Abstract
The single-chained sphingolipid sphingosine is an essential structural lipid and signaling molecule. Abnormal sphingosine metabolism is observed in several diseases, including cancer, diabetes, and Alzheimer’s. Despite its biological importance, there is a lack of tools for detecting sphingosine in living cells. This is likely due to the broader challenge of developing highly selective and live-cell compatible affinity probes for hydrophobic lipid species. In this work, we have developed a small molecule fluorescent turn-on probe for labeling sphingosine in living cells. We demonstrate that this probe exhibits a dose-dependent response to sphingosine and is able to detect endogenous pools of sphingosine. Using our probe, we successfully detected sphingosine accumulation in cells from patients with Niemann–Pick type C1 (NPC1), a lipid transport disorder in which increased sphingosine mediates disease progression. This work provides a simple and accessible method for the detection of sphingosine and should facilitate study of this critical signaling lipid in biology and disease.
Sphingolipids are a diverse class of lipids defined by their long-chain amino alcohol backbones. In eukaryotes, these lipids play essential roles in both membrane structure and cell signaling pathways.1 Several sphingolipid species such as ceramides, sphingomyelin, glucosylceramide, sphingosine, and sphingosine-1-phosphate have emerged as integral signaling molecules in cell proliferation,2 apoptosis,3 migration,4 inflammation,5 and intracellular trafficking.6 Due to their central role in cellular function, disruption of sphingolipid metabolism can have devastating biological effects. Altered sphingolipid levels are associated with several diseases such as diabetes,7 cancer,8 Alzheimer’s,9 and lysosomal storage diseases.10,11 Because of their biological and clinical importance, there is tremendous interest in detecting and quantifying sphingolipids in cells and biological samples. Mass spectrometry-based methods are the current standard for detecting cellular sphingolipids.12,13 These techniques have made it possible to accurately measure the abundance of specific sphingolipids in a wide range of samples but often require sophisticated instrumentation and preclude the non-destructive analysis of lipids in living cells. Alternatively, fluorescently labeled lipids have been used to indirectly study sphingolipid localization in cells but provide no information about endogenous lipid concentrations.14,15 Recently, methods have been developed for the live-cell imaging of sphingomyelin using fluorescent protein fusions of the sphingomyelin binding protein lysenin; however, the extension of this approach to other sphingolipids has been limited.16,17 Sphingosine (Sph) and sphinganine (Spa) are single-chained sphingolipids which not only serve as the backbones of more complex sphingolipids but also have important biological signaling activity.18,19 They play essential roles in cellular function and diseases such as cancer20 and Niemann–Pick disease type C (NPC).10 Unfortunately, dissecting the exact function and behavior of sphingosines in biology and disease has been difficult due to the lack of effective techniques for their imaging and detection in live cells. The development of such tools is critical for the biological understanding, diagnosis, and treatment of sphingolipid-associated diseases.
Sphingosine and sphinganine have unique terminal 1,2-amino alcohol functionality. Recent studies have shown that fatty acid salicylaldehyde esters and 1,2-amino alcohols, such as those found in Sph and Spa, can react selectively under biological conditions.21,22 We hypothesized that a fluorophore ester of salicylaldehyde could be used as a probe for the detection of endogenous Sph and Spa. Additionally, this probe could be made fluorogenic by functionalizing the salicylaldehyde auxiliary with a fluorescence quencher, diminishing the fluorescence of the fluorophore ester. Upon reaction with Sph or Spa, the fluorophore would be transferred from the salicylaldehyde scaffold to the sphingolipid base and become unquenched due to its decreased proximity to the quencher (Figure 1A).23 In mammalian cells, Sph is 10-fold more abundant than Spa and would be the predominant species detected by such a probe.24
Figure 1.
