Abstract
Increased moderate and vigorous physical activity (MVPA) is associated with better health outcomes in breast cancer survivors; yet, most are insufficiently active. Smartphone applications (apps) to promote MVPA have high scalability potential, but few evidence-based apps exist. The purpose is to describe the testing and usability of Fit2Thrive, a MVPA promotion app for breast cancer survivors. A user-centered, iterative design process was utilized on three independent groups of participants. Two groups of breast cancer survivors (group 1 n = 8; group 2: n = 14) performed app usability field testing by interacting with the app for ≥3 days in a free-living environment. App refinements occurred following each field test. The Post-Study System Usability Questionnaire (PSSUQ) and the User Version Mobile Application Rating Scale (uMARS) assessed app usability and quality on a 7- and 5-point scale, respectively, and women provided qualitative written feedback. A third group (n = 15) rated potential app notification content. Quantitative data were analyzed using descriptive statistics. Qualitative data were analyzed using a directed content analysis. The PSSUQ app usability score (M1= 3.8; SD = 1.4 vs. M2= 3.2; SD = 1.1; lower scores are better) and uMARS app quality score (M1 = 3.4; SD = 1.3 vs. M2= 3.4; SD = 0.6; higher scores are better) appeared to improve in Field Test 2. Group 1 participants identified app “clunkiness,” whereas group 2 participants identified issues with error messaging/functionality. Group 3 “liked” 53% of the self-monitoring, 71% of the entry reminder, 60% of the motivational, and 70% of the goal accomplishment notifications. Breast cancer survivors indicated that the Fit2Thrive app was acceptable and participants were able to use the app. Future work will test the efficacy of this app to increase MVPA.
Keywords: Exercise, mHealth, Interventions
“Increasing time spent in moderate-to-vigorous physical activity is associated with better health outcomes in breast cancer survivors. Even so, most breast cancer survivors do not meet current physical activity recommendations for moderate-to-vigorous physical activity. One potential solution to assist breast cancer survivors in increasing their physical activity is to provide remote access to a physical activity promotion intervention through a smartphone application (app). Our purpose was to describe the iterative process we completed to design, Fit2Thrive, a moderate-to-vigorous physical activity promotion app for breast cancer survivors. We recruited three separate groups of survivors to test app components and provide feedback on the useability and app quality, with updates being made to the app between each test group. At the end of this iterative design process, breast cancer survivors indicated that the Fit2Thrive app was acceptable, and participants were able to use the app. Our future work will test the efficacy of the app to increase moderate-to-vigorous physical activity among breast cancer survivors.”
Implications.
Practice: Breast cancer survivors indicated that the Fit2Thrive app had high acceptability and usability during initial development which provides valuable information on the efficacy of a user-centered developed physical activity promotion app in increasing physical activity behaviors in breast cancer survivors.
Policy: Smartphone applications to promote MVPA have high scalability potential; therefore, development of population-specific apps, such as the Fit2Thrive app, may increase MVPA participation and promote better health outcomes among breast cancer survivors.
Research: Future research will test the effectiveness of the Fit2Thrive app to promote physical activity in breast cancer survivors.
Introduction
Nearly 4 million breast cancer survivors live in the USA, and this number is expected to increase to almost 5 million over the next decade [1]. Increased moderate and vigorous intensity physical activity (MVPA) is consistently associated with improvements in health outcomes among breast cancer survivors including reduced treatment-related side effects, cancer recurrence, and mortality, and increased quality of life [2–5]. However, 85% of survivors do not meet public health recommendations for MVPA [6]. As the number of breast cancer survivors increases, it is critical to develop effective MVPA-promotion interventions to improve health and disease outcomes.
Mobile health (“mHealth”) MVPA interventions have demonstrated efficacy for increasing MVPA in populations such as older adults, children and adolescents, and patients with type-2 diabetes [7–9]. Furthermore, digital health behavior change interventions, including text messaging and website-based interventions, have been shown to significantly increase MVPA and decrease body mass index [10]. With recent estimates of smartphone ownership among cancer survivors ranging from 68% to 93%, based on age and educational attainment, mHealth interventions present an opportunity to reach a greater population [11–13]. Additionally, mHealth MVPA interventions and wearables are viewed as acceptable for promoting MVPA among breast cancer survivors [14–16]. To the best of our knowledge, there have been two completed, fully powered, randomized controlled trials of breast cancer survivors. One conducted by Lynch and colleagues using a wrist-worn wearable (Garmin Vivofit 2) coupled with an in-person behavioral feedback and goal setting sessions and telephone health coaching sessions [16]. The intervention group demonstrated a significant increase in MVPA compared with the waitlist control group (69 min/week [95% CI: 22, 116]). Uhm et al. compared the effect of an mHealth + pedometer intervention with an educational brochure intervention to increase MVPA in breast cancer survivors (N = 356). The Smart After Care App provided participants with their weekly MVPA goals in minutes/week and provided they are weekly exercise prescription including resistance training and stretching exercise videos. Total metabolic equivalents (METs) increased significantly from baseline to 12 weeks for both groups with no significant differences seen between the groups [17]. The remaining mHealth interventions that have been conducted among cancer survivors have been pilot studies based on smartphone or online platforms [18–21]. In light of these results, the widespread ownership of smartphones [22] and increasingly ubiquitous ownership of wearables given 1 in 5 Americans own a wearable device (i.e., smart watch or fitness tracker) [23], mHealth MVPA promotion interventions may be a scalable, less resource-intensive strategy than in-person interventions to reach breast cancer survivors [24].
