from frameshifting (Wang et al. 2018; Juszkiewicz et al. 2020a), although both the magnitude and directionality of frameshifting differ between yeast and humans. In humans, EDF1 also promotes recruitment of GIGYF2 and EIF4E2, which in turn reduce translation initiation on mRNAs with collided ribosomes (Juszkiewicz et al. 2020a; Sinha et al. 2020). The yeast homologs of GIGYF2, Smy2 and Syh1 are involved in the decay of mRNAs with a stalling sequence (Hickey et al. 2020), but it is unknown how they are recruited to these mRNAs.
Induction of global stress-response pathways is also mediated through ribosome collisions. In both yeast and humans, ribosome collisions result in activation of the Gcn2 kinase to effect a global reduction in translation initiation, as well as activation of the integrated stress response (ISR) (Meydan and Guydosh 2020; Wu et al. 2020; Pochopien et al. 2021; Yan and Zaher 2021), also known as the general amino acid control (GAAC) pathway in yeast (Hinnebusch 2005). The observation that key effectors of the ISR/GAAC pathway, Gcn1, Gcn20, Rbg2, and Gir2, bind collided ribosomes with Mbf1 (Pochopien et al. 2021) likely clarifies a previous observation that Mbf1 also modulates the induction of the ISR in yeast (Takemaru et al. 1998). In humans, the ribosome-associated MAPKKK ZAKα autophosphorylates during ribosome collisions, resulting in activation of stress-activated protein kinases p38 and cJun, which respectively cause cell cycle arrest and apoptosis (Sinha et al. 2020; Wu et al. 2020).
The idea that ribosome collisions are the essential signal to activate these quality control and stress response pathways is based on four lines of evidence. First, the global induction of ribosome collisions is sufficient to provoke Hel2-dependent ubiquitination of uS3, a hallmark of NGD (Simms et al. 2017b). Second, crucial regulators of quality control responses (Asc1/RACK1, uS3, and Mbf1/EDF1) occupy central positions in structures of collided ribosomes (disomes and trisomes), supporting their role in the regulation of quality control responses. For instance, yeast Asc1 (RACK1) (Kuroha et al. 2010) and uS3 (Simms et al. 2018; Wang et al. 2018) reside at the 40S–40S interface of collided ribosomes (Matsuo et al. 2017; Juszkiewicz et al. 2018; Ikeuchi et al. 2019; Sinha et al. 2020), and Mbf1 (EDF1) (Wang et al. 2018; Simms et al. 2019; Juszkiewicz et al. 2020a; Sinha et al. 2020) interacts with uS3 on the colliding ribosome (Sinha et al. 2020; Pochopien et al. 2021). Third, crucial regulators of quality control are specifically recruited to collided ribosomes, rather than monosomes. For instance, both Hel2 and ZNF598 act preferentially on disomic or trisomic ribosomes in vitro (Juszkiewicz et al. 2018; Ikeuchi et al. 2019; Matsuo et al. 2020), and both ZNF598 and EDF1 are specifically enriched on nuclease-resistant ribosome multimers compared to their relative abundance on monosomes (Juszkiewicz et al. 2020a; Sinha et al. 2020). Gcn1, an essential component of the ISR, also specifically binds collided ribosomes with Mbf1 (Pochopien et al. 2021; Yan and Zaher 2021). Fourth, frameshifting at CGA codon repeats in yeast, which occurs when Mbf1 or uS3 proteins are mutated (Wang et al. 2018), is critically dependent upon ribosome density on the mRNA and the position of the CGA codon repeats relative to the AUG start, as expected if collisions are required for the frameshift (Wolf and Grayhack 2015; Simms et al. 2019).
While the events, components and interactions of the NGD and ISR pathways have been studied extensively, there is far less known about pathways involving Mbf1 or EDF1, although both proteins affect frameshifting (Hendrick et al. 2001; Wang et al. 2018; Juszkiewicz et al. 2020a). We can infer much about the mechanisms by which Mbf1 prevents frameshifting based upon the structural analyses of collided ribosomes with and without Gcn1 (Sinha et al. 2020; Pochopien et al. 2021). Mbf1 binds the colliding ribosome through interactions with conserved residues of uS3 and also interacts directly with the mRNA entering the colliding ribosome, altering the path of the 3′ end of the mRNA, promoting interactions between the mRNA and h16 of the 40S, and likely locking the 40S head to prevent translocation, all of which are likely to inhibit frameshifting (Sinha et al. 2020; Pochopien et al. 2021). However, as noted above, in mammals, frameshifting is much less efficient and occurs in the −1 rather than the +1 direction when ribosomes stall and collide (Juszkiewicz et al. 2020a). Thus, additional factors may promote frameshifting at CGA codon pairs and other inhibitory pairs in yeast (Wang et al. 2018). We note that ribosomes exhibit multiple defects in decoding CGA–CGA and CGA–CCG codons, including a distorted conformation of mRNA in the ribosomal A site, both slow and incomplete elongation in vitro, and pausing of ribosomes with empty A sites at these pairs in vivo (Tesina et al. 2020). Thus, efficient +1 frameshifting at CGA–CGA codon pairs in yeast may be promoted by signals in addition to the ribosome collision, by proteins unique to yeast or by differences between yeast and humans in the relative efficiency of different response pathways, any or all of which could in turn affect the magnitude of frameshifting.
We set out to further understand the forces that promote and inhibit frameshifting and their relationship to other pathways regulated by ribosome collisions. To that end, we selected mutants that suppressed frameshifting at CGA–CGA codon pairs when Mbf1 was defective and identified a truncation mutation in the general elongation factor eEF3. We present evidence that the mutant form of eEF3 specifically reduces frameshifting, rather than reducing either inhibition by CGA codon pairs or the overall translation efficiency of an optimal reporter. Thus, we infer that frameshifting is driven by events in addition to the collision, since otherwise effects on stalling and the ensuing collision should equally impact CGA inhibition and frameshifting. We also selected mutants that promoted frameshifting when Mbf1 was functional and found mutations in GCN1. Moreover, we uncovered a synergistic interaction between uS3, the site of Mbf1 binding, and Gcn1, which binds collided ribosomes with Mbf1 (Pochopien et al. 2021). We find that Gcn1 modulates frameshifting in conjunction with Gcn20, which also binds collided ribosomes, but that Gcn2 and Gcn4 have much smaller media-dependent effects on frameshifting, suggesting a unique role for Gcn1 and Gcn20 on the collided ribosome distinct from their known role in the ISR pathway. Furthermore, we provide evidence that Mbf1, Gcn1, and Hel2 (the NGD regulator) all act to constrain eEF3 effects on frameshifting.
RESULTS
eEF3 plays a role in frameshifting at CGA codon repeats
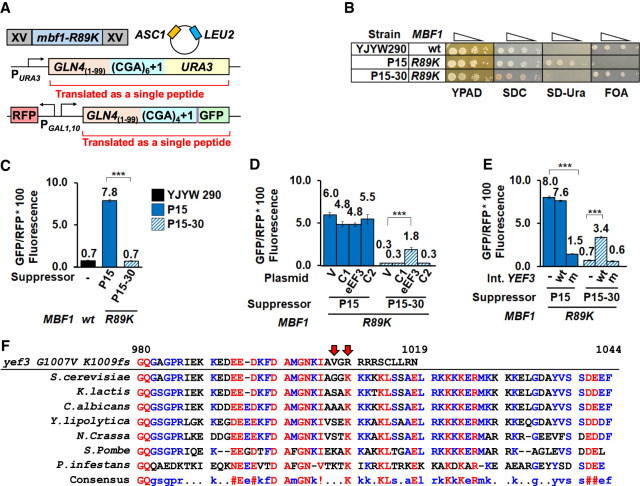
Based on the apparent differences between yeast and humans in both directionality and efficiency of frameshifting at collided ribosomes (Wang et al. 2018; Simms et al. 2019; Juszkiewicz et al. 2020a), we considered that frameshifting at CGA codon repeats in yeast might involve yeast-specific factors that promote frameshifting. If so, mutations in the corresponding genes could suppress the frameshifting at CGA codon repeats that is caused by defects in Mbf1. To obtain mutants that suppressed frameshifting caused by mbf1 mutations, we reversed a previous selection, which yielded the mbf1 mutants (Wang et al. 2018), using strains in which expression of both the URA3 and GFP genes require a +1 frameshift downstream from four to six adjacent CGA codons in chromosomally integrated reporters (Fig. 1A). In this background, mbf1 mutants (Wang et al. 2018) exhibited a Ura+ GFP+ phenotype, since ribosomes frameshift efficiently at CGA repeats in these mutants. We selected suppressors from strains bearing six different mbf1 alleles, including P15 bearing mbf1-R89K, because these strains exhibited different levels of frameshifting. To obtain frameshifting suppressors, we selected Ura− mutants based on resistance to 5-fluoro-orotic acid (FOAR), since Ura+ yeast convert FOA to the toxic compound fluorouracil (Boeke et al. 1984, 1987). To determine which of the FOAR mutants specifically affected frameshifting, we screened the mutants for reduced expression of the frameshifted GFP reporter, using the ratio of GFP/RFP to account for differences in the overall expression of the reporter between strains, as described previously (Dean and Grayhack 2012). To ensure mutants exhibited low levels of frameshifting we identified mutants with GFP/RFP ratios <60% of the parental strain.
FIGURE 1.
eEF3 plays a role in frameshifting at CGA codon repeats. (A) Schematic of the selection for mutants that suppress frameshifting at CGA codon repeats when Mbf1 is defective. In the P15 selection strain, CGA codon repeats plus a single nucleotide were inserted upstream of the URA3 and HA epitope-GFP coding regions; the strain also contains a plasmid-borne copy of ASC1 to avoid mutations in ASC1. The mbf1-R89K mutant in the P15 strain results in an Ura+ GFP+ phenotype due to efficient frameshifting at CGA codon repeats (Wang et al. 2018). Mutants that suppress frameshifting in the mbf1-R89K mutant were selected as FOA resistant mutants that also exhibited reduced GFP expression. (B) The P15 suppressor (P15–30) exhibits an Ura− FOA-resistant phenotype, unlike its parent P15, but like its grandparent (YJYW290) (see Wang et al. 2018). Serial dilutions of the indicated strains were grown at 30°C on rich media (YPAD), complete minimal media (SDC), minimal media lacking uracil (SD-Ura), and minimal media containing FOA. (C) Expression of the GLN4(1–99)-(CGA)4+1-GFP frameshifted reporter is significantly reduced in the P15–30 suppressor relative to its parent (P15). (D) Expression of the GLN4(1–99)-(CGA)4 + 1-GFP frameshifted reporter is partially restored in the P15–30 suppressor by addition of a plasmid bearing the YEF3 gene (encoding eEF3). GFP/RFP was measured in the P15 parent strain and the P15–30 suppressor strain bearing 2µ plasmids with either no insert (V, vector) or genomic inserts from the yeast tiling collection (Jones et al. 2008). eEF3: plasmid with the YEF3 gene; C1 and C2: plasmids with flanking chromosomal sequences. (E) Expression of the GLN4(1–99)-(CGA)4 + 1-GFP frameshifted reporter is modulated by replacement of YEF3 alleles in the chromosome. In the P15 strain, integration of mutant yef3-fs1009 into the chromosome results in reduced expression of frameshifted GFP/RFP while in the suppressor P15–30 strain, integration of wild-type YEF3 into the chromosome results in increased expression of frameshifted GFP/RFP. (F) Amino acid sequence of the carboxy-terminal region of eEF3 from S. cerevisiae was aligned with six evolutionarily distant Ascomycete fungi and a verified eEF3 from the Chromista P. infestans (Mateyak et al. 2018) using MultAlin (http://multalin.toulouse.inra.fr/multalin/) (Corpet 1988). The yef3 G1007V K1009fs mutation is shown above. Numbering above the sequences is based on S. cerevisiae eEF3. The color text represents the level of consensus for each residue (blue: 50%–90%, red: >90%). In all panels, (***) indicates P-value of <0.001.
In this study, we focus on one such mutant, P15–30, which had a mutated version of the gene encoding eEF3 (YEF3), based on whole-genome sequencing and targeted resequencing. We evaluated ten additional independent mutants, obtained from selection strains bearing different mbf1 alleles, by either whole-genome sequencing or targeted sequencing of YEF3, and found no additional mutations in YEF3. In P15–30, the YEF3 gene bears two mutations, a single amino acid change (G1007V) and a frameshift (K1009fs) which leads to premature termination and loss of 35 amino acids from the carboxyl terminus; we refer to the yef3-G1007V K1009fs mutations as yef3-fs1009 in this paper. As expected if frameshifting is reduced in the P15–30 suppressor, the P15–30 mutant failed to grow on media lacking uracil, unlike its mbf1-R89K parent P15, but similar to its MBF1+ grandparent YJYW290 (Fig. 1B; Wang et al. 2018). Similarly, the P15–30 suppressor and its grandparent exhibited low levels of frameshifted GFP/RFP relative to P15 (Fig. 1C).