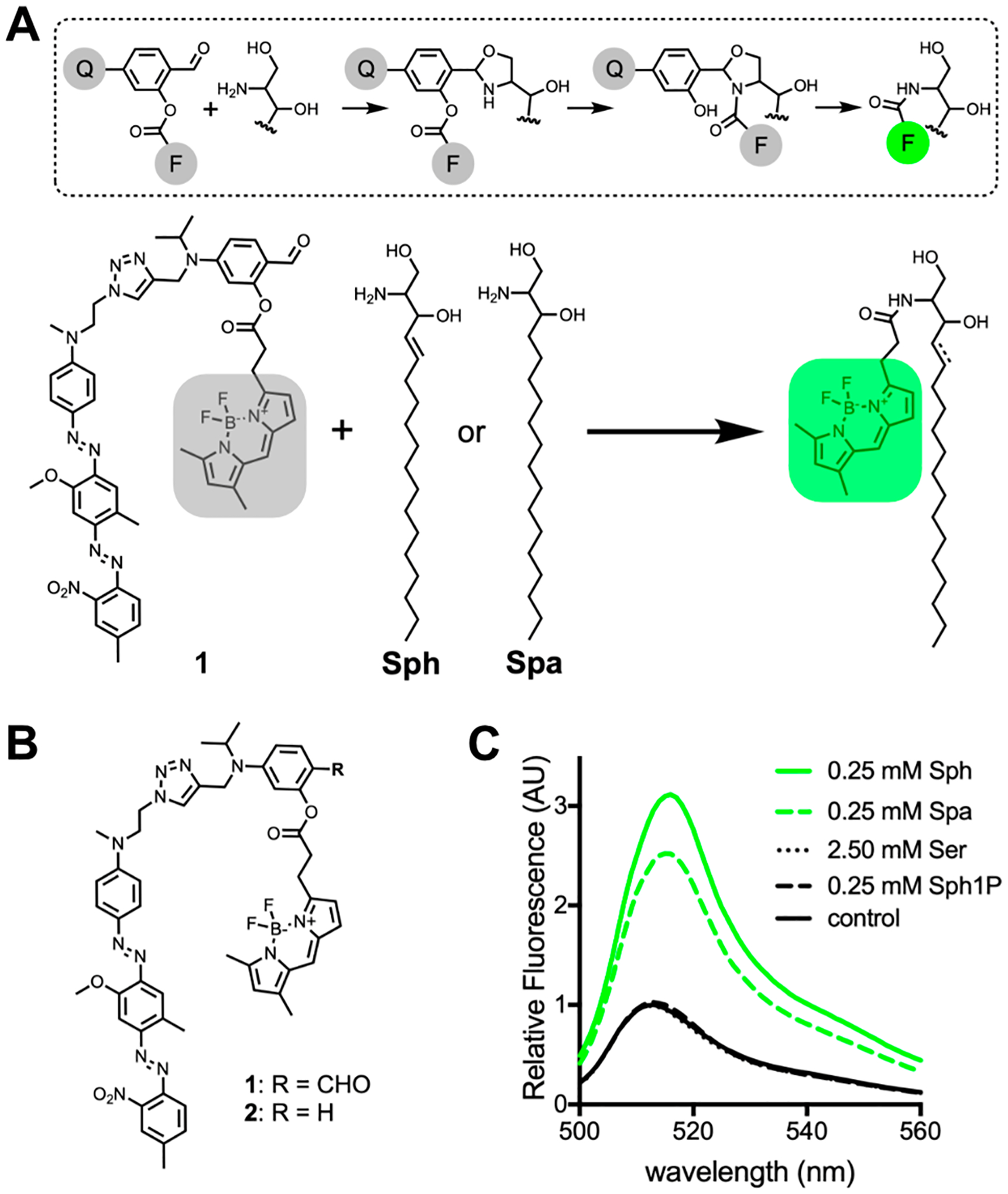
(A) Proposed mechanism for the reaction between the salicylaldehyde-containing probe (1) and Sph or Spa. During the reaction, the fluorescent dye “F” is covalently attached to the sphingolipid base and separated from quencher “Q”, resulting in a highly fluorescent lipid product. (B) Chemical structure of probes synthesized in this study. (C) Fluorescence emission spectra of samples containing 1 (5 μM) after 24 h incubation in a DOPC liposome solution containing Sph, Spa, serine (Ser), sphingosine-1-phosphate (Sph1P), or buffer (control).
In designing our probe, we chose Bodipy FL as the fluorophore because its neutral charge and lipophilicity enhance cell permeability and partitioning into biological membranes.25 As a quencher, we chose Black Hole Quencher-1 (BHQ-1) due to its high quenching efficiency at the 509 nm emission maximum of Bodipy FL (Figure S1).26 In previous work, we determined that 4-(diethylamino)salicylaldehyde esters provided optimal hydrolytic stability under physiological conditions.21 Therefore, we designed the reactive core of our probe 1 to include analogous 4-dialkylamino functionality (Figure 1B). Additionally, we synthesized a control probe 2, which is identical to 1 except that it lacks the aldehyde necessary for selective reaction with terminal 1,2-amino alcohol groups and thus should not react with sphingolipid bases in the cell (Figure 1B).21,22 Detailed synthetic procedures for 1 and 2 are provided in the Supporting Information. Using vesicles to model biological membranes, we tested the ability of compounds 1 and 2 to react with Sph and Spa under physiological conditions (pH 7.4, 37 °C). We found that 1 showed good stability (<4% hydrolysis over 24 h) and reacted with both Sph and Spa to form the expected fluorescently labeled lipids (Figures S2 and S3). Control probe 2 was stable under these conditions and showed no reaction with Sph or Spa over 24 h (Figure S4). To determine if our probe is compatible with common cellular lipids, we incubated 1 in vesicles composed of several naturally abundant lipid species for 24 h (Figure S5A–S5D).27 Under these conditions, we observed no ligation products between 1 and any of the tested lipids, although 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS) did appear to slightly accelerate background hydrolysis of the probe. Additionally, 1 did not form ligation products with free cysteine, ethanolamine, cysteamine, or cystamine at a concentration (1 mM) exceeding physiological levels,28–30 although some acceleration of probe hydrolysis was observed (Figure S5E–H).
