Abstract
Osteoporosis, a widespread skeletal disorder with a substantial economic load, is characterized by increased porosity of the bones resulting in vulnerability to fractures. When activated, the canonical Wnt signaling pathway results in osteoblastogenesis and bone formation. A Wnt ligand forms a complex with low-density lipoprotein receptor-related proteins 5 and 6 (Lrp5/6) and stimulates intracellular signaling cascades, leading to nuclear translocation of β-catenin and transcription of downstream molecules involved in osteoblast differentiation, maturation, and survival. Sclerostin (SOST), a glycoprotein produced by osteocytes, is an extracellular Wnt antagonist that blocks the binding of Wnt ligands to Lrp5/6, preventing the activation of the pathway and osteoblast-mediated bone formation subsequently. Inhibition of SOST represents a new therapeutic paradigm for the treatment of osteoporosis. Monoclonal antibodies to SOST include romosozumab, blosozumab, and setrusumab. With its unique dual effect of increasing bone formation (anabolic action) and decreasing bone resorption, the Food and Drug Administration approved romosozumab, a promising new treatment for postmenopausal osteoporosis. Its efficacy and safety have been established in trials. However, patients at high risk of cardiovascular or cerebrovascular events should not be prescribed romosozumab.
KEYWORDS: Osteoporosis, romosozumab, sclerostin, Wnt-β-catenin singling pathway, β-catenin
INTRODUCTION
Labeled a silent and insidious disorder with a heavy economic impact, osteoporosis is characterized by low bone mass and microarchitectural eroding, leading to bone fragility and fractures.[1] The World Health Organization ranks osteoporosis as second only to cardiovascular diseases as a global health issue.[2] Studies from India show a wide variation in the prevalence – ranging from as low as 8% to as high as 81%.[1,2,3,4,5,6] By 2050, about 8 lakh Indians are projected to suffer from osteoporotic hip fractures, which cause the highest morbidity and mortality.[7] Only one-fourth of older women with a fracture get either a bone mineral density (BMD) test done or a prescription for its treatment in the 6 months postfracture, signifying that it is an underdiagnosed and undertreated condition.[8]
Pharmacotherapy is the mainstay of prevention and treatment of postmenopausal osteoporosis. The goal is to prevent fractures, especially in patients at high risk. Both antiresorptive and anabolic agents have drawbacks. Besides the fear of side effects, high costs, inconvenient and complex regimens, and socioeconomic conditions have led to the problem of lack of treatment adherence.[9] Moreover, patients with severe osteoporosis need more aggressive strategies, creating a need for more options of anabolic drugs.[10]
The term “Wnt” is derived from the acronym between wingless (Wg) in Drosophila and Int1 in the mouse (Wg-type mouse mammary tumor virus integration site) after the identification of these two homologous genes. The Wnt canonical pathway has a central role in stimulating bone formation and inhibiting bone resorption.[11] Sclerostin (SOST) is the natural antagonist of this pathway. Genetic disorders (sclerosteosis and Van Buchem's disease) resulting in a deficiency of SOST showed high bone mass – paving the way for research on pharmacological modulation of this crucial pathway.[11] Romosozumab is a Food and Drug Administration (FDA)-approved monoclonal IgG2 antibody to Wnt antagonist SOST.
We searched Medline, Embase, Cochrane Library, Medscape, Google Scholar, and ClinicalTrials.gov for articles in the English language published in peer-reviewed journals with the terms “Wnt signaling pathway,” “β-catenin,” “osteoporosis,” “romosozumab,” and “SOST.” We screened all the journal articles published from 2015 to 2020 that discussed the function of the Wnt pathway in bone remodeling, the role of SOST, and the relevant clinical studies of romosozumab to write this review article.
THE BONE REMODELING PROCESS
Four phases distinguish bone turnover.[12,13] The activation phase comprises the recruitment of the osteoclasts. Under the influence of cytokines such as receptor activator of nuclear factor kappa-B (RANK) ligand (RANKL) and macrophage colony-stimulating factor, osteoclast progenitors fuse to form multinucleated osteoclasts, which attach to the bone surface and start resorption. Osteoprotegerin (OPG), a cytokine receptor secreted by osteoblasts, binds to RANKL and prevents the binding of RANKL to RANK, thereby inhibiting osteoclast differentiation and activation. It guards the bone against excessive resorption.
In the second (resorption) phase, the osteoclasts resorb bone. The third (reversal) phase involves apoptosis of the osteoclasts and recruitment of the osteoblasts; bone-forming osteoblasts are derived from mesenchymal stem cells through the activation of specific transcription factors such as activating transcription factor 4, osterix, and runt-related transcription factor 2. The final (formation) phase involves laying down a new organic bone matrix by osteoblasts called osteoid. This nonmineralized bone matrix forms the stable lamellar bone after mineralization.[14] Osteoblasts produce osteocalcin, a specific marker of osteoblast activity.[13]
The cycles of bone remodeling are separated by a rest phase when the surface of the newly formed bone is covered by osteocytes derived from osteoblasts. Apoptosis of osteocytes marks the transition from the resting phase to the resorption phase of the bone turnover.[12]
PHARMACOTHERAPY OF OSTEOPOROSIS
Anti-osteoporosis drugs are classified into two groups – antiresorptive drugs that suppress bone resorption (by inhibiting osteoclast activity) and anabolic drugs that enhance bone formation (by improving osteoblast activity) [Table 1].[12]
Table 1.
