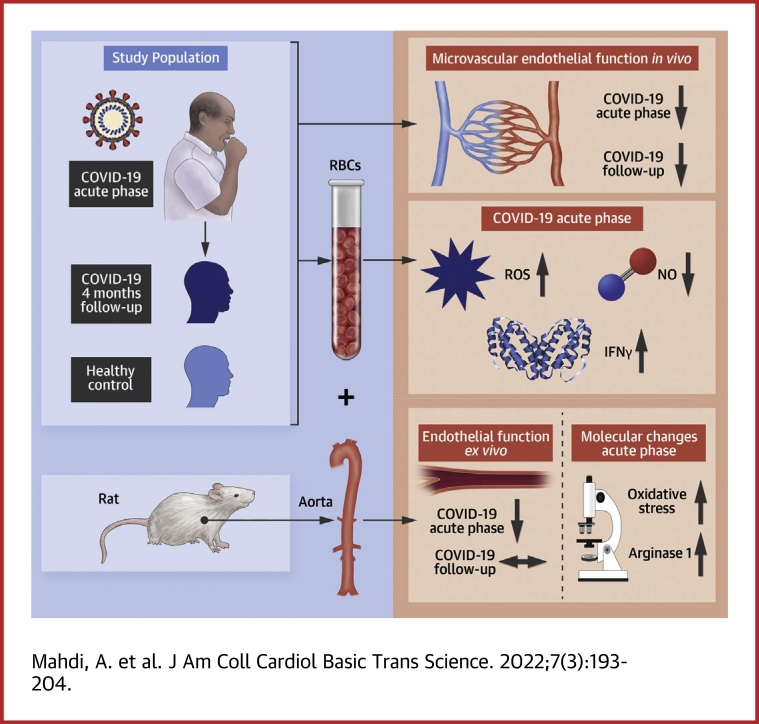
Visual Abstract
Key Words: arginase, COVID-19, endothelial dysfunction, nitric oxide, reactive oxygen species, red blood cells
Abbreviations and Acronyms: ACh, acetylcholine; C19-RBC, red blood cell from patients with COVID-19; EDR, endothelium-dependent relaxation; EIR, endothelium-independent relaxation; HNE, hydroxynonenal; H-RBC, red blood cell from healthy subjects; IFN, interferon; RBC, red blood cell; RHI, reactive hyperemia index; ROS, reactive oxygen species; SNP, sodium nitroprusside; TNF, tumor necrosis factor
Highlights
-
•
Patients hospitalized for COVID-19 display marked impairment in endothelial function, which is persistent following recovery from the acute infection.
-
•
RBCs from patients with COVID-19 impair vascular function through mechanisms involving increased arginase 1, ROS and IFNγ, and reduced NO bioactivity.
-
•
These data advance our understanding in COVID-19–associated vascular injury with a clear involvement of RBCs.
-
•
Targeting these mechanisms might provide a novel therapeutic strategy to alleviate vascular injury in patients with COVID-19.
Summary
Current knowledge regarding mechanisms underlying cardiovascular complications in patients with COVID-19 is limited and urgently needed. We shed light on a previously unrecognized mechanism and unravel a key role of red blood cells, driving vascular dysfunction in patients with COVID-19 infection. We establish the presence of profound and persistent endothelial dysfunction in vivo in patients with COVID-19. Mechanistically, we show that targeting reactive oxygen species or arginase 1 improves vascular dysfunction mediated by red blood cells. These translational observations hold promise that restoring the redox balance in red blood cells might alleviate the clinical complications of COVID-19–associated vascular dysfunction.
The ongoing COVID-19 caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV)-2 has created a global medical emergency of severe cases with multiple organ failures, including cardiovascular injury. Given the wide range of symptoms and the occurrence of multiorgan failure, it has been speculated that endothelial dysfunction with impaired tissue perfusion and a high prevalence of thromboembolism may represent a unifying pathological mechanism.1,2 Indeed, the term “endotheliitis” was introduced to describe endothelial injury in COVID-19, and recent position papers suggested that endothelial dysfunction, contributing to multiorgan failure and microvascular thrombosis, provides a framework for targeted treatment strategies.1,3 However, besides autopsy investigations showing disruption of the endothelial cell lining in patients with COVID-19,4,5 clinical and experimental evidence supporting the existence of endothelial dysfunction during ongoing COVID-19 infections are sparse.
Early in the pandemic, one hypothesis explaining endothelial injury was proposed to be via a direct viral entry of the virus through its decoy receptor angiotensin-converting enzyme 2 (ACE2).1 This has recently been challenged by the observation that ACE2 is not expressed in endothelial cells.6 Thus, it is more likely that endothelial injury arises because of systemic inflammation, which is supported by the observation that plasma isolated from patients with COVID-19 induces a toxic effect on cultured endothelial cells.7 These uncertainties emphasize the need to establish the presence of endothelial dysfunction in patients with ongoing COVID-19, irrespective of comorbidities, and for deeper insights into the mechanisms by which it is orchestrated. Furthermore, longitudinal studies assessing endothelial function in patients recovered from COVID-19 are needed to assess whether endothelial injury also contributes to long-term complications.
Another cell type suggested to be affected in COVID-19 is the red blood cell (RBC). RBCs are equipped with a fascinating battery of antioxidants, which are able to resist oxidative insult for maintenance of functional integrity and cell structure.8 In certain circumstances with excess in oxidative stress, the RBCs may switch to a redox balance promoting oxidative stress and become harmful for both resident and circulatory adjacent cell types.8 Epidemiological data support the notion that elevated red cell distribution width represents an independent predictor for mortality from COVID-19.9 RBCs from patients with COVID-19 also display alterations in the lipid architecture, increased oxidative metabolites, and reduction in antioxidants.10 The functional implications of these changes in COVID-19 are unknown, however. We recently demonstrated that oxidative stress is substantially increased in RBCs from patients with type 2 diabetes and that this alteration induces endothelial dysfunction through upregulation of arginase 1 and reactive oxygen species (ROS).11,12 It is therefore reasonable to hypothesize that altered function of RBCs in COVID-19 is involved in the development of endothelial dysfunction via increased oxidative stress and arginase 1. Indeed arginase 1 has been implicated in the pathogenesis of COVID-19.13
Based on the preceding, this study was designed to test the hypothesis that patients hospitalized for COVID-19 have impairment in endothelial function and that RBCs from those patients contribute to vascular injury through alterations in redox signaling. We observed a pronounced impairment in endothelial function in patients with COVID-19 in vivo irrespective of underlying comorbidities. Furthermore, RBCs, but not plasma, from these patients induced impairment in both endothelium-dependent and -independent relaxation through mechanisms involving oxidative stress and arginase 1. After recovery from the infection, endothelial dysfunction persisted, whereas RBC-induced vascular dysfunction was absent. These observations support the role of the RBC as an important mediator of endothelial dysfunction, which is sustained also after recovery from the acute phase despite normalized erythrocyte function. These results not only support the hypothesis that endothelial dysfunction is prominent in COVID-19, but more importantly provide evidence for a previously unrecognized mechanism underlying vascular dysfunction in patients with ongoing COVID-19 infection.
