Abstract
We report a Plasmodium vivax infection in a Rwandan child misdiagnosed with Plasmodium falciparum and administered artemether-lumefantrine. Antigen detection revealed an absence of P falciparum histidine-rich protein 2 (HRP2) and presence of Plasmodium vivax lactate dehydrogenase. Nested and real-time polymerase chain reactions verified that the sample only contained P vivax deoxyribonucleic acid.
Keywords: microscopy, misdiagnosis, multiplex, PCR, Plasmodium vivax
A child presenting to a health facility in Rwanda was diagnosed with Plasmodium falciparummalaria, but was later found to have a P. vivaxinfection by laboratory assays. Further surveys should investigate P. vivaxprevalence in Rwanda.
A recent household survey in Rwanda estimated that Plasmodium falciparum causes 97% of malaria infections, whereas Plasmodium malariae and Plasmodium ovale each cause 1%–2% of remaining infections (Rwandan National Malaria Control Program, Aline Uwimana, 2018, unpublished data). The last reported case of Plasmodium vivax of assumed Rwandan origin occurred in 2005 in a Japanese traveler returning to Japan after traveling to Rwanda [1]. Diagnosis and speciation of malaria in Rwanda at health facilities are mainly performed using microscopy, but combination rapid diagnostic tests (RDTs) utilizing detection of pan-Plasmodium lactate dehydrogenase and P falciparum antigen histidine-rich protein 2 (HRP2) are used in community health centers and at larger health facilities when microscopy is not available. According to the World Health Organization (WHO) [2], the recommended treatment of children and adults with uncomplicated P falciparum infection is a 3-day course of an artemisinin-based combination therapy (ACT). In Rwanda, artemether-lumefantrine and dihydroartemisinin-piperaquine are the first- and second-line treatments for P falciparum malaria. Unlike P falciparum, P vivax and P ovale parasites can form dormant stages in the liver, known as the hypnozoites, which can reactivate weeks to years after the primary infection. To achieve a radical cure—the elimination of all parasites—in uncomplicated P vivax infections, the WHO recommends a 3-day course of an ACT to eliminate blood-stage parasites and a 14-day course of primaquine to clear hypnozoites [2]. In this study, we report the case of a 4-year-old boy from Masaka, Rwanda, with P vivax infection who was misdiagnosed with P falciparum monoinfection and given a 3-day course of treatment with artemether-lumefantrine.
METHODS
Patient Enrollment
The patient was enrolled in an ongoing antimalarial therapeutic efficacy study (TES) approved by the Rwanda National Ethics Committee (reference 195/RNEC/2017) [3]. Informed consent was obtained from the accompanying parent/guardian at the time of enrollment, and a dried blood spot (DBS) specimen was collected on filter paper.
Treatment and Monitoring of Infection
To diagnose malaria and determine parasitemia in the patient, thin and thick blood smears were prepared and stained with 5% Giemsa. Each slide was independently read by 2 microscopists who were trained and supervised by WHO Level 1-certified laboratory technicians from the Rwandan National Reference Laboratory. Asexual parasite density per microliter of blood was calculated as the average of the 2 readings using the number of infected red blood cells per 200 white blood cells and multiplied accordingly.
Multiplex Assays
Multiplex antigen detection of 4 antigens—P falciparum HRP2/3, P vivax lactate dehydrogenase (PvLDH), pan-Plasmodium antigens LDH, and aldolase—was performed on whole blood eluted from DBS as described [4]. Antigen detection using anti-PvLDH capture antibodies has previously been shown to be specific for P vivax and not reactive to P malariae or P ovale antigens [5]. The P vivax isolate included in this study as a control was isolated from an Indian patient in February 2021, and prior photo-induced electron transfer polymerase chain reaction (PET-PCR) analysis determined this sample to have a cycle threshold value of 32.69 with P vivax primers.
A second multiplex assay was used to capture immunoglobulin (Ig)G that recognize recombinant merozoite surface protein 1 (MSP1) 19-kD antigens from P falciparum, P vivax, P ovale, and P malariae. The anti-MSP119 IgG capture assay has previously been validated to be species specific and was performed as described previously [6]. This work was reviewed by the US Centers for Disease Control and Prevention (CDC), determined to be nonresearch (program evaluation), and conducted consistent with applicable federal law and CDC policy.