Classification of drugs approved for postmenopausal osteoporosis
| Drug | Route | Mechanism | Adverse effects | |
|---|---|---|---|---|
| Antiresorptive drugs | a). SERMs Raloxifene Bazedoxifene |
Oral, daily | Decrease osteoclastogenesis | Risk of DVT/PE, Hot flashes, Leg cramps |
| b). Bisphosphonates Alendronate Ibandronate Pamidronate Risedronate Zoledronic Acid |
Oral-daily, weekly or monthly Intravenous-quarterly or yearly |
Impair osteoclast function | Gastrointestinal side effects, Esophagitis, Flu-like symptoms (iv administration), ONJ, AFF | |
| c). Calcitonin | Subcutaneous/ Intramuscular-every other day Nasal Spray-daily |
Decrease osteoclastic activity | Facial flushing, Hypocalcemia, Allergic reactions | |
| d). Denosumab (Antibody to RANKL) | Subcutaneous, Six monthly | Inhibit osteoclastic activity | Hypocalcemia, Cellulitis, musculoskeletal pain, ONJ, AFF | |
| Anabolic agents | a). PTH analogues Teriparatide Abaloparatide |
Subcutaneous, daily | Increase osteoblastic activity | Leg cramps, Headache, Hypercalcemia, Urolithiasis, Postural hypotension, Risk of osteosarcoma |
| b).Sclerostin antibody Romosozumab |
Subcutaneous, monthly | Increase osteoblastic activity and decrease osteoclastogenesis | Arthralgia, Nasopharyngitis, Injection-site reactions, Risk of cardiac events |
AFF - atypical femoral fracture, DVT - deep vein thrombosis, ONJ - osteonecrosis of jaw, PE - pulmonary embolism, PTH - parathyroid hormone, RANKL - receptor activator of nuclear factor kappa - B ligand
Antiresorptive drugs include estrogen, selective estrogen receptor modulators such as raloxifene, bisphosphonates (BPNs), denosumab, and calcitonin. The use of menopausal hormone therapy needs careful risk–benefit assessment.[15] The European Medicines Agency (EMA) does not recommend calcitonin for postmenopausal osteoporosis, citing its linkage with cancer. However, the FDA recommends its use in osteoporotic women at least 5 years postmenopausal, when other therapies have failed. Its short-term use is recommended – primarily to relieve pain in vertebral fractures.[15] A combination of conjugated estrogens with bazedoxifene (tissue-selective estrogen complex) is recommended if vasomotor symptoms exist. Denosumab, a human monoclonal antibody (MAb) to RANKL, blocks the binding of RANKL to RANK. BPNs are the first-line drugs and comprise second-generation drugs – alendronate (ALN), ibandronate, and pamidronate, and third-generation drugs – risedronate and zoledronate.[15] The cost-effectiveness of BPNs has been established. However, side effects linked to long-term treatment, such as atypical long bone fractures and osteonecrosis of the jaw, have led to poor initiation and noncompliance.[16] Moreover, antiresorptive drugs cannot rebuild the lost bone, creating a need for an anabolic medication.[16]
Anabolic agents include parathyroid hormone (PTH) and its analogs teriparatide and abaloparatide. Injected daily, PTH (intermittent PTH or iPTH) or its peptide fragment PTH1-34 (teriparatide) exhibits anabolic action by increasing osteoblastic activity.[16,17] Teriparatide administration is inconvenient as it needs daily subcutaneous injections and storage in the refrigerator.[12] A prior administration of an antiresorptive drug decreased the BMD response to teriparatide, signifying that the sequence of using anabolic and antiresorptive therapies may impact the skeletal response.[17] Moreover, the potential risk of osteosarcoma limits the use of teriparatide to 2 years; it may increase bone resorption beyond that.[18] Requiring daily subcutaneous injections, abaloparatide is a synthetic analog of PTH-related protein, proven superior to teriparatide.[19] While BPN and denosumab treatment is associated with 40%–70% reductions in vertebral fractures, abaloparatide decreased the incidence of new vertebral fracture by 86%.[20]
Pharmacological treatment is advocated for persons aged ≥50 years with a history of a spine or hip fracture, a T-score of ≤2.5, and a high fracture risk.[8,15] For postmenopausal women, treatment with BPN or denosumab for 5 years is appropriate.[8] Anabolic therapy is recommended in severe osteoporosis, failure or intolerability to other drugs, and glucocorticoid-induced osteoporosis.[17] While sequential treatment of teriparatide (anabolic) followed by an antiresorptive medication is approved, combination therapy is not endorsed due to less supportive data, high cost, and side effects.[8] Teriparatide, denosumab, or zoledronic acid is considered for those at high fracture risk unable to use oral therapy.[8] Current guidelines suggest a 5-year treatment with a BPN, followed by a reassessment. Drug holidays should be considered after 6–10 years of therapy for patients at a higher fracture risk. Teriparatide or raloxifene can be used for high-risk patients during drug holidays.[8]
WNT/β-CATENIN SIGNALING SYSTEM
The Wnt signaling system is a developmentally conserved pathway that plays a pivotal role in organogenesis in embryonic development, tissue homeostasis, and tissue repair post injuries.[21] Activation of the Wnt/β-catenin pathway promotes osteoblastogenesis by stimulating osteoblast differentiation and inhibiting osteoclastogenesis by the RANK/RANKL signaling pathway.