Methods
Seventeen patients with moderate COVID-19 infection and 27 age- and sex-matched healthy subjects were included in the current study. Inclusion criteria for patients with COVID-19 were as follows: age >18 years, polymerase chain reaction (PCR)-verified SARS-CoV-2 infection within the preceding 14 days, pulmonary COVID-19–associated interstitial infiltrates on radiographs, requiring in-hospital care, oxygen demand during hospital stay, and hospital arrival within the preceding 14 days. Exclusion criteria were as follows: type 1 or 2 diabetes, myocardial infarction within the past 6 months, acute kidney injury, chronic kidney disease, pregnancy, ongoing malignancy, >1 cardiopulmonary comorbidity, unwillingness to participate, need for intensive care, mechanical ventilation or noninvasive ventilation. The patients with COVID-19 underwent evaluation of microvascular digital endothelial function with pulse amplitude tonometry and expressed as reactive hyperemia index (RHI).14 Simultaneously, blood sampling following an overnight fasting during the hospitalization period (acute phase) and at follow-up 4 months later was performed. All patients were invited to the follow-up visit for which no specific inclusion or exclusion criteria were applied. Healthy controls were examined once. RBCs were isolated from whole blood after a washing procedure and incubated to a final hematocrit of ∼45% with isolated aortic rings from wild-type rats for 18 hours.11 Endothelium-dependent (EDR) and -independent relaxations (EIR) were subsequently evaluated by application of acetylcholine (ACh) and sodium nitroprusside (SNP), respectively, in isolated organ chambers. Separate aortic segments that underwent similar incubation protocol were fixed and immunohistochemically stained for arginase 1 and 4-hydroxynonenal (4-HNE). Isolated RBCs were collected for measurements of ROS with electron paramagnetic resonance11 and nitrate export with high-performance liquid chromatography.15,16 The reader is referred to the Supplemental Appendix for a detailed methods section.
Statistical analyses
Power calculation was performed for the primary endpoint RHI. Based on our reference material consisting of 28 healthy subjects and an estimated difference in RHI between groups of 0.5, an SD of 0.4, and enrollment ratio of 1:1, 14 observations in each group were needed to detect a significant difference between groups at a significance level of 5% and 80% power. Normality was checked with D’Agostino Pearson’s test. Clinical and experimental continuous data are reported as mean ± SD or as medians with 25th and 75th percentiles (Q1-Q3) depending on normality. Categorical data are reported as numbers and %. Cytokine data are reported as median and Q1-Q3 or as numbers. Differences between 2 groups were determined with either paired or unpaired Student's t-test and Wilcoxon signed-rank test or Mann-Whitney depending on normality. Differences between more than 2 groups were determined with 1-way analysis of variance (ANOVA) with appropriate post hoc test depending on normality and matching. Concentration-response curves were analyzed with stacked ordinary 2-way ANOVA for independent observations and with repeated measures for both concentration and relaxation for dependent observations. Categorical data were analyzed with Fisher’s exact test. All tests for specific analyses are described in the Figure legends; n denotes the number of subjects recruited for each experimental condition. All experimental conditions, which involved the addition of pharmacological drugs or compounds, are presented as paired observations. Two-tailed P < 0.05 was considered statistically different for all analyses. All analyses were carried out with GraphPad Prism version 7.02 (GraphPad Software, Inc).
Results
Study subjects
Study subject characteristics at inclusion are shown in Table 1. Patients were included a median of 4 days after arrival to the hospital and 12 days since onset of first symptom. All patients had verified pulmonary changes on radiographs with infiltrates associated with COVID-19 infection. One patient suffered from pulmonary embolism at inclusion. Eight of the patients had 1 comorbidity. All patients received anticoagulant medication, and most received corticosteroids during hospitalization. Anticoagulants were continued until 2 to 4 weeks following discharge according to local guidelines. All subjects had an oxygen demand during the hospital stay and 13 had it at inclusion. A subgroup of 10 patients was scheduled for a follow-up visit. Hemodynamic parameters and laboratory status for those subjects are shown both at inclusion and follow-up in Supplemental Table 1. Follow-up time between the 2 occasions was in the range of 110 to 138 days. The patients had stable hemodynamic parameters and were normoxic both at inclusion and at follow-up, although slightly improved oxygen saturation at follow-up. Almost all derangements in laboratory parameters were normalized at follow-up (Supplemental Table 1).
Table 1.