Although there are many “of the shelf” MVPA smartphone apps available, a recent review found only one-fifth contained quality content featuring behavior change techniques that maximize usability, safety, and impact for people affected by cancer [25]. Additionally, key findings from our prior mixed methods work which included themes related to (a) the importance of the app being relevant to the breast cancer survivor population, (b) ease of use, (c) integration with wearable activity trackers, and (d) providing survivors with a sense of accomplishment informed the development and tailoring of the Fit2Thrive app for breast cancer survivors [14]. The Fit2Thrive app was developed to promote the safe adoption and maintenance of MVPA for this population. We are currently testing the efficacy of the Fit2Thrive app in the Fit2Thrive trial [26], a randomized trial using the Multiphase Optimization Strategy (MOST) [27] to test five technology supported intervention components to increase MVPA, which included receipt of a standard app, deluxe app, app notifications, coaching calls, and social support from a buddy. One of the components to be tested as part of the Fit2Thrive intervention trial is a comparison of the efficacy of a “standard” version of the Fit2Thrive app versus a “deluxe” version of the app that included additional features that may increase physical activity participation. The “standard” version includes Fitbit integration, self-monitoring features, and educational content [26]. The “deluxe” version included additional features including a newsfeed, planning tool, and goal setting challenges [26]. Another component tested was tailored text messages (on/off) that are embedded in the Fit2Thrive app.
The purpose of the present study was to evaluate the usability of the Fit2Thrive smartphone app, including all standard and deluxe app features, and the acceptability of app notification content prior to implementation of the mobile phone app component in the Fit2Thrive intervention. In this paper, we describe our iterative process and present findings on (a) usability including functionality, aesthetics, and engagement and (b) preferences for app notification content.
Methods
Study design
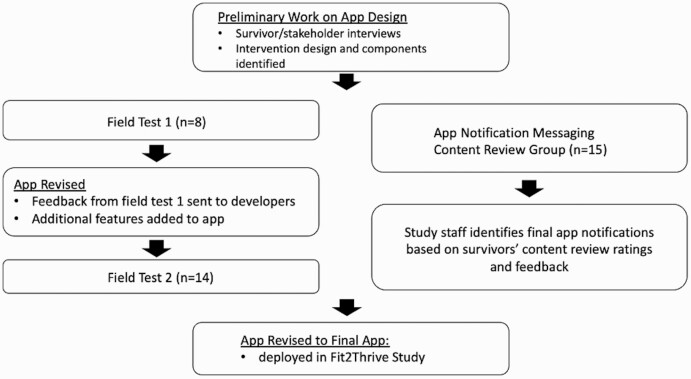
A user-centered, iterative design process was utilized over 18 months. This included iterative evaluation of app components using two groups of field tests with refinement between successive field tests. Additionally, we conducted a review of potential content for app notifications in a third group of breast cancer survivors. Figure 1 details the full, iterative app development and refinement process.
Fig 1 .
App design process overview.
Prototype development
Initial app features were identified in order to target social cognitive theory constructs (i.e., self-efficacy, goal setting, self-monitoring, and barrier/facilitators) and final features were selected based on breast cancer survivor (N = 96, 55.8 + 10.2 years old, highly educated (83.7% college degree or greater), and majority of Stage I or II breast cancer diagnosis (88.1%) interviews and responses to an online questionnaire regarding app feature preferences [14]. Five themes emerged from these interviews: (a) importance of relevance to breast cancer survivors, (b) easy to use, (c) integration with wearable activity trackers, (d) provide a sense of accomplishment, and (e) variability in desired level of structure and personalization. Questionnaire data provided process information including addressing questions such as (a) what type of educational information would be important to have, (b) what activity feedback would you like, and (c) what would you like the frequency of reminder messages to be. Greater details on app feature selection have been published previously [14]. These data were then used by a multidisciplinary team as the foundation to develop a minimally viable test prototype of the Fit2Thrive app. The app prototype was examined for usability and perceived usefulness.
Fit2Thrive app description
Overview.
Because the purpose of the Fit2Thrive trial is to test five technology-supported intervention strategies for increasing MVPA in breast cancer survivors, two versions of the Fit2Thrive App were developed to determine which was more efficacious for increasing physical activity: the standard or deluxe app. The app was built with several modules that could be turned on or off depending on participants’ group membership.
Standard App Features.
Standard app features were (a) self-monitoring and (b) tracking of physical activities and were chosen to target the social cognitive theory construct self-monitoring [28,29]. These features were implemented via full integration with a Fitbit activity tracker which included automatic syncing of data from the Fitbit app to the Fit2Thrive app or manual entry of activities using the “Add Activity” function when the Fitbit is not worn or does not appropriately capture the activity. Manual entry of activity included selecting a physical activity using the search function to search a library of all relevant moderate and vigorous activities from the compendium of physical activities [30]. To promote self-monitoring, participants were provided four separate modules. First, participants received visualizations (charts and graphs) of progress towards their weekly MVPA minute goal in the “Physical Activity This Week “module. They were provided with feedback on their daily activity in the “Physical Activity Today” module which included total MVPA minutes with these minutes broken down into moderate and vigorous as well as the minutes as obtained from the Fitbit or manual entry. There was also a “My Progress” module, which provided detailed feedback on activity from the previous 4 weeks including minutes, steps, and distance. Finally, the app included a “Fit Lessons” module that targeted education and provided educational materials on safely increasing physical activity, physical activity guidelines, and effective behavior change strategies.
Deluxe app features.
The deluxe app included the standard app modules targeting self-monitoring and tracking physical activity plus the following additional modules with the intention to target additional social-cognitive constructs, goal-setting, and self-efficacy: (a) “Today’s Goal”; (b) “This Week’s Challenge”; and (c) “My Fit News.” The “Today’s Goal” module is a weekly goal setting tool that included a scheduler allowing participants to schedule physical activities on specific days/times and provided them reminders [28,29]. The “This Week’s Challenge” module allowed participants to enroll in individual weekly challenges that set a daily activity “challenge” specific to minutes (e.g., 5 min of MVPA/day challenge) or steps (e.g., the 7,000 steps/day challenge) with progressively harder challenges becoming unlocked as easier ones were achieved. The “MyFit News” module included two pre-scheduled weekly posts: (a) “Survivors Spotlights” and (b) “Fit Studies.” “Survivor’s Spotlights” highlighted a survivor who had successfully become active including how she started becoming more active, what her exercise routine looks like, her motivation, tips to stay motivated and overcome barriers, and any advice to share with other survivors’. The “Fit Studies” included a lay summary of physical activity research in breast cancer survivors and featured topics such as benefits of tai chi/yoga, benefits of reducing sedentary behavior, psychological benefits of exercise, and cognitive benefits of exercise.