Wnt signaling pathways are classified as the canonical Wnt-β-catenin pathway (involving β-catenin) and the noncanonical pathways (not involving β-catenin). The canonical Wnt-β-catenin pathway plays an important role in adult skeletal homeostasis and bone remodeling.[22] Binding of a Wnt ligand to a specific Frizzled family receptor (G protein-coupled receptor) and a low-density lipoprotein receptor-related protein 5 and 6 co-receptor(Lrp5/6) leads to a series of cellular changes resulting in inhibition of the function of the destruction complex, assembled by a scaffolding protein known as axin along with other proteins – disheveled (Dsh or Dvl), adenomatous polyposis coli, casein kinase 1 alpha, and glycogen synthase kinase 3 beta (GSK3 β). GSK3 β phosphorylates β-catenin. When inhibited, it is unable to phosphorylate β-catenin. Unphosphorylated β-catenin is not degraded, causing the accumulation of β-catenin molecules within the cell. After they enter the nucleus, they bind to the T-cell/lymphoid enhancer factor leading to transcription from Wnt-responsive genes promoting bone formation [Figure 1]. On the other hand, when an extracellular antagonist like SOST binds, the Wnt ligand cannot bind to the Frizzled family receptor. Subsequently, the activated destruction complex(axin-GSK3 β protein assembly) phosphorylates β-catenin. Phosphorylated β-catenin is then ubiquitinated and broken down by a proteasome. The β-catenin molecules do not enter the nucleus, and Wnt-responsive genes are not activated, leading to decreased bone formation and increased bone resorption.[21,22]
Figure 1.
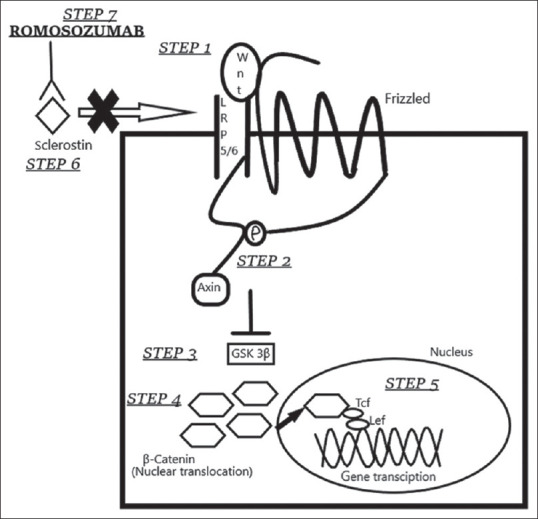
Mechanism of action of romosozumab. Step 1 – Binding of Wnt ligand to the frizzled family receptor (G protein-coupled receptor) and co-receptor low-density lipoprotein receptor-related proteins 5 and 6. Step 2 – The receptor complex inhibits axin-associated protein complex. Step 3 – The complex inhibits glycogen synthase kinase 3-beta, which is unable to phosphorylate β-catenin. Step 4 – This results in increased production of unphosphorylated β-catenin. Step 5 – β-catenin translocates to the nucleus and increases transcription of Wnt target genes with T-cell factor/lymphoid enhancer factor, resulting in increased bone formation. Step 6 – Sclerostin inhibits binding of Wnt ligand to the receptor and inhibits Wnt signaling pathway resulting in reduced bone formation. Step 7 – Romosozumab sclerostin MAb (MAb in place of Mab) inhibits the binding of sclerostin to the lipoprotein receptor-related proteins 5 and 6-frizzled receptor complex, thereby activating the Wnt signaling pathway and leading to increased bone formation
Thus, activating the canonical Wnt-β-catenin pathway leads to osteoblast precursors and osteogenesis by osteoblasts. Furthermore, increased β-catenin levels result in increased expression of OPG, leading to less binding of RANKL to RANK and reduced osteoclastogenesis and subsequent bone resorption.[12]
SCLEROSTIN AND ITS REGULATION
The SOST gene is localized on chromosome 17q12-q21 and codes for SOST.[23] Diseases with high bone mass such as sclerosteosis, craniodiaphyseal dysplasia, and Van Buchem's disease have mutations in the SOST gene resulting in defective SOST production.[23] This observation led to the exploration of the link between SOST and bone mass. A balance between bone formation and breakdown is mediated through SOST production. Secreted by osteocytes, it facilitates osteoblastic cell apoptosis through the activation of caspases and inhibits osteoblast differentiation-decreasing bone formation in areas where bone remodeling is unnecessary.[24] Mechanical forces (such as loading and exercise), microtrauma of bone, PTH, and estrogen inhibit the secretion of SOST by osteocytes and lead to increased bone formation.[23,24] On the other hand, bone morphogenetic proteins (2, 4, and 6), glucocorticoids, and calcitriol stimulate SOST secretion.[16] A positive relationship between circulating SOST levels and 25-OH-D and phosphate levels has been reported.[25] However, Cidem et al. demonstrated a decrease in serum SOST levels after Vitamin D treatment in Vitamin D-deficient adult females.[26] The correlation between Vitamin D and SOST secretion warrants further studies.