Deoxyribonucleic Acid Extraction and Genotyping
Total deoxyribonucleic acid (DNA) was extracted from a 6-mm DBS punch using the QIAamp Blood Mini Kit (QIAGEN Inc) according to the manufacturer’s protocol. Two PCR speciation assays, PET-PCR [7] and a nested PCR [8], were carried out as described previously. Amplification of pfmsp1, pfmsp2, pfhrp2, pfhrp3 exon 1-2, and exon 2 spanning regions by PCR was conducted as described previously [4]. To confirm consistent results, each PCR was repeated on a different day.
RESULTS
Case Report
On May 18, 2018, a 49-month-old boy arrived at a health center in Masaka, Rwanda, with clinical signs and symptoms suggestive of malaria infection, including fever. No manifestations of severe malaria were detected, and no underlying diseases, malnutrition, allergy to antimalarial medications, or any other ongoing prophylaxis were noted. The patient was determined to be anemic after a capillary blood sample revealed a hemoglobin level of 10.2 mg/dL as measured via HemoCue (HemoCue AB). The patient’s parasite density was determined to be 14200 p/µL blood. He was diagnosed with a P falciparum monoinfection and was enrolled in an ongoing TES and assigned a study identification number of R218. The patient was then admitted to the health center for 3 days of inpatient observation to ensure adherence to the treatment regimen, which comprised 6 doses of artemether-lumefantrine at hours 0, 8, 24, 36, 48, and 60. On day 3, upon completion of treatment, thin and thick smear were again taken and determined to be negative for malaria parasites, and the patient was discharged. As part of the TES protocol [3], the child returned for visits on days 7, 14, 21, and 28 postenrollment to provide additional blood samples for microscopy and DBS, but no infection was detected by microscopy during these visits.
Molecular Analysis and Plasmodium Species Identification
The HRP2-based RDTs are widely used for the diagnosis of P falciparum malaria in sub-Saharan Africa. However, pfhrp2 and pfhrp3 gene deletions in P falciparum allow parasites to evade detection by these RDTs. As part of an ongoing study to investigate the presence of pfhrp2 and pfhrp3 deletions in samples derived from African TESs, all DBS from the 2018 Rwanda TES were subjected to a multiplex antigen detection assay to determine the antigen carriage of P falciparum HRP2/3, P vivax lactate dehydrogenase (PvLDH), and pan-Plasmodium antigens LDH and aldolase.
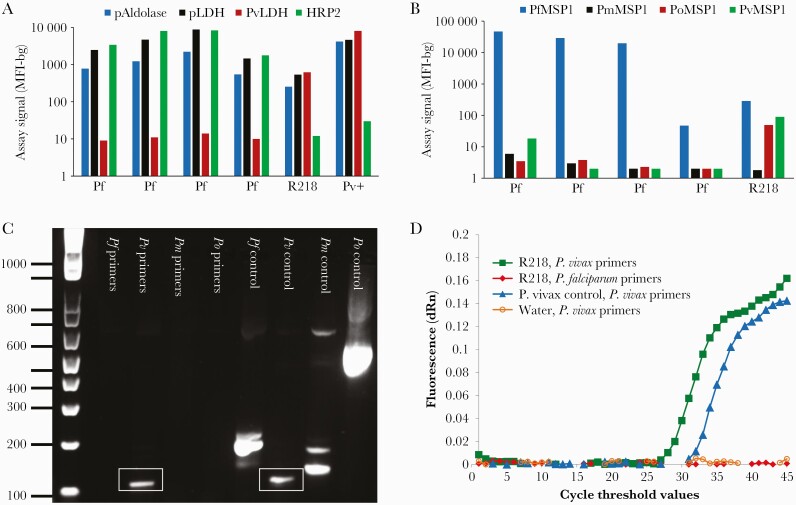
Of the patient samples collected as part of the 2018 Rwanda TES, the blood sample from our patient of interest showed a nonpositive low assay signal for HRP2 but high signals for the pan-Plasmodium antigens and PvLDH (Figure 1A). This profile is consistent with the antigen profile observed for a P vivax isolate obtained from a patient with PCR-confirmed P vivax infection from India. A separate IgG capture multiplex assay utilizing the 4 Plasmodium spp orthologs of the MSP1 19-kD antigen revealed that the same patient’s blood sample was positive for IgG antibodies recognizing the P falciparum, P ovale, and P vivax MSP1 antigens (Figure 1B).