Having confirmed the reactivity of 1 toward Sph and Spa, we next quantified the fluorescence turn-on of 1 in the presence of these sphingolipid bases and potentially competing biomolecules. We found that when 1 (5 μM) was incubated with Sph (0.25 mM) or Spa (0.25 mM) at 37 °C for 24 h, a 3- and 2.5-fold fluorescent turn-on respectively was observed. However, when 1 was incubated with serine (2.50 mM) or sphingosine-1-phosphate (Sph1P) (0.25 mM) under the same conditions, there was no change in fluorescence intensity as compared to untreated probe 1 (Figure 1C). These results agree with previous reports that, in the presence of phospholipid membranes, salicylaldehyde-modified lipids are only reactive toward molecules that are both lipophilic and contain 1,2-amino alcohol functionality.21 We were particularly encouraged to find that 1 did not react with Sph1P, which is identical in structure to sphingosine except that the primary alcohol is phosphorylated. The selective reaction and turn-on of our probe with Sph and Spa in membranes encouraged us to explore the utility of this tool in live cells.
As mentioned, Sph is roughly 10-fold more abundant than Spa in mammalian cells.24 Therefore, any observed response from probe 1 in live cells is likely attributable to Sph. For this reason, we chose to focus on Sph as the main analyte in our studies. To determine if 1 could react with Sph in live cells, we incubated HeLa cells with 1 (7.5 μM) for 2 h. We then exchanged cell media for new media containing varying concentrations of Sph. We found that after a 20 h incubation, cells treated with exogenous Sph showed a dose-dependent increase in fluorescence (Figure 2A, B). The turn-on of 1 in response to Sph was also confirmed in three additional cell lines (Figure S6). To ensure that the observed increase in fluorescence was due to the reaction of 1 with Sph, and not a biological effect of the added Sph, we performed the same experiment with control probe 2. We found that when cells were incubated with 2 (7.5 μM) and then treated with Sph, no significant difference in fluorescence was observed between nontreated cells and those treated with Sph (Figure 2A and 2C). Furthermore, to confirm that the observed increase in cellular fluorescence was the result of the generation of a labeled sphingosine product and not the release of free Bodipy FL carboxylic acid (BFL) from 1, we treated cells with the expected Bodipy FL-Sph product (3) and BFL (Figure S7A). We found that cells treated with 3 showed a membrane fluorescence pattern consistent with that observed in cells treated with 1 and sphingosine, while cells treated with BFL showed diffuse fluorescence, which did not stain cellular membranes (Figure S7B). These results indicate that 1 reacts with sphingosine in living cells to generate Bodipy FL-Sph in a dose-dependent manner.
Figure 2.
