Abstract
Different immunoassays using recombinant antigens or synthetic peptides were evaluated for the serodiagnosis of Chlamydia trachomatis infections. Antigens used included cysteine-rich outer membrane protein 2 (OMP2), heat shock protein 60, the polypeptide encoded by open reading frame 3 of the plasmid (pgp3), synthetic peptides derived from species-specific epitopes in variable domain IV of the major OMP (MOMP) (Labsystems, Helsinki, Finland), and a fragment of the total lipopolysaccharide (Medac, Hamburg, Germany). Because cross-reactions between chlamydial species have been reported, Chlamydia pneumoniae-specific antibodies were also determined by immunoassays (Labsystems). Responses obtained with serum samples from patients with well-defined diseases (i.e., urethral or endocervical samples from which C. trachomatis DNA was amplified) were compared to those obtained with samples from healthy blood donors. The best sensitivity (79%) associated with the best specificity (82%) was obtained when immunoglobulin G (IgG) responses to both MOMP and pgp3 were considered. The highest sensitivity (89%) was obtained with anti-OMP2 IgG, but the lowest specificity (57%) was obtained with this antibody, due to probable cross-reactivity with C. pneumoniae OMP2.
Among the different antigens used to detect antibodies to Chlamydia trachomatis, large differences in sensitivity and specificity can be observed (3). The cysteine-rich protein outer membrane protein 2 (OMP2) has been found to be a major immunogen in chlamydial infections in an assay using denatured and truncated fusion proteins (9). As OMP2 had not been compared to other Chlamydia antigens, we investigated whether it was more sensitive and specific than four other antigens previously found to have the best diagnostic values in the serodiagnosis of C. trachomatis infection (3). Two of these antigens were used in commercially available enzyme-linked immunosorbent assays (ELISA). One detected antibodies to species-specific epitopes in variable domain IV of the major OMP (MOMP) of C. trachomatis (Labsystems Research Laboratory, Helsinki, Finland). The other was based on an exclusively Chlamydia-specific recombinant fragment of the total lipopolysaccharide (LPS) (3-deoxy-d-manno-2-octulopyranosonic acid) (Medac GmbH, Hamburg, Germany). The two remaining antigens were recombinant proteins: heat shock protein 60 (hsp60) and the polypeptide encoded by open reading frame 3 of the plasmid (pgp3), used also in ELISA (3). They were prepared and tested in exactly the same way as OMP2, except that OMP2 was prepared under denaturing conditions.
As OMP2 is a highly conserved structural protein among all Chlamydia species, anti-C. pneumoniae-specific antibodies were also determined with commercially available ELISA (Labsystems Research Laboratory) to investigate potential cross-reactivity.
The sensitivity was evaluated for samples from patients with well-defined disease (i.e., urethral or endocervical samples from which C. trachomatis DNA was amplified), and the specificity was evaluated for those from healthy blood donors.
MATERIALS AND METHODS
Patients.
Serum samples were stored at −70°C until they were processed. The study subjects were categorized into one of the following two groups: group 1 subjects (n = 28; median age, 31 years; 25% were female) were patients with acute C. trachomatis urogenital infection as determined by C. trachomatis DNA amplification from urethral or endocervical samples by the Amplicor test (Roche Diagnostic Systems, Branchburg, N.J.); group 2 subjects (n = 56; median age, 45 years; 30% were female) were healthy blood donors.
Recombinant protein preparation.
hsp60 and pgp3 antigen preparation was done as previously described (3). OMP2 was prepared in the same way. Template DNA for the PCR was obtained from C. trachomatis serovar D, strain UW-3/Cx, purchased from the American Type Culture Collection (catalogue no. VR-885). A 1,644-bp fragment (GenBank accession no. M23001) was amplified (1). Oligonucleotides used as primers were designed with 5′ NcoI (5′-CATGccatggACAAACTCATCAGACGAGCAGTGACG-3′) and 3′ BamHI (5′-CGggatccATAGATGTGTGTATTCTCTGTATCA-3′) restriction endonuclease sites (lowercase letters) and were synthesized by Microsynth (Balgach, Switzerland). The pQE-60 vector (Qiagen, Chatsworth, Calif.) was chosen to append a six-histidine tag to the C termini for large-scale purification via nickel chelate affinity chromatography. In order to change an incorrect base introduced at the cloning step, in vitro site-directed mutagenesis was performed with a QuickChange site-directed mutagenesis kit from Stratagene. For the amplification reaction, 5′- and 3′-end oligonucleotide primers containing the desired mutation were 5′-AGAAATTAACCATGAACAAACTCATCAGACGAGC-3′, where the boldface A indicates the desired mutation and 5′-GCTCGTCTGATGAGTTTGTTCATGGTTAATTTCT-3′, respectively.