STUDIES WITH SCLEROSTIN ANTIBODIES
In development, humanized IgG MAbs to SOST are romosozumab [Figure 1], blosozumab, and setrusumab.
Bone formation is of two types – remodeling-based formation (RBF) or modeling-based formation (MBF). RBF involves both resorption and formation; MBF takes place directly on quiescent surfaces without the process of resorption. The initial, though transient, effect of romosozumab is the activation of quiescent bone lining cells of both cancellous and cortical bone, resulting in bone formation on the surface not resorbed.[27] This is followed by a fading of MBF with a persistent antiresorptive effect and RBF resulting in a slower but progressive increase in BMD.
CLINICAL STUDIES
In the Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) study, two groups received either romosozumab (210 mg subcutaneously once a month) or placebo for 1 year followed by another 1 year of open-label treatment with denosumab (60 mg every 6 months) [Table 2].[28] At two years, there was a 75% relative reduction in the risk of new vertebral fracture in the romosozumab–denosumab group compared to the placebo group. FRAME was the first study to indicate that treatment with an anabolic agent followed by an antiresorptive drug was more effective than starting with antiresorptive drug therapy alone. Two-year buildup with romosozumab was reported to equate the effect of 7 years of denosumab treatment.[29,30]
Table 2.
Phase 3 clinical trials with romosozumab
| Name of Trial | Number of Patients | Drug Groups | Duration | Primary Efficacy Outcome | ||
|---|---|---|---|---|---|---|
| 1.FRAME [55] | 7180 | Romo210 | 12 months | New VFR | CFR | NVFR |
| (Postmenopausal women) | Placebo | 0.5% | 1.6% | 1.6% | ||
| 1.8% | 2.5% | 2.1% | ||||
| RRR-↓73% | RRR-↓36% | RRR-↓25% | ||||
| 2.ARCH [59] | 4093 | Romo210 | 12 months | RRR-↓37% | RRR-↓28% | RRR-↓26% |
| (Postmenopausal Women) | ALN 70, PO | |||||
| 3.STRUCTURE [60] | 436 | Romo210 | 12 months | LS BMD | TH BMD | FN BMD |
| (Postmenopausal Women) | Teri 20 μg | 9.8% | 2.9% | 3.2% | ||
| OD,SC | 5.4% | -0.5% | -0.2% | |||
| (P<0.0001) | (P<0.0001) | |||||
| 4.BRIDGE[61] | 245 (men) | Romo210 | 12 months | LS BMD | TH BMD | FN BMD |
| Placebo | 12.1% | 2.5% | 2.2% | |||
| 1.2% | -0.5% | -0.2% | ||||
| (P<0.001) | (P<0.001) | (P<0.001) | ||||
ALN-alendronate; BMD-bone mineral density, expressed as percentage change from baseline; CFR-clinical fracture risk; FN-femoral neck; LS-lumbar spine; New VFR-new vertebral fracture risk; NVF-non vertebral fracture risk; OD-once daily; PO-per oral; RRR-relative risk reduction; Romo-romosozumab 210 mg subcutaneously, monthly; SC-subcutaneous; Teri- teriparatide; TH-total hip;↓ - decrease
In the FRAME EXTENSION study, relative risk reduction in romosozumab versus placebo for 12 months followed by 24 months of denosumab for both the groups was 66% for new vertebral fracture, 27% for clinical fracture, and 21% for nonvertebral fracture.[31] The Active-Controlled FRAME at High Risk (ARCH) compared the effects of romosozumab (210 mg monthly) with oral ALN (70 mg weekly) for 12 months, followed by open-label ALN therapy in both the treatment groups for up to additional 2 years. In the romosozumab group, the risk of new vertebral and clinical fractures was lower [Table 2]. The ARCH study was the first trial to establish the superiority of a novel treatment of osteoporosis over the first-line BPNs, fractures being the primary endpoint. At two years, the reduction of new vertebral, clinical, nonvertebral, and hip fractures in the romosozumab-ALN group compared to the ALN-ALN group was 48%, 27%, 19%, and 38%, respectively.[32]
The STRUCTURE trial was a Phase 3, open-label, active-controlled study comparing romosozumab (210 mg subcutaneous once monthly) to teriparatide (20 μg once daily) in postmenopausal osteoporosis with a history of an oral BPN use for at least 3 years [Table 2].[33]
In the BRIDGE trial, men received romosozumab (210 mg subcutaneously monthly) or matched placebo for 12 months, with concomitant calcium and Vitamin D administration daily. The levels of bone formation marker procollagen type 1 N-terminal propeptide (P1NP) increased early, and the levels of bone resorption marker C-terminal telopeptide remained lower than levels in the placebo group throughout the study [Table 2].[34]
Phase 2 studies have been completed for blosozumab. Setrusumab is currently being evaluated in osteogenesis imperfecta.[35] SOST antibodies are tested in fracture healing, periodontitis, and implant fixation.[36]
ADVERSE EFFECTS
Simultaneous mineralization of the new bone matrix and inhibition of bone resorption leading to mild and transient decreases in serum calcium (hypocalcemia), serum phosphorus, and reciprocal increases in PTH have been observed.[37] The risk of hypocalcemia is more in severe renal impairment and patients on dialysis. A sufficient intake of calcium and Vitamin D should be ensured. Contraindications for the use of romosozumab are hypocalcemia and hypersensitivity reactions to it. Concerns with SOST inhibition include bony overgrowth as seen in sclerosteosis and Van Buchem's disease and extraskeletal effects, given the diverse role of the Wnt signaling pathway in the development and homeostasis of adult tissues.[25] It should also be avoided in skeletal metastases and Paget's disease.