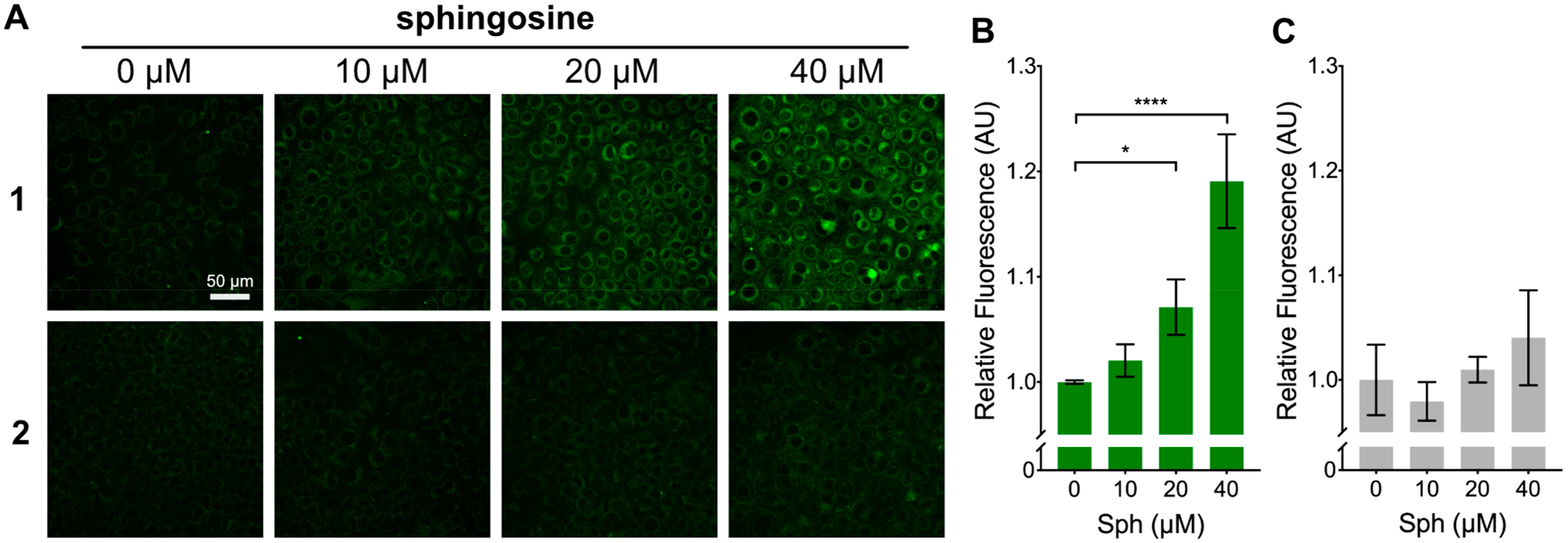
(A) Fluorescence microscopy images of HeLa cells treated with 1 and 2 and exposed to a range of Sph concentrations (0–40 μM) for 20 h. (B and C) Quantified fluorescence response of 1 and 2 within large populations of cells after treatment with Sph (0–40 μM). Values are reported as means ± SD. Statistically significant changes in fluorescence are indicated as determined by one-way ANOVA: *P < 0.05, ****P < 0.0001.
We next sought to apply this probe to the detection of endogenous levels of sphingosine in cells. We treated HeLa cells with 1 or 2 (20 μM) and imaged before and after a 16 h incubation. An increase in fluorescence (17.4%) was readily detected in cells treated with 1 over the course of the experiment (Figure 3A). In contrast, control probe 2 showed only a slight increase in fluorescence (4.7%), which was significantly less than probe 1 (Figure 3A, B). These results suggested that 1 is sensitive enough to detect native sphingosine in cells and may be used for probing differences in the sphingosine levels of cells.
Figure 3.
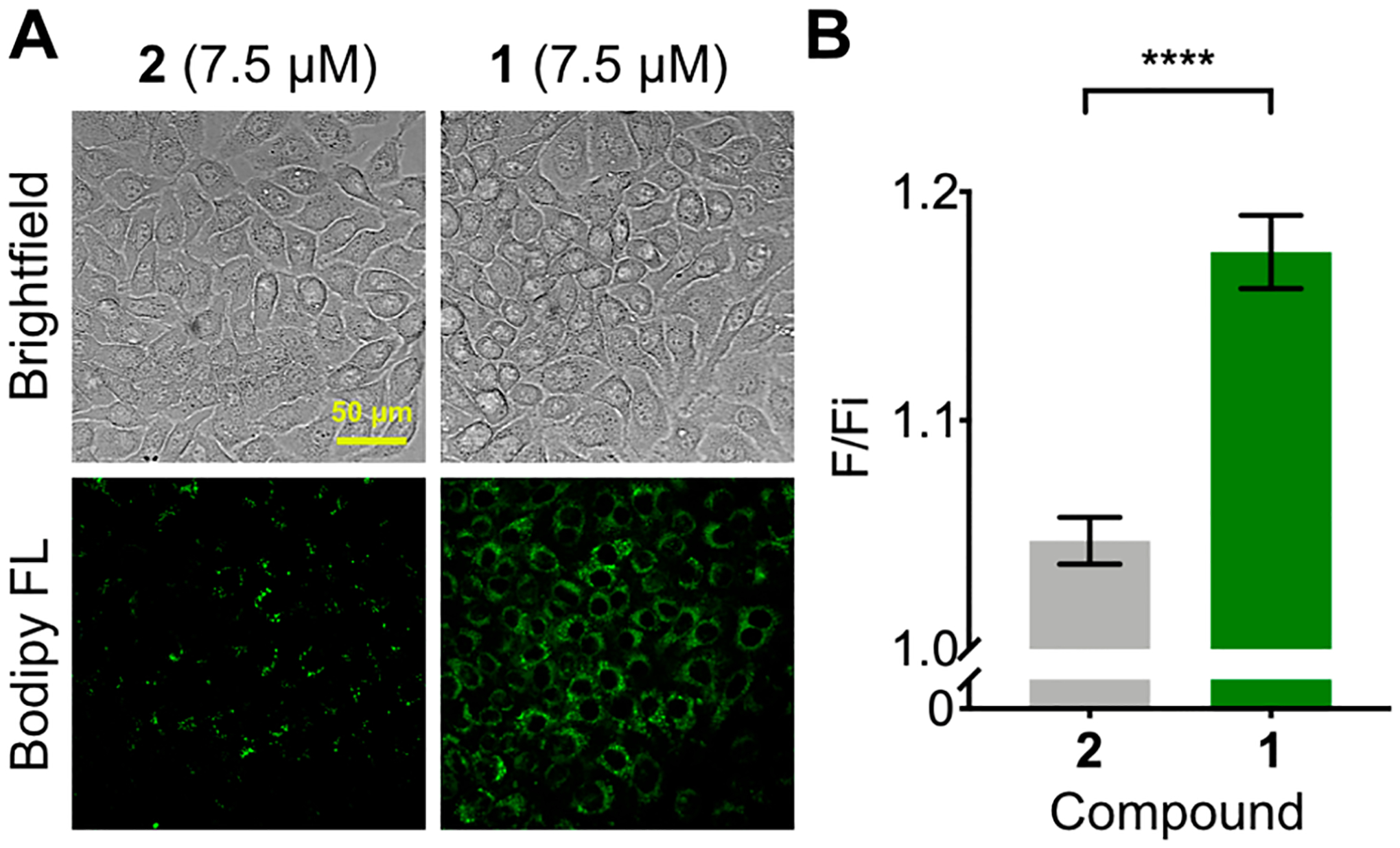
(A) Fluorescence microscopy images of endogenous sphingosine in HeLa cells treated with probe 1 and control probe 2 for 16 h. (B) Quantified fluorescence turn-on of 1 and 2 (final fluorescence [F]/ initial fluorescence [Fi]) within large populations of cells over 24 h. Values are reported as means ± SD. Significance was determined using an unpaired t test: ****P < 0.0001.