Fit2Thrive app notifications.
A second component to be tested in the Fit2Thrive trial was whether receiving messages or notifications through the Fit2Thrive app as an additional feature targeting the social cognitive theory construct outcome expectations, to increase physical activity [26,28,29]. Participants who were randomized to receive the messaging component would receive between one and five automated messages daily. Message categories included enhancing social cognitive theory constructs, motivational, goal accomplishment, and reminders to sync Fitbit or add manual activities. Those not randomized to receive notifications only received the reminder notifications. In this paper, we only describe survivors’ preference ratings of the notification message content and do not test functionality of participants receiving the notifications.
Recruitment
All study procedures were approved by the university institutional review board. Women were recruited via email from the Love Research Army (formerly Army of Women), a nationwide registry of women interested in participating in breast-cancer related research. Inclusion criteria included: female, 18 years of age or older, previous diagnosis of Stage I-III breast cancer, ≥3 month post-primary treatment (i.e., surgery, chemotherapy, and radiation), could speak, read, and write in English, owned a smartphone, and had access to a computer with Internet. Further details on recruitment and screening are provided elsewhere [14]. We further restricted the eligibility criteria for app field-testing to a convenience sample of those who owned a Fitbit (n = 22; n = 8 iOS users for field test 1; n = 6 android users; and n = 8 iOS users for field test 2) from the original sample (n = 96) so Fitbit integration features could be tested. Participants in field test 1 were limited to iOS users because the Fit2Thrive android prototype had not been developed. Finally, a third subsample (n = 15) was randomly selected to complete app notification content review from the original sample (n = 96) using a pre-populated computer algorithm. Prior to participation, all participants completed an informed consent approved by the university’s institutional review board.
Procedures
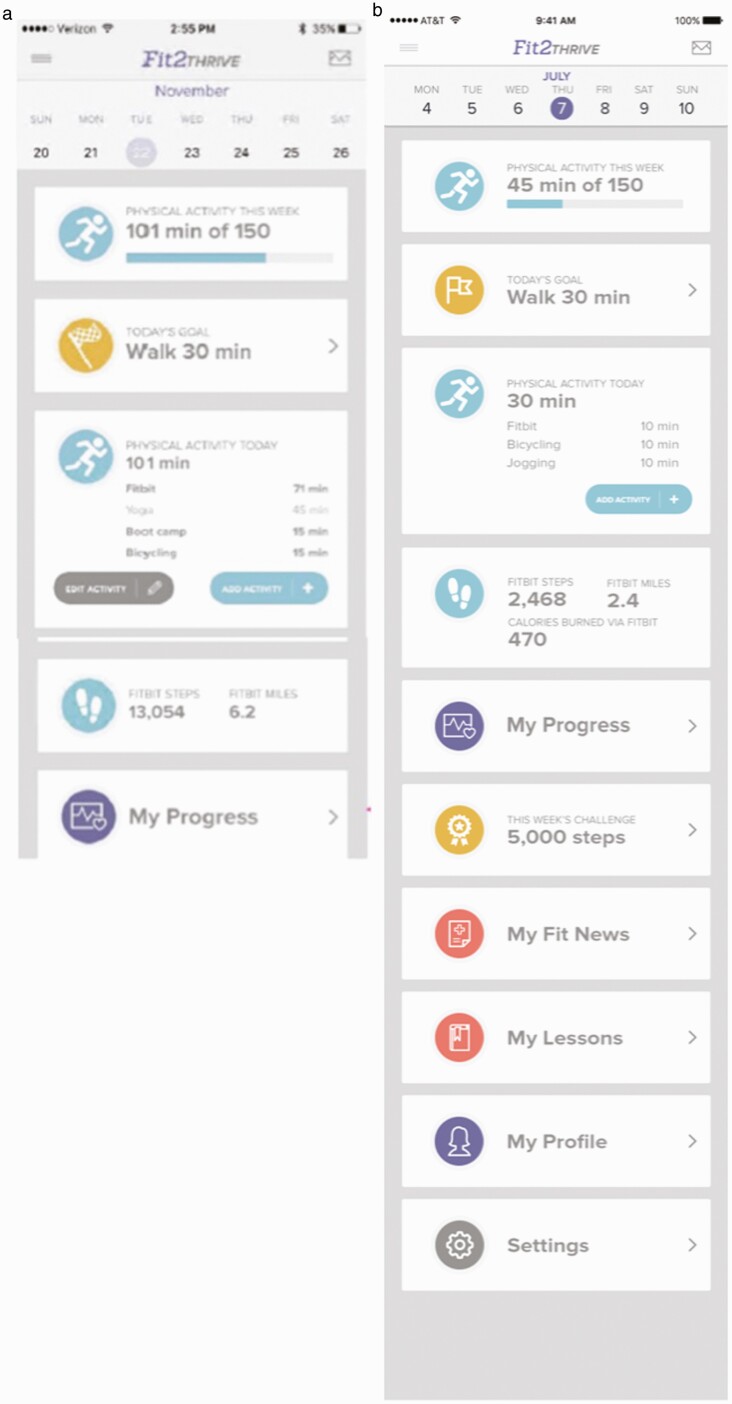
Participants selected to complete field-testing were e-mailed instructions for downloading and using the Fit2Thrive app and connecting their Fitbit to the Fit2Thrive app. Participants were asked to use the app for at least 3 days and spend approximately 30 to 60 min interacting with the app each day. They were also instructed to provide feedback to developers in real time regarding their opinions on app features and functionality. Participants were explicitly instructed not to change their usual physical activity patterns as part of the field test, as the purpose of the field test was solely to evaluate the application’s functioning and ease of use in everyday life. Participants in field test 1 tested all of the “standard” app modules including Fitbit integration and all tracking, self-monitoring, and feedback modules (i.e., progress, manually adding/editing activities, and activity tracking). Participant feedback was compiled and reviewed by the study team and developer team and expert judgements were made about what changes to make to the app. Participants in field test 2 tested a revised version of the “standard” app based on feedback from field test 1 participants as well as the “deluxe” app features (goal-setting tool and challenges) that had not yet been developed for field test 1. The differences in app features noted above are illustrated in Fig. 2. Figure 2a presents an image of the Fit2Thrive app home screen during field test 1 and Fig. 2b shows the home screen during field test 2. The Fit News feature was not tested during this phase since it was a noninteractive feature.