To confirm that the yef3-fs1009 mutation in P15–30 was responsible for the suppression of frameshifting, we first showed that the mutation was recessive, in that a diploid of P15–30 obtained by mating to a MATα mbf1Δ strain restored frameshifting to the same level as the similarly mated P15 parent (Supplemental Fig. S1). Exogenous expression of wild-type YEF3 (Jones et al. 2008) in the P15–30 suppressor did result in an increase in frameshifted GFP/RFP from 0.3 to 1.8 (Fig. 1D), but did not restore frameshifted GFP/RFP to parental levels of 4.8–5.5 (P15 transformants). To quantitatively determine the effect of the yef3 mutation on frameshifting suppression, we replaced the chromosomal yef3-fs1009 allele in P15–30 with wild-type YEF3 kanR and found that expression of the frameshifted reporter increased fivefold (0.7 to 3.4 GFP/RFP) while replacement with a yef3-fs1009 kanR construct did not increase GFP/RFP (Fig. 1E). Likewise, we found that replacement of the chromosomal YEF3 in the P15 parent with yef3-fs1009 kanR resulted in a fivefold reduction in GFP/RFP (8.0 to 1.5) (Fig. 1E). Thus, we conclude that the mutation of YEF3 is both necessary and sufficient to suppress frameshifting in the mbf1-R89K mutant, although the original P15–30 mutant strain may bear secondary mutations that contribute to the suppression of frameshifting.
eEF3 is one of four essential translation factors in yeast, which act during each round of translation to facilitate the steps required for elongation: acceptance of aminoacyl-tRNA into the A site of the ribosome, formation of the peptide bond, translocation of the mRNA with its cognate tRNAs from the A and P sites to the P and E sites, and release of deacyl-tRNA from the E site (Dever and Green 2012; Dever et al. 2016). Unlike the other three elongation factors, which are conserved in all kingdoms, eEF3 is highly conserved (Supplemental Fig. S2) in fungi as well as in other single-celled eukaryotes such as oomycetes (a phylogenetic lineage including some algae), but has no known homolog in mammals or bacteria (Belfield et al. 1995; Mateyak et al. 2018). eEF3, a member of the ribosome-associated family of ATP-binding cassette (ABC) ATPases (Sandbaken et al. 1990; Andersen et al. 2006; Murina et al. 2019), promotes the late stages of tRNA translocation and facilitates the release of deacyl-tRNA from the E-site (Triana-Alonso et al. 1995; Ranjan et al. 2021). eEF3 is positioned on the ribosome to assist movement of the L1 stalk, providing a structural model for its function of promoting E site release (Andersen et al. 2006; Ranjan et al. 2021). However, the function of the carboxy-terminal domain in which the G1007V and K1009fs mutations are found is unknown. This domain (residues 981–1044) (Fig. 1F) was not resolved in either structure (Andersen et al. 2006; Ranjan et al. 2021) and is dispensable for the essential function of eEF3 (Anand et al. 2006; Andersen et al. 2006). However, this domain, which is also reported to have ribosome binding activity (Kambampati and Chakraburtty 1997) and associates with polyribosomes (Visweswaraiah et al. 2012), contains three highly conserved lysine blocks (Fig. 1F) that are removed due to the K1009fs mutation.
Translation function and eEF3 levels are affected by the frameshifting suppressor mutant
To determine which aspects of eEF3 function were affected by the yef3-fs1009 mutation we analyzed the growth of strains with wild-type MBF1 and either wild-type YEF3 or the yef3-fs1009 mutation. To eliminate the effects of other mutations in the P15 and P15-30 strains, we introduced the YEF3 and yef3-fs1009 alleles into wild-type BY4741 yeast strains, precisely replacing the chromosomal YEF3 locus by integrating constructs with YEF3 (wild-type or mutant) fused to a K. lactis URA5 gene followed by excision of the K. lactis URA5 marker using selection on FOA-containing media (Boeke et al. 1984, 1987). We next integrated various MBF1 alleles into these strains for the experiments described below.
If the yef3-fs1009 mutation affects an important function of eEF3 in translation, then the yef3-fs1009 mutation might be expected to alter either growth rate or sensitivity to translation inhibitors. Mutations in YEF3 (one located between its two ABC domains and one in the chromodomain) are known to result in sensitivity to the aminoglycoside paromomycin (Anand et al. 2003; Sasikumar and Kinzy 2014). Indeed, we found that three independent isolates of strains bearing the yef3-fs1009 mutation grew slowly on rich media at all temperatures, showing an exacerbated growth defect at high temperatures (Fig. 2A). Furthermore, the yef3-fs1009 mutants were more sensitive at 37°C to both anisomycin, which inhibits peptidyl transferase activity (Grollman 1967) and paromomycin, which relaxes decoding specificity resulting in increased misreading (Fig. 2B; Fourmy et al. 1996; Fan-Minogue and Bedwell 2008). Thus, we infer that the mutant eEF3 results in a translation defect.
FIGURE 2.
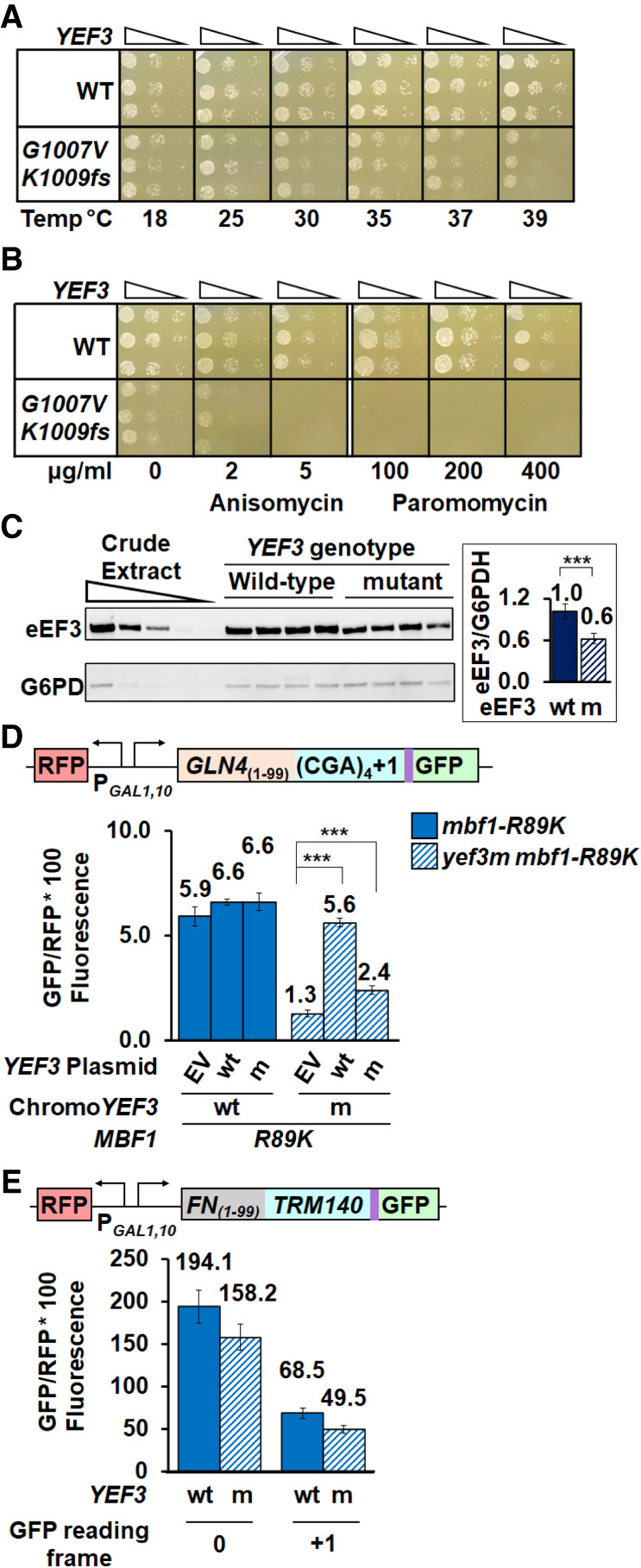
The yef3-fs1009 mutation results in a temperature-dependent growth defect, sensitivity to translation inhibitors and reduced amounts of eEF3. (A) The yef3-fs1009 mutation results in a growth defect that is exacerbated at higher temperatures. Serial dilutions of YEF3 wild-type and yef3-fs1009 strains were grown on rich media (YPAD) at the indicated temperatures. (B) The yef3-fs1009 mutation confers sensitivity to translation inhibitors anisomycin and paromomycin. Serial dilutions of strains in (A) were grown on rich (YPAD) media at 37°C with indicated concentrations of anisomycin or paromomycin. (C) Strains with the yef3-fs1009 mutation have reduced levels of eEF3 protein compared to otherwise isogenic strains with wild-type YEF3. Crude extracts, separated by SDS-PAGE, were subjected to western analysis with anti-eEF3 and anti-glucose-6-phosphate dehydrogenase (G6PDH) antibodies and quantified using Image J (https://imagej.nih.gov/ij/). (D) Increased copies of the yef3-fs1009 allele result in increased frameshifting in the yef3-fs1009 mbf1-R89K mutant, but have no effect in YEF3 mbf1-R89K strains. GFP/RFP expression from the (CGA)4+1 reporter was examined in YEF3 and yef3-fs1009 strains bearing CEN plasmids with no insert (EV), YEF3 (wt), or yef3-fs1009 (m). (E) The yef3-fs1009 mutation does not suppress programmed frameshifting in TRM140 mRNA. (**) P < 0.01 ≥ 0.001. (***) P < 0.001.
Since the carboxy-terminal domain of eEF3 itself is not essential (Anand et al. 2006; Andersen et al. 2006), we considered that mutant phenotypes might be caused by reduced eEF3 protein levels. Indeed, we found a reduction in both antibody-reactive eEF3 in the mutant, to 60% of that in wild-type (Fig. 2C) and an apparent reduction in size and intensity of a Coomassie-stained band likely corresponding to eEF3 (Supplemental Fig. S3A). The antibody-reactive eEF3 likely sets a lower limit for mutant eEF3 protein levels as the polyclonal antibody could recognize a carboxy-terminal epitope not present in the mutant protein.
To determine if the frameshifting suppression observed in the yef3-fs1009 mutant was due to limiting amounts of eEF3 protein, we examined the effect of increased expression of the mutant and wild-type eEF3 on frameshifted GFP/RFP and on eEF3 levels. As expected, expression of wild-type YEF3 from a LEU2 CEN plasmid in the yef3-fs1009 mutant nearly completely restored expression of the frameshifted reporter from 1.3 GFP/RFP with the empty vector to 5.6 GFP/RFP (a 4.3-fold increase), 95% of that in a YEF3 wild-type strain with an empty vector (Fig. 2D), confirming that the mutant is recessive. In contrast, increased expression of yef3-fs1009 to levels exceeding those in wild-type (Supplemental Fig. S3B) had a much reduced effect, resulting in 2.4 GFP/RFP (a 1.8-fold increase relative to the vector control), 41% of that in a YEF3 wild-type strain with an empty vector (Fig. 2D). We conclude that the effects of the yef3-fs1009 mutant are due to two effects, one that is due to reduced expression and a separate effect that is due to reduced function (and cannot be restored with increased amounts).
Since the E site at which eEF3 acts (Triana-Alonso et al. 1995; Ranjan et al. 2021) has been implicated in programmed frameshifting (Marquez et al. 2004; Sanders and Curran 2007; Devaraj et al. 2009), we considered that the yef3-fs1009 mutant might generally affect different classes of frameshifting. To test this idea, we compared the frameshifting efficiency of two native yeast +1 frameshifting signals, TRM140 and TY1, in wild-type yeast and the yef3-fs1009 mutant. While the site of frameshifting at these two sites is identical, CUU–AGG–C (Belcourt and Farabaugh 1990; Asakura et al. 1998; Farabaugh et al. 2006), the TRM140 site is a remarkably efficient frameshifting signal (D'Silva et al. 2011), perhaps due to sites upstream of the frameshift at which ribosomes collide (Meydan and Guydosh 2020). We observed highly efficient TRM140 frameshifting in both wild-type and yef3-fs1009 mutant strains based on frameshifted GFP/RFP levels relative to in-frame expression, 68.5 compared to 194.1 in wild-type (35%) and 49.5 compared to 158.2 in the mutant (31%) (Fig. 2E). Thus, there was little difference in relative frameshifting between YEF3 wt and the yef3-fs1009 mutant. Similarly, there was little difference in frameshifted GFP/RFP with the TY1 signal although the level of frameshifted protein was much less than with TRM140 (Supplemental Fig. S3C). In addition, we tested frameshifting efficiency at a −1 HIV-1 frameshifting site because the E site tRNA has been specifically implicated in frameshifting efficiency here (Leger et al. 2007). However, frameshifting at this site is not affected in the yef3-fs1009 mutant (Supplemental Fig. S3D). Thus, suppression of frameshifting at CGA codon repeats by this mutant form of eEF3 is unlikely due to an inherent defect in frameshifting ability.
eEF3 modulates frameshifting caused by defects in either Mbf1 or ribosomal protein S3
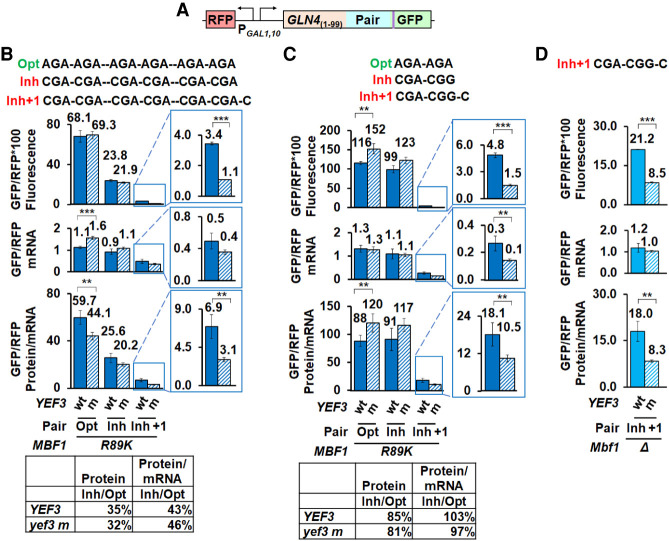
Reading frame maintenance at collided ribosomes depends upon the extraribosomal protein Mbf1 and its interaction with uS3 in the colliding ribosome, as mutations in RPS3 (encoding yeast uS3) that affect this interface result in frameshifting (Wang et al. 2018; Juszkiewicz et al. 2020a; Sinha et al. 2020; Pochopien et al. 2021). To ascertain the nature of the suppression by the yef3-fs1009 mutant, we examined both the types of frameshifting mutations suppressed by yef3-fs1009 as well as the effects of yef3-fs1009 on the levels of frameshifted protein (GFP fluorescence), mRNA and the ratio of GFP to mRNA from the GLN4(1–99)-(CGA)4+1-GFP reporter (Fig. 3A). To this end, we examined the ability of the yef3-fs1009 mutation to suppress frameshifting in four mutants: mbf1-R89K, mbf1Δ, RPS3-K108N and RPS3-S104Y (Fig. 3B–E).
FIGURE 3.
