Abstract
Background
Over the past several years, hyperdilute calcium hydroxylapatite (CaHA) has emerged as an effective modality for improving skin quality and managing laxity in the face, arms, hands, neck, décolletage, upper arms, abdomen, buttocks, and upper legs, as well as for treating cellulite and striae. Whereas undiluted CaHA is used to provide volume, hyperdilute CaHA is distributed across a much larger surface area in a more superficial plane to stimulate neocollagenesis and elastin formation over time. The absence of lymphocytic infiltrates and predominance of type 1 collagen in the tissue response to CaHA make hyperdilute CaHA a valuable tool for nonsurgical skin tightening.
Objectives
The aim of this study was to provide practical step-by-step guidance on patient selection, dilution practices, and optimal injection technique to facilitate incorporation of the technique into clinical practice.
Methods
Over the course of 3 regional meetings in the United States, 12 expert physician injectors participated in live webinars as part of a continuing medical education program.
Results
The practical guidance in this manuscript is based upon the most frequently requested information by audience members and the information considered critical for success by the authors.
Conclusions
The minimally invasive nature of filler injection results in little downtime, making this treatment particularly appealing. The recommendations presented are consistent with previously published consensus guidelines on hyperdilute CaHA but are intended to serve as “how-to” guidance based on the experience of expert injectors who have successfully treated the face and body.
Level of Evidence: 4

Calcium hydroxylapatite (CaHA, Radiesse; Merz North America, Raleigh, NC) is a filler currently approved by the US FDA for the correction of moderate to severe facial wrinkles and folds, as well as to correct volume loss in the dorsum of the hands.1 Used undiluted as a volumizing agent, CaHA is highly viscous and has a high elastic modulus, properties that make it well suited to deeper placement, particularly in the supraperiosteal plane, where it can serve as a liquid implant to support overlying structures.2 The CaHA microspheres in the filler have a well-documented ability to induce both neocollagenesis and elastogenesis via a histiocytic and fibroblastic response, which persists even once CaHA is diluted 1:6.3-5 When CaHA is diluted at 1:1, it retains some volumizing properties. When CaHA is hyperdiluted at or beyond 1:2 (eg, 1.5 mL of CaHA + 3.0 mL of diluent), the viscoelastic properties are changed dramatically. Hyperdilute CaHA is no longer a volumizing agent, but rather a suspension of microspheres that is easily distributed over larger surface areas of the face and body, where they serve to promote neocollagenesis and elastogenesis, thereby improving skin elasticity, pliability, and dermal thickness (Table 1).5
Table 1.
Properties of CaHA Dilutions and Recommended Uses
| Dilution | Recommended Use | Properties | Treatment goal |
|---|---|---|---|
| None | Facial rejuvenation; supraperiosteal and subdermal placement | High viscosity (remains in place); high elastic modulus (stiffness) | Biostimulatory effects can improve skin quality secondarily |
| 1:1 (1.5 mL CaHA + 1.5 mL diluent) |
Hand rejuvenation; facial rejuvenation; placement in the subdermal plane | Intermediate viscosity and stiffness | Primary goal is volume restoration with ease of product distribution |
| ≥1:2 (1.5 mL CaHA + ≥3.0 mL diluent) |
Skin tightening in the face and body; treatment of cellulite and striae; placement is in the subcutaneous plane at the dermal/subcutaneous junction | Negligible lifting properties (biostimulation only) | Primary goal is skin tightening. Hyperdilution permits even distribution of microspheres over a larger surface area |
CaHA, calcium hydroxylapatite.
To date, 2 consensus guideline articles on the use of CaHA as a skin-tightening agent have been published,6,7 each of which includes a review of the rationale and evidence supporting the practice across a range of anatomic areas. These articles also provide excellent guidance surrounding the dilutions best used for each area and provide potential injection vectors for both needle- and cannula-based techniques. However, since the publication of these guidelines, questions from injectors hoping to include hyperdilute CaHA in their practices continue to surface, and practical guidance on how to begin is needed. Thus, the topics covered in the consensus guidelines are not the primary focus of this article. Rather, we seek to provide step-by-step guidance for getting started with CaHA for minimally invasive skin tightening in clinical practice.
