Supplemental Digital Content is Available in the Text. The hypoalgesic impact of cardiovascular regulatory systems on evoked pain responsiveness in those with chronic pain is conveyed via the indirect effects of psychological distress.
Keywords: Chronic pain, Heart rate variability, Baroreceptor sensitivity, Psychological distress, Pain sensitivity, Pain tolerance
Abstract
Introduction:
Chronic pain (CP) patients often display lower heart rate variability (HRV) and baroreceptor sensitivity (BRS), which are associated with increased evoked pain intensity and decreased pain tolerance.
Objective:
The purpose of this study was to test whether the association between low levels of HRV and BRS and increased evoked pain responsiveness in individuals with CP is mediated by psychological distress and whether this mediation is sex dependent.
Methods:
The sample consisted of 877 participants in Wave 6 of the Tromsø population study who reported clinically meaningful CP. Resting HRV and BRS parameters were derived from continuous beat-to-beat blood pressure recordings. Psychological distress was assessed using the Hopkins Symptom Checklist-10. After cardiovascular assessment, participants completed a 106-second cold pressor task (3°C bath), which assessed cold pressor pain intensity (CPI) and cold pressor pain tolerance (CPT).
Results:
In the full CP sample, mediation analyses showed significant indirect effects, without direct effects, of HRV and BRS on both CPT and CPI via psychological distress. When stratified by sex, significant indirect effects via psychological distress were only found in males for the impact of rMSSD on CPT, the impact of SDNN on CPT, and the impact of BRS on CPT via psychological distress. Moderated mediation analyses revealed that there were no significant sex differences in the indirect effects of HRV and BRS on both CPT and CPI via psychological distress.
Conclusions:
The hypoalgesic impact of cardiovascular regulatory systems on evoked pain responses is conveyed via the indirect effects of psychological distress.
1. Introduction
Baroreceptor sensitivity (BRS) and heart rate variability (HRV) are among the most important indexes of cardiovascular and autonomic health in those suffering from chronic pain (CP).2,8,51 BRS assesses efficiency of the baroreflex, a homeostatic feedback loop important for maintaining healthy blood pressure levels,39 whereas HRV captures variability in the time interval between successive heartbeats.34,40,43 The standard deviation of the average normal-to-normal interbeat intervals (SDNN) and the root mean square of successive RR interval differences (rMSSD) are specific HRV parameters associated with a subset of vagal fibers that modulate their activity in response to physiological changes.37,41,62,63
Meta-analysis65 indicates that CP is associated with reduced resting rMSSD,8 which can be associated with poor fear inhibition,68 failure to recognize safety cues,62 and hypervigilance61 manifesting in worry and rumination.6,49 Significant differences in SDNN have also been found in individuals with fibromyalgia, neuropathic pain, and orofacial pain when compared with healthy controls.65 Elevated HRV and BRS are linked to optimized cardiac regulation,34,43,60 emotion regulation,35,63 meaning in life,11 and increased pain resiliency and lower pain sensitivity in healthy individuals.19,24,26,51 Lower SDNN and rMSSD are associated with greater pain intensity in those with CP.3,8 The experience of more intense pain in those with CP may be interfering with parasympathetic regulatory activity.17,20,53
Weekly elevations of pain and stress in CP patients predict increased psychological distress; conversely, greater positive affect predicts lower pain intensity.51,71 Psychological distress, which is often comorbid with CP,67 could contribute to reduced HRV and BRS and thus might influence both evoked pain tolerance and intensity.8 Moreover, sex differences have also been found47 in associations between cardiovascular function and pain responsiveness.
The current study aimed to test whether the impact of HRV and BRS on cold pressor pain tolerance and intensity is mediated by psychological distress and sex dependent in participants reporting CP. We hypothesized that levels of psychological distress would significantly mediate the impact of rMSSD, SDNN, and BRS on cold pressor pain intensity and tolerance in those with CP. Furthermore, we hypothesized that there would be significant differences between men and women in the extent to which psychological distress mediates the relationship between HRV and BRS measures and evoked pain responsiveness.
2. Methods
2.1. Design
The Tromsø Study has been an ongoing epidemiological study of chronic disease prevalence, health issues, and symptoms in Norway. To date, 7 surveys (6–7 years apart) have been conducted. Tromsø 6 provided data for the current study and included assessment of sociodemographic, psychosocial, and health-related factors as well as a standardized cold pressor protocol.15 The study and analysis were approved by the Data Inspectorate of Norway, the Regional Committee of Medical and Health Research Ethics of Northern Norway (#8885, December 10, 2019), and complies with the Declaration of Helsinki. Each participant gave written informed consent before participation.
2.2. Sample
For Tromsø 6 (2007–2008), 19,762 men and women were invited and 12,982 of them (65.7%) aged 30 to 87 years participated. The sample was recruited from 4 different groups: (1) all previous attendees in the second visit of Tromsø 4 (1994–1995), (2) a 10% random sample of individuals aged 30 to 39 years, (3) all inhabitants aged 40 to 42 years and 60 to 87 years, and (4) a 40% random sample of inhabitants aged 43–59 years.15 All participants in Tromsø 6 were asked to participate in a cold pressor test to evaluate acute pain responsiveness; because of capacity problems, some participants left the testing site without being examined.
Within the total potential sample available for this study, 1,143 participants reported experiencing clinically meaningful CP, operationalized as participants reporting that: (1) they were currently experiencing persistent pain that had lasted for ≥3 months, (2) the pain was experienced daily, and (3) the pain had a usual severity of ≥3/10 on a 0 to 10 pain intensity scale (described below).48 From this group, a final sample (n = 877) was selected based on the following criteria: (1) age 30–65 years; (2) continuous BP data sufficient to derive HRV and/or BRS values; (3) valid cold pressor pain intensity ratings and cold pressor pain tolerance times and; (4) valid reports of negative affect on the Hopkins Symptom Checklist-10. Individuals not meeting the criteria for CP or other study criteria were excluded from the study sample. Figure 1 shows the CP population selection process and Table 1 displays the sociodemographic characteristics of the final sample analyzed.
Figure 1.
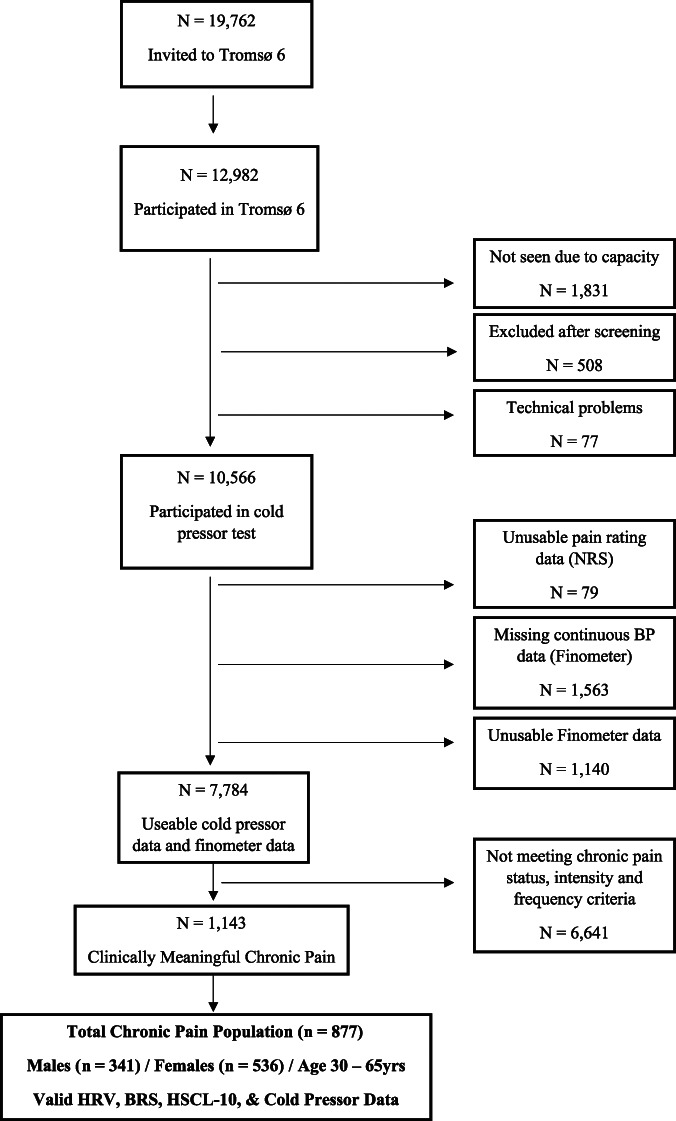
Chronic pain population selection from participants of the Tromsø 6 population survey.
