Abstract
Substantial evidence from studies in humans suggests the amygdala is pivotal for anxiety. Findings from animal models and translational studies suggests the bed nucleus of the stria terminalis (BNST) is also critical for anxiety and the anticipation of unpredictable threat in adults. However, it remains unknown whether the BNST is involved in unpredictable threat anticipation in children. Forty-two 8–10-year-olds completed resting state fMRI scans and an unpredictable threat fMRI task in which they were trained to associate cues with images. Intrinsic connectivity analyses were performed to establish functional BNST and amygdala networks. BNST and amygdala activation to cues and images was tested. Significant findings were followed by task-based functional connectivity analyses. Children showed evidence for BNST and amygdala intrinsic connectivity that was similar to previous patterns observed in adults. In response to unpredictable cues relative to neutral face cues, children had a significant amygdala response but no response in the BNST. The amygdala, but not the BNST, also showed a significantly greater response to fear face images relative to neutral images. Thus, unpredictable threat activated the amygdala, but not BNST, in children. This finding is contrary to studies showing robust BNST activation to unpredictable threat in adults and may suggest that the BNST’s role in threat processing emerges later in development.
Keywords: fear, anxiety, extended amygdala, uncertainty, childhood
Introduction
Anxiety disorders are the most prevalent disorders in childhood and adolescence with a staggering total of 15%-30% of children and adolescents being diagnosed with an anxiety disorder before reaching adulthood (Abbafati et al., 2020; Bittner et al., 2007; Woodward & Fergusson, 2001). Anxiety disorders arise early in development with symptoms emerging between 12 and 14 years of age (Merikangas et al., 2010). For some, the nature of anxiety disorders is persistent and chronic. Childhood anxiety disorders are also associated with an increased risk of anxiety, depression, substance abuse, educational deficiencies, and suicidality in adulthood (Kim-Cohen et al., 2003; Pine, Cohen, Gurley, Brook, & Ma, 1998; Soto-Sanz et al., 2019; Woodward & Fergusson, 2001). A better understanding of the neural basis of normative fear and anxiety in children has the potential to inform the development of novel treatments and early detection of anxiety disorders.
Fear is a normative response to threat that appears early in development. Many key phases of development are marked by noticeable changes in what stimuli generate a fear response including changes in behavior, cognition, arousal, and subjective feelings (Scarr & Salapatek, 2016). For example, a fear of strangers arises toward the end of the first year of life (Waters, Matas, & Sroufe, 1975) and a fear of the dark emerges around preschool-age (Gullone, 2000). Converging rodent and human research has identified the amygdala as a pivotal region underlying the detection of imminent threat in the environment and generates the cascade of fear responses (Davis, 1992; Feinstein, 2013; Inman et al., 2020; Tovote, Fadok, & Lüthi, 2015). Overall, the amygdala matures early in development, consistent with the early behavioral expression of fear (Blackford & Pine, 2016; Leppänen & Nelson, 2012), although research suggests that amygdala subnuclei networks may develop at different rates (Gabard-Durnam et al., 2014). Seminal studies of fear in adults, including fear conditioning and responses to threatening stimuli, have confirmed the amygdala’s role in normative fear processing and shown amygdala hyperactivity in anxiety disorders (Janiri et al., 2020; Shackman & Fox, 2021; Shin & Liberzon, 2010). Consistent with findings in adults, anxious children show hyperactive amygdala responses to threat-related stimuli (Blackford & Pine, 2016; Strawn et al., 2014).
Recent studies of anxiety have focused on the anticipation of potential or unpredictable threat. Heightened arousal experienced during threat anticipation, especially when the threat is uncertain or ambiguous, is a core mechanism underlying anxiety (Barlow, 2000; Grupe & Nitschke, 2013). Findings from animal models suggest that although responses to predictable or phasic threat are mediated by the amygdala, sustained responses to unpredictable or potential threat are also driven by the bed nucleus of the stria terminalis (BNST; see reviews by Avery, Clauss, & Blackford, 2016; Davis, Walker, Miles, & Grillon, 2010; Fox, Oler, Tromp, Fudge, & Kalin, 2015; Goode & Maren, 2017; Lebow & Chen, 2016). Some studies in humans also show distinct BNST and amygdala responses (Alvarez, Chen, Bodurka, Kaplan, & Grillon, 2011; Clauss, Avery, Benningfield, & Blackford, 2019; Herrmann et al., 2016; Klumpers et al., 2015; McMenamin, Langeslag, Sirbu, Padmala, & Pessoa, 2014; Somerville, Whalen, & Kelley, 2010). However, it is important to note that the amygdala and BNST are highly connected and other studies show overlapping roles for the amygdala and BNST during threat anticipation (Fox & Shackman, 2019; Hur et al., 2020; Shackman & Fox, 2016). Together these findings highlight that our understanding of the amygdala and BNST’s function during threat anticipation is continuing to evolve.
Studies in adults show that anxiety is associated with BNST responses and connectivity. In adults, trait anxiety correlates with BNST activation (Somerville et al., 2013, 2010) and connectivity (Brinkmann et al., 2018) and anxiety symptoms correlate with BNST connectivity (Andreescu et al., 2015; Clauss et al., 2019). In addition, people with an anxiety diagnosis demonstrate heightened BNST activation to unpredictable threat (Brinkmann et al., 2018; Figel et al., 2019; Straube, Mentzel, & Miltner, 2007) and altered BNST connectivity (Torrisi et al., 2019). To our knowledge, there has only been one study of unpredictable threat anticipation in children; anxious children showed a heightened amygdala response during unpredictable threat anticipation compared to non-anxious children (Williams et al., 2014). BNST activation to unpredictable threat was not observed in that study in the exploratory whole analysis; however, the BNST is a small subcortical region that may not be detected when whole brain cluster corrections are used. Therefore, it remains unclear if the BNST plays a role in threat anticipation or uncertainty in children.