METHODS
As part of a continuing medical education (CME) event series, a team of 12 experts in plastic surgery and dermatology was identified, the authors of this article participated in 1 of 3 regional meetings in the United States on the role of hyperdilute CaHA for improving skin quality and firmness. Due to the SARS-CoV-2 pandemic, there was minimal in-person attendance at the meetings. Rather, each of the meetings was livestreamed to pre-registered physicians and physician extenders, who were permitted to submit questions throughout the meeting. The meetings took place between June and August 2020. The number of webinar attendees totaled >900 for the 3 meetings, and >400 individual queries from audience members were collected, in addition to the questions gleaned from >600 chat entries. Based upon the most frequently encountered questions and identified stumbling blocks for new injectors of hyperdilute CaHA, topics for this article were identified, including the following: patient identification and education, dilution practices, treatment planning, plane of injection, updates on injection technique, and safety.
For each of the identified topics, video footage of the meetings was reviewed and draft guidance generated. Each of the authors reviewed the guidance, and only statements with ≥80% agreement are included here. In instances where there was not complete agreement, the reasons underlying the exceptions are noted. All patients treated as a part of this activity provided written informed consent and were treated in agreement with the principles outlined in the Declaration of Helsinki.
RESULTS
Patient Selection and Education
Hyperdilute CaHA may be used to improve skin quality and address laxity in the face, arms, hands, neck, décolletage, upper arms, abdomen, buttocks, and upper legs, and to treat cellulite or striae.6,7 In contrast to undiluted CaHA, hyperdilute CaHA should be thought of as a minimally invasive skin-tightening modality and used in situations for which improvement of skin thickness and quality will further the patient’s aesthetic goals. As with other tightening methods, a proper diagnosis is required for optimal patient selection. The diagnosing clinician must be able to differentiate issues resulting from volume loss or lipodystrophy vs those that can be addressed by improving skin quality. Just as injection of undiluted CaHA filler in the midface can result in composite volumization and lifting of the tissues of the lower face,8 but generally not optimal management of skin laxity in the lower face, treatment of the skin in the lower face with hyperdilute CaHA cannot remedy ptosis due to collapsed fat pads in the midface. In general, hyperdilute CaHA is not appropriate in areas for which the apparent laxity is caused by excess fat (abdomen and thighs) or lipodystrophy (upper arms). For treatment of the thighs and abdomen in particular, the patient should be near their ideal body weight. In our experience, the most common presenting complaint of ideal candidates for hyperdilute CaHA is crepey skin, but patients may also present with complaints of sagging, unevenness in skin texture, or cellulite and/or striae.
Setting patient expectations is of particular importance for hyperdilute CaHA because many patients are familiar with the product’s use as a volumizing filler. It is important that patients understand that the effect of treatment will not be immediately visible and that the induced collagenesis does not produce an appreciable effect for approximately 4 to 6 weeks. To encourage patient understanding of the nature of the treatment effect, expert panelists often present treatment as a series of injections over time, recommending between 2 and 3 sessions approximately 6 weeks apart. This helps patients to recognize that the results take time, and optimal results may require more than a single session. Most often, 2 or 3 treatments are needed for an optimal effect depending on the collagen-producing capacity of the patient and the severity of the skin laxity. Because treatment outcome is contingent upon the patient’s ability to produce collagen, treatment of more mature patients may require additional sessions or treatment with complementary modalities. In our experience, the duration of treatment effect with hyperdilute CaHA is about 1 year before patients generally request retreatment. At this time, the results have not fully diminished, and optimal correction is generally reached with fewer treatment sessions than were required at initial treatment.