Table 1.
Sociodemographic sample characteristics overall and by sex.
| Characteristic | Category | Total (n = 877) | Females (n = 536) | Males (n = 341) | Average difference (95% CI) | P |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD), range | Mean (SD), range | ||||
| Age (y) | 52.8 (8.7) | 52.8 (8.7), 30–65 | 53.0 (8.9), 33–65 | 0.19 (−1.00, 1.37) | 0.759 | |
| HSCL-10 | 14.9 (5.1) | 15.5 (5.3) | 14.0 (4.5) | −1.51 (−2.21, −0.81) | <0.001 | |
| Average pain intensity, past week (0–10) | 5.3 (1.6) | 5.4 (1.6), 3–10 | 5.2 (1.6), 3–10 | −0.19 (−0.41, 0.02) | 0.080 | |
| Number of pain locations (1–14) | 4.1 (2.6) | 4.7 (2.7), 1–14 | 3.1 (2.0), 1–12 | −1.59 (−1.91, −1.27) | <0.001 | |
| Pain duration (y), median (IQR) | 8.0 (14.0), 0.2–96 | 10.0 (15.0), 0.3–55.0 | 6.0 (12.5), 0.2–96 | −4.00 (−6.35, −1.35) | 0.001 | |
| BMI (kg/m2) | 27.9 (4.7) | 27.6 (5.1), 17.8–49.0 | 28.3 (4.2), 17.9–43.7 | 0.80 (0.18, 1.41) | 0.012 | |
| WHR | 0.9 (0.1) | 0.9 (0.1), 0.7–1.3 | 1.0 (0.1), 0.8–1.2 | 0.08 (0.07, 0.09) | <0.001 | |
| SBP (mm Hg) | 131.3 (19.1), 85–196 | 128.3 (19.5), 85–196 | 136.1 (17.6), 92–193 | 7.83 (5.33, 10.33) | <0.001 | |
| DBP (mm Hg) | 78.1 (10.2), 47–118 | 75.3 (9.2), 47–106 | 82.4 (10.2), 54–118 | 7.16 (5.85, 8.47) | <0.001 | |
| Daily coffee consumption (cups) | 4.9 (3.3) | 4.4 (2.8), 0–20 | 5.7 (3.7), 0–20 | 1.38 (0.91, 1.84) | <0.001 | |
| Daily alcohol consumption (units) | 1.6 (0.8) | 1.4 (0.6), 1–4 | 1.9 (1.0), 1–5 | 0.54 (0.42, 0.66) | <0.001 | |
| Exercise days/week | 3.5 (1.1) | 3.6 (1.0), 1–5 | 3.3 (1.2), 1–5 | −0.34 (−0.49, −0.19) | <0.001 |
| n (%) | n (%) | n (%) | ||||
|---|---|---|---|---|---|---|
| Smoking | Never | 256 (29.2) | 157 (29.3) | 99 (29.0) | 0.99 (0.73, 1.33) | 0.694 |
| Before, not now | 384 (43.8) | 237 (44.2) | 147 (43.1) | 0.96 (0.73, 1.26) | ||
| Sometimes | 56 (6.4) | 77 (14.4) | 26 (7.6) | 1.39 (0.81, 2.40) | ||
| Daily | 181 (20.6) | 100 (18.8) | 69 (20.2) | 0.96 (0.69, 1.34) | ||
| Snuff or chewing tobacco | Never | 793 (91.7) | 511 (97.3) | 282 (82.9) | 0.23 (0.14, 0.38) | <0.001 |
| Before, not now | 23 (2.7) | 4 (0.8) | 19 (5.6) | 7.85 (2.65, 23.27) | ||
| Sometimes | 27 (3.1) | 6 (1.1) | 21 (6.2) | 5.80 (2.32, 14.52) | ||
| Daily | 22 (2.5) | 4 (0.8) | 18 (5.3) | 7.41 (2.49, 22.09) | ||
| Education | Primary/secondary | 239 (27.4) | 138 (25.9) | 101 (29.8) | 1.21 (0.90, 1.64) | 0.001 |
| High school | 339 (38.9) | 218 (40.9) | 121 (35.7) | 0.80 (0.61, 1.06) | ||
| University (1–3 y) | 150 (17.2) | 77 (14.4) | 73 (21.5) | 1.62 (1.14, 2.31) | ||
| University (≥4 y) | 144 (16.5) | 100 (18.8) | 44 (13.0) | 0.65 (0.44, 0.95) | ||
| Occupation | Fulltime | 411 (47.2) | 207 (39.1) | 204 (59.8) | 2.37 (1.79, 3.12) | <0.001 |
| Part time | 121 (13.9) | 102 (19.3) | 19 (5.6) | 0.25 (0.15, 0.42) | ||
| Unemployed | 13 (1.5) | 6 (1.1) | 7 (2.1) | 1.85 (0.62, 5.56) | ||
| Housekeeping | 11 (1.3) | 9 (1.7) | 2 (0.6) | 0.35 (0.07, 1.61) | ||
| Retired | 305 (35.1) | 199 (37.6) | 106 (51.6) | 0.76 (0.57, 1.02) | ||
| Student | 9 (1.0) | 6 (1.1) | 3 (0.9) | 0.78 (0.20, 3.16) | ||
| BP medication | Yes | 166 (19.2) | 92 (17.4) | 74 (22.0) | 1.35 (0.96, 1.89) | 0.089 |
| Lipid-lowering medication | Yes | 102 (11.7) | 52 (9.8) | 50 (14.7) | 1.59 (1.05, 2.40) | 0.029 |
| Heart disease medication | Yes | 52 (6.0) | 21 (4.0) | 31 (9.1) | 2.41 (1.36, 4.26) | 0.002 |
| Diabetic medication | Yes | 35 (4.1) | 18 (3.4) | 17 (5.1) | 1.51 (0.76, 2.95) | 0.238 |
| Analgesics | Yes | 222 (26.1) | 145 (28.0) | 77 (23.1) | 0.77 (0.56, 1.06) | 0.109 |
| Antidepressants | Yes | 43 (5.2) | 34 (6.7) | 9 (2.8) | 0.40 (0.19, 0.84) | 0.012 |
| Anxiolytics | Yes | 31 (3.7) | 27 (5.3) | 4 (1.2) | 0.22 (0.08, 0.63) | 0.002 |
Probability values refer to comparisons between sexes based on independent sample t test (continuous measures) or Pearson χ2 test (categorical measures).