The current study aimed to examine BNST and amygdala responses during threat anticipation in children. We chose the 8-10-year-old range to study children who can easily complete fMRI tasks and with the goal to reduce heterogeneity due to age and pubertal changes. We hypothesized that children would show similar patterns to adults, with amygdala activation to predictable threat and BNST activation to unpredictable threat. In addition, because the BNST has yet to be investigated in children, to our knowledge, we also aimed to establish the BNST intrinsic connectivity network in children.
Methods
Participants
Fifty children were recruited from the Vanderbilt University Medical Center and surrounding community through the use of flyers, e-mails, and research recruitment databases. Children were eligible for the study if they were 8-10 years old, had no cognitive deficits, had no contraindications to MRI scanning, and had no current or past psychiatric diagnoses. The presence of current or past psychiatric diagnoses was determined using the Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (Kaufman et al., 1997). The Kaufman Brief Intelligence Test (Kaufman & Kaufman, 1990) was used to measure intelligence quotient. Of the initial sample, eight children were excluded due to failure to accurately perform the task (n = 4), missing data due to technology or scanner failures (n = 3), or a structural anomaly (n = 1).
There were 42 children (16 female, 31%) in the final analytic sample, who were primarily White (90.5%) with a mean age of 9.87 years (SD = .92). The sample reported here partially overlaps with a previously reported study (Clauss, Benningfield, Rao, & Blackford, 2016). The Vanderbilt University Institutional Review Board approved this study, and all participants and parents provided written informed consent. Financial compensation was provided.
Threat Anticipation Task
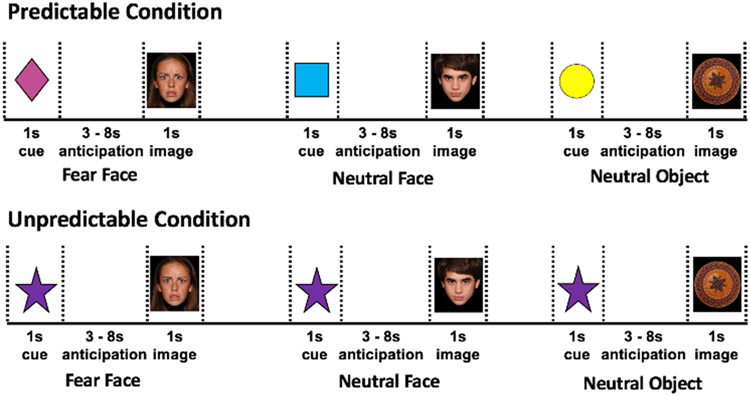
Anticipation of threat and response to threat were assessed using fMRI during a slow event-related design-cued anticipation task (Figure 1), similar to a task previously used in adults (Clauss et al., 2019). Children were trained to associate three cues (colored shapes) with three different images (fear face, neutral face, neutral object). Fear faces were used as the threat stimuli based on previous studies that have shown robust amygdala responses to fear faces (Gee et al., 2013; Guyer et al., 2008) as well as higher ratings of fear and faster reaction times relative to neutral faces (Guyer et al., 2008). The fear and neutral faces were used from the National Institute of Mental Health Child Emotional Faces Dataset (Egger et al., 2011). The neutral objects provided a neutral non-face control and were round, nonsocial objects with similar sizing, shape, and luminescence to the faces acquired from several sources, including the International Affective Picture System image set (Lang, Bradley, & Cuthbert, 2008), purchased images, and publicly available images. The task included four predictable runs where the trained cues were always followed by trained images. A fifth, unpredictable run was untrained and always presented last; participants saw a novel, untrained cue that was randomly followed by a fear face, neutral face, or neutral object. Each run had 24 trials (8 fear face, 8 neutral face, 8 neutral object) consisting of a cue presentation (1 second) and an image presentation (1 sec). Both the cue and image were followed by a blank screen (3-8 seconds, jittered). To optimize the task, the presentation order of the cues was randomly determined. The jittered interval was based on a gaussian distribution from 3-8 seconds, with each interval randomly assigned following each cue and image event. To measure attention to the task, children were asked to press a button each time they saw a cue or image. Children were removed from the analysis if they missed more than 50% of the button pushes (n = 4). Individual functional runs were excluded if the button push accuracy was less than 50% (n = 5 had 1 run removed, n = 2 had 2 runs removed), and individual events were excluded if the button was not pressed during the cue or image event.
Figure 1.
Cued anticipation task. Participants were trained to associated three cues (colored shapes) with three different events: 1) predictable fear face (pink triangle); 2) predictable neutral face (blue square), 3) predictable neutral object (yellow circle). Within the unpredictable block, a novel, untrained, unpredictable cue was presented that may be followed by one of the three images (fear face, neutral face, neutral object).
MRI Data Collection
Each child completed a mock MRI scan prior to the visit to familiarize the child with the scanner. The MRI scans were collected at Vanderbilt University Institute of Imaging Science (VUIIS) on a Phillips 3T Intera Achieva MRI scanner. T1-weighted anatomical images were acquired using the following sequence parameters: 256 mm FOV, 189 slices, 0.8 × 0.8 × 0.9 mm slice thickness, 9.1 ms TR, 4.9 ms TE. Seven minutes of resting state fMRI data (rsfMRI) were obtained approximately 20 minutes after entering the scanner, following structural MRI data collection, but prior to any functional MRI task. Participants were instructed to “close their eyes and relax but try not to fall asleep”. Resting State images were acquired using the following parameters: 2 second TR; 35 millisecond TE, 1.8 SENSE; 240 mm FOV; 3 x 3 mm in plane resolution using an 80 x 80 matrix (reconstructed to 128 x 128). Each volume contained 28 4 mm slices (acquisition voxels = 3 mm x 3 mm x 4 mm). For the task, functional (echo-planar imaging [EPI]) data were acquired using a sequence optimized for the amygdala and orbitofrontal cortex with the following parameters: 38 slices, 240 mm FOV, 2 s TR; 28 ms TE, 3 x 3 x 3.20 mm voxels, and an axial oblique acquisition, tilted -15°, anterior higher than posterior, relative to the intercommisural plane.