One special patient population that was repeatedly discussed and warrants attention here comprises patients who present with surface irregularities or lumpiness following liposuction. In these patients, contour irregularities are accompanied by fibrosis and scarring under the skin, which can make it difficult for the cannula to pass through the plane of injection. This can make it more difficult both to inject product evenly and maintain the plane of injection. Although treatment of patients with sufficiently thick skin may reduce the appearance of unevenness by minimizing shadowing and will improve laxity and skin quality, it is important that patient expectations are tempered and patients understand that treatment will not fully resolve this issue. Generally, patients are advised that a series of hyperdilute CaHA treatments may improve the rippled appearance of the postliposuction abdomen by about 50%.
Dilution Practices
Detailed recommendations on dilutions for each anatomic area are described in detail elsewhere and should be referenced as a guide.6,7 In general, the dilution is based on skin thickness, with more dilute product used for thinner skin to avoid visibility and/or palpability. A 1:2 or 1:3 dilution is best for normal skin, 1:4 for thin skin, and 1:6 for atrophic skin. Most often, this translates to injecting a 1:2 dilution in the face, a 1:2 to 1:3 dilution in the neck and décolletage, and 1:3 in most of the body, unless the skin is thin or atrophic, in which case the product should be diluted further.
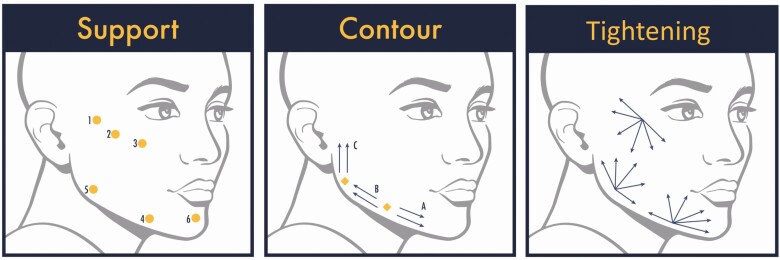
Dilutions at or beyond 1:2 should not be expected to provide volume. During discussion, the panelists reviewed applications for a dilution of ≤1:1 (diluted CaHA). When diluted at 1:1, CaHA provides volume and promotes tissue remodeling, but care must be taken to avoid placement that is too superficial so that product is not visible. In most cases, dilute CaHA is better suited for injection in deeper planes as a volumizing agent with modified flow properties. Thus, dilute CaHA (≤1:1) is best used in situations for which slightly deeper placement is needed and the primary goal of treatment is revolumization, such as the treatment of the dorsum of the hands, acne scars, or focal treatment of cellulite. Product placement, plane of injection, and injection pattern vary depending on the treatment goal (Figure 1). When 1:1 diluted CaHA is used in the face, it should be placed in the subdermal plane according to the same technique as shown for either contouring or tightening.
Figure 1.
Injection patterns for nondilute and hyperdilute CaHA. For nondiluted product, the primary goal of treatment is volumization, and placement is supraperiosteal or subdermal. For hyperdilute CaHA, the goal of treatment is skin tightening, and placement is in the subcutaneous plane. Illustration created by James Silvera, reproduced with permission from xMedica, LLC (Alpharetta, GA). CaHA, calcium hydroxylapatite.
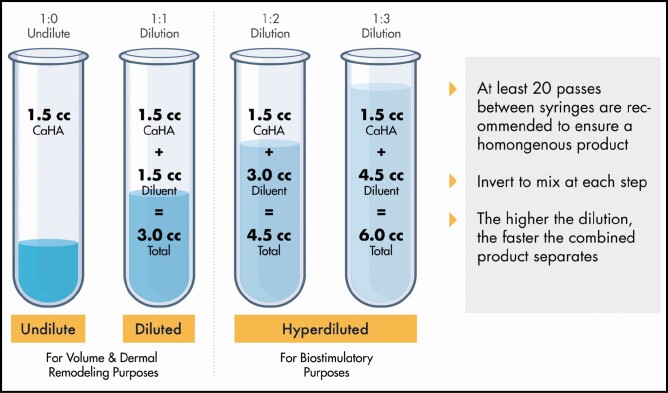
When preparing dilutions, it is important to remember that the final dilution includes the sum of all parts. For example, a 1:4 dilution contains a total of 5 parts: 1.5 mL of undiluted CaHA and 6 mL of diluent. The desired dilution may be achieved with the use of a large-volume (5- to 10-mL) syringe, which contains the entirety of the diluent needed. Following injection of the CaHA into the syringe containing the diluent, the product is mixed by attaching a second Luer-Lock syringe via a female-to-female adapter, and making at least 20 passes between syringes to ensure homogenization. Figure 2 illustrates the volumes needed for dilutions up to 1:4. Hyperdilution of the CaHA carboxymethyl cellulose gel carrier renders it unable to hold the CaHA particles in suspension, and the CaHA microspheres will precipitate. It is important to ensure that the mixture is homogeneous before reloading the hyperdilute product into the 1.5-mL CaHA syringe and between introducing the syringe to new injection ports.