BMI, body mass index; DBP, diastolic blood pressure; HSCL-10, Hopkins Symptom Checklist-10; SBP, systolic blood pressure.
2.3. Apparatus and assessment
2.3.1. Chronic pain assessment
All participants rated their usual CP intensity on a 0 to 10 numeric rating scale, anchored with “no pain” and “worst pain imaginable.” Participants also reported all body locations in which they experienced CP (from a list of 14 locations; yes/no format). The number of reported pain locations was summed, creating a variable reflecting the total number of CP locations (range: 1–14). Pain locations included head, jaw, neck, back, shoulder, arm, hand, hip, leg, foot, chest, stomach, genitals, and skin. Pain duration was recorded in years.
2.3.2. Psychological distress assessment
The Hopkins Symptom Checklist-25 (HSCL-25)13 assesses symptoms of anxiety and depression using 25 items on a 4-point scale (“not at all” to “extremely”). The shortening of the HSCL-25 to the 10-item HSCL-10 does not adversely affect specificity or sensitivity and has been validated in the Norwegian population.29 It consists of 2 subscales, anxiety (5 items) and depression (5 items), with these 2 subscales aggregated into a total score assessing overall psychological distress.59
2.3.3. Cardiovascular assessment
A noninvasive beat-to-beat blood pressure (BP) monitor (Finometer Pro; Finapres Medical Systems, Amsterdam, The Netherlands) was used to assess BP, BRS, and HRV via continuous examination of the arterial pressure wave in the middle finger of the nondominant hand and analysis of the pulse wave data (as an estimate of the R-R interval).52 Matlab R2015 was used for Finometer data preprocessing, artifact correction, and data formatting as well as for BRS derivation. The Finometer data were cleaned for technical errors using a threshold-based recording rejection method that removed nonphysiological values and sporadic artifacts.12 The resting HRV and BRS values reported here for each participant were recorded during the seated rest period before the cold pressor test was conducted.
2.3.3.1. Heart rate variability
The current analyses focused on the standard deviation of the NN intervals (SDNN) (in milliseconds) and the root mean square of successive RR interval differences (rMSSD) (milliseconds). All participant HRV data were processed using the RHRV module (version 4.0) within the R statistical package.38 Continuous resting HRV data were recorded during a 30-second resting assessment period. rMSSD is a reliable HRV measure under ultrashort recording windows (≤1 minute)16 and is not confounded by respiratory effects.34,50
2.3.3.2. Baroreceptor sensitivity
Baroreflex sensitivity (BRS) values (in milliseconds/mm Hg) were derived using the sequence technique based on procedures described previously.66 The sequence technique assesses spontaneous BRS in the time domain and has been used in numerous previous studies.25,69 This technique identifies spontaneous ramps in BP (ie, progressive increases or decreases in BP) that are associated with concordant changes in the R-R interval. Sequence method BRS data derived using R-R interval estimations using the pulse wave from finger plethysmograph devices (like the Finometer used in the current study) have been found to correspond well with BRS measures derived using ECG recordings when obtained under short resting conditions.10 Continuous resting BRS data were recorded during a 30-second assessment period.
2.3.4. Cold pressor assessment
A 3°C circulating water bath (Julabo PF40-HE; JULABO Labortechnik GmbH, Seelbach, Germany), connected to a 13-L external plexiglass container with a flow of 22 L/min, was used in the cold pressor test. The procedure began by having participants seated in a comfortable chair with instructions to relax for 30 seconds. Participants were asked to submerge their dominant hand up to the wrist in the cold water and keep their hand still without clenching or making a fist. They were instructed to continue until their pain tolerance was reached or the full test was completed (106 seconds). During the cold pressor test, participants rated their pain intensity on a verbal numeric rating scale (NRS) every time they heard a recorded voice say “now,” by calling out a number from 0 through 10, with 0 representing “no pain” and 10 representing “worst pain imaginable.” These ratings of cold pressor pain intensity (CPI) were obtained 4 seconds after cold pressor onset and every 9 seconds thereafter for a total of 12 ratings. Cold pressor pain tolerance (CPT) time (in seconds) was recorded when the hand was removed from the water.
2.4. Procedure
Participants completed 2 questionnaires64 including items regarding various health issues, symptoms and diseases (medical and psychological), and medication use. Physical examinations, performed by trained personnel, included measurements of height, weight, waist and hip measurements, and resting systolic and diastolic blood pressure (SBP/DBP). Height and weight were measured in cm and kg, respectively. Body mass index (BMI) was calculated as weight in kg/m2. Waist and hip circumferences were measured in cm, and waist-to-hip ratio was calculated as waist (cm)/hip (cm).
A single study technician conducted all laboratory testing procedures with participants seated. The cardiovascular and cold pressor assessment procedure began with participants resting quietly for 5 minutes as the cold pressor test was described and the Finometer Pro device was placed and calibrated. Continuous resting cardiovascular data were then recorded for a 30-second resting baseline assessment period followed by the cold pressor task. Resting oscillometric BP measurements (SBP/DBP) were then obtained in a separate, adjacent room at least 25 minutes after the cold pressor test as in previous population studies.48,56 Participants remained seated and completed the HSCL-10 during this time.
2.5. Statistical analyses
Sociodemographic sample characteristics (Table 1) are presented as mean with standard deviation for continuous variables and frequencies with percentages for categorical variables. Descriptive statistics (Table 2) are provided for HRV, BRS, CPT, and CPI overall and by sex. Differences by sex were tested using independent sample t-tests. A χ2 test was used to test sex differences for categorical variables. Pearson correlational analyses were conducted to examine pairwise associations between HRV, BRS, HSCL-10, CPT, and CPI variables (Table 3A). All primary mediation analyses were conducted1 with Stata/MP version 16.1 while further analyses were performed in IBM SPSS version 26 for Windows.14 For CPI, the average of 12 pain ratings during the cold pressor task was calculated for each participant. The expectation-maximization algorithm was used to impute any missing values for CPI before calculating the average NRS pain rating for each participant.
Table 2.
Descriptive statistics for HRV, BRS, CPT, and CPI overall and by sex.
| Total (n = 561–877) | Females (n = 344–536) | Males (n = 217–341) | Difference (95% CI) (n = 653–870) | P (two independent samples t test) | |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| CPT (s) | 85.0 (31.0) | 81.9 (32.4) | 89.9 (28.1) | 7.95 (3.88, 12.01) | <0.001 |
| CPI (0–10) | 6.7 (2.3) | 6.9 (2.2) | 6.3 (2.3) | −0.62 (−0.93, −0.31) | <0.001 |
| rMSSD (ms) | 29.5 (23.4) | 29.6 (23.2) | 29.4 (23.7) | −0.21 (−3.40, 2.99) | 0.901 |
| SDNN (ms) | 38.6 (25.6) | 38.1 (25.8) | 39.4 (25.3) | 1.39 (−2.08, 4.86) | 0.435 |
| BRS (ms/mm Hg) | 11.4 (16.1) | 11.2 (14.7) | 11.8 (18.1) | 0.55 (−2.12, 3.22) | 0.672 |
Probability values refer to comparisons between sexes.