Fig 2 .
(a) Field test group 1 Fit2Thrive app home page and (b) field test group 2 Fit2Thrive app home page.
Measures
Demographic and disease characteristics
Participants self-reported demographic (age, race, and education) and disease characteristics (stage, treatment received, time since diagnosis, and self-rated health) through an online questionnaire completed upon study enrollment. Additionally, participants self-rated their smartphone/app proficiency by answering the single question, “What is your perceived level of proficiency in the use of your smartphone and smartphone mobile apps?” with responses ranging from 1 (unskilled) through 5 (expert).
Post-field test surveys
Participants reported overall satisfaction and ease of using the app through two online surveys at the completion of the app testing period. The Post-Study System Usability questionnaire (PSSUQ) is a 19-item questionnaire assessing usability characteristics which participants rated on a scale from 1 (strongly agree) to 7 (strongly disagree) with a not applicable option. The overall score was calculated by averaging the scores from the 7 points of the scale. In addition, three subscales were calculated: system usefulness, information quality, and interface quality. Lower scores represent better performance and satisfaction. Normative scores are provided by subscale to determine whether answers fall within expected norm scores. Originally developed to rate users satisfaction with computer systems in 1980, evidence has continued to show the PSSUQ’s reliability (r = 0.96) across technological advancements through the years [31].
The User Version of the Mobile Application Rating Scale (uMARS) is a 20-item measure used to classify and assess the quality of mobile apps [32]. The uMARS has three parts: (a) four, objective quality subscales—engagement, functionality, aesthetics, and information; (b) a subjective quality scale; and (c) one subscale to measure user’s perceived impact of the evaluated app. In part 1, participants rated responses to the items on a scale ranging from 1 to 5 (1: Inadequate, 2: Mostly Inadequate, 3: OK, 4: Moderately good, and 5: Excellent) with a not applicable option added. Higher scores indicated better ratings. In part 2, participants rated subjective quality questions on a scale ranging from 1 to 5 with higher scores indicating better ratings but labels differed by question. For part 3, participants rated perceived impact on a 1 (strongly disagree) to 5 (strongly agree) likert scale. The uMARS has exhibited high internal consistency for all subscales (α = 0.90) [32].
Qualitative feedback
Participants reported any issues with app functionality or feedback on features in real time via the beta version of the app, email, or one free response item following completion of the PSSUQ and uMARS: “Please provide any further comments about the Fit2Thrive app.” Three researchers (S.P., P.S., and L.A-.G.) independently reviewed the responses and recorded comment themes. Independent review themes were assessed, and any discrepancies were settled by further discussion and consensus among all researchers to comprise final comment themes reported.
App notification content review survey
This survey provided participants with three options asking them to indicate whether they (a) liked, (b) disliked, or (c) felt neutral about the content of potential app notifications that users could receive via the Fit2Thrive app. Participants were asked to rate 40 positive/motivational messages, 10 self-monitoring messages, 34 weekly goal-accomplishment messages, and 5 entry reminder messages.
Statistical analysis
All quantitative data were analyzed in SPSS version 26 (IBM Corp., Chicago, IL). Frequencies and descriptive statistics were used to analyze participant characteristics and participant responses to the PUSSQ and uMARS questionnaires. Qualitative data from written participant responses were recorded, summarized, and analyzed using directed content analyses [33].
Results
Participants
A total of eight participants completed the field test 1, 14 participants completed field test 2, and 15 participants completed the content review. All three field test groups were comprised of independent samples of participants. The average age for all participants (N = 37) was 55.6 ± 11.2 years old. Most participants were white (94.6%) and highly educated (89.2% college graduate or greater). Fifty-nine percent of participants reported stage 1 breast cancer diagnosis, 32.4% reported stage 2, and 8.1% reported a stage 3 diagnosis. On average, participants were 31.7 ± 15.4 months since their diagnosis. All participants reported having received surgery as part of their treatment plan in addition to 48.6% having received chemotherapy, 64.9% having received radiation therapy, and 67.6% having received endocrine/hormone therapy. Overall, 94.6% of participants self-rated their overall health status as “good” or better. Participants self-reported that they engaged in an average of 124.1 ± 106.1 min per week of MVPA. Finally, participants rated their perceived level of smartphone/smartphone mobile app proficiency as intermediate (35.1%), advanced (40.5%), or expert (24.3%). Participant characteristics broken down by field test group are presented in Table 1.
Table 1 |.