Figure 2.
Dilution of calcium hydroxylapatite to 1:1, 1:2, and 1:3 with key procedural notes. Illustration created by James Silvera, reproduced with permission from xMedica, LLC (Alpharetta, GA).
For CaHA + lidocaine, the diluent used is generally sterile saline, without additional lidocaine. For standard CaHA, the diluent should contain some lidocaine (1%-2%) for patient comfort. However, it is important to remember that the threshold for lidocaine toxicity is 7 mg/kg with epinephrine and 3 mg/kg without. A 2% lidocaine solution contains 20 mg/mL. Thus, for a 70-kg person, the maximum lidocaine exposure without epinephrine is 210 mg, the amount of lidocaine in 10.5 mL of 2% lidocaine diluent.
Treatment Planning
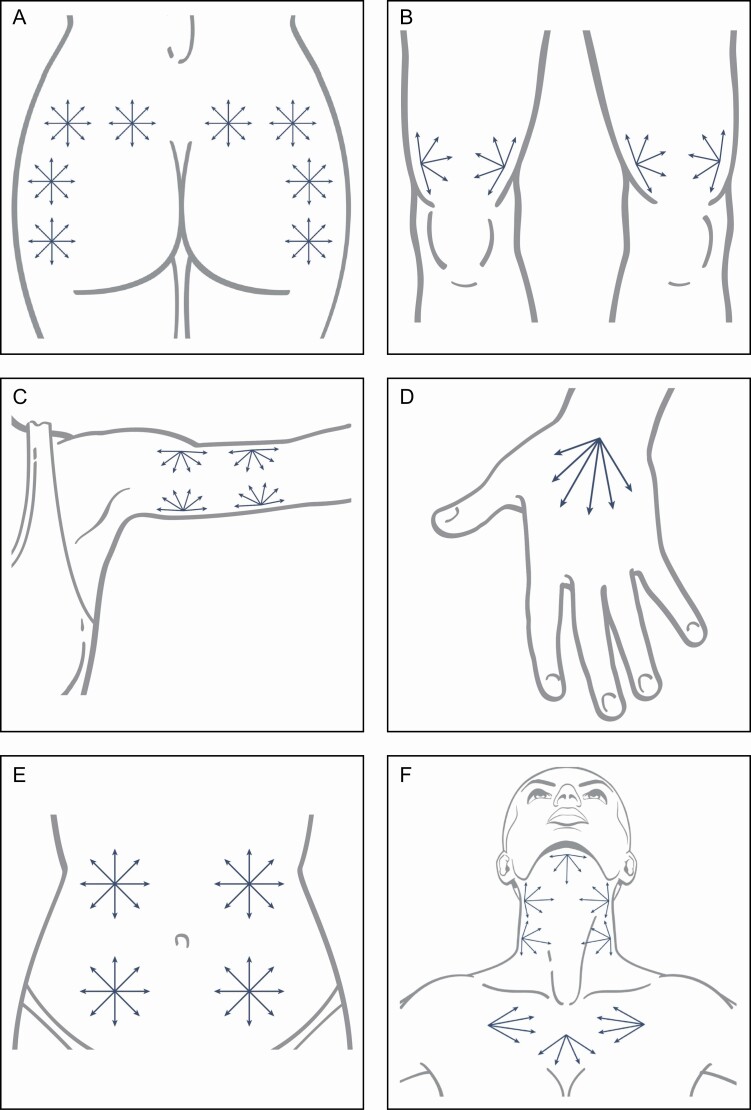
Although it is important to customize treatment for the needs of individual patients, representative treatment maps with potential vectors are presented in Figure 3. Regardless of the area to be treated, a treatment goal with hyperdilute CaHA is to minimize the number of entry ports for the surface area to be covered. For this reason, we recommend injecting with a cannula (22-25G) rather than a needle for most treatments. We favor the use of cannulas because they may be passed through the plane of the subcutaneous-dermal junction easily, and the placement of the product is more than sufficiently precise. Exceptions may include focal treatment of acne scars and individual cellulite dimples with diluted product (1:1) or hyperdiluted product (1:2). However, passage of a cannula underneath cellulite dimples or scars can disrupt the fibrous septae, similar to subcision, which may further improve textural irregularities beyond the biostimulatory effect of CaHA. This effect has also been noted when treating the neck, where passage of the cannula underneath transverse neck lines during product placement can improve treatment outcome, possibly due to subcision of fibrous septae. It is important to disinfect the skin along potential patterns of injection with hypochlorous acid or other antimicrobial skin preparation. At each port site, topical lidocaine may be applied or 0.5 mL of 2% lidocaine with epinephrine (1:100,000 dilution) injected prior to making the insertion site with the guide needle. It should be noted that it is helpful to use a guide needle larger than the cannula (eg, 23G needle for 25G cannula) and to orient the needle in the same direction desired for cannula advancement when making the port at the insertion site. No lidocaine is needed along the treatment vectors. Patients should experience minimal discomfort with cannula insertion and passes, and additional lidocaine may help alleviate patient pain.
