LETTER
Timely and accurate diagnostic testing is a critical component of the public health response to coronavirus (CoV) disease 2019 (COVID-19). Antigen (Ag) tests are used widely in many countries to provide rapid, economical, and accessible point-of-care testing (1). The vast majority of antigen tests detect nucleocapsid (N) protein, a structural protein that displays less variation than the spike (S) protein across different severe acute respiratory syndrome (SARS)-CoV-2 lineages. Although antigen tests are less sensitive than reverse transcription-PCR (RT-PCR) tests, their ability to quickly detect individuals with high viral loads provides clinical and public health utility in many countries, including Australia, where antigen tests have recently been approved for self-testing (2). As new variants arise, including the recent SARS-CoV-2 Omicron variant, it is essential to rapidly assess the performance of diagnostic assays. Here, in order to assess and compare the abilities of antigen tests to detect the Delta and Omicron variants, we performed a rapid assessment of 10 commercially available antigen tests.
To evaluate analytical sensitivity, we used representative Delta and Omicron isolates cultured from clinical samples (see the supplemental material). Isolates were grown in Calu-3 cells, as described in the supplemental material. For each variant, we constructed a dilution series of quantified virus, ranging from ∼109 to 105 copies/mL, corresponding to cycle threshold (Ct) values of ∼18 to ∼29 from an in-house RT-PCR for the N gene (3). Testing was performed according to the manufacturers’ provided instructions for use (IFU). Where no specific protocol was provided, a 1:1 dilution of sample to extraction buffer was prepared and added to the test kit. Interpretations of antigen test results were performed as per the manufacturers’ instructions, and results of all tests were recorded independently by two scientists. All antigen testing was conducted in quadruplicate and under biosafety level 3 conditions using live virus.
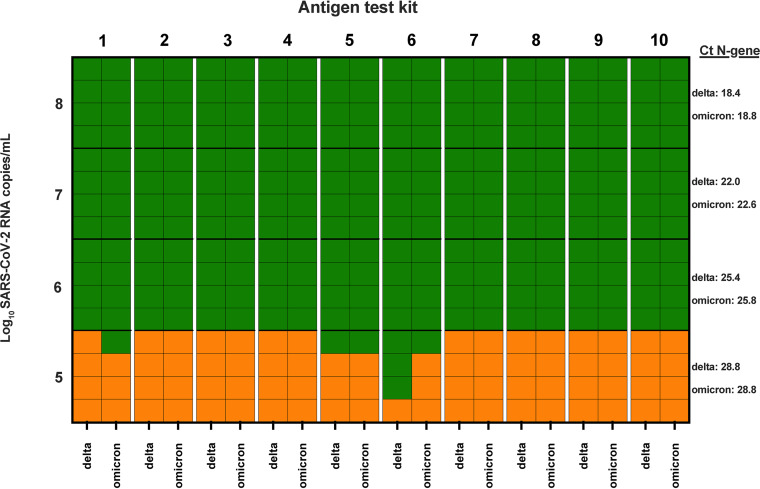
Overall, the analytical sensitivities of the 10 antigen kits were similar for both the Delta and Omicron variants (Fig. 1). All 10 kits were able to detect Delta at 6.50 log10 copies/mL (Ct, 25.4) and Omicron at 6.39 log10 copies/mL (Ct, 25.8), consistent with the results of previous work on the analytical sensitivities of antigen kits (4). None of the 10 kits consistently detected either Delta or Omicron at the lowest dilutions (5.23 log10 copies/mL, with a Ct of 28.8 [Delta]; 5.33 log10 copies/mL, with a Ct of 28.8 [Omicron]).
FIG 1.