MRI Data Processing and Analysis
Resting State MRI.
Resting state fMRI data were analyzed with the CONN toolbox (Whitfield-Gabrieli & Nieto-Castanon, 2012; version 19.c) with standard pipeline (motion correct, slice timing, scrubbing, normalization, 6 mm smoothing, band-pass filter .008-.09 Hz). At least 50% of data frames had to be low motion to be included in the resting state analysis. All children had at least 74% low motion frames in this sample (range 74%-100%, mean = 95%, median = 98%). For each participant, the blood oxygenated level dependent (BOLD) time series was estimated as the average time series for all voxels in the left and right BNST and amygdala. The BNST was defined using our published mask (Avery et al., 2014, Supplemental Figure 1) and the amygdala was defined using the Harvard-Oxford atlas thresholded at a 50% probability (Desikan et al., 2006, Supplemental Figure 1). Given concerns about introducing spurious negative correlations (Murphy, Birn, Handwerker, Jones, & Bandettini, 2009; Saad et al., 2012), global signal was not removed. Second-level analyses across participants were performed. A whole brain SPM cluster-correction (voxel p < .00001, FWE α =.01) was used.
In order to provide a visual comparison between resting state connectivity of the BNST and amygdala in children with adults, we used a previously published dataset (Avery et al., 2014). The resting state connectivity analyses had already been performed for that study. The findings are displayed in Figure 1 for illustrative purposes.
Task Based Functional MRI.
Task fMRI data preprocessing was performed in SPM. Preprocessing steps included the following: slice time correction; motion correction; co-registration to the functional image; normalization of functional scans to the SPM EPI template; and smoothing (6 mm). For each participant, scans were checked for data quality: functional and structural data were visually inspected for artifacts, coverage of brain regions, and signal dropout.
Individual participant GLMs were estimated with four cue types (unpredictable, predictable fear face, predictable neutral face, predictable neutral object) and six image types (unpredictable fear face, unpredictable neutral face, unpredictable neutral object, predictable fear face, predictable neutral face, predictable neutral object) conditions. To control for motion, we used the Robust Weighted Least Squares (rWLS) toolbox (Diedrichsen & Shadmehr, 2005) which uses standard robust methods to weight the contribution of each volume using the inverse of the variance, thereby reducing the statistical influence of motion outliers without removing data or disrupting the temporal sequence of the data. The performance of rWLS was reviewed by visually comparing the inverse variance maps across time for each participant. Activation in the left and right BNST and amygdala were computed for each cue and image condition as average percent signal change using MarsBar (Brett, Anton, Valabregue, & Poline, 2002).
Exploratory whole brain functional connectivity analyses were performed for any significant activation findings. Functional connectivity was calculated using beta series correlations (Rissman, Gazzaley, & D’Esposito, 2004) with BNST or amygdala as the seed regions. A Fisher r-to-z transformation was applied to the correlations to provide a normal distribution. A slightly liberal voxel-wise α = .005 and a whole brain SPM cluster-correction α = .05 were used for these exploratory analyses. The anatomical locations were determined using parcellations based on the automated anatomical labeling atlas (Tzourio-Mazoyer et al., 2002) and verified with visual inspection by JUB and BF.
Statistical Analysis
Tests of BNST and amygdala activation were performed for both anticipation (cues) and viewing (images), based on previous findings that unpredictability can impact image processing (Clauss et al., 2019; Williams et al., 2014). For cues, linear mixed models were performed with cue type (unpredictable, predictable fear face, predictable neutral face, predictable neutral object) as the fixed factor and child as the random factor. For images, linear mixed models were performed with image type (predictable, unpredictable) and image valence (fear face, neutral face, neutral object) as the fixed factors and child as the random factor. Hemisphere was included as a covariate in all analyses. Post-hoc tests were performed following significant main effects and interactions. Analyses were performed in SAS (SAS Studio, release 3.8, SAS Institute Inc., Cary, NC).
Results
Intrinsic Connectivity
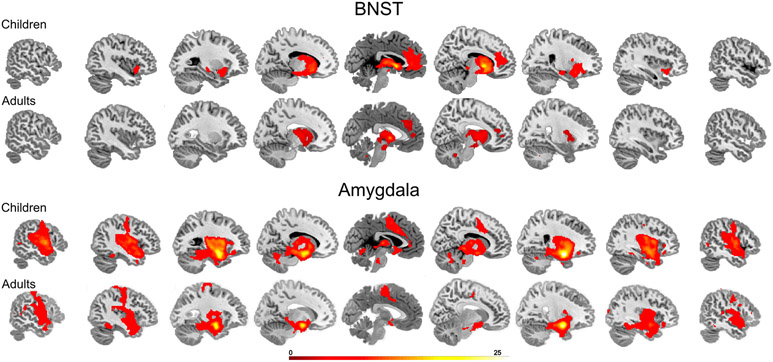
Given the lack of prior studies of the BNST in young children, we first investigated whether there is evidence for BNST intrinsic connectivity. Children showed strong BNST connectivity with the anterior insula, thalamus, and the rostral anterior cingulate cortex (Figure 2; Supplemental Table 1). To provide a comparison with adults, we have included resting state connectivity from our previously published study (Avery et al., 2014), illustrated in Figure 2. A visual comparison shows similar patterns between children and adults, which provides preliminary validation for investigations of the BNST in children.
Figure 2.