Participant characteristics [mean (SD) or n(%)]
| Variable | Field test 1 (n = 8) | Field test 2 (n = 14) | Content review (n = 15) |
|---|---|---|---|
| Age (years) | 55.3 (11.8) | 53.9 (10.4) | 57.5 (12.0) |
| Race [n(%), white] | 8 (100%) | 13 (93%) | 15 (100%) |
| Education (n, college education or greater) | 8 (100%) | 12 (86%) | 13 (87%) |
| Stage | |||
| 1 | 5 (63%) | 8 (57%) | 9 (60%) |
| 2 | 2 (25%) | 6 (43%) | 4 (27%) |
| 3 | 1 (13%) | 2 (13%) | |
| Treatment | |||
| Received surgery | 8 (100%) | 14 (100%) | 15 (100%) |
| Received chemotherapy | 3 (38%) | 7 (50%) | 8 (53%) |
| Received radiation | 4 (50%) | 8 (57%) | 12 (80%) |
| Endocrine/hormone therapy | 5 (63%) | 11 (79%) | 9 (60%) |
| Time since diagnosis (months) | 32.6 (15.3) | 35.4 (17.7) | 27.6 (12.8) |
| Self-rated health status | |||
| Fair | 2 (14%) | ||
| Good | 5 (63%) | 9 (64%) | 5 (33%) |
| Very good | 2 (25%) | 2 (14%) | 9 (60%) |
| Excellent | 1 (13%) | 1 (7%) | 1 (7%) |
| Physical activity (min/week) | 108.8 (88.8) | 92.1 (73.9) | 162.0 (131.2) |
| Perceived level of smartphone/smartphone app proficiency | |||
| Intermediate | 4 (50%) | 5 (36%) | 4 (27%) |
| Advanced | 2 (25%) | 6 (43%) | 7 (47%) |
| Expert | 2 (25%) | 3 (21%) | 4 (27%) |
PSSUQ results
PSSUQ results are detailed in Table 2. The overall average PSSUQ score was 3.8 ± 1.4 for field test 1 and 3.2 ± 1.1 for field test 2. When subscales are examined, the best ratings were reported for interface quality (field test 1: 3.3 ± 2.3; field test 2: 2.9 ± 1.2), followed by system usefulness (field test 1: 3.7 ± 1.7; field test 2: 3.2 ± 1.1), and finally information quality (field test 1: 4.1 ± 1.4; field test 2: 3.7 ± 1.2).
Table 2 |.
Field test results for the Post-Study System Usability Questionnaire
| App quality mean scores | Average rating (1–7) | |||
| Field test 1 | SD | Field test 2 | SD | |
| Overall score | 3.8 | 1.4 | 3.2 | 1.1 |
| System Usability | 3.7 | 1.7 | 2.8 | 1.3 |
| Information Quality | 4.1 | 1.4 | 3.7 | 1.2 |
| Interface Quality | 3.3 | 2.3 | 2.9 | 1.2 |
Scores range from 1 to 7 and lower scores indicate higher satisfaction.
uMARS results
uMARs results are detailed in Table 3. Both field test 1 and field test 2 participants completed this survey. The overall app quality mean score was 3.4 ± 1.3 for field test 1 and 3.4 ± 0.6 for field test 2. All subscale averages (i.e., engagement, functionality, aesthetics, and information) were greater than or equal to 3.0 (or a rating of “OK” or better). Field test 2 participants predominantly indicated that there were several people who would recommend this app to (43% of field test 2 participants). Participants in both field test 1 and field test 2 predominantly endorsed the question, would they use the app greater than 50 times over the next 12 months, with 57% of participants in field test 1 and 43% of participants in field test 2 indicating this frequency. Participants in both field-testing groups indicated that they would likely not pay for the app and the average overall star rating was 3.0 ± 1.0 for field test 1 and 2.9 ± 0.9 for field test 2 out of 5 stars.
Table 3 |.
Field test results for the user Mobile Application Rating Scale
| App quality mean scores | Average rating (1–5) | |||
| Field test 1 | SD | Field test 2 | SD | |
| App quality mean score | 3.4 | 1.3 | 3.4 | 0.6 |
| Engagement | 3.9 | 1.1 | 3.2 | 0.6 |
| Functionality | 3.0 | 1.2 | 3.3 | 1.2 |
| Aesthetics | 4.1 | 0.7 | 3.8 | 0.6 |
| Information | 3.9 | 0.7 | 3.5 | 0.8 |
| App Subjective Quality Score | 2.9 | 1.4 | 2.8 | 0.9 |
| Would you recommend this app to people who may benefit from it? (Overall Mean) | 3.0 | 1.5 | 2.9 | 1.0 |
| 1. I would not recommend this app to anyone (%) | 13% | 14% | ||
| 2. There are very few people I would recommend this app to (%) | 38% | 14% | ||
| 3. There are several people I would recommend this app to (%) | 13% | 43% | ||
| 4. There are many people I would recommend this app to (%) | 13% | 29% | ||
| 5. I would recommend this app to everyone (%) | 25% | 0% | ||
| How many times do you think you would use this app in the next 12 months if it was relevant to you? (Overall Mean) | 3.4 | 2.0 | 3.9 | 1.4 |
| 1. None | 29% | 14% | ||
| 2. 1–2 times | 14% | 0% | ||
| 3. 3–10 times | 0% | 7% | ||
| 4. 10–50 times | 0% | 36% | ||
| 5. >50 times | 57% | 43% | ||
| Would you pay for this app? (Response Options: 1 Definitely not—5 Definitely yes) | 2.3 | 1.6 | 1.6 | 0.8 |
| What is your overall (star) rating of the app? (Response Options: 1 Worst app—5 Best app) | 3.0 | 1.0 | 2.9 | 0.9 |
| App Specific: Perceived impact of the app on the likelihood to change target behavior | ||||
| Awareness | 3.0 | 0.9 | 4.1 | 0.8 |
| Knowledge | 3.2 | 1.5 | 3.5 | 1.1 |
| Attitudes | 3.7 | 1.2 | 3.6 | 0.9 |
| Intention to Change | 3.8 | 1.2 | 4.0 | 1.0 |
| Help Seeking | 2.7 | 1.2 | 3.2 | 1.2 |
| Behavior Change | 4.0 | 1.0 | 4.3 | 0.9 |
Scores range from 1 to 5 and higher scores indicate greater satisfaction.