Figure 1.
Detection of Plasmodium vivax antigens, antibodies, and deoxyribonucleic acid (DNA). (A) Bead-based multiplex antigen detection assay for pan-Plasmodium aldolase (pAldolase) and pan-Plasmodium lactate dehydrogenase (pLDH), P vivax lactate dehydrogenase (PvLDH), and Plasmodium falciparum histidine-rich protein 2 (HRP2) using whole blood eluted from dried blood spots. Results from 4 P falciparum infections from Rwanda (Pf) are compared with the Rwandan P vivax patient (R218) and a P vivax isolate obtained from an Indian patient (Pv+). Results are displayed as median fluorescence intensity minus background (MFI-bg) assay signal. (B) Capture of immunoglobulin (Ig)G from blood samples from 4 P falciparum-infected patients and the P vivax-infected patient R218 from the 2018 Rwandan therapeutic efficacy study (TES). Antigens used for IgG capture were merozoite surface protein 19 kD (MSP1) antigens from P falciparum, P malariae, P ovale spp, and P vivax. Data displayed as MFI-bg assay signal. (C) Agarose gel of nested polymerase chain reaction (PCR) products after amplification of DNA from the P vivax patient’s blood using P falciparum (Pf), P vivax (Pv), Plasmodium malariae (Pm), and Plasmodium ovale (Po) 18S primers compared with control DNA amplified with the same primers. Gel products in the box show the amplified P vivax DNA target. (D) Photo-induced electron transfer PCR (PET-PCR) amplification curves from DNA from the P vivax patient’s blood (R218) using P vivax 18S primers are shown in green squares. Controls presented include an Indian P vivax reference strain amplified using the same P vivax primers (blue triangles), the patient R218’s blood amplified using P falciparum primers (red diamonds), and a water nontemplate control amplified with P vivax primers (orange open circles). All PET-PCR curves are shown as the average of 3 replicates.
Due to these antigen and antibody assay results, additional PCR assays were performed on this sample due to suspicion of P vivax infection. Two repeat experiments of a PET-PCR (Figure 1D) and a nested PCR (Figure 1C) speciation assays both revealed the sample to be positive for Plasmodium genus and P vivax DNA only.
In addition to speciation, PCR amplification of pfmsp1, pfmsp2, pfhrp2, pfhrp3 exon 1–2, and exon 2 spanning regions [4] revealed this sample to be negative for all P falciparum genes tested. Genotyping for polymorphisms associated with drug resistance, including P falciparum kelch 13 and multidrug resistance-1 genes, was carried out as part of routine TES surveillance and also revealed a lack of P falciparum genes.
Upon confirmation by PCR that this patient was infected with P vivax, the thin smear obtained at the time of enrollment was re-examined by a WHO Level 1-certified microscopist who confirmed P vivax parasites by morphology as observation revealed that the cytoplasm of the parasite was fragmented and increased, with irregular ameboid appearance characteristic of P vivax.
DISCUSSION
In this study, we report a PCR-confirmed symptomatic P vivax infection for a 49-month-old Rwandan male who was misdiagnosed with P falciparum infection by microscopy and treated with artemether-lumefantrine. Treatment of a P vivax-infected patient without a 14-day course of primaquine puts the patient at risk for a relapse infection weeks to months later. Although this patient returned for weekly monitoring until day 28 postdiagnosis, we cannot be certain whether the child experienced a relapsed infection due to P vivax beyond the last observation date. One limitation of our report is the lack of previous travel history for the patient.
The most recent report of P vivax infections linked to Rwanda is the case of a man who returned to Japan from Rwanda with a P vivax infection in 2005 [1]. The WHO’s malaria country profile for Rwanda shows no P vivax cases in the country dating back to 2000 [9, 10]. A 2015 study that assessed the evidence of P vivax transmission in sub-Saharan Africa found 5 cases of travel-related P vivax infections where Rwanda was considered the probable country of origin but ultimately concluded evidence of P vivax infection in Rwanda to be weak [11]. The CDC’s Morbidity and Mortality Weekly reports of Plasmodium species responsible for malaria infection in persons returning to the United States after travel show only 2 non-PCR-confirmed cases of P vivax imported from Rwanda in 2017 and 2016 [12, 13].