CPT, cold pressor tolerance; CPI, cold pressor intensity, BRS, baroreflex sensitivity; rMSSD, root mean square of the successive differences of the R-R intervals; SDNN, standard deviation of R-R intervals.
Table 3.
Intercorrelations (Pearson r) among cardiovascular parameters, psychological distress, and cold pressor pain outcomes for overall chronic pain sample (n = 649–877; panel A), chronic pain males (n = 250–341; panel B) and chronic pain females (n = 403–536; panel C) in Tromsø 6.
| A. Total (n = 649-877) | rMSSD | SDNN | BRS | HSCL-10 | CPT | CPI |
|---|---|---|---|---|---|---|
| rMSSD | 0.868‡ | 0.322‡ | −0.081* | 0.028 | −0.020 | |
| SDNN | — | 0.269‡ | −0.065 | 0.029 | −0.035 | |
| BRS | — | — | −0.068 | 0.043 | −0.064 | |
| HSCL-10 | −0.079 | −0.069 | −0.053 | −0.198‡ | 0.180‡ | |
| CPT (sec) | 0.007 | 0.005 | 0.039 | −0.207‡ | −0.678‡ | |
| CPI | −0.008 | −0.002 | −0.054 | 0.198‡ | — |
| B. Males (n = 250-341) | rMSSD | SDNN | BRS | HSCL-10 | CPT | CPI |
|---|---|---|---|---|---|---|
| rMSSD | 0.886‡ | 0.452‡ | −0.141* | 0.081 | −0.055 | |
| SDNN | — | 0.369‡ | −0.135* | 0.072 | −0.031 | |
| BRS | — | — | −0.092 | 0.091 | −0.092 | |
| HSCL-10 | −0.139* | −0.152* | −0.086 | −0.158† | 0.106 | |
| CPT (s) | 0.081 | 0.073 | 0.107 | −0.161* | −0.669‡ | |
| CPI | −0.080 | −0.041 | −0.100 | 0.096 | — |
| C. Females (n = 403-536) | rMSSD | SDNN | BRS | HSCL-10 | CPT | CPI |
|---|---|---|---|---|---|---|
| rMSSD | 0.857‡ | 0.210‡ | −0.049 | −0.0002 | 0.002 | |
| SDNN | — | 0.196‡ | −0.019 | 0.002 | −0.032 | |
| BRS | — | — | −0.040 | 0.011 | −0.041 | |
| HSCL-10 | −0.057 | −0.032 | −0.045 | −0.199‡ | 0.202‡ | |
| CPT (s) | −0.033 | −0.028 | 0.001 | −0.228‡ | −0.666‡ | |
| CPI | 0.039 | 0.020 | −0.028 | 0.254‡ | — |
Partial correlations controlling for age and BMI (and sex in panel A) are shown in bold below the diagonal.
* P < 0.05.
† P < 0.01.
‡ P ≤ 0.001.
BRS, baroreflex sensitivity; CPT, cold pressor tolerance; CPI, cold pressor intensity; HSCL-10, Hopkins symptom checklist-10; rMSSD, root mean square of the successive differences of the R-R intervals; SDNN, standard deviation of R-R intervals.
To test associations between CPT/CPI and HRV/BRS, and between HSCL-10 and HRV/BRS independent of the influence of age, sex, and BMI, partial correlation analyses were performed for the total sample controlling for age, sex, and BMI (Table 3A). For each sex, the partial correlation coefficients were adjusted for age and BMI (Tables 3B and C). Associations between cardiovascular measures and clinical CP measures (usual pain intensity ratings and number of pain body sites) were evaluated using Pearson correlations (Table 4).
Table 4.
Correlations (Pearson r) between clinical chronic pain measures (pain intensity and number of chronic pain sites) and cardiovascular parameters, psychological distress, and cold pressor measures in the Tromsø 6 chronic pain population.
| Cardiovascular measures | Total (n = 651–877) | Females (n = 401–536) | Males (n = 250–341) | |||
|---|---|---|---|---|---|---|
| Usual pain intensity | Number of chronic pain sites | Usual pain intensity | Number of chronic pain sites | Usual pain intensity | Number of chronic pain sites | |
| rMSSD | −0.058 | −0.055 | −0.099* | −0.094* | 0.004 | 0.01 |
| SDNN | −0.057 | −0.099† | −0.103* | −0.121† | 0.020 | −0.04 |
| BRS | −0.077* | −0.005 | −0.127* | −0.059 | −0.008 | 0.10 |
| HSCL-10 | 0.155‡ | 0.270‡ | 0.195‡ | 0.263‡ | 0.069 | 0.19‡ |
| CPT | −0.110‡ | −0.090† | −0.105* | −0.056 | −0.103 | −0.05 |
| CPI | 0.104† | 0.052 | 0.120† | 0.004 | 0.062 | 0.04 |
Pearson correlation coefficients were given. Number of chronic pain sites = total number of pain locations.
BRS, baroreflex sensitivity; rMSSD, root mean square of the successive differences of the R-R intervals; SDNN, standard deviation of R-R intervals; CPT, cold pressor tolerance; CPI, cold pressor intensity; HSCL-10, Hopkins Symptom Checklist-10.
P < 0.05.
P < 0.01.
P ≤ 0.001.
To test our mediation hypothesis (Fig. 2), structural equation modeling (SEM) in Stata was used to examine the direct and indirect (via HSCL-10 scores as the mediator) effects of HRV and BRS on CPT and CPI for the whole sample (n = 877) (Table 5A) and within each sex subgroup (n = 341 males and n = 536 females) (Tables 5B and C). Because the various HRV and BRS measures were significantly correlated in the overall sample (Table 3A), each mediation model evaluated included only a single HRV or BRS measure to avoid issues of multicollinearity. The primary mediation analyses used a series of hierarchical linear regressions (embedded in SEM); CPT/CPI were specified as the dependent variables, HRV/BRS as the independent variables, and HSCL-10 as the proposed mediator.
Figure 2.
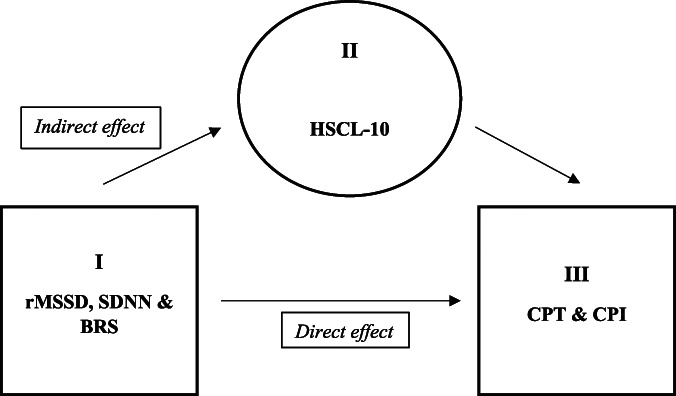
Mediation model of the hypothesized indirect effect of HRV (rMSSD and SDNN) and BRS on cold pressor pain intensity (CPI) and tolerance (CPT) via psychological distress levels (HSCL-10). HRV, heart rate variability.
Table 5.