Similarly, all participant’s average responses to the app specific questions (awareness, knowledge, attitudes, intention to change, help seeking, and behavior change) or their perception of the impact the app will have on the changing physical activity behaviors were greater than 3.0, on a five-point likert scale ranging from 1 (strongly disagree) to 5 (strongly agree).
Free response results
Field test 1 participants’ general comments addressed issues with “clunkiness” of the app functions in addition to difficulties syncing self-monitoring features. Additionally, participants noted that the app, in its current state, was basic in comparison to the Fitbit app or too similar to the Fitbit app. An example of one participant’s feedback, “The app at the current time is pretty basic. Not that much different from the Fitbit App.”
Field test 2 participants’ comments included issues with functioning errors and lack of obvious instructions or feedback. For example, participants identified syncing or formatting errors encountered while using the app. One participant noted, “Overall I enjoyed using the app. It didn’t sync with my Fitbit accurately at all times. My main issue with Fitbit and this app were how many of the activities I engage in didn’t record steps.” They also indicated that some functions of the app were not intuitive, such as feedback on completing challenges, setting goals, and identifying current goals. Finally, two field test 2 participants asked for greater inclusion of breast cancer survivor-specific content within the app with one participant noting, “I did not see any specific reference to breast cancer.”
Content review survey
Survivors in the content review group indicated that they “liked” 53% of the self-monitoring messages, with 26% of total responses neutral and 21% “dislike.” For the MVPA entry reminder or reminder to sync notifications, responses indicated that they liked 71% of the notifications, had neutral feelings towards 17% of notifications, and “disliked” 12% notifications. For the positive motivational notifications, participants indicated 60% “liked,” 26% neutral, and 13% “dislike” responses. Finally, for the weekly goal accomplishment notifications, participants rated 70% “liked,” 22% neutral, and 9% “disliked.” A few example notifications from each category with corresponding participant ratings are reported in Table 4.
Table 4 |.
Notification messaging content review (n = 15)
| Message | Dislike | Like | Neutral |
| Self-monitoring notification | |||
| “It seems it’s been a few days since you’ve exercised. Let’s change that! What activity can you engage in today?” | 20% | 67% | 13% |
| “You seem to have been busy these past few days, but let’s remember to make exercise a priority. Make a plan to finish your week strong and hit that goal!” | 7% | 53% | 40% |
| “You’ve been a little quiet about your activity this week! Staying active isn’t easy, but you are strong and you can do this! Let’s get back out there!” | 13% | 67% | 20% |
| “It seems you haven’t been very active these past few days, let’s make fitness a priority the rest of this week and get going!” | 40% | 60% | |
| “We haven’t seen any exercise in X days! Remember what you’re working towards, and stay positive. Let’s see you finish this week strong!” | 33% | 40% | 27% |
| “Hey! We haven’t heard from you in a while. Let’s make a plan to fit in your daily activity and hit those goals.” | 27% | 60% | 13% |
| Positive Motivational Notifications | |||
| “Regularly exercising isn’t easy, but the more you do it, the easier it will be, and the better you’ll feel!” | 93% | 7% | |
| “Exercise has been shown to decrease stress levels. Even just 10–15 minutes can have a relaxing effect!” | 80% | 20% | |
| “Low on energy today? Exercise can be re-energizing for your mind and body. Try going for a 10-minute walk and see how you feel!” | 80% | 20% | |
| “Don’t let yourself feel weak or lazy. You are strong and powerful, and a workout will remind you who’s boss!” | 60% | 33% | 7% |
| “Sleep in your workout clothes instead of your pajamas. One less excuse not to workout in the morning!” | 47% | 20% | 27% |
| “Your Fit2Thrive peers are exercising and progressing towards their goals. Check in with yourself; are you on the same page?”* | 53% | 20% | 20% |
| Weekly Goal Accomplishment | |||
| After reaching halfway of the goal for week 19 “Way to hit that halfway point! Only 75 more minutes of activity this week, keep it up!” | 93% | 7% | |
| After reaching 75% of the goal for week 3 “Way to hit 75% of your weekly goal. Only 15 minutes of MVPA left, let’s do this!” | 93% | 7% | |
| After reaching the goal for week 5 “Way to accomplish your goal for this week! Keep it up, we’re almost halfway through the 12 week training program!” | 93% | 7% | |
| After reaching the goal for week 8 “You’ve accomplished this week’s goal, congrats! You’ve had a great 2 months in the Fit2Thrive program! Congratulations on this major milestone!” | 93% | 7% | |
| After reaching halfway of the goal for week 15 “You’ve reached halfway! Don’t sell yourself short and finish up your weekly goal!” | 53% | 13% | 33% |
| On the Monday of week 16 and no physical activity was recorded for week 15 “You did not make any progress towards your goal last week. Make sure you are setting aside some time to be active this wek so you don’t sell yourself short!” | 47% | 33% | 20% |
| After reaching the goal for week 1 “You’ve achieved your MVPA goal for Week 1! What a great way to kick off the program! But remember, you’re not done until you track all of your progress in the app!” | 33% | 53% | 13% |
| Entry Reminder/Reminder to Sync | |||
| “Recording your activity each day can help keep you on track. Have you recorded today’s activity minutes yet?” | 7% | 73% | 20% |
| “Have you recorded today’s physical activity in Fit2Thrive? Make sure to do this before the end of the day!” | 7% | 60% | 33% |
| “It’s not too late to enter today’s activities in Fit2Thrive, if you haven’t already done so!” | 27% | 53% | 20% |
| “Finished with your physical activity for today? Make sure to record it in Fit2Thrive!” | 13% | 73% | 13% |
| “Just a friendly reminder to enter your activities in Fit2Thrive!” | 7% | 93% |
*One participant preferred not to answer this question.