Intrinsic connectivity networks for the BNST (top panel) and amygdala (bottom panel) for children and adults (cluster corrected p < .01). Adult data was reproduced and modified with journal permissions from Avery et al., 2014.
To provide a comprehensive assessment of both the BNST and amygdala, we also conducted intrinsic connectivity of the amygdala (Figure 2; Supplemental Table 2). The amygdala was connected with the insula, dorsal anterior cingulate cortex, hippocampus, thalamus, middle temporal cortex, and superior temporal cortex. To provide a comparison with adults, we performed resting state connectivity analyses with the same adult sample (Avery et al., 2014). The visual comparison suggests that the pattern of amygdala connectivity is also similar between children and adults.
Threat Anticipation
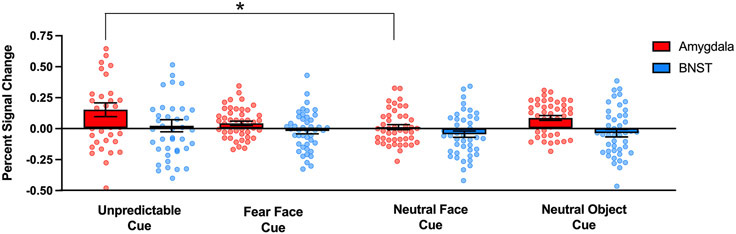
To determine whether children show BNST or amygdala responses during threat anticipation, we performed linear mixed models to test for effects of cue type. Children did not engage the BNST in response to the cues (Figure 3; no main effect of cue type). For the amygdala, there was a significant main effect of cue type (F (3,41) = 4.71, p = .007; Figure 3). The post-hoc analyses revealed significantly stronger amygdala activation for the unpredictable cues relative to the neutral face cues (t (41) = 2.40, p < .001; Figure 3).
Figure 3.
BNST and amygdala activation during threat anticipation. Bar graphs with dot plot overlays represent the extracted percent signal change for the BNST (blue) and amygdala (red) during cues. The significant results from the post-hoc analysis of amygdala cue type is highlighted with an asterisk (p < 0.05). Error bars represent ±1 standard deviation from the mean.
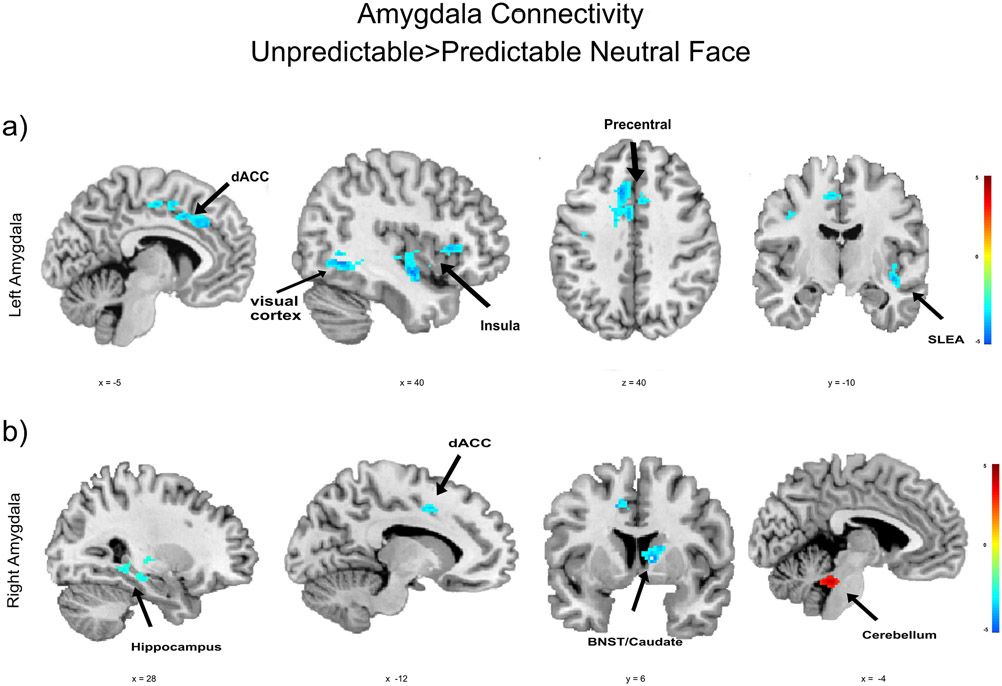
We performed exploratory task-based functional connectivity analysis to characterize the amygdala network involved in unpredictable threat anticipation. The results are shown in Table 1 and Figure 4. During the unpredictable cue (vs. neutral face cue), the left amygdala had weaker connectivity with a large cluster comprising the sublenticular extended amygdala (SLEA), anterior and posterior insula, putamen, and caudate head, as well as clusters in the dorsal anterior cingulate cortex (dACC), dorsal posterior cingulate cortex, precentral gyrus, fusiform gyrus, and visual cortex. For the right amygdala, there was weaker connectivity during unpredictable threat relative to predictable neutral face cues with clusters in the BNST/caudate, dorsal anterior cingulate cortex/precentral gyrus, hippocampus, and fusiform gyrus. Right amygdala connectivity was stronger during unpredictable relative to predictable neutral face cues in a region comprising the cerebellum and midbrain. There were no regions with stronger left amygdala connectivity during unpredictable cue relative to the neutral face cue.
Table 1.