Baseline Characteristics
| Healthy (n = 27) | COVID-19 (n = 17) | P Value | |
|---|---|---|---|
| Age, y | 54 ± 11 | 54 ± 12 | 0.932a |
| Male | 17 (63) | 11 (65) | >0.99b |
| Days since symptoms | N/A | 12 (10-16) | |
| In-hospital day at inclusion | N/A | 4 (3-8) | |
| Smokers | 0 (0) | 1 (6) | 0.39b |
| BMI | 24 (23-26) | 28 (25-31) | <0.001c |
| 30-35 kg/m2 | 1 (4) | 5 (29) | 0.025b |
| >35 kg/m2 | 0 (0) | 1 (6) | 0.39b |
| X-ray changes | N/A | 17 (100) | |
| Pulmonary embolism | N/A | 1 (6) | |
| Comorbidities | |||
| Any | 0 | 8 (47) | <0.001b |
| Hypertension | 5 (29) | ||
| Previous myocardial infarction | 1 (6) | ||
| Asthma or COPD | 2 (12) | ||
| General medication | |||
| ACEi/ARB | - | 6 (35) | |
| Statins | - | 2 (12) | |
| Aspirin | - | 1 (6) | |
| Inhaled corticosteroids | - | 2 (12) | |
| COVID-19 medication | |||
| Corticosteroids | - | 13 (76) | |
| LMWH | - | 15 (88) | |
| Anti FX | - | 2 (12) | |
| Antibiotics | - | 3 (18) | |
| Remdesivir | - | 1 (6) | |
| Hemodynamic parameters at inclusion | |||
| SBP, mm Hg | 123 ± 13 | 117 ± 12 | 0.125a |
| DBP, mm Hg | 78 ± 8 | 74 ± 12 | 0.238a |
| Heart rate, beats/min | 63 ± 7 | 74 ± 16 | 0.004a |
| Respiratory rate, breaths/min | - | 19 ± 3 | |
| Oxygen saturation, % | - | 95 ± 3 | |
| Oxygen requirements anytime | N/A | 17 (100) | |
| Max oxygen requirement, L/min | N/A | 2.5 (2.0-4.0) | |
| HFNC anytime | N/A | 4 (24) | |
| Oxygen demand in total, d | N/A | 8 (3-9) | |
| Oxygen requiring at inclusion | N/A | 13 (76) | |
| Liters/min among those | N/A | 1.5 (1.0-2.0) | |
| HFNC at inclusion | N/A | 2 (12) | |
| Erythrocyte indices | |||
| Hemoglobin, g/L | 142 (133-147) | 136 (129-141) | 0.044c |
| EVF | 0.41 ± 0.03 | 0.39 ± 0.03 | 0.017a |
| MCV, fL | 89 (87-92) | 87 (84-89) | 0.015c |
| MCH, pg | 30 (29-31) | 30 (29-31) | 0.270c |
| RBC count, 1012/L | 4.6 ± 0.3 | 4.6 ± 0.4 | 0.623a |
| Thrombocytes, 109/L | 234 (192-278) | 301 (240-421) | 0.007c |
| Leukocytes, 109/L | 5.0 (4.3-5.9) | 9.5 (5.3-11.2) | <0.001c |
| Lymphocytes, 109/L | - | 1.2 (0.9-1.8) | |
| Creatinine, μmol/L | 78 ± 16 | 61 ± 13 | <0.001a |
| CRP, mg/L | |||
| At inclusion | 0.6 (0.3-1.0) | 36 (12-94) | <0.001c |
| Peak | N/A | 111 ± 59 | |
| ASAT, μkat/L | - | 0.94 ± 0.46 | |
| ALAT, μkat/L | - | 1.21 (0.88-1.41) | |
| Albumin, g/L | - | 29 ± 4 | |
| Procalcitonin, μg/L | - | 0.14 (0.08-0.19) | |
| LD, μkat/L | - | 5.5 (4.4-6.8) | |
| D-dimer, mg/L | - | 0.74 (0.33-0.94) | |
| Ferritin, μg/L | - | 1,629 ± 1,080 | |
| Troponin T elevation, >4 ng/L | - | 11 (65) | |
| Mean value among those, ng/L | - | 7.2 ± 1.4 |
Values are mean ± SD, n (%), or median (Q1-Q3).
ACEi = angiotensin-converting enzyme inhibitor; ALAT = alanine amino transaminase; Anti FX = anti-factor X; ARB = angiotensin receptor blocker; ASAT = aspartate aminotransferase; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; DBP = diastolic blood pressure; EVF = erythrocyte volume fraction; HFNC = high flow nasal cannula; LD = lactate dehydrogenase; LMWH = low molecular weight heparin; MCH = mean corpuscular hemoglobin; MCV = mean corpuscular volume; mmHg = millimeter mercury; N/A = not applicable; RBC = red blood cell; SBP = systolic blood pressure.
Unpaired t-test.
Fisher’s exact test.
Mann-Whitney test. Reference values are as follows: ALAT <1.1 μkat/L, albumin 34-45 g/L, ASAT <0.76 μkat/L, D-Dimer <0.5 mg/L for age <51 years and <0.71 mg/L for age >50 years, ferritin 30-350 μg/L, LD <3.5 μkat/L, lymphocytes 1.1-3.5·109/L, procalcitonin <0.5 μg/L, troponin T <15 ng/L.
Presence of endothelial dysfunction in the acute phase of COVID-19
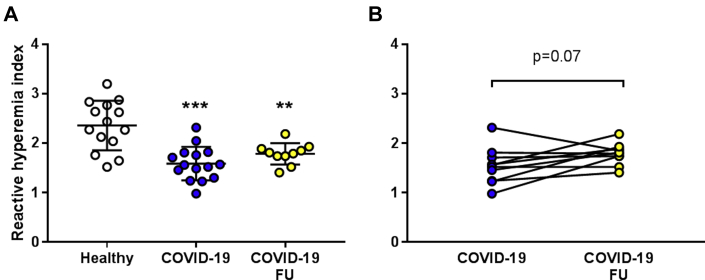
Previous reports from in situ postmortem studies have reported disruption of the endothelial cell lining in microvascular beds in patients with COVID-19.4,5 To confirm that this is reflected by endothelial dysfunction in vivo, we assessed microvascular digital endothelial function. RHI was ∼33% lower in patients with COVID-19 in the acute phase compared with healthy control subjects (Figure 1A). Furthermore, RHI in patients with COVID-19 remained significantly impaired and did not significantly improve at follow-up 4 months after the infection (Figures 1A and 1B). There was no difference in RHI between patients with COVID-19 with (n = 6) and without comorbidities (n = 9) (Supplemental Figure 1A). RHI was significantly lower among the patients with COVID-19 without comorbidities than in the healthy subjects (P < 0.001).
Figure 1.