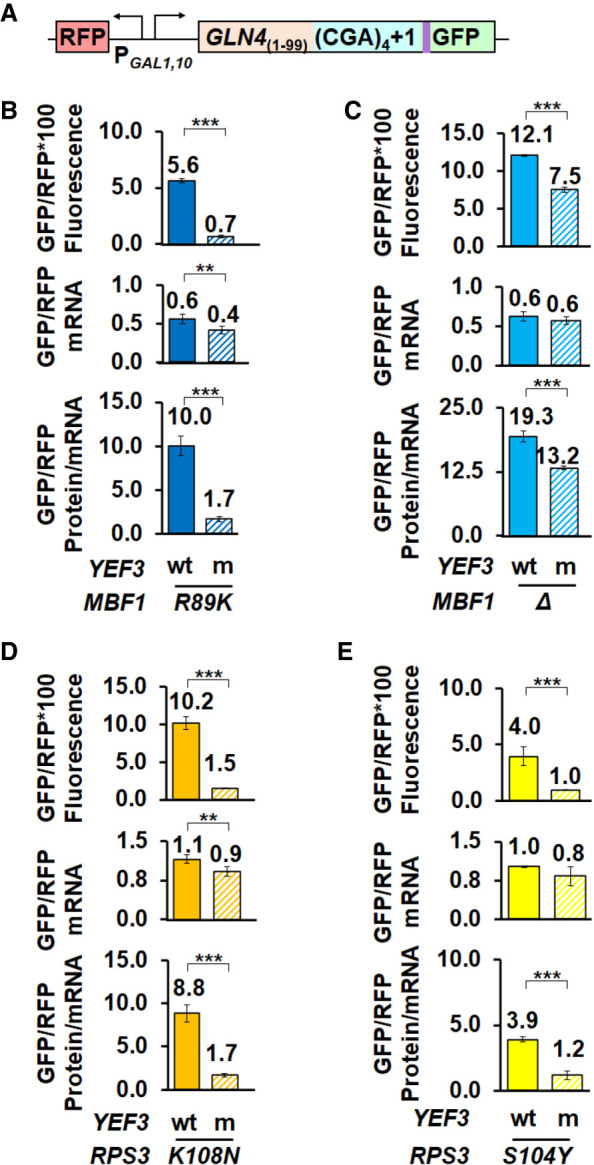
The yef3-fs1009 mutation suppresses frameshifting at CGA codon repeats when the frame quality control system is compromised by defects in MBF1 or RPS3. (A) Schematic of RFP and GLN4(1–99)-(CGA)4 + 1-GFP reporter used in these analyses. (B–E) The yef3-fs1009 mutation suppresses frameshifting caused by the mbf1-R89K mutation (B), by deletion of MBF1 (C), by the RPS3-K108N mutation (D) or by the RPS3-S104Y mutation (E). In each case, the yef3-fs1009 mutation results in significantly reduced levels of both frameshifted protein and protein per mRNA and in two cases results in small but significant reductions in mRNA. (**) P < 0.01 ≥ 0.001. (***) P < 0.001.
The yef3-fs1009 mutant suppressed frameshifting caused by mutations in either MBF1 or RPS3, albeit with some differences in effectiveness. The yef3-fs1009 mutant had major effects on frameshifted protein per mRNA for each of the three-point mutations (mbf1-R89K, RPS3-K108N, and RPS3-S104Y). In the mbf1-R89K strains, the yef3-fs1009 mutation resulted in a 5.9-fold reduction in frameshifted GFP/RFP per mRNA (10.0 to 1.7) (Fig. 3B). Similarly, in the RPS3-K108N and RPS3-S104Y strains, the yef3-fs1009 mutation resulted in 5.2- and 3.3-fold reductions in frameshifted GFP/RFP per mRNA (8.8 to 1.7 and 3.9 to 1.2) (Fig. 3D,E). In contrast, in mbf1Δ strains, the yef3-fs1009 mutation had a much smaller 1.5-fold effect on frameshifted GFP/RFP (19.3 in the YEF3 mbf1Δ strain compared to 13.2 in the yef3-fs1009 mbf1Δ strain) (Fig. 3C), suggesting that Mbf1 presence is required to inhibit eEF3-mediated frameshifting. We note that in most cases, the yef3 mutant also exhibited a small (but in some cases significant) reduction in mRNA levels (Fig. 3B–E), which could indicate increased mRNA decay. However, in no case did the reduction in mRNA account for the reduced amount of frameshifted protein. Thus we conclude that yef3-fs1009 suppressed ribosomal frameshifting at CGA codon repeats in a manner independent of the identity of the gene or particular mutation in that gene that allowed frameshifting. The reduced effectiveness of the yef3 mutant in the complete absence of Mbf1 protein is consistent with the idea that Mbf1 functions to prevent eEF3 from assisting frameshifting of the stalled ribosome in a collided ribosome complex (see below).
eEF3 has specific effects on frameshifting, rather than CGA inhibition
eEF3 acts in each cycle of translation and its depletion in yeast cells altered both the rate-limiting step in translation and codon discrimination (Ranjan et al. 2021). Thus, we considered that the yef3-fs1009 mutant could exert its effects by altering the stall at CGA–CGA codon pairs, or by altering overall ribosome availability and thus impacting the frequency of the ribosome collisions that lead to both inhibition and frameshifting (Simms et al. 2017a; Simms et al. 2019). To test these possibilities, we examined the effects of the yef3-fs1009 mutant on in-frame expression of reporters with inhibitory (CGA–CGA) codon pairs and the corresponding optimal (AGA–AGA) codon pairs. We expected to observe a reduction in CGA inhibition in the mutant strain, if either the stall or collisions at CGA–CGA codon pairs were reduced in the yef3-fs1009 mutant. We might observe a reduction in expression of the optimal reporter, if the overall rate of initiation was reduced in the yef3-fs1009 mutant. We performed these experiments in reconstructed strains bearing mbf1-R89K mutations to assess frameshifting of a related reporter in parallel.
We found little difference between the yef3-fs1009 and wild-type YEF3 strains in the expression of either the inhibitory or optimal in-frame reporters (Fig. 4A,B). CGA inhibition as measured by GFP levels from the CGA reporter relative to those from the AGA reporter were 35% in theYEF3 wild-type strain (23.8 to 68.1 GFP/RFP fluorescence) and 32% in the yef3-fs1009 strain (21.9 to 69.3 GFP/RFP fluorescence) (Fig. 4B); similarly, GFP/RFP protein per mRNA levels were 43% (25.6 to 59.7) and 46% (20.2 to 44.1), respectively. As expected, the yef3-fs1009 mutation suppressed frameshifting in the GLN4(1–99)-(CGA–CGA)3 +1-GFP reporter (6.9 to 3.1 GFP/RFP protein per mRNA) (Fig. 4B). Moreover, frameshifting in both YEF3+ and yef3-fs1009 mutant is far less efficient than in-frame read through (11.6% in YEF3+ and 7.0% in yef3 mutant protein/mRNA). We verified that CGA inhibition was also substantial in yef3-fs1009 strains bearing either wild-type MBF1 (Supplemental Fig. S4A) or mbf1Δ (Supplemental Fig. S4B). Thus, CGA–CGA codon pairs are inhibitory in the yef3-fs1009 strains, suggesting that ribosomes stall and collide in the mutant strain, consistent with a specific defect in frameshifting caused by the mutation.
FIGURE 4.
The yef3-fs1009 mutation suppresses frameshifting with two different inhibitory codon combinations, but has only small effects on in-frame expression of reporters with optimal or inhibitory codons. (A) Schematic of RFP and GLN4(1–99)-codon insert-GFP reporters used in these analyses. (B) The yef3-fs1009 mutation suppresses frameshifting in a mbf1-R89K mutant bearing a reporter with three copies of CGA–CGA codon pairs but does not relieve or enhance CGA inhibition from in-frame reporters. Levels of GFP/RFP protein (fluorescence), mRNA and protein/mRNA are similar from in-frame reporters with optimal (AGA–AGA) or inhibitory (CGA–CGA) codon pairs, but levels of protein and protein/mRNA levels are significantly different from the analogous frameshifted reporter with a (CGA–CGA)3 +1 insert. In-frame expression of inhibitory (CGA) reporter relative to optimal (AGA) reporter is shown below. (C) The yef3-fs1009 mutation suppresses frameshifting in an mbf1-R89K mutant with a reporter bearing a single CGA–CGG inhibitory pair, but does not affect CGA–CGG inhibition. In-frame expression of inhibitory (CGA–CGG) reporters relative to optimal (AGA–AGA) reporters is similar in YEF3 and the yef3-fs1009 mutant strains (table), although levels of GFP/RFP protein and protein/mRNA from both in-frame reporters are greater in the yef3-fs1009 mutant. In contrast, both GFP/RFP protein and protein/mRNA levels from the frameshifted reporter are reduced in the yef3-fs1009 mutant. (D) The yef3-fs1009 mutation suppresses frameshifting in an mbf1Δ mutant with a reporter bearing a single CGA–CGG inhibitory pair. (**) P < 0.01 ≥ 0.001. (***) P < 0.001.
One might consider that the primary effect of the mutant eEF3 protein at CGA–CGA codon pairs is to allow ribosomes to abort translation rather than frameshift, since a large fraction of ribosomes fail to translate through the strongly inhibitory sequences (Matsuo et al. 2017; Sitron et al. 2017). To assess whether the mutant eEF3 really suppresses frameshifting or simply results in stalled ribosomes aborting translation, we examined the ability of the yef3-fs1009 mutant to suppress frameshifting at a high frameshifting site with minimal inhibitory effects. We had previously determined that ribosomes frameshift efficiently at a single CGA–CGG–C site (Wang et al. 2018). We found that this CGA–CGG–C site is minimally inhibitory, in that expression of the in-frame GFP/RFP protein with the inhibitory codon pair is 85% that of the reporter with the optimal codon pair in the mbf1-R89K YEF3+ strain (99.0 to 116 GFP/RFP fluorescence) and 81% in the mbf1-R89K yef3-fs1009 mutant (123 to 152 GFP/RFP fluorescence) (Fig. 4C) [compared to 35% (23.8 to 68.1) and 32% (21.9 to 69.3), respectively, with the (CGA–CGA)3 reporter (Fig. 4B)]. Nevertheless, we find that the frameshifted GFP/RFP protein at the CGA–CGG–C site is significantly reduced in the yef3-fs1009 mutant (1.5 GFP/RFP fluorescence) relative to the YEF3 wt (4.8 GFP/RFP fluorescence) (Fig. 4C). Suppression is still apparent in the frameshifted protein/mRNA, although it is clear that significant reduction in mRNA also occurred in the yef3-fs1009 mutant relative to the wild-type (Fig. 4C). We also examined frameshifting suppression at this site in a set of mbf1Δ mutants, since the original suppression in these mutants had been less robust. Again, we found that both frameshifted protein and protein per mRNA were significantly reduced in the yef3-fs1009 mutant (Fig. 4D). Thus, we infer that frameshifting suppression caused by this mutant eEF3 is likely not dependent upon aborting translation at a high rate.
Integrated stress response regulators Gcn1 and Gcn20 inhibit frameshifting at CGA codon repeats
To understand frameshifting during ribosome collisions, we previously obtained mutants that allow frameshifting at CGA codon repeats in wild-type strains and identified mutations in MBF1 and RPS3 (Wang et al. 2018). To identify additional genes in this process, we repeated the selection and screen for Ura+ GFP+ mutants in an MBF1 strain in which expression of both URA3 and GFP requires frameshifting (Supplemental Fig. S5A; Wang et al. 2018), but which also carried plasmid-borne copies of both MBF1 and ASC1 to avoid recessive mutations in these genes. Among the Ura+ GFP+ mutants that passed this screen, whole-genome sequencing yielded seven independent mutations in GCN1 (Supplemental Table S1). GCN1 encodes a key regulator of the Integrated Stress Response pathway (Garcia-Barrio et al. 2000; Sattlegger and Hinnebusch 2000; Hinnebusch 2005), known to bind to polyribosomes (with Gcn20) as an essential component of its activation of Gcn2, the eIF2α kinase that initiates the ISR (Marton et al. 1997; Sattlegger and Hinnebusch 2005). The role of Gcn1 in reading frame maintenance at collided ribosomes is particularly interesting for two reasons: (i) Gcn1 and Gcn20 bind collided ribosomes with Mbf1 (Pochopien et al. 2021), and (ii) Gcn1 and eEF3 share extensive homology in their ribosome binding domains (Marton et al. 1993). Gcn1 and eEF3 likely compete with each other for binding to the ribosome, based on both functional evidence that overproduction of eEF3 reduces activation of the ISR pathway (Visweswaraiah et al. 2012) and structural analyses showing similar interactions of the eEF3-like region of Gcn1 on the stalled ribosome and eEF3 with ES39S of 18S rRNA, eS19 and uS13 (Pochopien et al. 2021; Ranjan et al. 2021).
To study the role of Gcn1 in frameshifting at CGA codon repeats, we constructed gcn1Δ mutants and assayed frameshifting with our (CGA)4+1 frameshifting reporter (Fig. 5A). We observed an increase in frameshifted GFP/RFP in the gcn1Δ strains (from 0.5 to 1.9 GFP/RFP), but serendipitously discovered an amplification of frameshifting in RPS3-S104Y gcn1Δ mutants, a sixfold increase over that seen within the RPS3-S104Y single mutant (from 3.3 to 20.2 GFP/RFP) (Fig. 5B). The expression of GCN1 on a LEU2 CEN plasmid in the gcn1Δ or gcn1Δ RPS3-S104Y strains returned GFP/RFP expression of the frameshifted reporter to levels observed in the wild-type or RPS3-S104Y strains, but had little or no effect on the GFP/RFP in either wild-type or RPS3-S104Y strains (Fig. 5B). Similarly, overexpression of wild-type RPS3 partially suppressed frameshifting in strains with the RPS3-S104Y mutation, while expression of the RPS3-K108E mutated uS3 exacerbated frameshifting in all strains, particularly those with a gcn1Δ mutation (Supplemental Fig. S5B), providing additional evidence that the mutated uS3 protein is responsible for the enhanced frameshifting. We demonstrated that the gcn1Δ mutation resulted in an increase in frameshifting rates rather than stabilization of the mRNA, as levels of frameshifted protein per mRNA increased from 2.2 and 7.3 in the gcn1Δ and RPS3-S104Y single mutants to 25.7 in the gcn1Δ RPS3-S104Y double mutant (Supplemental Fig. S5C). We confirmed that Gcn1 and uS3 proteins have specific roles in frameshifting, by showing, as we did above for the yef3-fs1009 mutants, that the gcn1Δ and RPS3-S104Y mutants have little effect on CGA inhibition with in-frame reporters (Supplemental Fig. S5D).