Amygdala Connectivity During Anticipation
| MNI Coordinates |
Unpredictable Cue |
Neutral Face Cue |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Hemi- sphere |
k | t | x | y | z | Mean | SD | Mean | SD |
| Predictable Neutral Face > Unpredictable Threat Cues | ||||||||||
| Left amygdala | ||||||||||
| Dorsal anterior cingulate cortex | L | 335 | 4.67 | −10 | 22 | 40 | 1.91 | 1.11 | 2.86 | 1.24 |
| Anterior insula/ Posterior insula/ SLEA/ Putamen/ Caudate head | R | 676 | 4.66 | 40 | −10 | −12 | 2.14 | 0.93 | 3.09 | 1.21 |
| Fusiform gyrus | R | 335 | 4.55 | 40 | −58 | −6 | 0.90 | 1.20 | 1.83 | 0.93 |
| Visual cortex | L | 147 | 4.25 | −24 | −64 | −2 | 0.96 | 1.24 | 1.88 | 1.06 |
| Visual cortex | R | 422 | 4.22 | 24 | −52 | −4 | 1.04 | 1.17 | 1.99 | 1.07 |
| Dorsal posterior cingulate cortex | L | 138 | 3.77 | −6 | −12 | 52 | 1.36 | 1.39 | 2.23 | 1.26 |
| Precentral gyrus | L | 71 | 3.60 | −42 | −4 | 32 | 1.27 | 1.07 | 2.13 | 1.16 |
| Right Amygdala | ||||||||||
| BNST/caudate | R | 138 | 5.45 | 10 | 6 | 4 | 5.00 | 1.29 | 5.95 | 1.31 |
| Dorsal anterior cingulate/ Precentral gyrus | L | 217 | 4.34 | −20 | 0 | 44 | 0.90 | 1.06 | 1.77 | 1.19 |
| Fusiform gyrus | L | 79 | 4.28 | −36 | −66 | −6 | 0.47 | 1.08 | 1.39 | 1.37 |
| Posterior Hippocampus | R | 128 | 4.18 | 34 | −20 | −12 | 1.30 | 1.27 | 2.20 | 1.11 |
| Middle temporal gyrus | L | 73 | 4.08 | −58 | −12 | −14 | 0.63 | 1.22 | 1.42 | 1.10 |
| Hippocampus/ Parahippo-campal gyrus | R | 189 | 3.84 | 18 | −34 | 0 | 1.43 | 1.18 | 2.34 | 1.06 |
| Occipital cortex | R | 142 | 3.76 | 38 | −60 | −8 | .66 | 1.04 | 1.50 | 1.01 |
| Anterior hippocampus | L | 80 | 3.59 | −20 | −10 | −12 | 1.33 | 1.24 | 2.20 | 1.20 |
| Unpredictable Threat > Predictable Neutral Face Cue | ||||||||||
| Right amygdala | ||||||||||
| Cerebellum | L/R | 195 | 4.26 | −2 | −36 | −28 | 0.35 | 1.12 | −.54 | 1.15 |
Note. SLEA = sublenticular extended amygdala.
Figure 4.
Amygdala connectivity during the anticipation of threat when comparing unpredictable to neutral face cues cluster corrected p < 0.05. a) Left amygdala connectivity b) Right amygdala connectivity. dACC: dorsal anterior cingulate cortex; SLEA: sublenticular extended amygdala.
Threat Image Viewing
To determine whether children show BNST or amygdala responses when viewing the images following cues, we performed linear mixed models to test for effects of image type and image valence. Similar to the cue findings, children did not show a BNST response to the images and there were no significant main effects or interactions with image type or valence (Figure 5).
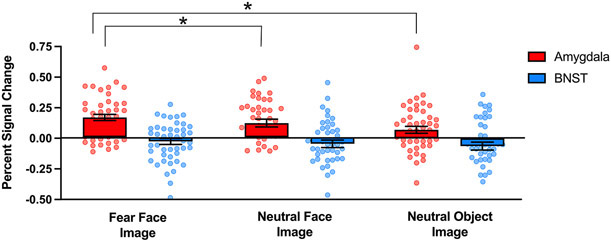
Figure 5.
BNST and amygdala activation during image viewing. Bar graphs with dot plot overlays represent the extracted percent signal change for the BNST (blue) and amygdala (red) during image viewing. Image valence (fear face, neutral face, neutral object) is averaged across image type (predictable, unpredictable). Significant results from the post-hoc analyses of amygdala image valence are highlighted with asterisks (p < 0.05). Error bars represent ±1 standard deviation from the mean.
For the amygdala, activation differed by image valence (F (2,41) = 10.21, p < .001). As shown in Figure 5, the amygdala showed stronger activation to fear faces compared to both neutral faces (t (41) = 2.04, p = 0.05) and neutral objects (t (41) = 4.50, p < 0.001). There were no significant main effects of image type or type x valence interactions.
Exploratory functional connectivity analyses of the amygdala were performed for the fear face vs. neutral face image contrast and the fear face vs. neutral object contrast. The results are provided in Table 2 and Supplemental Figure 2. For the fear vs. neutral face images, there was significantly stronger connectivity between the left amygdala and the dorsal anterior cingulate when viewing fear faces. During fear face viewing, the left amygdala had weaker connectivity with clusters in the fusiform gyrus and putamen relative to the neutral face image. The right amygdala had weaker connectivity with the fusiform gyrus during fear face image viewing relative to the neutral face image. None of the connectivity differences reached the statistical threshold for the fear face versus neutral object image contrasts.
Table 2.