Patients With COVID-19 Infection Have Impaired Endothelial Function
(A) Microvascular digital endothelial function expressed as reactive hyperemia index (RHI) in healthy subjects (n = 14) and patients with COVID-19 in the acute phase of the infection (n = 15) and at follow-up (FU) 4 months later (n = 10). (B) RHI in a subgroup of patients with COVID-19 evaluated both in the acute phase of the infection and at follow-up 4 months later (n = 10 each). Significant differences were analyzed using 1-way analysis of variance (ANOVA) and Tukey’s multiple comparison post hoc test in A and paired Student's t-test in B; ∗∗P < 0.01, ∗∗∗P < 0.001 vs healthy. Values are expressed as mean ± SD (A) or individual dot plots (B).
RBCs from patients with COVID-19 induce vascular dysfunction in the acute phase
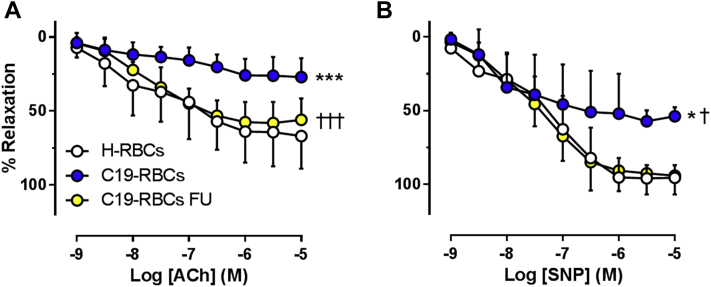
To elucidate the involvement of the RBC as a mediator of vascular dysfunction in patients with COVID-19, EDR and EIR were evaluated in rat aortic rings following incubation with RBCs from healthy subjects and patients with COVID-19 in the acute phase. EDR was significantly impaired following incubation with RBCs from patients with COVID-19 (C19-RBCs) compared with RBCs from healthy controls (H-RBCs) (Figure 2A). EIR was also impaired following incubation with C19-RBCs, especially at the higher concentrations of SNP. The effect of RBCs on EDR and EIR did not differ between the subjects with and those without comorbidities (Supplemental Figures 1B and 1C). By contrast, incubation for 1 hour or 18 hours with plasma isolated from patients with COVID-19 did not affect endothelial function (Supplemental Figures 2A and 2B). This suggests that C19-RBCs per se induce impairment in EDR and EIR in the acute phase, irrespective of the presence of comorbidities. It is further unlikely that the observations are caused by hemolysis, as free hemoglobin in plasma did not differ between healthy subjects and patients with COVID-19 (Supplemental Figure 3).
Figure 2.
C19-RBCs Impair Endothelium-Dependent and -Independent Relaxations
(A) Endothelium-dependent relaxation (n = 7-14) evoked by acetylcholine (ACh) and (B) endothelium-independent relaxation (n = 6-7) evoked by sodium nitroprusside (SNP) in rat aorta following 18 hours of incubation with red blood cells (RBCs) from either healthy subjects (H-RBCs) or patients with COVID-19 in the acute phase (C19-RBCs) or at follow-up (C19-RBCs FU). ∗∗∗P < 0.001 vs H-RBCs, †††P < 0.001 vs C19-RBCs, ∗P < 0.05 vs H-RBCs, †P < 0.05 vs C19-RBCs FU with 2-way analysis of variance and Tukey’s multiple comparison post hoc test (A and B). Values are expressed as mean and SD.
C19-RBCS induce endothelial dysfunction through vascular arginase 1 and ROS
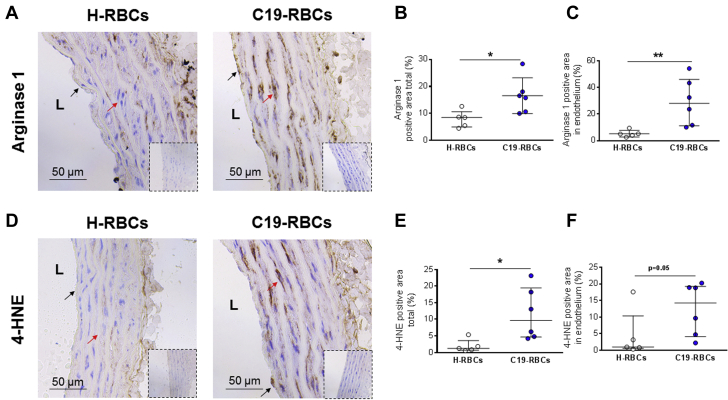
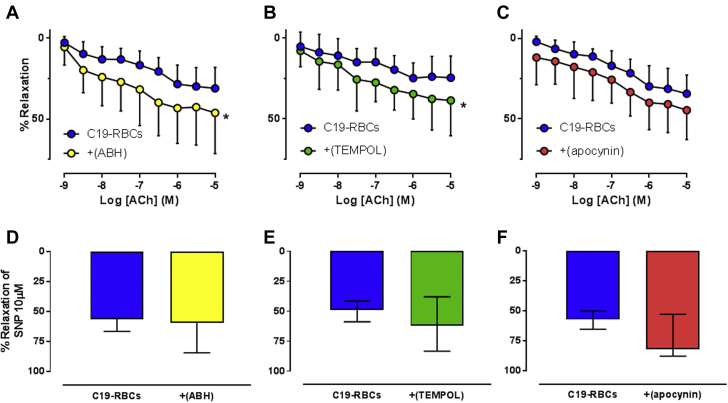
Arginase, which is known to be a regulator of endothelial function and nitric oxide (NO) bioactivity, has been implicated in COVID-19.13 Based on this, we hypothesized that arginase and ROS contribute to endothelial dysfunction induced by C19-RBCs. Following incubation of aortas with C19-RBCs, the expression of arginase 1 was increased both in endothelial cells and smooth muscle cells (Figures 3A to 3C). 4-HNE, an alpha, beta-unsaturated hydroxyalkenal that is produced by lipid peroxidation in cells and used as a stable marker for oxidative stress, was also upregulated in both endothelial cells and smooth muscle cells by C19-RBCs (Figures 3D to 3F). To elucidate the functional implications of these changes, we applied the arginase inhibitor 2(S)-amino-6-boronohexanoic acid (ABH), the superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL), or the NOX inhibitor (apocynin). Following the 18-hour co-incubation with C19-RBCs, ABH (Figure 4A) and TEMPOL (Figure 4B), but not apocynin (Figure 4C) significantly attenuated the impairment in endothelial function induced by C19-RBCs. None of the inhibitors affected EIR (Figures 4D to 4F). This suggests that C19-RBCs increase vascular arginase and oxidative stress, which results in impaired endothelial function.