The Duffy blood group antigens are the main invasion receptors on reticulocytes and erythrocytes for P vivax merozoites. In Rwanda, Duffy negativity is highly prevalent, with an estimated frequency of >95% [14]. Because consent for human DNA genotyping was not agreed upon by the patient/guardian at the time of enrollment, the Duffy status of this patient is unknown. However, P vivax can still cause symptomatic malaria in Duffy-negative individuals through the P vivax ligands and host receptor molecules that allow merozoite invasion of Duffy negative erythrocytes [15]. Plasmodium vivax infection of Duffy-negative individuals has been reported across sub-Saharan Africa, including in Mali, Benin, Cameroon, Equatorial Guinea, Kenya, and Angola, all countries with a predominantly Duffy-negative population [15].
CONCLUSIONS
Although confirmatory diagnosis by microscopy or RDT is recommended before initiation of antimalarial treatment [2], malaria microscopists may have difficulty identifying low-parasite density and mixed infections—which would be especially pertinent in a country where multiple Plasmodium species are endemic [16]. In addition, a mixed P falciparum/P vivax clinical infection could be misdiagnosed as P falciparum only if an RDT based on HRP2-only was being used or if a microscopist identified P falciparum parasites and failed to continue monitoring for parasites from other species. Therefore, increased awareness and routine performance evaluation of microscopists in P falciparum predominant areas is beneficial to ensure accurate diagnoses of infections causing clinical disease.
Acknowledgments
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was funded by the U.S. President’s Malaria Initiative and the Atlanta Research and Education Foundation. This research was supported in part by appointments through the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Culleton R, Coban C, Zeyrek FY, et al. The origins of African Plasmodium vivax; insights from mitochondrial genome sequencing. PLoS One 2011; 6:e29137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Guidelines for the Treatment of Malaria . 3rd ed. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 3. Uwimana A, Umulisa N, Venkatesan M, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 2021. doi: 10.1016/S1473-3099(21)00142-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herman C, Huber CS, Jones S, et al. Multiplex malaria antigen detection by bead-based assay and molecular confirmation by PCR shows no evidence of Pfhrp2 and Pfhrp3 deletion in Haiti. Malar J 2019; 18:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogier E, Nace D, Ljolje D, et al. Capture and detection of Plasmodium vivax lactate dehydrogenase in a bead-based multiplex immunoassay. Am J Trop Med Hyg 2020; 102:1064–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Priest JW, Plucinski MM, Huber CS, et al. Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP119 subunit proteins in multiplexed serologic assays. Malar J 2018; 17:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lucchi NW, Narayanan J, Karell MA, et al. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 2013; 8:e56677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA.. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg 1999; 60:687–92. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. WHO Malaria Country Profile - Sierra Leone 2018. Geneva, Switzerland: World Health Organization; 2018. [Google Scholar]
- 10. World Health Organization. WHO Malaria country profile - Rwanda 2013. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 11. Howes RE, Reiner RC Jr., Battle KE, et al. Plasmodium vivax Transmission in Africa. PLoS NeglTrop Dis 2015; 9:e0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mace KE, Lucchi NW, Tan KR.. Malaria surveillance – United States, 2017. MMWR Surveill Summ 2021; 70:1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mace KE, Arguin PM, Lucchi NW, Tan KR.. Malaria surveillance – United States, 2016. MMWR Surveill Summ 2019; 68:1–35. [DOI] [PubMed] [Google Scholar]
- 14. Twohig KA, Pfeffer DA, Baird JK, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS NeglTrop Dis 2019; 13:e0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gunalan K, Sa JM, Moraes Barros RR, et al. Transcriptome profiling of Plasmodium vivax in Saimiri monkeys identifies potential ligands for invasion. Proc Natl Acad Sci USA 2019; 116:7053–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yitbarek T, Nega D, Tasew G, et al. Performance evaluation of malaria microscopists at defense health facilities in Addis Ababa and its surrounding areas, Ethiopia. PLoS One 2016; 11:e0166170. [DOI] [PMC free article] [PubMed] [Google Scholar]