Mediation analyses evaluating the total direct effect and indirect effect (via psychological distress) of cardiovascular parameters on cold pressor pain tolerance and intensity in the overall Tromsø 6 chronic pain population (panel A; n = 877), in men only (panel B; n = 341), in women only (panel C; n = 536), and between men and women (panel D; n = 877).
| A. Overall chronic pain population (n = 877) | |||
|---|---|---|---|
| Cardiovascular parameter | Cold pressor outcome | With imputing missing data (total n = 877) | |
| Direct effect (95% CI) | Indirect/mediated effect via HSCL-10 (95% CI) | ||
| rMSSD | CPT | 0.016 (−0.063, 0.095) | 0.022* (0.007, 0.042) |
| CPI | −0.0005 (−0.007, 0.006) | −0.001* (−0.003, −0.0004) | |
| SDNN | CPT | 0.020 (−0.057, 0.092) | 0.016* (0.0006, 0.034) |
| CPI | −0.002 (−0.007, 0.004) | −0.001* (−0.002, −0.00003) | |
| BRS | CPT | 0.053 (−0.075, 0.135) | 0.027* (0.007, 0.055) |
| CPI | −0.007 (−0.016, 0.002) | −0.002* (−0.003, −0.0005) | |
| B. Male chronic pain participants only (n = 341) | |||
|---|---|---|---|
| Cardiovascular parameter | Cold pressor outcome | With imputing missing data (n = 341 men) | |
| Direct effect (95% CI) | Indirect/mediated effect via HSCL-10 (95% CI) | ||
| rMSSD | CPT | 0.071 (−0.043, 0.171) | 0.024* (0.003, 0.053) |
| CPI | −0.004 (−0.014, 0.006) | −0.001 (−0.003, 0.0003) | |
| SDNN | CPT | 0.057 (−0.057, 0.160) | 0.022* (0.002, 0.052) |
| CPI | −0.002 (−0.011, 0.008) | −0.001 (−0.003, 0.0002) | |
| BRS | CPT | 0.125 (0.048, 0.364) | 0.022* (0.002, 0.073) |
| CPI | −0.011 (−0.032, −0.002) | −0.001 (−0.005, 0.0004) | |
| C. Female chronic pain participants only (n = 536) | |||
|---|---|---|---|
| Cardiovascular parameter | Cold pressor outcome | With imputing missing data (n = 536 women) | |
| Direct effect (95% CI) | Indirect/mediated effect via HSCL-10 (95% CI) | ||
| rMSSD | CPT | −0.014 (−0.123, 0.090) | 0.015 (−0.006, 0.045) |
| CPI | 0.001 (−0.007, 0.008) | −0.001 (−0.003, 0.0004) | |
| SDNN | CPT | −0.002 (−0.101, 0.090) | 0.006 (−0.019, 0.031) |
| CPI | −0.002 (−0.009, 0.005) | −0.0004 (−0.002, 0.001) | |
| BRS | CPT | −0.002 (−0.276, 0.138) | 0.024 (−0.022, 0.076) |
| CPI | −0.004 (−0.019, 0.013) | −0.002 (−0.004, 0.002) | |
| D. Moderated mediation analysis for chronic pain participants (n = 877) | |||
|---|---|---|---|
| Cardiovascular parameter | Cold pressor outcome | With imputing missing data (n = 877) | |
| Difference in mediated effects between males and females | Percentile method: bootstrapped 95% CI for the difference | ||
| rMSSD | CPT | 0.0093 | (−0.0252, 0.0434) |
| CPI | −0.00028 | (−0.0026, 0.0020) | |
| SDNN | CPT | 0.0162 | (−0.0155, 0.0510) |
| CPI | −0.00079 | (−0.0031, 0.0013) | |
| BRS | CPT | −0.00224 | (−0.0512, 0.0668) |
| CPI | 0.00036 | (−0.0043, 0.0037) | |
BRS, baroreflex sensitivity; rMSSD, root mean square of the successive differences of the R-R intervals; SDNN, standard deviation of R-R intervals.
P < 0.05.
To account for missing data in the independent HRV and BRS variables (the latter was missing in ≈26% of the total n = 877 sample) that could have influenced the overall mediation results, SEM with full information maximum likelihood was applied first to impute missing data (assuming missing at random) and thereafter fitted to the path models (3 linear regression models). Additionally, SEM with maximum likelihood as an estimation method without imputing missing data was also carried out. The results without imputing missing data were valid if the missing values were Missing Completely at Random.
In our analysis, an indirect (mediated) effect was considered significant if the 95% confidence interval (CI) did not contain zero. For the mediation analyses, bootstrap methods (percentile and bias-corrected) were used to estimate the path coefficients and 95% CIs, which were based on 2000 random samples with replacements from the original sample (overall and by sex). Additive difference in mediated effects between males and females was tested for significance by subtracting the mediated effects of males from those of females for each bootstrap. The percentile bootstrap CI was derived after 2000 bootstraps for the difference in mediated effects between sexes. All mediation analyses were adequately powered based on previously published empirical power estimates for percentile and bias-corrected bootstrap methodology for large sample sizes.8,18
3. Results
3.1. Sample characteristics
Sample characteristics are summarized overall and by sex in Table 1. The CP population comprised more females (n = 536; 61.1%) than males (n = 341; 38.9%) yet both sexes were of similar age. Average past week clinical chronic pain intensity was statistically similar across both sexes and moderate in intensity. Widespread pain was significantly more common in females than males. Furthermore, females reported significantly higher psychological distress than males (average difference [95% CI] P-value: −1.51 [−2.21, −0.81], P < 0.001) and had pain for a longer duration. Males had higher alcohol and coffee consumption as well as higher average SBP and DBP but exercised less than females. HRV, BRS, and cold pressor outcomes are summarized overall and by sex in Table 2. Mean CPT was significantly higher in males than in females (average difference [95% CI] P-value: 7.95 [3.88, 12.01], P < 0.001) and as expected, the opposite was true for CPI (−0.62 [−0.93, −0.31], P < 0.001). There were no significant differences in BRS, SDNN, or rMSSD parameters between the sexes.
3.2. Pearson and partial correlations between heart rate variability, baroreflex sensitivity, and pain-related outcomes
Pearson and partial intercorrelations between cardiovascular parameters, psychological distress, and cold pressor pain outcomes for the entire CP population and separately, for males and females, are summarized in Table 3. Partial correlations showed that after controlling for potential confounds of age, sex, and BMI in the entire CP sample, psychological distress (HSCL-10) was significantly and inversely correlated with CPT (r = −0.207, P < 0.001) and positively correlated with CPI (r = 0.180, P < 0.001)—both representing small effect sizes. Partial correlations in males controlling for age and BMI revealed significant negative correlations (small effect sizes) between psychological distress and rMSSD, SDNN, and CPT. For females, psychological distress displayed a significant negative correlation with CPT and a positive correlation with CPI (see Appendix Table I for exact P-values, available as supplemental digital content at http://links.lww.com/PR9/A147). Both of these findings indicated small effect sizes.