Figure 3.
Potential patterns of injection for the body, including the buttock (A), knees (B), upper arm (C), hand (D), abdomen (E), and neck and décolletage (F). Patterns shown are for injection with a cannula. Illustration created by James Silvera, reproduced with permission from xMedica, LLC (Alpharetta, GA).
Plane of Injection
Hyperdilute CaHA should always be placed immediately below the dermis in the dermal-subdermal plane. Placement that is too superficial within the dermal plane can lead to visibility. Achieving an even distribution is important because this prevents product visibility and allows CaHA to exert its biostimulatory effect over the entirety of the treated area. Product accumulation can be avoided by maintaining the correct plane during injection and by avoiding injection over concentric muscles (including those where the dermis inserts directly into muscle as in the periorbital, perioral, nipple, and genital areas) and directly on top of the fibrous connective tissue of the dynamic joints in the knee and elbow. We also recommend against injecting hyperdilute CaHA above the arcus marginalis and in the lips. Although the unique property of periosteal collagen stimulation offers an additional benefit of CaHA for forehead contouring, this area is best addressed by an experienced injector. Overall, we do not recommend instructing the patient to massage the treated area after treatment. The product should be placed in the desired location during treatment and the microspheres spread evenly, and any needed massage should be performed by the physician at the time of placement.
Updates on Injection Technique
During the meetings, the following treatment areas were identified as being of particular interest to attendees as well as areas where there have been minor changes to technique since the publication of the consensus guidelines. Within each of the videos, the injection technique shown may be applied to other treatment areas.
Décolletage
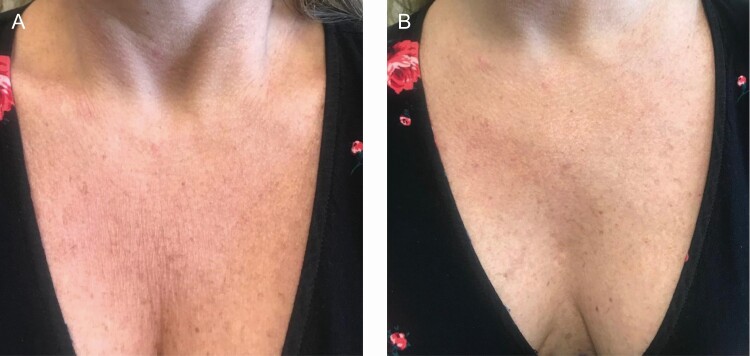
The décolletage is one of the most frequently treated areas in patients, generally for sun damage or etched rhytids in the skin. Most often, this area is treated with a 1:3 dilution, with the treatment vectors planned according to the individual needs of the patient. A short video of the décolletage procedure, including the neck is shown in Video 1, available online at www.aestheticsurgeryjournal.com, and before-and-after images are shown in Figure 4. Within the video, the patient’s neck, including transverse neck lines, is also treated.