Amygdala Connectivity during Image Viewing
| MNI Coordinates |
Fear Face Image |
Neutral Face Image |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain Region | Hemi- sphere |
k | t | x | y | z | Mean | SE | Mean | SE |
| Fear Face Image > Neutral Face Image | ||||||||||
| Left amygdala | ||||||||||
| Dorsal anterior cingulate cortex | R | 133 | 4.04 | 24 | 18 | 38 | 1.96 | 1.37 | 1.16 | 1.32 |
| Dorsal anterior cingulate cortex | L | 105 | 3.82 | −18 | 18 | 38 | 1.94 | 1.13 | 1.07 | 1.59 |
| Neutral Face Image > Fear Face Image | ||||||||||
| Left amygdala | ||||||||||
| Fusiform gyrus / middle temporal gyrus | R | 258 | 4.64 | 40 | −66 | −2 | 1.17 | 1.40 | 3.46 | 1.55 |
| Putamen | R | 82 | 3.38 | 28 | −8 | 4 | 2.59 | 1.62 | 3.46 | 1.55 |
| Right amygdala | ||||||||||
| Fusiform gyrus / middle temporal gyrus | R | 102 | 4.03 | 38 | −66 | −6 | 0.75 | 1.31 | 1.54 | 1.45 |
Discussion
The current study examined BNST and amygdala responses to predictable and unpredictable threat in children. To our knowledge, this is the first neuroimaging study of BNST function in children. The major study finding was that the BNST was not engaged during unpredictable threat anticipation, which is in stark contrast to numerous studies in healthy adults. In fact, there was little BNST activation during any of the task conditions in children, despite evidence for a BNST intrinsic network. Instead, in children, unpredictable threat elicited a robust amygdala response. These findings show that children, like adults (Clauss et al., 2019), are sensitive to unpredictable threat cues, but that the underlying neural mechanism may differ. The findings from the present study provide an important first step to understanding the role of the amygdala and BNST in the normative threat processes that are thought to go awry in anxiety disorders.
First, we investigated BNST intrinsic connectivity in children. To our knowledge, BNST connectivity has yet to be established in children, therefore, this finding provided an important foundation for investigating BNST responses to threat. Children had a robust BNST intrinsic network that showed a similar pattern to our previously published adult sample (Avery et al., 2014), suggesting that the intrinsic BNST network may be relatively mature by ages 8-10 years. We also examined the amygdala intrinsic network and found evidence for a similar pattern in children and adults. Previous studies have found weaker amygdala connectivity in young children (ages 7-9) relative to young adults (Qin, Young, Supekar, Uddin, & Menon, 2012) but that most of the amygdala intrinsic connections are developed by age 12 (Gabard-Durnam et al., 2014). It will be important to directly test for age-related similarities in BNST connectivity in a future systematic investigation. Investigations of the similarities and differences in BNST and amygdala age-related changes in connectivity may also be of value to informing our understanding of developmental changes in threat processing.
Next, we investigated the role of BNST in children during unpredictable threat anticipation. The major study finding was that anticipation of unpredictable threat engaged the amygdala, but not the BNST. Multiple studies have demonstrated BNST responses to the anticipation of unpredictable threat in adults (e.g., Alvarez et al., 2011; Choi et al., 2012; Clauss et al., 2019; Grupe et al., 2013; Herrmann et al., 2016; Klumpers et al., 2015, 2017; McMenamin et al., 2014). Although children demonstrated BNST intrinsic connectivity patterns that appeared similar to adults, our findings suggest that the BNST’s role in processing unpredictable threat is not present in children. However, the findings of an amygdala response is consistent with a previous study in a similar age group that found increased amygdala activation during unpredictable threat in children with anxiety disorders (Williams et al., 2014). In the present study, the heightened amygdala activation to unpredictable threat cues was accompanied with lower amygdala connectivity during unpredictable threat relative predictable neutral face cues in multiple brain regions including the dorsal anterior cingulate cortex (dACC), insula, sublenticular extended amygdala/BNST/caudate, hippocampus, fusiform gyrus, and visual cortex. Many of these regions are components of the amygdala intrinsic network and were more activated in response to the predictable neutral face cue. Thus, the finding of less connectivity during unpredictable threat relative to neutral face anticipation, may indicate that the anticipation of unpredictable threat disrupted connectivity with brain regions that typically regulate amygdala responses.
There are several possible explanations for the lack of BNST activation to unpredictable threat in this study. One possibility is that there are developmental changes in BNST function, such that the BNST’s response to unpredictable threat emerges later in development. Studies of BNST volume in humans and studies of BNST function in other species suggest that BNST volume and function change across development and are impacted by puberty and sex hormones (Amano et al., 2017; Chung, De Vries, & Swaab, 2002; del Abril, Segovia, & Guillamón, 1987). Another possible explanation is developmental differences in the amount of threat experienced by children, relative to adults, from the fear face stimuli. Although one previous study found that youth and adults had similar ratings and behavioral responses to fear faces (Guyer et al., 2008), it will be important for future studies to further explore children’s experiences of threat to different types of stimuli. Finally, it is possible that children interpreted the unpredictable cue condition differently than adults. Children are active, dynamic learners that are continually refining their understanding of the world through experiences and interactions with their environment. Therefore, the cognitive interpretation of and subjective experience of unpredictable threat may differ in children. Future studies should aim to create tasks that provide a systematic investigation of developmental differences in predictable and unpredictable threat processing.
The current study had some limitations. First, the study age range was narrowly defined to reduce heterogeneity. The limited age range, however, prevented explicit tests of age-related changes in BNST function. Longitudinal studies will be important for determining when BNST responses to unpredictable threat emerge. Second, the unpredictable threat run was always presented last and children were not trained on the unpredictable cues. The goal of the task design was to maximize unpredictability and we previously used a similar task in adults; however, the limitations are that the inferences are confounded by time effects and also precision of estimate, with fewer events in the unpredictable condition. Third, the current study lacks independent measures of threat-elicited distress or arousal throughout the task which limits the ability to infer that the stimuli were threatening. Finally, the statistical threshold used for the exploratory whole-brain connectivity analyses was somewhat liberal; future studies will need to replicate these findings, ideally in larger samples with more conservative statistical thresholds.
In summary, the current findings show that in children, the amygdala, but not the BNST, is engaged by unpredictable threat anticipation. This pattern is opposite to the pattern observed in adults, which raises questions about the normative development of the neural basis of fear and anxiety. A greater understanding of the contributions of the BNST and amygdala to threat processing throughout development is critical for identifying the mechanism of childhood anxiety disorders.