FIGURE 5.
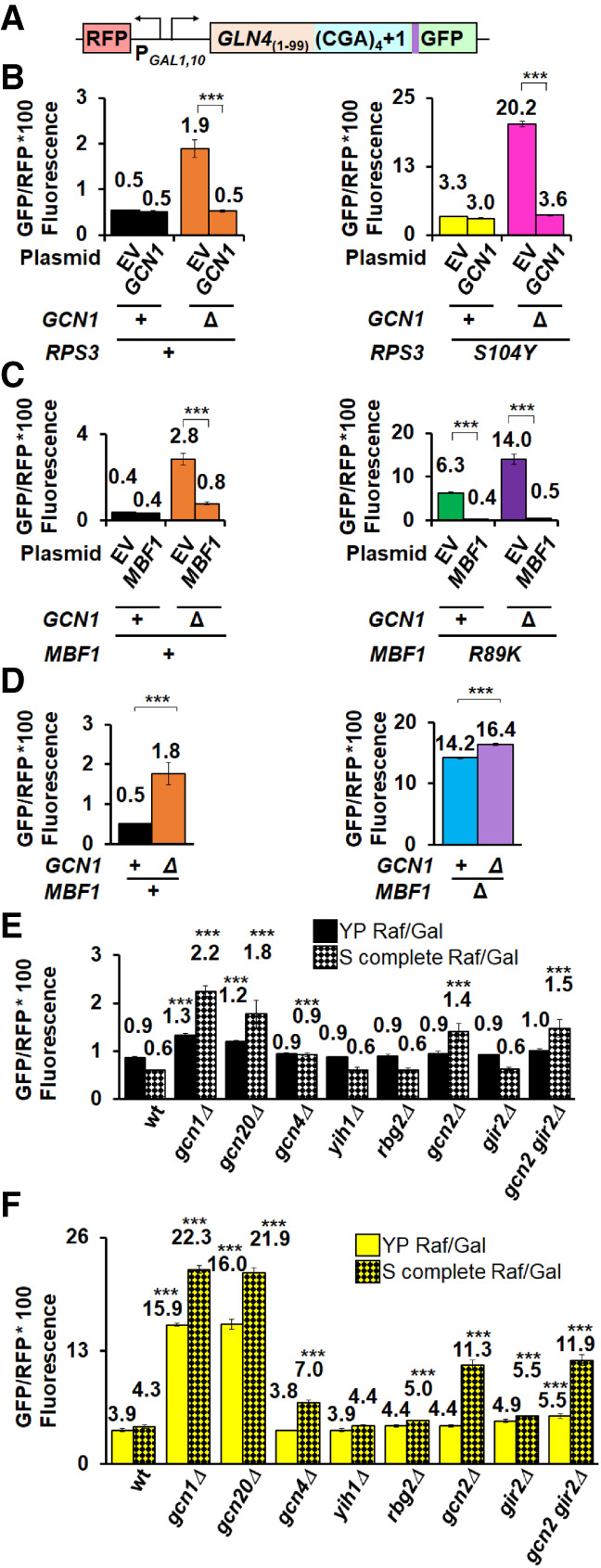
Gcn1 and Gcn20 antagonize frameshifting, but their effects are not mediated primarily by the ISR/GAAC pathway. (A) Schematic of RFP and GLN4(1–99)-(CGA)4 + 1-GFP reporter used in these analyses. (B) Deletion of GCN1 alone or in combination with the RPS3-S104Y mutation results in increased expression of the frameshifted reporter relative to appropriate parent strains. Expression of GCN1 suppresses the frameshifting in the gcn1Δ mutant and in the gcn1Δ RPS3-S104Y double mutant but has no effect on frameshifting in the RPS3-S104Y single mutant. (C) Deletion of GCN1 combined with the mbf1-R89K mutation results in increased expression of the frameshifted reporter relative to either single mutation. Expression of MBF1 suppresses frameshifting in both the mbf1-R89K mutant and in the gcn1Δ mbf1-R89K double mutant. (D) Deletion of GCN1 has only minor effects on the expression of the frameshifted reporter in combination with a deletion of MBF1. (E,F) Deletion of either GCN1 or GCN20 in a wild-type (E) or RPS3-S104Y mutant (F) results in increased expression of the frameshifted reporter when cells are grown in rich (solid bars) or minimal media (checkered bars). Deletions of either GCN2 or GCN4, encoding components of the ISR pathway, result in increased frameshifting only when cells are grown in minimal media (checkered bars). Deletions of other genes encoding proteins that modulate the ISR pathway (YIH1) or interact with Gcn1 on the collided ribosome (GIR2, RBG2) have minimal effects on frameshifting in both conditions. (***) P < 0.001.
Similarly, we observed increased frameshifting with gcn1Δ mbf1-R89K double mutants (14.0 GFP/RFP), compared to either single mutant (2.8; 6.3 GFP/RFP), which was similarly complemented by expression of MBF1 (Fig. 5C). In contrast, we observed a relatively small increase in frameshifting in gcn1Δ mbf1Δ mutants relative to mbf1Δ mutants alone (Fig. 5D). Thus, Mbf1 protein must be present for Gcn1 to effectively modulate frameshifting.
In the course of this analysis, we noted that effects of gcn1Δ on frameshifting were generally larger in minimal media than in rich media (Compare Fig. 5D to Supplemental Fig. S5E), although in all cases the effects of the gcn1Δ mutation were significant (P < 0.001). Thus, the selection for frameshifting mutants on minimal media lacking uracil may have facilitated the detection of the gcn1 mutations.
Since Gcn1 is a key component of the ISR pathway, we examined the effects of other components in this pathway to find out if frameshifting is modulated by induction of the pathway. Induction of the ISR pathway involves Gcn1 and Gcn20-dependent activation of the Gcn2 kinase, which in turn phosphorylates eIF2α, reducing translation initiation and causing induction of the Gcn4 transcriptional regulator, which modifies expression of more than 500 yeast genes (Jia et al. 2000; Natarajan et al. 2001). Moreover, two other proteins interact with Gcn1 on collided ribosomes: Gir2, a competitor of Gcn2, and Rbg2, a ribosome binding GTPase, (Wout et al. 2009; Pochopien et al. 2021). If frameshifting depends predominantly upon the induction of the ISR pathway, we expected that deletion of some of these components alone or in combination with an RPS3-S104Y mutation would yield an increase in frameshifting. We measured frameshifted GFP/RFP in both wild-type and RPS3-S104Y mutants with deletions in various ISR components (Fig. 5E,F). We found that deletions of GCN1 or GCN20 resulted in significantly increased expression of the frameshifted reporter alone or in combination with the RPS3-S104Y mutation in both rich and minimal media (Fig. 5E,F), and that effects of gcn20Δ were complemented by a plasmid-borne copy of GCN20 (Supplemental Fig. S5F). Deletion of the other two genes encoding proteins (Gir2 or Rbg2) that bind the leading stalled ribosome had only small effects on frameshifting (Fig. 5E,F). Surprisingly, deletion of GCN2 or GCN4 had little or no effect (with or without the RPS3-S104Y mutation) in rich media, but did result in some increase in frameshifting (both with or without the RPS3-S104Y mutation) in minimal media (Fig. 5E,F); these effects were generally smaller than those seen with deletions of GCN1 or GCN20. We also examined the effects of a combined deletion of GIR2 and GCN2 since these two proteins are thought to compete for ribosome access (Wout et al. 2009), but their combined deletion had no greater effect on the expression of the frameshifted reporter than the gcn2Δ mutant (Fig. 5E,F). Thus, we conclude that Gcn1 and Gcn20 exert their effects on frameshifting primarily through the complex on the ribosome, but that the induction of the ISR pathway assists in reading frame maintenance under some circumstances.
Gcn1 and Hel2 quality control components constrain eEF3-dependent frameshifting
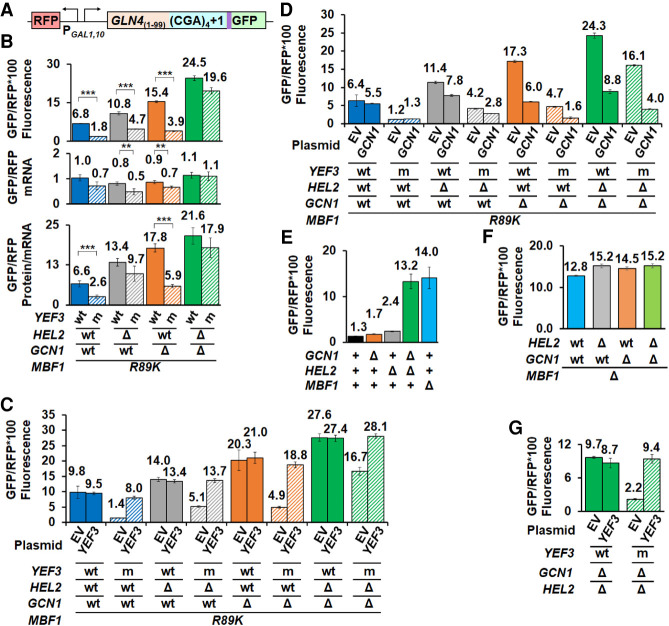
We considered that Gcn1 and Hel2 might compete with eEF3 for access to the collided ribosome, based on the evidence of functional and physical competition between Gcn1 and eEF3 (Visweswaraiah et al. 2012; Pochopien et al. 2021; Ranjan et al. 2021) as well as evidence of functional competition between the Gcn1 ISR and Hel2 NGD pathways, such that an increase in activation of the ISR occurs if Hel2 is missing (Meydan and Guydosh 2020; Yan and Zaher 2021). Specifically, we thought that if Hel2 and Gcn1 do constrain frameshifting by impeding eEF3, the mutant eEF3 protein might not compete well with either one or both of Hel2 and Gcn1. If so, we expected that efficient frameshifting would be restored in the mbf1-R89K yef3-fs1009 mutant if the appropriate regulator (Hel2 or Gcn1) was inactivated. To this end, we constructed mbf1-R89K hel2Δ and mbf1-R89K gcn1Δ single mutants and mbf1-R89K hel2Δ gcn1Δ double mutants in YEF3 and yef3-fs1009 strains and assessed frameshifting in these strains (Fig. 6A,B). Indeed, we found that levels of frameshifted protein in the mbf1-R89K hel2Δ gcn1Δ yef3-fs1009 mutant were both high (19.6 GFP/RFP) and fairly similar to that in the corresponding mbf1-R89K hel2Δ gcn1Δ YEF3 strain (24.5 GFP/RFP) (80%) (Fig. 6B). In contrast, in the mbf1-R89K HEL2+ GCN1+ yef3-fs1009 parent mutant, frameshifting was low (1.8 GFP/RFP) and only 26% that of its corresponding YEF3 strain (1.8 to 6.8 GFP/RFP). Thus, ribosomes using this mutant eEF3 can frameshift if Gcn1 and Hel2 are removed.
FIGURE 6.
eEF3 competes with Hel2 and Gcn1 to regulate frameshifting at CGA codon repeats. (A) Schematic of RFP and GLN4(1–99)-(CGA)4 + 1-GFP reporter used in these analyses. (B) The suppression of frameshifting due to the yef3-fs1009 mutation is nearly lost in mutants lacking both HEL2 and GCN1. Similar effects are observed with removal of HEL2 alone. (C,D) Complementation of mutants with plasmid-born YEF3 (C) or GCN1 (D). (E) High levels of frameshifting are observed in hel2Δ gcn1Δ mutants despite the presence of a functional MBF1 gene. (F) The high level of frameshifting in strains lacking Mbf1 is not substantially affected by deletion of HEL2 (gray), GCN1 (orange), or HEL2 and GCN1 (green). (G) The yef3-fs1009 mutation suppresses the high levels of frameshifting in the gcn1Δ hel2Δ mutant. Frameshifting suppression in the yef3-fs1009 mutant is complemented with addition of a CEN plasmid bearing YEF3.
We also note that Hel2 appears to play a larger role in the competition than Gcn1. The deletion of HEL2 resulted in an increase in the relative frameshifted protein in the mbf1-R89K hel2Δ yef3-fs1009 mutant to 43% (compared to 26%) that of the corresponding YEF3 strain (4.7 to 10.8 GFP/RFP) (Fig. 6B). Furthermore, the hel2Δ mutants also displayed differences in mRNA levels that further increased the apparent frameshifting per mRNA in the mbf1-R89K hel2Δ yef3-fs1009 mutant [9.7 to 13.4 (GFP/RFP fluorescence)/(GFP/RFPmRNA)] (Fig. 6B). In contrast, deletion of GCN1 resulted in no increase in the relative frameshifted protein (26%; 3.9 to 15.4 GFP/RFP), but these interpretations are complicated because the gcn1Δ mutants also display significantly higher levels of frameshifting than the parents.
Standard complementation experiments revealed unexpected insights into relationships among these systems (Fig 6C,D; Supplemental Fig. S6A,B). As expected, the expression of wild-type YEF3 in all strains with the yef3-fs1009 mutation resulted in increased frameshifting to similar levels as the corresponding YEF3 strain (Fig. 6C). Expression of either GCN1 or HEL2 resulted in reduced frameshifting in the corresponding gcn1Δ or hel2Δ mutants (Fig. 6D; Supplemental Fig. S6A). Surprisingly, the expression of GCN1 also reduced frameshifting in hel2Δ mutants (from 11.4 to 7.8 GFP/RFP), but not in wild-type (Fig. 6D); likewise, expression of HEL2 reduced frameshifting in gcn1Δ mutants (from 17.3 to 13.4 GFP/RFP) (Supplemental Fig. S6A). The apparent cross complementation is consistent with a strong relationship between these pathways, as suggested by previous results (Meydan and Guydosh 2020; Yan and Zaher 2021). Perhaps most surprisingly, we found that while expression of wild-type MBF1 fully suppressed frameshifting in HEL2 GCN1 or single mutant strains, expression of MBF1 only partially suppressed frameshifting in gcn1Δ hel2Δ mutants bearing either YEF3 wild-type or the yef3-fs1009 mutation (Supplemental Fig. S6B).