The most common adverse events in the clinical trials were nasopharyngitis, arthralgia, injection-site reactions (pain and erythema), and headache. The romosozumab group showed more cardiovascular side effects in the ARCH study (2.5% in the romosozumab group vs. 1.9% in the ALN group), though the trial included older participants with a higher baseline prevalence of cardiovascular risk.[38] Furthermore, a meta-analysis found that romosozumab increased the four-point major adverse cardiovascular events.[39] There is no explanation for a biological basis for cardiovascular risk as patients suffering from sclerosteosis or Van Buchem's disease do not show any trend in cardiac events.[40] Recent guidelines state that romosozumab should be avoided in patients at a high risk of cardiovascular disease or cerebrovascular disease. Its product label has a black box warning regarding the potential risk of adverse cardiac events such as myocardial infarction, stroke, and cardiovascular death.
Clinical studies also report an increase in cataracts (2.1% vs. 1.6%).[41]
McClung et al. report loss of benefit of romosozumab soon after the termination of therapy in postmenopausal osteoporosis. The two groups tested received 2-year treatment with romosozumab followed by either denosumab or placebo for a further 1 year. Romosozumab treatment led to a continued increase in BMD over 2 years with additional gains in those who changed over to denosumab, whereas BMD decreased to pretreatment levels in those shifted to placebo.[42] The cause of reversal of BMD values may be a reduction in osteoblast progenitors or a compensatory increase in other inhibitors of bone formation such as Dickkopf-related protein 1.
Anti-romosozumab antibodies, seen in up to 20% of patients in the 1st year of treatment, affected neither its safety nor efficacy.[31] It should not be used in pregnant and lactating females. Animal toxicity studies indicate that romosozumab does not pose a carcinogenic risk.[43]
NEED FOR PHARMACOVIGILANCE
There are many safety concerns with romosozumab. One event of osteonecrosis of the jaw and atypical femur fracture has been reported in the romosozumab study group. This may be due to the inhibition of bone resorption – a component of the dual-mode of action of romosozumab.[41] Concomitant administration of drugs associated with ONJ (corticosteroids, chemotherapy, BPNs, denosumab, and angiogenesis inhibitors) may increase the risk of developing ONJ. For evaluation of atypical femur fracture, any thigh or groin pain should be reported.[41] Serious infections were reported in elderly patients aged over 75 years (1.9% vs. 1.3%). Postauthorization surveillance studies are underway to clarify and quantify these serious adverse events.
REGULATORY ASPECTS OF THE NOVEL ANTIBODY
The first approval for romosozumab came from Japan in January 2019. The US FDA approved romosozumab in April 2019. However, the European Commission, acting via EMA, delayed the approval as it required more data on the serious cardiovascular complications reported in the trials. After restricting its use to severe osteoporosis at high risk of fracture, marketing authorization was granted by EMA and Health Canada in October 2019. Table 3 outlines the indications for the use of romosozumab. In India, the Central Drugs Standard Control Organization (CDSCO) has approved the drug, and is available on prescription.[44]
Table 3.