Figure 3.
C19-RBCs Induce Upregulation of Vascular Arginase 1 and 4-HNE
Representative immune-histochemical images for arginase 1 (A) and 4-hydroxynonenal (4-HNE) (D) in aortic rings incubated with either H-RBCs or C19-RBCs for 18 hours. Immunoglobulin G controls are presented in inserts for each experimental condition. Quantitative analyses of arginase 1 and 4-HNE positivity of the total area (B and E) (n = 5-6) and the endothelial lining (C and F) (n = 5-6). L = luminal side of the vessel, black arrows = endothelial cells, and red arrows = smooth muscle cells. ∗P < 0.05, ∗∗P < 0.01 with Mann-Whitney test. Values are expressed as median (Q1-Q3). Abbreviations as in Figure 2.
Figure 4.
Vascular Arginase and Superoxide Mediate Endothelial Dysfunction Induced by C19-RBCs
Endothelium-dependent relaxation induced by acetylcholine (ACh) in rat aorta following incubation of C19-RBCs in the absence or presence of (A) the arginase inhibitor 2(S)-amino-6-boronohexanoic acid (ABH) (n = 8), (B) the superoxide dismutase mimetic 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL) (n = 7), or (C) the NOX inhibitor apocynin (n = 7). Endothelium-independent relaxation was evaluated by 1 dose of sodium nitroprusside (SNP) at the end of the experiment in the absence or presence of (D) ABH (n = 8), (E) TEMPOL (n = 7), or (F) apocynin (n = 7). ( ) indicates that compounds were applied after the 18-hour incubation to selectively target the vasculature. ∗P < 0.05 vs C19-RBCs with 2-way analysis of variance matched for both concentration and relaxation (A, B, C), paired Student's t-test (D), or Wilcoxon test (E, F). Values are expressed as mean and SD (A-D) or median (Q1-Q3) (E, F).
Endothelial dysfunction in COVID-19 is driven by an increase in RBC-derived ROS
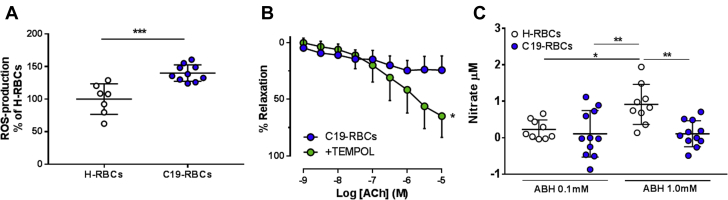
To further elucidate the mechanisms by which changes within the RBCs contribute to endothelial dysfunction, we measured ROS production in RBCs. Indeed, we found that ROS production is increased in C19-RBCs compared with H-RBCs (Figure 5A). Attenuation of superoxide in RBCs by application of TEMPOL (before the 18-hour incubation) significantly attenuated endothelial dysfunction induced by C19-RBCs (Figure 5B). Because ROS production reciprocally regulates NO bioactivity, we hypothesized that export of NO bioactivity from C19-RBCs is compromised compared with H-RBCs. Because export of NO bioactivity from RBCs is tightly regulated by arginase, ABH was used to increase NO bioactivity with extracellular levels of the NO metabolite nitrate as the readout according to previous results.16 There was no difference in baseline nitrate production between the groups (Supplemental Figure 4). Extracellular nitrate levels were significantly increased following incubation of H-RBCs with ABH, whereas they were completely unchanged following incubation of C19-RBCs (Figure 5C). These observations suggest that export of NO bioactivity is markedly compromised in C19-RBCs.
Figure 5.
Increased ROS and Decreased NO Bioactivity in RBCs Contribute to Endothelial Dysfunction in COVID-19
(A) Production of reactive oxygen species (ROS) with electron paramagnetic resonance in H-RBCs (n = 7) and C19-RBCs (n = 10) expressed as percentage of H-RBCs. (B) Endothelium-dependent relaxation induced by ACh in rat aorta following incubation of C19-RBCs in the absence or presence of the superoxide dismutase mimetic TEMPOL (n = 8). (C) Change in nitrate levels with high-performance liquid chromatography in the supernatant following incubation of H-RBCs and C19-RBCs with the arginase inhibitor ABH at concentrations indicated (n = 9-11). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 with unpaired Student's t-test (A), 2-way analysis of variance (ANOVA) matched for both concentration and relaxation (B), and 1-way ANOVA with Holm-Sidak’s post hoc test (C). Values are expressed as mean ± SD (A, C) or mean and SD (B). Other abbreviations as in Figures 2 and 4.
Interferonγ triggers endothelial dysfunction induced by RBCS
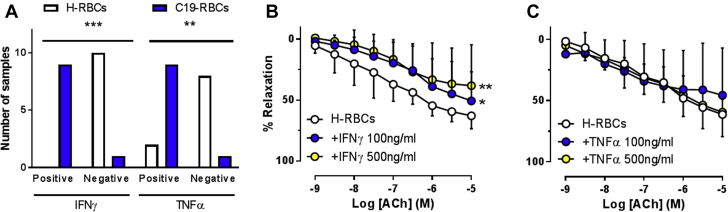
As the cytokine storm is clearly a major contributor to illness in COVID-19, we investigated the impact of cytokines on the impairment in endothelial function induced by RBCs. RBCs have been proposed to represent a dynamic reservoir of cytokines.17 We investigated whether this represents a putative mechanism behind the observed interaction between RBCs and endothelial function in COVID-19. We performed a Luminex technology-based 27-plex profiling of proinflammatory cytokines and chemokines in RBC lysates. The expression patterns of the various cytokines and chemokines are listed in Supplemental Table 2. Only interferon γ (IFNγ) and tumor necrosis factor α (TNFα) significantly differed in the number of positive samples, with higher frequency of detectable samples in C19-RBC lysates compared with H-RBC lysates (Figure 6A). To test whether this has functional implications on vascular reactivity, H-RBCs were pretreated with these cytokines with subsequent evaluation of vascular reactivity. Pre-incubation of H-RBCs for 2 hours with IFNγ (Figure 6B) but not TNFα (Figure 6C) induced a significant impairment in EDR. Levels of interleukin (IL)-9 (Supplemental Figure 5A) and macrophage inflammatory protein (MIP)-1β (Supplemental Figure 5B) were higher in C19-RBCs compared with H-RBCs. However, pre-incubation of IL-9 or MIP-1β with H-RBCs did not affect endothelial function (Supplemental Figures 5C and 5D). These observations collectively suggest that IFNγ is one of the key triggers of RBC-induced endothelial dysfunction in COVID-19.