Figure 4.
A 48-year-old female treated with 4.5 mL of a 1:2 dilution of CaHA (1.5 mL CaHA, 2.5 mL saline, and 0.5 mL 1:100,000 epinephrine with 2% lidocaine) injected with a 25G 2-inch cannula. The patient is shown at baseline (A) and 8 weeks after a single treatment (B). Note the improvement in texture and elimination of vertical lines. CaHA, calcium hydroxylapatite.
Buttocks
For the buttocks in particular, it is important to clarify for the patient the difference between smoothing and lift from skin tightening and augmentation. In somewhat of a departure from previous recommendations, panelists agree that they do not often treat the medial lower buttock. However, it is not uncommon to treat cellulite depressions with CaHA (1:1) in this area. Video 2, available online at www.aestheticsurgeryjournal.com, shows treatment of the buttock, including mild cellulite. Before-and-after images are shown in Figure 5.
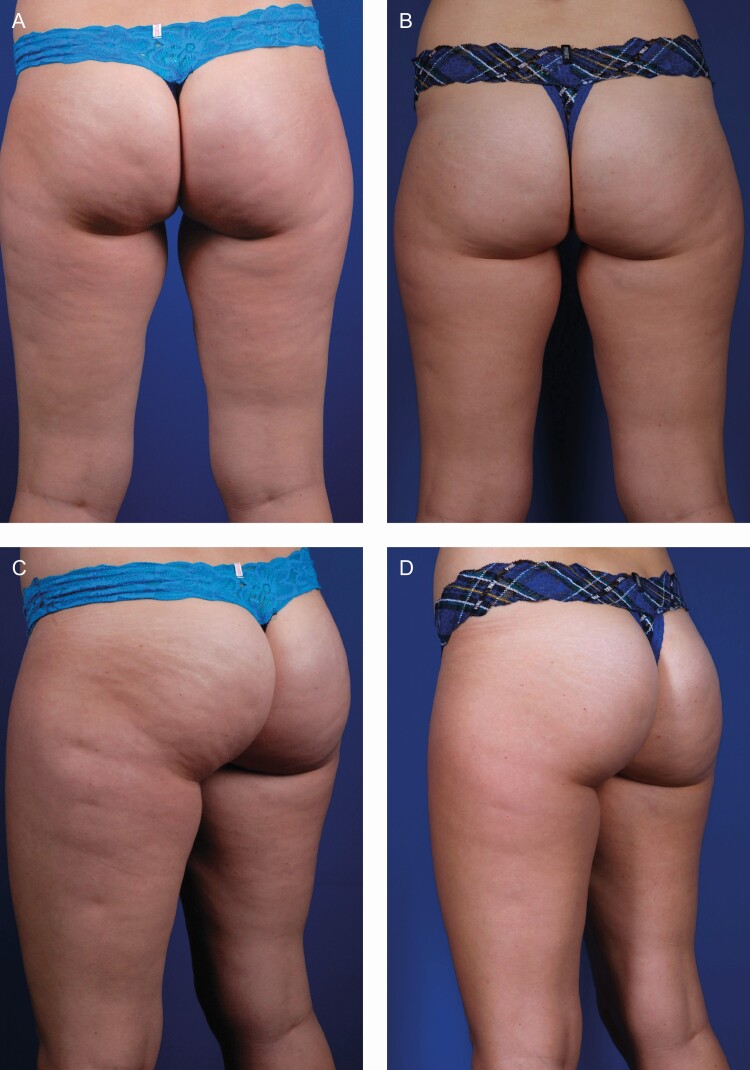
Figure 5.