Changes in cellular sphingolipid levels occur in several diseases and often play a functional role in disease progression.31–33 Niemann–Pick type C1 (NPC1) is a lysosomal storage disorder caused by a mutation in the NPC1 gene, which encodes a large integral membrane protein (NPC1).34 The exact function of the NPC1 protein is not known, but when mutated in NPC1, it results in the accumulation of several lipids, including sphingosine, which plays a key role in promoting the disease phenotype.10 Patients with NPC1 feature a wide range of different neurological and systemic symptoms which differ from patient to patient, making an accurate diagnosis difficult. Current diagnostic standards include filipin staining and DNA sequencing, but these tests are not always confirmative.35 Therefore, a probe for detecting increases in cellular sphingosine could be a valuable tool for the diagnosis of NPC1 and similar disorders. To test the ability of 1 to detect increased sphingosine in NPC1, we cultured fibroblasts derived from healthy and NPC1 patients. We incubated these cells in the presence of 1 (7.5 μM) for 24 h.
We found that NPC1 cells showed a significantly higher fluorescence signal as compared to healthy fibroblasts (Figure 4). In line with our fluorescence results, we found that the cell line derived from an NPC1 patient had higher levels of Sph compared to healthy fibroblasts (Figure S8). Analysis of the same samples showed that Spa levels were elevated in NPC1 cells, but, as expected, were roughly 10-fold less abundant than Sph, suggesting the majority of the fluorescence increase was due to increased Sph levels (Figure S8).
Figure 4.