Given the poor suppression of frameshifted protein by MBF1 wild-type in the gcn1Δ hel2Δ mbf1-R89K mutants (Supplemental Fig. S6B), we considered the possibility that Mbf1 requires the function of either Hel2 or Gcn1 to work efficiently and thus is unable to prevent frameshifting when neither Hel2 nor Gcn1 is present. We tested this idea and indeed found very high levels of frameshifting in a gcn1Δ hel2Δ MBF1 strain, similar to those in an mbf1Δ mutant (Fig. 6E). Thus, we infer that either Mbf1 requires the action of Hel2 or Gcn1 to maintain the reading frame; or that Hel2 and Gcn1 have an independent function in reading frame maintenance. If Hel2 and Gcn1 have independent roles from Mbf1, we would expect that frameshifting in a gcn1Δ hel2Δ mbf1Δ strain would substantially exceed that in an mbf1Δ strain (i.e., at least an additive increase in frameshifting). This is not true (Fig. 6F); frameshifting in the triple mutant is only slightly greater (15.2 GFP/RFP) than in the single mutant (12.8 GFP/RFP). However, the effects of Hel2 and Gcn1 on frameshifting can still be observed in the mbf1Δ yef3-fs1009 mutant, as we observed that either a hel2Δ or gcn1Δ mutation eliminated all effects of the yef3-fs1009 suppressor on frameshifting in the mbf1Δ mutant (Supplemental Fig. S6C). Thus, Mbf1 requires either the Gcn1 complex or Hel2 to prevent eEF3-dependent frameshifting.
To find out if Mbf1 alone antagonizes eEF3 in the absence of Gcn1 and Hel2, we asked if frameshifting in a gcn1Δ hel2Δ MBF1 strain was modulated by YEF3. Indeed, this yef3-fs1009 mutant strain exhibited reduced levels of frameshifted GFP/RFP (2.2 compared to 9.7), which was restored by the expression of wild-type YEF3 (Fig. 6G). Overall, we infer that Hel2, Gcn1, and Mbf1 each constrain the frameshifting driven by eEF3 on collided ribosomes, with each regulator setting off distinct events, and that Mbf1 relies on Hel2 or Gcn1 to act on and remove ribosomes that would otherwise frameshift. Moreover, eEF3 is integral to the frameshifting event.
DISCUSSION
We showed here that frameshifting at collided ribosomes requires functions of the general translation elongation factor eEF3 and is restrained by multiple aspects of the quality control systems, including not only Mbf1, but also the ISR/GAAC regulators Gcn1 and Gcn20, and the NGD regulator Hel2. We deduce that wild-type eEF3 protein is required for frameshifting at CGA codon repeats, based primarily on the finding that the yef3-fs1009 mutation in the gene encoding eEF3 suppresses frameshifting at CGA codon repeats when Mbf1 is defective. We infer that eEF3 has a specific role in frameshifting, rather than simply mediating its effects on frameshifting through effects on ribosome stalls or collisions, based on two observations. First, the yef3-fs1009 mutant does not affect CGA–CGA inhibition, an argument that the yef3-fs1009 mutant specifically affects frameshifting, rather than the ribosome collisions or stalls that are necessary for both frameshifting and CGA–CGA inhibition (Letzring et al. 2013; Simms et al. 2017b, 2019; Sitron et al. 2017). Second, the yef3-fs1009 mutant also suppresses frameshifting at a site that produced little overall inhibition of expression, an argument that strong inhibition is not required for the yef3-fs1009 mutant's effects. We infer that eEF3 effects do not absolutely depend upon any specific quality control component that inhibits frameshifting, as the yef3-fs1009 mutant suppressed frameshifting in mutants that were simultaneously defective in two of the three quality control regulators (hel2Δ gcn1Δ and mbf1-R89K gcn1Δ double mutants). Finally, we argue that the yef3-fs1009 mutant's effects are not due to a specific interaction with the Mbf1-R89K protein, since the yef3-fs1009 mutant suppressed frameshifting caused by different defects in either Mbf1 or uS3. Thus, the general elongation factor eEF3 is specifically required for frameshifting at collided ribosomes. Our results provide the first evidence of a direct involvement of the general translation apparatus in the events occurring when ribosomes collide, and the first evidence of a unique role for eEF3 in ribosome collisions.
The involvement of eEF3 in frameshifting in yeast may help to explain differences between yeast and humans in the magnitude and directionality of frameshifting during ribosome collisions (Juszkiewicz et al. 2020a), since mammals do not have an eEF3 homolog (Belfield et al. 1995; Mateyak et al. 2018). For example, if mammals do have a protein or RNA that assists with the removal of the E site tRNA, that component may not participate in frameshifting during ribosome collisions.
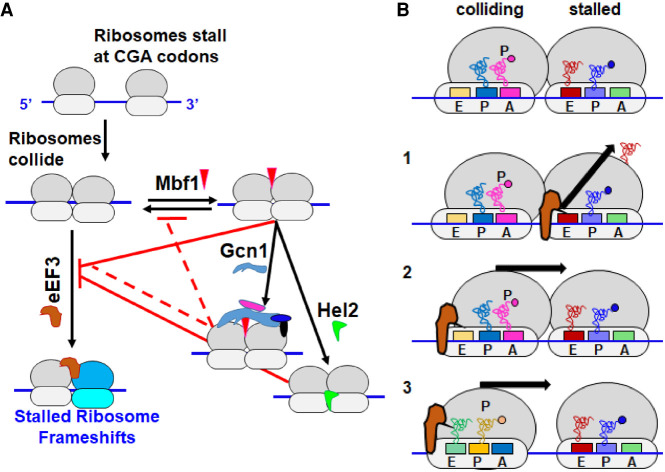
The relationships between different pathways activated by ribosome collisions are complex, exhibiting redundancy and competition. Our results are consistent with a system with opposing forces in which eEF3 acts to promote frameshifting on collided ribosomes lacking Mbf1, while Mbf1 holds the mRNA and 40S head with Gcn1 and Gcn20 (Sinha et al. 2020; Pochopien et al. 2021) and Hel2 works to remove the stalled ribosome. The interplay between these components is illustrated in the model in Figure 7A. We showed here that frameshifting is constrained not only by Mbf1, but also by the ISR components Gcn1 and Gcn20, as well as the NGD regulator Hel2. We infer that either Gcn1 or Hel2 function is required to maintain the reading frame in MBF1+ cells as ribosomes frameshift efficiently in hel2Δ gcn1Δ MBF1+ mutants in which Mbf1 is present. We infer that Gcn1 and Hel2 act downstream from Mbf1 to support its function in reading frame maintenance as frameshifting in an mbf1Δ mutant is nearly as robust as in the triple mbf1Δ gcn1Δ hel2Δ mutant. However, Mbf1 does act even in the absence of HEL2 and GCN1 to suppress the frameshifting in the yef3-fs1009 mutant, implying that Mbf1 is present and functional in this mutant. We infer that the effects of eEF3 are held in check by the combined actions of Mbf1, Hel2, and Gcn1/Gcn20, based on the observations that either removal of MBF1 or mutations in all three regulators (mbf1-R89K hel2Δ gcn1Δ) result in a large increase in frameshifting in the yef3-fs1009 mutant. The redundancy in the three sets of regulators (Mbf1, Gcn1, and Hel2) that all work to restrain frameshifting at the collided ribosome demonstrates the extensive coordination between the translational quality control systems, which allows a plasticity of the response dependent upon the particular problem.
FIGURE 7.
Models of eEF3 functions at collided ribosomes. (A) Mbf1 and other quality control regulators oppose eEF3-mediated frameshifting at collided ribosomes. We propose that eEF3 can act on collided ribosomes prior to Mbf1 binding, but that Mbf1 interaction prevents the action of eEF3. Gcn1/Gcn20/Gir2/Rbg2 binding to the Mbf1-bound collided ribosome (Pochopien et al. 2021) likely assists Mbf1 or blocks the interaction of eEF3 due to homologous ribosome binding sites (Marton et al. 1993; Visweswaraiah et al. 2012). Hel2-mediated ubiquitination results in disassembly of the stalled ribosome resulting in depletion of the pool of collided ribosomes. Gcn1 and Hel2 also prevent disassociation of Mbf1 from the collided ribosome, an event which might allow eEF3 to access this ribosome and promote frameshifting. (B) Three models for the mechanisms by which eEF3 may induce frameshifting on collided ribosome. Model 1: eEF3 could effect dissociation of the E site tRNA from the stalled ribosome. Model 2: eEF3 could bind the hybrid collided ribosome to finish the translocation reaction into a POST state, which would increase the force on the mRNA. Model 3: eEF3 could be responsible for driving the collided ribosomes into close contact, closing the gap that traps the colliding ribosome in the hybrid state.
The molecular role of eEF3 in translation informs speculation about the likely defect in eEF3 function caused by the yef3-fs1009 mutation. eEF3 participates in the translocation reaction and in removal of the E site tRNA, based on biochemical, structural and ribosome profiling analysis (Triana-Alonso et al. 1995; Ranjan et al. 2021), and has also been implicated in coupling between the exit of the tRNA from the E site and the delivery of tRNA to the A site of the ribosome (Uritani and Miyazaki 1988; Kamath and Chakraburtty 1989; Triana-Alonso et al. 1995; Anand et al. 2003). If the yef3-fs1009 mutation primarily results in a reduction in the effective amount of functional eEF3 protein, we might expect the mutant to display defects in these activities. The proposal that the mutation affects the amount of functional protein is consistent with observations that the mutation is recessive, confers sensitivity to translational inhibitors, and is at least partially rescued by increased expression of the mutant form of the protein. However, one alternative is that the effects of the yef3-fs1009 mutation are due to the loss of the conserved carboxy-terminal domain reported to have ribosome binding activity (Kambampati and Chakraburtty 1997) and possibly to interact with eEF1A (Anand et al. 2003, 2006); if so, the mutant might primarily affect a specific interaction between eEF3 and collided ribosomes.
We think there are three reasonable models for the proposed role of eEF3 in promoting frameshifting (Fig. 7B). First, eEF3 might work to cause ejection of the E site tRNA from the stalled ribosome, resulting in a ribosome with a single P site tRNA which itself has weak base-pairing interactions. In the yeast collided ribosome structure on the CGA–CCG stalling reporter, the stalled ribosome is found in the POST state with tRNAs in the P and E site (Ikeuchi et al. 2019). eEF3 binds tightly to ribosomes in the POST state and promotes the ejection of the E site tRNA (Ranjan et al. 2021). Thus, this model is based on the known activities of eEF3, and it is easy to envision that the extent of frameshifting would depend upon the fraction of stalled ribosomes in which the E site tRNA has been ejected. Second, eEF3 could act on the colliding ribosome to drive it into the leading stalled ribosome and create additional strain on the mRNA. The colliding ribosome has been found in the hybrid state with A/P and P/E tRNAs in an incomplete translocation step and the idea is that the stalled ribosome prevents it from completing translocation (Ikeuchi et al. 2019), but this ribosome, which lacks eEF2, may be an excellent substrate for eEF3 (Ranjan et al. 2021). Third, eEF3 could act on the colliding ribosome just prior to the actual collision to drive it into closer approximation to the stalled ribosome. We note that Meydan and Guydosh (Meydan and Guydosh 2020) found both 58 and 61 nucleotide (nt) disome footprints that differ at their 5′ ends, suggesting different spacing between collided ribosomes. One possibility is that Hel2 and Gcn1 can bind the more widely spaced disomes, but that these disomes do not drive efficient frameshifting because there is less tension on the mRNA in the wider configuration. eEF3, as part of its normal function in elongation, could drive the collided and stalled ribosomes into close approximation (the 58 nt disome) and this close configuration might drive frameshifting.
We also found evidence that key regulators of the ISR pathway Gcn1 and Gcn20, which bind collided ribosomes with Mbf1 (Pochopien et al. 2021), play a role in reading frame maintenance. To our knowledge, the reading frame maintenance role of Gcn1 and Gcn20 is the only known case in which their function is not completely tied to ISR induction. The effects of deleting GCN1 depend on the status of Mbf1 (or uS3), as deletion of GCN1 has relatively small effects on frameshifting when Mbf1 and uS3 are functional and has much greater effects on frameshifting when Mbf1 or uS3 are compromised, but has no additional effect on frameshifting when Mbf1 is removed. Moreover, Gcn1 has no direct contacts with Mbf1, although the architecture of the collided ribosomes containing Gcn1 (and its binding partners) is more compact than that of the collided ribosomes stalled on CGA–CCG (Pochopien et al. 2021). A parsimonious explanation for all of these data is that while Mbf1 is essential to prevent frameshifting, the effects of Gcn1 (and its binding partners) on the overall architecture of the collided ribosome either stabilize Mbf1 or facilitate its function on the collided ribosome. Our initial expectation was that the role of Gcn1/Gcn20 might be to compete directly with eEF3 since there is evidence of such a competition, and eEF3 shares extensive homology with ribosome binding domains in Gcn1 and Gcn20 (Marton et al. 1993; Visweswaraiah et al. 2012). However, we did not find the expected increase in frameshifting in the yef3-fs1009 mutant when GCN1 or GCN20 were deleted.