Measurement of immunoglobulin G (IgG) and IgA antibodies against six-His-tagged recombinant proteins by ELISA and commercially available ELISA.
Experimental conditions were as previously described (3).
Calculations.
Sensitivity, specificity, positive predictive value, negative predictive value, false-positive and false-negative rates, and agreement were calculated as described previously (2).
RESULTS
Analysis of purified recombinant proteins.
Protein solubility was determined according to the Qiagen protocol. OMP2 was found to be present with insoluble matter and was therefore purified under denaturing conditions.
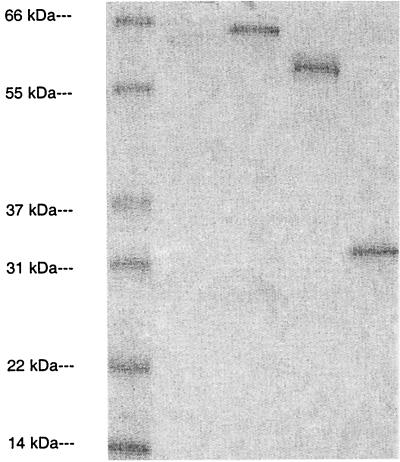
After migration by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining (Fig. 1), purified proteins showed a band representing the expected apparent molecular weight.
FIG. 1.
The separation of expressed recombinant proteins was performed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and proteins were visualized after Coomassie blue staining. Lanes from left to right: molecular mass standards, OMP2, hsp60, and pgp3.
Diagnostic values of different antibody determinations.
When each assay was considered individually, the highest sensitivity was obtained with anti-OMP2 IgG (89%), but this antibody also showed the lowest specificity (57%); the highest specificity (89%) but the lowest sensitivity (57%) was obtained with anti-pgp3 IgG (Table 1).
TABLE 1.
Best sensitivities, specificities, positive and negative predictive values, false-positive and -negative values, and agreement obtained for the different anti-Chlamydia antibody assaysa
| Anti-Chlamydia IgG assay | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | False-positive rate (%) | False-negative rate (%) | Agreement (%) |
|---|---|---|---|---|---|---|---|
| Anti-LPS | 82 | 70 | 58 | 89 | 30 | 18 | 74 |
| Anti-MOMP | 61 | 84 | 65 | 81 | 16 | 39 | 76 |
| Anti-hsp60 | 61 | 77 | 57 | 80 | 23 | 39 | 71 |
| Anti-pgp3 | 57 | 89 | 73 | 81 | 11 | 43 | 79 |
| Anti-OMP2 | 89 | 57 | 51 | 91 | 43 | 11 | 68 |
Positive patients were 28 patients with C. trachomatis urogenital infection, and negative individuals were 56 healthy blood donors.
When different combinations of two antibodies were tested, the same sensitivity of 89% was obtained in all combinations using anti-OMP2 IgG but the specificity was ≤64%. The best combination of specificity (82%) and sensitivity (79%) was observed when the number of individuals with IgG responses to MOMP and pgp3 was considered. Results with these combined antibodies had the best agreement (81%) (Table 2).
TABLE 2.
Best sensitivities, specificities, positive and negative predictive values, false-positive and -negative values, and agreement obtained for different combinations of anti-Chlamydia antibody assaysa
| Anti-Chlamydia IgG assays | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | False-positive rate (%) | False-negative rate (%) | Agreement (%) |
|---|---|---|---|---|---|---|---|
| Anti-LPS and anti-MOMP | 82 | 61 | 51 | 87 | 39 | 18 | 68 |
| Anti-LPS and anti-hsp60 | 82 | 59 | 50 | 87 | 41 | 18 | 67 |
| Anti-LPS and anti-pgp3 | 89 | 64 | 56 | 92 | 36 | 11 | 73 |
| Anti-LPS and anti-OMP2 | 89 | 43 | 44 | 89 | 57 | 11 | 58 |
| Anti-MOMP and anti-hsp60 | 71 | 66 | 51 | 82 | 34 | 29 | 68 |
| Anti-MOMP and anti-pgp3 | 79 | 82 | 69 | 88 | 18 | 21 | 81 |
| Anti-MOMP and anti-OMP2 | 89 | 55 | 50 | 91 | 45 | 11 | 67 |
| Anti-hsp60 and anti-pgp3 | 75 | 73 | 58 | 85 | 27 | 25 | 74 |
| Anti-hsp60 and anti-OMP2 | 89 | 52 | 48 | 91 | 48 | 11 | 64 |
| Anti-pgp3 and anti-OMP2 | 89 | 57 | 51 | 91 | 43 | 11 | 68 |
Positive patients were 28 patients with C. trachomatis urogenital infection, and negative individuals were 56 healthy blood donors.