| Postmenopausal women with osteoporosis at high risk of fracture, defined as those with |
|---|
| 1). History of an osteoporotic fracture |
| 2). Multiple risk factors for fracture (Age, low BMI, low BMD scores at hip or spine, falls, use of glucocorticoids, smoking, alcohol >3 units per day, prolonged immobility, chronic diseases, rheumatoid arthritis) |
| 3). Failure of first-line anti-osteoporosis therapy |
| 4). Intolerance to anti-osteoporotic therapy |
BMI - body mass index, BMD - bone mineral density
HEALTH ECONOMIC ANALYSIS
Economic evaluation is integral to decision-making and allocating limited health resources. Globally, the annual health-care costs of osteoporosis run in billions of dollars.[36] The key drivers of cost-effectiveness are baseline fracture risk, drug efficacy, drug cost, and medication adherence.[45]
The wholesale acquisition cost of romosozumab is $1825/dose, similar to that of abaloparatide and less expensive than teriparatide.[46] Although studies with romosozumab are few, the cost-effectiveness of bone-forming agents has been established.[47] In one such study, abaloparatide/ALN produced an incremental cost-effectiveness ratio (ICER) of $333266/quality-adjusted life years (QALYs) relative to placebo/ALN over 10 years.[48] In a study by Söreskog et al., compared to ALN alone (5 years), romosozumab (1 year) followed by ALN treatment (4 years) was associated with 0.089 additional QALYs, resulting in an ICER of €33,732.[49] Romosozumab/ALN had more QALYs and a lower cost than teriparatide for 2 years of treatment when using the Patient Access Scheme (PAS) price for romosozumab.[50]
Fracture liaison services are a cost-effective, multidisciplinary care manager-based program, wherein a dedicated health-care worker ensures proper management of the patient after a fragility fracture.[51] This preventive health-care model has been initiated in a few Indian cities.
PLACE IN OSTEOPOROSIS THERAPY
Romosozumab has demonstrated good anti-fracture efficacy, and it fulfills the unmet need for a bone-forming agent. Table 4 discusses the advantages and disadvantages of romosozumab. It is now recommended that anabolic drug use, limited to 12 months, should be followed by treatment with antiresorptive drugs. More real-world data will establish its place in the pharmacotherapy of postmenopausal osteoporosis.
Table 4.
Advantages and disadvantages of sclerostin antibody in osteoporosis
| Advantages of sclerostin modulating therapy in postmenopausal osteoporosis: |
| 1. Dual effect of increase in bone formation and decreases bone resorption |
| 2. Rapid increases in bone mineral density |
| 3. Monthly subcutaneous administration increases compliance |
| 4. No risk of osteosarcoma |
| 5. Well tolerated in renal insufficiency |
| 6. No limit to use (unlike PTH based anabolic therapies) |
| Disadvantages of sclerostin modulating therapy in postmenopausal osteoporosis: |
| 1. Loss of anabolic effect after stopping treatment; Needs transition to antiresorptive drug after use |
| 2. Increased risk of cardiovascular complications (avoid in history of myocardial infarction or stroke) |
| 3. Contraindicated in skeletal metastases, Paget’s disease, hypersensitivity, hypocalcemia |
| 4. Long-term effects are not known |
| 5. Costly |
CONCLUSION
Osteoporosis, highly prevalent in postmenopausal women, adversely affects their quality of life. The current treatments have low patient compliance. Secreted Wnt glycoproteins help regulate cell-to-cell communication during embryogenesis and adult tissue homeostasis. The glycoprotein SOST is an inhibitor of the Wnt/β-catenin signaling pathway. Inhibition of SOST is a novel strategy to develop anabolic drugs for postmenopausal osteoporosis. Once-a-month administration of SOST MAb romosozumab stimulates modeling and remodeling-based bone formation. It is recommended for severe osteoporosis in postmenopausal women. Subjects at high risk of cardiovascular events should avoid romosozumab. Impressive increases in BMD and bone strength as well as significant reductions in the risk of fractures along with proven safety, make FDA-approved romosozumab, a first-in-class anabolic drug, a welcome addition to the armamentarium of anti-osteoporotic drugs.
New scientific information added by this review
This review highlights the application of a “bench-to-bedside approach” in the treatment of osteoporosis. It is an update on the recent advances in the development of drugs targeting the Wnt signaling pathway, such as anti-SOST antibodies. Exemplified by romosozumab, these anabolic therapies for postmenopausal osteoporosis offer hope to patients unable to use first-line BPNs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Kadam NS, Chiplonkar SA, Khadilkar AV, Khadilkar VV. Prevalence of osteoporosis in apparently healthy adults above 40 years of age in Pune City, India. Indian J Endocrinol Metab. 2018;22:67–73. doi: 10.4103/ijem.IJEM_438_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khadilkar AV, Mandlik RM. Epidemiology and treatment of osteoporosis in women: An Indian perspective. Int J Womens Health. 2015;7:841–50. doi: 10.2147/IJWH.S54623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thulkar J, Singh S, Sharma S, Thulkar T. Preventable risk factors for osteoporosis in postmenopausal women: Systematic review and meta-analysis. J Midlife Health. 2016;7:108–13. doi: 10.4103/0976-7800.191013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaki O, Rai SK, Kashid M, Chakrabarty BK. Prevalence of osteoporosis in peri- and post-menopausal women in slum areas of Mumbai, India. J Midlife Health. 2018;9:117–22. doi: 10.4103/jmh.JMH_84_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgohain B, Phukan P, Sarma K. Prevalence of osteoporosis among vulnerable adults residing in the northeastern region of India: A preliminary report from a tertiary care referral hospital. J OrthopTraumatol Rehabil. 2017;9:84–7. [Google Scholar]