The biological significance of putting Mbf1 into a functional unit with Gcn1, Gcn20, Rbg2, and Gir2 on the collided ribosome is not immediately obvious. We think it is not likely to be a coincidental pairing, since Mbf1 affects induction of the ISR (Takemaru et al. 1998), and Gcn1 (and Gcn20) affects frameshifting. One idea is that the relationship is used to measure the prevalence of collisions. In that light, we note that Mbf1 is far more abundant in cells (85,474 molecules per cell) than either Gcn1 or Gcn20 (9432 and 13,281 molecules per cell) (Kulak et al. 2014), an imbalance that could result in reduced ability to prevent frameshifting in cells in which the number of collisions exceeds the capacity of the ISR regulators. We speculate that just such a stochastic excess of collisions in minimal media explains the roles of Gcn2 and Gcn4 in these conditions. High levels of ribosome collisions would occur if cells experience a deficit in one metabolite, perhaps exceeding the capacity of Gcn1 (and Gcn20) and Hel2. Induction of the ISR pathway would then reduce initiation, reduce collisions and allow the existing Gcn1 and Gcn20 to prevent frameshifting. Failure to induce the ISR response (with gcn2Δ or gcn4Δ) might thus exacerbate the difficulties in reading frame maintenance at collided ribosomes.
Many aspects of the relationships between the translation machinery and the quality control systems remain to be investigated, including the extent to which different pathways are activated by distinct signals. For example, Yan and Zaher (2021) demonstrated that while ribosome collisions activate both the NGD and ISR pathways, induction of the ISR, but not NGD, is much more efficient with treatments that leave an empty A site in the stalled ribosome. Thus, the NGD and ISR pathways are activated in slightly different ways. One attractive possibility is that frameshifting is driven by a distinct subset of collided ribosomes, perhaps those in which the E site tRNA from the stalled ribosome has been ejected or those in which the collided ribosomes are in close apposition.
MATERIALS AND METHODS
Strains, plasmids, and oligonucleotides
Strains, plasmids, and oligonucleotides used in this study are listed in Supplemental Tables S2–S4. Parents for yeast strains used in this study were BY4741 (MATa his3Δ leu2Δ0 met15Δ0 ura3Δ0) or BY4742 (MATα his3Δ leu2Δ0 lys2Δ0 ura3Δ0) (Open Biosystems). RNA-ID reporters were constructed as previously described and integrated at the ADE2 locus, using a MET15 marker in MATa strains and an S. pombe HIS5 marker in MATα strains (Dean and Grayhack 2012; Wolf and Grayhack 2015; Gamble et al. 2016; Wang et al. 2018). The mbf1-R89K suppressor P15–30 was obtained from YJYW290 (MATa mbf1-R89K GLN4(1–99)-(CGA)6+1-URA3; RFP-GAL1,10 promoter-GLN4(1–99)-(CGA)4+1-GFP-MET15 [LEU2 ASC1]) (Wang et al. 2018).
To obtain Leu− derivatives of P15 and P15–30, cell cultures were grown overnight in YPD, diluted to achieve ∼400 cells/0.1 mL, plated onto YPD, incubated for 2 d at 30°C and replica plated to SD-Leu and YPD plates. Six Leu− colonies were isolated from each strain, streaked for single colonies on YPD and tested for growth on YPD, YPG, SD-Leu, SD-Ura, and SD complete plates at 30°C.
To construct yeast strains in which the 3′ end of the YEF3 coding sequence was replaced, we assembled integrating plasmids in which base pairs 1640–3135 of the YEF3 gene (wt or yef3 G1007V K1009 fs) extending to +305 in the 3′ UTR were fused to a selectable marker (kanR or K. lactis URA5), and then followed by 207 base pairs of the YEF3 3′ UTR. Plasmids bearing either wild-type YEF3 (pELB1306) or yef3-fs1009 (pELB1310) coding sequences (nt 1640 through nt 305 of YEF3 3′ UTR) were fused to a kanR marker in pLB1264, which was derived from pEJYW279, a modified Bluescript vector with a kanR marker (Wang et al. 2018) by cloning 207 bp of YEF3 3′ UTR (OLB239) into the NheI and NotI sites. The chromosomal YEF3 gene (YEF3 wild-type or yef3 G1007V K1009fs) was PCR amplified (oligos OLB236 and OLB247) and cloned into pELB1264 between XmaI and AatII to create pELB1306 (wt) and pELB1310 (yef3 G1007V K1009fs). These plasmids were digested with XmaI and NotI, followed by linear transformation into P15, P15–30, YJYW2578, and YLB5853. The YEF3 gene in the resulting yeast strains was sequence verified.
Similarly, plasmids bearing either wild-type YEF3 (pELB1274) or yef3-fs1009 (pELB1278) were assembled by fusion to K. lactis URA5 in pELB1258, which was derived from pECB1330 (a modified Bluescript vector with a K. lactis URA5 marker) by insertion of the first 207 base pairs of YEF3 3′ UTR (OLB239) between NotI and NheI. The chromosomal YEF3 gene (YEF3 wild-type and yef3 G1007V K1009fs) was PCR amplified from 1640 bp in the YEF3 coding sequence to 305 bases of 3′ UTR (oligos OLB235 and OLB237) and cloned into ELB1258 between MluI and SacI sites. Following MluI and Not1 digestion, YEF3 and yef3-fs1009 were integrated into BY4741 by linear transformation. Both the integrating plasmids and YEF3 alleles in the resulting Ura+ strains were sequence verified. FOA resistant isolates were selected to obtain strains in which the K. lactis URA5 marker was removed.
MBF1 alleles were introduced into Ura− derivatives of the YEF3 strains (YLB5691 YEF3 wt and YLB5715 yef3 G1007V K1009fs) by linear transformation of XmaI and NheI digested pEJYW279 (MBF1-HA), pEJYW344 (MBF1-StrepII) (Wang et al. 2018), and pELB1418 (mbf1-R89K-HA). To construct the mbf1-R89K-HA plasmid (pELB1418), base pairs 230–411 of the coding sequence from mbf1-R89K (OLB256) were cloned into the BamHI and AatII sites of pEJYW279. YEF3 strains with MBF1 deletions were constructed by PCR amplification of mbf1Δ:kanR with OEVN015 and OJYW125, followed by linear transformation into YLB5691 and YLB5715. The MBF1 alleles from these strains were verified by sequencing.
The plasmids YEF3 CEN LEU2 (EEVN250) and yef3-fs1009 CEN LEU2 (EEVN246) were constructed in two steps to insert the entire coding sequence of YEF3 (wt or yef3 G1007V K1009fs) with flanking sequences from −714 to +305. In the first step, a gene block (gbEP03) bearing sequences −714 to −652, restriction sites Mlu1 and Xba1, and sequences +245 to +305, followed by restriction site AatII were cloned into PstI and EcoRI sites of AVA581 (Alexandrov et al. 2006) to produce EEVN237. The YEF3 containing plasmids EEVN250 (wt) and EEVN246 (yef3 G1007V K1009fs) were constructed using Gibson Assembly (Gibson et al. 2009) of the MluI-XbaI digested EEVN237, a PCR product from −714 to base pair 226 in the YEF3 ORF (using oligonucleotides OEP152 and OEP153 to amplify BY4741 DNA) and Msc1-AatII digested ELB1314 (wt YEF3) or ELB1319 (yef3 G1007V K1009fs) to supply the 3286 bp YEF3 sequences from 166 in the YEF3 ORF through +305. Each clone (EEVN250 and EEVN246) was sequence verified.
The GCN1 CEN LEU2 plasmid EEVN129 was constructed in two steps to insert GCN1 with flanking sequences (−804 to +341) into the vector AVA581 (Alexandrov et al. 2006). In the first step, a gene block bearing sequences −804 to −342, restriction sites NruI and XmaI, and sequences 7980 in the GCN1 ORF to +341 in 3′ UTR was cloned into PstI and EcoRI sites of AVA581 (EEVN109). The GCN1 containing plasmid EEVN129 was obtained by gap repair in yeast, following transformation of BY4741 with NruI and XmaI digested EEVN109, plasmids were isolated using Zymoprep Yeast Plasmid Miniprep II kit, transformed into E. coli, and isolated by Qiagen minipreps. After verification of the presence of the GCN1 coding sequence by PCR (OEP063 and OEP064) and restriction digestion, plasmids were tested for functional complementation by transformation into YEVN1004 bearing gcn1Δ HIS3 and selection on 3-aminotriazole (Hilton et al. 1965; Klopotowski and Wiater 1965). The complementing clone EEVN129 was sequence verified.
Deletions of MBF1, HEL2, GCN2, GCN20, GCN4, YIH1, RBG2, and GIR2 were constructed by standard methods using the genomic yeast deletion collection (Giaever et al. 2002) or plasmid cassettes bearing resistance markers (Wach et al. 1994; Goldstein and McCusker 1999; Gueldener et al. 2002).
Selection for mutants which suppress frameshifting when MBF1 is defective and identification of mutations
FOA resistant (FOAR) mutants were selected from independent cultures of strains bearing one of six different mbf1 alleles which allow frameshifting: mbf1-R89K and mbf1-K64E mutations in YJYW290, and mbf1-R89G, mbf1-R61T, mbf1-I85T, and mbf1-S86P mutations in YJYW331 (Wang et al. 2018). Strains were grown overnight in three mL YPD at 30°C, harvested, washed twice with sterile water, and resuspended in 1 mL to OD600 0.7. Approximately two million cells were plated on SD-Ura plates containing 50 µg/mL uracil and 500 µg/mL of 5-fluoroorotic acid (FOA) (Boeke et al. 1987). The selection plates were grown at 30°C, 33°C, and 37°C for up to 9 d and several single colonies were initially picked at different times and temperatures. Single colonies of FOA resistant mutants were streaked onto SD-Ura plates containing 50 µg/mL uracil and 500 µg/mL of FOA. Single colonies from each streak were grown in YP Raf/Gal, spotted onto SD-Ura plates to determine if the mutants displayed an Ura− phenotype and then analyzed by flow cytometry to measure frameshifted GFP and RFP expression. Ura− mutants with GFP/RFP values <60% of the parent, GFP values <65% of the parent and RFP values <125% of the parent were considered likely frameshifting suppressors. Two independent mutants were selected for further study from each mbf1 allele, including from the P15 strain with the mbf1-R89K mutation.
To identify relevant mutations in mbf1-R89K (YJYW290-15) suppressor P15–30, whole-genome sequencing was performed on DNA isolated from approximately 30 OD600 yeast cells using Lucigen MasterPure Yeast DNA Purification kit (Lucigen catalog: MMPY80200) according to the manufacturer's directions. Purified DNA (200 µL TE, pH8) was treated with 2 µL RNase A (10 mg/mL stock) at room temperature for 1 h, followed by treatment with PCA (Invitrogen 15593-031), then precipitation and washing with ethanol. The pellet was dried and resuspended in 30 µL sterile dH2O. Whole-genome sequencing was performed by the UR Genomics Research Center.
Candidate mutations were initially identified from whole-genome sequencing by direct comparison with the whole-genome sequence of their parent strain, followed by exclusion of putative mutations in which the number of wild-type reads exceeded 10 reads or the number of wild-type reads exceeded the number of mutant reads. In the P15–30 mutant, this procedure resulted in nine mutations with passing scores in sequence quality and 15 putative mutations with low-quality reads. The five genes with candidate mutations that occurred in coding sequences and were not synonymous mutations were considered as likely candidates. YEF3 was prioritized for analysis as none of the other genes had obvious connections to translation (two dubious ORFs, one transcription factor and one recombination enzyme).
Analysis of yeast growth
Appropriate control strains and two to four independent isolates of each strain being tested were grown overnight at 30°C in rich media (YPAD or YP Raf/Gal). The strains were diluted in sterile water to obtain 0.5 OD600 (for four spot tests) or 0.05 OD600 (for three spot tests), followed by 10-fold serial dilutions in sterile water. Two microliters of diluted cells were spotted onto the indicated plates and grown at various temperatures for a minimum of 2 d.
Western blotting
Cells from 100 mL YP Raf/Gal culture were grown to an OD600 of 0.8–1.2, harvested by centrifugation and resuspended in 120 µL–160 µL extraction buffer (20 mM Hepes pH 7.5, 1 M NaCl, 5% Glycerol, 2 mM 2-mercaptoethanol [BME] 1 mM pefabloc, 2.5 µg/mL leupeptin, and 2.5 µg/mL pepstatin) (Alexandrov et al. 2004) and 0.5 mm Zirconia/Silca beads (BioSpec #11079105z) and lysed with vortex (five repeats of 1 min vortex followed by 1 min in ethanol-ice) essentially as described previously (Gelperin et al. 2005). The cell lysates were collected by centrifugation at 4°C for 10 min at 13,000 RPM. The crude extracts were separated by SDS-PAGE on 4%–20% Criterion TGX precast midi protein gels (BioRad #5671094), transferred to a 0.2 µm nitrocellulose membrane (BioRad #1620112) and blotted as described previously (Gelperin et al. 2005). eEF3 protein was detected with anti-eEF3 antibody (Kerafast ED7003) and Glucose-6-phosphate dehydrogenase (G-6-PDH) with anti-G-6-PDH antibody (Sigma A9521). Blots were probed first with anti-eEF3 and anti-G-6-PDH antibodies and then with IgG Goat anti-Rabbit (BioRad 170-6515) and developed with Pierce ECL Plus Western Blotting Substrate kit (Thermo Scientific 32132).
The eEF3 antibody was raised against full-length eEF3 and could cross react with Hef3, a paralog of eEF3, or with other members of the ABCF protein family, such as New1. However, the HEF3 gene is not expressed in vegetative growth (Maurice et al. 1998; Sarthy et al. 1998) and the New1 protein is ∼15 kDa larger than eEF3.
Coomassie stained gel
Crude extracts of the given strains were separated on 4%–20% Criterion TGX Precast Midi Protein Gels (BioRad #5671094). The gel was washed in fixing solution (40% ethanol, 10% acetic acid) for 15 min and rinsed in deionized water three times for 5 min each. The gel was stained in QC Colloidal Coomassie Stain (BioRad #1610803) for 17–20 h, followed by destaining in deionized water for 3 h, changing the water every hour.
Flow cytometry
Yeast strains containing modified RNA-ID reporters were grown at least 24 h prior to analysis at 30°C in YP media (for strains without a plasmid), or appropriate synthetic drop-out media (for strains with a plasmid), containing 2% raffinose + 2% galactose + 80 mg/L Ade. The cell culture was diluted 6 h before analysis such that the culture had a final OD600 between 0.8–1.1. Analytical flow cytometry and downstream analysis were performed for four to six independent isolates of each strain (outliers were rejected using a Q-test with >90% confidence level) as previously described (Dean and Grayhack 2012). Background “GFP” fluorescence from a reporter expressing RFP but lacking a GFP start codon was ∼0.3 to 0.7 in all experiments, similar to that in a strain completely lacking the GFP RFP reporter. P-values were calculated using a one-tailed or two-tailed homoscedastic or heteroscedastic t-test in Excel, as indicated in the source data for relevant figures.