Comparison of IgG responses to OMP2 with responses to other antigens.
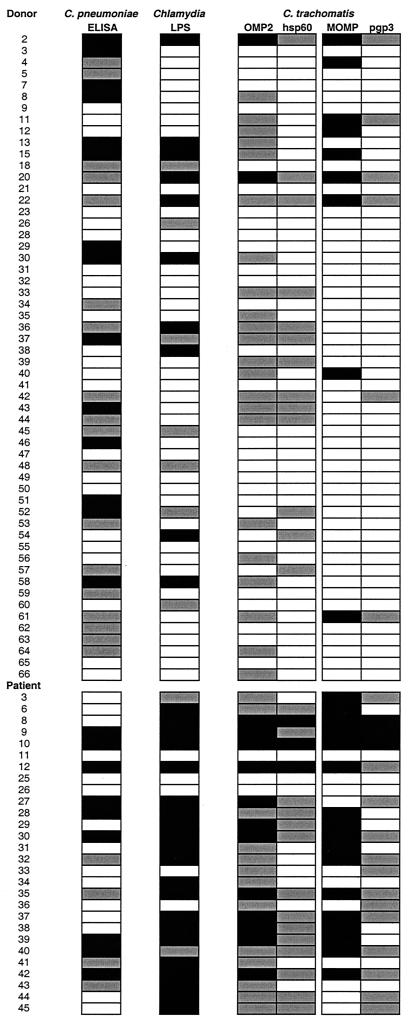
To determine what types of response accompanied IgG antibodies to OMP2, we examined the binding of IgG to the C. pneumoniae antigen and to genus-specific (LPS) and highly conserved (hsp60) antigens, as well as to C. trachomatis MOMP and pgp3, for each individual (Fig. 2). This analysis showed that among 49 samples positive for anti-OMP2 IgG, 18 (37%) had no anti-C. trachomatis MOMP or anti-pgp3 antibodies. Three samples (6%) had only anti-OMP2 IgG, three had only anti-C. pneumoniae antibodies (donors 8, 53, and 64), three had only IgG to a genus-specific (LPS) or highly conserved (hsp60) antigen (donors 33 and 39 and patient 34), and nine (18%) had IgG to both C. pneumoniae antigen and LPS or hsp60 antigen, in addition to anti-OMP2 IgG antibodies.
FIG. 2.
Sera from healthy blood donors or C. trachomatis-infected patients tested for anti-C. pneumoniae IgG (ELISA kit from Labsystems), anti-Chlamydia LPS antibodies (ELISA kit from Medac), and anti-C. trachomatis antibodies (ELISA kit for anti-MOMP antibodies from Labsystems; anti-OMP2, anti-hsp60, and anti-pgp3 antibody assays were developed in our laboratory). For anti-C. pneumoniae IgG, high reactivities (>100) are depicted as black boxes and lower reactivities (>45 to 100) are depicted as gray boxes. For anti-LPS, anti-MOMP, anti-OMP2, anti-hsp60, and anti-pgp3 IgG, high reactivities (result greater than or equal to the cutoff value with an optical density of >1.0) are depicted as black boxes and lower reactivities (result greater than or equal to the cutoff value with an optical density of <1.0) are depicted as gray boxes. For all tests, reactivities below the cutoff values are depicted as white boxes.
It should also be noted that among 13 donors with anti-hsp60 IgG, 9 (69%) had no anti-C. trachomatis MOMP or anti-pgp3 antibodies.
DISCUSSION
In order to improve the reliability of serological tests in the diagnosis of C. trachomatis infection, we compared the diagnostic values of different antigens used in immunoassays. The highest sensitivities (89 and 82%) were observed for IgG reactivity to OMP2 and LPS, respectively, but these antibodies also showed the lowest specificities (57 and 70%, respectively). These results confirm observations by Mygind et al. of OMP2 (9) and previous observations of LPS (3, 4). These low specificities were attributed to the high prevalence of anti-C. pneumoniae antibodies in the population (6, 8) and the fact that OMP2 (5, 10) and LPS are genus-specific antigens. The fact that 37% of anti-OMP2-positive samples had no anti-C. trachomatis MOMP or pgp3 antibodies supports the hypothesis that another species of Chlamydia (pneumoniae or maybe psittaci) might be the triggering bacterium.