- 6.Hiremath RN, Yadav AK, Ghodke S, Yadav J, Latwal S, Kotwal A. Osteoporosis among household women: A growing but neglected phenomenon. Med J Armed Forces India. 2018;74:5–10. doi: 10.1016/j.mjafi.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung CL, Ang SB, Chadha M, Chow ES, Chung YS, Hew FL, et al. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos Sarcopenia. 2018;4:16–21. doi: 10.1016/j.afos.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, et al. Clinician's guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25:2359–81. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiligsmann M, Cornelissen D, Vrijens B, Abrahamsen B, Al-Daghri N, Biver E, et al. Determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment: Results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Osteoporosis Foundation (IOF) Osteoporos Int. 2019;30:2155–65. doi: 10.1007/s00198-019-05104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rachner TD, Hofbauer LC, Göbel A, Tsourdi E. Novel therapies in osteoporosis: PTH-related peptide analogs and inhibitors of sclerostin. J Mol Endocrinol. 2019;62:R145–54. doi: 10.1530/JME-18-0173. [DOI] [PubMed] [Google Scholar]
- 11.Fabre S, Funck-Brentano T, Cohen-Solal M. Anti-sclerostin antibodies in osteoporosis and other bone diseases. J Clin Med. 2020;9:3439. doi: 10.3390/jcm9113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka S. Molecular understanding of pharmacological treatment of osteoporosis. EFORT Open Rev. 2019;4:158–64. doi: 10.1302/2058-5241.4.180018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langdahl B, Ferrari S, Dempster DW. Bone modeling and remodeling: Potential as therapeutic targets for the treatment of osteoporosis. Ther Adv Musculoskelet Dis. 2016;8:225–35. doi: 10.1177/1759720X16670154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahney CS, Zondervan RL, Allison P, Theologis A, Ashley JW, Ahn J, et al. Cellular biology of fracture healing. J Orthop Res. 2019;37:35–50. doi: 10.1002/jor.24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meeta M, Harinarayan CV, Marwah R, Sahay R, Kalra S, Babhulkar S. Clinical practice guidelines on postmenopausal osteoporosis: *An executive summary and recommendations – Update 2019-2020. J Midlife Health. 2020;11:96–112. doi: 10.4103/jmh.JMH_143_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suen PK, Qin L. Sclerostin, an emerging therapeutic target for treating osteoporosis and osteoporotic fracture: A general review. J Orthop Translat. 2016;4:1–13. doi: 10.1016/j.jot.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas AV, LeBoff MS. Osteoanabolic agents for osteoporosis. J Endocr Soc. 2018;2:922–32. doi: 10.1210/js.2018-00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Padhi D, Allison M, Kivitz AJ, Gutierrez MJ, Stouch B, Wang C, et al. Multiple doses of sclerostin antibody romosozumab in healthy men and postmenopausal women with low bone mass: A randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2014;54:168–78. doi: 10.1002/jcph.239. [DOI] [PubMed] [Google Scholar]
- 19.Russow G, Jahn D, Appelt J, Märdian S, Tsitsilonis S, Keller J. Anabolic therapies in osteoporosis and bone regeneration. Int J Mol Sci. 2018;20:83. doi: 10.3390/ijms20010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khosla S, Hofbauer LC. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. doi: 10.1016/S2213-8587(17)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huybrechts Y, Mortier G, Boudin E, Van Hul W. WNT signaling and bone: Lessons from skeletal dysplasias and disorders. Front Endocrinol (Lausanne) 2020;11:165. doi: 10.3389/fendo.2020.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal P, Singh P, Bey A, Gupta ND. Sclerostin and occlusion: A brief review. J Indian Soc Periodontol. 2015;19:11–3. doi: 10.4103/0972-124X.145785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pietrzyk B, Smertka M, Chudek J. Sclerostin: Intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv Clin Exp Med. 2017;26:1283–91. doi: 10.17219/acem/68739. [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietrzyk B, Wyskida K, Ficek J, Kolonko A, Ficek R, Więcek A, et al. Relationship between plasma levels of sclerostin, calcium-phosphate disturbances, established markers of bone turnover, and inflammation in haemodialysis patients. Int Urol Nephrol. 2019;51:519–26. doi: 10.1007/s11255-018-2050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cidem M, Karacan I, Arat NB, Zengi O, Ozkaya M, Guzel SP, et al. Serum sclerostin is decreased following vitamin D treatment in young vitamin D-deficient female adults. Rheumatol Int. 2015;35:1739–42. doi: 10.1007/s00296-015-3294-1. [DOI] [PubMed] [Google Scholar]