Supplementary Material
Acknowledgments
Support for this project was provided by the National Institutes of Health (F30-MH097344 to JAC, T32MH018921 to JAC, BF, EAF), the Vanderbilt Institute for Clinical and Translational Research (1-UL-1-TR000445 from the National Center for Research Resources/NIH), Jack Martin MD Research Professorship in Psychopharmacology (JUB), the Vanderbilt University Institute for Imaging Science and the Vanderbilt Department of Psychiatry and Behavioral Sciences.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abbafati C, Abbas KM, Abbasi-Kangevari M, Abd-Allah F, Abdelalim A, Abdollahi M, … May V (2020). Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet, 396, 1135–1159. 10.1016/j.bbr.2020.113084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, & Grillon C (2011). Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage, 55(1), 389–400. 10.1016/j.neuroimage.2010.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Shindo S, Yoshihara C, Tsuneoka Y, Uki H, Minami M, & Kuroda KO (2017). Development-dependent behavioral change toward pups and synaptic transmission in the rhomboid nucleus of the bed nucleus of the stria terminalis. Behavioural Brain Research, 325, 131–137. 10.1016/j.bbr.2016.10.029 [DOI] [PubMed] [Google Scholar]
- Andreescu C, Mennin D, Tudorascu D, Sheu LK, Walker S, Banihashemi L, & Aizenstein H (2015). The many faces of anxiety-neurobiological correlates of anxiety phenotypes. Psychiatry Research - Neuroimaging, 234(1), 96–105. 10.1016/j.pscychresns.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, & Blackford JU (2014). BNST neurocircuitry in humans. NeuroImage, 91, 311–323. 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SNN, Clauss JAA, & Blackford JUU (2016). The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology, 41(1), 126–141. 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH (2000). Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist, 55(November), 1247–1263. 10.1037/0003-066X.55.11.1247 [DOI] [PubMed] [Google Scholar]
- Bittner A, Egger HL, Erkanli A, Jane Costello E, Foley DL, & Angold A (2007). What do childhood anxiety disorders predict? Journal of Child Psychology and Psychiatry and Allied Disciplines, 48(12), 1174–1183. 10.1111/j.1469-7610.2007.01812.x [DOI] [PubMed] [Google Scholar]
- Blackford JU, & Pine DS (2016). Neural substrates of childhood anxiety disorders. A review of neuroimaging findings. Child and Adolescent Psychiatric Clinics of North America, 16(1), 501–525. 10.1016/j.chc.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, & Poline J-B (2002). Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2-6, 2002, Sendai, Japan. [Google Scholar]
- Brinkmann L, Buff C, Feldker K, Neumeister P, Heitmann CY, Hofmann D, … Straube T (2018). Inter-individual differences in trait anxiety shape the functional connectivity between the bed nucleus of the stria terminalis and the amygdala during brief threat processing. NeuroImage, 166, 110–116. 10.1016/j.neuroimage.2017.10.054 [DOI] [PubMed] [Google Scholar]
- Choi JM, Padmala S, & Pessoa L (2012). Impact of state anxiety on the interaction between threat monitoring and cognition. NeuroImage, 59(2), 1912–1923. 10.1016/j.neuroimage.2011.08.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WCJ, De Vries GJ, & Swaab DF (2002). Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood. Journal of Neuroscience, 22(3), 1027–1033. 10.1523/jneurosci.22-03-01027.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM, & Blackford JU (2019). Social anxiety is associated with BNST response to unpredictability. Depression and Anxiety, 36, 666–675. 10.1002/da.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Benningfield MM, Rao U, & Blackford JU (2016). Altered prefrontal cortex function marks heightened anxiety risk in children. Journal of the American Academy of Child and Adolescent Psychiatry, 55(9). 10.1016/j.jaac.2016.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–375. 10.1146/annurev.ne.15.030192.002033 [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, & Grillon C (2010). Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology, 35(1), 105–135. 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Abril A, Segovia S, & Guillamón A (1987). The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Developmental Brain Research, 32(2), 295–300. 10.1016/0165-3806(87)90110-6 [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, & Shadmehr R (2005). Detecting and adjusting for artifacts in fMRI time series data. NeuroImage, 27(3), 624–634. 10.1016/j.neuroimage.2005.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, & Angold A (2011). The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–156. 10.1002/mpr.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS (2013). Lesion studies of human emotion and feeling. Current Opinion in Neurobiology, 23(3), 304–309. 10.1016/j.conb.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Figel B, Brinkmann L, Buff C, Heitmann CY, Hofmann D, Bruchmann M, … Straube T (2019). Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. NeuroImage: Clinical, 22(101735). 10.1016/j.nicl.2019.101735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DPM, Fudge JL, & Kalin NH (2015). Extending the amygdala in theories of threat processing. Trends in Neurosciences, 38(5), 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, & Shackman AJ (2019). The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neuroscience Letters, 693(November 2017), 58–67. 10.1016/j.neulet.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, … Tottenham N (2014). The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. NeuroImage, 95, 193–207. 10.1016/j.neuroimage.2014.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110(39), 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, & Maren S (2017). Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learning and Memory, 24(9), 480–491. 10.1101/lm.044206.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, & Nitschke JB (2013). Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nature Reviews Neuroscience, 14(7), 488–501. 10.1038/nrn3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Oathes DJ, & Nitschke JB (2013). Dissecting the anticipation of aversion reveals dissociable neural networks. Cerebral Cortex, 23(8), 1874–1883. 10.1093/cercor/bhs175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullone E (2000). The development of fear: A century of research. Clinical Psychology Review, 4, 429–451. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Monk CS, McClure-Tone EB, Nelson EE, Roberson-Nay R, Adler A, … Ernst M (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–1582. 10.1162/jocn.2008.20114.A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann MJ, Boehme S, Becker MPI, Tupak SV, Guhn A, Schmidt B, … Straube T (2016). Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Human Brain Mapping, 37(3), 1091–1102. 