RT-qPCR
mRNA measurements with reverse transcription (RT) and quantitative PCR were performed as described previously (Gamble et al. 2016).
Plasmid transformation
Yeast strains bearing plasmids were transformed as previously described (Schiestl and Gietz 1989).
Linear transformation
Yeast strains bearing RNA-ID reporters and chromosomal deletions were obtained by linear transformation as previously described (Gietz and Woods 2002).
Alignment
Amino acid sequence alignments were obtained using multAlin (Corpet 1988; http://multalin.toulouse.inra.fr/multalin/).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
Supplementary Material
ACKNOWLEDGMENTS
We thank Eric Phizicky, Christina Brule, Thareendra De Zoysa, Dmitri Ermolenko, Alayna Hauke, Erin Marcus, Elaine Sia, Monika Tasak, and Yi-Tao Yu for discussions of the science, and Eric Phizicky for comments on the manuscript. We thank the University of Rochester Genomics Research Center for high-throughput sequencing, including library construction, sequencing, and primary data analysis for this study, and the URMC Flow Cytometry Resource for technical support. This work was supported by National Insitutes of Health (NIH) grant R01 GM118386 to E.J.G. L.B.H. was also supported by an NIH T32 Training Grant in Cellular, Biochemical and Molecular Sciences (GM68411).
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.078964.121.
Freely available online through the RNA Open Access option.
MEET THE FIRST AUTHOR
Lisa Houston.

Meet the First Author(s) is a new editorial feature within RNA, in which the first author(s) of research-based papers in each issue have the opportunity to introduce themselves and their work to readers of RNA and the RNA research community. Lisa Houston is the first author of this paper, “Frameshifting at collided ribosomes is modulated by elongation factor eEF3 and by integrated stress response regulators Gcn1 and Gcn20.” Lisa is a graduate student in the laboratory of Elizabeth Grayhack in the Department of Biochemistry and Biophysics at the University of Rochester, with a research focus on frameshifting that occurs at CGA codon repeats in the yeast Saccharomyces cerevisiae.
What are the major results described in your paper and how do they impact this branch of the field?
The major result of this work shows that when translation stalls at CGA codon repeats in the yeast S. cerevisiae, the general translation factor eEF3 promotes frameshifting. However, Mbf1 and key regulators of both the integrated stress response (Gcn1 and Gcn20) and No-Go decay (Hel2) work with Mbf1 to oppose eEF3-mediated frameshifting, and thus work to maintain the reading frame. This work shows that the quality control systems work together to maintain the essential balance of translation. Moreover, the elongation factors that work during each cycle of translation play a crucial role in frameshifting during collisions.
What led you to study RNA or this aspect of RNA science?
I decided to research translation and RNA because I found that it presented clear and elegant problems that related to crucial and basic biological processes. In addition, our laboratory had previously found that CGA codon repeats reduce translation output in S. cerevisiae. Further, we found that ribosomes frameshift at CGA codon repeats when the key quality control regulator Asc1 is missing. More recently, my laboratory mate Jiyu Wang showed that Mbf1 works with Asc1 and ribosomal protein uS3 to maintain the reading frame during ribosome collisions at CGA codon repeats. Other investigators from the Green and Hegde laboratories found that Mbf1 specifically associates with the collided ribosomes and that there were differences between yeast and humans in frameshifting by collided ribosomes. I wanted to understand not only what prevents frameshifting at CGA codon repeats, but also what drives frameshifting during ribosome collisions, especially considering the differences in frameshifting between S. cerevisiae and mammals.
What are some of the landmark moments that provoked your interest in science or your development as a scientist?
I have had several great science teachers who have encouraged me to push through and go after my dreams. When I went to college, I found another field I was interested in, and I graduated with my bachelor's degree in that field. After 14 years of being away from school, my husband encouraged me to go back to school to study a field I have always loved. When I went back to college, Dr. Olga Kopp was particularly influential. Her encouragement and confidence in me were pivotal in my decision to pursue my doctorate.
If you were able to give one piece of advice to your younger self, what would that be?
My path to graduate school has been long and unique. I have three daughters, and when I started graduate school my youngest was in elementary school and my oldest was just starting high school. This has presented some concerns and additional stresses. While balancing work and family life is a challenge for all graduate students, I also had to balance my daughter's homework, extracurricular activities, and even teaching two daughters how to drive. I would advise myself to not get discouraged by the time it takes to achieve my goals, keep moving forward, don't give up. Be patient with yourself and the timing of things. I certainly don't regret going back to school and the sacrifices have definitely been worth it.
REFERENCES
- Alexandrov A, Vignali M, LaCount DJ, Quartley E, de Vries C, De Rosa D, Babulski J, Mitchell SF, Schoenfeld LW, Fields S, et al. 2004. A facile method for high-throughput co-expression of protein pairs. Mol Cell Proteomics 3: 934–938. 10.1074/mcp.T400008-MCP200 [DOI] [PubMed] [Google Scholar]
- Alexandrov A, Chernyakov I, Gu W, Hiley SL, Hughes TR, Grayhack EJ, Phizicky EM. 2006. Rapid tRNA decay can result from lack of nonessential modifications. Mol Cell 21: 87–96. 10.1016/j.molcel.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Anand M, Chakraburtty K, Marton MJ, Hinnebusch AG, Kinzy TG. 2003. Functional interactions between yeast translation eukaryotic elongation factor (eEF) 1A and eEF3. J Biol Chem 278: 6985–6991. 10.1074/jbc.M209224200 [DOI] [PubMed] [Google Scholar]
- Anand M, Balar B, Ulloque R, Gross SR, Kinzy TG. 2006. Domain and nucleotide dependence of the interaction between Saccharomyces cerevisiae translation elongation factors 3 and 1A. J Biol Chem 281: 32318–32326. 10.1074/jbc.M601899200 [DOI] [PubMed] [Google Scholar]
- Andersen CB, Becker T, Blau M, Anand M, Halic M, Balar B, Mielke T, Boesen T, Pedersen JS, Spahn CM, et al. 2006. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature 443: 663–668. 10.1038/nature05126 [DOI] [PubMed] [Google Scholar]
- Asakura T, Sasaki T, Nagano F, Satoh A, Obaishi H, Nishioka H, Imamura H, Hotta K, Tanaka K, Nakanishi H, et al. 1998. Isolation and characterization of a novel actin filament-binding protein from Saccharomyces cerevisiae. Oncogene 16: 121–130. 10.1038/sj.onc.1201487 [DOI] [PubMed] [Google Scholar]
- Belcourt MF, Farabaugh PJ. 1990. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell 62: 339–352. 10.1016/0092-8674(90)90371-K [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfield GP, Ross-Smith NJ, Tuite MF. 1995. Translation elongation factor-3 (EF-3): an evolving eukaryotic ribosomal protein? J Mol Evol 41: 376–387. 10.1007/BF01215185 [DOI] [PubMed] [Google Scholar]
- Boeke JD, LaCroute F, Fink GR. 1984. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet 197: 345–346. 10.1007/BF00330984 [DOI] [PubMed] [Google Scholar]
- Boeke JD, Trueheart J, Natsoulis G, Fink GR. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 154: 164–175. 10.1016/0076-6879(87)54076-9 [DOI] [PubMed] [Google Scholar]
- Brandman O, Hegde RS. 2016. Ribosome-associated protein quality control. Nat Struct Mol Biol 23: 7–15. 10.1038/nsmb.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J, et al. 2012. A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151: 1042–1054. 10.1016/j.cell.2012.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, Weiss B. 2020. Ribosome pausing, a dangerous necessity for co-translational events. Nucleic Acids Res 48: 1043–1055. 10.1093/nar/gkz763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16: 10881–10890. 10.1093/nar/16.22.10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean KM, Grayhack EJ. 2012. RNA-ID, a highly sensitive and robust method to identify cis-regulatory sequences using superfolder GFP and a fluorescence-based assay. RNA 18: 2335–2344. 10.1261/rna.035907.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj A, Shoji S, Holbrook ED, Fredrick K. 2009. A role for the 30S subunit E site in maintenance of the translational reading frame. RNA 15: 255–265. 10.1261/rna.1320109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Green R. 2012. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harb Perspect Biol 4: a013706. 10.1101/cshperspect.a013706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Kinzy TG, Pavitt GD. 2016. Mechanism and regulation of protein synthesis in Saccharomyces cerevisiae. Genetics 203: 65–107. 10.1534/genetics.115.186221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova LN, Kuroha K, Tatematsu T, Inada T. 2009. Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J Biol Chem 284: 10343–10352. 10.1074/jbc.M808840200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doma MK, Parker R. 2006. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440: 561–564. 10.1038/nature04530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orazio KN, Green R. 2021. Ribosome states signal RNA quality control. Mol Cell 81: 1372–1383. 10.1016/j.molcel.2021.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Orazio KN, Wu CC, Sinha N, Loll-Krippleber R, Brown GW, Green R. 2019. The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during No Go Decay. Elife 8: 49117. 10.7554/eLife.49117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Silva S, Haider SJ, Phizicky EM. 2011. A domain of the actin binding protein Abp140 is the yeast methyltransferase responsible for 3-methylcytidine modification in the tRNA anti-codon loop. RNA 17: 1100–1110. 10.1261/rna.2652611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan-Minogue H, Bedwell DM. 2008. Eukaryotic ribosomal RNA determinants of aminoglycoside resistance and their role in translational fidelity. RNA 14: 148–157. 10.1261/rna.805208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh PJ, Kramer E, Vallabhaneni H, Raman A. 2006. Evolution of +1 programmed frameshifting signals and frameshift-regulating tRNAs in the order Saccharomycetales. J Mol Evol 63: 545–561. 10.1007/s00239-005-0311-0 [DOI] [PubMed] [Google Scholar]
- Fourmy D, Recht MI, Blanchard SC, Puglisi JD. 1996. Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science 274: 1367–1371. 10.1126/science.274.5291.1367 [DOI] [PubMed] [Google Scholar]
- Gamble CE, Brule CE, Dean KM, Fields S, Grayhack EJ. 2016. Adjacent codons act in concert to modulate translation efficiency in yeast. Cell 166: 679–690. 10.1016/j.cell.2016.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Barrio M, Dong J, Ufano S, Hinnebusch AG. 2000. Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2α kinase GCN2 is required for GCN2 activation. EMBO J 19: 1887–1899. 10.1093/emboj/19.8.1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzia A, Jafarnejad SM, Meyer C, Chapat C, Gogakos T, Morozov P, Amiri M, Shapiro M, Molina H, Tuschl T, et al. 2017. The E3 ubiquitin ligase and RNA-binding protein ZNF598 orchestrates ribosome quality control of premature polyadenylated mRNAs. Nat Commun 8: 16056. 10.1038/ncomms16056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelperin DM, White MA, Wilkinson ML, Kon Y, Kung LA, Wise KJ, Lopez-Hoyo N, Jiang L, Piccirillo S, Yu H, et al. 2005. Biochemical and genetic analysis of the yeast proteome with a movable ORF collection. Genes Dev 19: 2816–2826. 10.1101/gad.1362105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6: 343–345. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Gietz RD, Woods RA. 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol 350: 87–96. 10.1016/S0076-6879(02)50957-5 [DOI] [PubMed] [Google Scholar]
- Glover ML, Burroughs AM, Monem PC, Egelhofer TA, Pule MN, Aravind L, Arribere JA. 2020. NONU-1 encodes a conserved endonuclease required for mRNA translation surveillance. Cell Rep 30: 4321–4331.e4. 10.1016/j.celrep.2020.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AL, McCusker JH. 1999. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast 15: 1541–1553. [DOI] [PubMed] [Google Scholar]
- Grollman AP. 1967. Inhibitors of protein biosynthesis. II. Mode of action of anisomycin. J Biol Chem 242: 3226–3233. 10.1016/S0021-9258(18)95953-3 [DOI] [PubMed] [Google Scholar]
- Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH. 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30: e23. 10.1093/nar/30.6.e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick JL, Wilson PG, Edelman II, Sandbaken MG, Ursic D, Culbertson MR. 2001. Yeast frameshift suppressor mutations in the genes coding for transcription factor Mbf1p and ribosomal protein S3: evidence for autoregulation of S3 synthesis. Genetics 157: 1141–1158. 10.1093/genetics/157.3.1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey KL, Dickson K, Cogan JZ, Replogle JM, Schoof M, D'Orazio KN, Sinha NK, Hussmann JA, Jost M, Frost A, et al. 2020. GIGYF2 and 4EHP inhibit translation initiation of defective messenger RNAs to assist ribosome-associated quality control. Mol Cell 79: 950–962.e6. 10.1016/j.molcel.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton JL, Kearney PC, Ames BN. 1965. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys 112: 544–547. 10.1016/0003-9861(65)90093-7 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. 2005. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59: 407–450. 10.1146/annurev.micro.59.031805.133833 [DOI] [PubMed] [Google Scholar]
- Ikeuchi K, Tesina P, Matsuo Y, Sugiyama T, Cheng J, Saeki Y, Tanaka K, Becker T, Beckmann R, Inada T. 2019. Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. EMBO J 38: e100276. 10.15252/embj.2018100276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia MH, Larossa RA, Lee JM, Rafalski A, Derose E, Gonye G, Xue Z. 2000. Global expression profiling of yeast treated with an inhibitor of amino acid biosynthesis, sulfometuron methyl. Physiol Genomics 3: 83–92. 10.1152/physiolgenomics.2000.3.2.83 [DOI] [PubMed] [Google Scholar]