Analytical sensitivities of lateral flow devices against SARS-CoV-2 Delta and Omicron variants. Ten lateral flow devices were tested against 10-fold dilutions (1:100 to 1:100,000) of SARS-CoV-2 Delta and Omicron variants in quadruplicate. Green boxes indicate where SARS-CoV-2 antigen was detected for a single replicate, and orange boxes indicate where a negative result was observed. Mean Ct values from three replicates for each dilution were calculated using in-house RT-PCR for the N gene. The registered test names and manufacturers of the lateral flow devices included were as follows: (1) PanBio COVID-19 Ag rapid test device (nasal), Abbott Rapid Diagnostics Jena GmbH (Germany); (2) NowCheck COVID-19 antigen test, BioNote Inc. (Republic of Korea); (3) Roche SARS-CoV-2 rapid antigen test, SD Biosensor Inc. (Republic of Korea); (4) Standard Q COVID-19 Ag test, SD Biosensor Inc. (Republic of Korea); (5) SureScreen Diagnostics COVID-19 antigen rapid test cassette, BTNX Inc. (Canada); (6) VivaDiag SARS-CoV-2 Ag rapid test, VivaChek Biotech (Hangzhou) Co. Ltd. (China); (7) Wantai SARS-CoV-2 Ag rapid test (colloidal gold), Beijing Wantai Biological Pharmacy Enterprise Co. Ltd. (China); (8) Testsea SARS-CoV-2 antigen test kit, Hangzhou Testsea Biotechnology Co. Ltd. (China); (9) InnoScreen COVID-19 antigen rapid test device, Innovation Scientific Pty. Ltd. (Australia); and (10) LYHER novel coronavirus (COVID-19) antigen test kit (colloidal gold), Hangzhou Laihe Biotech Co. Ltd. (China).
Our results provide valuable rapid in vitro data on the abilities of antigen tests to detect the Omicron variant and are consistent with other work demonstrating the effectiveness of antigen tests for SARS-CoV-2 variants (5). Given the large investments made in antigen testing in many countries and the increasing reliance on these tests to inform clinical and public health action, ongoing postmarket validation, including assessment of clinical sensitivity against new variants, is essential to ensure optimal test selection and deployment.
ACKNOWLEDGMENTS
This work was supported by the Department of Health, Victoria, Australia.
We declare no competing interests.
Footnotes
Supplemental material is available online only.
Contributor Information
Deborah A. Williamson, Email: deborah.williamson@unimelb.edu.au.
Alexander J. McAdam, Boston Children's Hospital
REFERENCES
- 1.Mina MJ, Peto TE, García-Fiñana M, Semple MG, Buchan IE. 2021. Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19. Lancet 397:1425–1427. 10.1016/S0140-6736(21)00425-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Therapeutic Goods Administration (TGA). 2021. COVID-19 rapid antigen self-tests that are approved in Australia. TGA, Woden, Australia. https://www.tga.gov.au/covid-19-rapid-antigen-self-tests-are-approved-australia. Accessed 15 December 2021. [Google Scholar]
- 3.Caly L, Druce J, Roberts J, Bond K, Tran T, Kostecki R, Yoga Y, Naughton W, Taiaroa G, Seemann T, Schultz MB, Howden BP, Korman TM, Lewin SR, Williamson DA, Catton MG. 2020. Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia. Med J Aust 212:459–462. 10.5694/mja2.50569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corman VM, Haage VC, Bleicker T, Schmidt ML, Mühlemann B, Zuchowski M, Jo WK, Tscheak P, Möncke-Buchner E, Müller MA, Krumbholz A, Drexler JF, Drosten C. 2021. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study. Lancet Microbe 2:e311–e319. 10.1016/S2666-5247(21)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekliz M, Adea K, Essaidi-Laziosi M, Sacks JA, Escadafal C, Kaiser L, Eckerle I. 24 November 2021. SARS-CoV-2 antigen-detecting rapid tests for the delta variant. Lancet Microbe 10.1016/S2666-5247(21)00302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Download jcm.02479-21-s0001.pdf, PDF file, 0.1 MB (114.2KB, pdf)