Table 4 indicates that in the full sample (n = 877), SDNN showed a small but significant inverse association with the number of chronic pain sites, and BRS showed a similar inverse association with usual pain intensity. Other associations between clinical pain outcomes and cardiovascular measures were not significant. Psychological distress showed a significant positive correlation with both usual pain intensity (r = 0.155, P < 0.001) and number of chronic pain sites (r = 0.270, P < 0.001). CPT exhibited significant inverse associations with both usual pain intensity and number of chronic pain sites, whereas CPI had a significant positive correlation only with usual pain intensity. All these associations represented small effect sizes. In females, small but significant inverse associations were noted between rMSSD and SDNN and both usual pain intensity and number of chronic pain sites. BRS was inversely associated only with usual pain intensity. Psychological distress showed notable positive correlations with usual pain intensity (r = 0.195, P < 0.001) and number of chronic pain sites (r = 0.263, P < 0.001). Additionally, usual pain intensity was associated significantly with both CPT (inverse) and CPI (positive). All the significant associations noted in females represented small effect sizes. In males, there were no significant associations found among the cardiovascular parameters, clinical pain parameters, and experimental pain parameters except for a significant positive correlation between the number of CP sites and psychological distress (see Appendix, Table II for exact P-values, available as supplemental digital content at http://links.lww.com/PR9/A147).
3.3. Mediation analyses
To evaluate our primary hypothesis, we performed a mediation analysis (Fig. 2) for the full CP sample (n = 877) which tested for the indirect effects of HRV and BRS on cold pressor pain intensity (CPI) and tolerance (CPT) via psychological distress (HSCL-10). As shown in Table 5A, these analyses showed that the direct effect of HRV and BRS on CPT and CPI were nonsignificant. However, significant indirect-only mediation was found for the impact of rMSSD, SDNN, and BRS on CPI and CPT via psychological distress for the entire CP population.
Parallel analyses using non-imputed data indicated that in the full sample, indirect-only (mediated) effects for rMSSD and BRS remained, although the indirect effects for SDNN were no longer significant (see Appendix, Table IIIA, available as supplemental digital content at http://links.lww.com/PR9/A147). Even though SDNN and rMSSD are intercorrelated, SDNN has been shown to be less reliable under short recording windows.41,55 The significant indirect-only effects of SDNN and rMSSD on CPT in males using imputed data remained significant in analyses using non-imputed data, although only direct effects were noted between BRS and CPT in analyses using non-imputed data (Appendix, Table IIIB, available as supplemental digital content at http://links.lww.com/PR9/A147).
To evaluate hypothesized sex differences in these mediation effects, our sample was stratified by sex and mediation analysis was rerun separately for males and females. In males (Table 5B), mediation analyses revealed significant indirect-only mediation effects for the impact of rMSSD, SDNN, and BRS on CPT via psychological distress. In addition to these indirect-only mediated effects, analyses in CP males also revealed significant complementary mediation (mediation with direct effect) for the impact of BRS on CPT via psychological distress. No significant direct or indirect effects via psychological distress were found in mediation analyses carried out in CP females (see Table 5C and Appendix Table IIIC, available as supplemental digital content at http://links.lww.com/PR9/A147). Finally, a moderated mediation analysis for our entire CP population (n = 877) (Table 5D) was performed to determine whether the hypothesized mediation model was significantly different between CP males and females (see Appendix Table IIID for mediation without imputing data, available as supplemental digital content at http://links.lww.com/PR9/A147). Findings showed that sex did not significantly moderate the indirect effects of any of the cardiovascular variables on CPT or CPI via psychological distress (ie, the 95% CI for the sex difference in the mediated effects contained zero).
In summary, significant indirect-only mediation was found for the impact of rMSSD and SDNN on CPT in the entire CP population. When stratified by sex, both significant indirect and direct effects of BRS on CPT were noted in males (ie, complementary mediation). Moderated mediation analyses indicated that there were no significant sex differences between males and females for the impact of rMSSD, SDNN, and BRS on CPT and CPI via psychological distress.
4. Discussion
The role psychological distress plays in mediating the impact of HRV and BRS on evoked pain responsiveness in individuals with CP has remained unclear.23,32,57 The current study tested whether: (1) associations between rMSSD, SDNN, and BRS and responses to the cold pressor pain task were mediated by psychological distress and (2) whether that mediation was dependent on sex.
Our findings indicate that psychological distress significantly mediated the impact of rMSSD, SDNN, and BRS on cold pressor pain tolerance (CPT) and intensity (CPI) for those diagnosed with CP. These mediated effects occurred in the absence of any significant direct effects (ie, indirect-only mediation72). When stratified by sex, psychological distress significantly mediated associations between both HRV and BRS measures and CPT in males (ie, complementary mediation72). Females with CP exhibited no statistically significant mediation effects for psychological distress. Contrary to our secondary hypothesis, a formal moderated mediation test of these apparent sex differences did not reveal significant sex moderation effects.
The absence of sex moderation effects for our mediation model is surprising given that females display significantly higher psychological distress. Contrary to results regarding evoked pain responsiveness, analyses by sex revealed that significant inverse associations between HRV, BRS, and CP intensity were observed only in females. The mechanisms by which CP weakens these associations which are commonly found among psychological distress, autonomic tone, and pain remain unclear.3,7,65 Males and females had statistically comparable HRV and BRS levels, so any apparent sex differences do not appear to be driven by baseline differences in these cardiovascular measures.
Although complementary mediation (both direct and indirect effects) was found for links between BRS and CPT in males with CP, most of the significant indirect effects of HRV and BRS on evoked pain responses via psychological distress occurred in the absence of significant direct effects (indirect-only mediation). The presence of significant indirect-only mediation (mediated effects in the absence of significant direct associations between independent and dependent variables) has been well recognized in prior statistical literature.22,42,45,58,72 Recent research has shown that step 1 in the study by Baron and Kenny4 is not a requirement for mediation45; several methodological studies have shown that mediated effects can be statistically significant even when the total effects are not.18,27,36,46
The general absence of significant direct associations in the current work may relate to previous findings, suggesting that hypoalgesia related to resting HRV is significantly reduced in individuals with CP8,33,38 relative to healthy pain-free populations.31 Limited work indicates that direct enhancement of HRV may be hypoalgesic in individuals free of CP28,44 and with those suffering from conditions defined by autonomic imbalance/dysregulation21,54,70—a characteristic of CP.3 While speculative, CP may have reduced the magnitude of direct HRV- and BRS-related hypoalgesia sufficiently enough to leave only the indirect effects conveyed via psychological distress that were observed in this current study.
The current study has several limitations. Due to the large population size and time constraints during data collection, ultrashort HRV and BRS recording periods (30 seconds) were used. Ultrashort HRV recordings prevented analyses of HRV parameters,30,34 such as high-frequency HRV, which are deemed unreliable under such short recording windows.16,34,50 Furthermore, reliance on pulse wave-derived HRV values, rather than ECG-derived HRV values, can potentially lead to overestimation.8,56 Not accounting for caffeine consumption, physical exercise intensity, specific cardioactive medication use, and nicotine intake immediately before HRV participant recording sessions may have influenced our results.8,34,55 It also remains unclear whether descending modulation of pain and HRV may have been cognitively confounded in our study during the cold pressor task.5 Finally, it is important to note that all significant correlations in this study indicated small effect sizes,9 so clinical importance of these effects remains unclear.
4.1. Conclusion
In conclusion, this study found that in a large CP population sample of wide age range, the impact of HRV and BRS on evoked pain tolerance and intensity was not direct. Rather, the impact of HRV and BRS on evoked pain responses was conveyed indirectly via psychological distress, with these mediated effects not differing significantly by sex. Future work to enhance our understanding of the mechanisms accounting for indirect-only vs complementary mediation effects appears warranted.
Disclosures
The authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A147.
Supplementary Material
Acknowledgements
This project was supported by a doctorate scholarship received from the South-East Regional Health Authority of Norway. The authors thank the Tromsø population survey committee members and study practitioners for their general support with data access and preparation.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
Contributor Information
Stephen Bruehl, Email: stephen.bruehl@vumc.org.