Discussion
The preparation phase of MOST is critical to developing an effective, feasible, and scalable behavioral intervention. The purpose of this study was to describe and report the results of the user-centered, iterative design process to develop and test the Fit2Thrive mobile app for use in a multicomponent physical activity intervention in breast cancer survivors. Overall, participants rated the app usability, including functionality, engagement, and aesthetics as “OK” or better. Importantly, participants rated that they “strongly agreed” the app had potential to change physical activity behavior in breast cancer survivors. These ratings conveyed confidence to the research team in the final app used in the subsequent physical activity intervention among breast cancer survivors that was updated based on feedback from the field testing before deployment.
Our field test consisted of two field tests groups with researchers and developers revising the app between field test 1 and field test 2 to incorporate field test 1 feedback and add additional app features. Keeping in mind that lower scores indicate better performance and satisfaction on a 7-point scale, overall PSSUQ results and each PSSUQ subscale result were lower (or appeared to improve) at field test 2 compared to field test 1, but were unable to test significance. uMARS results averaged at least a 3.0, corresponding to “OK,” or higher (on a five-point scale with higher score indicating better ratings) on overall scores and all subscale scores at field test 1 and field test 2. Furthermore, participants rated the app-specific behavior change subscale the highest with an average score of 4.0 ± 1.0 for field test 1 and 4.3 ± 0.9 for field test 2 on a 5-point scale, indicating that field test users believed use of the app would change physical activity behavior in breast cancer survivors, the intended app purpose. Collectively, these results suggest that the user-centered iterative process employed was successful in eliciting app satisfaction and performance.
While the use of a user-centered iterative design process to ensure that reliable, effective products are being tested through behavior change interventions may seem time-consuming, producing an app that users ultimately do not find meeting their needs is a much larger waste of resources in the long run. Additionally, there is a further elongation in the cycle of research dissemination [34]. Our results revealed that participants found the app useful and could see its potential even as they reported possible improvements and areas where the app could meet their needs to a greater degree. For example, in both field test groups, participants rated that the app could change physical activity behaviors. Although using commercially available apps may provide an “off the shelf” generalizable option compared to app development which remains a time consuming and intensive process, a large limitation looms in that companies often unexpectedly introduce proprietary updates or data changes that may significantly alter an intervention. Future research should additionally investigate the usefulness of a “hybrid” model, for example, an academic-industry partnership, in developing and disseminating apps to promote physical activity among specific populations.
This study had many strengths. We used a user-centered design approach to ensure that the app design was relevant and usable by the intended population group, breast cancer survivors. Our field tests were conducted in a free-living setting providing a greater reflection of real-world difficulties that might arise. Finally, we employed an iterative design process that allowed for integration of feedback and revision of the app prior to deploying it in a large-scale behavioral intervention. This provided us with additional confidence that any success or breakdown of the app intervention component was not due to poor usability of the app. Limitations include restricted generalizability as our sample was mostly white and college educated. In addition, we recruited only participants who already owned a Fitbit for the two field tests. That eligibility criterion may have biased participants to be more critical of the Fit2Thrive app since fitness apps were not as novel to them as to someone who was not already familiar with the Fitbit app. While the Fit2Thrive app and Fitbit app have some similarities, such as providing feedback on activity, the Fit2Thrive app incorporated additional features such as enrolling in physical activity challenges or goal setting including a scheduler to schedule activity into your day that would have been new for participants. Finally, our sample size was small; however, prior research suggests that 85% of usability issues can be identified with five people [35] and free responses indicated saturation within and between field testing groups.
Conclusions and Future Directions
We employed an iterative, user-centered app development process that included conducting two field testing groups and an app notification rating survey. Our process ensured a critical component of the Fit2Thrive physical activity behavior change intervention, and the Fit2Thrive app was empirically evaluated by the target population. Future research will test the effectiveness of the app to promote physical activity in breast cancer survivors using the MOST framework. Results from this trial will provide valuable information on the efficacy of a user-centered–developed physical activity promotion app in increasing physical activity behaviors in breast cancer survivors.
Funding
This study was funded by the National Cancer Institute grant number K07CA196840, awarded to Siobhan Phillips and the Robert H. Lurie Comprehensive Cancer Center.
Compliance with Ethical Standards
Conflicts of Interest: All authors declare that they have no conflicts of interest.
Author Contributions
WAW, PS, KLG: data management, formal analysis, interpretation, manuscript drafting and revisions; LA-G: formal analysis, interpretation, manuscript drafting and revisions; MW: interpretation, manuscript drafting and revisions; EC, EI: data management, interpretation, manuscript drafting and revisions; BS: conceptualization, methodology, resources, manuscript drafting and revisions; KSC, RA, DC, FP: conceptualization, methodology, manuscript drafting and revisions; SMP: conceptualization, methodology, data analysis and interpretation, resources, and manuscript drafting and revisions.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Also, this article does not contain any studies with animals performed by any of the authors.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Transparency Statement
This study was not formally registered. The analysis plan was not formally pre-registered. De-identified data from this study are not available in a public archive. De-identified data from this study will be made available (as allowable according to the institutional IRB standards) by emailing the corresponding author. Some of the materials used to conduct the study are presented in a public archive: uMARS https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4920963/#!po=62.5000; PSSUQ: https://www.tandfonline.com/doi/pdf/10.1080/10447318.2002.9669130.