- 27.Boyce RW, Niu QT, Ominsky MS. Kinetic reconstruction reveals time-dependent effects of romosozumab on bone formation and osteoblast function in vertebral cancellous and cortical bone in cynomolgus monkeys. Bone. 2017;101:77–87. doi: 10.1016/j.bone.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–43. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 29.Kerschan-Schindl K. Romosozumab: A novel bone anabolic treatment option for osteoporosis? Wien Med Wochenschr. 2020;170:124–31. doi: 10.1007/s10354-019-00721-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosman F, Crittenden DB, Ferrari S, Khan A, Lane NE, Lippuner K, et al. FRAME study: The foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J Bone Miner Res. 2018;33:1219–26. doi: 10.1002/jbmr.3427. [DOI] [PubMed] [Google Scholar]
- 31.Lewiecki EM, Dinavahi RV, Lazaretti-Castro M, Ebeling PR, Adachi JD, Miyauchi A, et al. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: Results of the frame extension study. J Bone Miner Res. 2019;34:419–28. doi: 10.1002/jbmr.3622. [DOI] [PubMed] [Google Scholar]
- 32.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417–27. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 33.Langdahl BL, Libanati C, Crittenden DB, Bolognese MA, Brown JP, Daizadeh NS, et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet. 2017;390:1585–94. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 34.Lewiecki EM, Blicharski T, Goemaere S, Lippuner K, Meisner PD, Miller PD, et al. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J Clin Endocrinol Metab. 2018;103:3183–93. doi: 10.1210/jc.2017-02163. [DOI] [PubMed] [Google Scholar]
- 35.Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, et al. BPS804 anti-sclerostin antibody in adults with moderate osteogenesis imperfecta: Results of a randomized phase 2a trial. J Bone Miner Res. 2017;32:1496–504. doi: 10.1002/jbmr.3143. [DOI] [PubMed] [Google Scholar]
- 36.MacNabb C, Patton D, Hayes JS. Sclerostin antibody therapy for the treatment of osteoporosis: Clinical prospects and challenges. J Osteoporos. 2016;2016:6217286. doi: 10.1155/2016/6217286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370:412–20. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 38.Solling AS, Harslof T, Langdahl B. The clinical potential of romosozumab for the prevention of fractures in postmenopausal women with osteoporosis. Ther Adv Musculoskel Dis. 2018;10:105–15. doi: 10.1177/1759720X18775936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv F, Cai X, Yang W, Gao L, Chen L, Wu J, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: Systematic review and meta- analysis. Bone. 2020;130:115121. doi: 10.1016/j.bone.2019.115121. [DOI] [PubMed] [Google Scholar]
- 40.Fuggle NR, Cooper C, Harvey NC, Al-Daghri N, Brandi ML, Bruyere O, et al. Assessment of cardiovascular safety of anti-osteoporosis drugs. Drugs. 2020;80:1537–52. doi: 10.1007/s40265-020-01364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evenity: EPAR Public Assessment Report. European Medicines Agency. 2019. [Last accessed on 2021 Feb 05]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/evenity .
- 42.McClung MR, Brown JP, Diez-Perez A, Resch H, Caminis J, Meisner P, et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: A randomized, double-blind, phase 2, parallel group study. J Bone Miner Res. 2018;33:1397–406. doi: 10.1002/jbmr.3452. [DOI] [PubMed] [Google Scholar]
- 43.Chouinard L, Felx M, Mellal N, Varela A, Mann PJ, Jolette J, et al. Carcinogenicity risk assessment of romosozumab: A review of scientific weight-of-evidence and findings in a rat lifetime pharmacology study. Regul Toxicol Pharmacol. 2016;81:212–22. doi: 10.1016/j.yrtph.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Recommendations of the SEC (Analgesic & Rheumatology) Made in its 63rd Meeting Held on 22.07.2020 & 23.07.2020 at CDSCO HQ New Delhi. [Last accessed on 2021 Feb 05]. Available from: https://www.cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadCommitteeFiles/63rdSECAnalgesic%20&%20Rheumatology22-07-2020pdf.
- 45.Li N, Cornelissen D, Silverman S, Pinto D, Si L, Kremer I, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181–209. doi: 10.1007/s40273-020-00965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller SA, St Onge EL, Whalen KL. Romosozumab: A novel agent in the treatment for postmenopausal osteoporosis. J Pharm Technol. 2021;37:45–52. doi: 10.1177/8755122520967632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Hanlon CE, Parthan A, Kruse M, Cartier S, Stollenwerk B, Jiang Y, et al. A model for assessing the clinical and economic benefits of bone-forming agents for reducing fractures in postmenopausal women at high, near-term risk of osteoporotic fracture. Clin Ther. 2017;39:1276–90. doi: 10.1016/j.clinthera.2017.05.348. [DOI] [PubMed] [Google Scholar]
- 48.Le QA, Hay JW, Becker R, Wang Y. Cost-effectiveness analysis of sequential treatment of abaloparatide followed by alendronate versus teriparatide followed by alendronate in postmenopausal women with osteoporosis in the United States. Ann Pharmacother. 2019;53:134–43. doi: 10.1177/1060028018798034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Söreskog E, Lindberg I, Kanis JA, Åkesson KE, Willems D, Lorentzon M, et al. Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int. 2021;32:585–94. doi: 10.1007/s00198-020-05780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis S, Simpson E, Hamilton J, James MM, Rawdin A, Wong R, et al. Denosumab, raloxifene, romosozumab and teriparatide to prevent osteoporotic fragility fractures: A systematic review and economic evaluation. Health Technol Assess. 2020;24:1–314. doi: 10.3310/hta24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanis JA, Cooper C, Rizzoli R, Reginster JY. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