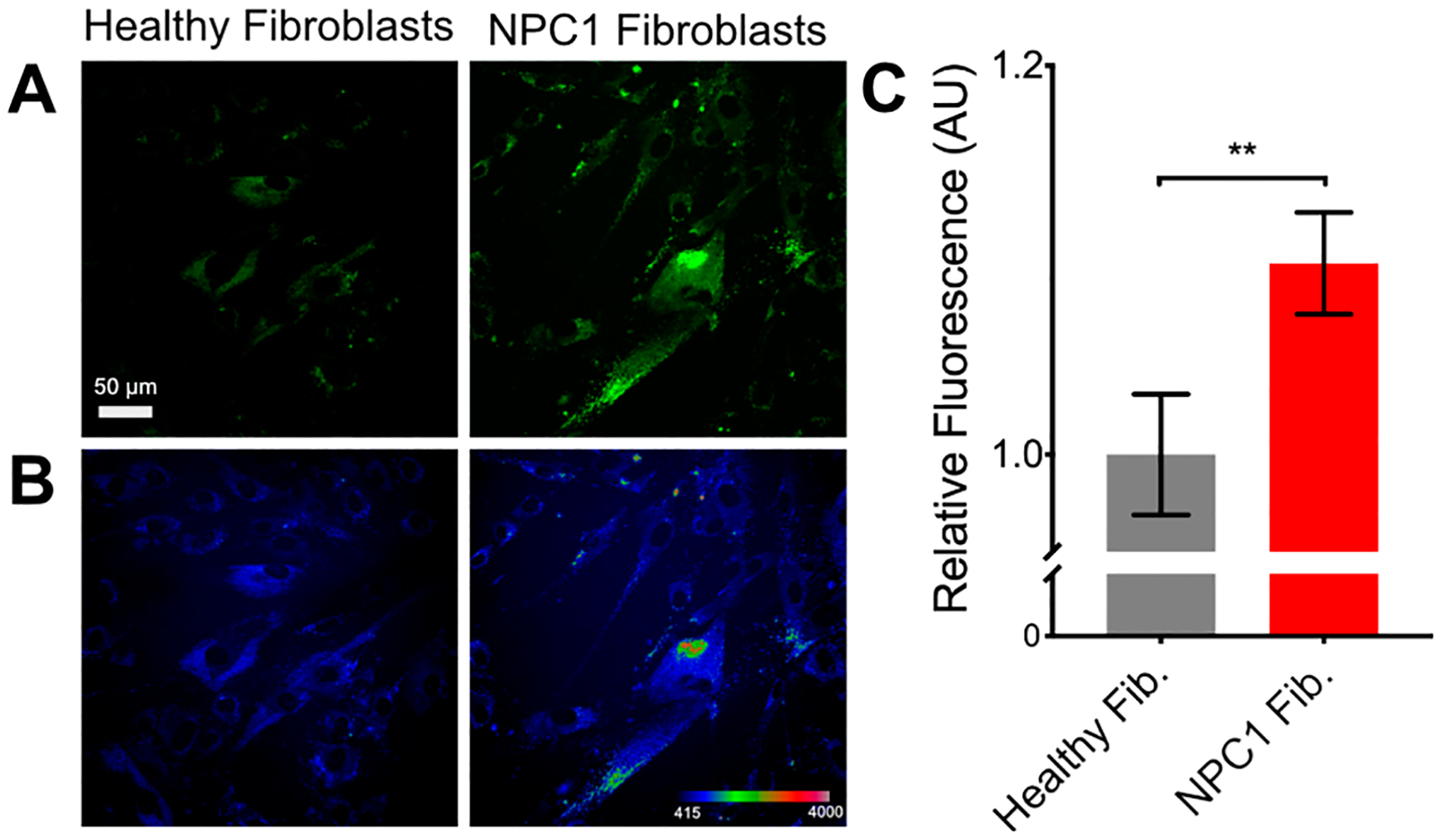
(A) Fluorescence microscopy images of Niemann–Pick disease type C1 (NPC1) patient-derived fibroblasts treated with probe 1 (7.5 μM) for 24 h. (B) Pixel intensity map of images in panel A. (C) Quantified fluorescence response of 1 within large populations of cells. Values are reported as means ± SD. Significance was determined using an unpaired t test: **P < 0.01.
In summary, we developed the first chemical probe for the fluorogenic detection of Sph in living cells. The probe allows for the concentration-dependent detection of Sph in cultured mammalian cells using standard fluorescence microscopy techniques.
While this probe is sufficient to detect biologically relevant changes in Sph levels, it has limitations which will be the subject of future study. First, 1 is subject to hydrolysis under biological conditions, which contributes to substantial fluorescence background. Second, the reaction rate of our probe is sluggish and requires long incubation times to illicit a sufficient fluorescence response. Third, quenching of Bodipy FL by BHQ1 in our probe is not complete, which may be improved by utilizing different fluorophore–quencher pairs. Finally, 1 has a low dynamic range, resulting in a small difference in fluorescence intensity between normal cells and cells with gross changes in Sph levels. In future work, we plan to explore different probe designs to improve probe stability and performance. We envision that this straightforward approach for the detection of specific sphingolipids will facilitate their study in biology and may hold promise as a diagnostic tool for lipid storage disorders like NPC1 and Gaucher disease.11
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from the National Institute of Health (DP2DK111801 and R01 CA234245). A.K.R. thanks the NIH/NCI for his support though the Ruth L. Kirschstein National Research Service Award (T32 CA009523). E.W.L. thanks the NIH for her support (T32 EB009380).