Another antigen commonly used in immunoassays is hsp60. It is another conserved protein, and C. trachomatis hsp60 and C. pneumoniae hsp60 have been shown to be immunologically similar (7, 11). In the present study, anti-hsp60 IgG determination appears less specific (77%) than anti-MOMP IgG (84%) or anti-pgp3 IgG (89%) determination, and again, in the cases of four donors, anti-hsp60 IgG was associated with anti-C. pneumoniae IgG but not with anti-C. trachomatis MOMP or pgp3. These observations suggest that cross-reactivity with C. pneumoniae could be responsible for some anti-hsp60 responses.
A higher specificity was observed here than in a previous study, which also evaluated serum responses in patients with acute C. trachomatis infections and healthy blood donors (3), except with anti-hsp60 IgG. The difference might be explained by the fact that the healthy blood donor population of the present study was older (median age, 45 years, compared to 28 years for the blood donors of the previous study) and had a lower incidence of C. trachomatis infection.
In conclusion, the anti-OMP2 antibody test appears of marginal usefulness due to its nonspecificity. Two specific serological assays using MOMP and pgp3 as antigens are apparently able to discriminate among various Chlamydia species and may serve to avoid both overestimating and underestimating the prevalence of C. trachomatis infection.
ACKNOWLEDGMENTS
The technical assistance of Yvette Froment and Ursula Spenato is gratefully acknowledged.
This research was supported by grant no. 32-47299.96 from the Fonds National Suisse de la Recherche Scientifique.
REFERENCES
- 1.Allen J E, Stephens R S. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989;171:285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bas S, Cunningham T, Kvien T K, Glennas A, Melby K, Vischer T L. The value of isotype determination of serum antibodies against Chlamydia for the diagnosis of Chlamydia reactive arthritis. Br J Rheumatol. 1996;35:542–547. doi: 10.1093/rheumatology/35.6.542. [DOI] [PubMed] [Google Scholar]
- 3.Bas S, Muzzin P, Ninet B, Bornand J E, Scieux C, Vischer T L. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J Clin Microbiol. 2001;39:1368–1377. doi: 10.1128/JCM.39.4.1368-1377.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bas S, Vischer T L. Chlamydia trachomatis antibody detection and diagnosis of reactive arthritis. Br J Rheumatol. 1998;37:1054–1059. doi: 10.1093/rheumatology/37.10.1054. [DOI] [PubMed] [Google Scholar]
- 5.Freidank H M, Herr A S, Jacobs E. Identification of Chlamydia pneumoniae-specific protein antigens in immunoblots. Eur J Clin Microbiol Infect Dis. 1993;12:947–951. doi: 10.1007/BF01992171. [DOI] [PubMed] [Google Scholar]
- 6.Karvonen M, Tuomilehto J, Pitkaniemi J, Naukkarinen A, Saikku P. Chlamydia pneumoniae IgG antibody prevalence in south-western and eastern Finland in 1982 and 1987. Int J Epidemiol. 1994;23:176–184. doi: 10.1093/ije/23.1.176. [DOI] [PubMed] [Google Scholar]
- 7.Kikuta L C, Puolakkainen M, Kuo C-C, Campbell L A. Isolation and sequence analysis of the Chlamydia pneumoniae GroE operon. Infect Immun. 1991;59:4665–4669. doi: 10.1128/iai.59.12.4665-4669.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo C-C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mygind P, Christiansen G, Persson K, Birkelund S. Analysis of the humoral immune response to Chlamydia outer membrane protein 2. Clin Diagn Lab Immunol. 1998;5:313–318. doi: 10.1128/cdli.5.3.313-318.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagar E A, Schachter J, Bavoil P, Stephens R S. Differential human serologic response to two 60,000 molecular weight Chlamydia trachomatis antigens. J Infect Dis. 1990;162:922–927. doi: 10.1093/infdis/162.4.922. [DOI] [PubMed] [Google Scholar]
- 11.Yuan Y, Lyng K, Zhang Y-X, Rockey D D, Morrison R P. Monoclonal antibodies define genus-specific, species-specific, and cross-reactive epitopes of the chlamydial 60-kilodalton heat shock protein (hsp60): specific immunodetection and purification of chlamydial hsp60. Infect Immun. 1992;60:2288–2296. doi: 10.1128/iai.60.6.2288-2296.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]