10.1002/hbm.23088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur J, Smith JF, DeYoung KA, Anderson AS, Kuang J, Kim HC, … Shackman AJ (2020). Anxiety and the neurobiology of temporally uncertain threat anticipation. Journal of Neuroscience, 40(41), 7949–7964. 10.1523/JNEUROSCI.0704-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman CS, Manns JR, Bijanki KR, Bass DI, Hamann S, Drane DL, … Feinstein JS (2020). Human amygdala stimulation effects on emotion physiology and emotional experience. Neuropsychologia, 145(March 2018), 106722. 10.1016/j.neuropsychologia.2018.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, … Frangou S (2020). Shared neural phenotypes for mood and anxiety disorders: A meta-analysis of 226 task-related functional imaging studies. JAMA Psychiatry, 77(2). 10.1001/jamapsychiatry.2019.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (1990, January). Kaufman Brief Intelligence Test. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, & Poulton R (2003). Prior juvenile diagnoses in adults with mental disorder. Archives of General Psychiatry, 60(7), 709. 10.1001/archpsyc.60.7.709 [DOI] [PubMed] [Google Scholar]
- Klumpers F, Kroes MC, Heitland I, Everaerd D, Akkermans SEA, Oosting RS, … Baas JMP (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biological Psychiatry, 78(8), 582–589. 10.1016/j.biopsych.2014.07.034 [DOI] [PubMed] [Google Scholar]
- Klumpers F, Kroes MCW, Baas J, & Fernández G (2017). How human amygdala and bed nucleus of the stria terminalis may drive distinct defensive responses. The Journal of Neuroscience, 37(40), 3830–16. 10.1523/JNEUROSCI.3830-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (2008). International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville: University of Florida, Gainesville, FL. [Google Scholar]
- Lebow MA, & Chen A (2016). Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Molecular Psychiatry, 21, 450–463. 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppänen JM, & Nelson CA (2012). Early development of fear processing. Current Directions in Psychological Science, 21(3), 200–204. 10.1177/0963721411435841 [DOI] [Google Scholar]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, & Pessoa XL (2014). Network organization unfolds over time during periods of anxious anticipation. Journal of Neuroscience, 34(34), 11261–11273. 10.1523/JNEUROSCI.1579-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime prevalence of mental disorders in U.S. adolescents: Results from the national comorbidity survey replication-adolescent supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 49(10), 980–989. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, & Bandettini PA (2009). The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage, 44(3), 893–905. 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, & Ma Y (1998). The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry, 55(1), 56–64. 10.1001/archpsyc.55.1.56 [DOI] [PubMed] [Google Scholar]
- Qin S, Young CB, Supekar K, Uddin LQ, & Menon V (2012). Immature integration and segregation of emotion-related brain circuitry in young children. Proceedings of the National Academy of Sciences of the United States of America, 109(20), 7941–7946. 10.1073/pnas.1120408109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, & D’Esposito M (2004). Measuring functional connectivity during distinct stages of a cognitive task. NeuroImage, 23(2), 752–763. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, & Cox RW (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2(1), 25–32. 10.1089/brain.2012.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarr S, & Salapatek P (2016). Patterns of fear development during infancy. Merrill-Palmer Quarterly of Behavior and Development, 16(1), 53–90. [Google Scholar]
- Shackman AJ, & Fox AS (2016). Contributions of the central extended amygdala to fear and anxiety. Journal of Neuroscience, 36(31), 8050–8063. 10.1523/JNEUROSCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, & Fox AS (2021). Two decades of anxiety neuroimaging research: New insights and a look to the future. American Journal of Psychiatry, 178(2), 106–109. 10.1176/appi.ajp.2020.20121733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, & Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology, 35(1), 169–191. 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, & Kelley WM (2013). Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex, 23(1), 49–60. 10.1093/cercor/bhr373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, & Kelley WM (2010). Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biological Psychiatry, 68(5), 416–424. 10.1016/j.biopsych.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Sanz V, Castellví P, Piqueras JA, Rodríguez-Marín J, Rodríguez-Jiménez T, Miranda-Mendizábal A, … Alonso J (2019). Internalizing and externalizing symptoms and suicidal behaviour in young people: a systematic review and meta-analysis of longitudinal studies. Acta Psychiatrica Scandinavica, Vol. 140, pp. 5–19. 10.1111/acps.13036 [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, & Miltner WHR (2007). Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. NeuroImage, 37(4), 1427–1436. 10.1016/j.neuroimage.2007.06.023 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Dominick KC, Patino LR, Doyle CD, Picard LS, & Phan KL (2014). Neurobiology of pediatric anxiety disorders. Current Behavioral Neuroscience Reports, 1(3), 154–160. 10.1007/s40473-014-0014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S, Alvarez GM, Gorka AX, Fuchs B, Geraci M, Grillon C, & Ernst M (2019). Resting-state connectivity of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in clinical anxiety. Journal of Psychiatry and Neuroscience, 44(5), 313–323. 10.1503/jpn.180150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, & Lüthi A (2015). Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience, 16(6). 10.1038/nrn3945 [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Waters E, Matas L, & Sroufe LA (1975). Infants’ reactions to an approaching Stranger: Description, validation, and functional significance of wariness. Child Development, 46(2), 348–356. [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, & Nieto-Castanon A (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- Williams LE, Oler J. a, Fox AS, McFarlin DR, Rogers GM, Jesson M. Al,… Kalin NH (2014). Fear of the unknown: uncertain anticipation reveals amygdala alterations in childhood anxiety disorders. Neuropsychopharmacology, 40(6), 1428–1435. 10.1038/npp.2014.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, & Fergusson DM (2001). Life course outcomes of young people with anxiety disorders in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry, 40(9), 1086–1093. 10.1097/00004583-200109000-00018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.