- Joazeiro CAP. 2017. Ribosomal stalling during translation: providing substrates for ribosome-associated protein quality control. Annu Rev Cell Dev Biol 33: 343–368. 10.1146/annurev-cellbio-111315-125249 [DOI] [PubMed] [Google Scholar]
- Jones GM, Stalker J, Humphray S, West A, Cox T, Rogers J, Dunham I, Prelich G. 2008. A systematic library for comprehensive overexpression screens in Saccharomyces cerevisiae. Nat Methods 5: 239–241. 10.1038/nmeth.1181 [DOI] [PubMed] [Google Scholar]
- Juszkiewicz S, Hegde RS. 2017. Initiation of quality control during Poly(A) translation requires site-specific ribosome ubiquitination. Mol Cell 65: 743–750.e4. 10.1016/j.molcel.2016.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszkiewicz S, Chandrasekaran V, Lin Z, Kraatz S, Ramakrishnan V, Hegde RS. 2018. ZNF598 is a quality control sensor of collided ribosomes. Mol Cell 72: 469–481.e7. 10.1016/j.molcel.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszkiewicz S, Slodkowicz G, Lin Z, Freire-Pritchett P, Peak-Chew SY, Hegde RS. 2020a. Ribosome collisions trigger cis-acting feedback inhibition of translation initiation. Elife 9: e60038. 10.7554/eLife.60038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszkiewicz S, Speldewinde SH, Wan L, Svejstrup JQ, Hegde RS. 2020b. The ASC-1 complex disassembles collided ribosomes. Mol Cell 79: 603–614.e8. 10.1016/j.molcel.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath A, Chakraburtty K. 1989. Role of yeast elongation factor 3 in the elongation cycle. J Biol Chem 264: 15423–15428. 10.1016/S0021-9258(19)84845-7 [DOI] [PubMed] [Google Scholar]
- Kambampati R, Chakraburtty K. 1997. Functional subdomains of yeast elongation factor 3. Localization of ribosome-binding domain. J Biol Chem 272: 6377–6381. 10.1074/jbc.272.10.6377 [DOI] [PubMed] [Google Scholar]
- Klopotowski T, Wiater A. 1965. Synergism of aminotriazole and phosphate on the inhibition of yeast imidazole glycerol phosphate dehydratase. Arch Biochem Biophys 112: 562–566. 10.1016/0003-9861(65)90096-2 [DOI] [PubMed] [Google Scholar]
- Kulak NA, Pichler G, Paron I, Nagaraj N, Mann M. 2014. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat Methods 11: 319–324. 10.1038/nmeth.2834 [DOI] [PubMed] [Google Scholar]
- Kuroha K, Akamatsu M, Dimitrova L, Ito T, Kato Y, Shirahige K, Inada T. 2010. Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep 11: 956–961. 10.1038/embor.2010.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Dulude D, Steinberg SV, Brakier-Gingras L. 2007. The three transfer RNAs occupying the A, P and E sites on the ribosome are involved in viral programmed -1 ribosomal frameshift. Nucleic Acids Res 35: 5581–5592. 10.1093/nar/gkm578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzring DP, Wolf AS, Brule CE, Grayhack EJ. 2013. Translation of CGA codon repeats in yeast involves quality control components and ribosomal protein L1. RNA 19: 1208–1217. 10.1261/rna.039446.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyumkis D, Oliveira dos Passos D, Tahara EB, Webb K, Bennett EJ, Vinterbo S, Potter CS, Carragher B, Joazeiro CA. 2014. Structural basis for translational surveillance by the large ribosomal subunit-associated protein quality control complex. Proc Natl Acad Sci 111: 15981–15986. 10.1073/pnas.1413882111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez V, Wilson DN, Tate WP, Triana-Alonso F, Nierhaus KH. 2004. Maintaining the ribosomal reading frame: the influence of the E site during translational regulation of release factor 2. Cell 118: 45–55. 10.1016/j.cell.2004.06.012 [DOI] [PubMed] [Google Scholar]
- Marton MJ, Crouch D, Hinnebusch AG. 1993. GCN1, a translational activator of GCN4 in Saccharomyces cerevisiae, is required for phosphorylation of eukaryotic translation initiation factor 2 by protein kinase GCN2. Mol Cell Biol 13: 3541–3556. 10.1128/mcb.13.6.3541-3556.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton MJ, Vazquez de Aldana CR, Qiu H, Chakraburtty K, Hinnebusch AG. 1997. Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF2α kinase GCN2. Mol Cell Biol 17: 4474–4489. 10.1128/MCB.17.8.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateyak MK, Pupek JK, Garino AE, Knapp MC, Colmer SF, Kinzy TG, Dunaway S. 2018. Demonstration of translation elongation factor 3 activity from a non-fungal species, Phytophthora infestans. PLoS One 13: e0190524. 10.1371/journal.pone.0190524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Ikeuchi K, Saeki Y, Iwasaki S, Schmidt C, Udagawa T, Sato F, Tsuchiya H, Becker T, Tanaka K, et al. 2017. Ubiquitination of stalled ribosome triggers ribosome-associated quality control. Nat Commun 8: 159. 10.1038/s41467-017-00188-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Tesina P, Nakajima S, Mizuno M, Endo A, Buschauer R, Cheng J, Shounai O, Ikeuchi K, Saeki Y, et al. 2020. RQT complex dissociates ribosomes collided on endogenous RQC substrate SDD1. Nat Struct Mol Biol 27: 323–332. 10.1038/s41594-020-0393-9 [DOI] [PubMed] [Google Scholar]
- Maurice TC, Mazzucco CE, Ramanathan CS, Ryan BM, Warr GA, Puziss JW. 1998. A highly conserved intraspecies homolog of the Saccharomyces cerevisiae elongation factor-3 encoded by the HEF3 gene. Yeast 14: 1105–1113. [DOI] [PubMed] [Google Scholar]
- Meydan S, Guydosh NR. 2020. Disome and trisome profiling reveal genome-wide targets of ribosome quality control. Mol Cell 79: 588–602.e6. 10.1016/j.molcel.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meydan S, Guydosh NR. 2021. A cellular handbook for collided ribosomes: surveillance pathways and collision types. Curr Genet 67: 19–26. 10.1007/s00294-020-01111-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murina V, Kasari M, Takada H, Hinnu M, Saha CK, Grimshaw JW, Seki T, Reith M, Putrins M, Tenson T, et al. 2019. ABCF ATPases involved in protein synthesis, ribosome assembly and antibiotic resistance: structural and functional diversification across the tree of life. J Mol Biol 431: 3568–3590. 10.1016/j.jmb.2018.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan K, Meyer MR, Jackson BM, Slade D, Roberts C, Hinnebusch AG, Marton MJ. 2001. Transcriptional profiling shows that Gcn4p is a master regulator of gene expression during amino acid starvation in yeast. Mol Cell Biol 21: 4347–4368. 10.1128/MCB.21.13.4347-4368.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochopien AA, Beckert B, Kasvandik S, Berninghausen O, Beckmann R, Tenson T, Wilson DN. 2021. Structure of Gcn1 bound to stalled and colliding 80S ribosomes. Proc Natl Acad Sci 118: e2022756118. 10.1073/pnas.2022756118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan N, Pochopien AA, Chih-Chien Wu C, Beckert B, Blanchet S, Green R MVR, Wilson DN. 2021. Yeast translation elongation factor eEF3 promotes late stages of tRNA translocation. EMBO J 40: e106449. 10.15252/embj.2020106449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Horikawa W, Ito K. 2015. Inhibiting K63 polyubiquitination abolishes no-go type stalled translation surveillance in Saccharomyces cerevisiae. PLoS Genet 11: e1005197. 10.1371/journal.pgen.1005197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samatova E, Daberger J, Liutkute M, Rodnina MV. 2020. Translational control by ribosome pausing in bacteria: how a non-uniform pace of translation affects protein production and folding. Front Microbiol 11: 619430. 10.3389/fmicb.2020.619430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbaken MG, Lupisella JA, DiDomenico B, Chakraburtty K. 1990. Protein synthesis in yeast. Structural and functional analysis of the gene encoding elongation factor 3. J Biol Chem 265: 15838–15844. 10.1016/S0021-9258(18)55474-0 [DOI] [PubMed] [Google Scholar]
- Sanders CL, Curran JF. 2007. Genetic analysis of the E site during RF2 programmed frameshifting. RNA 13: 1483–1491. 10.1261/rna.638707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy AV, McGonigal T, Capobianco JO, Schmidt M, Green SR, Moehle CM, Goldman RC. 1998. Identification and kinetic analysis of a functional homolog of elongation factor 3, YEF3 in Saccharomyces cerevisiae. Yeast 14: 239–253. [DOI] [PubMed] [Google Scholar]
- Sasikumar AN, Kinzy TG. 2014. Mutations in the chromodomain-like insertion of translation elongation factor 3 compromise protein synthesis through reduced ATPase activity. J Biol Chem 289: 4853–4860. 10.1074/jbc.M113.536201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger E, Hinnebusch AG. 2000. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J 19: 6622–6633. 10.1093/emboj/19.23.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger E, Hinnebusch AG. 2005. Polyribosome binding by GCN1 is required for full activation of eukaryotic translation initiation factor 2α kinase GCN2 during amino acid starvation. J Biol Chem 280: 16514–16521. 10.1074/jbc.M414566200 [DOI] [PubMed] [Google Scholar]
- Schiestl RH, Gietz RD. 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16: 339–346. 10.1007/BF00340712 [DOI] [PubMed] [Google Scholar]
- Shao S, Hegde RS. 2014. Reconstitution of a minimal ribosome-associated ubiquitination pathway with purified factors. Mol Cell 55: 880–890. 10.1016/j.molcel.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, von der Malsburg K, Hegde RS. 2013. Listerin-dependent nascent protein ubiquitination relies on ribosome subunit dissociation. Mol Cell 50: 637–648. 10.1016/j.molcel.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen PS, Park J, Qin Y, Li X, Parsawar K, Larson MH, Cox J, Cheng Y, Lambowitz AM, Weissman JS, et al. 2015. Protein synthesis. Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains. Science 347: 75–78. 10.1126/science.1259724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Hudson BH, Mosior JW, Rangwala AS, Zaher HS. 2014. An active role for the ribosome in determining the fate of oxidized mRNA. Cell Rep 9: 1256–1264. 10.1016/j.celrep.2014.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Thomas EN, Zaher HS. 2017a. Ribosome-based quality control of mRNA and nascent peptides. Wiley Interdiscipl Rev RNA 8: 10.1002/wrna.1366. 10.1002/wrna.1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Yan LL, Zaher HS. 2017b. Ribosome collision is critical for quality control during no-go decay. Mol Cell 68: 361–373.e5. 10.1016/j.molcel.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Kim KQ, Yan LL, Qiu J, Zaher HS. 2018. Interactions between the mRNA and Rps3/uS3 at the entry tunnel of the ribosomal small subunit are important for no-go decay. PLoS Genet 14: e1007818. 10.1371/journal.pgen.1007818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Yan LL, Qiu JK, Zaher HS. 2019. Ribosome collisions result in +1 frameshifting in the absence of no-go decay. Cell Rep 28: 1679–1689.e4. 10.1016/j.celrep.2019.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha NK, Ordureau A, Best K, Saba JA, Zinshteyn B, Sundaramoorthy E, Fulzele A, Garshott DM, Denk T, Thoms M, et al. 2020. EDF1 coordinates cellular responses to ribosome collisions. Elife 9: e58828. 10.7554/eLife.58828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitron CS, Park JH, Brandman O. 2017. Asc1, Hel2, and Slh1 couple translation arrest to nascent chain degradation. RNA 23: 798–810. 10.1261/rna.060897.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy E, Leonard M, Mak R, Liao J, Fulzele A, Bennett EJ. 2017. ZNF598 and RACK1 regulate mammalian ribosome-associated quality control function by mediating regulatory 40S ribosomal ubiquitylation. Mol Cell 65: 751–760.e4. 10.1016/j.molcel.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru K, Harashima S, Ueda H, Hirose S. 1998. Yeast coactivator MBF1 mediates GCN4-dependent transcriptional activation. Mol Cell Biol 18: 4971–4976. 10.1128/MCB.18.9.4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesina P, Lessen LN, Buschauer R, Cheng J, Wu CC, Berninghausen O, Buskirk AR, Becker T, Beckmann R, Green R. 2020. Molecular mechanism of translational stalling by inhibitory codon combinations and poly(A) tracts. EMBO J 39: e103365. 10.15252/embj.2019103365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Alonso FJ, Chakraburtty K, Nierhaus KH. 1995. The elongation factor 3 unique in higher fungi and essential for protein biosynthesis is an E site factor. J Biol Chem 270: 20473–20478. 10.1074/jbc.270.35.20473 [DOI] [PubMed] [Google Scholar]
- Uritani M, Miyazaki M. 1988. Role of yeast peptide elongation factor 3 (EF-3) at the AA-tRNA binding step. J Biochem 104: 118–126. 10.1093/oxfordjournals.jbchem.a122405 [DOI] [PubMed] [Google Scholar]
- Visweswaraiah J, Lee SJ, Hinnebusch AG, Sattlegger E. 2012. Overexpression of eukaryotic translation elongation factor 3 impairs Gcn2 protein activation. J Biol Chem 287: 37757–37768. 10.1074/jbc.M112.368266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10: 1793–1808. 10.1002/yea.320101310 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou J, Yang Q, Grayhack EJ. 2018. Multi-protein bridging factor 1(Mbf1), Rps3 and Asc1 prevent stalled ribosomes from frameshifting. Elife 7: e39637. 10.7554/eLife.39637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AS, Grayhack EJ. 2015. Asc1, homolog of human RACK1, prevents frameshifting in yeast by ribosomes stalled at CGA codon repeats. RNA 21: 935–945. 10.1261/rna.049080.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wout PK, Sattlegger E, Sullivan SM, Maddock JR. 2009. Saccharomyces cerevisiae Rbg1 protein and its binding partner Gir2 interact on polyribosomes with Gcn1. Eukaryot Cell 8: 1061–1071. 10.1128/EC.00356-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Peterson A, Zinshteyn B, Regot S, Green R. 2020. Ribosome collisions trigger general stress responses to regulate cell fate. Cell 182: 404–416.e4. 10.1016/j.cell.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LL, Zaher HS. 2021. Ribosome quality control antagonizes the activation of the integrated stress response on colliding ribosomes. Mol Cell 81: 614–628.e4. 10.1016/j.molcel.2020.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.