References
- 1. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 2. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Cmaj. 2006;175(1):34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007;16(1):104–121. [DOI] [PubMed] [Google Scholar]
- 4. Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87–100. [DOI] [PubMed] [Google Scholar]
- 5. Fong DY, Ho JW, Hui BP, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. Bmj. 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arem H, Mama SK, Duan X, Rowland JH, Bellizzi KM, Ehlers DK. Prevalence of Healthy Behaviors among Cancer Survivors in the United States: how far have we come? Cancer Epidemiol Biomarkers Prev. 2020;29(6):1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elavsky S, Knapova L, Klocek A, Smahel D. Mobile health interventions for physical activity, sedentary behavior, and sleep in adults aged 50 years and older: a systematic literature review. J Aging Phys Act. 2019;27(4):565–593. [DOI] [PubMed] [Google Scholar]
- 8. Böhm B, Karwiese SD, Böhm H, Oberhoffer R. Effects of mobile health including wearable activity trackers to increase physical activity outcomes among healthy children and adolescents: systematic review. JMIR Mhealth Uhealth. 2019;7(4):e8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poppe L, De Bourdeaudhuij I, Verloigne M, et al. Efficacy of a self-regulation-based electronic and mobile health intervention targeting an active lifestyle in adults having type 2 diabetes and in adults aged 50 years or older: two randomized controlled trials. J Med Internet Res. 2019;21(8):e13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts AL, Fisher A, Smith L, Heinrich M, Potts HWW. Digital health behaviour change interventions targeting physical activity and diet in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2017;11(6):704–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. St George SM, Noriega Esquives B, Agosto Y, et al. Development of a multigenerational digital lifestyle intervention for women cancer survivors and their families. Psychooncology. 2020;29(1):182–194. [DOI] [PubMed] [Google Scholar]
- 12. Potdar R, Thomas A, DiMeglio M, et al. Access to internet, smartphone usage, and acceptability of mobile health technology among cancer patients. Support Care Cancer. 2020;28(11):5455–5461. [DOI] [PubMed] [Google Scholar]
- 13. Allicock M, Kendzor D, Sedory A, et al. A pilot and feasibility mobile health intervention to support healthy behaviors in African American Breast Cancer Survivors. J Racial Ethn Health Disparities. 2021;8(1):157–165. [DOI] [PubMed] [Google Scholar]
- 14. Phillips SM, Courneya KS, Welch WA, et al. Breast cancer survivors’ preferences for mHealth physical activity interventions: findings from a mixed methods study. J Cancer Surviv. 2019;13(2):292–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McNeil J, Brenner DR, Stone CR, et al. Activity tracker to prescribe various exercise intensities in breast cancer survivors. Med Sci Sports Exerc. 2019;51(5):930–940. [DOI] [PubMed] [Google Scholar]
- 16. Lynch BM, Nguyen NH, Moore MM, et al. A randomized controlled trial of a wearable technology-based intervention for increasing moderate to vigorous physical activity and reducing sedentary behavior in breast cancer survivors: the ACTIVATE Trial. Cancer. 2019;125(16):2846–2855. [DOI] [PubMed] [Google Scholar]
- 17. Uhm KE, Yoo JS, Chung SH, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2017;161(3):443–452. [DOI] [PubMed] [Google Scholar]
- 18. Roberts AL, Potts HW, Koutoukidis DA, Smith L, Fisher A. Breast, prostate, and colorectal cancer survivors’ experiences of using publicly available physical activity mobile apps: qualitative study. JMIR Mhealth Uhealth. 2019;7(1):e10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Short CE, Finlay A, Sanders I, Maher C. Development and pilot evaluation of a clinic-based mHealth app referral service to support adult cancer survivors increase their participation in physical activity using publicly available mobile apps. BMC Health Serv Res. 2018;18(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ainsworth MC, Pekmezi D, Bowles H, et al. Acceptability of a mobile phone app for measuring time use in breast cancer survivors (life in a day): mixed-methods study. JMIR Cancer. 2018;4(1):e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Forbes CC, Blanchard CM, Mummery WK, Courneya KS. A pilot study on the motivational effects of an internet-delivered physical activity behaviour change programme in Nova Scotian cancer survivors. Psychol Health. 2017;32(2):234–252. [DOI] [PubMed] [Google Scholar]
- 22. Pew Research Center [Internet]. Available from: https://www.pewresearch.org/internet/fact-sheet/mobile/. Date accessed 22 October 2020.
- 23. Center PR [Internet]. Available from: https://www.pewresearch.org/fact-tank/2020/01/09/about-one-in-five-americans-use-a-smart-watch-or-fitness-tracker/. Date accessed 22 October 2020.
- 24. Harvey EJ, Rubin LF, Smiley SL, Zhou Y, Elmasry H, Pearson JL. Mobile phone ownership is not a serious barrier to participation in studies: descriptive study. JMIR Mhealth Uhealth. 2018;6(2):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martín Payo R, Harris J, Armes J. Prescribing fitness apps for people with cancer: a preliminary assessment of content and quality of commercially available apps. J Cancer Surviv. 2019;13(3):397–405. [DOI] [PubMed] [Google Scholar]
- 26. Phillips SM, Collins LM, Penedo FJ, et al. Optimization of a technology-supported physical activity intervention for breast cancer survivors: Fit2Thrive study protocol. Contemp Clin Trials. 2018;66:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collins LM. Optimization of Behavioral, Biobehavioral, and Biomedical Interventions. Cham, Switzerland: Springer International Publishing; 2018. [Google Scholar]
- 28. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stacey FG, James EL, Chapman K, Courneya KS, Lubans DR. A systematic review and meta-analysis of social cognitive theory-based physical activity and/or nutrition behavior change interventions for cancer survivors. J Cancer Surviv. 2015;9(2):305–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. [DOI] [PubMed] [Google Scholar]
- 31. Lewis JR. Psychometric evaluation of the PSSUQ using data from five years of usability studies. Int J Hum-Comput Int. 2002;14(3–4):463–88. [Google Scholar]
- 32. Stoyanov SR, Hides L, Kavanagh DJ, Wilson H. Development and validation of the User Version of the Mobile Application Rating Scale (uMARS). JMIR Mhealth Uhealth. 2016;4(2):e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. [DOI] [PubMed] [Google Scholar]
- 34. Rabin BA, Glasgow RE, Kerner JF, Klump MP, Brownson RC. Dissemination and implementation research on community-based cancer prevention: a systematic review. Am J Prev Med. 2010;38(4):443–456. [DOI] [PubMed] [Google Scholar]
- 35. van der Weegen S, Verwey R, Tange HJ, Spreeuwenberg MD, de Witte LP. Usability testing of a monitoring and feedback tool to stimulate physical activity. Patient Prefer Adherence. 2014;8:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]