A 27-year-old female treated with 6.0 mL of a 1:3 dilution of CaHA (1.5 mL CaHA and 4.5 mL of saline) per visit, per side, injected with a 22G cannula. The patient is shown at baseline (A, C) and 16 weeks after 3 treatments spaced 6 weeks apart (B, D). CaHA, calcium hydroxylapatite.
Upper Arms
The upper arms are another frequently requested area. Although the treatment maps presented in Figure 3 include an image showing potential vectors, the placement of the vectors should be adjusted to accommodate the needs of the individual patient. In addition, in our experience additional vectors placed on the posterior side of the arm can further improve outcomes by helping to lift descended tissue on the underside of the arm. For patients with excessive lipodystrophy, results may be suboptimal because skin tightening is often insufficient to overcome the mechanical load of the descended tissue. Video 3, available online at www.aestheticsurgeryjournal.com, shows an injection of the upper arms.
Safety and Postprocedure Care
As with any injectable filler, prevention is the most important aspect of mitigating the risk of adverse events. It is important that the injector understand the physiochemical properties of the filler, and possess an in-depth knowledge of anatomy, including resident nerves and vasculature. With hyperdilute CaHA, the injector should never inject the suspension as a large bolus in a single area because this can cause compression of surrounding vessels. In areas of volume deficit, such as cellulite, small bolus injections of 1:1 dilution CaHA are acceptable.
Treatment with hyperdilute CaHA is minimally invasive and typically requires no or minimal anesthesia apart from topical or small injections of lidocaine at the injection port sites. For this reason, the treatment necessitates almost no down time. In our experience, the most common side effect is bruising, most often in the knees and buttock at the cannula insertion pilot sites. Bruising occurs in approximately 3% to 5% of patients. Swelling may also occur, most commonly in the knees and hands. When treating the hands, we recommend against treating the patient with more than 1 syringe of CaHA diluted 1:1 (total volume of 3 mL) per hand to avoid swelling.
To date, none of us have observed delayed-onset reactions to the CaHA microspheres. Although this is anecdotal, the absence of late-onset events may be due, in part, to the fact that the primary type of collagen stimulated by CaHA is type 1, rather than type 3, which is more often associated with the process of fibrosis.3
Use With Other Treatments
Neuromodulators may be injected on the same day as hyperdilute CaHA to treat the platysma and reduce the appearance of transverse neck lines or platysmal banding. Injection along transverse neck lines is shown in Video 1. Injection of neuromodulators is generally carried out before hyperdilute CaHA is placed and may be done on the same day. Additional biostimulatory treatments may also be used to further stimulate collagen and improve skin quality. Devices may include microfocused ultrasound with visualization, microneedling, microneedling with radiofrequency, platelet-rich plasma, or treatment with fractional lasers. Additional biostimulatory modalities should be considered based on patient aesthetic goals or in cases in which the skin is atrophic and has been treated with a 1:6 dilution. In these instances, cotreatment further improves collagenesis though a complementary mechanism and may have a synergistic effect. Recently published guidelines describe optimal timing and combination of these modalities.6
CONCLUSIONS
It should be noted that this article was developed based on extensive collective clinical experience, not formal clinical study. There remains a need for a formal controlled study to better understand the nature and duration of patient satisfaction following treatment with different dilutions in different anatomic areas. Nevertheless, hyperdilute CaHA represents an effective nonsurgical mode of skin tightening with minimal downtime. The technique is straightforward and versatile and can be applied to both the face and body. Over time, the biostimulatory CaHA microspheres induce collagen and improve the skin’s mechanical properties, yielding excellent results.