Lien My Diep, Email: l.m.diep@medisin.uio.no.
Leiv A. Rosseland, Email: l.a.rosseland@medisin.uio.no.
Audun Stubhaug, Email: astubhau@ous-hf.no.
Henrik B. Jacobsen, Email: heboja@ous-hf.no.
References
- [1].Advanced multiprocessing capabilities. Stata. Available at: www.stata.com/statamp/. Accessed July 15, 2021. [Google Scholar]
- [2].Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol 2006;10:229–40. [Google Scholar]
- [3].Barakat A, Vogelzangs N, Licht CM, Geenen R, MacFarlane GJ, de Geus EJ, Smit JH, Penninx BWJH, Dekker J. Dysregulation of the autonomic nervous system and its association with the presence and intensity of chronic widespread pain. Arthritis Care Res 2012;64:1209–16. [DOI] [PubMed] [Google Scholar]
- [4].Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Personal Soc Psychol 1986;51:1173. [DOI] [PubMed] [Google Scholar]
- [5].Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology 2008;23:371–80. [DOI] [PubMed] [Google Scholar]
- [6].Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. J Psychosom Res 2006;60:113–24. [DOI] [PubMed] [Google Scholar]
- [7].Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neurosci Biobehav Rev 2004;28:395–414. [DOI] [PubMed] [Google Scholar]
- [8].Bruehl S, Olsen RB, Tronstad C, Sevre K, Burns JW, Schirmer H, Nielsen CS, Stubhaug A, Rosseland LA. Chronic pain-related changes in cardiovascular regulation and impact on comorbid hypertension in a general population: the tromsø study. PAIN 2018;159:119–27. [DOI] [PubMed] [Google Scholar]
- [9].Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum, 1988; 75–108. [Google Scholar]
- [10].Coppieters I, Cagnie B, Nijs J, van Oosterwijck J, Danneels L, De Pauw R, Meeus M. Effects of stress and relaxation on central pain modulation in chronic whiplash and fibromyalgia patients compared to healthy controls. Pain Physician 2016;19:119–30. [PubMed] [Google Scholar]
- [11].Dang K, Kirk MA, Monette G, Katz J, Ritvo P. Meaning in life and vagally-mediated heart rate variability: evidence of a quadratic relationship at baseline and vagal reactivity differences. Int J Psychophysiology 2021;165:101–11. [DOI] [PubMed] [Google Scholar]
- [12].Deegan BM, O'Connor M, Lyons D, OLaighin G. A new blood pressure and heart rate signal analysis technique to assess orthostatic hypotension and its subtypes. Conf Proc IEEE Eng Med Biol Soc 2007;2007:935–8. [DOI] [PubMed] [Google Scholar]
- [13].Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci 1974;19:1–15. [DOI] [PubMed] [Google Scholar]
- [14].Downloading IBM SPSS statistics 26, 2020. Available at: www.ibm.com/support/pages/downloading-ibm-spss-statistics-26. [Google Scholar]
- [15].Eggen AE, Mathiesen EB, Wilsgaard T, Jacobsen BK, Njolstad I. The sixth survey of the Tromso Study (Tromso 6) in 2007-08: collaborative research in the interface between clinical medicine and epidemiology: study objectives, design, data collection procedures, and attendance in a multipurpose population-based health survey. Scand J Pub Lic Health 2013;41:65–80. [DOI] [PubMed] [Google Scholar]
- [16].Esco MR, Flatt AA. Ultra-short-term heart rate variability indexes at rest and post-exercise in athletes: evaluating the agreement with accepted recommendations. J Sports Sci Med 2014;13:535. Accessed July 15, 2021. [PMC free article] [PubMed] [Google Scholar]
- [17].Evans S, Seidman LC, Tsao JC, Lung KC, Zeltzer LK, Naliboff BD. Heart rate variability as a biomarker for autonomic nervous system response differences between children with chronic pain and healthy control children. J Pain Res 2013;6:449–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fritz MS, Cox MG, MacKinnon DP. Increasing statistical power in mediation models without increasing sample size. Eval Health Professions 2015;38:343–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Frøkjaer JB, Bergmann S, Brock C, Madzak A, Farmer AD, Ellrich J, Drewes AM. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterology Motil 2016;28:592–8. [DOI] [PubMed] [Google Scholar]
- [20].Geenen R, Bijlsma JW. Deviations in the endocrine system and brain of patients with fibromyalgia: cause or consequence of pain and associated features?. Ann N Y Acad Sci 2010;1193:98–110. [DOI] [PubMed] [Google Scholar]
- [21].Gold MR, Van Veldhuisen DJ, Hauptman PJ, Borggrefe M, Kubo SH, Lieberman RA, Milasinovic G, Berman BJ, Djordjevic S, Neelagaru S, Schwartz PJ. Vagus nerve stimulation for the treatment of heart failure: the INOVATE-HF trial. J Am Coll Cardiol 2016;68:149–58. [DOI] [PubMed] [Google Scholar]
- [22].Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Publications, 2017. [Google Scholar]
- [23].Hoffmann A, Ettinger U, del Paso GA, Duschek S. Executive function and cardiac autonomic regulation in depressive disorders. Brain Cogn 2017;118:108–17. [DOI] [PubMed] [Google Scholar]
- [24].Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep 2014;1:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jackson T, Iezzi T, Gunderson J, Nagasaka T, Fritch A. Gender differences in pain perception: the mediating role of self-efficacy beliefs. Sex Roles 2002;47:561–8. [Google Scholar]
- [26].Juel J, Brock C, Olesen SS, Madzak A, Farmer AD, Aziz Q, Frøkjær JB, Drewes AM. Acute physiological and electrical accentuation of vagal tone has no effect on pain or gastrointestinal motility in chronic pancreatitis. J pain Res 2017;10:1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kenny DA, Judd CM. Power anomalies in testing mediation. Psychol Sci 2014;25:334–9. [DOI] [PubMed] [Google Scholar]
- [28].Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology 2000;55:1167–71. [DOI] [PubMed] [Google Scholar]
- [29].Kleppang AL, Hagquist C. The psychometric properties of the Hopkins Symptom Checklist-10: a Rasch analysis based on adolescent data from Norway. Fam Pract 2016;33:740–5. [DOI] [PubMed] [Google Scholar]
- [30].Koenig J, Falvay D, Clamor A, Wagner J, Jarczok MN, Ellis RJ, Weber C, Thayer JF. Pneumogastric (vagus) nerve activity indexed by heart rate variability in chronic pain patients compared to healthy controls: a systematic review and meta-analysis. Pain physician 2016;19:E55–78. [PubMed] [Google Scholar]
- [31].Koenig J, Jarczok MN, Ellis RJ, Hillecke TK, Thayer JF. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur J Pain 2014;18:301–14. [DOI] [PubMed] [Google Scholar]
- [32].Koenig J, Kemp AH, Beauchaine TP, Thayer JF, Kaess M. Depression and resting state heart rate variability in children and adolescents—a systematic review and meta-analysis. Clin Psychol Rev 2016;46:136–50. [DOI] [PubMed] [Google Scholar]
- [33].Koenig J, Loerbroks A, Jarczok MN, Fischer JE, Thayer JF. Chronic Pain and Heart Rate Variability in a Cross-Sectional Occupational Sample: Evidence for Impaired Vagal Control. Clin J Pain 2016;32:218–25. [DOI] [PubMed] [Google Scholar]
- [34].Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Front Psychol 2017;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Larkin KT, Tiani AG, Brown LA. Cardiac vagal tone and stress. InOxford Res Encyclopedia Neurosci 2021. [Google Scholar]