Figure 6.
IFNγ Triggers Endothelial Dysfunction Induced by RBCs
Number of samples with detectable levels (positive) vs undetectable levels (negative) of interferon γ (IFNγ) and tumor necrosis factor α (TNFα) in H-RBC and C19-RBC lysates (A). Effect of 2-hour pre-incubation of H-RBCs with (B) IFNγ (n = 6), and (C) TNFα (n = 4) on endothelium-dependent relaxations induced by ACh. (A) ∗∗P < 0.01, ∗∗∗P < 0.001 with Fisheŕs exact test. (B, C) ∗P < 0.05, ∗∗P < 0.01 vs H-RBCs with 2-way analysis of variance matched for both concentration and relaxation and Dunnett’s post hoc test. Values are expressed as numbers (A) or mean and SD (B, C). Abbreviations as in Figure 2.
In vivo endothelial function and ex vivo RBC function at follow-up
To determine the dynamics of endothelial and RBC dysfunction after the acute phase of the infection, a subgroup of the patients (n = 10) was reexamined after a mean follow-up period of 4 months. The rationale for choosing 4 months as follow-up time was for the RBCs to undergo 1 cycle of renewal, as the mean circulatory life span of a RBC is 120 days. Interestingly, RHI remained markedly impaired at follow-up (Figure 1A) and it was slightly but not significantly improved compared with during the acute phase (Figure 1B). By contrast, C19-RBCs collected at follow-up did not affect EDR or EIR (Figures 2A and 2B). In fact, both EDR and EIR of aorta incubated with C19-RBCs at follow-up were similar to those observed after incubation with H-RBCs. Levels of free hemoglobin in plasma did not differ between the acute phase and at follow-up (Supplemental Figure 3).
Discussion
The present study demonstrates the presence of profound and persistent endothelial dysfunction in patients with moderately severe COVID-19 (ie, not requiring intensive care and with isolated respiratory failure). We further demonstrate that alterations in RBC function, but not plasma, characterized by increased ROS formation, mediate impaired endothelial function via upregulation of vascular arginase 1 and oxidative stress in the acute phase. Last, we reveal that 4 cytokines are increased in C19-RBCs and that IFNγ might be the trigger of these events.
Given the wide range of symptoms arising from COVID-19, it has been speculated that many of the cardiovascular complications are attributed to endothelial injury.1,3 Whether vascular dysfunction and more specifically endothelial dysfunction actually is present in patients with ongoing COVID-19 infection has not been unequivocally demonstrated, however. The mechanisms by which such injury occurs are also poorly understood. Evidence provided so far is limited to autopsy studies,4,5 evaluation of endothelial function several weeks after a positive PCR test,18 and assessment of microvascular function19,20 or density21 in severely ill patients with multiple cardiovascular comorbidities. Alterations in severe COVID-19 may be confounded by multiple comorbidities or intensive care interventions, such as anesthetic drugs and mechanical ventilation. It is therefore of importance that we were able to demonstrate the presence of profound endothelial dysfunction in patients with moderate severity of COVID-19. This was observed not only during active disease, but also 4 months later, independent of other comorbidities, and despite that most of the patients received anti-inflammatory glucocorticoids and all received anticoagulants. Interestingly, evaluation of endothelial function in the current study was performed in an extrapulmonary vascular bed in patients suffering from only respiratory symptoms. This fact supports the idea that COVID-19 has systemic effects even though the clinical presentation does not involve signs from extrapulmonary vascular beds. In fact, previous studies have established the association between peripheral microvascular endothelial dysfunction and systemic endothelial dysfunction.22,23 The impairment in microvascular digital endothelial function in this cohort of patients with COVID-19 in the acute phase is even more pronounced than that previously detected using the same methodology in patients with type 2 diabetes having micro- and macrovascular complications.14 This illustrates the severity of microvascular endothelial dysfunction among these patients with COVID-19.
The implications of structural9 and oxidative10 alterations in RBCs in patients with COVID-19 for vascular function have previously not been investigated. We here show that the RBC is an important mediator of vascular dysfunction during the acute phase of the infection. RBCs from patients with COVID-19 with ongoing infection induced pronounced impairment in endothelial function. Interestingly, after 1 cycle of RBC renewal (120 days), the detrimental effect of RBCs on vascular function was absent. This finding suggests that RBCs are severely affected and contribute to the development of endothelial dysfunction in the acute phase of the infection. Although the RBC function is normalized at follow-up, most likely caused by the production of new cells, the endothelial injury induced during the acute phase is persistent for at least 4 months.
The mechanism behind the vascular injury induced by C19-RBCs in the acute phase was investigated ex vivo. C19-RBCs induced upregulation of arginase 1 and increased oxidative stress in endothelial cells and smooth muscle cells. Excess in endothelial arginase 1 is known to reduce NO bioavailability and uncouple endothelial NO synthase (eNOS) with subsequent increment in ROS production.24 The observation that inhibition of vascular arginase or ROS attenuated endothelial dysfunction induced by RBCs suggests that RBCs induce endothelial dysfunction through upregulation of arginase 1 and ROS. This observation is in line with recent studies indicating that arginase is upregulated in patients with COVID-19.13,25 Interestingly, the effect of plasma from patients with COVID-19 did not affect endothelial function, further confirming the specific involvement of RBCs. This is in contrast to a previous study showing decreased endothelial cell viability after 1-hour incubation with plasma from patients with COVID-19 both with a mild disease and from patients requiring intensive care.7 However, the experimental readouts and possible influence of comorbidities differ between the studies, which might explain the different outcomes.