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c06652.
Supporting figures, detailed procedures, and spectral data (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.0c06652
The authors declare no competing financial interest.
Contributor Information
Andrew K. Rudd, Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, California 92093, United States.
Neel Mittal, Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, California 92093, United States.
Esther W. Lim, Department of Bioengineering, University of California San Diego, La Jolla, California 92093, United States
Christian M. Metallo, Department of Bioengineering, University of California San Diego, La Jolla, California 92093, United States
Neal K. Devaraj, Department of Chemistry and Biochemistry, University of California San Diego, La Jolla, California 92093, United States.
REFERENCES
- (1).Pruett ST; Bushnev A; Hagedorn K; Adiga M; Haynes CA; Sullards MC; Liotta DC; Merrill AH Thematic Review Series: Sphingolipids. Biodiversity of Sphingoid Bases (“Sphingosines”) and Related Amino Alcohols. J. Lipid Res 2008, 49, 1621–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Spiegel S; Milstien S Sphingosine-1-Phosphate: An Enigmatic Signalling Lipid. Nat. Rev. Mol. Cell Biol 2003, 4, 397–407. [DOI] [PubMed] [Google Scholar]
- (3).Ségui B; Andrieu-Abadie N; Jaffrézou J-P; Benoist H; Levade T Sphingolipids as Modulators of Cancer Cell Death: Potential Therapeutic Targets. Biochim. Biophys. Acta, Biomembr 2006, 1758, 2104–2120. [DOI] [PubMed] [Google Scholar]
- (4).Spiegel S Sphingosine 1-Phosphate Signaling: Providing Cells with a Sense of Direction. Trends Cell Biol 2002, 12, 236–242. [DOI] [PubMed] [Google Scholar]
- (5).Maceyka M; Spiegel S Sphingolipid Metabolites in Inflammatory Disease. Nature 2014, 510, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Sillence D; Platt F Glycosphingolipids in Endocytic Membrane Transport. Semin. Cell Dev. Biol 2004, 15, 409–416. [DOI] [PubMed] [Google Scholar]
- (7).Hla T; Kolesnick R C16:0-Ceramide Signals Insulin Resistance. Cell Metab 2014, 20, 703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Morad SAF; Cabot MC Ceramide-Orchestrated Signalling in Cancer Cells. Nat. Rev. Cancer 2013, 13, 51–65. [DOI] [PubMed] [Google Scholar]
- (9).He X; Huang Y; Li B; Gong C-X; Schuchman EH Deregulation of Sphingolipid Metabolism in Alzheimer’s Disease. Neurobiol. Aging 2010, 31, 398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lloyd-Evans E; Morgan AJ; He X; Smith DA; Elliot-Smith E; Sillence DJ; Churchill GC; Schuchman EH; Galione A; Platt FM Niemann–Pick Disease Type C1 Is a Sphingosine Storage Disease That Causes Deregulation of Lysosomal Calcium. Nat. Med 2008, 14, 1247–1255. [DOI] [PubMed] [Google Scholar]
- (11).Schonauer S; Körschen HG; Penno A; Rennhack A; Breiden B; Sandhoff K; Gutbrod K; Dörmann P; Raju DN; Haberkant P; Gerl MJ; Brügger B; Zigdon H; Vardi A; Futerman AH; Thiele C; Wachten D Identification of a Feedback Loop Involving β-Glucosidase 2 and Its Product Sphingosine Sheds Light on the Molecular Mechanisms in Gaucher Disease. J. Biol. Chem 2017, 292, 6177–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Sullards MC; Liu Y; Chen Y; Merrill AH Analysis of Mammalian Sphingolipids by Liquid Chromatography Tandem Mass Spectrometry (LC-MS/MS) and Tissue Imaging Mass Spectrometry (TIMS). Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2011, 1811, 838–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Jones EE; Dworski S; Canals D; Casas J; Fabrias G; Schoenling D; Levade T; Denlinger C; Hannun YA; Medin JA; Drake RR On-Tissue Localization of Ceramides and Other Sphingolipids by MALDI Mass Spectrometry Imaging. Anal. Chem 2014, 86, 8303–8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lipsky NG; Pagano RE Sphingolipid Metabolism in Cultured Fibroblasts: Microscopic and Biochemical Studies Employing a Fluorescent Ceramide Analogue. Proc. Natl. Acad. Sci. U. S. A 1983, 80, 2608–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Pagano RE; Martin OC; Kang HC; Haugland RP A Novel Fluorescent Ceramide Analogue for Studying Membrane Traffic in Animal Cells: Accumulation at the Golgi Apparatus Results in Altered Spectral Properties of the Sphingolipid Precursor. J. Cell Biol 1991, 113, 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Shogomori H; Kobayashi T Lysenin: A Sphingomyelin Specific Pore-Forming Toxin. Biochim. Biophys. Acta, Gen. Subj 2008, 1780, 612–618. [DOI] [PubMed] [Google Scholar]