- [36].MacKinnon DP, Cheong J, Pirlott AG. Statistical mediation analysis. In H. Cooper, P. M. Camic, D. L. Long, A. T. Panter, D. Rindskopf, & K. J. Sher (Eds.), APA handbook of research methods in psychology, Vol. 2. Research designs: Quantitative, qualitative, neuropsychological, and biological. Washington, D.C.: American Psychological Association, 2012. pp. 313–331. [Google Scholar]
- [37].Marmerstein JT, McCallum GA, Durand DM. Direct measurement of vagal tone in rats does not show correlation to HRV. Scientific Rep 2021;11:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martínez CA, Quintana AO, Vila XA, Touriño MJ, Rodríguez-Liñares L, Presedo JM, Penín AJ. Heart rate variability analysis with the R package RHRV. Germany: Springer International Publishing, 2017. [Google Scholar]
- [39].Mason H, Vandoni M, Debarbieri G, Codrons E, Ugargol V, Bernardi L. Cardiovascular and respiratory effect of yogic slow breathing in the yoga beginner: what is the best approach?. Evid Based Complement Altern. Med. 2013;2013:743504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Matuz A, van der Linden D, Kisander Z, Hernádi I, Kázmér K, Csathó Á. Enhanced cardiac vagal tone in mental fatigue: analysis of heart rate variability in Time-on-Task, recovery, and reactivity. Plos one 2021;16:e0238670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].McCraty R, Shaffer F. Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med 2015;4:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Memon MA, Cheah JH, Ramayah T, Ting H, Chuah F. Mediation analysis issues and recommendations. J Appl Struct Equation Model 2018;2:1–9. [Google Scholar]
- [43].Nahman‐Averbuch H, Sprecher E, Jacob G, Yarnitsky D. The relationships between parasympathetic function and pain perception: the role of anxiety. Pain Pract 2016;16:1064–72. [DOI] [PubMed] [Google Scholar]
- [44].Ness TJ, Fillingim RB, Randich A, Backensto EM, Faught E. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain 2000;86:81–5. [DOI] [PubMed] [Google Scholar]
- [45].O'Rourke HP, MacKinnon DP. Reasons for testing mediation in the absence of an intervention effect: a research imperative in prevention and intervention research. J Stud alcohol Drugs 2018;79:171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O'Rourke HP, MacKinnon DP. When the test of mediation is more powerful than the test of the total effect. Behav Res Methods 2015;47:424–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Olsen RB, Bruehl S, Nielsen CS, Rosseland LA, Eggen AE, Stubhaug A. Gender differences in blood pressure–related hypoalgesia in a general population: the Tromsø Study. J Pain 2013;14:699–708. [DOI] [PubMed] [Google Scholar]
- [48].Olsen RB, Bruehl S, Nielsen CS, Rosseland LA, Eggen AE, Stubhaug A. Chronic pain and cardiovascular stress responses in a general population: the Tromsø study. J Behav Med 2014;37:1193–201. [DOI] [PubMed] [Google Scholar]
- [49].Ottaviani C, Shapiro D, Davydov DM, Goldstein IB, Mills PJ. The autonomic phenotype of rumination. Int J Psychol 2009;72:267–75. [DOI] [PubMed] [Google Scholar]
- [50].Paccione CE, Diep LM, Stubhaug A, Jacobsen HB. Motivational nondirective resonance breathing versus transcutaneous vagus nerve stimulation in the treatment of fibromyalgia: study protocol for a randomized controlled trial. Trials 2020;21:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Paccione CE, Jacobsen HB. Motivational nondirective resonance breathing as a treatment for chronic widespread pain. Front Psychol 2019;10:1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Parati G, Casadei R, Groppelli A, Di RM, Mancia G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 1989;13:647–55. [DOI] [PubMed] [Google Scholar]
- [53].Pittig A, Arch JJ, Lam CW, Craske MG. Heart rate and heart rate variability in panic, social anxiety, obsessive–compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol 2013;87:19–27. [DOI] [PubMed] [Google Scholar]
- [54].Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, DiCarlo LA, Ardell JL, Rector TS, Amurthur B, KenKnight BH. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 2014;20:808–16. [DOI] [PubMed] [Google Scholar]
- [55].Quintana DS, Alvares GA, Heathers JA. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry 2016;6:e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schafer A, Vagedes J. How accurate is pulse rate variability as an estimate of heart rate variability? A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int J Cardiol 2013;166:15–29. [DOI] [PubMed] [Google Scholar]
- [57].Schumann A, Andrack C, Baer KJ. Differences of sympathetic and parasympathetic modulation in major depression. Prog Neuro-Psychopharmacology Biol Psychiatry 2017;79:324–31. [DOI] [PubMed] [Google Scholar]
- [58].Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods 2002;7:422. [PubMed] [Google Scholar]
- [59].Strand BH, Dalgard OS, Tambs K, Rognerud M. Measuring the mental health status of the Norwegian population: a comparison of the instruments SCL-25, SCL-10, SCL-5 and MHI-5 (SF-36). Nord J Psychiatry 2003;57:113–8. [DOI] [PubMed] [Google Scholar]
- [60].Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 1996;93:1043–65. [PubMed] [Google Scholar]
- [61].Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology 2005;30:1050–8. [DOI] [PubMed] [Google Scholar]
- [62].Thayer JF. Heart rate variability: a neurovisceral integration model. Encycl Neurosci 2009;2009:1041–7. [Google Scholar]
- [63].Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 2000;61:201–16. [DOI] [PubMed] [Google Scholar]
- [64].The tromsø study: Available at: www.tromsostudy.com. Accessed May 20, 2020.
- [65].Tracy LM, Ioannou L, Baker KS, Gibson SJ, Georgiou-Karistianis N, Giummarra MJ. Meta-analytic evidence for decreased heart rate variability in chronic pain implicating parasympathetic nervous system dysregulation. Pain 2016;157:7–29. [DOI] [PubMed] [Google Scholar]
- [66].Turk DC, Okifuji A. Does sex make a difference in the prescription of treatments and the adaptation to chronic pain by cancer and non-cancer patients? PAIN 1999;82:139–48. [DOI] [PubMed] [Google Scholar]
- [67].Velly AM, Mohit S. Epidemiology of pain and relation to psychiatric disorders. Prog Neuro-Psychopharmacology Biol Psychiatry 2018;87:159–67. [DOI] [PubMed] [Google Scholar]
- [68].Wendt J, Neubert J, Koenig J, Thayer JF, Hamm AO. Resting heart rate variability is associated with inhibition of conditioned fear. Psychophysiology 2015;52:1161–66. [DOI] [PubMed] [Google Scholar]
- [69].Wise EA, Price DD, Myers CD, Heft MW, Robinson ME. Gender role expectations of pain: relationship to experimental pain perception. PAIN 2002;96:335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, Klein H, Stolen C, Meyer S, Stein KM, Ramuzat A. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J 2015;36:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol 2005;73:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zhao X, Lynch JG, Jr, Chen Q. Reconsidering Baron and Kenny: myths and truths about mediation analysis. J consumer Res 2010;37:197–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A147.