A feature of RBCs is their central involvement in redox regulation with prominent pro-oxidative as well as antioxidant capacities.8 Thus, an increase in oxidative stress by the RBCs may have important biological effects. It is therefore of interest that we found a clear increase in ROS production in C19-RBCs compared with H-RBCs. This switch in redox balance toward a pro-oxidative phenotype in COVID-19 contributes to the development of endothelial dysfunction, which was evident from the rescue of endothelial function by application of a superoxide dismutase mimetic. This is corroborated by recent observations that oxidative metabolites are markedly elevated in C19-RBCs.10 However, the levels of antioxidants in C19-RBCs are largely intact. Interestingly, one of the few antioxidants that are reduced in C19-RBCs, is superoxide dismutase. This, together with our observations, suggests that the oxidative phenotype is driven by not only increased superoxide anion radical but also a decrease in its anti-oxidative capacity. The increased formation of ROS is also reflected by the attenuated export of nitrate, the stable metabolite of NO, and a marker of NO bioactivity, from C19-RBCs. In these experiments, arginase, the reciprocal inhibitor of NO production in RBCs,16 was inhibited to enhance the formation and export of NO bioactivity. In accordance with previous results,16 nitrate was markedly increased in the supernatant following incubation of H-RBCs with the arginase inhibitor. By contrast, there was no increase in nitrate levels in the supernatant from C19-RBCs in the presence of arginase inhibitor. It is still under debate how and in which form RBCs are able to export NO.12 Together with the increased ROS production, one possibility is that uncoupling of eNOS with attenuated NO formation results in less production of nitrate. Another possible explanation is that NO is exported in the form of nitrate, which is reduced in C19-RBCs.
A prominent feature of COVID-19 is the release of cytokines from immune cells. To investigate whether cytokines initiate RBC-induced endothelial dysfunction, a cytokine profiling in H-RBCs and C19-RBCs was performed. Several cytokines were present in RBCs, but only 4 differed in the expression profile between the groups, and only IFNγ was found to induce endothelial dysfunction following incubation with H-RBCs, suggesting that IFNγ might be a key driver of RBC-induced vascular injury. To our knowledge, this is the first time a cytokine profiling has been performed in RBCs from patients with COVID-19 and provides support that RBCs are affected by the cytokine storm. This opens up the possibility that RBCs may be carriers of cytokines and contribute to vascular damage in COVID-19. Our findings concerning IFNγ are supported by the observation that IFNγ, among other cytokines, is substantially elevated in patients with moderately severe COVID-19.26 This is also in line with the follow-up observations that the RBCs do not induce alterations in vascular function after 1 cycle of renewal of RBCs (4 months) when the cytokine storm has passed. Although we tried to avoid a carry-over effect by the cytokines by a washing step before the co-incubation of the RBCs with aortic rings, it cannot be excluded that some IFNγ remains and directly affects the aorta. However, pre-incubation of TNFα, which is known to alter endothelial function,27 did not affect endothelial function in our pre-incubation protocols, which makes a possible carry-over effect unlikely.
C19-RBCs were found to disrupt both endothelium-dependent and -independent (smooth muscle cell) function. Previous studies have demonstrated that RBCs induce selective impairment in endothelial function without affecting smooth muscle cell function in type 2 diabetes.11 Given the different nature of the 2 diseases in which type 2 diabetes is a slowly progressing disease with changes developing over years or decades, whereas COVID-19 is an acute infection with rapid onset, it is likely that the mechanisms are different. Another possibility is that ROS formation by RBCs also attenuates the pharmacological effect of the exogenous NO donor SNP, which results in impaired vascular smooth muscle cell relaxation.
Study limitations
Although RHI evaluations were performed in vivo and the results were in line with the ex vivo effects of the RBCs from the same patients, the degree and contribution of RBCs to vascular dysfunction in vivo remains unclear. Another limitation is that we were not able to evaluate endothelium-independent function in vivo in our cohort of patients because of the risk for hemodynamic side effects and aerosol generation during administration of sublingual nitroglycerine. The healthy group may not represent the ideal control group, because it cannot be excluded that inflammatory states associated with viral infections other than COVID-19 might trigger similar changes. Because of the dramatically reduced incidence of influenza and other viral infections with the implementation of restrictions related to COVID-19, it has not been possible to recruit such control subjects for fresh blood sampling needed for these studies. However, given the endothelial involvement and specific changes in COVID-19 compared with other viral infections, such as influenza,4 it is likely that the changes observed are specific for COVID-19. Furthermore, although previous investigations have confirmed that there are a negligible amount of other cell types following the RBC isolation protocol,16,28 it cannot be excluded that a few remaining white blood cells or platelets contribute to the vascular dysfunction induced by the C19-RBC suspension. Finally, the RBC-induced endothelial function was investigated in rat aorta, which may be different from human vessels. However, our previous study demonstrated that dysfunctional RBCs induce a similar degree of endothelial dysfunction in human mammary arteries,11 indicating that the rat aorta is representative for this type of investigation.
Conclusions
The present study advances our knowledge regarding vascular complications in COVID-19 by demonstrating pronounced and persistent endothelial dysfunction and unravelling a previously unrecognized role of RBCs in the pathophysiology of vascular dysfunction. Targeting RBC dysfunction may provide a novel therapeutic strategy to combat cardiovascular complications in patients infected with COVID-19. Furthermore, our data reinforce the implementation of evaluating not only endothelial function as an important surrogate endpoint but also indices of RBC dysfunction in patients with COVID-19, especially considering the sustained endothelial injury. The results may lay the foundation for the identification of novel pharmacological targets for treating vascular complications arising from the infection. This may include treatment strategies that result in restoration of the redox balance in the RBCs, such as targeted antioxidants, arginase inhibition, and boosting NO bioavailability.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Cardiovascular complications have arisen as major clinical problems in patients with COVID-19. A unifying pathological mechanism underlying these complications may be caused by endothelial injury for which the mechanisms are poorly understood. We here provide evidence for the presence of endothelial dysfunction in patients with COVID-19 mediated by RBCs.
TRANSLATIONAL OUTLOOK: These observations provide mechanistic insights and a possible target for preventing vascular dysfunction in COVID-19.