Disclosures
Dr Lorenc is a consultant for Allergan (Irvine, CA), Galderma (Fort Worth, TX), Merz North America (Raleigh, NC), Suneva (San Diego, CA), and Thermi (Irvine, TX). Dr Black is an investigator, speaker, and consultant for Allergan, Galderma Aesthetics, Merz North America, Revance Therapeutics (Nashville, TN), and Evolus (Newport Beach, CA). Dr Cheung is on the advisory board for Merz North America and ISDIN (Barcelona, Spain) and is a trainer for Merz and Suneva. Dr Chiu is a consultant for Allergan, Evolus, Revance, Obagi (Long Beach, CA), Burt’s Bees (Durham, NC), and Revance, as well as a researcher for Allergan and Cynosure (Westford, MA). Dr Del Campo is a speaker for Aerolase (Tarrytown, NY) and Merz, and is a consultant for Salt Facial (Cardiff by the Sea, CA) and Merz. Dr Durkin is a paid consultant for Merz Aesthetics, the Musculoskeletal Transplant Foundation (Edison, NJ), BTL Aesthetics (Boston, MA), and Prollenium (Aurora, ON, Canada). Dr Graivier is an investigator for Endo Aesthetics (Malvern, PA), Galderma, and Ideal Implants (Irving, TX); a speaker for Revanesse (Aurora, ON, Canada); and is on the advisory board for Merz North America and Endo Aesthetics. Dr Green discloses that he performs clinical trials and is a speaker/on the advisory board for Allergan, Brickell Biotech (San Diego, CA), Croma (Leobendorf, Austria), Cutera (Brisbane, CA), Endo Aesthetics, Galderma, Merz, Pulse Biosciences (Hayward, CA), and Revance; and holds stock/ownership in Candesant (San Francisco, CA) and Illustris (Irvine, CA). Dr Kwok is a speaker, trainer, and on the advisory board for Galderma, Cartessa (Mellville, NY), and Merz North America; and has an ownership/investment interest in Healeon Medical (Solana Bech, CA). Dr Marcus is a speaker and on the advisory board for Allergan, Galderma, Merz North America, and Evolus; is a trainer for Galderma, Merz, Sinclair Pharma (San Diego, CA), and Evolus, and has done clinical research for Galderma. Dr Werschler is a speaker, investigator, and consultant/advisor for Allergan, Merz North America, Suneva, Galderma, Revance, and Endo Aesthetics. Dr Rammos declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
Funding was provided by Merz North America (Raleigh, NC) and was administered by xMedica, LLC (Alpharetta, GA).
REFERENCES
- 1. Radiesse [instructions for use]. Franksville, WI: Merz North America, Inc.; 2020. [Google Scholar]
- 2. Meland M, Groppi C, Lorenc ZP. Rheological properties of calcium hydroxylapatite with integral lidocaine. J Drugs Dermatol. 2016;15(9):1107-1110. [PubMed] [Google Scholar]
- 3. Berlin AL, Hussain M, Goldberg DJ. Calcium hydroxylapatite filler for facial rejuvenation: a histologic and immunohistochemical analysis. Dermatol Surg. 2008;34(Suppl 1):S64-S67. [DOI] [PubMed] [Google Scholar]
- 4. Marmur ES, Phelps R, Goldberg DJ. Clinical, histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler. J Cosmet Laser Ther. 2004;6(4):223-226. [DOI] [PubMed] [Google Scholar]
- 5. Yutskovskaya YA, Kogan EA. Improved neocollagenesis and skin mechanical properties after injection of diluted calcium hydroxylapatite in the neck and décolletage: a pilot study. J Drugs Dermatol. 2017;16(1):68-74. [PubMed] [Google Scholar]
- 6. de Almeida AT, Figueredo V, da Cunha ALG, et al. . Consensus recommendations for the use of hyperdiluted calcium hydroxyapatite (Radiesse) as a face and body biostimulatory agent. Plast Reconstr Surg Glob Open. 2019;7(3):e2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldie K, Peeters W, Alghoul M, et al. . Global consensus guidelines for the injection of diluted and hyperdiluted calcium hydroxylapatite for skin tightening. Dermatol Surg. 2018;44(Suppl 1):S32-S41. [DOI] [PubMed] [Google Scholar]
- 8. Lorenc ZP, Lee JC. Composite volumization of the aging face: supra-periosteal space as the foundation for optimal facial rejuvenation. J Drugs Dermatol. 2016;15(9):1136-1141. [PubMed] [Google Scholar]