- (17).Abe M; Makino A; Hullin-Matsuda F; Kamijo K; Ohno-Iwashita Y; Hanada K; Mizuno H; Miyawaki A; Kobayashi T A Role for Sphingomyelin-Rich Lipid Domains in the Accumulation of Phosphatidylinositol-4,5-Bisphosphate to the Cleavage Furrow during Cytokinesis. Mol. Cell. Biol 2012, 32, 1396–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lingwood D; Simons K Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [DOI] [PubMed] [Google Scholar]
- (19).Pruett ST; Bushnev A; Hagedorn K; Adiga M; Haynes CA; Sullards MC; Liotta DC; Merrill AH Thematic Review Series: Sphingolipids. Biodiversity of Sphingoid Bases (“Sphingosines”) and Related Amino Alcohols. J. Lipid Res 2008, 49, 1621–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ogretmen B Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Rudd AK; Devaraj NK Traceless Synthesis of Ceramides in Living Cells Reveals Saturation-Dependent Apoptotic Effects. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 7485–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Li X; Lam HY; Zhang Y; Chan CK Salicylaldehyde Ester-Induced Chemoselective Peptide Ligations: Enabling Generation of Natural Peptidic Linkages at the Serine/Threonine Sites. Org. Lett 2010, 12, 1724–1727. [DOI] [PubMed] [Google Scholar]
- (23).Hodges HL; Brown RA; Crooks JA; Weibel DB; Kiessling LL Imaging Mycobacterial Growth and Division with a Fluorogenic Probe. Proc. Natl. Acad. Sci. U. S. A 2018, 115, 5271–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Lieser B; Liebisch G; Drobnik W; Schmitz G Quantification of Sphingosine and Sphinganine from Crude Lipid Extracts by HPLC Electrospray Ionization Tandem Mass Spectrometry. J. Lipid Res 2003, 44, 2209–2216. [DOI] [PubMed] [Google Scholar]
- (25).Kowada T; Maeda H; Kikuchi K BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells. Chem. Soc. Rev 2015, 44, 4953–4972. [DOI] [PubMed] [Google Scholar]
- (26).Chevalier A; Renard P-Y; Romieu A Straightforward Synthesis of Bioconjugatable Azo Dyes. Part 1: Black Hole Quencher-1 (BHQ-1) Scaffold. Tetrahedron Lett 2014, 55, 6759–6763. [Google Scholar]
- (27).van Meer G; Voelker DR; Feigenson GW Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol 2008, 9, 112–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Bergström J; Fürst P; Norée LO; Vinnars E Intracellular Free Amino Acid Concentration in Human Muscle Tissue. J. Appl. Physiol 1974, 36, 693–697. [DOI] [PubMed] [Google Scholar]
- (29).Patel D; Witt SN Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxid. Med. Cell. Longevity 2017, 2017, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Qiu L; Zhang M; Tonks I; Kay G; Parsons PG; Sturm RA; Gardiner B Inhibition of Melanin Synthesis by Cystamine in Human Melanoma Cells. J. Invest. Dermatol 2000, 114, 21–27. [DOI] [PubMed] [Google Scholar]
- (31).Kolter T; Sandhoff K Sphingolipid Metabolism Diseases. Biochim. Biophys. Acta, Biomembr 2006, 1758, 2057–2079. [DOI] [PubMed] [Google Scholar]
- (32).Ryland LK; Fox TE; Liu X; Loughran TP; Kester M Dysregulation of Sphingolipid Metabolism in Cancer. Cancer Biol. Ther 2011, 11, 138–149. [DOI] [PubMed] [Google Scholar]
- (33).Wymann MP; Schneiter R Lipid Signalling in Disease. Nat. Rev. Mol. Cell Biol 2008, 9, 162–176. [DOI] [PubMed] [Google Scholar]
- (34).Vanier M; Millat G Niemann–Pick Disease Type C. Clin. Genet 2003, 64, 269–281. [DOI] [PubMed] [Google Scholar]
- (35).Patterson MC; Hendriksz CJ; Walterfang M; Sedel F; Vanier MT; Wijburg F Recommendations for the Diagnosis and Management of Niemann–Pick Disease Type C: An Update. Mol. Genet. Metab 2012, 106, 330–344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