Funding Support and Author Disclosures
This work was supported by the Swedish Research Council (2020-01372), the Swedish Heart and Lung Foundation (20190266, 20190341, 20200326, and 20210062), and the Stockholm County Council ALF (20190031). Dr Mahdi is supported by the Swedish Heart and Lung Foundation (20190267). Dr Collado is supported by a Novo Nordisk postdoctoral fellowship run-in partnership with Karolinska Institutet. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The technical assistance of David Ersgård, Marita Wallin, and Carina Nihlén is gratefully acknowledged. The authors thank the nurses at the COVID-19 wards at Karolinska University Hospital for help with blood sampling.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. The authors have no conflict of interest to declare. For more information, visit the Author Center.
Appendix
For supplemental materials, methods, and references section as well as supplemental tables and figures, please see the online version of this paper.
Contributor Information
Ali Mahdi, Email: ali.mahdi@ki.se.
John Pernow, Email: john.pernow@ki.se.
Appendix
References
- 1.Evans P.C., Rainger G.E., Mason J.C., et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–2184. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Libby P., Luscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41:3038–3044. doi: 10.1093/eurheartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCracken I.R., Saginc G., He L., et al. Lack of evidence of angiotensin-converting enzyme 2 expression and replicative infection by SARS-CoV-2 in human endothelial cells. Circulation. 2021;143:865–868. doi: 10.1161/CIRCULATIONAHA.120.052824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rauch A., Dupont A., Goutay J., et al. Endotheliopathy is induced by plasma from critically ill patients and associated with organ failure in severe COVID-19. Circulation. 2020;142:1881–1884. doi: 10.1161/CIRCULATIONAHA.120.050907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahdi A., Cortese-Krott M.M., Kelm M., Li N., Pernow J. Novel perspectives on redox signaling in red blood cells and platelets in cardiovascular disease. Free Radic Biol Med. 2021;168:95–109. doi: 10.1016/j.freeradbiomed.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Foy B.H., Carlson J.C.T., Reinertsen E., et al. Association of red blood cell distribution width with mortality risk in hospitalized adults with SARS-CoV-2 Infection. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas T., Stefanoni D., Dzieciatkowska M., et al. Evidence of structural protein damage and membrane lipid remodeling in red blood cells from COVID-19 patients. J Proteome Res. 2020;19:4455–4469. doi: 10.1021/acs.jproteome.0c00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z., Mahdi A., Tratsiakovich Y., et al. Erythrocytes from patients with type 2 diabetes induce endothelial dysfunction via arginase I. J Am Coll Cardiol. 2018;72:769–780. doi: 10.1016/j.jacc.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Pernow J., Mahdi A., Yang J., Zhou Z. Red blood cell dysfunction: a new player in cardiovascular disease. Cardiovasc Res. 2019;115:1596–1605. doi: 10.1093/cvr/cvz156. [DOI] [PubMed] [Google Scholar]
- 13.Falck-Jones S., Vangeti S., Yu M., et al. Functional monocytic myeloid-derived suppressor cells increase in blood but not airways and predict COVID-19 severity. J Clin Invest. 2021;131 doi: 10.1172/JCI144734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafnsson A., Bohm F., Settergren M., Gonon A., Brismar K., Pernow J. The endothelin receptor antagonist bosentan improves peripheral endothelial function in patients with type 2 diabetes mellitus and microalbuminuria: a randomised trial. Diabetologia. 2012;55:600–607. doi: 10.1007/s00125-011-2415-y. [DOI] [PubMed] [Google Scholar]
- 15.Montenegro M.F., Sundqvist M.L., Nihlen C., Hezel M., Carlstrom M., Weitzberg E., Lundberg J.O. Profound differences between humans and rodents in the ability to concentrate salivary nitrate:Implications for translational research. Redox Biol. 2016;10:206–210. doi: 10.1016/j.redox.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J., Gonon A.T., Sjoquist P.O., Lundberg J.O., Pernow J. Arginase regulates red blood cell nitric oxide synthase and export of cardioprotective nitric oxide bioactivity. Proc Natl Acad Sci U S A. 2013;110:15049–15054. doi: 10.1073/pnas.1307058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karsten E., Breen E., Herbert B.R. Red blood cells are dynamic reservoirs of cytokines. Sci Rep. 2018;8:3101. doi: 10.1038/s41598-018-21387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratchford S.M., Stickford J.L., Province V.M., et al. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol. 2021;320:H404–H410. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabioni L., De Lorenzo A., Lamas C., Muccillo F., Castro-Faria-Neto H.C., Estato V., Tibirica E. Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients:Evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res. 2021;134:104119. doi: 10.1016/j.mvr.2020.104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tehrani S., Gille-Johnson P. Microvascular dysfunction in patients with critical COVID-19, a pilot study. Shock. 2021;56:964–968. doi: 10.1097/SHK.0000000000001803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rovas A., Osiaevi I., Buscher K., et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24:145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R., Jr., Kuvin J.T., Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 23.Di Serafino L., Mangiacapra F., Pyxaras S., et al. Relationship between peripheral arterial reactive hyperemia and the index of myocardial resistance in patients undergoing invasive coronary angiography. Int J Cardiol. 2021;333:8–13. doi: 10.1016/j.ijcard.2021.02.085. [DOI] [PubMed] [Google Scholar]
- 24.Mahdi A., Kovamees O., Pernow J. Improvement in endothelial function in cardiovascular disease. Is arginase the target? Int J Cardiol. 2020;301:207–214. doi: 10.1016/j.ijcard.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Rees C.A., Rostad C.A., Mantus G., et al. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2101708118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghazavi A., Ganji A., Keshavarzian N., Rabiemajd S., Mosayebi G. Cytokine profile and disease severity in patients with COVID-19. Cytokine. 2021;137:155323. doi: 10.1016/j.cyto.2020.155323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang P., Ba Z.F., Chaudry I.H. Administration of tumor necrosis factor-alpha in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266:H2535–H2541. doi: 10.1152/ajpheart.1994.266.6.H2535. [DOI] [PubMed] [Google Scholar]
- 28.Hanson M.S., Stephenson A.H., Bowles E.A., Sridharan M., Adderley S., Sprague R.S. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol. 2008;295:H786–H793. doi: 10.1152/ajpheart.00349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.